Synthesis and Characterization of Carboxymethyl Chitosan Nanosponges with Cyclodextrin Blends for Drug Solubility Improvement
Abstract
1. Introduction
2. Results and Discussion
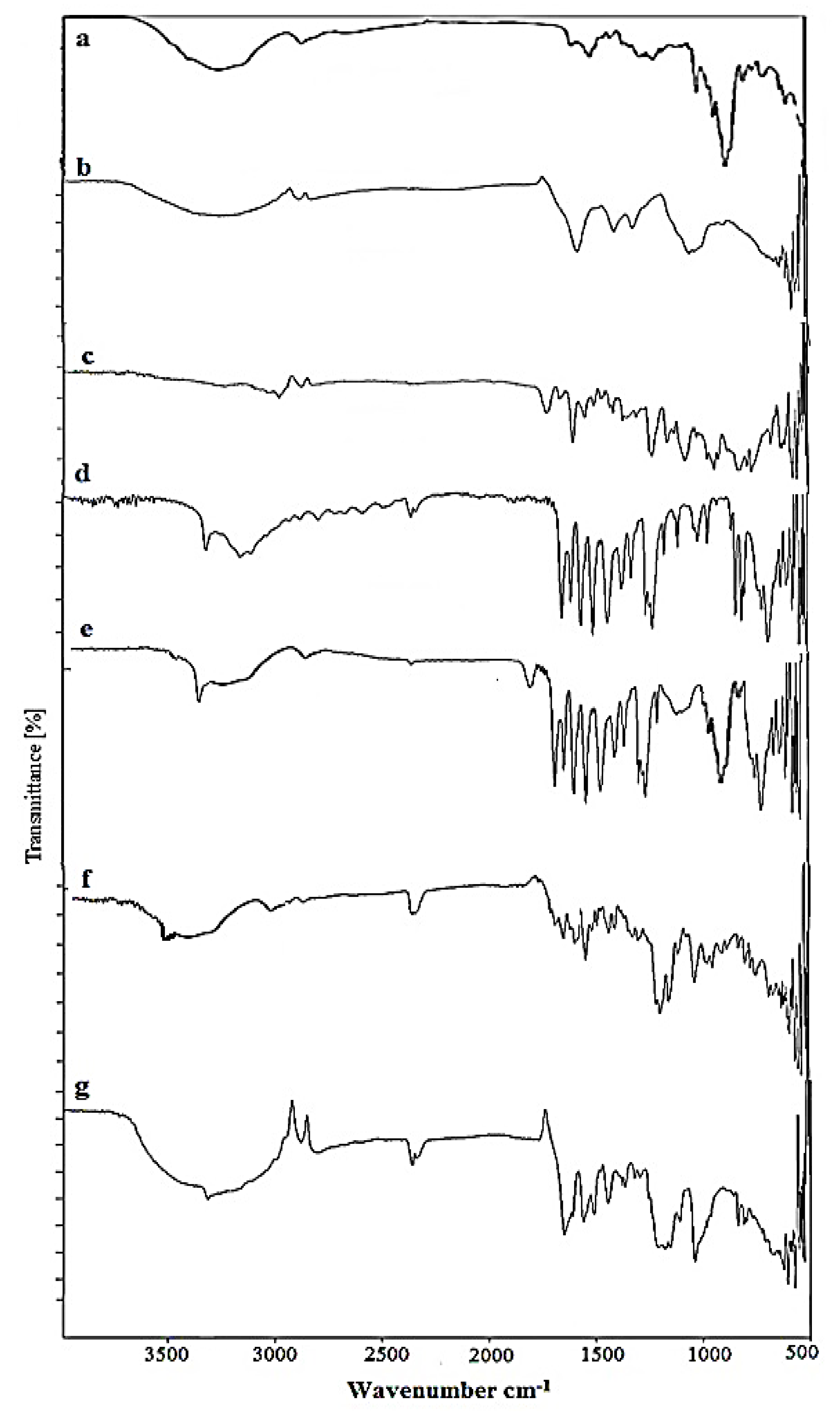
2.1. FTIR Analysis of Nanosponges
2.2. Powder X-ray Diffraction Analysis
2.3. TGA Analysis of Nanosponges
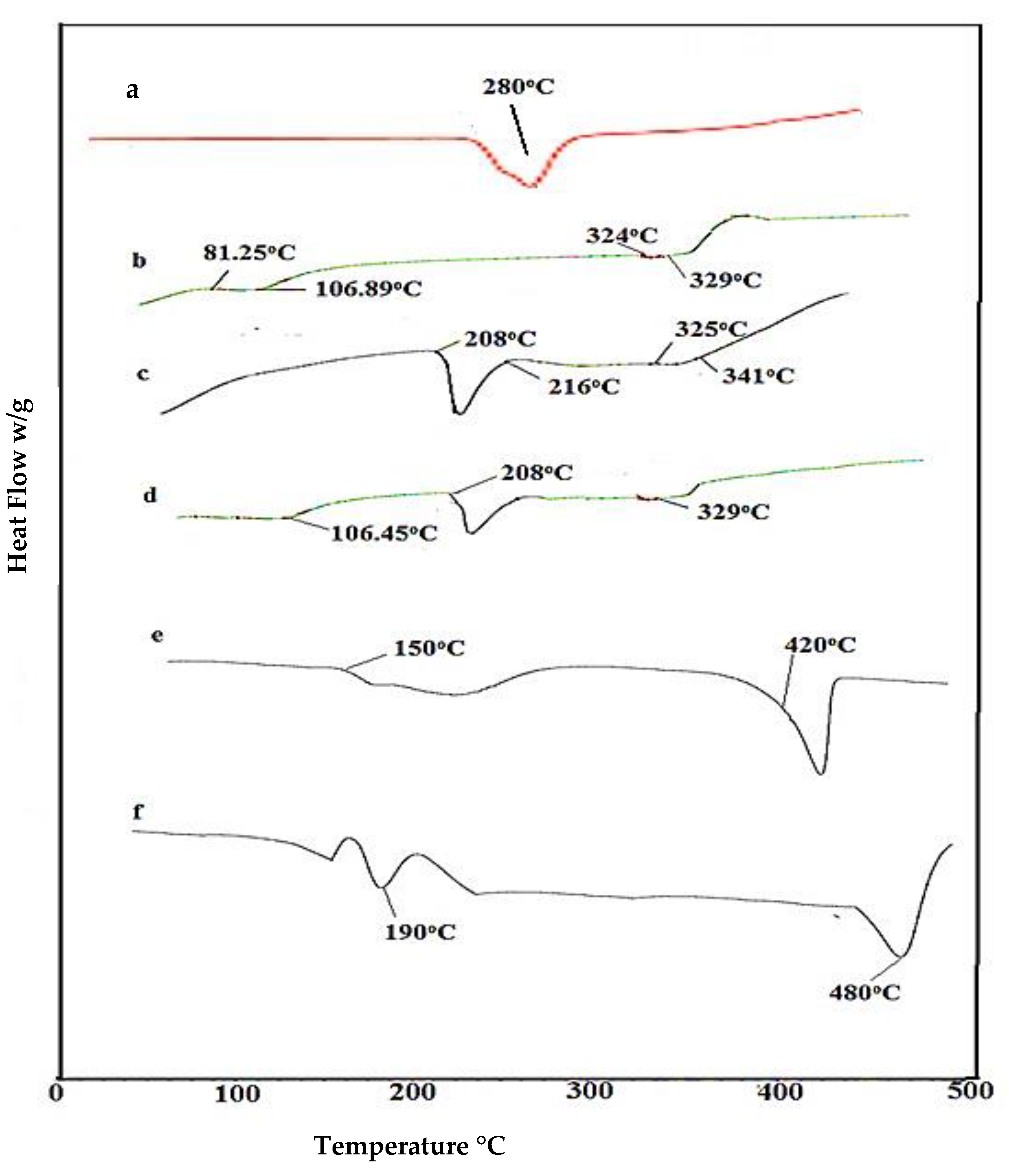
2.4. DSC Analysis of Nanosponges
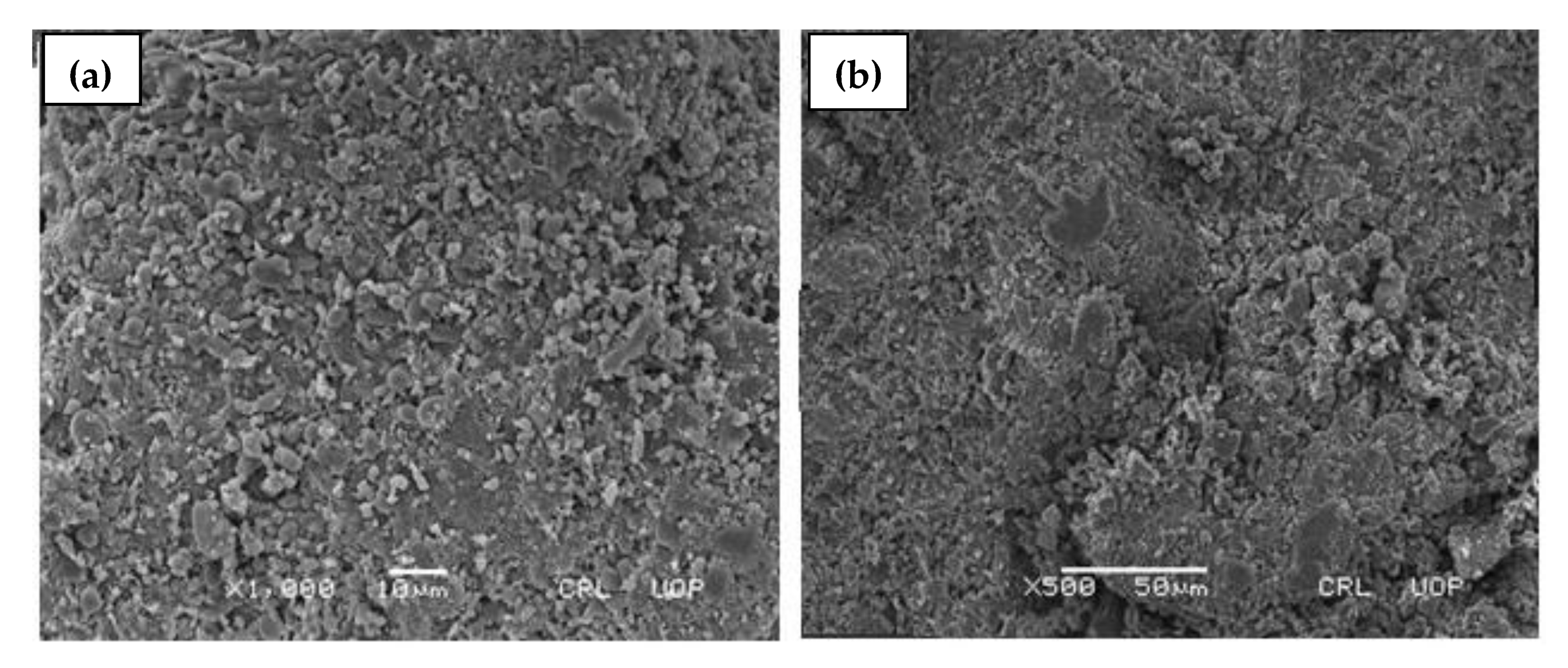
2.5. Scanning Electron Microscopy (SEM)
2.6. Particle Size Analysis
2.7. Drug Entrapment Efficiency (DEE %) and Drug Loaded contents (DLC%)
2.8. Solubilization Efficiency
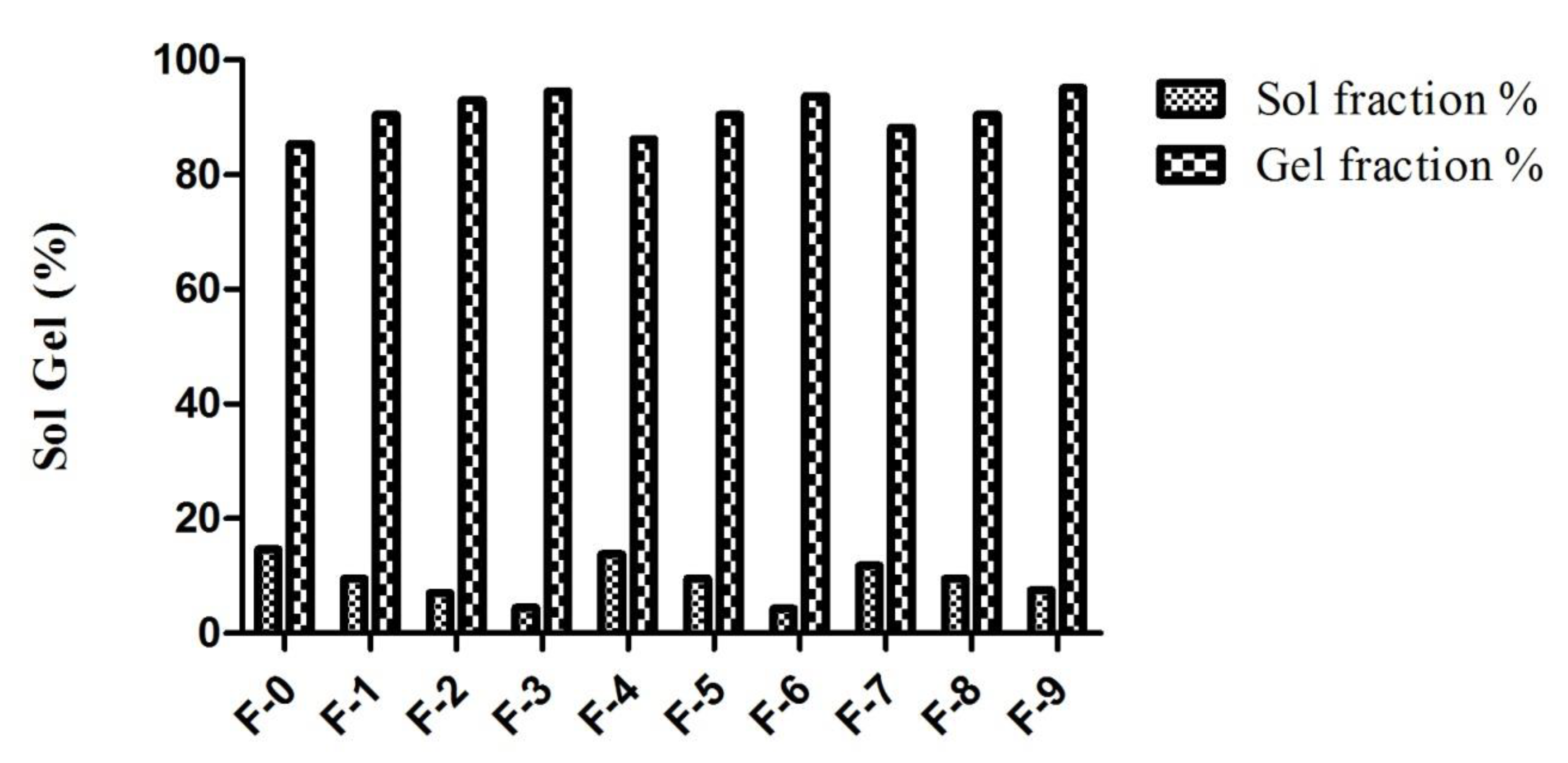
2.9. Sol Gel Analysis
2.10. Swelling Analysis
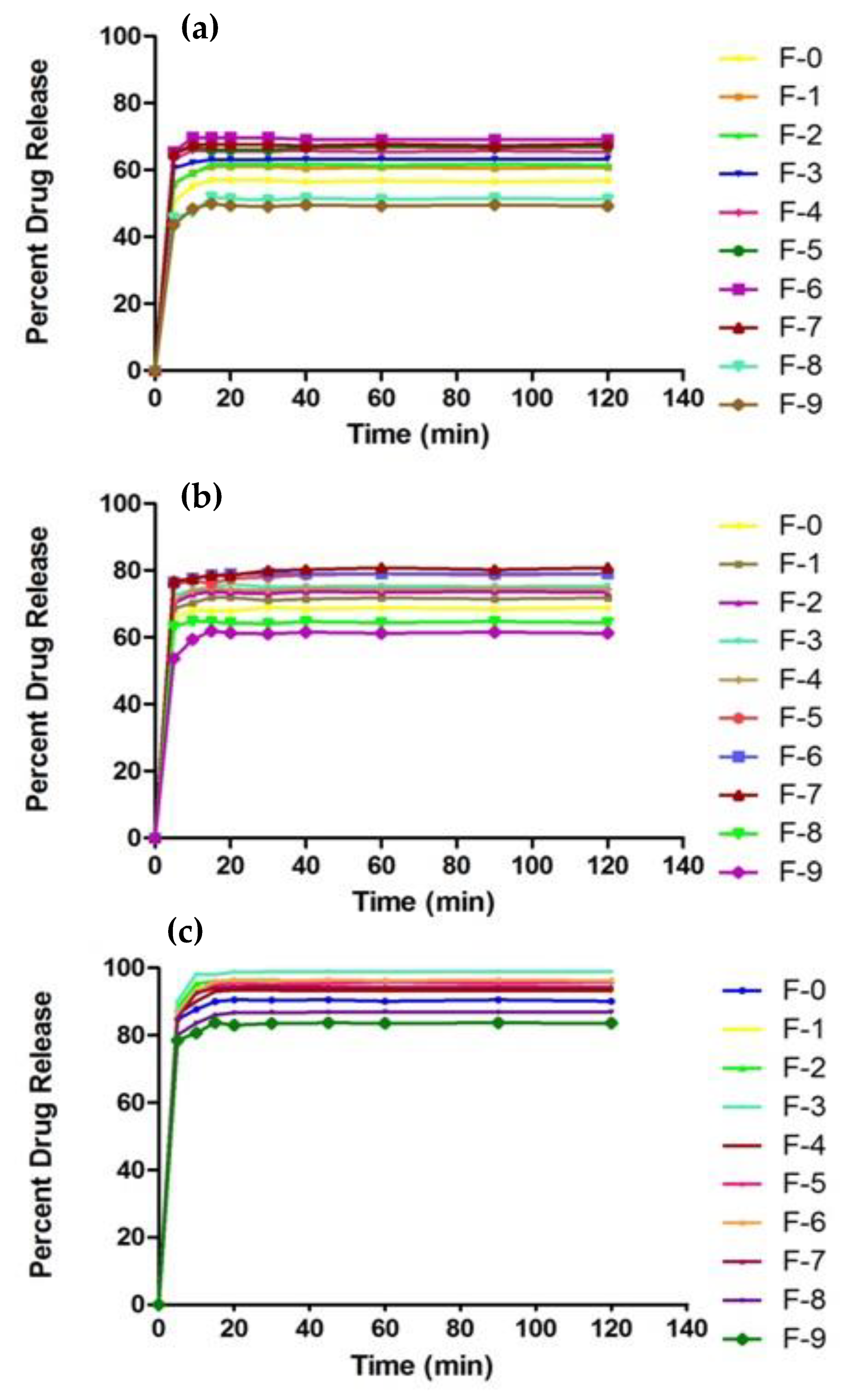
2.11. In Vitro Drug Release Studies
2.12. In Vivo Toxicity Study
2.12.1. Clinical manifestations
2.12.2. Blood Analysis
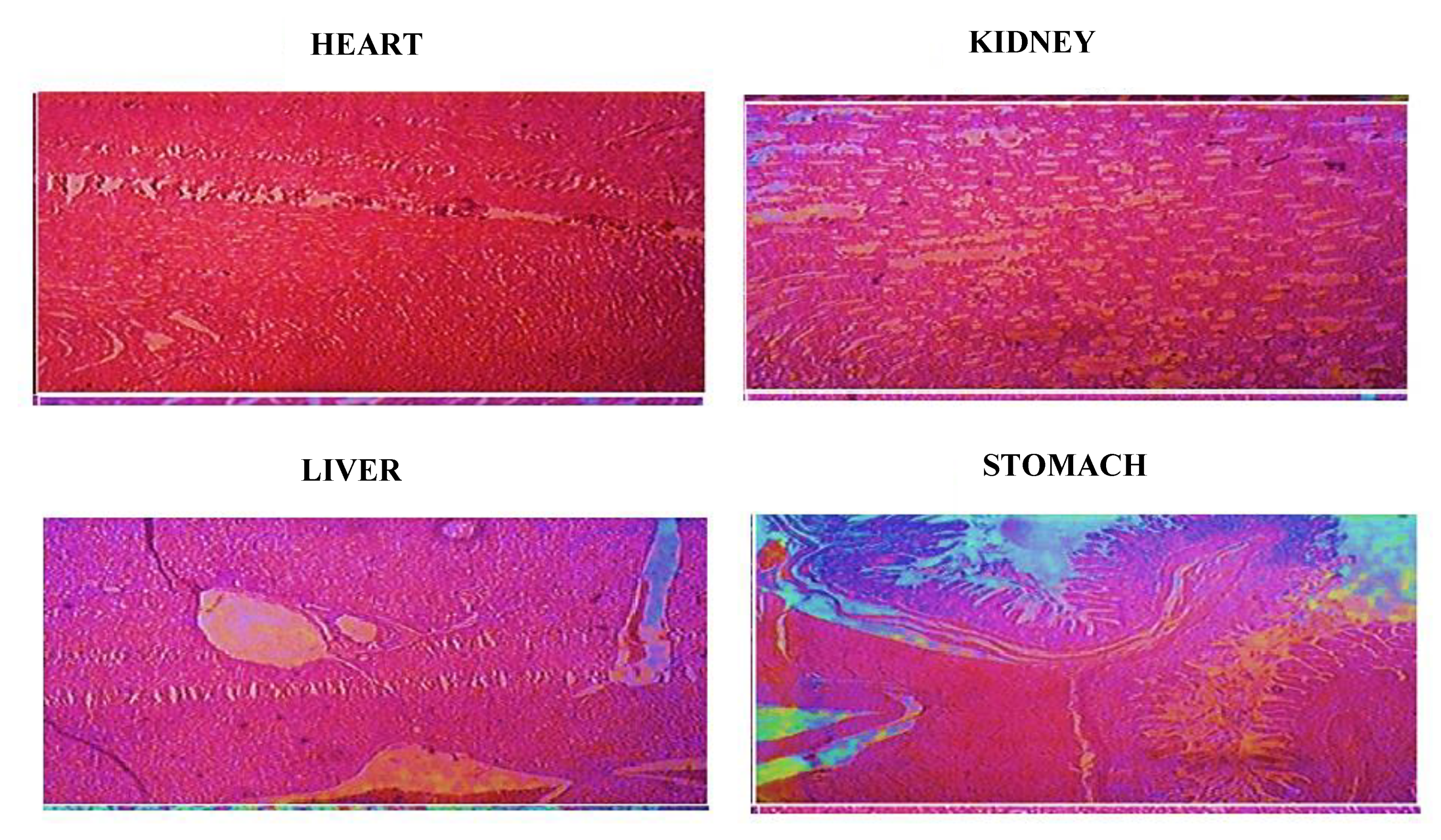
2.12.3. Histopathological Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
Fabrication of βCD-CMC g-poly (AMPS) Nanosponges
4.3. Characterization of Fabricated Nanosponges
4.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
4.3.2. Thermo-Gravimetric Analysis (TGA)
4.3.3. Differential Scanning Calorimeter (DSC)
4.3.4. Powder X-ray Diffraction (PXRD Analysis
4.3.5. Particle Size Analysis
4.3.6. Scanning Electron Microscopy
4.3.7. Solubilization Efficiency
4.3.8. Sol Gel Analysis
4.3.9. Swelling Studies
4.3.10. Drug Loading Studies
4.3.11. Percent Drug-Loaded Contents (%DLC) and Percent Drug Entrapment Efficiency (%DEE)
4.3.12. In Vitro Drug Release Studies
4.3.13. In Vivo Toxicity Studies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, J.; Shen, Q.; Fang, L. Sulfobutylether-β-cyclodextrin/chitosan nanoparticles enhance the oral permeability and bioavailability of docetaxel. Drug Dev. Ind. Pharm. 2013, 39, 1010–1019. [Google Scholar] [CrossRef]
- Ringel, I.; Horwitz, S.B. Studies with RP 56976 (Taxotere): A Semisynthetic Analogue of Taxol. JNCI J. Natl. Cancer Inst. 1991, 83, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.J.; Rivory, L. Clinical Pharmacokinetics of Docetaxel. Clin. Pharmacokinet. 1999, 36, 99–114. [Google Scholar] [CrossRef]
- Dou, J.; Zhang, H.; Liu, X.; Zhang, M.; Zhai, G. Preparation and evaluation in vitro and in vivo of docetaxel loaded mixed micelles for oral administration. Colloids Surf. B Biointerfaces 2014, 114, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Duchêne, D.; Ponchel, G.; Wouessidjewe, D. Cyclodextrins in targeting: Application to nanoparticles. Adv. Drug Deliv. Rev. 1999, 36, 29–40. [Google Scholar] [CrossRef]
- Valerian, T.; Kenny, B. Introduction and general overview of cyclodextrin chemistry. J. Chem. Rev. 1998, 98, 1731. [Google Scholar]
- Del Valle, E. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Trotta, F.; Dianzani, C.; Caldera, F.; Mognetti, B.; Cavalli, R. The application of nanosponges to cancer drug delivery. Expert Opin. Drug Deliv. 2014, 11, 931–941. [Google Scholar] [CrossRef]
- Roux, M.; Perly, B.; Djedaini-Pilard, F. Self-assemblies of amphiphilic cyclodextrins. Eur. Biophys. J. 2007, 36, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Kayaman-Apohan, N.; Akyürek, E. Synthesis and drug-release properties of biodegradable hydrogels having β-cyclodextrin. Polym. Bull. 2013, 70, 2151–2167. [Google Scholar] [CrossRef]
- Loftsson, T.; Duchêne, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, H.S.; Al-Sa’Bani, A.F.; Darmokoesoemo, H. N,O-Carboxymethyl Chitosan: An Innovation in New Natural Preservative from Shrimp Shell Waste with a Nutritional Value and Health Orientation. Procedia Food Sci. 2015, 3, 35–51. [Google Scholar] [CrossRef]
- Zhu, G.-Q.; Wang, F.-G.; Liu, Y.-Y.; Gao, Q.-C. Properties of water-soluble carboxymethyl chitosan film modified by hydrophobic poly(propylene glycol). Chem. Pap. 2013, 67, 423–428. [Google Scholar] [CrossRef]
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef]
- Tong, D.; Maitland, G.; Trusler, M.; Fennell, P. Solubility of carbon dioxide in aqueous blends of 2-amino-2-methyl-1-propanol and piperazine. Chem. Eng. Sci. 2013, 101, 851–864. [Google Scholar] [CrossRef]
- Wen, Y.; Wei, B.; Cheng, D.; An, X.; Ni, Y. Stability enhancement of nanofibrillated cellulose in electrolytes through grafting of 2-acrylamido-2-methylpropane sulfonic acid. Cellulose 2017, 24, 731–738. [Google Scholar] [CrossRef]
- Mahmood, A.; Ahmad, M.; Sarfraz, R.M.; Minhas, M.U. β-CD based hydrogel microparticulate system to improve the solubility of acyclovir: Optimization through in-vitro, in-vivo and toxicological evaluation. J. Drug Deliv. Sci. Technol. 2016, 36, 75–88. [Google Scholar] [CrossRef]
- Selvamuthukumar, S.; Anandam, S.; Krishnamoorthy, K.; Rajappan, M. Nanosponges: A novel class of drug delivery system-review. J. Pharm. Pharm. Sci. 2012, 15, 103–111. [Google Scholar]
- David, F. Nanosponge drug delivery system more effective than direct injection. Pharm. Dev. Technol. 2011, 16, 367–376. [Google Scholar]
- Trotta, F.; Tumiatti, V.; Cavalli, R.; Rogero, C.; Mognetti, B.; Berta, G. Cyclodextrin-based nanosponges as a vehicle for antitumoral drugs. WO 2009, 3656, A1. [Google Scholar]
- Marchettini, P.; Stuart, A.O.; Mohamed, F.; Yoo, D.; Sugarbaker, P.H. Docetaxel: Pharmacokinetics and tissue levels after intraperitoneal and intravenous administration in a rat model. Cancer Chemother. Pharmacol. 2002, 49, 499–503. [Google Scholar] [CrossRef]
- Yin, Y.-M.; Cui, F.-D.; Mu, C.-F.; Choi, M.-K.; Kim, J.S.; Chung, S.-J.; Shim, C.-K.; Kim, D.-D. Docetaxel microemulsion for enhanced oral bioavailability: Preparation and in vitro and in vivo evaluation. J. Control. Release 2009, 140, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Krauland, A.H.; Alonso, M.J. Chitosan/cyclodextrin nanoparticles as macromolecular drug delivery system. Int. J. Pharm. 2007, 340, 134–142. [Google Scholar] [CrossRef]
- Maestrelli, F.; Garcia-Fuentes, M.; Mura, P.; Alonso, M.J. A new drug nanocarrier consisting of chitosan and hydoxypropylcyclodextrin. Eur. J. Pharm. Biopharm. 2006, 63, 79–86. [Google Scholar] [CrossRef]
- Trapani, A.; Lopedota, A.; Franco, M.; Cioffi, N.; Ieva, E.; Garcia-Fuentes, M.; Alonso, M.J. A comparative study of chitosan and chitosan/cyclodextrin nanoparticles as potential carriers for the oral delivery of small peptides. Eur. J. Pharm. Biopharm. 2010, 75, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Thakur, K.; Kush, P.; Jain, U.K. Docetaxel loaded chitosan nanoparticles: Formulation, characterization and cytotoxicity studies. Int. J. Biol. Macromol. 2014, 69, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Ahmad, M.; Sarfraz, R.M.; Minhas, M.U. Development of Acyclovir Loaded β-Cyclodextrin-g-Poly Methacrylic Acid Hydrogel Microparticles: An In Vitro Characterization. Adv. Polym. Technol. 2018, 37, 697–705. [Google Scholar] [CrossRef]
- Sarfraz, R.M.; Ahmad, M.; Mahmood, A.; Ijaz, H. Development, In Vitro and In Vivo Evaluation of pH Responsive β-CD-Comethacrylic Acid-Crosslinked Polymeric Microparticulate System for Solubility Enhancement of Rosuvastatin Calcium. Polym. Technol. Eng. 2018, 57, 1175–1187. [Google Scholar] [CrossRef]
- Anitha, A.; Maya, S.; Deepa, N.; Chennazhi, K.; Nair, S.; Tamura, H.; Jayakumar, R. Efficient water soluble O-carboxymethyl chitosan nanocarrier for the delivery of curcumin to cancer cells. Carbohydr. Polym. 2011, 83, 452–461. [Google Scholar] [CrossRef]
- Vaghani, S.S.; Patel, M.M.; Satish, C.S.; Patel, K.M.; Jivani, N.P. Synthesis and characterization of carboxymethyl chitosan hydrogel: Application as site specific delivery for lercanidipine hydrochloride. Bull. Mater. Sci. 2012, 35, 1133–1142. [Google Scholar] [CrossRef]
- An, N.T.; Thien, D.T.; Dong, N.T.; Le Dung, P. Water-soluble N-carboxymethylchitosan derivatives: Preparation, characteristics and its application. Carbohydr. Polym. 2009, 75, 489–497. [Google Scholar] [CrossRef]
- Rastogi, P.K.; Krishnamoorthi, S.; Ganesan, V. Synthesis, characterization, and ion exchange voltammetry study on 2-acrylamido-2-methylpropane sulphonic acid and N-(hydroxymethyl) acrylamide-based copolymer. J. Appl. Polym. Sci. 2012, 123, 929–935. [Google Scholar] [CrossRef]
- Azmeera, V.; Adhikary, P.; Krishnamoorthi, S. Synthesis and Characterization of Graft Copolymer of Dextran and 2-Acrylamido-2-methylpropane Sulphonic Acid. Int. J. Carbohydr. Chem. 2012, 2012, 209085. [Google Scholar] [CrossRef]
- Guo, Y.; Shalaev, E.; Smith, S. Physical stability of pharmaceutical formulations: Solid-state characterization of amorphous dispersions. TrAC Trends Anal. Chem. 2013, 49, 137–144. [Google Scholar] [CrossRef]
- Fang, G.; Tang, B.; Liu, Z.; Gou, J.; Zhang, Y.; Xu, H.; Tang, X. Novel hydrophobin-coated docetaxel nanoparticles for intravenous delivery: In vitro characteristics and in vivo performance. Eur. J. Pharm. Sci. 2014, 60, 1–9. [Google Scholar] [CrossRef]
- Tsao, J.-Y.; Tsai, H.-H.; Wu, C.-P.; Lin, P.-Y.; Su, S.-Y.; Chen, L.-D.; Tsai, F.-J.; Tsai, Y. Release of paeonol-β-CD complex from thermo-sensitive poly(N-isopropylacrylamide) hydrogels. Int. J. Pharm. 2010, 402, 123–128. [Google Scholar] [CrossRef]
- Farag, R.K.; Mohamed, R.R. Synthesis and Characterization of Carboxymethyl Chitosan Nanogels for Swelling Studies and Antimicrobial Activity. Molecules 2013, 18, 190–203. [Google Scholar] [CrossRef]
- Shoukat, H.; Pervaiz, F.; Noreen, S.; Nawaz, M.; Qaiser, R.; Anwar, M. Fabrication and evaluation studies of novel polyvinylpyrrolidone and 2-acrylamido-2-methylpropane sulphonic acid-based crosslinked matrices for controlled release of acyclovir. Polym. Bull. 2020, 77, 1869–1891. [Google Scholar] [CrossRef]
- Khan, K.U.; Minhas, M.U.; Badshah, S.F.; Sohail, M.; Sarfraz, R.M. β-cyclodextrin modification by cross-linking polymerization as highly porous nanomatrices for olanzapine solubility improvement; synthesis, characterization and bio-compatibility evaluation. J. Drug Deliv. Sci. Technol. 2021, 67, 102952. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, H.-M. Crosslinked carboxymethylchitosan-g-poly(acrylic acid) copolymer as a novel superabsorbent polymer. Carbohydr. Res. 2006, 341, 887–896. [Google Scholar] [CrossRef]
- Kabiri, K.; Azizi, A.; Zohuriaan, M.M.; Bagheri, M.G.; Bouhendi, H.; Jamshidi, A. Super-alcogels based on 2-acrylamido-2-methylpropane sulphonic acid and poly (ethylene glycol) macromer. Iran. Polym. J. 2011, 20, 175–183. [Google Scholar]
- Malik, N.S.; Ahmad, M.; Minhas, M.U. Cross-linked β-cyclodextrin and carboxymethyl cellulose hydrogels for controlled drug delivery of acyclovir. PLoS ONE 2017, 12, e0172727. [Google Scholar] [CrossRef]
- Sharif, Q.-U.; Sohail, M.; Ahmad, M.; Minhas, M.U.; Khan, S.; Khan, S.; Kousar, M. Novel polymeric composites based on carboxymethyl chitosan and poly(acrylic acid): In vitro and in vivo evaluation. J. Mater. Sci. Mater. Electron. 2017, 28, 147. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Mohamed, R.R.; Eltaweel, S.H.; Seoudi, R.S. Crosslinked poly (vinyl alcohol)/carboxymethyl chitosan hydrogels for removal of metal ions and dyestuff from aqueous solutions. J. Appl. Polym. Sci. 2012, 123, 3459–3469. [Google Scholar] [CrossRef]
- Asghar, S.; Akhtar, N.; Minhas, M.U.; Khan, K.U. Bi-polymeric Spongy Matrices through Cross-linking Polymerization: Synthesized and Evaluated for Solubility Enhancement of Acyclovir. AAPS PharmSciTech 2021, 22, 181. [Google Scholar] [CrossRef]
- Zhang, C. and A.J. Easteal, Study of free-radical copolymerization of N-isopropylacrylamide with 2-acrylamido-2-methyl-1-propanesulphonic acid. J. Appl. Polym. Sci. 2003, 88, 2563–2569. [Google Scholar] [CrossRef]
- Cheng, W.-M.; Hu, X.-M.; Wang, D.-M.; Liu, G.-H. Preparation and Characteristics of Corn Straw-Co-AMPS-Co-AA Superabsorbent Hydrogel. Polymers 2015, 7, 2431–2445. [Google Scholar] [CrossRef]
- Rao, M.; Bajaj, A.; Khole, I.; Munjapara, G.; Trotta, F. In vitro and in vivo evaluation of β-cyclodextrin-based nanosponges of telmisartan. J. Incl. Phenom. Macrocycl. Chem. 2013, 77, 135–145. [Google Scholar] [CrossRef]
- Jinno, J.-I.; Kamada, N.; Miyake, M.; Yamada, K.; Mukai, T.; Odomi, M.; Toguchi, H.; Liversidge, G.G.; Higaki, K.; Kimura, T. Effect of particle size reduction on dissolution and oral absorption of a poorly water-soluble drug, cilostazol, in beagle dogs. J. Control. Release 2006, 111, 56–64. [Google Scholar] [CrossRef]
- Hecq, J.; Deleers, M.; Fanara, D.; Vranckx, H.; Amighi, K. Preparation and characterization of nanocrystals for solubility and dissolution rate enhancement of nifedipine. Int. J. Pharm. 2005, 299, 167–177. [Google Scholar] [CrossRef]
- Khan, K.U.; Akhtar, N.; Minhas, M.U. Poloxamer-407-Co-Poly (2-Acrylamido-2-Methylpropane Sulfonic Acid) Cross-linked Nanogels for Solubility Enhancement of Olanzapine: Synthesis, Characterization, and Toxicity Evaluation. AAPS PharmSciTech 2020, 21, 141. [Google Scholar] [CrossRef]
- Badshah, S.F.; Akhtar, N.; Minhas, M.U.; Khan, K.U.; Khan, S.; Abdullah, O.; Naeem, A. Porous and highly responsive cross-linked β-cyclodextrin based nanomatrices for improvement in drug dissolution and absorption. Life Sci. 2021, 267, 118931. [Google Scholar] [CrossRef]
- Penjuri, S.C.B.; Ravouru, N.; Damineni, S.; Bns, S.; Poreddy, S.R. Formulation and Evaluation of Lansoprazole Loaded Nanosponges. Turk. J. Pharm. Sci. 2016, 13, 304–310. [Google Scholar] [CrossRef]
- Tiwari, G.; Tiwari, R.; Rai, A.K. Cyclodextrins in delivery systems: Applications. J. Pharm. Bioallied Sci. 2010, 2, 72–79. [Google Scholar] [CrossRef]
- Khan, K.U.; Minhas, M.U.; Badshah, S.F.; Suhail, M.; Ahmad, A.; Ijaz, S. Overview of nanoparticulate strategies for solubility enhancement of poorly soluble drugs. Life Sci. 2022, 291, 120301. [Google Scholar] [CrossRef]
- Darandale, S.S.; Vavia, P.R. Cyclodextrin-based nanosponges of curcumin: Formulation and physicochemical characterization. J. Incl. Phenom. Macrocycl. Chem. 2013, 75, 315–322. [Google Scholar] [CrossRef]
- Torne, S.; Darandale, S.; Vavia, P.; Trotta, F.; Cavalli, R. Cyclodextrin-based nanosponges: Effective nanocarrier for Tamoxifen delivery. Pharm. Dev. Technol. 2013, 18, 619–625. [Google Scholar] [CrossRef]
- Trotta, F.; Cavalli, R. Characterization and Applications of New Hyper-Cross-Linked Cyclodextrins. Compos. Interfaces 2009, 16, 39–48. [Google Scholar] [CrossRef]
- Khalid, Q.; Ahmad, M.; Minhas, M.U. Synthesis of β-cyclodextrin hydrogel nanoparticles for improving the solubility of dexibuprofen: Characterization and toxicity evaluation. Drug Dev. Ind. Pharm. 2017, 43, 1873–1884. [Google Scholar] [CrossRef]
- Ali, L.; Ahmad, M.; Usman, M.; Yousuf, M. Controlled release of highly water-soluble antidepressant from hybrid copolymer poly vinyl alcohol hydrogels. Polym. Bull. 2014, 71, 31–46. [Google Scholar] [CrossRef]
- Ali, L.; Ahmad, M.; Usman, M. Evaluation of cross-linked hydroxypropyl methylcellulose graft-methacrylic acid copolymer as extended release oral drug carrier. Cell. Chem. Technol. 2015, 49, 143–151. [Google Scholar]
- Ranjha, N.M.; Qureshi, U.F. Preparation and characterization of crosslinked acrylic acid/hydroxypropyl methyl cellulose hydrogels for drug delivery. Int. J. Pharm. Pharm. Sci. 2014, 6, 410. [Google Scholar]
- Wang, W.; Wang, A. Synthesis and swelling properties of pH-sensitive semi-IPN superabsorbent hydrogels based on sodium alginate-g-poly (sodium acrylate) and polyvinylpyrrolidone. Carbohydr. Polym. 2010, 80, 1028–1036. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Liu, Y.-L.; Chang, Y.; Higuchi, A.; Freeman, B.D. Effect of UV intensity on structure, water sorption, and transport properties of crosslinked N-vinyl-2-pyrrolidone/N,N′-methylenebisacrylamide films. J. Membr. Sci. 2010, 348, 47–55. [Google Scholar] [CrossRef]
- Khanum, H.; Ullah, K.; Murtaza, G.; Khan, S.A. Fabrication and in vitro characterization of HPMC-g-poly(AMPS) hydrogels loaded with loxoprofen sodium. Int. J. Biol. Macromol. 2018, 120, 1624–1631. [Google Scholar] [CrossRef]
- Jain, S.; Ancheria, R.K.; Shrivastava, S.; Soni, S.L.; Sharma, M. An overview of nanogel–novel drug delivery system. Asian J. Pharm. Res. Dev. 2019, 7, 47–55. [Google Scholar] [CrossRef]
- Bode, C.; Kranz, H.; Fivez, A.; Siepmann, F. Often neglected: PLGA/PLA swelling orchestrates drug release: HME implants. J. Control. Release 2019, 306, 97–107. [Google Scholar] [CrossRef]
- Taleb, M.A.; Alkahtani, A.; Mohamed, S.K. Radiation synthesis and characterization of sodium alginate/chitosan/hydroxyapatite nanocomposite hydrogels: A drug delivery system for liver cancer. Polym. Bull. 2015, 72, 725–742. [Google Scholar] [CrossRef]
- Minhas, M.U.; Ahmad, M.; Khan, S.; Ali, L.; Sohail, M. Synthesis and characterization of β-cyclodextrin hydrogels: Crosslinked polymeric network for targeted delivery of 5-fluorouracil. Drug Deliv. 2016, 9, 10. [Google Scholar]
- Suhail, M.; Khan, A.; Rosenholm, J.; Minhas, M.; Wu, P.-C. Fabrication and Characterization of Diclofenac Sodium Loaded Hydrogels of Sodium Alginate as Sustained Release Carrier. Gels 2021, 7, 10. [Google Scholar] [CrossRef]
- Anbarasan, S.; Brundha, B.A.; Pazhanisamy, P. Synthesis and swelling behavior of poly (NCA-co-AM/AMPS Na) hydrogels. J. Chem. 2010, 3, 571–575. [Google Scholar]
- Sohail, M.; Ahmad, M.; Minhas, M.U.; Ali, L.; Khalid, I.; Rashid, H. Controlled delivery of valsartan by cross-linked polymeric matrices: Synthesis, in vitro and in vivo evaluation. Int. J. Pharm. 2015, 487, 110–119. [Google Scholar] [CrossRef]
- Atta, A.M.; El Wahab, Z.H.A.; El Shafey, Z.A.; Zidan, W.I.; Akl, Z.F. Characterization and Evaluation of Acrylic Acid Co-2-acrylamido-2-methylpropane-1-sulfonic Acid Hydrogels for Uranium Recovery. J. Dispers. Sci. Technol. 2010, 31, 1415–1422. [Google Scholar] [CrossRef]
- Rao, K.S.V.K.; Ha, C.-S. pH Sensitive hydrogels based on acryl amides and their swelling and diffusion characteristics with drug delivery behavior. Polym. Bull. 2009, 62, 167–181. [Google Scholar] [CrossRef]
- Ravindra, S.; Mohan, Y.M.; Varaprasad, K.; Reddy, N.N.; Vimala, K.; Raju, K.M. Surfactant-Modified Poly(acrylamide- co -acrylamido propane sulphonic acid) Hydrogels. Int. J. Polym. Mater. 2009, 58, 278–296. [Google Scholar] [CrossRef]
- Mutar, M.A.; Radia, N.D. Controlled release from crosslinked polyacrylic acid as drug delivery theophylline. Iraqi Nat. J. Chem. 2012, 45, 67–85. [Google Scholar]
- Chavda, H.; Patel, C. Effect of crosslinker concentration on characteristics of superporous hydrogel. Int. J. Pharm. Investig. 2011, 1, 17–21. [Google Scholar] [CrossRef]
- Song, M.; Li, L.; Zhang, Y.; Chen, K.; Wang, H.; Gong, R. Carboxymethyl-β-cyclodextrin grafted chitosan nanoparticles as oral delivery carrier of protein drugs. React. Funct. Polym. 2017, 117, 10–15. [Google Scholar] [CrossRef]
- Sari, L.M.; Suyatna, F.D.; Subita, G.P.; Auerkari, E.I. Acute dermal toxicity study of areca catechu linn. Extract in sprague-dawley rats. Asian J. Pharm. Clin. Res. 2016, 9, 209. [Google Scholar] [CrossRef][Green Version]
- Onwusonye, J.C.; Muritala, I.K.; Odu, D.A.; Uzomba, N.I.; Ogbonnaya, M.; Duruamaku, C.C. Oral acute toxicity study of methanol leaf extracts of Croton Zambesicus in mice. Merit. Res. J. Pharm. Pharm. Sci. 2016, 2, 1–5. [Google Scholar]
- Ali, R.; Jaimini, A.; Nishad, D.K.; Mittal, G.; Chaurasia, O.P.; Kumar, R.; Bhatnagar, A.; Singh, S.B. Acute and sub acute toxicity and efficacy studies of Hippophae rhamnoides based herbal antioxidant supplement. Indian J. Pharmacol. 2012, 44, 504. [Google Scholar]
- Barkat, K.; Ahmad, M.; Minhas, M.U.; Khalid, I.; Nasir, B. Development and characterization of pH-responsive polyethylene glycol-co-poly(methacrylic acid) polymeric network system for colon target delivery of oxaliplatin: Its acute oral toxicity study. Adv. Polym. Technol. 2018, 37, 1806–1822. [Google Scholar] [CrossRef]
- de Lima, F.F.; Traesel, G.K.; Menegati, S.E.L.T.; dos Santos, A.C.; Souza, R.I.C.; DE Oliveira, V.S.; Argandoña, E.J.S.; Cardoso, C.A.L.; Oesterreich, S.A.; Vieira, M.D.C. Acute and subacute oral toxicity assessment of the oil extracted from Attalea phalerata Mart ex Spreng. pulp fruit in rats. Food Res. Int. 2017, 91, 11–17. [Google Scholar] [CrossRef]
- Shende, P.; Kulkarni, Y.A.; Gaud, R.; Deshmukh, K.; Cavalli, R.; Trotta, F.; Caldera, F. Acute and Repeated Dose Toxicity Studies of Different β-Cyclodextrin-Based Nanosponge Formulations. J. Pharm. Sci. 2015, 104, 1856–1863. [Google Scholar] [CrossRef]
- Tanveer, S.; Ahmad, M.; Minhas, M.U.; Ahmad, A.; Khan, K.U. Chitosan-PVA-co-poly (2-Acrylamido-2-Methylpropane Sulfonic Acid) Cross-linked Hybrid IPN-Nanogels for Transdermal Delivery of Ondansetron; Synthesis, Characterization and Toxicological Evaluation. Polym. Technol. Mater. 2021, 60, 1–22. [Google Scholar] [CrossRef]
- Khan, K.U.; Minhas, M.U.; Sohail, M.; Badshah, S.F.; Abdullah, O.; Khan, S.; Munir, A. Synthesis of PEG-4000-co-poly (AMPS) nanogels by cross-linking polymerization as highly responsive networks for enhancement in meloxicam solubility. Drug Dev. Ind. Pharm. 2021, 47, 465–476. [Google Scholar] [CrossRef]
- Khan, S.; Ranjha, N.M. Effect of degree of cross-linking on swelling and on drug release of low viscous chitosan/poly(vinyl alcohol) hydrogels. Polym. Bull. 2014, 71, 2133–2158. [Google Scholar] [CrossRef]





| S/No | Formulation Code | Average Particle Size of Nanosponges (nm) |
|---|---|---|
| 01 | F-0 | 195 ± 3 |
| 02 | F-1 | 213 ± 2 |
| 03 | F-2 | 238 ± 5 |
| 04 | F-3 | 250 ± 4 |
| 05 | F-4 | 224 ± 3 |
| 06 | F-5 | 209 ± 4 |
| 07 | F-6 | 211 ± 4 |
| 08 | F-7 | 218 ± 3 |
| 09 | F-8 | 205 ± 4 |
| 10 | F-9 | 199 ± 5 |
| Formulation Code | Varying Component | Entrapment Efficiency % | Drug Loaded Contents % | Sol Fraction % | Gel Fraction % |
|---|---|---|---|---|---|
| F-0 | Without CMC | 75.33 | 82.70 | 14.64 | 85.36 |
| F-1 | β-CD & CMC | 79.26 | 83.67 | 9.56 | 90.44 |
| F-2 | 83.10 | 87.58 | 7.06 | 92.94 | |
| F-3 | 87.41 | 91.74 | 4.46 | 94.54 | |
| F-4 | AMPS | 78.64 | 86.32 | 13.80 | 86.20 |
| F-5 | 81.74 | 88.92 | 9.56 | 90.44 | |
| F-6 | 85.32 | 89.32 | 4.26 | 93.74 | |
| F-7 | MBA | 76.73 | 86.57 | 11.76 | 88.24 |
| F-8 | 74.44 | 83.62 | 9.56 | 90.44 | |
| F-9 | 71.40 | 80.43 | 7.50 | 95.11 |
| Observations | Group I (Control) | Group II (Nanosponges Treated) |
|---|---|---|
| Signs of illness | Nil | Nil |
| Body weight (g) | ||
| Pretreatment | 233 ± 1.06 | 234 ± 1.03 |
| Day 1 | 233 ± 1.07 | 232 ± 0.63 |
| Day 7 | 234 ± 0.92 | 233 ± 0.57 |
| Day 14 | 235 ± 1.05 | 234 ± 1.03 |
| Water intake (mL) | ||
| Pretreatment | 37.40 ± 2.05 | 40.50 ± 2.15 |
| Day 1 | 39.31 ± 1.10 | 38.66 ± 1.40 |
| Day 7 | 36.13 ± 2.04 | 41.21 ± 2.52 |
| Day 14 | 38.97 ± 1.71 | 39.24 ± 1.31 |
| Food intake (g) | ||
| Pretreatment | 15 ± 1.51 | 17 ± 1.24 |
| Day 1 | 17 ± 1.01 | 16 ± 1.01 |
| Day 7 | 16 ± 1.61 | 18 ± 1.51 |
| Day 14 | 16 ± 1.21 | 17 ± 2.01 |
| Ocular Toxicity | Not observed | Not observed |
| Simple irritation | Not observed | Not observed |
| Dermal toxicity | Not observed | Not observed |
| Mortality rate | Not observed | Not observed |
| Parameters/Tests | Group I (Control) | Group II (Treated with Nanosponges) |
|---|---|---|
| Hemoglobin (g/dL) | 13.51 ± 0.39 | 13.38 ± 0.40 |
| Red blood cells × 106/mm3 | 5.16 ± 0.41 | 5.36 ± 0.51 |
| White blood cells × 109/L | 6.89 ± 0.12 | 6.71 ± 0.30 |
| Platelets × 109/L | 4.11 ± 2.01 | 4.16 ± 2.02 |
| Neutrophils (%) | 55.40 ± 3.95 | 57.11 ± 2.11 |
| Lymphocytes (%) | 38.50 ± 2.08 | 41.21 ± 1.92 |
| Monocytes (%) | 3.60 ± 0.31 | 3.40 ± 0.41 |
| Mean corpuscular volume (%) | 83.00 ± 2.30 | 82.04 ± 2.50 |
| Mean corpuscular hemoglobin pg/cells | 23 ± 2.75 | 25 ± 2.13 |
| Mean corpuscular hemoglobin concentration (%) | 35.10 ± 1.21 | 33.21 ± 2.41 |
| Formulation Code | βCD (g/100 g) | CMC (g/100 g) | AMPS (g/100 g) | MBA (g/100 g) | APS/SMS (g/100 g) |
|---|---|---|---|---|---|
| F-0 | 2 | - | 24 | 8 | 0.8/0.8 |
| F-1 | 0.4 | 0.4 | 24 | 8 | 0.8/0.8 |
| F-2 | 0.8 | 0.8 | 24 | 8 | 0.8/0.8 |
| F-3 | 1.2 | 1.2 | 24 | 8 | 0.8/0.8 |
| F-4 | 0.4 | 0.4 | 16 | 8 | 0.8/0.8 |
| F-5 | 0.4 | 0.4 | 24 | 8 | 0.8/08 |
| F-6 | 0.4 | 0.4 | 32 | 8 | 0.8/0.8 |
| F-7 | 0.4 | 0.4 | 24 | 6 | 0.8/0.8 |
| F-8 | 0.4 | 0.4 | 24 | 8 | 0.8/0.8 |
| F-9 | 0.4 | 0.4 | 24 | 10 | 0.8/0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizvi, S.S.B.; Akhtar, N.; Minhas, M.U.; Mahmood, A.; Khan, K.U. Synthesis and Characterization of Carboxymethyl Chitosan Nanosponges with Cyclodextrin Blends for Drug Solubility Improvement. Gels 2022, 8, 55. https://doi.org/10.3390/gels8010055
Rizvi SSB, Akhtar N, Minhas MU, Mahmood A, Khan KU. Synthesis and Characterization of Carboxymethyl Chitosan Nanosponges with Cyclodextrin Blends for Drug Solubility Improvement. Gels. 2022; 8(1):55. https://doi.org/10.3390/gels8010055
Chicago/Turabian StyleRizvi, Syeda Sadia Batool, Naveed Akhtar, Muhammad Usman Minhas, Arshad Mahmood, and Kifayat Ullah Khan. 2022. "Synthesis and Characterization of Carboxymethyl Chitosan Nanosponges with Cyclodextrin Blends for Drug Solubility Improvement" Gels 8, no. 1: 55. https://doi.org/10.3390/gels8010055
APA StyleRizvi, S. S. B., Akhtar, N., Minhas, M. U., Mahmood, A., & Khan, K. U. (2022). Synthesis and Characterization of Carboxymethyl Chitosan Nanosponges with Cyclodextrin Blends for Drug Solubility Improvement. Gels, 8(1), 55. https://doi.org/10.3390/gels8010055






