Green In Situ Synthesis of Silver Nanoparticles-Peptide Hydrogel Composites: Investigation of Their Antibacterial Activities
Abstract
1. Introduction
2. Results and Discussion
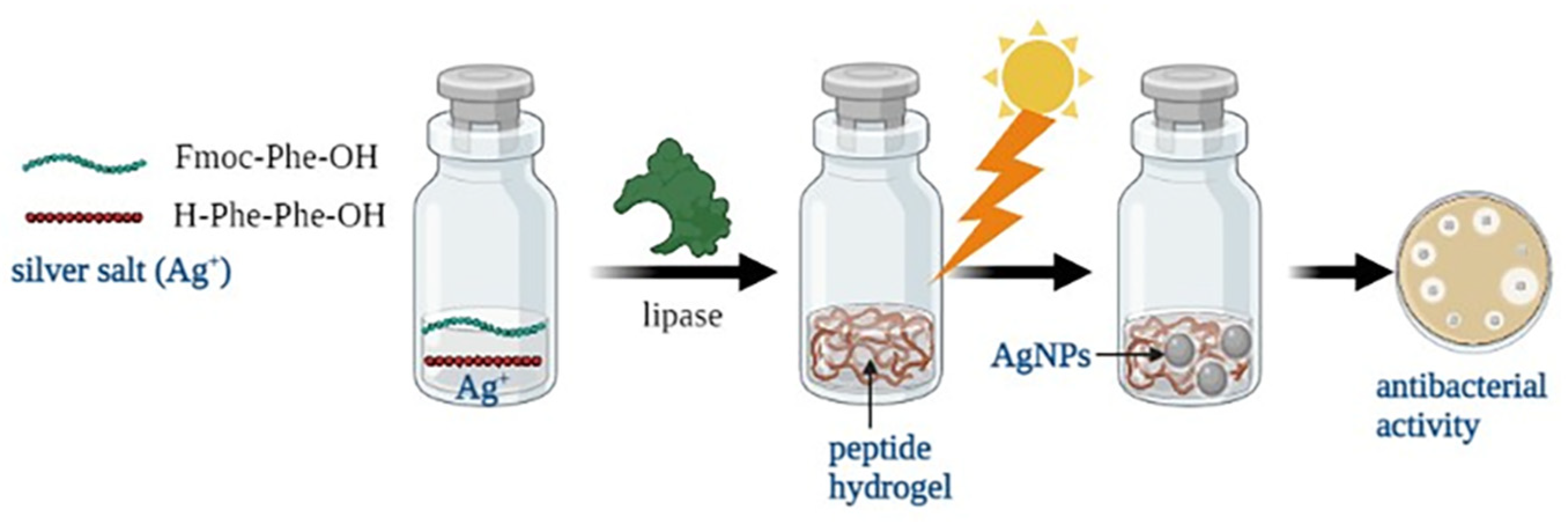
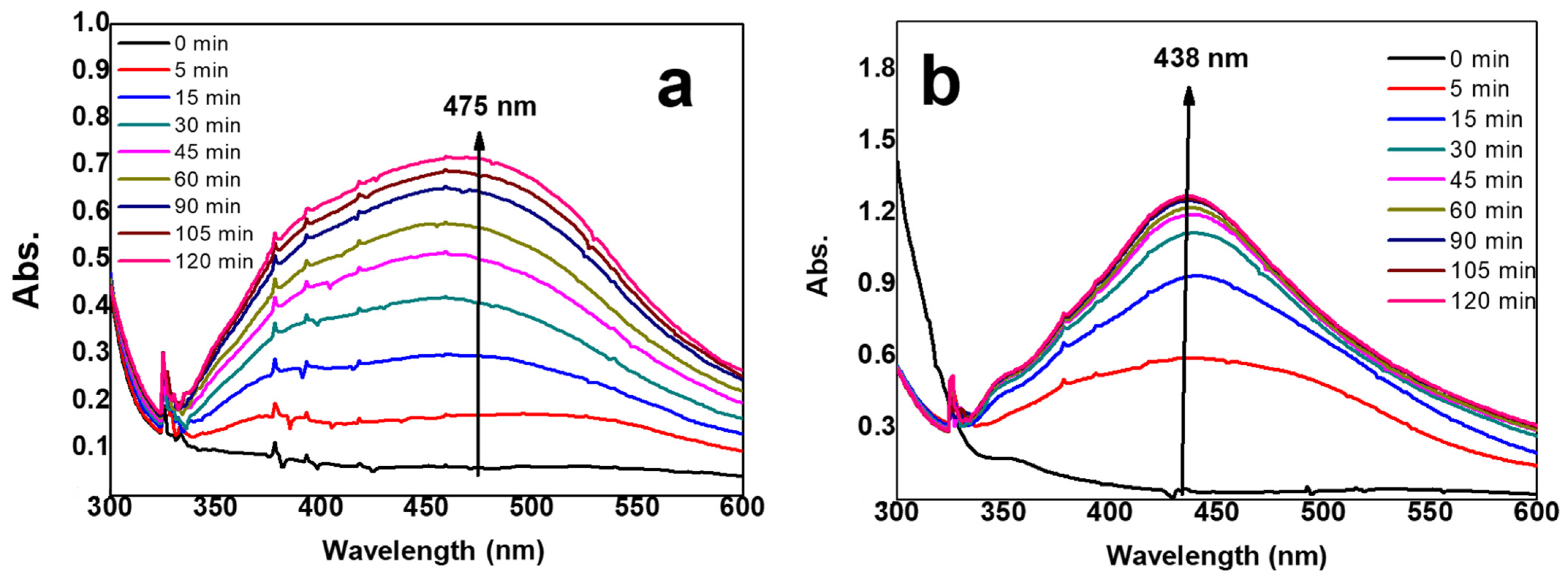
2.1. Preparation of AgNPs/Hydrogel Composites
2.2. Inductively Coupled Plasma-Atomic Emission Spectrometry (ICP-OES)
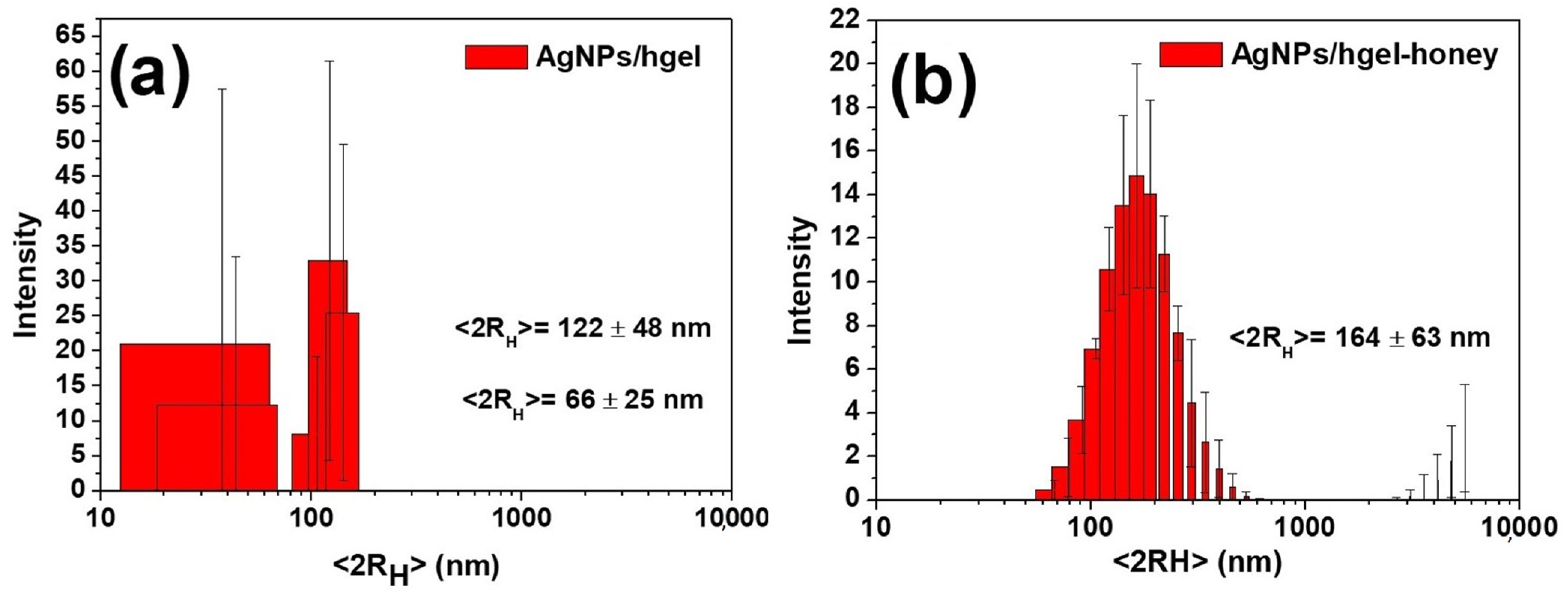
2.3. Dynamic Light Scattering (DLS)
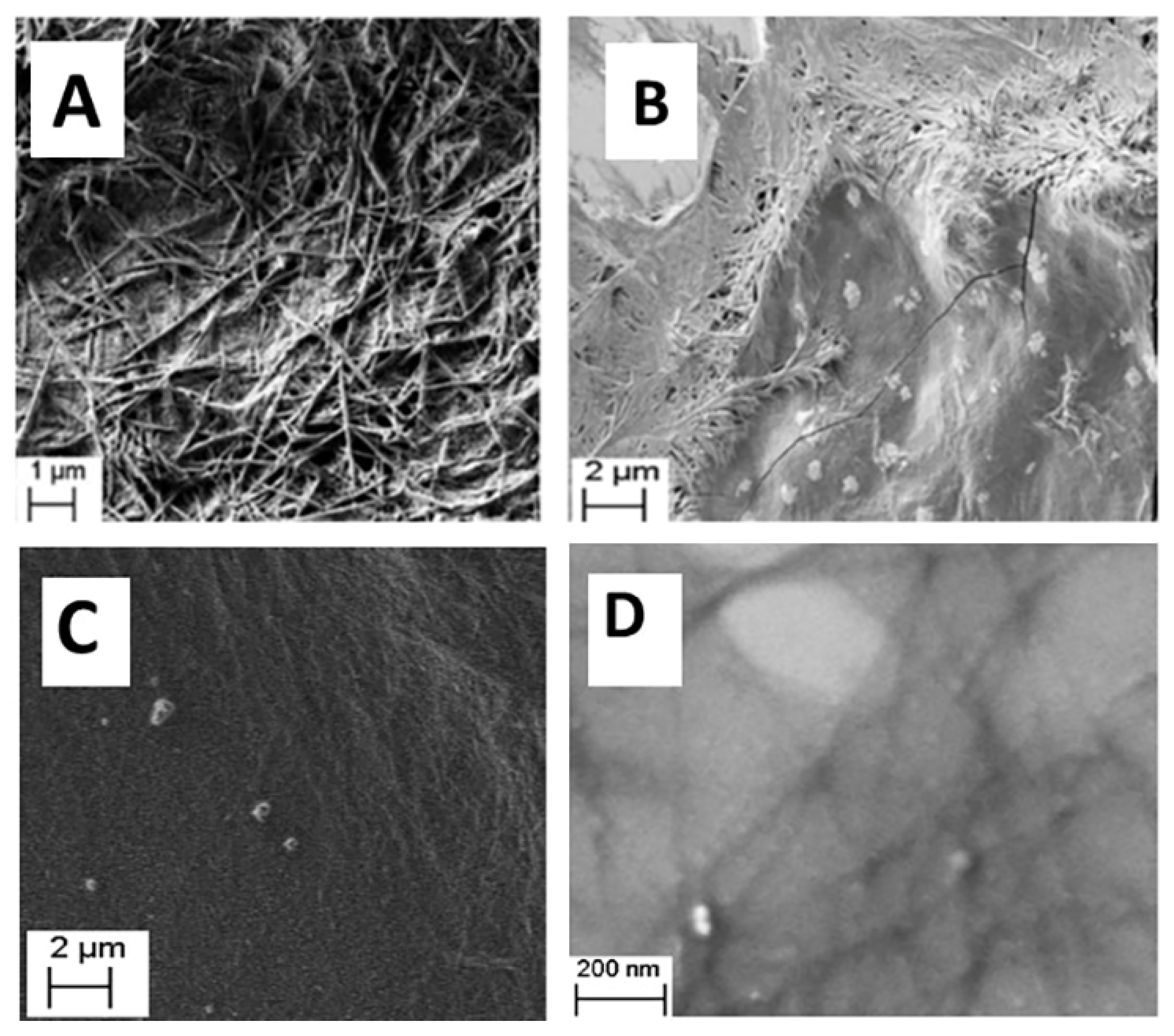
2.4. SEM Measurement
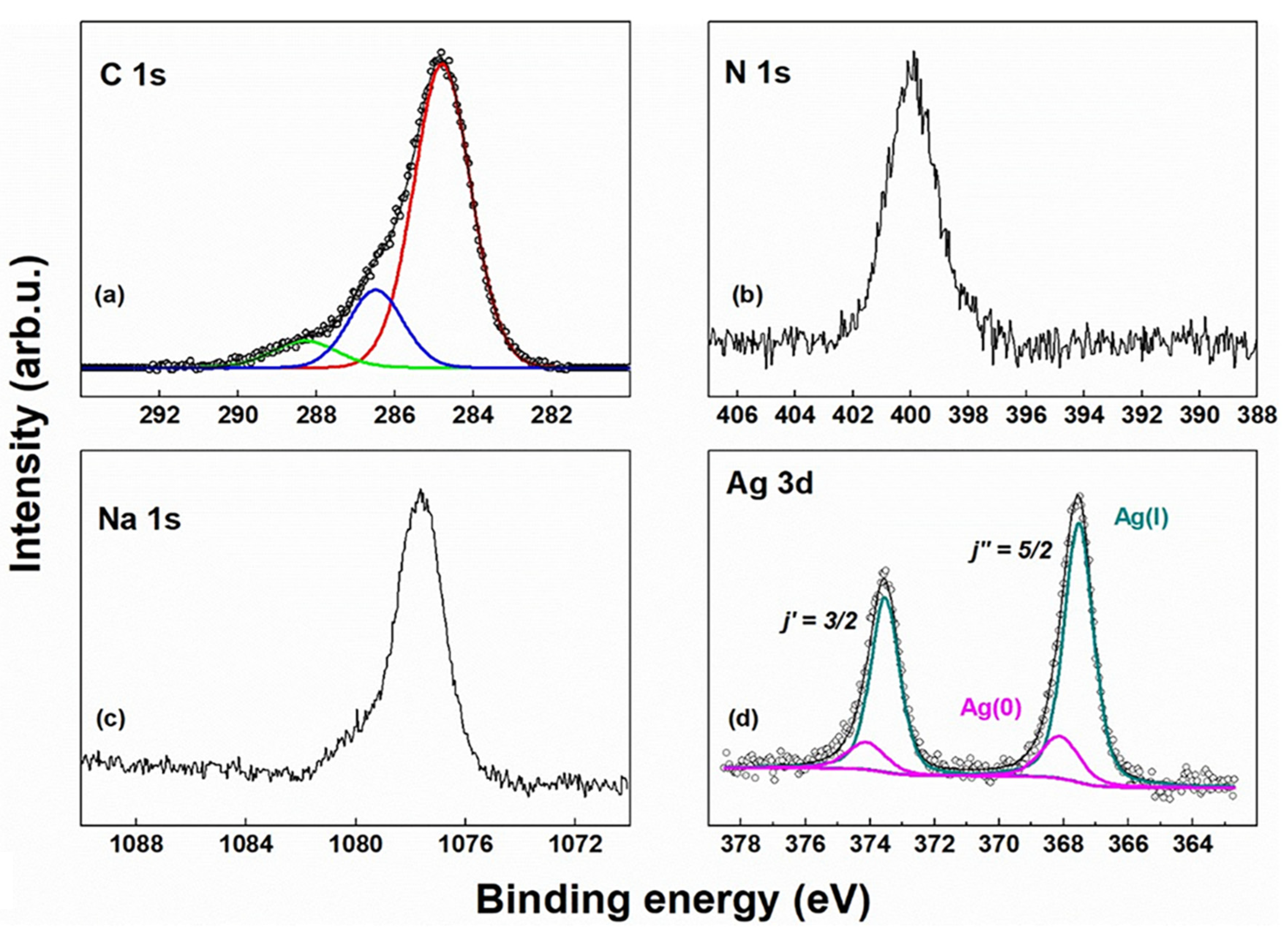
2.5. X-ray Photoelectron Spectroscopy (XPS)
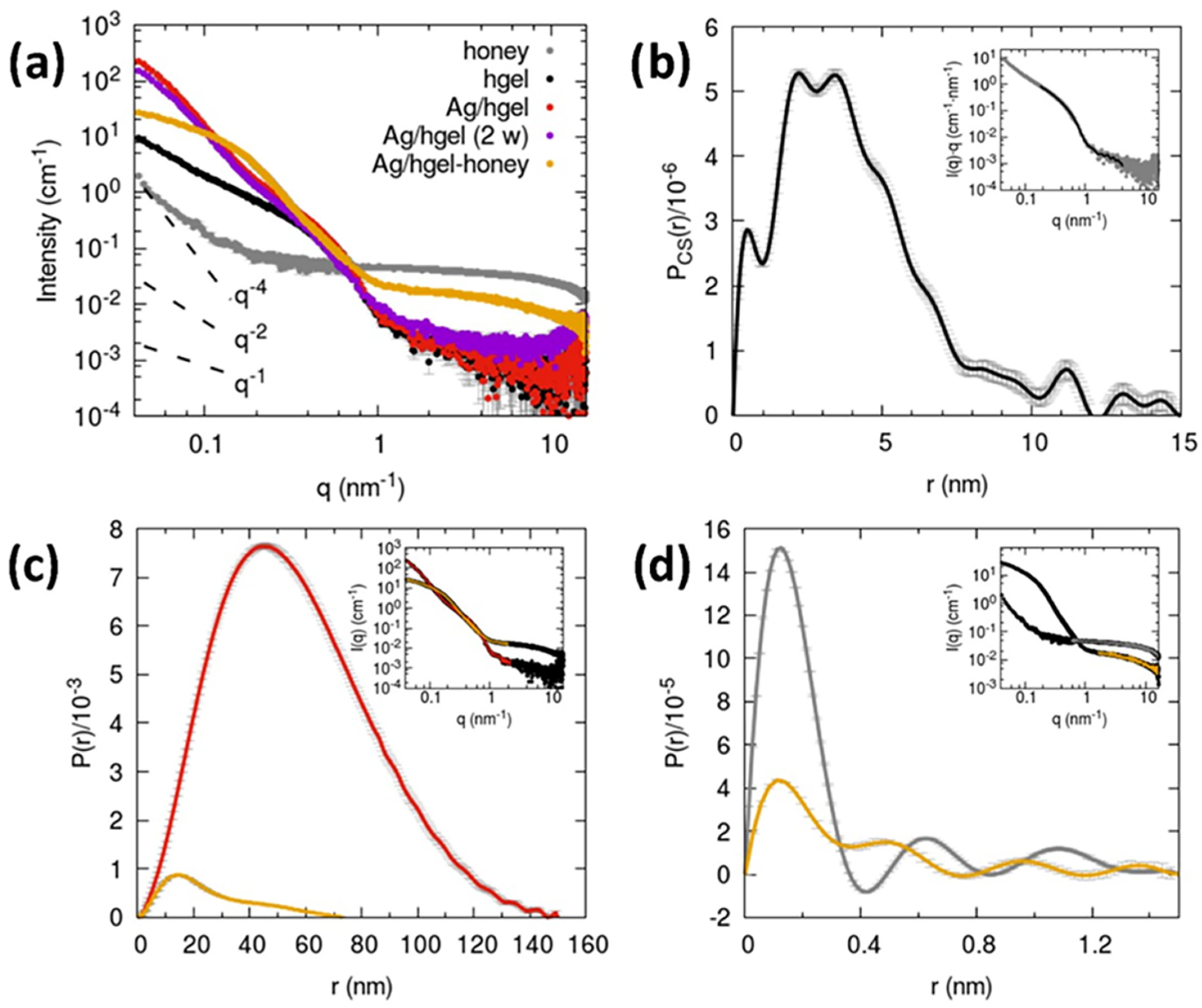
2.6. X-ray Small Angle Scattering (SAXS)
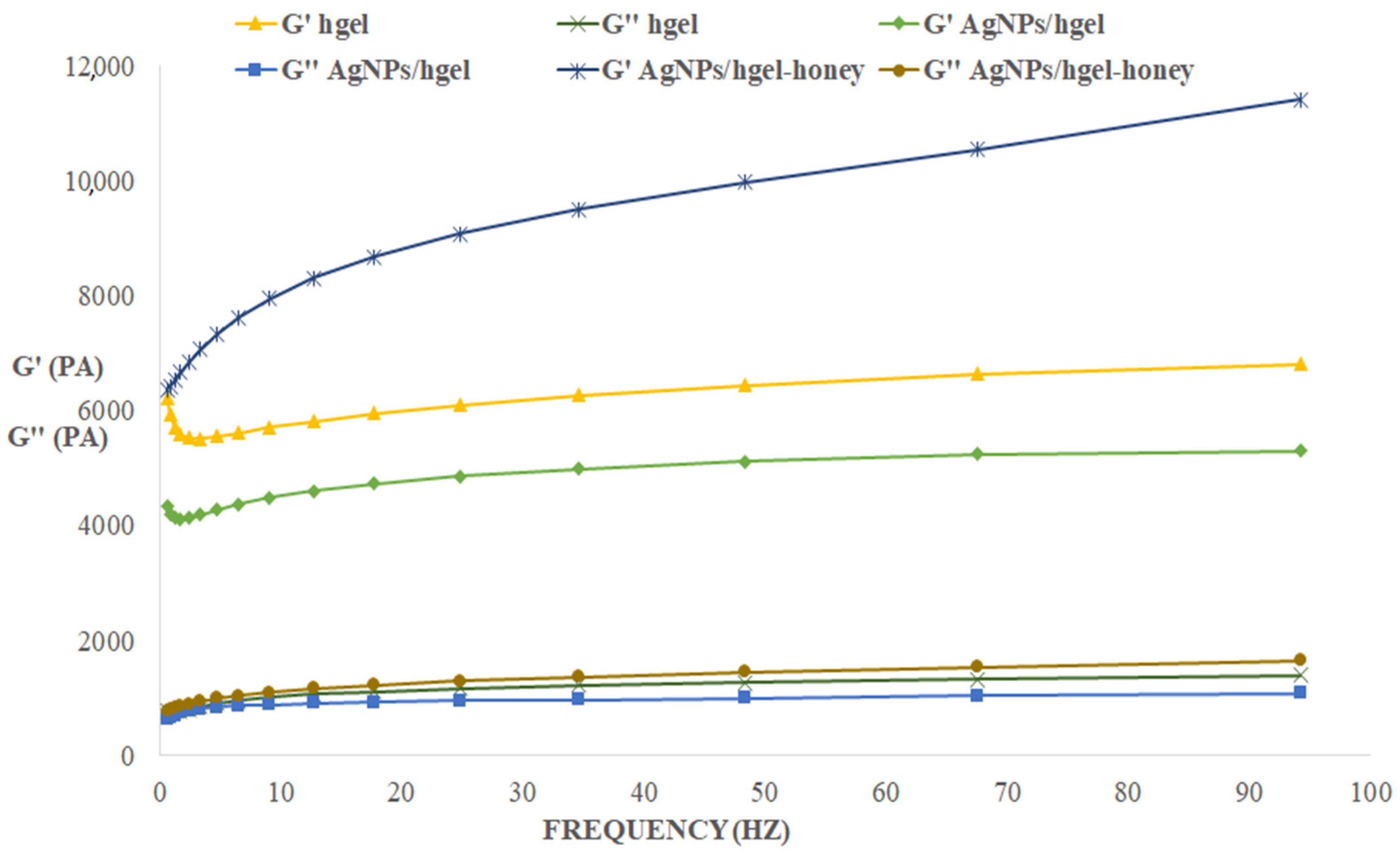
2.7. Rheological Studies
2.8. Swelling Ability
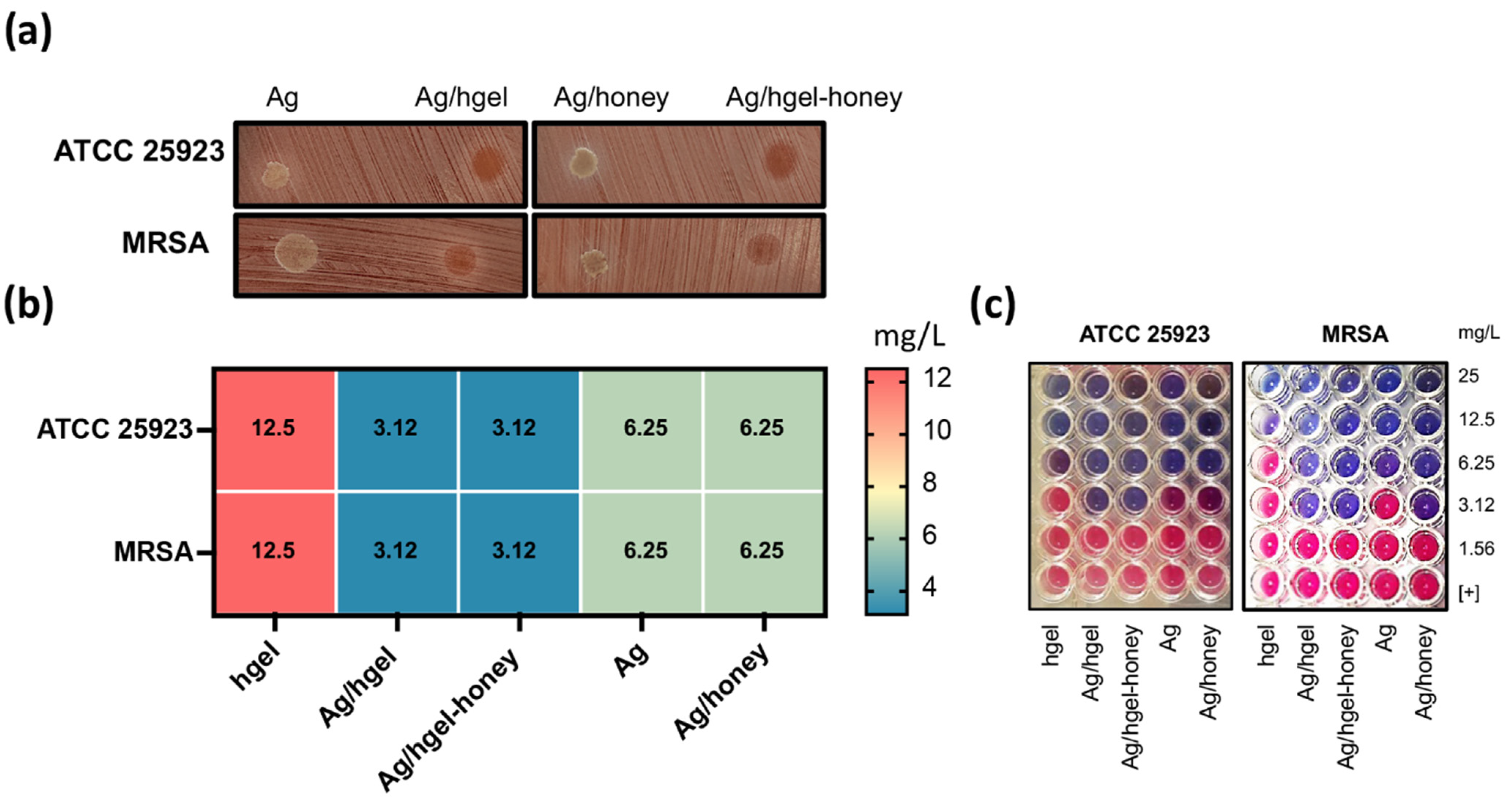
2.9. Antibacterial Studies
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Biosynthesis of Hydrogel Composites
4.3. UV-Vis Spectroscopy and Dynamic Light Scattering (DLS)
4.4. Electron Microscopy Studies
4.5. X-ray Photoelectron Spectroscopy (XPS)
4.6. Inductively Coupled Plasma-Atomic Emission Spectrometry (ICP-AES)
4.7. Small Angle X-ray Scattering (SAXS)
4.8. Rheological Measurements
4.9. Swelling Ability
4.10. Test Microorganisms Used in This Study
4.11. Plate Inhibition Zone Assay to Test for Antimicrobial Activity
4.12. Determination of Minimal Inhibitory Concentration (MIC)
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sharma, S.; Barman, P.; Joshi, S.; Preet, S.; Saini, A. Multidrug resistance crisis during COVID-19 pandemic: Role of anti-microbial peptides as next-generation therapeutics. Colloids Surf. B Biointerfaces 2022, 211, 112303. [Google Scholar] [CrossRef] [PubMed]
- Slavin, Y.N.; Ivanova, K.; Hoyo, J.; Perelshtein, I.; Owen, G.; Haegert, A.; Lin, Y.-Y.; LeBihan, S.; Gedanken, A.; Häfeli, U.O.; et al. Novel Lignin-capped silver nanoparticles against multidrug-resistant bacteria. ACS Appl. Mater. Interfaces 2021, 13, 22098–22109. [Google Scholar] [CrossRef] [PubMed]
- Binaymotlagh, R.; Chronopoulou, L.; Haghighi, F.H.; Fratoddi, I.; Palocci, C. Peptide-based hydrogels: New materials for biosensing and biomedical applications. Materials 2022, 15, 5871. [Google Scholar] [CrossRef]
- Seferji, K.A.; Susapto, H.H.; Khan, B.K.; Rehman, Z.U.; Abbas, M.; Emwas, A.-H.; Hauser, C.A.E. Green synthesis of silver-peptide nanoparticles generated by the photoionization process for anti-biofilm application. ACS Appl. Bio Mater. 2021, 4, 8522–8535. [Google Scholar] [CrossRef]
- de Kraker, M.E.A.; Stewardson, A.J.; Harbarth, S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med. 2016, 13, e1002184. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Jaiswal, S.; Sodhi, K.K.; Shree, P.; Singh, D.K.; Agrawal, P.K.; Shukla, P. Antibiotics bioremediation: Perspectives on its ecotoxicity and resistance. Environ. Int. 2019, 124, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Hrvatin, V. Combating antibiotic resistance: New drugs or alternative therapies? Can. Med. Assoc. J. 2017, 189, E1199. [Google Scholar] [CrossRef][Green Version]
- Bonetti, L.; Fiorati, A.; D’Agostino, A.; Pelacani, C.M.; Chiesa, R.; Farè, S.; De Nardo, L. Smart methylcellulose hydrogels for pH-triggered delivery of silver nanoparticles. Gels 2022, 8, 298. [Google Scholar] [CrossRef]
- Vazquez-Muñoz, R.; Meza-Villezcas, A.; Fournier, P.G.J.; Soria-Castro, E.; Juarez-Moreno, K.; Gallego-Hernández, A.L.; Bogdanchikova, N.; Vazquez-Duhalt, R.; Huerta-Saquero, A. Enhancement of antibiotics antimicrobial activity due to the silver nanoparticles impact on the cell membrane. PLoS ONE 2019, 14, e0224904. [Google Scholar] [CrossRef]
- Zannella, C.; Shinde, S.; Vitiello, M.; Falanga, A.; Galdiero, E.; Fahmi, A.; Santella, B.; Nucci, L.; Gasparro, R.; Galdiero, M.; et al. Antibacterial activity of indolicidin-coated silver nanoparticles in oral disease. Appl. Sci. 2020, 10, 1837. [Google Scholar] [CrossRef]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, H.; Huo, K.; Cui, L.; Zhang, W.; Ni, H.; Zhang, Y.; Wu, Z.; Chu, P.K. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials 2011, 32, 5706–5716. [Google Scholar] [CrossRef] [PubMed]
- Ülkür, E.; Oncul, O.; Karagoz, H.; Yeniz, E.; Çeliköz, B. Comparison of silver-coated dressing (ActicoatTM), chlorhexidine acetate 0.5% (Bactigrass®), and fusidic acid 2% (Fucidin®) for topical antibacterial effect in methicillin-resistant staphylococci-contaminated, full-skin thickness rat burn wounds. Burns 2005, 31, 874–877. [Google Scholar] [CrossRef]
- Atiyeh, B.S.; Costagliola, M.; Hayek, S.N.; Dibo, S.A. Effect of silver on burn wound infection control and healing: Review of the literature. Burns 2007, 33, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Yadollahi, M.; Farhoudian, S.; Namazi, H. One-pot synthesis of antibacterial chitosan/silver bio-nanocomposite hydrogel beads as drug delivery systems. Int. J. Biol. Macromol. 2015, 79, 37–43. [Google Scholar] [CrossRef]
- Mathew, S.; Prakash, A.; Radhakrishnan, E.K. Sunlight mediated rapid synthesis of small size range silver nanoparticles using zingiber officinale rhizome extract and its antibacterial activity analysis. Inorg. Nano Metal Chem. 2018, 48, 139–145. [Google Scholar] [CrossRef]
- Amaladhas, T.P.; Usha, M.; Naveen, S. Sunlight induced rapid synthesis and kinetics of silver nanoparticles using leaf extract of achyranthes aspera L. and their antimicrobial applications. Adv. Mater. Lett. 2013, 4, 779–785. [Google Scholar] [CrossRef]
- Annadhasan, M.; SankarBabu, V.R.; Naresh, R.; Umamaheswari, K.; Rajendiran, N. A Sunlight-induced rapid synthesis of silver nanoparticles using sodium salt of n-cholyl amino acids and its antimicrobial applications. Colloids Surf. B Biointerfaces 2012, 96, 14–21. [Google Scholar] [CrossRef]
- Li, J.; Xing, R.; Bai, S.; Yan, X. Recent advances of self-assembling peptide-based hydrogels for biomedical applications. Soft Matter 2019, 15, 1704–1715. [Google Scholar] [CrossRef]
- Chronopoulou, L.; Di Nitto, A.; Papi, M.; Parolini, O.; Falconi, M.; Teti, G.; Muttini, A.; Lattanzi, W.; Palmieri, V.; Ciasca, G. Biosynthesis and physico-chemical characterization of high performing peptide hydrogels@ graphene oxide composites. Colloids Surf. B Biointerfaces 2021, 207, 111989–112000. [Google Scholar] [CrossRef]
- Chronopoulou, L.; Daniele, M.; Perez, V.; Gentili, A.; Gasperi, T.; Lupi, S.; Palocci, C. A physico-chemical approach to the study of genipin crosslinking of biofabricated peptide hydrogels. Process Biochem. 2018, 70, 110–116. [Google Scholar] [CrossRef]
- Fusco, G.; Chronopoulou, L.; Galantini, L.; Zerillo, A.; Rasik, Z.M.; Antiochia, R.; Favero, G.; D’Annibale, A.; Palocci, C.; Mazzei, F. Evaluation of novel fmoc-tripeptide based hydrogels as immobilization supports for electrochemical biosensors. Microchem. J. 2018, 137, 105–110. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, D.; He, X.; Ni, Y.; Che, L.; Wu, J.; Wu, B.; Wang, Y.; Wang, S.; Sha, D.; et al. Cationic peptide-based salt-responsive antibacterial hydrogel dressings for wound healing. Int. J. Biol. Macromol. 2021, 190, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Casolaro, M.; Casolaro, I.; Akimoto, J.; Ueda, M.; Ueki, M.; Ito, Y. Antibacterial properties of silver nanoparticles embedded on polyelectrolyte hydrogels based on α-amino acid residues. Gels 2018, 4, 42. [Google Scholar] [CrossRef] [PubMed]
- Varaprasad, K.; Mohan, Y.M.; Ravindra, S.; Reddy, N.N.; Vimala, K.; Monika, K.; Sreedhar, B.; Raju, K.M. Hydrogel–silver nanoparticle composites: A new generation of antimicrobials. J. Appl. Polym. Sci. 2010, 115, 1199–1207. [Google Scholar] [CrossRef]
- Li, M.; Jiang, X.; Wang, D.; Xu, Z.; Yang, M. In situ reduction of silver nanoparticles in the lignin based hydrogel for enhanced antibacterial application. Colloids Surf. B Biointerfaces 2019, 177, 370–376. [Google Scholar] [CrossRef]
- Lustosa, A.K.; De Jesus Oliveira, A.C.; Quelemes, P.V.; Plácido, A.; Da Silva, F.V.; Oliveira, I.S.; De Almeida, M.P.; Amorim, A.D.; Delerue-Matos, C.; De Oliveira, R.D.; et al. In situ synthesis of silver nanoparticles in a hydrogel of carboxymethyl cellulose with phthalated-cashew gum as a promising antibacterial and healing agent. Int. J. Mol. Sci. 2017, 18, 2399. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kaur, H. Sprayed In-situ synthesis of polyvinyl alcohol/chitosan loaded silver nanocomposite hydrogel for improved antibacterial effects. Int. J. Biol. Macromol. 2020, 145, 950–964. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Senapati, K.; Panda, S.S.; Bhattacharya, P.; Jana, S.; Mandal, S.M.; Basak, A. π-Stacking assisted redox active peptide–gallol conjugate: Synthesis of a new generation of low-toxicity antimicrobial silver nanoparticles. RSC Adv. 2016, 6, 85254–85260. [Google Scholar] [CrossRef]
- Adhikari, B.; Banerjee, A. Short-peptide-based hydrogel: A template for the in situ synthesis of fluorescent silver nanoclusters by using sunlight. Chem. A Eur. J. 2010, 16, 13698–13705. [Google Scholar] [CrossRef]
- Arab, W.; Niyas, A.M.; Seferji, K.A.; Susapto, H.H.; Hauser, C.A.E. Evaluation of peptide nanogels for accelerated wound healing in normal micropigs. Front. Nanosci. Nanotech. 2018, 4, 1–9. [Google Scholar] [CrossRef]
- Mohd Zohdi, R.; Abu Bakar Zakaria, Z.; Yusof, N.; Mohamed Mustapha, N.; Abdullah, M.N.H. Gelam (Melaleuca spp.) honey-based hydrogel as burn wound dressing. Evid. Based Complement. Altern. Med. 2012, 2012, 843025–843032. [Google Scholar] [CrossRef]
- Bonsignore, G.; Patrone, M.; Martinotti, S.; Ranzato, E. “Green” biomaterials: The promising role of honey. J. Funct. Biomater. 2021, 12, 72. [Google Scholar] [CrossRef]
- Briggs, D.; Seah, M.P. Practical surface analysis, auger and X-ray Photoelectron Spectroscopy. In Practical Surface Analysis; Wiley: Hoboken, NJ, USA, 1990. [Google Scholar]
- Prieto, P.; Nistor, V.; Nouneh, K.; Oyama, M.; Abd-Lefdil, M.; Díaz, R. XPS study of silver, nickel and bimetallic silver–nickel nanoparticles prepared by seed-mediated growth. Appl. Surf. Sci. 2012, 258, 8807–8813. [Google Scholar] [CrossRef]
- Kurhade, S.T.; Momin, M.; Khanekar, P.; Mhatre, S. Novel biocompatible honey hydrogel wound healing sponge for chronic ulcers. Int. J. Drug Deliv. 2014, 5, 353–361. [Google Scholar]
- Sivori, F.; Cavallo, I.; Kovacs, D.; Guembe, M.; Sperduti, I.; Truglio, M.; Pasqua, M.; Prignano, G.; Mastrofrancesco, A.; Toma, L.; et al. Role of extracellular DNA in dalbavancin activity against methicillin-resistant Staphylococcus aureus (MRSA) biofilms in patients with skin and soft tissue infections. Microbiol. Spectr. 2022, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, E.G.; Petroni, G.; Mancini, D.; Geri, A.; Di Palma, L.; Ascenzioni, F. Development of electroactive and anaerobic ammonium-oxidizing (Anammox) biofilms from digestate in microbial fuel cells. Biomed. Res. Int. 2015, 2015, 351014–351024. [Google Scholar] [CrossRef]
- Di Domenico, E.G.; Cavallo, I.; Sivori, F.; Marchesi, F.; Prignano, G.; Pimpinelli, F.; Sperduti, I.; Pelagalli, L.; Di Salvo, F.; Celesti, I.; et al. Biofilm production by carbapenem-resistant klebsiella pneumoniae significantly increases the risk of death in oncological patients. Front. Cell. Infect. Microbiol. 2020, 10, 561741–561753. [Google Scholar] [CrossRef] [PubMed]
| Samples | Swelling Degree (q) |
|---|---|
| hgel | 62.18 ± 0.35 |
| AgNPs/hgel | 75.99 ± 0.65 |
| AgNPs/hgel-honey | 79.11 ± 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binaymotlagh, R.; Del Giudice, A.; Mignardi, S.; Amato, F.; Marrani, A.G.; Sivori, F.; Cavallo, I.; Di Domenico, E.G.; Palocci, C.; Chronopoulou, L. Green In Situ Synthesis of Silver Nanoparticles-Peptide Hydrogel Composites: Investigation of Their Antibacterial Activities. Gels 2022, 8, 700. https://doi.org/10.3390/gels8110700
Binaymotlagh R, Del Giudice A, Mignardi S, Amato F, Marrani AG, Sivori F, Cavallo I, Di Domenico EG, Palocci C, Chronopoulou L. Green In Situ Synthesis of Silver Nanoparticles-Peptide Hydrogel Composites: Investigation of Their Antibacterial Activities. Gels. 2022; 8(11):700. https://doi.org/10.3390/gels8110700
Chicago/Turabian StyleBinaymotlagh, Roya, Alessandra Del Giudice, Silvano Mignardi, Francesco Amato, Andrea Giacomo Marrani, Francesca Sivori, Ilaria Cavallo, Enea Gino Di Domenico, Cleofe Palocci, and Laura Chronopoulou. 2022. "Green In Situ Synthesis of Silver Nanoparticles-Peptide Hydrogel Composites: Investigation of Their Antibacterial Activities" Gels 8, no. 11: 700. https://doi.org/10.3390/gels8110700
APA StyleBinaymotlagh, R., Del Giudice, A., Mignardi, S., Amato, F., Marrani, A. G., Sivori, F., Cavallo, I., Di Domenico, E. G., Palocci, C., & Chronopoulou, L. (2022). Green In Situ Synthesis of Silver Nanoparticles-Peptide Hydrogel Composites: Investigation of Their Antibacterial Activities. Gels, 8(11), 700. https://doi.org/10.3390/gels8110700











