New Zwitterionic Polymer as a Highly Effective Salt- and Calcium-Resistant Fluid Loss Reducer in Water-Based Drilling Fluids
Abstract
1. Introduction
2. Results and Discussion
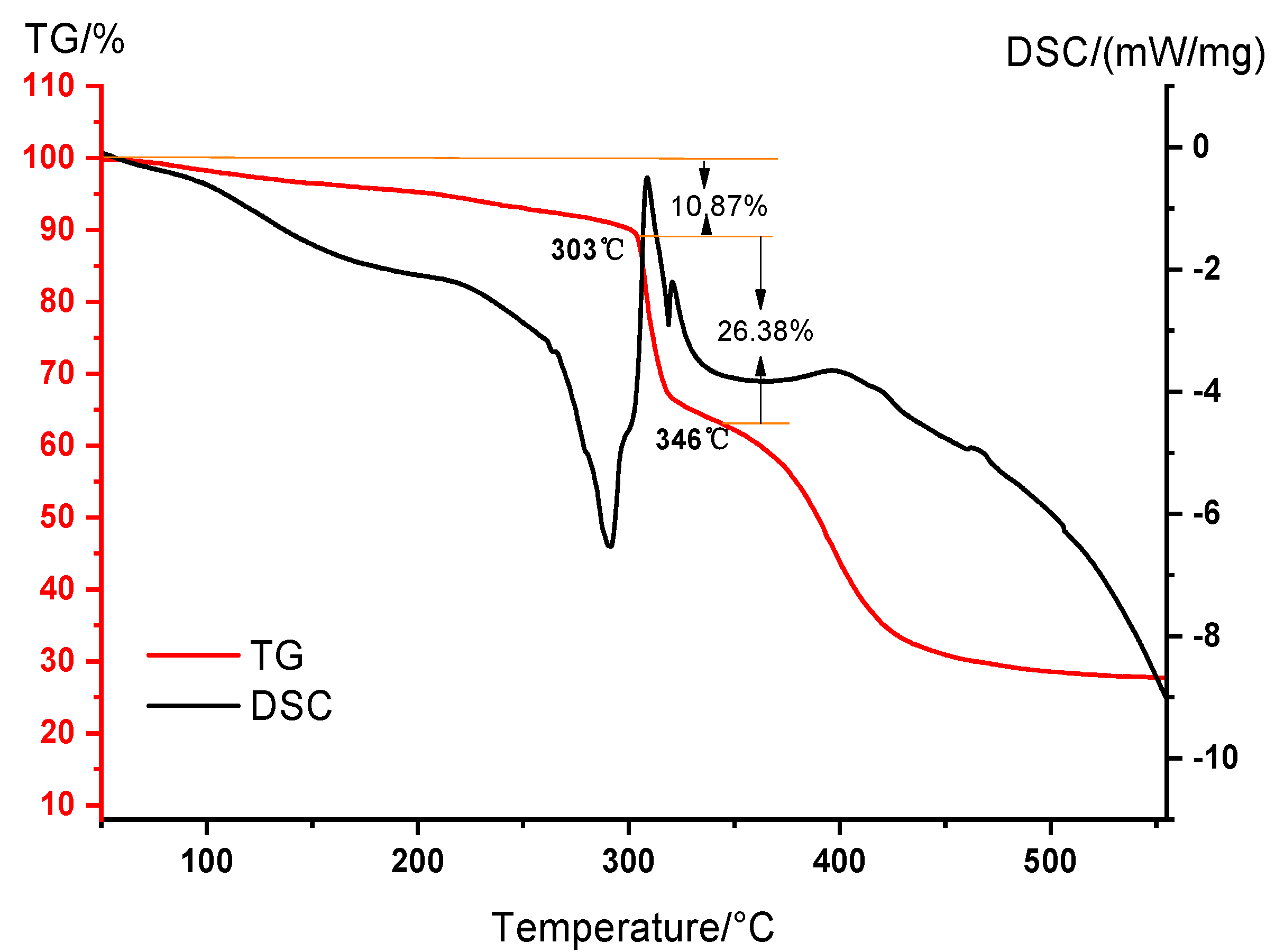
2.1. Characterization of DNDAP Copolymer
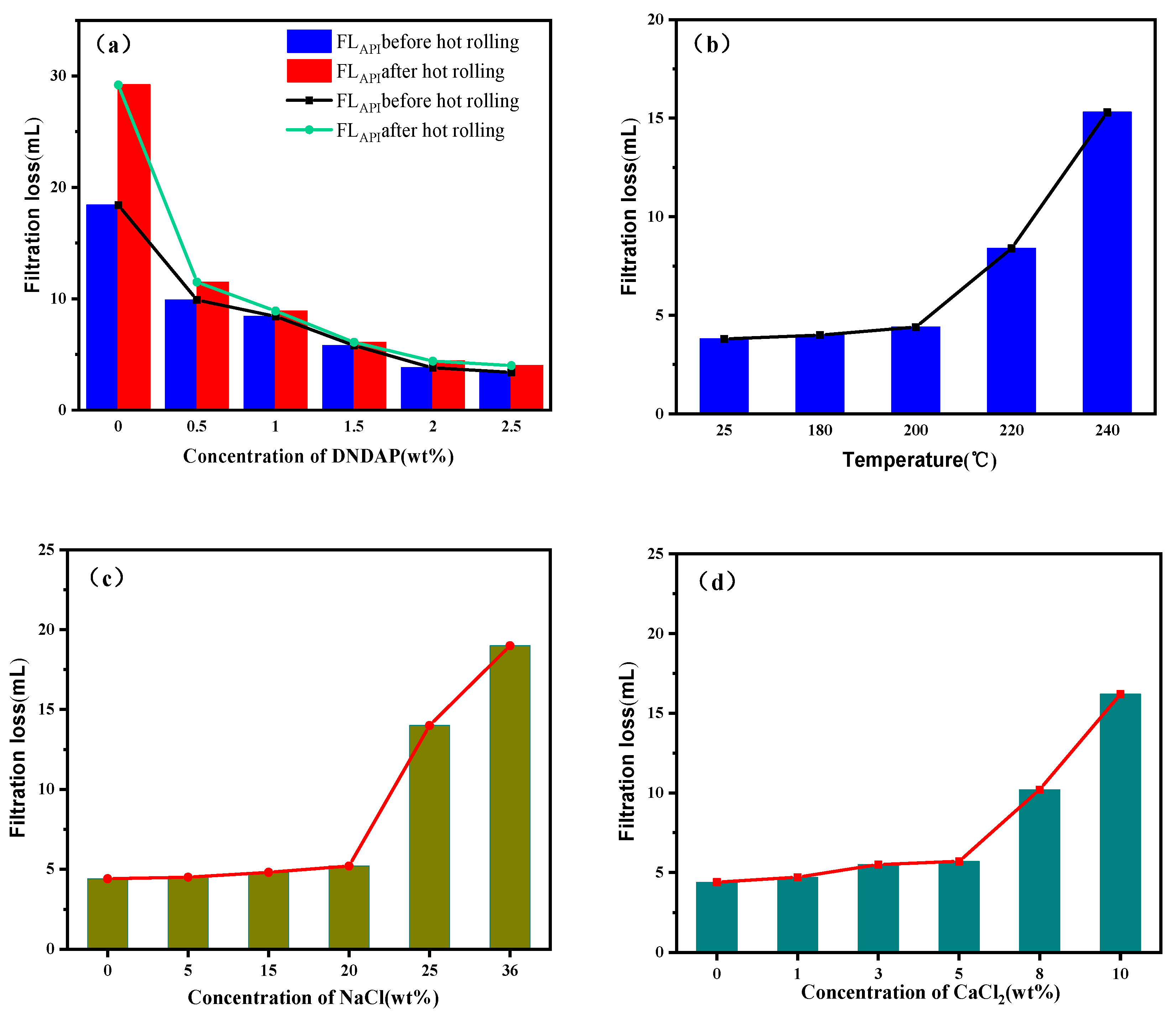
2.2. Filtration Loss Reduction Performance of DNDAP in the WBDF
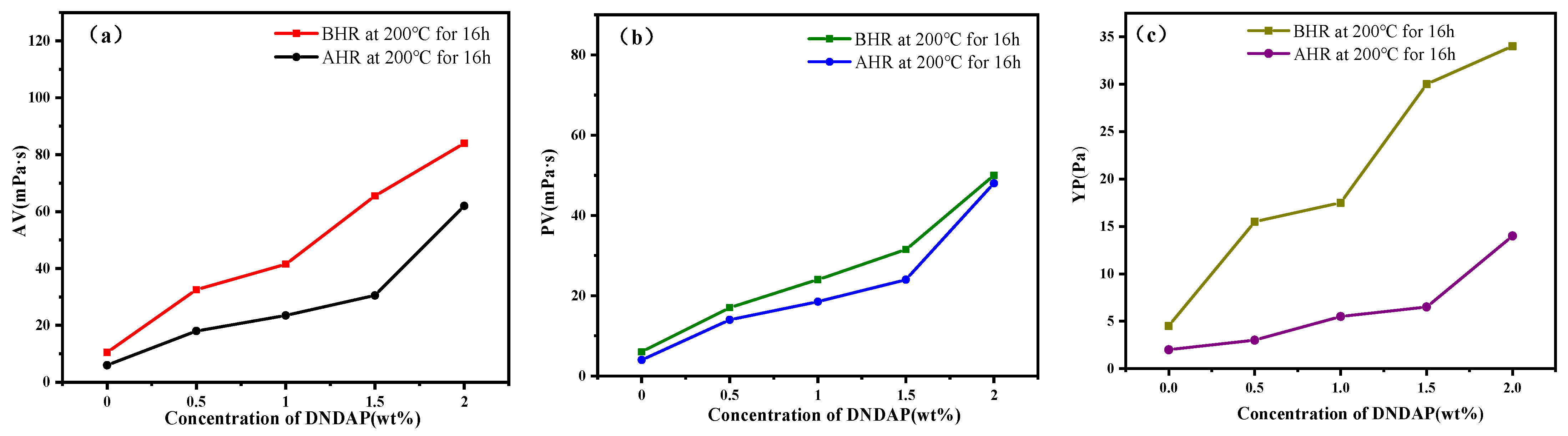
2.3. Rheological Properties of DNDAP-Based Drilling Fluid
2.4. Mechanism Analysis
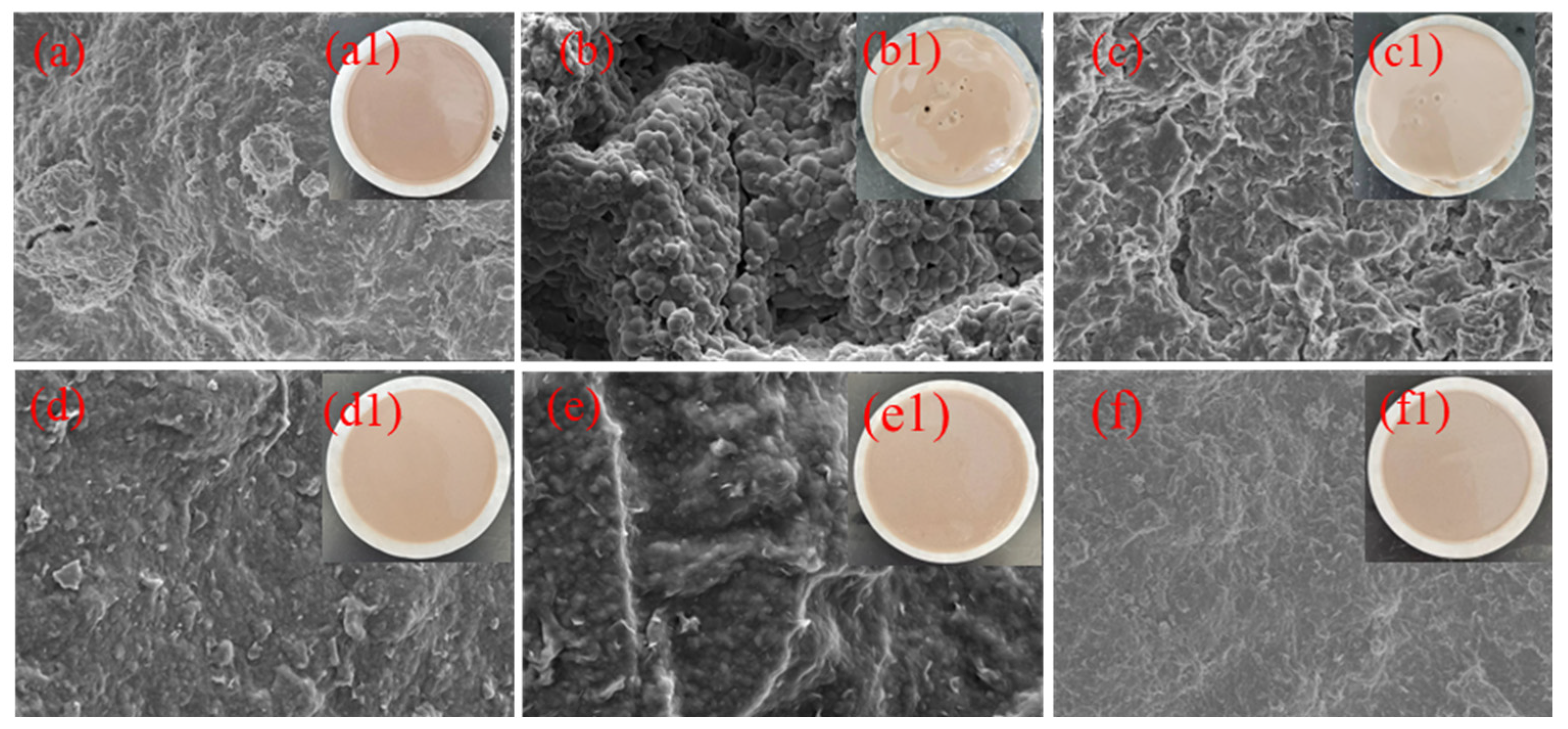
2.4.1. Morphological and Elemental Analysis of Mud Cake Surface
2.4.2. Particle Size Distribution Test
2.4.3. Zeta Potential Test
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Copolymer DNDAP
4.3. Characterization of DNDAP
4.4. Preparation of the WBDF
4.5. Filtration Performance and Rheological Properties of DNDAP/WBDF
4.6. Mechanism Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Apaleke, A.S.; Al-Majed, A.; Hossain, M.E. Drilling fluid: State of the art and future trend. In Proceedings of the North Africa Technical Conference and Exhibition, Cairo, Egypt, 20–22 February 2012. [Google Scholar]
- Bland, R.G.; Mullen, G.A.; Gonzalez, Y.N.; Harvey, F.E.; Pless, M.L. HPHT drilling fluid challenges. In Proceedings of the IADC/SPE Asia Pacific Drilling Technology Conference and Exhibition, Bangkok, Thailand, 13–15 November 2006. [Google Scholar]
- Ferreira, C.C.; Teixeira, G.T.; Lachter, E.R.; Nascimento, R.S.V. Partially hydrophobized hyperbranched polyglycerols as non-ionic reactive shale inhibitors for water-based drilling fluids. Appl. Clay Sci. 2016, 132, 122–132. [Google Scholar] [CrossRef]
- Sepehri, S.; Soleyman, R.; Varamesh, A.; Valizadeh, M.; Nasiri, A. Effect of synthetic water-soluble polymers on the properties of the heavy water-based drilling fluid at high pressure-high temperature (HPHT) conditions. J. Pet. Sci. Eng. 2018, 166, 850–856. [Google Scholar] [CrossRef]
- Tchameni, A.P.; Zhao, L.; Frimpong, I.K.; Nagre, R.D. Investigating the effect of high thermal–saline conditions on the rheological properties of waste vegetable oil biodiesel-based emulsion mud. J. Pet. Explor. Prod. Technol. 2018, 8, 155–164. [Google Scholar] [CrossRef]
- Salami, O.T.; Plank, J. Preparation and properties of a dispersing fluid loss additive based on humic acid graft copolymer suitable for cementing high temperature (200 °C) oil wells. J. Appl. Polym. Sci. 2013, 129, 2544–2553. [Google Scholar] [CrossRef]
- Mao, H.; Qiu, Z.; Shen, Z.; Huang, W. Hydrophobic associated polymer based silica nanoparticles composite with core–shell structure as a filtrate reducer for drilling fluid at utra-high temperature. J. Pet. Sci. Eng. 2015, 129, 1–14. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, T. Hydrocarbon habitat of the offshore Bohai Basin, China. Mar. Pet. Geol. 2004, 21, 691–708. [Google Scholar] [CrossRef]
- Elward-Berry, J.; Darby, J.B. Rheologically stable, nontoxic, high-temperature, water-based drilling fluid. SPE Drill. Complet. 1997, 12, 158–162. [Google Scholar] [CrossRef]
- Ma, Y.; Cai, X.; Yun, L.; Li, Z.; Li, H.; Deng, S.; Zhao, P. Practice and theoretical and technical progress in exploration and development of Shunbei ultra-deep carbonate oil and gas field, Tarim Basin, NW China. Pet. Explor. Dev. 2022, 49, 1–20. [Google Scholar]
- Segad, M.; Jonsson, B.; Åkesson, T.; Cabane, B. Ca/Na montmorillonite: Structure, forces and swelling properties. Langmuir 2010, 26, 5782–5790. [Google Scholar] [CrossRef]
- Ma, X.; Zhu, Z.; Shi, W.; Hu, Y. Synthesis and application of a novel betaine-type copolymer as fluid loss additive for water-based drilling fluid. Colloid Polym. Sci. 2017, 295, 53–66. [Google Scholar] [CrossRef]
- Geng, Y.; Sun, J.; Wang, J.; Wang, R.; Yang, J.; Wang, Q.; Ni, X. Modified Nanopolystyrene as a Plugging Agent for Oil-Based Drilling Fluids Applied in Shale Formation. Energy Fuels 2021, 35, 16543–16552. [Google Scholar] [CrossRef]
- Katende, A.; Boyou, N.V.; Ismail, I.; Chung, D.Z.; Sagala, F.; Hussein, N.; Ismail, M.S. Improving the performance of oil based mud and water based mud in a high temperature hole using nanosilica nanoparticles. Colloids Surf. A 2019, 577, 645–673. [Google Scholar] [CrossRef]
- Akpan, E.U.; Enyi, G.C.; Nasr, G.; Yahaya, A.A.; Ahmadu, A.A.; Saidu, B. Water-based drilling fluids for high-temperature applications and water-sensitive and dispersible shale formations. J. Pet. Sci. Eng. 2019, 175, 1028–1038. [Google Scholar] [CrossRef]
- Saleh, T.A. Advanced trends of shale inhibitors for enhanced properties of water-based drilling fluid. Upstream Oil Gas Technol. 2022, 8, 100069. [Google Scholar] [CrossRef]
- Dias, F.T.G.; Souza, R.R.; Lucas, E.F. Influence of modified starches composition on their performance as fluid loss additives in invert-emulsion drilling fluids. Fuel 2015, 140, 711–716. [Google Scholar] [CrossRef]
- Warren, B.; van der Horst, P.; Stewart, W. Application of amphoteric cellulose ethers in drilling fluids. In Proceedings of the International Symposium on Oilfield Chemistry, Houston, TX, USA, 5–7 February 2003. [Google Scholar]
- Menezes, R.R.; Marques, L.N.; Campos, L.A.; Ferreira, H.S.; Santana LN, L.; Neves, G.A. Use of statistical design to study the influence of CMC on the rheological properties of bentonite dispersions for water-based drilling fluids. Appl. Clay Sci. 2010, 49, 13–20. [Google Scholar] [CrossRef]
- Chang, X.; Sun, J.; Xu, Z.; Zhang, F.; Wang, J.; Lv, K.; Dai, Z. A novel nano-lignin-based amphoteric copolymer as fluid-loss reducer in water-based drilling fluids. Colloids Surf. A 2019, 583, 123979. [Google Scholar] [CrossRef]
- Li, M.C.; Wu, Q.; Song, K.; Lee, S.; Jin, C.; Ren, S.; Lei, T. Soy protein isolate as fluid loss additive in bentonite–water-based drilling fluids. ACS Appl. Mater. Interfaces 2015, 7, 24799–24809. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Y.; Ding, M.; Fan, X.; Hu, J.; Chen, Y.; Li, J.; Li, Z.; Liu, W. A 4arm-PEG macromolecule crosslinked chitosan hydrogels as antibacterial wound dressing. Carbohydr. Polym. 2022, 77, 118871. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, R.; Zhang, L.; Guan, X. Molecular insights into the adsorption of chloride ions in calcium silicate hydrate gels: The synergistic effect of calcium to silicon ratio and sulfate ion. Microporous Mesoporous Mater. 2022, 345, 112248. [Google Scholar] [CrossRef]
- Shi, T.; Liu, Y.; Zhao, X.; Wang, J.; Zhao, Z.; Corr, D.J.; Shah, S.P. Study on mechanical properties of the interfacial transition zone in carbon nanofiber-reinforced cement mortar based on the PeakForce tapping mode of atomic force microscope. J. Build. Eng. 2022, 61, 105248. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Lu, J.; Ding, M.; Chen, Y. Synthesis and properties of poly (vinyl alcohol) hydrogels with high strength and toughness. Polym. Test. 2022, 108, 107516. [Google Scholar] [CrossRef]
- Li, X.; Jiang, G.; He, Y.; Chen, G. Novel starch composite fluid loss additives and their applications in environmentally friendly water-based drilling fluids. Energy Fuels 2021, 35, 2506–2513. [Google Scholar] [CrossRef]
- Shan, W.; Ma, J.; Jiang, G.; Sun, J.; An, Y. An Inverse Emulsion Polymer as a Highly Effective Salt-and Calcium-Resistant Fluid Loss Reducer in Water-Based Drilling Fluids. ACS Omega 2022, 7, 16141–16151. [Google Scholar] [CrossRef]
- Liu, F.; Jiang, G.; Peng, S.; He, Y.; Wang, J. Amphoteric polymer as an anti-calcium contamination fluid-loss additive in water-based drilling fluids. EnergyFuels 2016, 30, 7221–7228. [Google Scholar] [CrossRef]
- O’Bryan, G.; Wong, B.M.; McElhanon, J.R. Stress sensing in polycaprolactone films via an embedded photochromic compound. ACS Appl. Mater. Interfaces 2010, 2, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Minkin, V.I. Photo-, thermo-, solvato-, and electrochromic spiroheterocyclic compounds. Chem. Rev. 2004, 104, 2751–2776. [Google Scholar] [CrossRef]
- Wu, Y.M.; Zhang, B.Q.; Wu, T.; Zhang, C.G. Properties of the forpolymer of N-vinylpyrrolidone with itaconic acid, acrylamide and 2-acrylamido-2-methyl-1-propane sulfonic acid as a fluid-loss reducer for drilling fluid at high temperatures. Colloid Energy Polym. Sci. 2001, 279, 836–842. [Google Scholar] [CrossRef]
- Wang, G.; Jiang, G.; Yang, J.; Yang, L.; Li, X.; He, Y.; Chang, X. Novel N, N-dimethylacrylamide copolymer containing multiple rigid comonomers as a filtrate reducer in water-based drilling fluids and mechanism study. J. Appl. Polym. Sci. 2021, 138, 51001. [Google Scholar] [CrossRef]
- Tchameni, A.P.; Xie, B.; Liu, W.; Li, Y.; Zhao, L.; Luo, M. Amphoteric tetramer as a filtration-loss reducer in low-solid phase water-based drilling fluids under high thermal-saline conditions. J. Dispersion Sci. Technol. 2021, 42, 920–933. [Google Scholar] [CrossRef]
- Tiemeyer, C.; Plank, J. Working mechanism of a high temperature (200 °C) synthetic cement retarder and its interaction with an AMPS®-based fluid loss polymer in oil well cement. J. Appl. Polym. Sci. 2012, 124, 4772–4781. [Google Scholar] [CrossRef]
- Bai, X.; Yang, Y.; Xiao, D.; Pu, X.; Wang, X. Synthesis, characterization, and performance evaluation of the AM/AMPS/DMDAAC/SSS quadripolymer as a fluid loss additive for water-based drilling fluid. J. Appl. Polym. Sci. 2015, 132, 41762. [Google Scholar] [CrossRef]
- Liu, L.; Sun, J.; Wang, R.; Qu, Y.; Liu, F.; Yang, J.; Huang, H. Synthesis of a new high temperature and salt resistant zwitterionic filtrate reducer and its application in water-based drilling fluid. Colloids Surf. A 2022, 651, 129730. [Google Scholar] [CrossRef]
- Liu, L.; Pu, X.; Tao, H.; Deng, Q.; Luo, A. Synthesis and characterization of comb-shaped copolymer as a filtration reducer and comparison with counterparts. RSC Adv. 2018, 8, 11424–11435. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Pu, X.; Rong, K.; Yang, Y. Comb-shaped copolymer as filtrate loss reducer for water-based drilling fluid. J. Appl. Polym. Sci. 2018, 135, 45989. [Google Scholar] [CrossRef]
- Yang, J.; Sun, J.; Wang, R.; Zhao, Z. Laponite-polymer composite as a rheology modifier and filtration loss reducer for water-based drilling fluids at high temperature. Colloids Surf. A 2022, 655, 130261. [Google Scholar] [CrossRef]



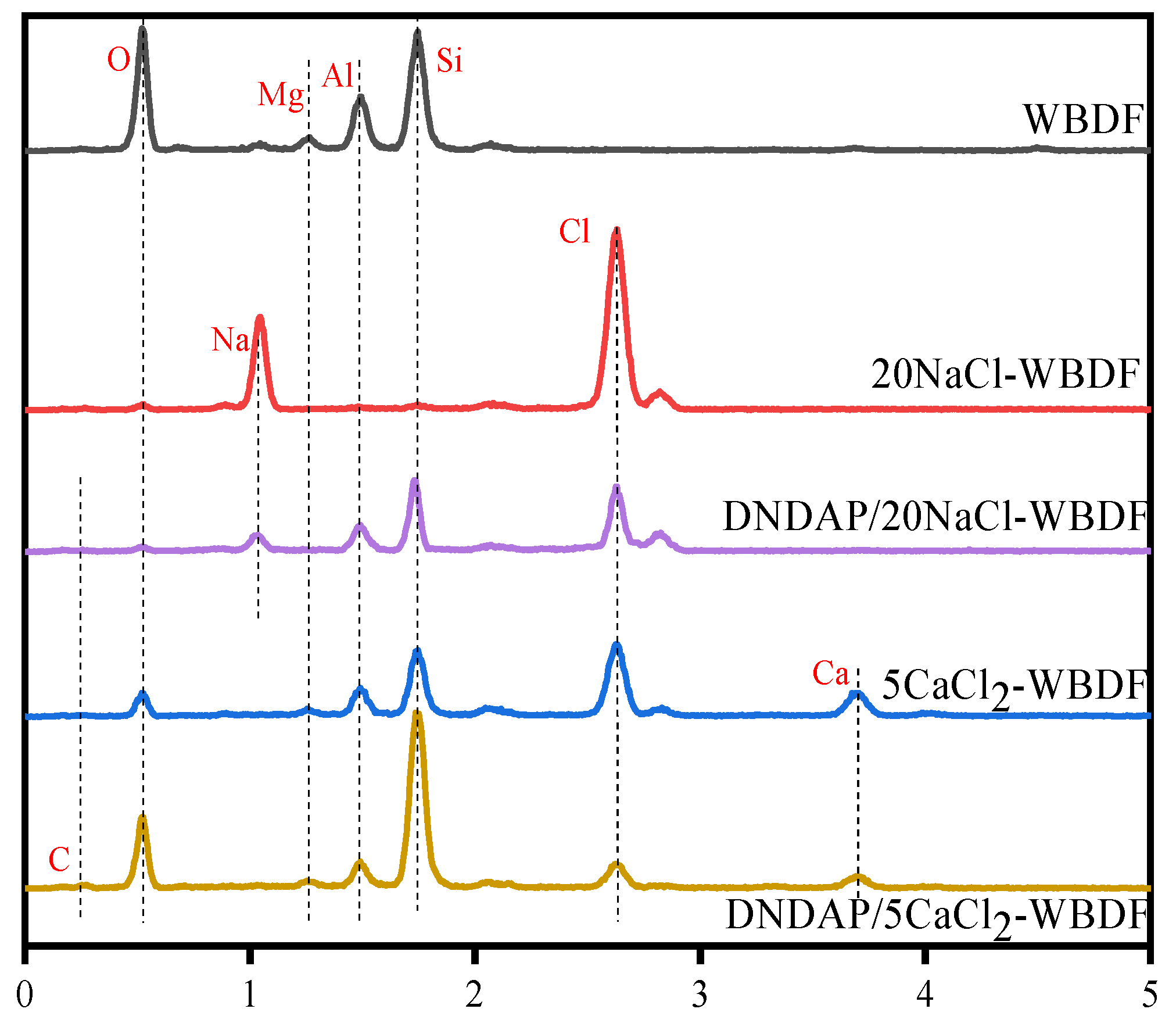

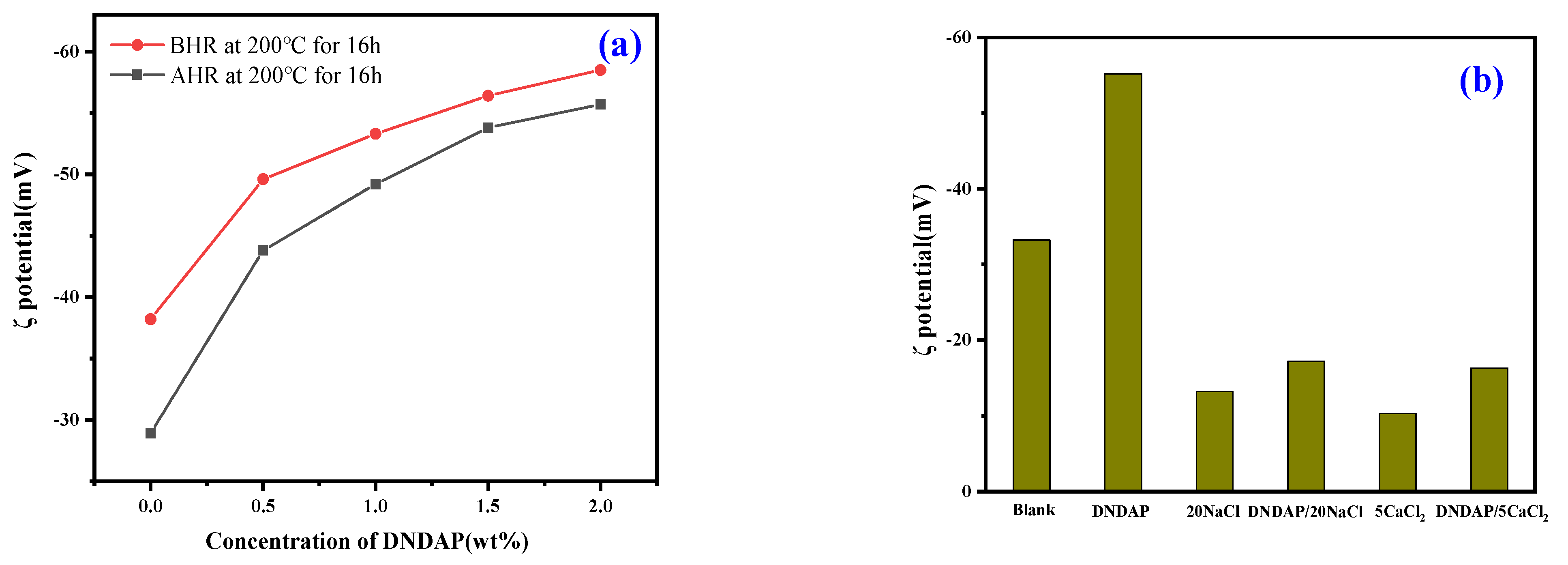

| C (Bentonite) (wt%) | C (Na+) (wt%) | C (Ca2+) (wt%) | T (℃) | FLAPI (mL) |
|---|---|---|---|---|
| 4 | 0 | 0 | 25 | 18.4 |
| 200 | 29.2 | |||
| 20 | 0 | 25 | 84.6 | |
| 200 | 138.2 | |||
| 0 | 5 | 25 | 57.6 | |
| 200 | 143.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Sun, J.; Wang, R.; Liu, F.; Gao, S.; Yang, J.; Ren, H.; Qu, Y.; Cheng, R.; Geng, Y.; et al. New Zwitterionic Polymer as a Highly Effective Salt- and Calcium-Resistant Fluid Loss Reducer in Water-Based Drilling Fluids. Gels 2022, 8, 735. https://doi.org/10.3390/gels8110735
Liu L, Sun J, Wang R, Liu F, Gao S, Yang J, Ren H, Qu Y, Cheng R, Geng Y, et al. New Zwitterionic Polymer as a Highly Effective Salt- and Calcium-Resistant Fluid Loss Reducer in Water-Based Drilling Fluids. Gels. 2022; 8(11):735. https://doi.org/10.3390/gels8110735
Chicago/Turabian StyleLiu, Luman, Jinsheng Sun, Ren Wang, Fan Liu, Shifeng Gao, Jie Yang, Han Ren, Yuanzhi Qu, Rongchao Cheng, Yuan Geng, and et al. 2022. "New Zwitterionic Polymer as a Highly Effective Salt- and Calcium-Resistant Fluid Loss Reducer in Water-Based Drilling Fluids" Gels 8, no. 11: 735. https://doi.org/10.3390/gels8110735
APA StyleLiu, L., Sun, J., Wang, R., Liu, F., Gao, S., Yang, J., Ren, H., Qu, Y., Cheng, R., Geng, Y., & Feng, Z. (2022). New Zwitterionic Polymer as a Highly Effective Salt- and Calcium-Resistant Fluid Loss Reducer in Water-Based Drilling Fluids. Gels, 8(11), 735. https://doi.org/10.3390/gels8110735





