Photosensitive Hydrogel-Based Embolic Agent Treatment of Wide-Necked Aneurysms: Preliminary Animal Results
Abstract
1. Introduction
2. Results
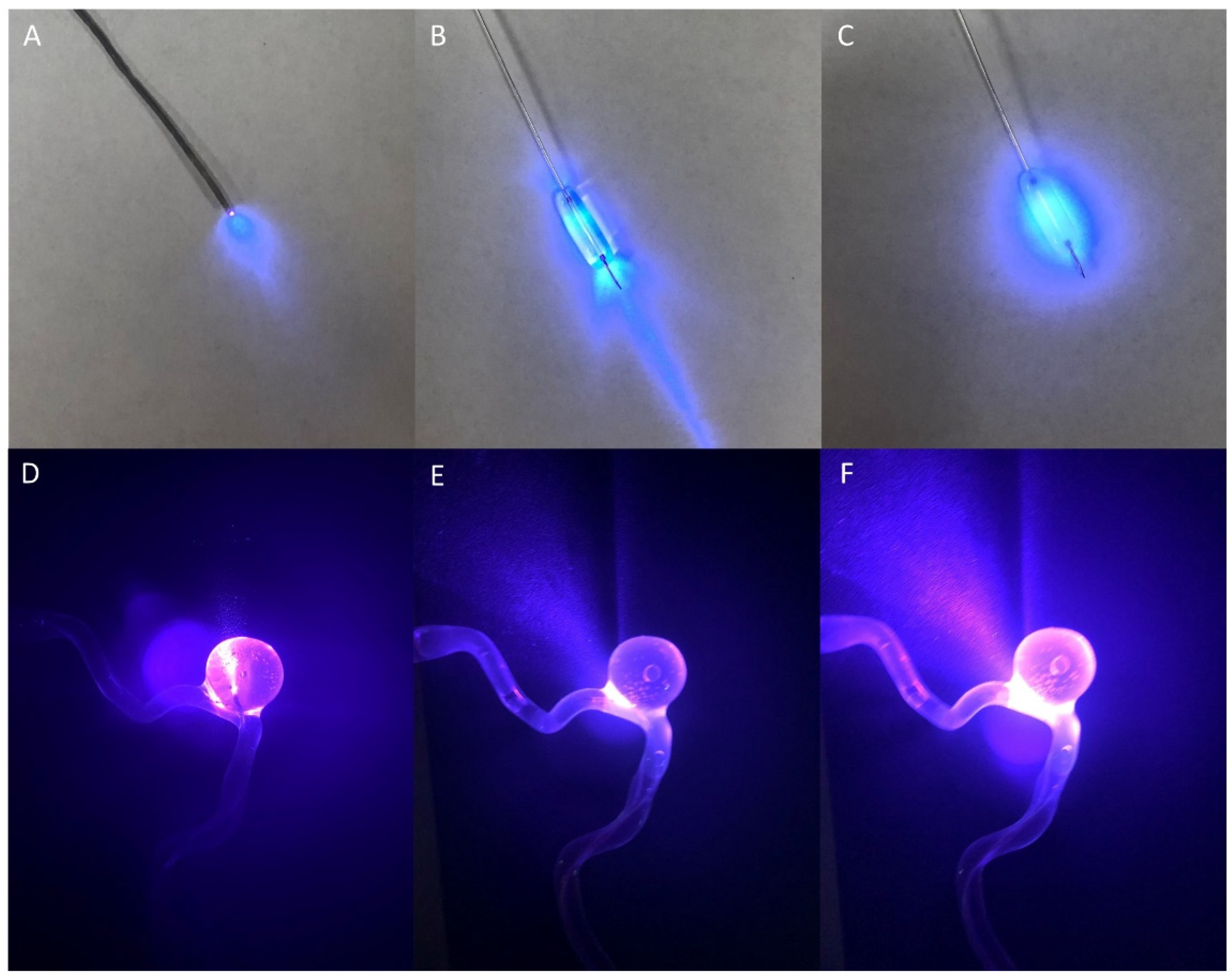
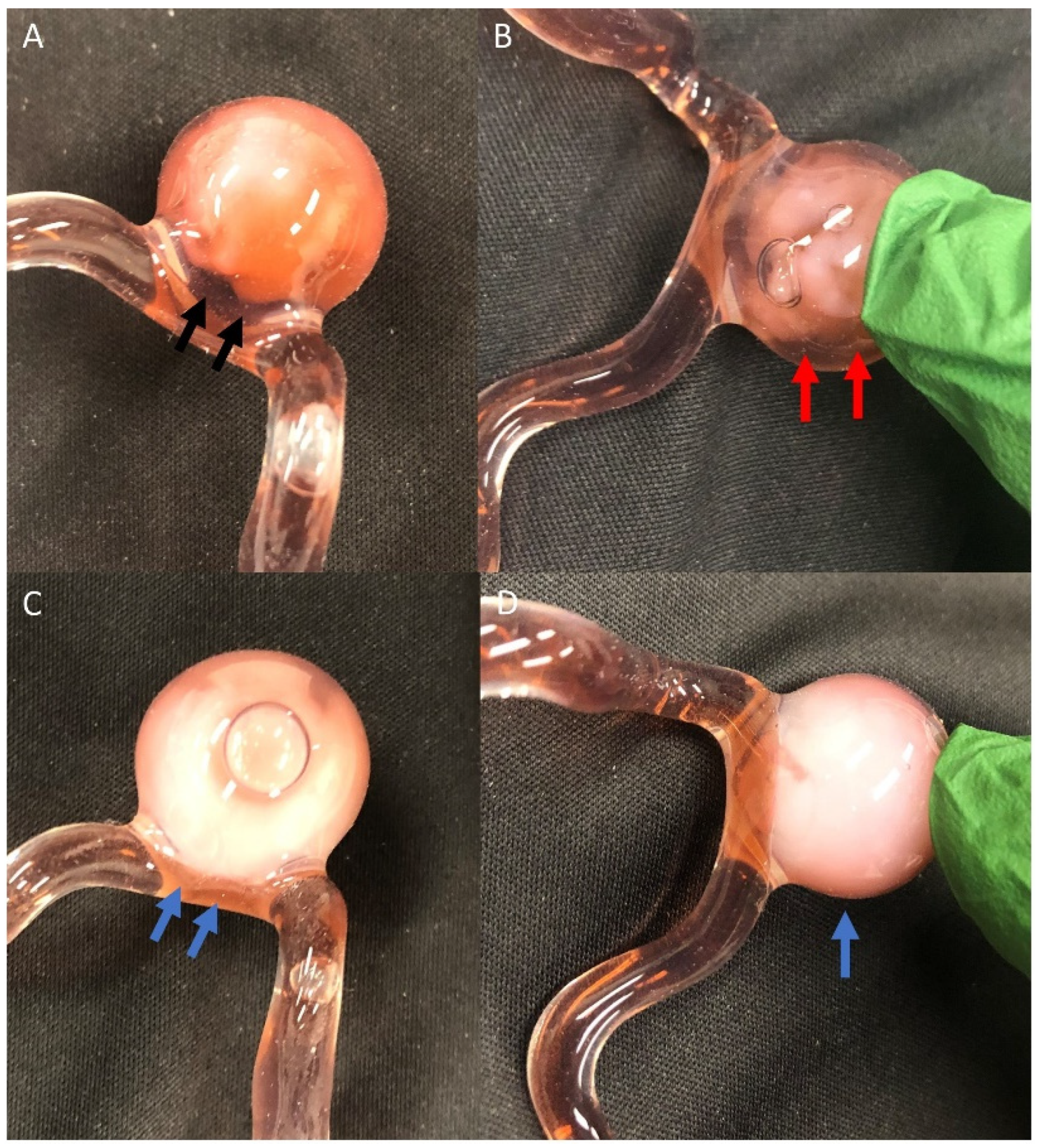
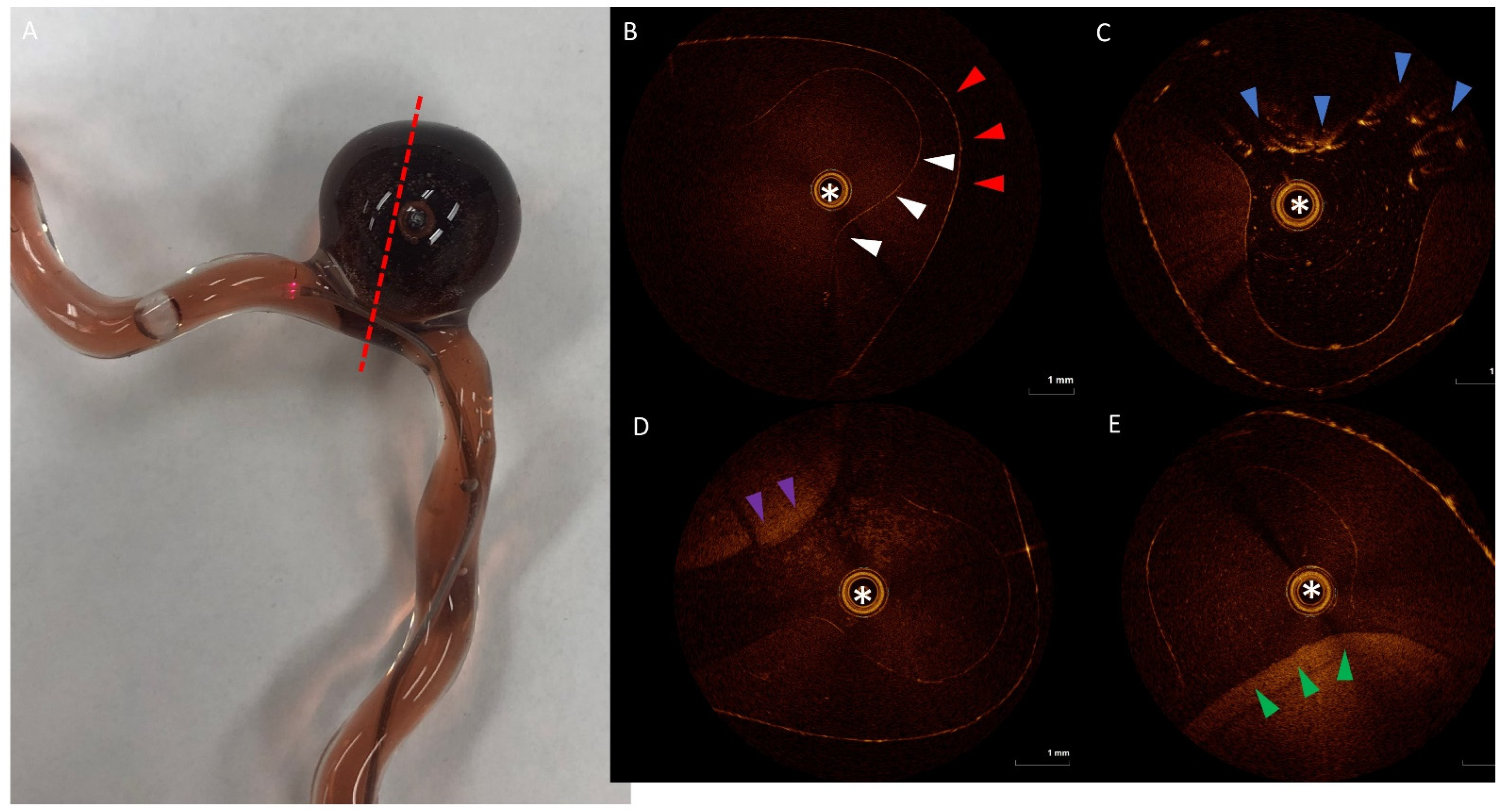
2.1. 3D Model
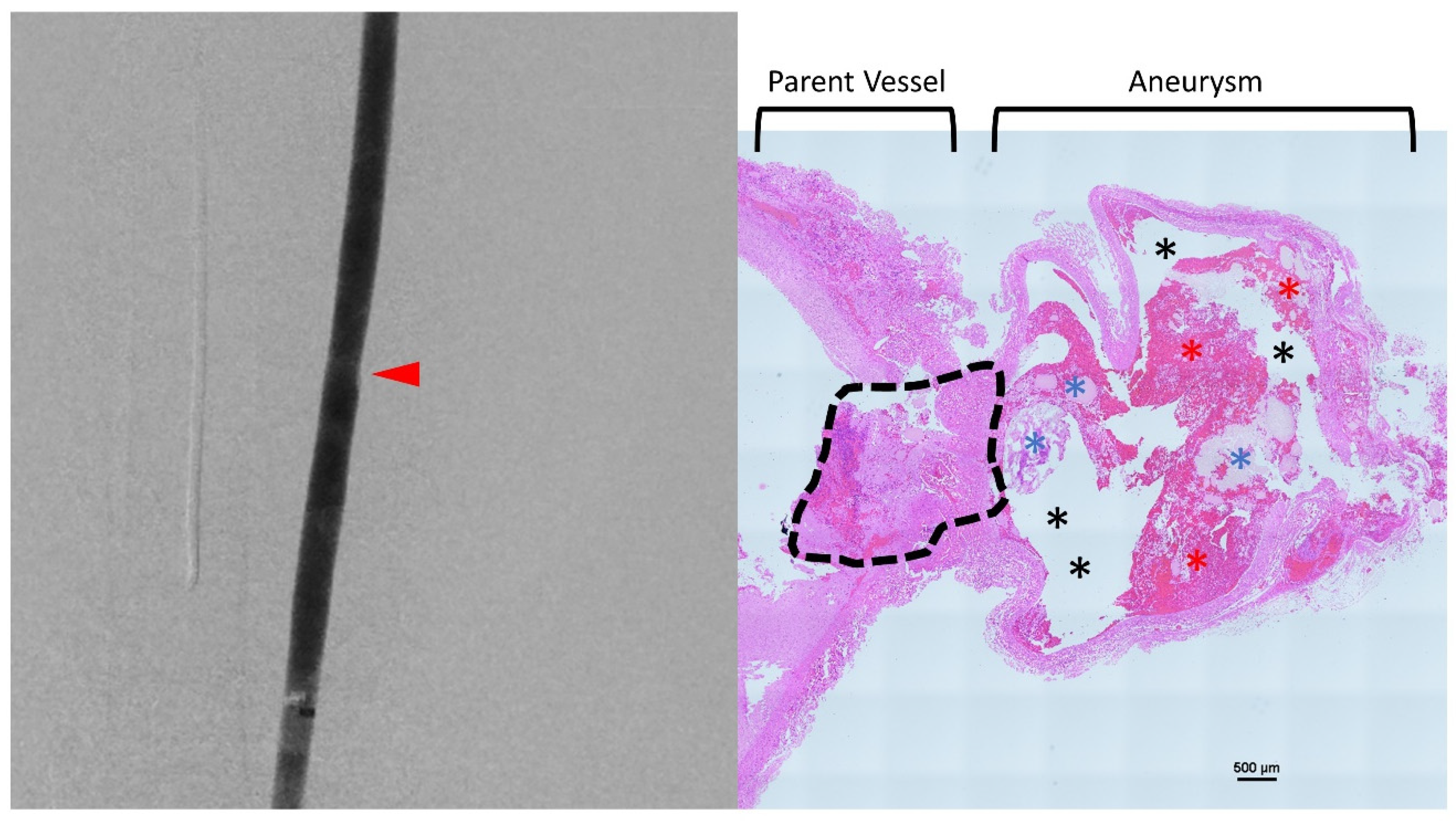
2.2. Animal Model
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
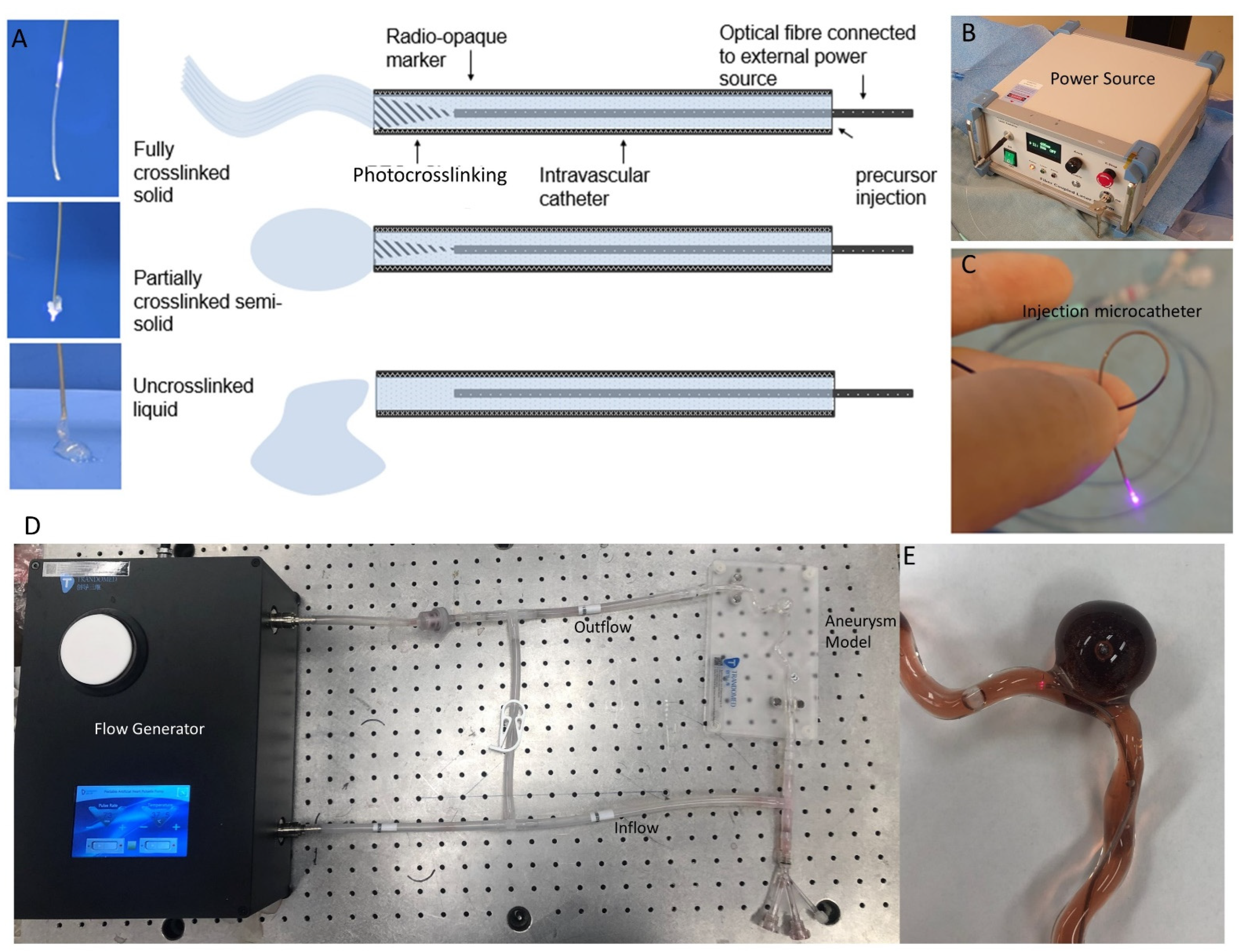
5.2. Novel Embolic Agent and Delivery Strategy
5.3. 3D Model Testing with OCT
5.4. Animal Model Testing
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Disclosures
References
- Macdonald, R.L.; Schweizer, T.A. Spontaneous subarachnoid haemorrhage. Lancet 2017, 389, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, A.J.; Birks, J.; Clarke, A.; Sneade, M.; Kerr, R.S.C. The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18 year follow-up of the UK cohort of the International Subarachnoid Aneurysm Trial (ISAT). Lancet 2015, 385, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lin, H.; Summers, R.; Yang, M.; Cousins, B.G.; Tsui, J. Current Treatment Strategies for Intracranial Aneurysms: An Overview. Angiology 2018, 69, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.; Guilbert, F.; Weill, A.; Georganos, S.A.; Juravsky, L.; Lambert, A.; Lamoureux, J.; Chagnon, M.; Roy, D. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke 2003, 34, 1398–1403. [Google Scholar] [CrossRef]
- Kawanabe, Y.; Sadato, A.; Taki, W.; Hashimoto, N. Endovascular Occlusion of Intracranial Aneurysms with Guglielmi Detachable Coils: Correlation Between Coil Packing Density and Coil Compaction. Acta Neurochir. 2001, 143, 451–455. [Google Scholar] [CrossRef]
- Sluzewski, M.; van Rooij, W.J.; Slob, M.J.; Bescós, J.O.; Slump, C.H.; Wijnalda, D. Relation between aneurysm volume, packing, and compaction in 145 cerebral aneurysms treated with coils. Radiology 2004, 231, 653–658. [Google Scholar] [CrossRef]
- Tamatani, S.; Ito, Y.; Abe, H.; Koike, T.; Takeuchi, S.; Tanaka, R. Evaluation of the stability of aneurysms after embolization using detachable coils: Correlation between stability of aneurysms and embolized volume of aneurysms. AJNR Am. J. Neuroradiol. 2002, 23, 762–767. [Google Scholar]
- Uchiyama, N.; Kida, S.; Nomura, M.; Hasegawa, M.; Yamashima, T.; Yamashita, J.; Matsui, O. Significance of Volume Embolization Ratio as a Predictor of Recanalization on Endovascular Treatment of Cerebral Aneurysms with Guglielmi Detachable Coils. Interv. Neuroradiol. 2000, 6 (Suppl. 1), 59–63. [Google Scholar] [CrossRef]
- Cloft, H.J.; Kallmes, D.F. Aneurysm packing with HydroCoil Embolic System versus platinum coils: Initial clinical experience. AJNR Am. J. Neuroradiol. 2004, 25, 60–62. [Google Scholar]
- Reinges, M.H.T.; Krings, T.; Drexler, A.Y.; Ludolph, A.; Sellhaus, B.; Bovi, M.; Geibprasert, S.; Agid, R.; Scherer, K.; Hans, F.J. Bare, bio-active and hydrogel-coated coils for endovascular treatment of experimentally induced aneurysms. Long-term histological and scanning electron microscopy results. Interv. Neuroradiol. 2010, 16, 139–150. [Google Scholar] [CrossRef]
- Lanzino, G.; Kanaan, Y.; Perrini, P.; Dayoub, H.; Fraser, K. Emerging Concepts in the Treatment of Intracranial Aneurysms: Stents, Coated Coils, and Liquid Embolic Agents. Neurosurgery 2005, 57, 449–459. [Google Scholar] [CrossRef]
- Bendok, B.R.; Abi-Aad, K.R.; Ward, J.D.; Kniss, J.F.; Kwasny, M.J.; Rahme, R.J.; Aoun, S.G.; El Ahmadieh, T.Y.; El Tecle, N.E.; Zammar, S.G.; et al. The Hydrogel Endovascular Aneurysm Treatment Trial (HEAT): A Randomized Controlled Trial of the Second-Generation Hydrogel Coil. Neurosurgery 2020, 86, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Berenstein, A.; Song, J.K.; Niimi, Y.; Namba, K.; Heran, N.S.; Brisman, J.L.; Nahoum, M.C.; Madrid, M.; Langer, D.J.; Kupersmith, M.J. Treatment of cerebral aneurysms with hydrogel-coated platinum coils (HydroCoil): Early single-center experience. AJNR Am. J. Neuroradiol. 2006, 27, 1834–1840. [Google Scholar] [PubMed]
- Marchan, E.M.; Sekula, R.F.; Ku, A.; Williams, R.; O’Neill, B.R.; Wilberger, J.E.; Quigley, M.R. Hydrogel coil–related delayed hydrocephalus in patients with unruptured aneurysms. J. Neurosurg. 2008, 109, 186–190. [Google Scholar] [CrossRef]
- Harker, P.; Regenhardt, R.W.; Alotaibi, N.M.; Vranic, J.; Robertson, F.C.; Dmytriw, A.A.; Ku, J.C.; Koch, M.; Stapleton, C.J.; Leslie-Mazwi, T.M.; et al. The Woven EndoBridge device for ruptured intracranial aneurysms: International multicenter experience and updated meta-analysis. Neuroradiology 2021, 63, 1891–1899. [Google Scholar] [CrossRef] [PubMed]
- Mouchtouris, N.; Hasan, D.; Samaniego, E.A.; Al Saiegh, F.; Sweid, A.; Abbas, R.; El Naamani, K.; Tahir, R.; Zanaty, M.; Khanna, O.; et al. The Woven EndoBridge (WEB) device: Feasibility, techniques, and outcomes after FDA approval. J. Neurosurg. 2022, 136, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Pierot, L.; Gubucz, I.; Buhk, J.H.; Holtmannspötter, M.; Herbreteau, D.; Stockx, L.; Spelle, L.; Berkefeld, J.; Januel, A.C.; Molyneux, A.; et al. Safety and Efficacy of Aneurysm Treatment with the WEB: Results of the WEBCAST 2 Study. AJNR Am. J. Neuroradiol. 2017, 38, 1151–1155. [Google Scholar] [CrossRef]
- Ashour, R.; Ali Aziz-Sultan, M. Onyx HD-500 for embolization of cerebral aneurysms. Neurol. Res. 2014, 36, 363–367. [Google Scholar] [CrossRef]
- Brassel, F.; Meila, D. Evolution of Embolic Agents in Interventional Neuroradiology. Clin. Neuroradiol. 2015, 25 (Suppl. 2), 333–339. [Google Scholar] [CrossRef]
- Cekirge, H.S.; Saatci, I.; Geyik, S.; Yavuz, K.; Öztürk, H.; Pamuk, G. Intrasaccular combination of metallic coils and Onyx liquid embolic agent for the endovascular treatment of cerebral aneurysms. J. Neurosurg. 2006, 105, 706–712. [Google Scholar] [CrossRef]
- Dobashi, Y.; Ku, J.C.; Pasarikovski, C.; Ramjist, J.; Madden, J.D.; Walus, K.; Yang, V.X. Dynamically tunable intravascular catheter delivery of hydrogels for endovascular embolization. MRS Adv. 2021, 6, 66–71. [Google Scholar] [CrossRef]
- Ku, J.C.; Dobashi, Y.; Pasarikovski, C.R.; Ramjist, J.; Madden, J.D.; Walus, K.; Yang, V.X. Dynamic laser-based photomodulation of endovascular hydrogel embolization for the treatment of various cerebrovascular disorders. In Clinical and Translational Neurophotonics 2022; Yang, V.X.D., Kainerstorfer, J.M., Eds.; SPIE: Bellingham, WA, USA, 2022; Volume 7. [Google Scholar] [CrossRef]
- Stavropoulos, S.W.; Kim, H.; Clark, T.W.I.; Fairman, R.M.; Velazquez, O.; Carpenter, J.P. Embolization of Type 2 Endoleaks after Endovascular Repair of Abdominal Aortic Aneurysms with Use of Cyanoacrylate with or without Coils. J. Vasc. Interv. Radiol. 2005, 16, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Vollherbst, D.F.; Chapot, R.; Bendszus, M.; Möhlenbruch, M.A. Glue, Onyx, Squid or PHIL? Liquid Embolic Agents for the Embolization of Cerebral Arteriovenous Malformations and Dural Arteriovenous Fistulas. Clin. Neuroradiol. 2022, 32, 25–38. [Google Scholar] [CrossRef]
- Zanchetta, M.; Faresin, F.; Pedon, L.; Riggi, M.; Ronsivalle, S. Fibrin Glue Aneurysm Sac Embolization at the Time of Endografting. J. Endovasc. Ther. 2005, 12, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Avery, R.K.; Albadawi, H.; Akbari, M.; Zhang, Y.S.; Duggan, M.J.; Sahani, D.V.; Olsen, B.D.; Khademhosseini, A.; Oklu, R. An injectable shear-thinning biomaterial for endovascular embolization. Sci. Transl. Med. 2016, 8, 365ra156. [Google Scholar] [CrossRef] [PubMed]
- Barnett, B.P.; Gailloud, P. Assessment of EmboGel—A Selectively Dissolvable Radiopaque Hydrogel for Embolic Applications. J. Vasc. Interv. Radiol. 2011, 22, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Fatimi, A.; Zehtabi, F.; Lerouge, S. Optimization and characterization of injectable chitosan-iodixanol-based hydrogels for the embolization of blood vessels. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1551–1562. [Google Scholar] [CrossRef]
- Brennecka, C.R.; Preul, M.C.; Bichard, W.D.; Vernon, B.L. In vivo experimental aneurysm embolization in a swine model with a liquid-to-solid gelling polymer system: Initial biocompatibility and delivery strategy analysis. World Neurosurg. 2012, 78, 469–480. [Google Scholar] [CrossRef]
- Poupart, O.; Schmocker, A.; Conti, R.; Moser, C.; Nuss, K.M.; Grützmacher, H.; Mosimann, P.J.; Pioletti, D.P. In vitro Implementation of Photopolymerizable Hydrogels as a Potential Treatment of Intracranial Aneurysms. Front. Bioeng. Biotechnol. 2020, 8, 261. [Google Scholar] [CrossRef]
- Zhou, F.; Chen, L.; An, Q.; Chen, L.; Wen, Y.; Fang, F.; Zhu, W.; Yi, T. Novel Hydrogel Material as a Potential Embolic Agent in Embolization Treatments. Sci. Rep. 2016, 6, 32145. [Google Scholar] [CrossRef]
- Huynh, C.T.; Nguyen, Q.V.; Lym, J.S.; Kim, B.S.; Jae, H.J.; Kim, Y.I.; Lee, D.S. Intraarterial gelation of injectable cationic pH/temperature-sensitive radiopaque embolic hydrogels in a rabbit hepatic tumor model and their potential application for liver cancer treatment. RSC Adv. 2016, 6, 47687–47697. [Google Scholar] [CrossRef]
- Takao, H.; Murayama, Y.; Saguchi, T.; Ishibashi, T.; Ebara, M.; Irie, K.; Yoshioka, H.; Mori, Y.; Ohtsubo, S.; Viñuela, F.; et al. Endovascular Treatment of Experimental Cerebral Aneurysms Using Thermoreversible Liquid Embolic Agents. Interv. Neuroradiol. 2006, 12 (Suppl. 1), 154–157. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.V.; Lee, M.S.; Lym, J.S.; Kim, Y.; Jae, H.J.; Lee, D.S. pH-Sensitive sulfamethazine-based hydrogels as potential embolic agents for transcatheter vascular embolization. J. Mater. Chem. B 2016, 4, 6524–6533. [Google Scholar] [CrossRef] [PubMed]
- Brennecka, C.R.; Preul, M.C.; Becker, T.A.; Vernon, B.L. In vivo embolization of lateral wall aneurysms in canines using the liquid-to-solid gelling PPODA-QT polymer system: 6-month pilot study. J. Neurosurg. 2013, 119, 228–238. [Google Scholar] [CrossRef]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Cowie, A.C. Recent advances in photo-crosslinkable hydrogels for biomedical applications. Biotechniques 2019, 66, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.; Zheng, Z.; Liu, Y.; Ruan, C.; Pan, H.; Zhao, X. A shear-thinning adhesive hydrogel reinforced by photo-initiated crosslinking as a fit-to-shape tissue sealant. J. Mater. Chem. B 2019, 7, 6488–6499. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Choi, G.; Joo, K.; Cha, H.J.; Kim, J. Embolization of Vascular Malformations via In Situ Photocrosslinking of Mechanically Reinforced Alginate Microfibers using an Optical-Fiber-Integrated Microfluidic Device. Adv. Mater. 2021, 33, 2006759. [Google Scholar] [CrossRef]
- Poupart, O.; Conti, R.; Schmocker, A.; Pancaldi, L.; Moser, C.; Nuss, K.M.; Sakar, M.S.; Dobrocky, T.; Grützmacher, H.; Mosimann, P.J.; et al. Pulsatile Flow-Induced Fatigue-Resistant Photopolymerizable Hydrogels for the Treatment of Intracranial Aneurysms. Front. Bioeng. Biotechnol. 2021, 8, 619858. [Google Scholar] [CrossRef]
- Eljamel, S. Photodynamic applications in brain tumors: A comprehensive review of the literature. Photodiagnosis Photodyn. Ther. 2010, 7, 76–85. [Google Scholar] [CrossRef]
- Kaneko, S.; Fujimoto, S.; Yamaguchi, H.; Yamauchi, T.; Yoshimoto, T.; Tokuda, K. Photodynamic Therapy of Malignant Gliomas. Prog. Neurol. Surg. 2018, 32, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ku, J.C.; Dobashi, Y.; Ramjist, J.; Pasarikovski, C.R.; Yang, V.X.D. Optimizing conditions for light delivery for intracranial photodynamic therapy for high grade gliomas: A 3D model study of light dosimetry. In Optical Methods for Tumor Treatment and Detection: Mechanisms and Techniques in Photodynamic and Photobiomodulation Therapy XXX; Kessel, D.H., Hasan, T., Eds.; SPIE: Bellingham, WA, USA, 2022; Volume 37. [Google Scholar] [CrossRef]
- Nakai, K.; Morimoto, Y.; Arai, T.; Ito, H.; Kominami, M.; Matsuo, H.; Kikuchi, M. Application of low-intensity ultraviolet irradiation to the treatment for pharmacological vasoconstriction via a percutaneous transluminal approach. Front. Med. Biol. Eng. 1999, 9, 241–248. [Google Scholar] [PubMed]
- Jenkins, M.P.; Buonaccorsi, G.; MacRobert, A.; Bishop, C.C.R.; Bown, S.G.; McEwan, J.R. Intra-arterial photodynamic therapy using 5-ALA in a swine model. Eur. J. Vasc. Endovasc. Surg. 1998, 16, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Hu, X.; Hamblin, M.R. Ultraviolet blood irradiation: Is it time to remember “the cure that time forgot”? J. Photochem. Photobiol. B 2016, 157, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Brinjikji, W.; Ding, Y.H.; Kallmes, D.F.; Kadirvel, R. From bench to bedside: Utility of the rabbit elastase aneurysm model in preclinical studies of intracranial aneurysm treatment. J. Neurointerv. Surg. 2016, 8, 521–525. [Google Scholar] [CrossRef]
- Bouzeghrane, F.; Naggara, O.; Kallmes, D.F.; Berenstein, A.; Raymond, J. In Vivo Experimental Intracranial Aneurysm Models: A Systematic Review. Am. J. Neuroradiol. 2010, 31, 418–423. [Google Scholar] [CrossRef]
- Thompson, J.W.; Elwardany, O.; McCarthy, D.J.; Sheinberg, D.L.; Alvarez, C.M.; Nada, A.; Snelling, B.M.; Chen, S.H.; Sur, S.; Starke, R.M. In vivo cerebral aneurysm models. Neurosurg. Focus 2019, 47, E20. [Google Scholar] [CrossRef]
- Xu, H.; Casillas, J.; Krishnamoorthy, S.; Xu, C. Effects of Irgacure 2959 and lithium phenyl-2,4,6-trimethylbenzoylphosphinate on cell viability, physical properties, and microstructure in 3D bioprinting of vascular-like constructs. Biomed. Mater. 2020, 15, 055021. [Google Scholar] [CrossRef]
- Ku, J.C.; Pasarikovski, C.R.; Dobashi, Y.; Ramjist, J.; Priola, S.M.; Yang, V.X.D. Review of intraluminal optical coherence tomography imaging for cerebral aneurysms. Front. Photonics 2022, 3, 45. [Google Scholar] [CrossRef]
- Mascitelli, J.R.; Moyle, H.; Oermann, E.K.; Polykarpou, M.F.; Patel, A.A.; Doshi, A.H.; Gologorsky, Y.; Bederson, J.B.; Patel, A.B. An update to the Raymond–Roy Occlusion Classification of intracranial aneurysms treated with coil embolization. J. Neurointerv. Surg. 2015, 7, 496–502. [Google Scholar] [CrossRef]

| Animal | Size of Aneurysm (Height × Width), Neck Width (mm) | Light Irradiation Method | MRRC | Complications |
|---|---|---|---|---|
| Acute experiments: | ||||
| 1 | Aneurysm size 9 × 7 mm, neck width 6 mm | ICL | II | None |
| 2 | 8 × 6, 5 | ICL | II | Non-target embolization |
| 3 | 6 × 7, 7 | BLI | I | None |
| 4 | 8 × 7, 5 | BLI | I | None |
| Subacute experiments: | ||||
| 5 | 10 × 6, 5 | BLI | I | None |
| 6 | 8 × 5, 5 | BLI | I | None |
| 7 | 11 × 7, 6 | BLI | I | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ku, J.C.; Dobashi, Y.; Pasarikovski, C.R.; Ramjist, J.; Hamani, C.; Heyn, C.; Walus, K.; Yang, V.X.D. Photosensitive Hydrogel-Based Embolic Agent Treatment of Wide-Necked Aneurysms: Preliminary Animal Results. Gels 2022, 8, 788. https://doi.org/10.3390/gels8120788
Ku JC, Dobashi Y, Pasarikovski CR, Ramjist J, Hamani C, Heyn C, Walus K, Yang VXD. Photosensitive Hydrogel-Based Embolic Agent Treatment of Wide-Necked Aneurysms: Preliminary Animal Results. Gels. 2022; 8(12):788. https://doi.org/10.3390/gels8120788
Chicago/Turabian StyleKu, Jerry C., Yuta Dobashi, Christopher R. Pasarikovski, Joel Ramjist, Clement Hamani, Chinthaka Heyn, Konrad Walus, and Victor X. D. Yang. 2022. "Photosensitive Hydrogel-Based Embolic Agent Treatment of Wide-Necked Aneurysms: Preliminary Animal Results" Gels 8, no. 12: 788. https://doi.org/10.3390/gels8120788
APA StyleKu, J. C., Dobashi, Y., Pasarikovski, C. R., Ramjist, J., Hamani, C., Heyn, C., Walus, K., & Yang, V. X. D. (2022). Photosensitive Hydrogel-Based Embolic Agent Treatment of Wide-Necked Aneurysms: Preliminary Animal Results. Gels, 8(12), 788. https://doi.org/10.3390/gels8120788







