Properties of Grass Carp (Ctenopharyngodon idella) Collagen and Gel for Application in Biomaterials
Abstract
1. Introduction
2. Results and Discussion
2.1. Yields of Collagens
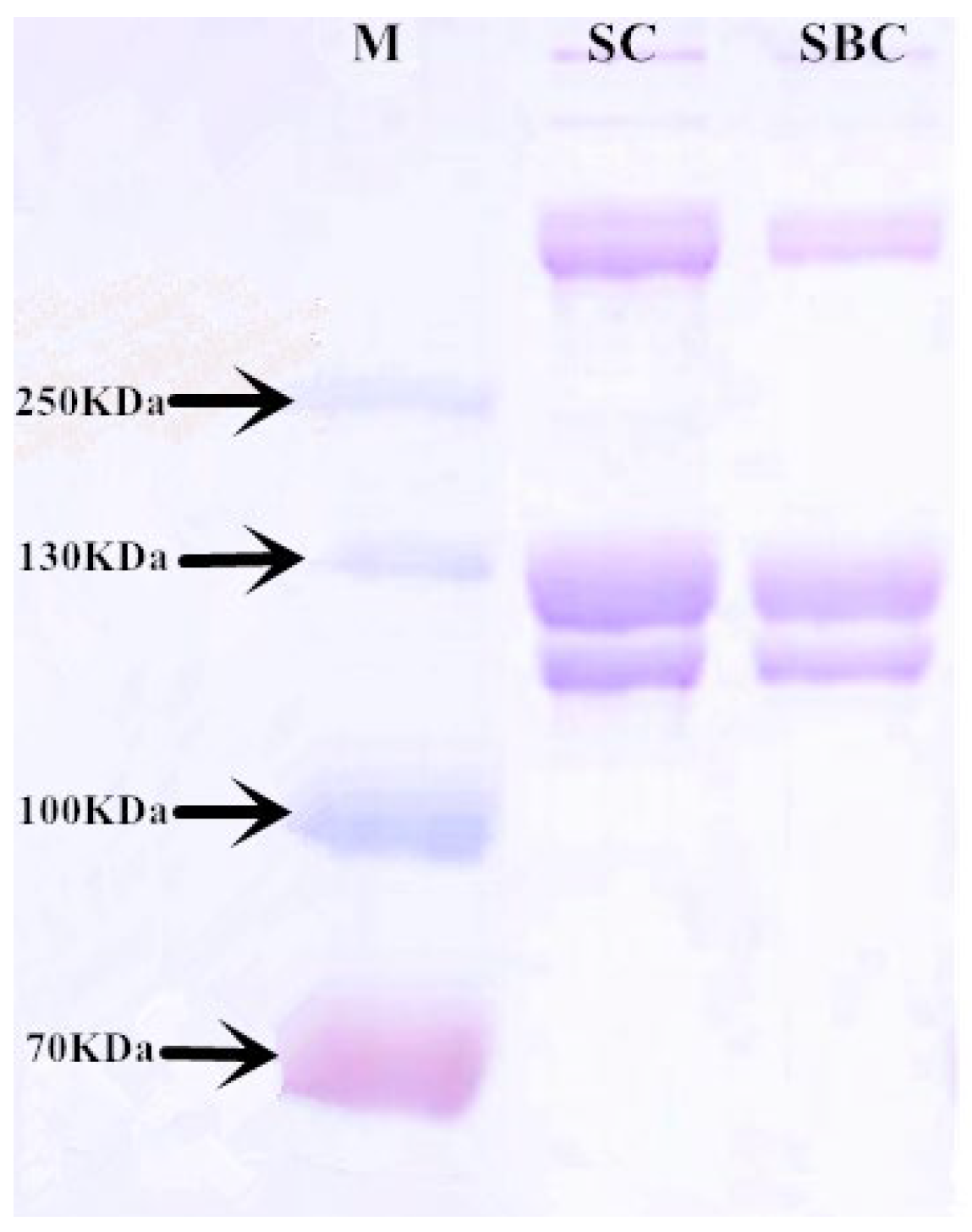
2.2. SDS-PAGE
2.3. Amino Acid Composition
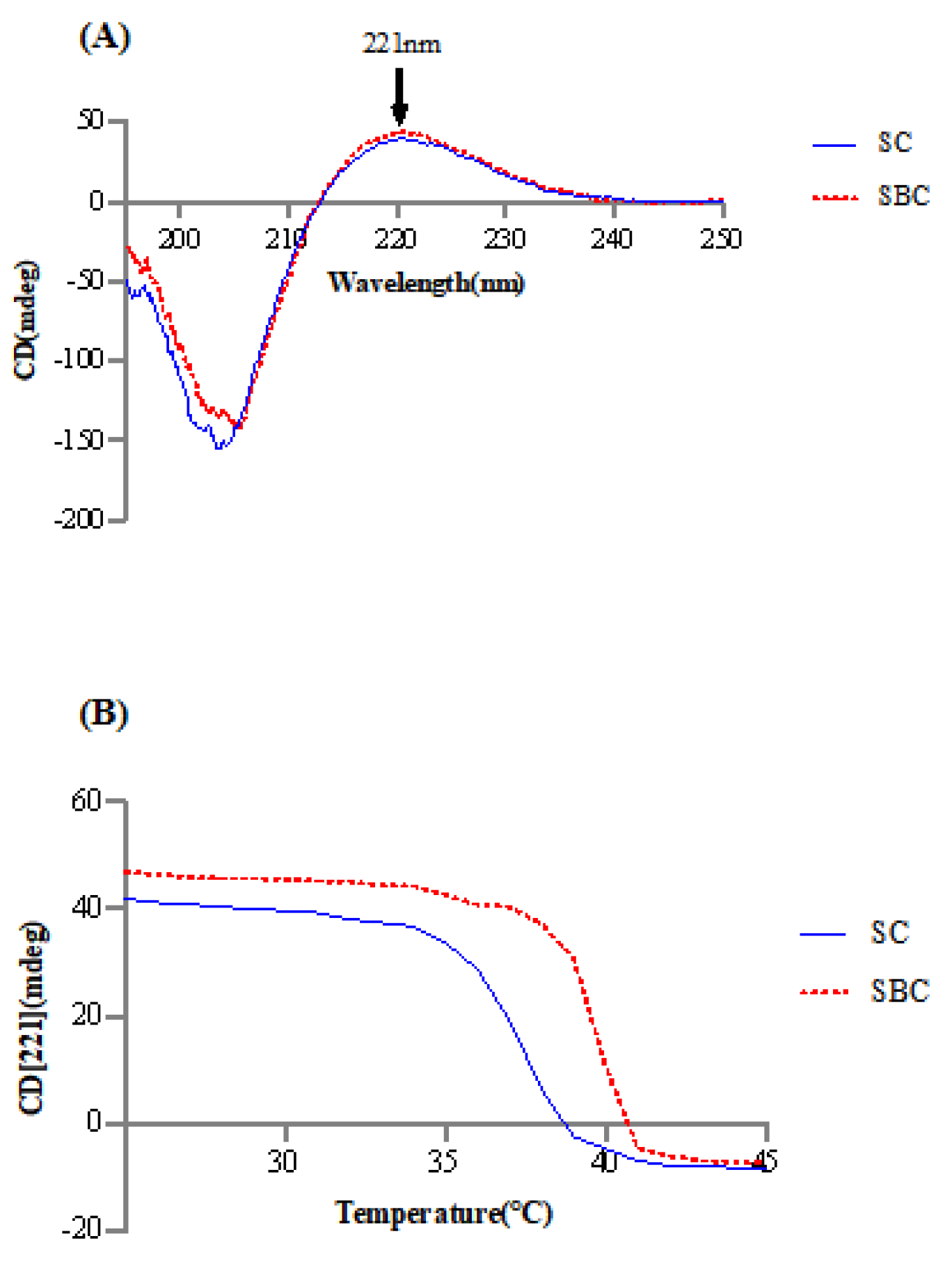
2.4. Thermal Stability
2.5. Antioxidant Activities
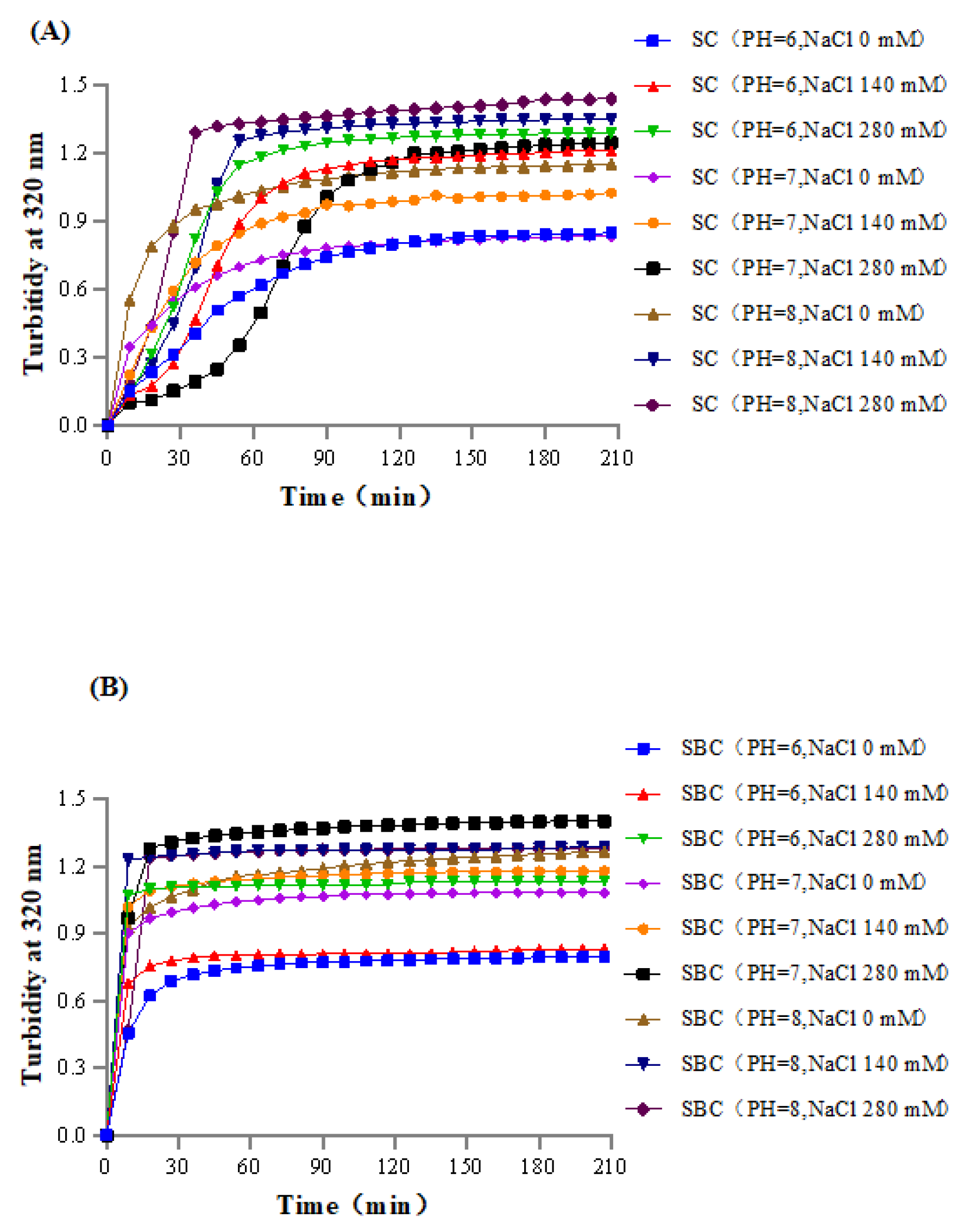
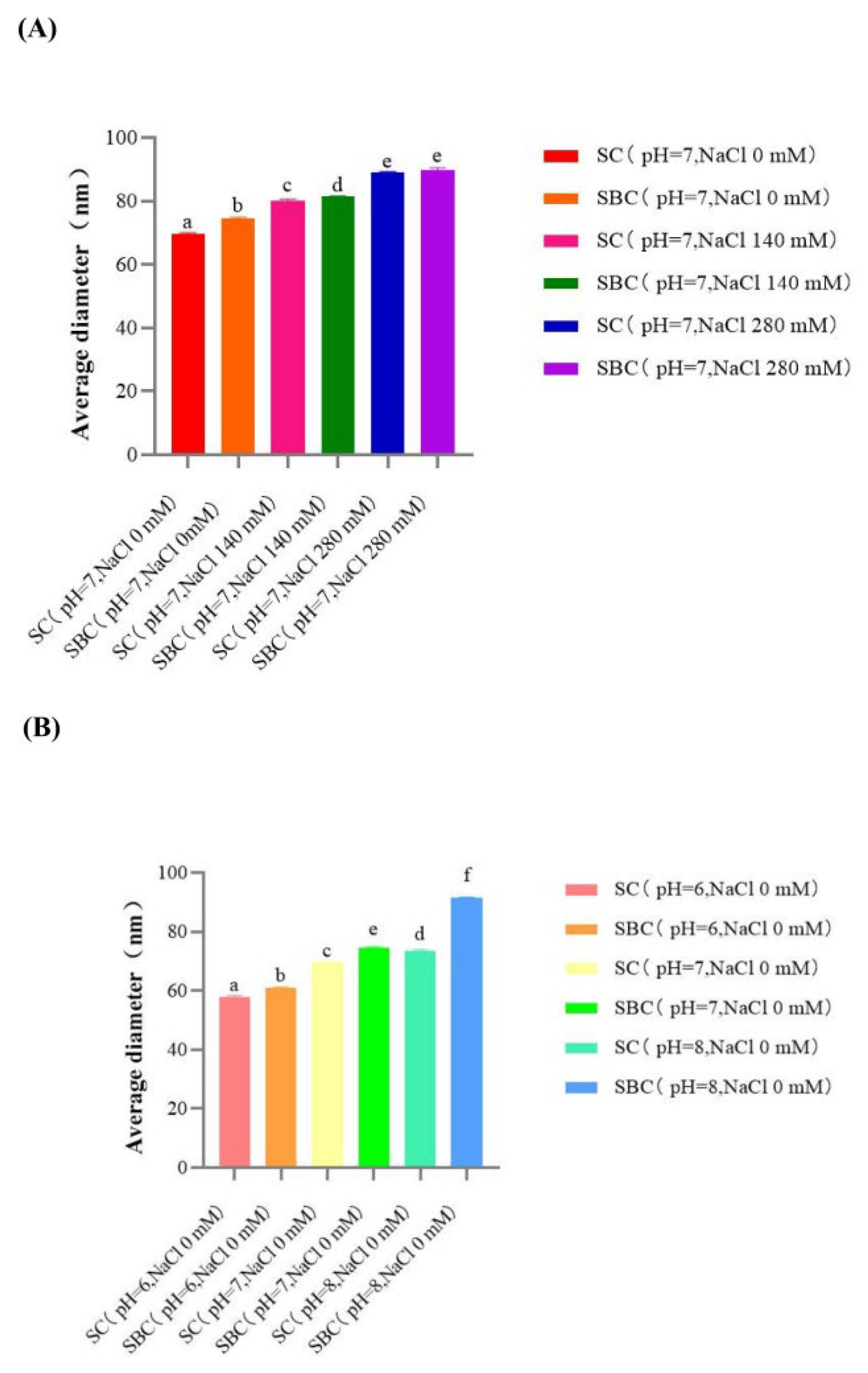
2.6. Collagen Fibril Formation In Vitro under Different Salinity and pH Conditions
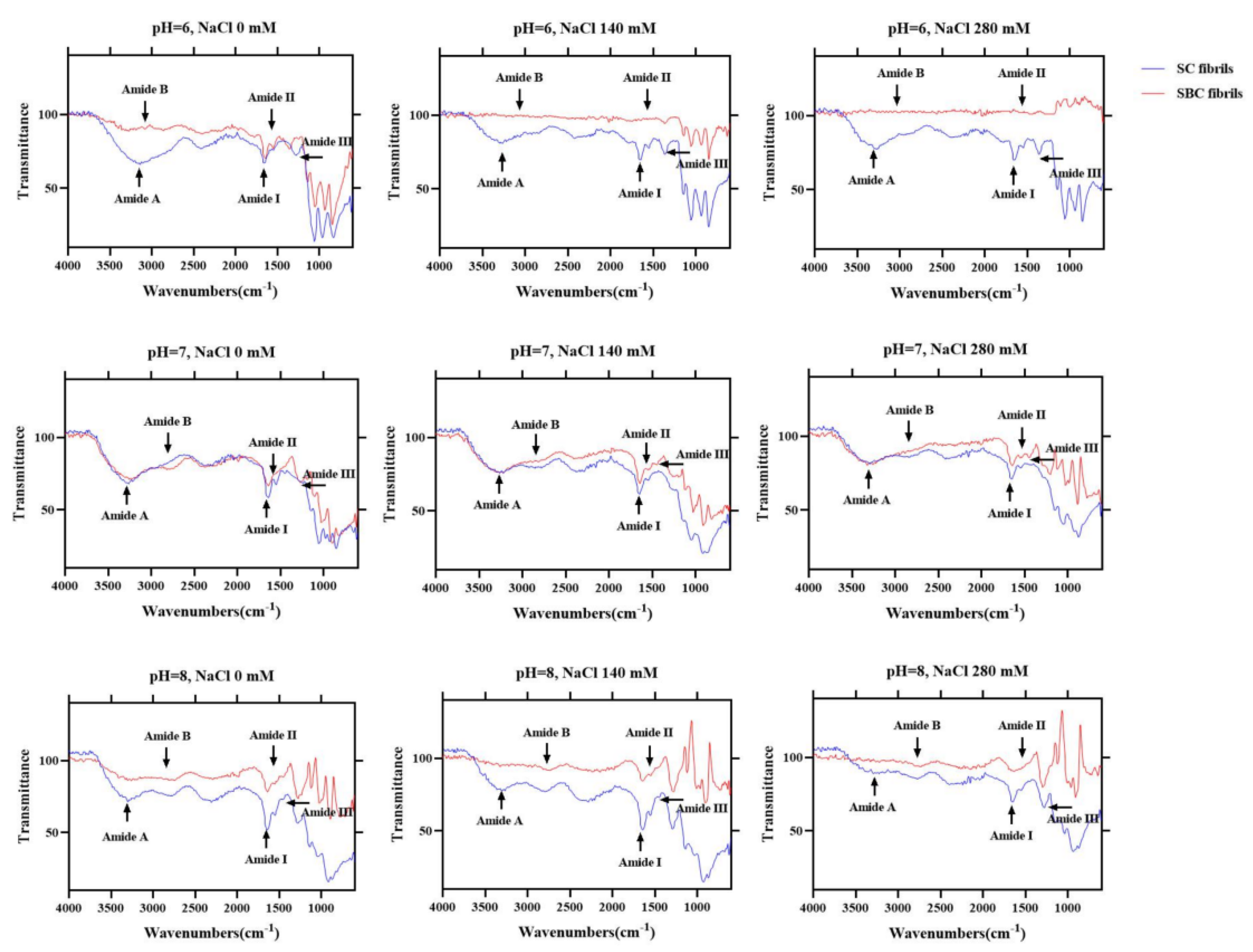
2.7. FTIR
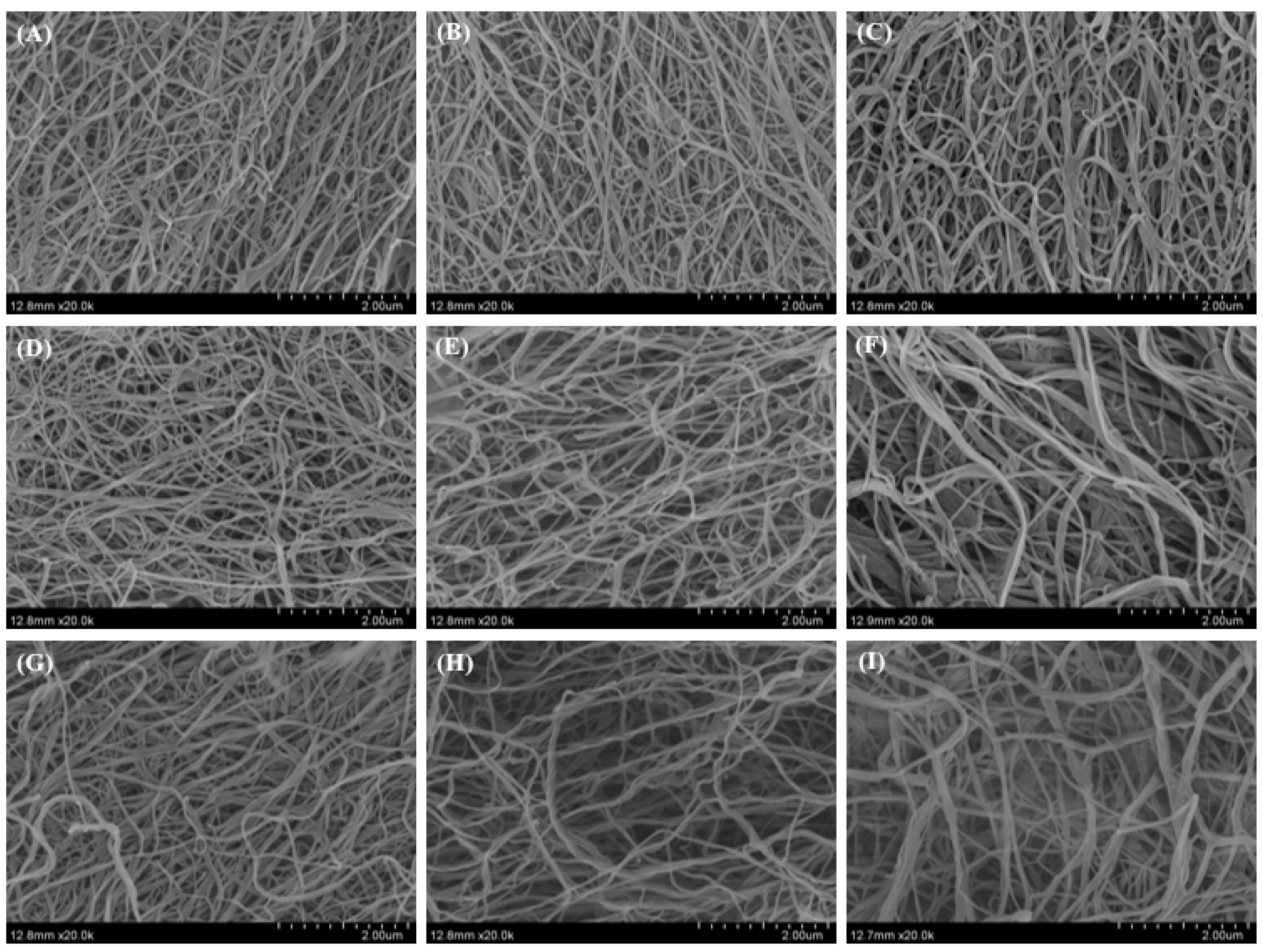
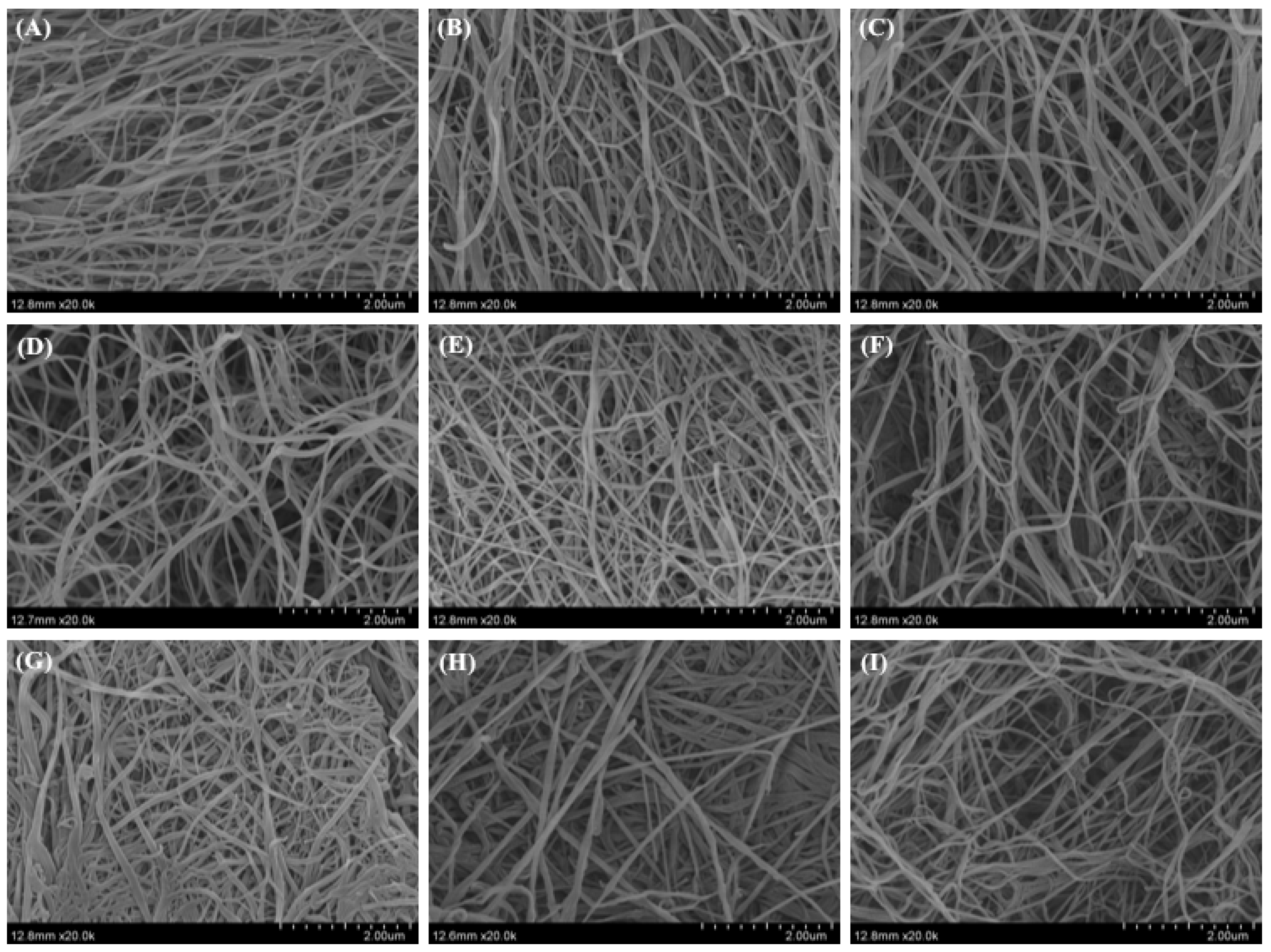
2.8. Morphology of Collagen Fibrils Formed In Vitro
2.9. SC Gel Formation and Property Analysis
2.9.1. SC Gel Formation
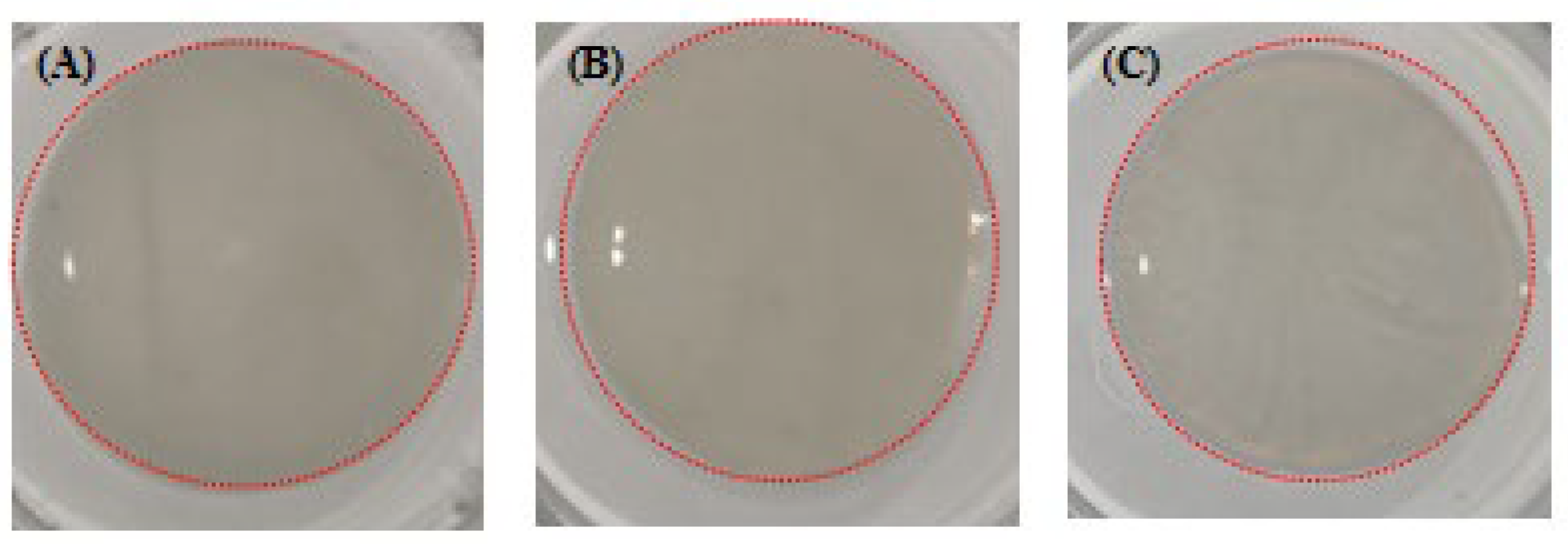
2.9.2. Chromatic Aberration
2.9.3. Texture
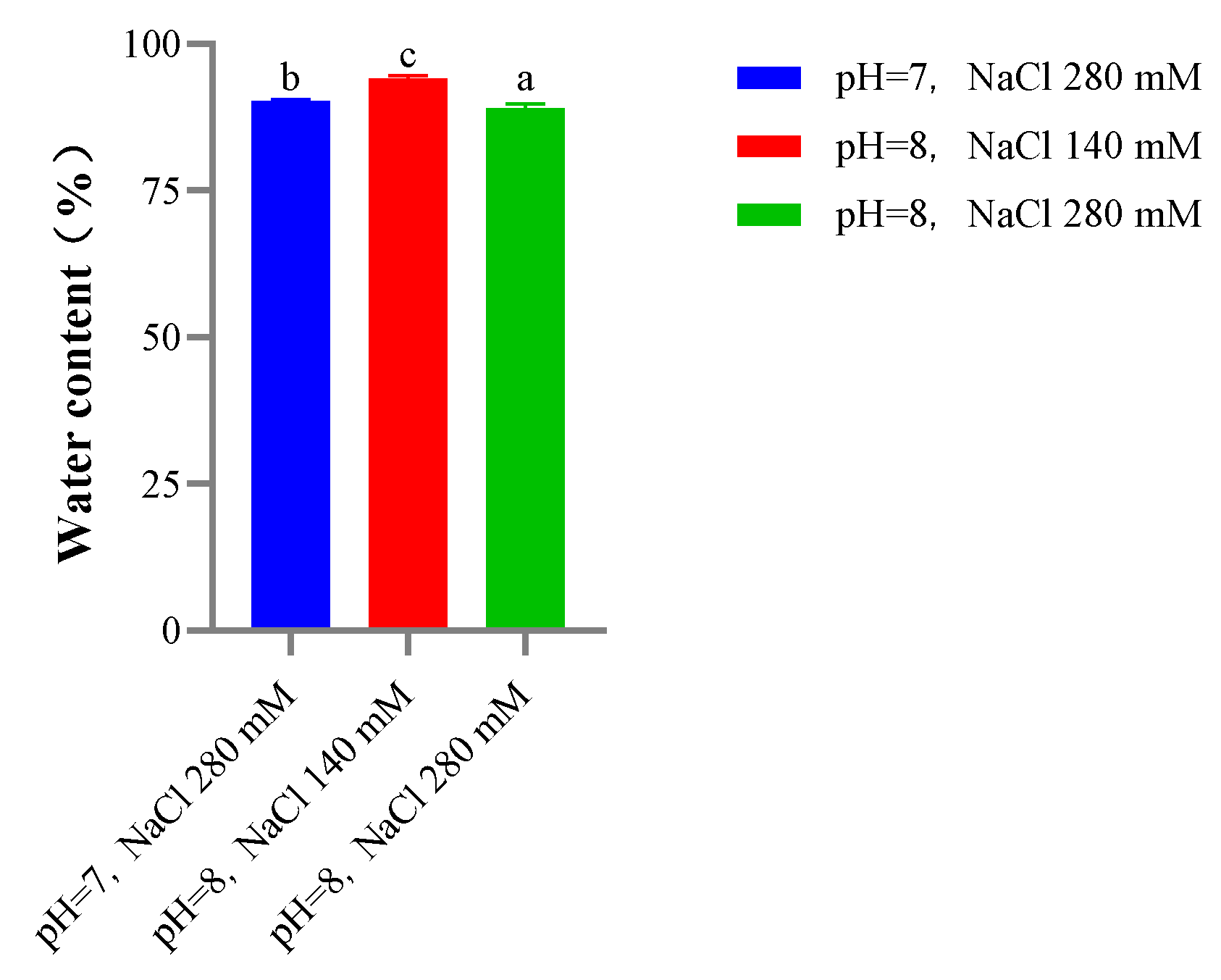
2.9.4. Water Content
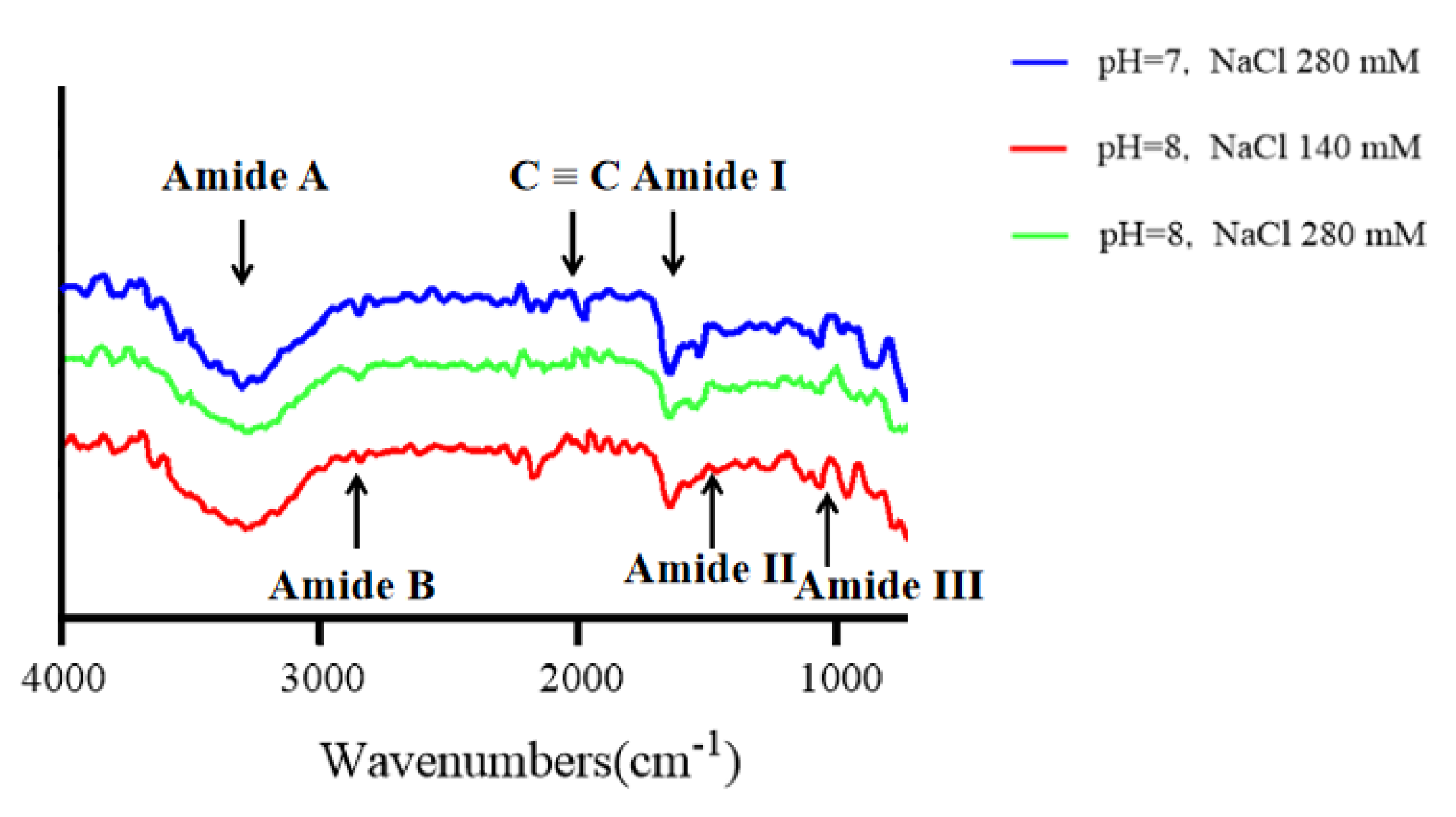
2.9.5. FTIR
3. Conclusions
4. Materials and Methods
4.1. Sample Collection and Processing
4.2. Isolation and Purification of Collagen
4.3. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
4.4. Amino Acid Analysis
4.5. Thermal Stability Analysis
4.6. Antioxidant Activity
4.7. Collagen Fibril Formation In Vitro
4.8. Fourier Transform Infrared (FTIR) Spectroscopy
4.9. Morphology of Collagen Fibrils Formed In Vitro
4.10. Collagen Gel Preparation and Property Analysis
4.10.1. Preparation of Gels
4.10.2. Chromatic Aberration Measurement
4.10.3. Texture Measurement
4.10.4. Water Content Measurement
4.10.5. FTIR
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Ookawa, M.; Tan, Y.; Ura, K.; Adachi, S.; Takagi, Y. Biochemical characterisation and assessment of fibril-forming ability of collagens extracted from Bester sturgeon Huso huso × Acipenser ruthenus. Food Chem. 2014, 160, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Therkildsen, M.; Aluko, R.E.; Lametsch, R. Exploration of collagen recovered from animal by-products as a precursor of bioactive peptides: Successes and challenges. Crit. Rev. Food Sci. Nutr. 2019, 59, 2011–2027. [Google Scholar] [CrossRef] [PubMed]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Characterisation of acid soluble collagen from skins of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 85, 81–89. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, W.; Li, G. Isolation and characterisation of collagens from the skin of largefin longbarbel catfish (Mystus macropterus). Food Chem. 2009, 115, 826–831. [Google Scholar] [CrossRef]
- Zhang, X.; Adachi, S.; Ura, K.; Takagi, Y. Properties of collagen extracted from Amur sturgeon Acipenser schrenckii and assessment of collagen fibrils in vitro. Int. J. Biol. Macromol. 2019, 137, 809–820. [Google Scholar] [CrossRef]
- Li, Y.; Yang, L.; Wu, S.; Chen, J.; Lin, H. Structural, functional, rheological, and biological properties of the swim bladder collagen extracted from grass carp (Ctenopharyngodon idella). LWT 2022, 153, 112518. [Google Scholar] [CrossRef]
- Yamada, S.; Yamamoto, K.; Ikeda, T.; Yanagiguchi, K.; Hayashi, Y. Potency of fish collagen as a scaffold for regenerative medicine. Biomed. Res. Int. 2014, 2014, 302932. [Google Scholar] [CrossRef]
- Li, C.; Duan, L.; Tian, Z.; Liu, W.; Li, G.; Huang, X. Rheological behavior of acylated pepsin-solubilized collagen solutions: Effects of concentration. Korea-Australia Rheol. J. 2015, 27, 287–295. [Google Scholar] [CrossRef]
- Sionkowska, A.; Lewandowska, K.; Adamiak, K. The Influence of UV Light on Rheological Properties of Collagen Extracted from Silver Carp Skin. Materials 2020, 13, 4453. [Google Scholar] [CrossRef]
- Liao, W.; Guanghua, X.; Li, Y.; Shen, X.R.; Li, C. Comparison of characteristics and fibril-forming ability of skin collagen from barramundi (Lates calcarifer) and tilapia (Oreochromis niloticus). Int. J. Biol. Macromol. 2018, 107, 549–559. [Google Scholar] [CrossRef]
- Martínez-Alvarez, O.; Chamorro, S.; Brenes, A. Protein hydrolysates from animal processing by-products as a source of bioactive molecules with interest in animal feeding: A review. Food Res. Int. 2015, 73, 204–212. [Google Scholar] [CrossRef]
- Pal, G.K.; Suresh, P.V. Sustainable valorisation of seafood by-products: Recovery of collagen and development of collagen-based novel functional food ingredients. Innov. Food Sci. Emerg. Technol. 2016, 37, 201–215. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, X.; Li, T.; Yang, H.; Zhang, H.; Regenstein, J.M.; Zhou, P. Extraction and characterization of acid- and pepsin-soluble collagens from the scales, skins and swim-bladders of grass carp (Ctenopharyngodon idella). Food Biosci. 2015, 9, 68–74. [Google Scholar] [CrossRef]
- Liu, D.; Wei, G.; Li, T.; Hu, J.; Lu, N.; Regenstein, J.M.; Zhou, P. Effects of alkaline pretreatments and acid extraction conditions on the acid-soluble collagen from grass carp (Ctenopharyngodon idella) skin. Food Chem. 2015, 172, 836–843. [Google Scholar] [CrossRef]
- Hu, J.; Li, T.; Liu, X.; Liu, D. Seasonal variation of acid-soluble collagens extracted from the swim bladders and skins of bighead carp (Hypophthalmichthys nobilis) and grass carp (Ctenopharyngodon idella). Food Biosci. 2016, 15, 27–33. [Google Scholar] [CrossRef]
- Meng, D.; Li, W.; Ura, K.; Takagi, Y. Effects of phosphate ion concentration on in-vitro fibrillogenesis of sturgeon type I collagen. Int. J. Biol. Macromol. 2020, 148, 182–191. [Google Scholar] [CrossRef]
- Meng, D.; Tanaka, H.; Kobayashi, T.; Hatayama, H.; Zhang, X.; Ura, K.; Yunoki, S.; Takagi, Y. The effect of alkaline pretreatment on the biochemical characteristics and fibril-forming abilities of types I and II collagen extracted from bester sturgeon by-products. Int. J. Biol. Macromol. 2019, 131, 572–580. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Toriumi, S.; Ura, K.; Takagi, Y. Feasibility of collagens obtained from bester sturgeon Huso huso × Acipenser ruthenus for industrial use. Aquaculture 2020, 529, 735641. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Li, G.; Shi, B.; Miao, Y.; Wu, X. Isolation and partial characterization of pepsin-soluble collagen from the skin of grass carp (Ctenopharyngodon idella). Food Chem. 2007, 103, 906–912. [Google Scholar] [CrossRef]
- Cruz-Lopez, H.; Rodriguez-Morales, S.; Enriquez-Paredes, L.M.; Villarreal-Gomez, L.J.; Olivera-Castillo, L.; Cortes-Santiago, Y.; Lopez, L.M. Comparison of collagen characteristic from the skin and swim bladder of Gulf corvina (Cynoscion othonopterus). Tissue Cell 2021, 72, 101593. [Google Scholar] [CrossRef]
- Song, Z.; Liu, H.; Chen, L.; Chen, L.; Zhou, C.; Hong, P.; Deng, C. Characterization and comparison of collagen extracted from the skin of the Nile tilapia by fermentation and chemical pretreatment. Food Chem. 2021, 340, 128139. [Google Scholar] [CrossRef] [PubMed]
- Kaewdang, O.; Benjakul, S.; Kaewmanee, T.; Kishimura, H. Characteristics of collagens from the swim bladders of yellowfin tuna (Thunnus albacares). Food Chem. 2014, 155, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Chang, S.K.C. Isolation and characterization of collagen extracted from channel catfish (Ictalurus punctatus) skin. Food Chem. 2018, 242, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-K.; Lin, T.-Y.; Su, H.-P. Extraction and characterisation of telopeptide-poor collagen from porcine lung. Food Chem. 2011, 124, 1583–1588. [Google Scholar] [CrossRef]
- Ramírez-Guerra, H.E.; Mazorra-Manzano, M.A.; Ezquerra-Brauer, J.M.; Carvajal-Millán, E.; Pacheco-Aguilar, R.; Lugo-Sánchez, M.E.; Ramírez-Suárez, J.C. Hydroxylysyl-pyridinoline occurrence and chemical characteristics of collagen present in jumbo squid (Dosidicus gigas) tissues. J. Food Compost. Anal. 2015, 44, 10–17. [Google Scholar] [CrossRef]
- Mirzapour-Kouhdasht, A.; Moosavi-Nasab, M.; Krishnaswamy, K.; Khalesi, M. Optimization of gelatin production from Barred mackerel by-products: Characterization and hydrolysis using native and commercial proteases. Food Hydrocoll. 2020, 108, 105970. [Google Scholar] [CrossRef]
- Sarbon, N.M.; Badii, F.; Howell, N.K. Purification and characterization of antioxidative peptides derived from chicken skin gelatin hydrolysate. Food Hydrocoll. 2018, 85, 311–320. [Google Scholar] [CrossRef]
- Rajapakse, N.; Mendis, E.; Jung, W.-K.; Je, J.-Y.; Kim, S.-K. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res. Int. 2005, 38, 175–182. [Google Scholar] [CrossRef]
- Feng, Y.-X.; Ruan, G.-R.; Jin, F.; Xu, J.; Wang, F.-J. Purification, identification, and synthesis of five novel antioxidant peptides from Chinese chestnut (Castanea mollissima Blume) protein hydrolysates. LWT 2018, 92, 40–46. [Google Scholar] [CrossRef]
- Sousa, R.O.; Alves, A.L.; Carvalho, D.N.; Martins, E.; Oliveira, C.; Silva, T.H.; Reis, R.L. Acid and enzymatic extraction of collagen from Atlantic cod (Gadus Morhua) swim bladders envisaging health-related applications. J. Biomater Sci. Polym. Ed. 2020, 31, 20–37. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, H.; Yang, F.; Jin, S.; Liu, C.; Zhang, L.; Huang, J.; Wang, S.; Yan, Z.; Cai, X.; et al. Physicochemical, antioxidant properties of giant croaker (Nibea japonica) swim bladders collagen and wound healing evaluation. Int. J. Biol. Macromol. 2019, 138, 483–491. [Google Scholar] [CrossRef]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Moroi, S.; Miura, T.; Tamura, T.; Zhang, X.; Ura, K.; Takagi, Y. Self-assembled collagen fibrils from the swim bladder of Bester sturgeon enable alignment of MC3T3-E1 cells and enhance osteogenic differentiation. Mater. Sci. Eng. C 2019, 104, 109925. [Google Scholar] [CrossRef] [PubMed]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 86, 325–332. [Google Scholar] [CrossRef]
- Chen, J.; Li, L.; Yi, R.; Xu, N.; Gao, R.; Hong, B. Extraction and characterization of acid-soluble collagen from scales and skin of tilapia (Oreochromis niloticus). LWT 2016, 66, 453–459. [Google Scholar] [CrossRef]
- Pati, F.; Adhikari, B.; Dhara, S. Isolation and characterization of fish scale collagen of higher thermal stability. Bioresour. Technol. 2010, 101, 3737–3742. [Google Scholar] [CrossRef]
- He, L.; Lan, W.; Cen, L.; Chen, S.; Liu, S.; Liu, Y.; Ao, X.; Yang, Y. Improving catalase stability by its immobilization on grass carp (Ctenopharyngodon idella) scale collagen self-assembly films. Mater. Sci. Eng. C 2019, 105, 110024. [Google Scholar] [CrossRef]
- Skopinska-Wisniewska, J.; Olszewski, K.; Bajek, A.; Rynkiewicz, A.; Sionkowska, A. Dialysis as a method of obtaining neutral collagen gels. Mater. Sci. Eng. C 2014, 40, 65–70. [Google Scholar] [CrossRef]
- Tian, H.; Ren, Z.; Shi, L.; Hao, G.; Chen, J.; Weng, W. Self-assembly characterization of tilapia skin collagen in simulated body fluid with different salt concentrations. Process Biochem. 2021, 108, 153–160. [Google Scholar] [CrossRef]
- Duan, L.; Xiang, H.; Yang, X.; Liu, L.; Tian, Z.; Tian, H.; Li, J. The influences of 3,4-dihydroxybenzaldehyde on the microstructure and stability of collagen fibrils. Polym. Degrad. Stab. 2019, 161, 198–205. [Google Scholar] [CrossRef]
- Tang, C.; Zhou, K.; Zhu, Y.; Zhang, W.; Xie, Y.; Wang, Z.; Zhou, H.; Yang, T.; Zhang, Q.; Xu, B. Collagen and its derivatives: From structure and properties to their applications in food industry. Food Hydrocoll. 2022, 131, 107748. [Google Scholar] [CrossRef]
- Mredha, M.T.I.; Kitamura, N.; Nonoyama, T.; Wada, S.; Goto, K.; Zhang, X.; Nakajima, T.; Kurokawa, T.; Takagi, Y.; Yasuda, K.; et al. Anisotropic tough double network hydrogel from fish collagen and its spontaneous in vivo bonding to bone. Biomaterials 2017, 132, 85–95. [Google Scholar] [CrossRef]
- Jiang, Y.; Lan, W.; Sameen, D.E.; Ahmed, S.; Qin, W.; Zhang, Q.; Chen, H.; Dai, J.; He, L.; Liu, Y. Preparation and characterization of grass carp collagen-chitosan-lemon essential oil composite films for application as food packaging. Int. J. Biol. Macromol. 2020, 160, 340–351. [Google Scholar] [CrossRef]
- Mirzapour-Kouhdasht, A.; Moosavi-Nasab, M.; Kim, Y.M.; Eun, J.B. Antioxidant mechanism, antibacterial activity, and functional characterization of peptide fractions obtained from barred mackerel gelatin with a focus on application in carbonated beverages. Food Chem. 2021, 342, 128339. [Google Scholar] [CrossRef]
- Shi, L.; Tian, H.; Wang, Y.; Hao, G.; Chen, J.; Weng, W. Effect of pH on properties of golden pompano skin collagen-based fibril gels by self-assembly in vitro. J. Sci. Food Agric. 2020, 100, 4801–4807. [Google Scholar] [CrossRef]
- Mredha, M.T.I.; Zhang, X.; Nonoyama, T.; Nakajima, T.; Kurokawa, T.; Takagi, Y.; Gong, J.P. Swim bladder collagen forms hydrogel with macroscopic superstructure by diffusion induced fast gelation. J. Mater Chem. B 2015, 3, 7658–7666. [Google Scholar] [CrossRef]



| Amino Acid Composition | SC | SBC |
|---|---|---|
| Asp | 50.83 ± 3.19 | 45.66 ± 3.06 |
| Thr | 23.87 ± 0.38 | 27.23 ± 1.02 * |
| Ser | 33.74 ± 0.54 | 29.73 ± 1.35 |
| Glu | 71.52 ± 1.22 | 71.38 ± 1.81 |
| Gly | 331.98 ± 5.68 | 325.61 ± 15.12 |
| Ala | 115.89 ± 2.08 | 116.92 ± 5.79 |
| Val | 40.48 ± 0.69 | 41.94 ±2.59 |
| Met | 27.33 ± 1.43 | 28.08 ± 5.39 |
| Ile | 19.02 ± 0.69 | 15.76 ± 6.73 |
| Leu | 33.61 ± 4.36 | 29.79 ± 7.77 |
| Phe | 12.18 ± 0.78 | 13.56 ± 0.32 |
| His | 6.40 ± 0.52 | 7.30 ± 0.35 |
| Lys | 23.20 ± 0.44 | 21.41 ± 1.55 |
| Arg | 51.92 ± 0.59 | 54.62 ± 6.09 |
| Pro | 100.32 ± 1.37 | 97.57 ± 3.44 |
| Hypro | 48.67 ± 0.91 | 61.13 ± 0.96 ** |
| Hylys | 9.05 ± 0.28 | 12.35 ± 0.40 ** |
| Imino acid | 148.99 ± 0.47 | 158.69 ± 3.81 |
| Collagen Fibril | SC | SBC |
|---|---|---|
| pH 6, NaCl 0 mM | 57.75 ± 0.33 | 61.01 ± 0.14 * |
| pH 6, NaCl 140 mM | 63.77 ± 0.29 | 67.73 ± 0.27 * |
| pH 6, NaCl 280 mM | 69.57 ± 0.26 | 73.41 ± 0.23 * |
| pH 7, NaCl 0 mM | 69.73 ± 0.16 | 74.55 ± 0.19 * |
| pH 7, NaCl 140 mM | 80.21 ± 0.25 | 81.31 ± 0.12 * |
| pH 7, NaCl 280 mM | 89.15 ± 0.14 | 89.77 ± 0.47 |
| pH 8, NaCl 0 mM | 73.47 ± 0.28 | 91.49 ± 0.15 * |
| pH 8, NaCl 140 mM | 83.75 ± 0.23 | 95.07 ± 0.67 * |
| pH 8, NaCl 280 mM | 93.60 ± 0.27 | 98.78 ± 0.13 * |
| SC Gel | pH 7, NaCl 280 mM | pH 8, NaCl 140 mM | pH 8, NaCl 280 mM |
|---|---|---|---|
| L | 73.47 ± 0.43 a | 71.49 ± 0.45 b | 68.40 ± 0.46 c |
| a | 6.32 ± 0.04 a | 7.18 ± 0.13 b | 6.48 ± 0.11 a |
| b | −5.56 ± 0.29 a | −0.58 ± 0.01 b | −6.17 ± 0.22 a |
| △L | −23.91 ± 0.43 a | −25.89 ± 0.31 b | −28.98 ± 0.46 c |
| △a | +0.40 ± 0.04 a | +1.27 ± 0.13 b | +0.67 ± 0.04 a |
| △b | −2.43 ± 0.10 a | +3.05 ± 0.17 b | −2.84 ± 0.15 a |
| △E | 24.01 ± 0.44 a | 23.46 ± 0.41 a | 29.42 ± 0.21 b |
| SC Gel | pH 7, NaCl 280 mM | pH 8, NaCl 140 mM | pH 8, NaCl 280 mM |
|---|---|---|---|
| Hardness | 925.91 ± 47.78 a | 666.91 ± 45.97 b | 1376.14 ± 72.12 c |
| Springiness | 0.82 ± 0.01 a | 0.94 ± 0.01 b | 0.93 ± 0.01 b |
| Cohesiveness | 0.32 ± 0.03 a | 0.41 ± 0.02 ab | 0.48 ± 0.038 b |
| Chewiness | 279.49 ± 15.429 a | 218.98 ± 4.88 b | 182.12 ± 3.33 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Z.; Zhang, Q.; Li, L.; Li, D.; Takagi, Y.; Zhang, X. Properties of Grass Carp (Ctenopharyngodon idella) Collagen and Gel for Application in Biomaterials. Gels 2022, 8, 699. https://doi.org/10.3390/gels8110699
Shen Z, Zhang Q, Li L, Li D, Takagi Y, Zhang X. Properties of Grass Carp (Ctenopharyngodon idella) Collagen and Gel for Application in Biomaterials. Gels. 2022; 8(11):699. https://doi.org/10.3390/gels8110699
Chicago/Turabian StyleShen, Zhiyuan, Qi Zhang, Li Li, Dapeng Li, Yasuaki Takagi, and Xi Zhang. 2022. "Properties of Grass Carp (Ctenopharyngodon idella) Collagen and Gel for Application in Biomaterials" Gels 8, no. 11: 699. https://doi.org/10.3390/gels8110699
APA StyleShen, Z., Zhang, Q., Li, L., Li, D., Takagi, Y., & Zhang, X. (2022). Properties of Grass Carp (Ctenopharyngodon idella) Collagen and Gel for Application in Biomaterials. Gels, 8(11), 699. https://doi.org/10.3390/gels8110699








