Herbal Fennel Essential Oil Nanogel: Formulation, Characterization and Antibacterial Activity against Staphylococcus aureus
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Characterization of Nanoparticles
2.1.1. Shape, Particle Size, Polydispersity Index and Zeta Potential
2.1.2. Drug Entrapment Efficiency (DEE%) of Nanoparticles
2.2. Characterization of FEO–PLGANPs Gel
2.2.1. Rheological Study of FEO–PLGANPs Gel
2.2.2. Drug Release Studies of FEO–PLGANPs Gel
2.2.3. In Vitro Cell Viability Assay
2.2.4. In Vitro Antibacterial Activity
2.2.5. Time-Kill Assays
2.2.6. Stability Study
3. Conclusions
4. Materials and Methods
4.1. Methods
Preparation of Nanoparticles
4.2. Physicochemical Characterization of Nanoparticles
4.2.1. Morphology of Nanoparticles
4.2.2. Particle Size, Polydispersity Index and Surface Charge of Nanoparticles
4.2.3. Drug Entrapment Efficiency (DEE%) of Nanoparticles
4.3. Preparation and Characterization of Hydrogel
Preparation of FEO–PLGANPs Gel
4.4. Characterization of FEO–PLGANPs Gel
4.4.1. Rheological Study of FEO–PLGANPs Gel
4.4.2. Drug Release Studies of FEO–PLGANPs Gel
4.4.3. Drug Delivery Kinetics
4.4.4. In Vitro Cell Viability Assay
4.4.5. In Vitro Antibacterial Activity
4.4.6. Time-Kill Kinetics Assay
4.4.7. Stability Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mei, L.; Zhu, S.; Yin, W.; Chen, C.; Nie, G.; Gu, Z.; Zhao, Y. Two-dimensional nanomaterials beyond graphene for antibacterial applications: Current progress and future perspectives. Theranostics 2020, 10, 757–781. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Nazeam, J.A.; Ragab, G.M.; El-Gazar, A.A.; El-Mancy, S.S.; Jamil, L.; Fayez, S.M. Topical Nano Clove/Thyme Gel against Genetically Identified Clinical Skin Isolates: In Vivo Targeting Behavioral Alteration and IGF-1/pFOXO-1/PPAR γ Cues. Molecules 2021, 26, 5608. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Zhang, F.; Cheng, W. The Mechanism of Bacterial Resistance and Potential Bacteriostatic Strategies. Antibiotics 2022, 11, 1215. [Google Scholar] [CrossRef]
- de Kraker, M.E.A.; Stewardson, A.J.; Harbarth, S. Will 10 Million People Die a Year due to Antimicrobial Resistance by 2050? PLoS Med. 2016, 13, e1002184. [Google Scholar] [CrossRef]
- Kang, S.-J.; Nam, S.H.; Lee, B.-J. Engineering Approaches for the Development of Antimicrobial Peptide-Based Antibiotics. Antibiotics 2022, 11, 1338. [Google Scholar] [CrossRef]
- Gill, A.A.S.; Singh, S.; Thapliyal, N.; Karpoormath, R. Nanomaterial-based optical and electrochemical techniques for detection of methicillin-resistant Staphylococcus aureus: A review. Mikrochim. Acta 2019, 186, 114. [Google Scholar] [CrossRef]
- Sun, J.; Uchiyama, S.; Olson, J.; Morodomi, Y.; Cornax, I.; Ando, N.; Kohno, Y.; Kyaw, M.M.T.; Aguilar, B.; Haste, N.M.; et al. Repurposed drugs block toxin-driven platelet clearance by the hepatic Ashwell-Morell receptor to clear Staphylococcus aureus bacteremia. Sci. Transl. Med. 2021, 13, eabd6737. [Google Scholar] [CrossRef]
- Singh, S.; Numan, A.; Somaily, H.H.; Gorain, B.; Ranjan, S.; Rilla, K.; Siddique, H.R.; Kesharwani, P. Nano-enabled strategies to combat methicillin-resistant Staphylococcus aureus. Mater. Sci. Eng. C 2021, 129, 112384. [Google Scholar] [CrossRef]
- Majumdar, S.; Srirangam, R. Solubility, Stability, Physicochemical Characteristics and In Vitro Ocular Tissue Permeability of Hesperidin: A Natural Bioflavonoid. Pharm. Res. 2009, 26, 1217–1225. [Google Scholar] [CrossRef]
- Sfeir, J.; Lefrançois, C.; Baudoux, D.; Derbré, S.; Licznar, P. In Vitro Antibacterial Activity of Essential Oils against Streptococcus pyogenes. Evid.-Based Complement. Altern. Med. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Miladinović, D.L.; Dimitrijević, M.V.; Mihajilov-Krstev, T.M.; Marković, M.S.; Ćirić, V.M. The significance of minor components on the antibacterial activity of essential oil via chemometrics. LWT 2020, 136, 110305. [Google Scholar] [CrossRef]
- Manion, C.R.; Widder, R.M. Essentials of essential oils. Am. J. Heal. Pharm. 2017, 74, e153–e162. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, N.; Kumari, A.; Raina, N.; Zakir, F.; Gupta, M. Prospects of essential oil loaded nanosystems for skincare. Phytomedicine Plus 2021, 2, 100198. [Google Scholar] [CrossRef]
- Anwar, F.; Ali, M.; Hussain, A.I.; Shahid, M. Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare Mill.) seeds from Pakistan. Flavour Fragr. J. 2009, 24, 170–176. [Google Scholar] [CrossRef]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.-H.; Khalel, K.I. Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare L.) and chamomile (Matricaria chamomilla L.). Ind. Crop. Prod. 2013, 44, 437–445. [Google Scholar] [CrossRef]
- Mutlu-Ingok, A.; Catalkaya, G.; Capanoglu, E.; Karbancioglu-Guler, F. Antioxidant and antimicrobial activities of fennel, ginger, oregano and thyme essential oils. Food Front. 2021, 2, 508–518. [Google Scholar] [CrossRef]
- Trifan, A.; Luca, S.V.; Greige-Gerges, H.; Miron, A.; Gille, E.; Aprotosoaie, A.C. Recent advances in tackling microbial multidrug resistance with essential oils: Combinatorial and nano-based strategies. Crit. Rev. Microbiol. 2020, 46, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, K.; Ivanova, A.; Ramon, E.; Hoyo, J.; Sanchez-Gomez, S.; Tzanov, T. Antibody-Enabled Antimicrobial Nanocapsules for Selective Elimination of Staphylococcus aureus. ACS Appl. Mater. Interfaces 2020, 12, 35918–35927. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Musaddiq, H.; Shahzad, M.I. Anti MRSA and Antiviral Evaluation of Nano Silver Against Avian Influenza virus. Front. Chem. Sci. 2020, 1, 59–66. [Google Scholar] [CrossRef]
- Rahimzadeh, G.; Gill, P.; Rezai, M.S. Cysteine/Histidine-Dependent Amidohydrolase/Peptidase (CHAP)-Displayed Nano Phages: Antimicrobial Function against Methicillin-Resistant Staphylococcus aureus (MRSA). Avicenna J. Med. Biotechnol. 2020, 12, 85–90. [Google Scholar] [PubMed]
- Hibbitts, A.; O’Leary, C. Emerging Nanomedicine Therapies to Counter the Rise of Methicillin-Resistant Staphylococcus aureus. Materials 2018, 11, 321. [Google Scholar] [CrossRef] [PubMed]
- Saqib, S.; Munis, M.F.H.; Zaman, W.; Ullah, F.; Shah, S.N.; Ayaz, A.; Farooq, M.; Bahadur, S. Synthesis, characterization and use of iron oxide nano particles for antibacterial activity. Microsc. Res. Tech. 2018, 82, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Siyadatpanah, A.; Norouzi, R.; Mirzaei, F.; Haghirosadat, B.F.; Nissapatorn, V.; Mitsuwan, W.; Nawaz, M.; Pereira, M.L.; Hosseini, S.A.; Montazeri, M.; et al. Green synthesis of nano-liposomes containing Bunium persicum and Trachyspermum ammi essential oils against Trichomonas vaginalis. J. Microbiol. Immunol. Infect. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) As biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Rezvantalab, S.; Keshavarz Moraveji, M. Microfluidic assisted synthesis of PLGA drug delivery systems. RSC Adv. 2019, 9, 2055–2072. [Google Scholar] [CrossRef]
- Zirak, N.; Maadani, A.; Salahinejad, E.; Abbasnezhad, N.; Shirinbayan, M. Fabrication, drug delivery kinetics and cell viability assay of PLGA-coated vancomycin-loaded silicate porous microspheres. Ceram. Int. 2021, 48, 48–54. [Google Scholar] [CrossRef]
- Maji, R.; Omolo, C.A.; Jaglal, Y.; Singh, S.; Devnarain, N.; Mocktar, C.; Govender, T. A transferosome-loaded bigel for enhanced transdermal delivery and antibacterial activity of vancomycin hydrochloride. Int. J. Pharm. 2021, 607, 120990. [Google Scholar] [CrossRef]
- Rojas, S.; Colinet, I.; Cunha, D.; Hidalgo, T.; Salles, F.; Serre, C.; Guillou, N.; Horcajada, P. Toward Understanding Drug Incorporation and Delivery from Biocompatible Metal–Organic Frameworks in View of Cutaneous Administration. ACS Omega 2018, 3, 2994–3003. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol. Rev. 2001, 53, 283–318. [Google Scholar] [PubMed]
- Mansur, H.S. Quantum dots and nanocomposites. Wiley Interdiscip Rev Nanomedicine Nanobiotechnology 2010. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.M.; Fatima, F.; Mohammed, A.B. Olive Oil Based Organogels for Effective Topical Delivery of Fluconazole: In-vitro Antifungal Study. J. Pharm. Res. Int. 2020, 32, 29–36. [Google Scholar] [CrossRef]
- Alshehri, S.; Imam, S.S. Formulation and evaluation of butenafine loaded PLGA-nanoparticulate laden chitosan nano gel. Drug Deliv. 2021, 28, 2348–2360. [Google Scholar] [CrossRef]
- Moinard-Chécot, D.; Chevalier, Y.; Briançon, S.; Beney, L.; Fessi, H. Mechanism of nanocapsules formation by the emulsion–diffusion process. J. Colloid Interface Sci. 2008, 317, 458–468. [Google Scholar] [CrossRef]
- Aldawsari, H.M.; Singh, S.; Alhakamy, N.A.; Bakhaidar, R.B.; Halwani, A.A.; Badr-Eldin, S.M. Gum Acacia Functionalized Colloidal Gold Nanoparticles of Letrozole as Biocompatible Drug Delivery Carrier for Treatment of Breast Cancer. Pharmaceutics 2021, 13, 1554. [Google Scholar] [CrossRef]
- Alqarni, M.H.; Foudah, A.I.; Alam, A.; Salkini, M.A.; Muharram, M.M.; Labrou, N.E.; Kumar, P. Development of Gum-Acacia-Stabilized Silver Nanoparticles Gel of Rutin against Candida albicans. Gels 2022, 8, 472. [Google Scholar] [CrossRef]
- Singh, S.; Alrobaian, M.M.; Molugulu, N.; Agrawal, N.; Numan, A.; Kesharwani, P. Pyramid-Shaped PEG-PCL-PEG Polymeric-Based Model Systems for Site-Specific Drug Delivery of Vancomycin with Enhance Antibacterial Efficacy. ACS Omega 2020, 5, 11935–11945. [Google Scholar] [CrossRef]
- Singh, S.; Aldawsari, H.M.; Alam, A.; Alqarni, M.H.S.; Ranjan, S.; Kesharwani, P. Synthesis and antimicrobial activity of vancomycin–conjugated zinc coordination polymer nanoparticles against methicillin-resistant staphylococcus aureus. J. Drug Deliv. Sci. Technol. 2022, 70, 103255. [Google Scholar] [CrossRef]
- Almeida, K.B.; Araujo, J.L.; Cavalcanti, J.F.; Romanos, M.T.V.; Mourão, S.C.; Amaral, A.C.F.; Falcão, D.Q. In vitro release and anti-herpetic activity of Cymbopogon citratus volatile oil-loaded nanogel. Rev. Bras. de Farm. 2018, 28, 495–502. [Google Scholar] [CrossRef]
- Barradas, T.N.; Senna, J.P.; Cardoso, S.A.; de holanda e Silva, K.G.; Mansur, C.R.E. Formulation characterization and in vitro drug release of hydrogel-thickened nanoemulsions for topical delivery of 8-methoxypsoralen. Mater. Sci. Eng. C 2018, 92, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Gaurav, C.; Goutam, R.; Rohan, K.N.; Sweta, K.T.; Abhay, C.S.; Amit, G.K. (Copper–curcumin) β-cyclodextrin vaginal gel: Delivering a novel metal–herbal approach for the development of topical contraception prophylaxis. Eur. J. Pharm. Sci. 2014, 65, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Wang, J.; Zhang, H.; Liu, S.; Wei, P.; Wang, H.; Xu, Z.; Fu, Q.; Zhang, K. MK2206 Enhances Cisplatin-Induced Cytotoxicity and Apoptosis in Testicular Cancer Through Akt Signaling Pathway Inhibition. Transl. Oncol. 2020, 13, 100769. [Google Scholar] [CrossRef]
- Yousef, J.M.; Danial, E.N. In Vitro Antibacterial Activity and Minimum Inhibitory Concentration of Zinc Oxide and Nano-particle Zinc oxide Against Pathogenic Strains. Int. J. Heal. Sci. 2012, 2, 38–42. [Google Scholar] [CrossRef]
- Appiah, T.; Boakye, Y.D.; Agyare, C. Antimicrobial Activities and Time-Kill Kinetics of Extracts of Selected Ghanaian Mushrooms. Evid.-Based Complement. Altern. Med. 2017, 2017, 1–15. [Google Scholar] [CrossRef]
- Robles, H. Tannic Acid. In Encyclopedia of Toxicology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 474–475. ISBN 9780123864543. [Google Scholar]
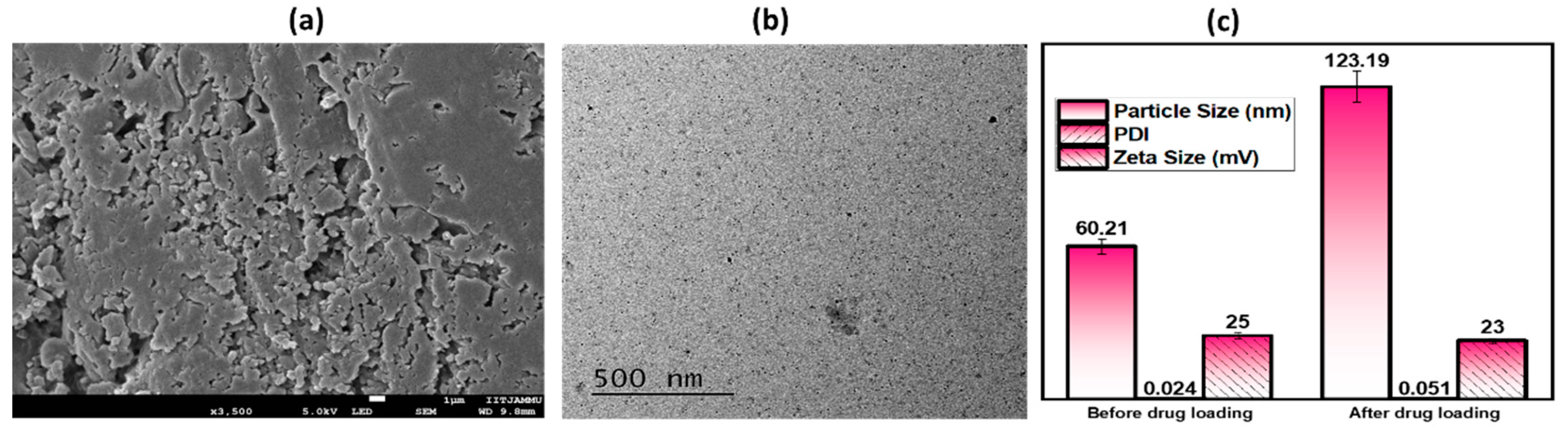
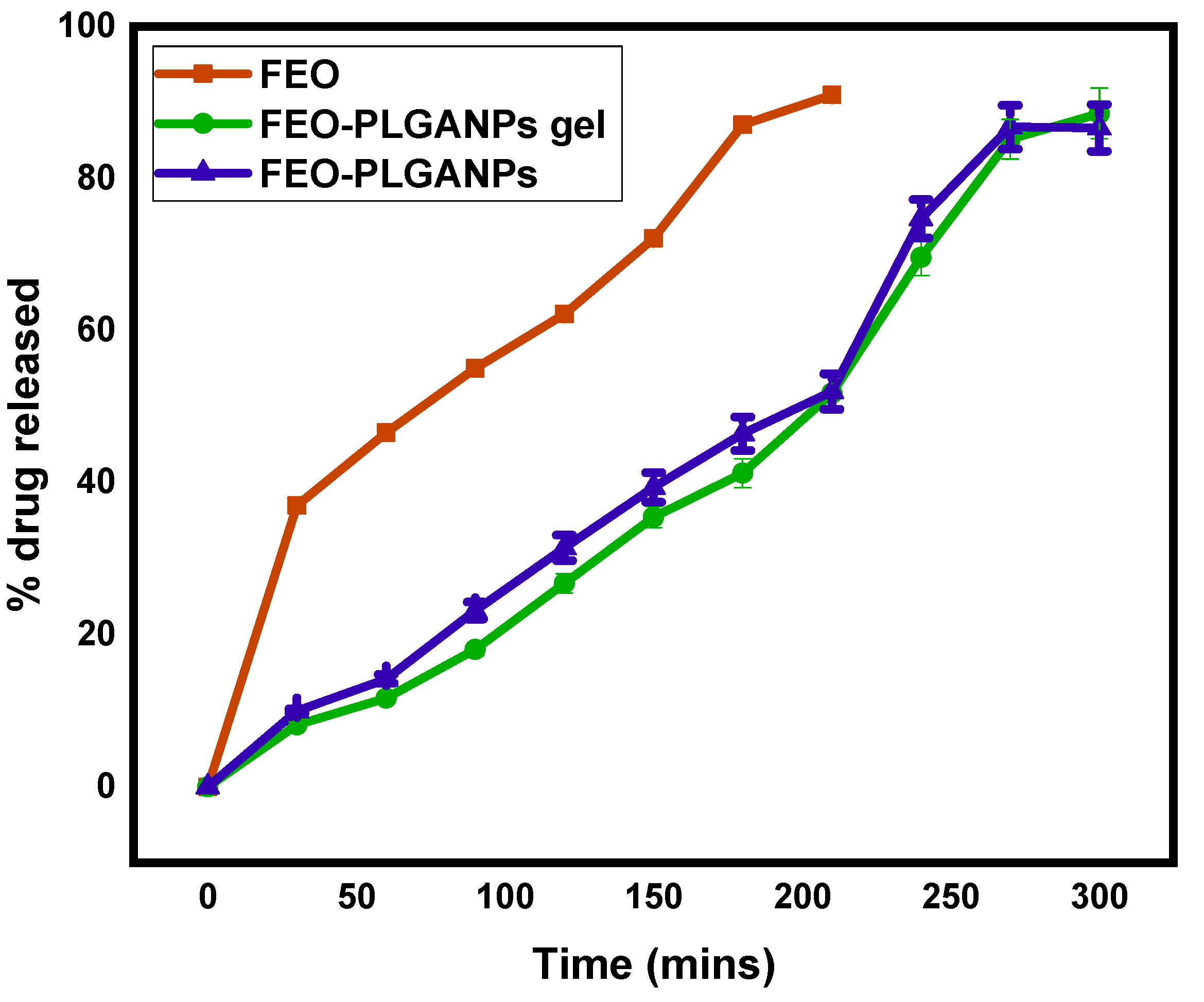
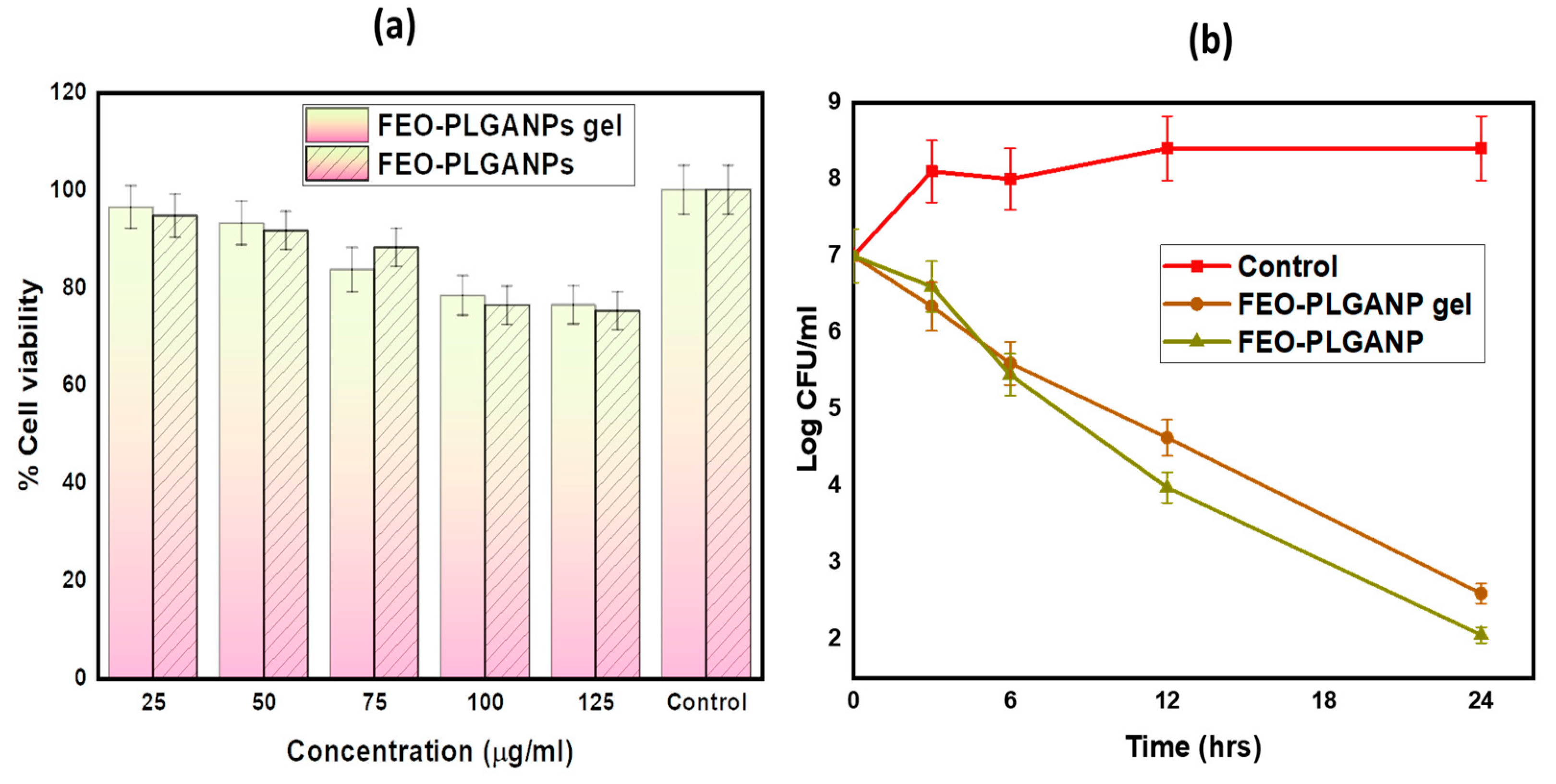
| Formulation | Zero Order | Higuchi | First Order | Kors–Peppas | Hixson |
|---|---|---|---|---|---|
| Bare FEO | 0.9145 | 0.9839 | 0.9239 | 0.9002 | 0.9506 |
| FEO–PLGANPs | 0.996 | 0.9117 | 0.9882 | 0.6994 | 0.9925 |
| FEO–PLGANPs gels | 0.9905 | 0.8827 | 0.9781 | 0.6591 | 0.9834 |
| Compound | MIC (µg/mL) |
|---|---|
| Vancomycin | 4.92 |
| FEO–PLGANPs gel | 3.12 |
| FEO–PLGANPs | 3.00 |
| PLGANPs | NA |
| FEO | 12.5 |
| Days | Color | Texture | Consistency | pH | EE% |
|---|---|---|---|---|---|
| 0 | Off-white | smooth | consistent | 6.0 | 66.4 |
| 15 | Off-white | smooth | consistent | 6.4 | 64.4 |
| 30 | Off-white | smooth | consistent | 6.7 | 62.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, A.; Foudah, A.I.; Salkini, M.A.; Raish, M.; Sawale, J. Herbal Fennel Essential Oil Nanogel: Formulation, Characterization and Antibacterial Activity against Staphylococcus aureus. Gels 2022, 8, 736. https://doi.org/10.3390/gels8110736
Alam A, Foudah AI, Salkini MA, Raish M, Sawale J. Herbal Fennel Essential Oil Nanogel: Formulation, Characterization and Antibacterial Activity against Staphylococcus aureus. Gels. 2022; 8(11):736. https://doi.org/10.3390/gels8110736
Chicago/Turabian StyleAlam, Aftab, Ahmed I. Foudah, Mohammad Ayman Salkini, Mohammad Raish, and Jyotiram Sawale. 2022. "Herbal Fennel Essential Oil Nanogel: Formulation, Characterization and Antibacterial Activity against Staphylococcus aureus" Gels 8, no. 11: 736. https://doi.org/10.3390/gels8110736
APA StyleAlam, A., Foudah, A. I., Salkini, M. A., Raish, M., & Sawale, J. (2022). Herbal Fennel Essential Oil Nanogel: Formulation, Characterization and Antibacterial Activity against Staphylococcus aureus. Gels, 8(11), 736. https://doi.org/10.3390/gels8110736