Effects of Sulfate and Sulfuric Acid on Efficiency of Geopolymers as Concrete Repair Materials
Abstract
1. Introduction
2. Results and Discussion
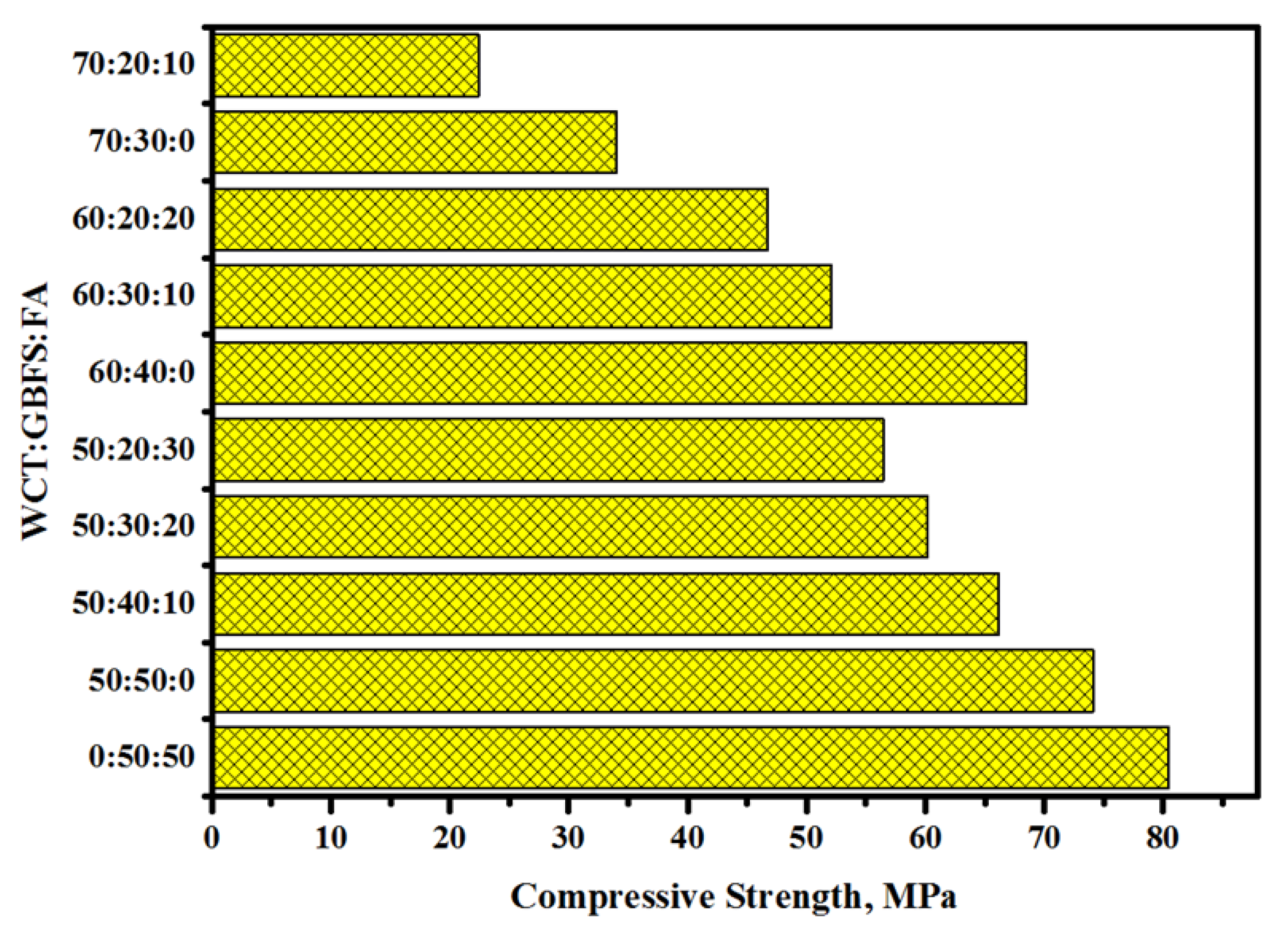
2.1. Compressive Strength (CS)
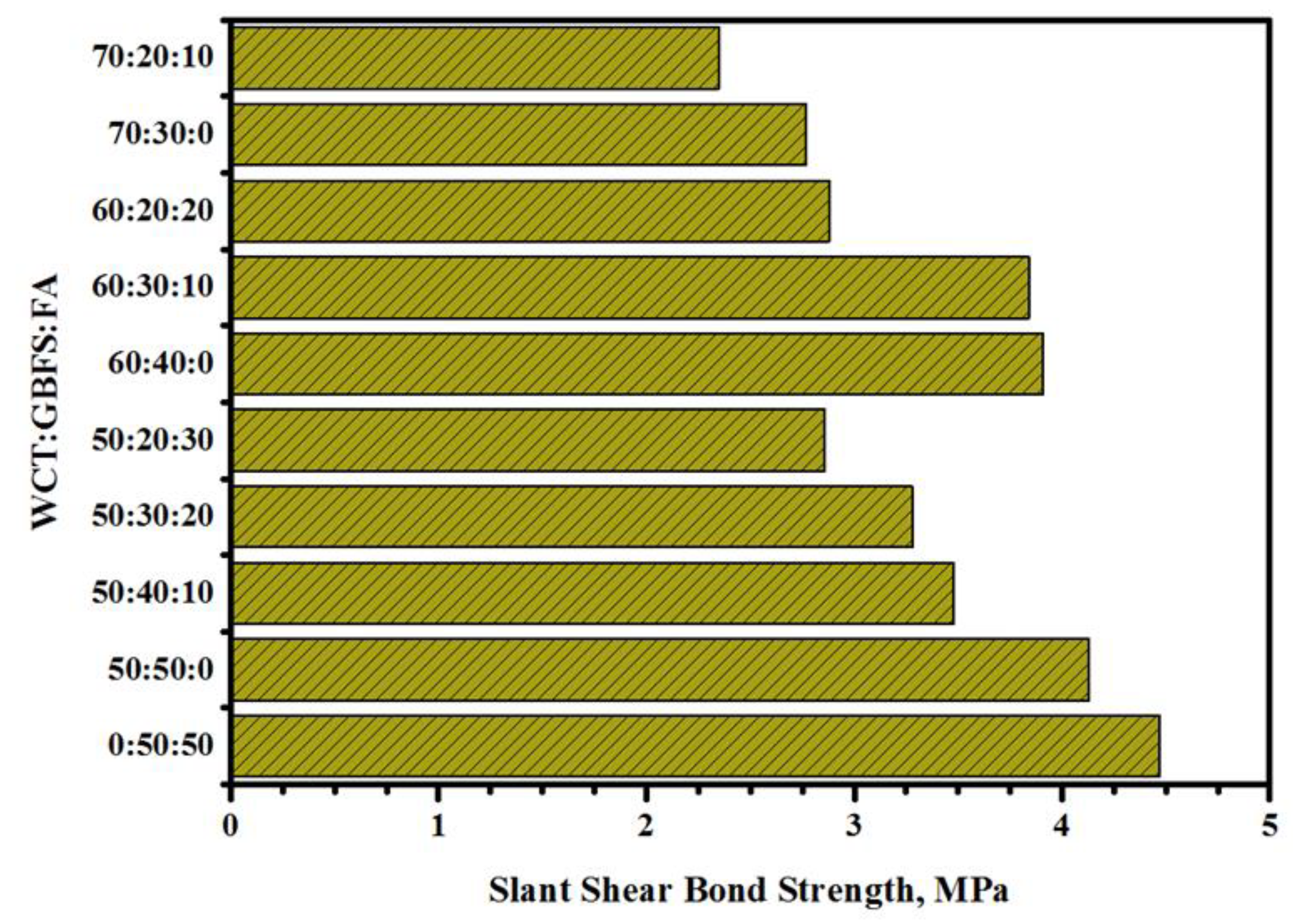
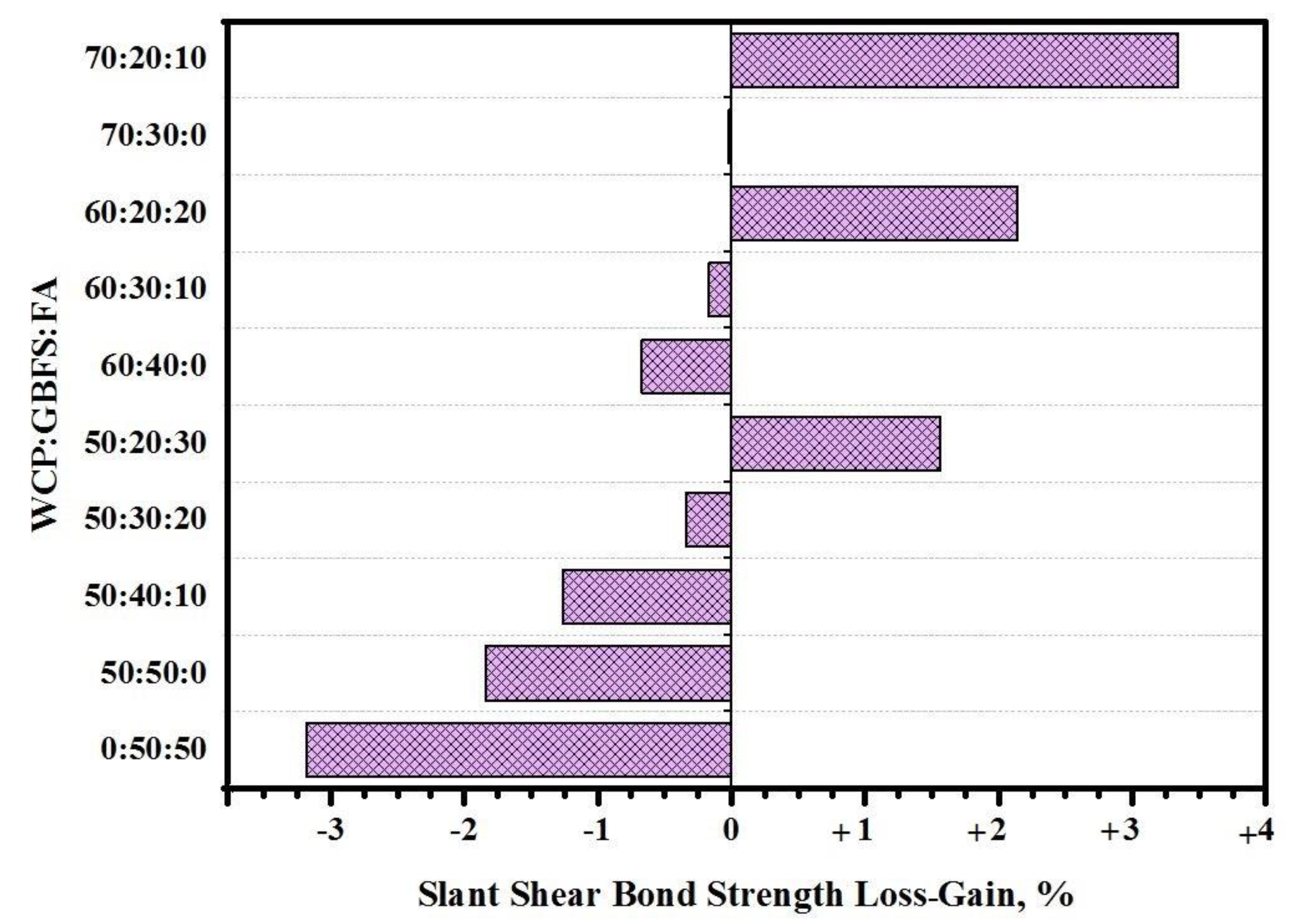
2.2. SSBS Test
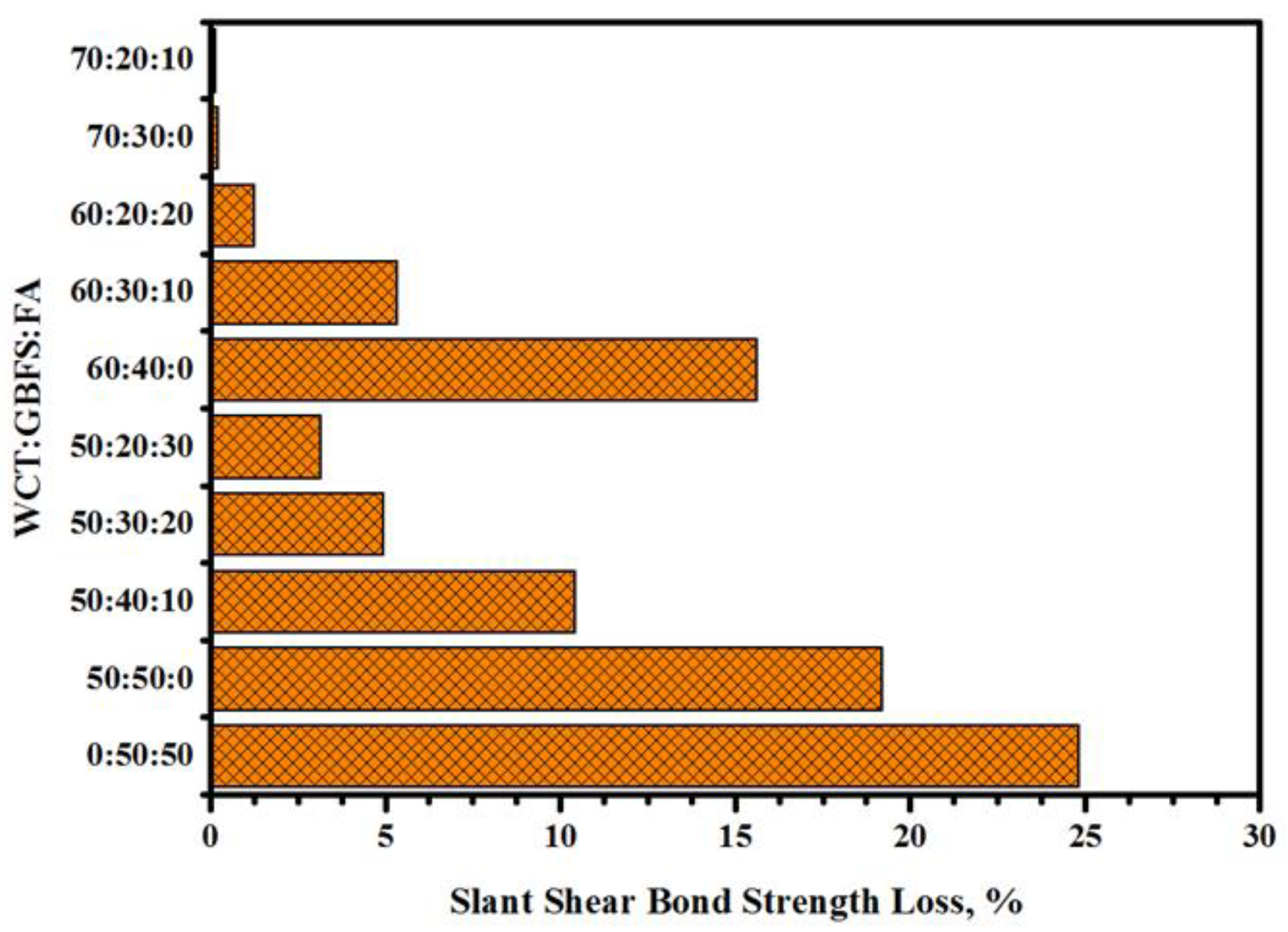
2.3. Resistance to Sulfuric Acid Attack
2.4. Resistance to Sulfate Attack
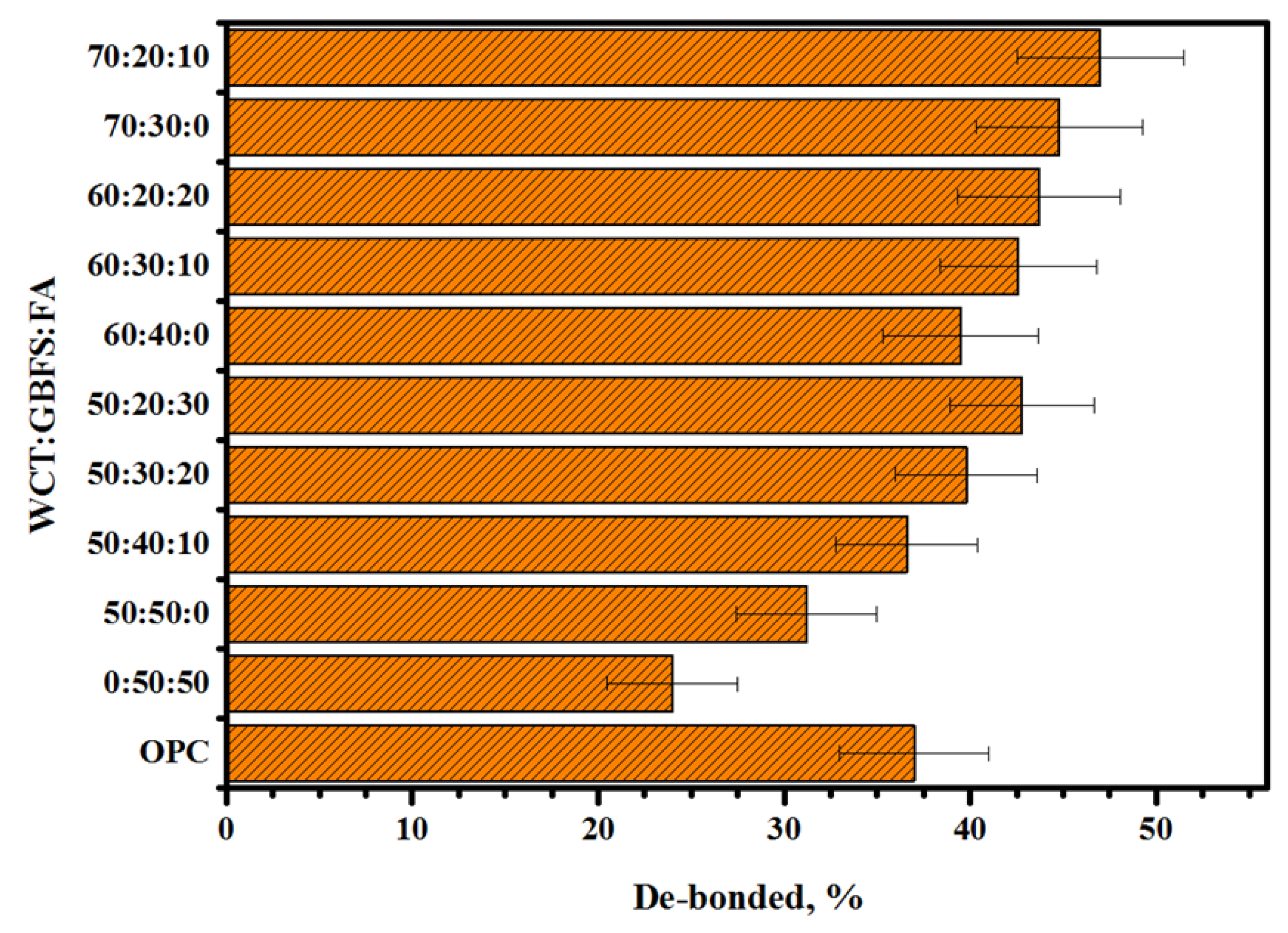
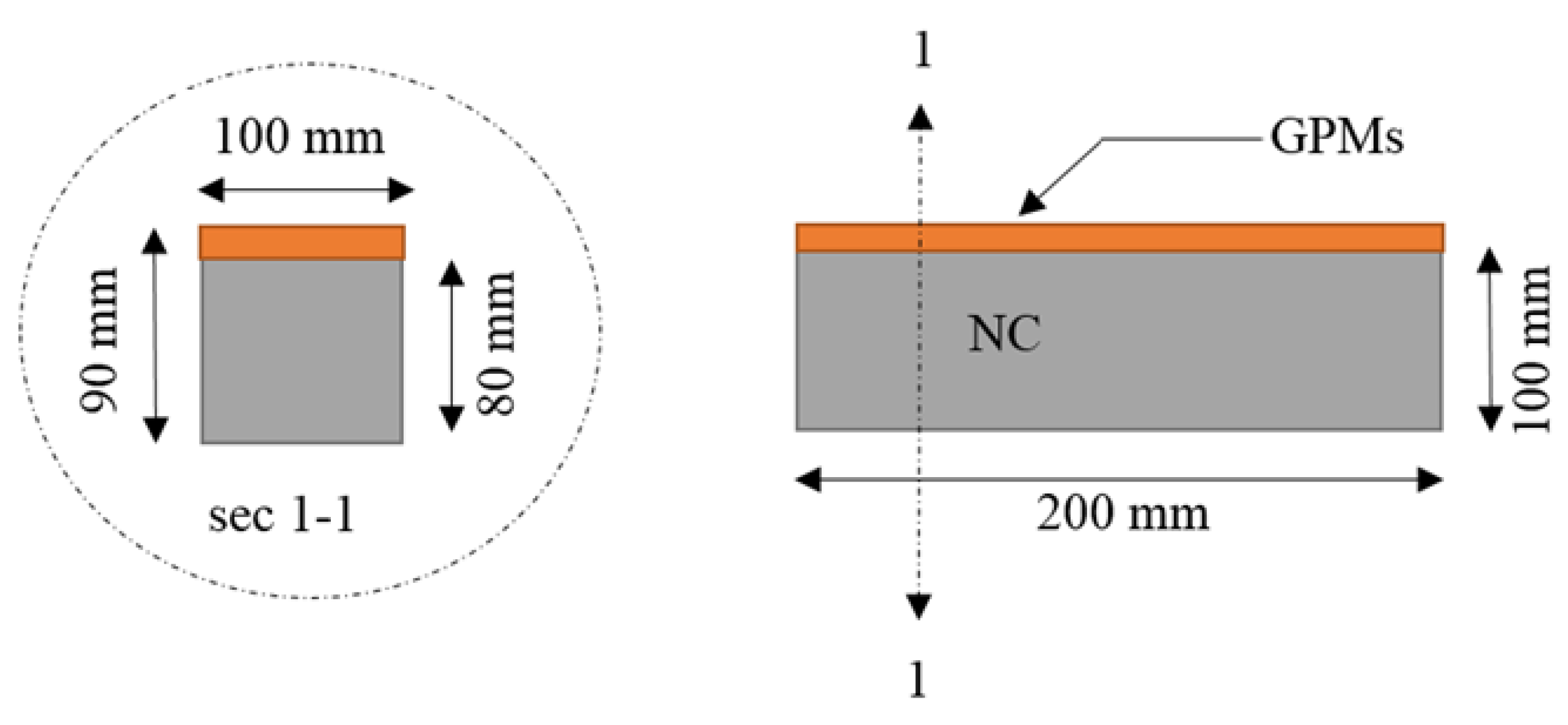
2.5. Compatibility between Concrete Substrate and GPMs
3. Conclusions
- i.
- Sustainable geopolymers as concrete repair materials were produced incorporating WCT, GBFS, and FA. The content of calcium in the design mix was significant where the main recourse of calcium (GBFS) was kept between 20 and 70%.
- ii.
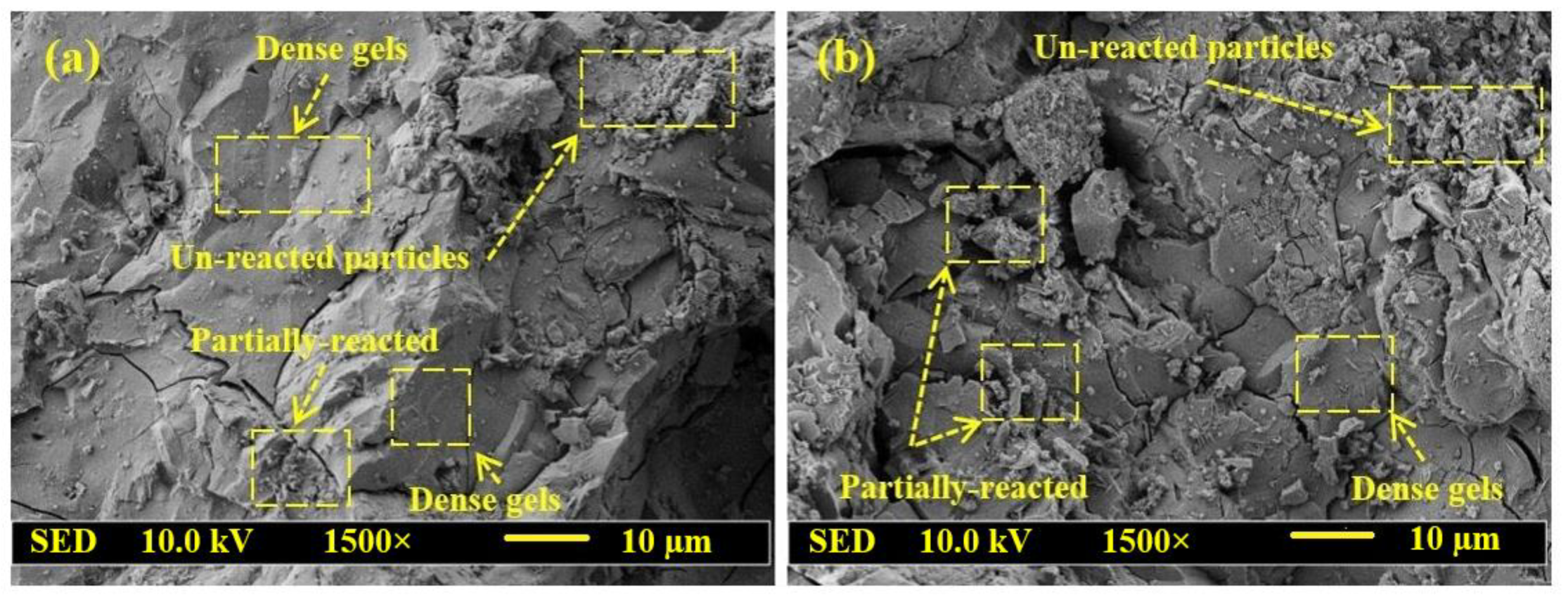
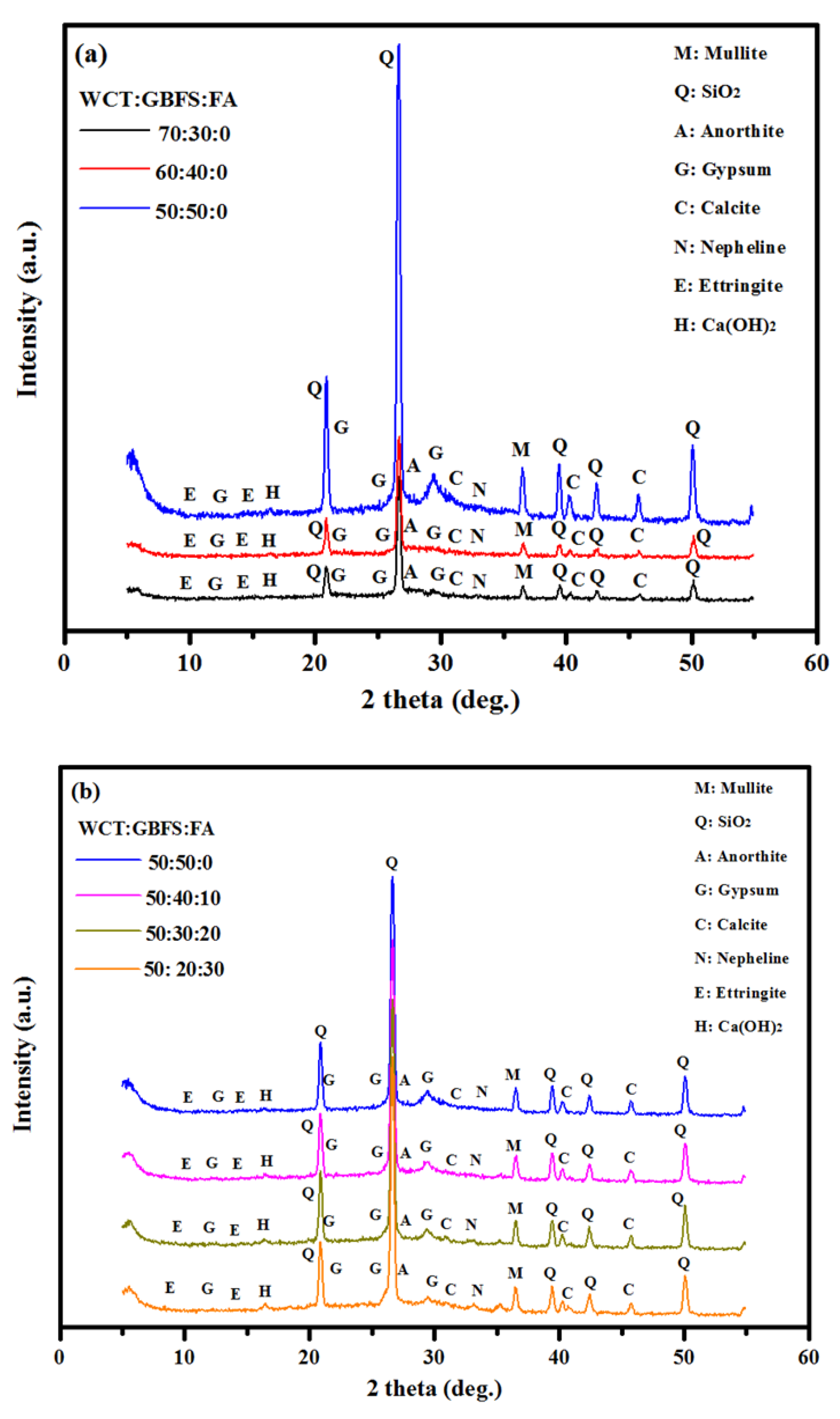
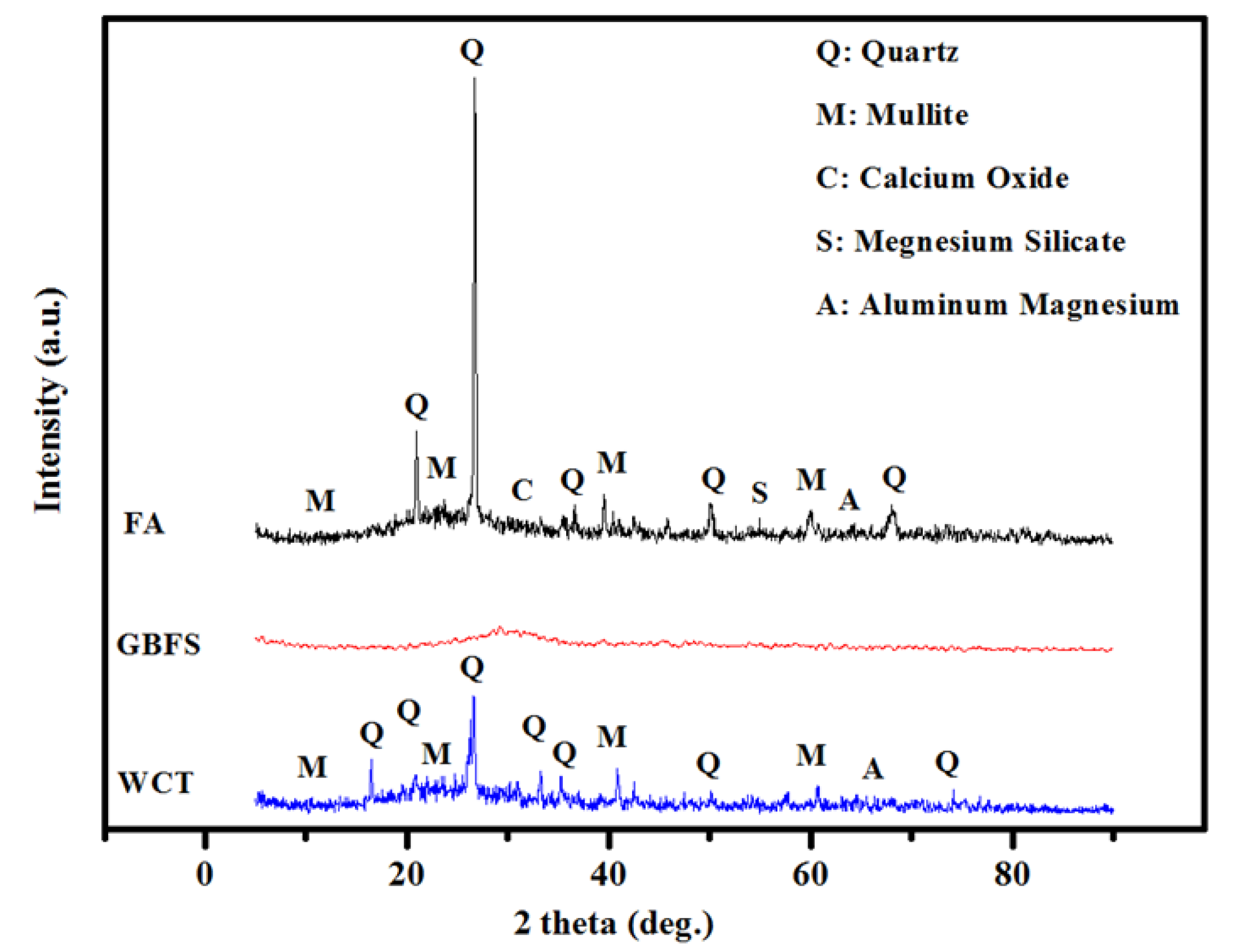
- Both the CS and SSBS of GPMs were decreased with the increase in WCT and FA content as GBFS replacements. At 28 days of age, GPMs made with 70% WCT and 20% GBFS showed lower bond strength than the control specimen. The XRD and SEM analyses showed that with the increase of WCT and FA content as GBFS replacements, GPMs became more porous and less homogeneous with lower amounts of N-A-S-H and C-A-S-H dense gels formation.
- iii.
- With the increase in WCT and fly ash as slag replacement, the performance of the composite specimens was enhanced and the loss in SSBS was reduced when exposed to sulfuric acid environments. Composite GPMs–NC (cylindrical specimens) prepared with 70% of WCT displayed excellent performance and achieved the highest resistance against sulfuric acid and sulfate attacks. It was claimed that the GPMs made with a high volume of WCT can be highly recommended as the sustainable repair materials for aggressive environments.
- iv.
- XRD and SEM microstructures analyses of GPMs exhibited that, with the increase in WCT level as a GBFS replacement, the formation of gypsum and ettringite in the geopolymer matrix were restricted, which in turn reduced the internal cracks and deterioration of the proposed repairing materials.
- v.
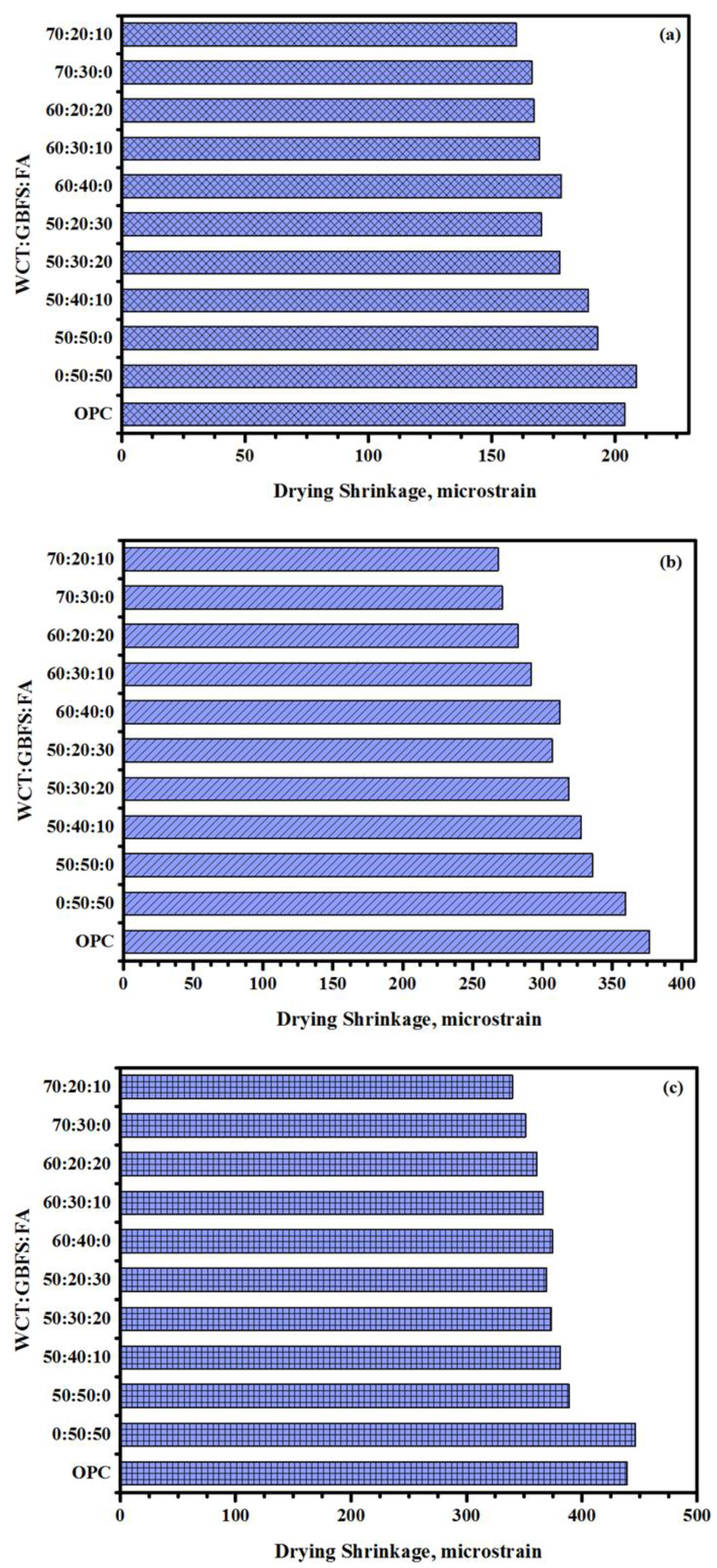
- The drying shrinkage of GPMs was reduced due to the inclusion of WCT as a GBFS replacement. All the specimens prepared with WCT as a GBFS replacement showed lower drying shrinkage compared to OPC-based NC. This reduction in the drying shrinkage could positively affect (increase) the durability performance (lower deterioration) of GPMs and GPMs–NC composites, thus increasing their life span because of the formation of fewer cracks in the mortars matrix.
- vi.
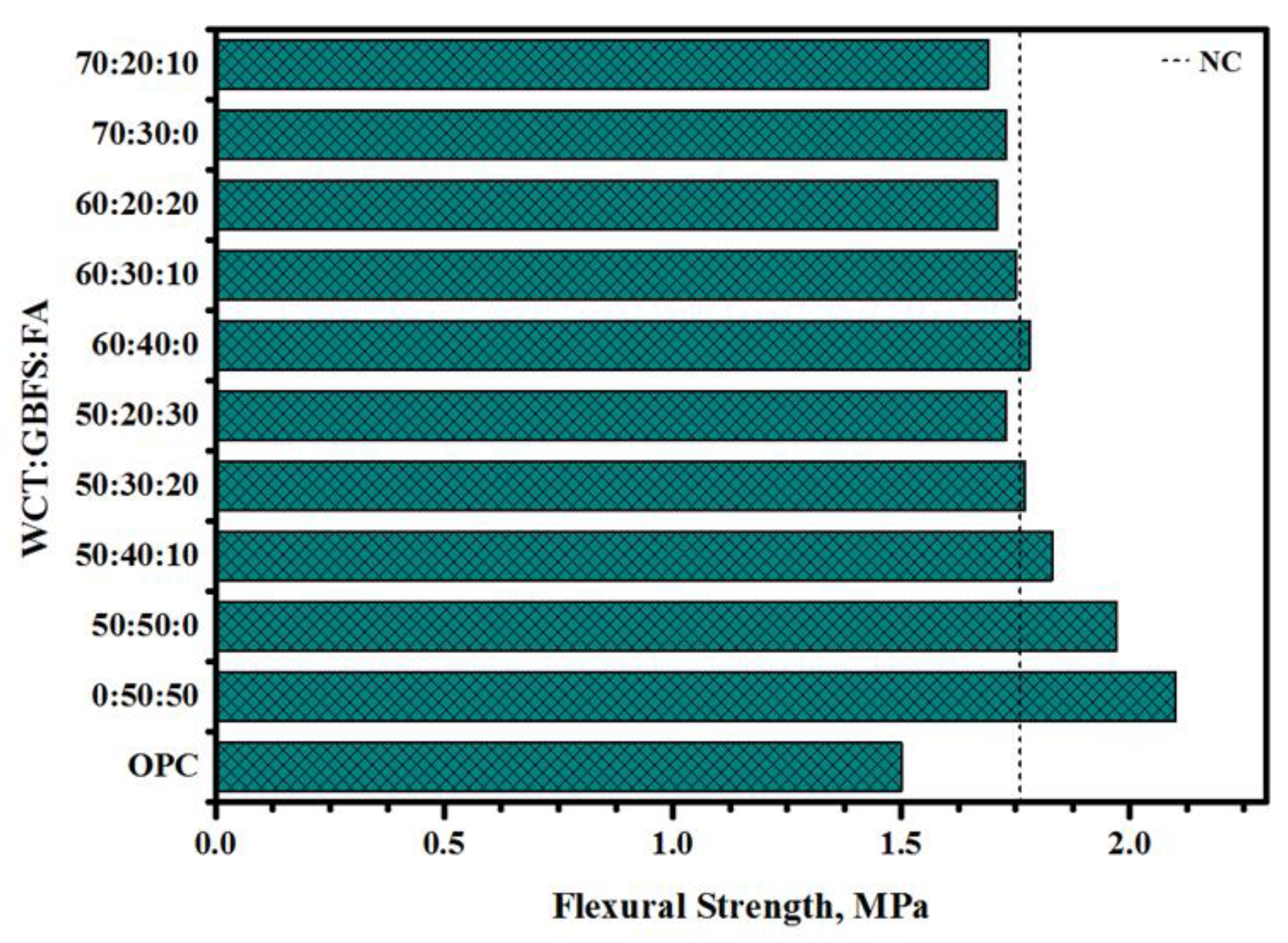
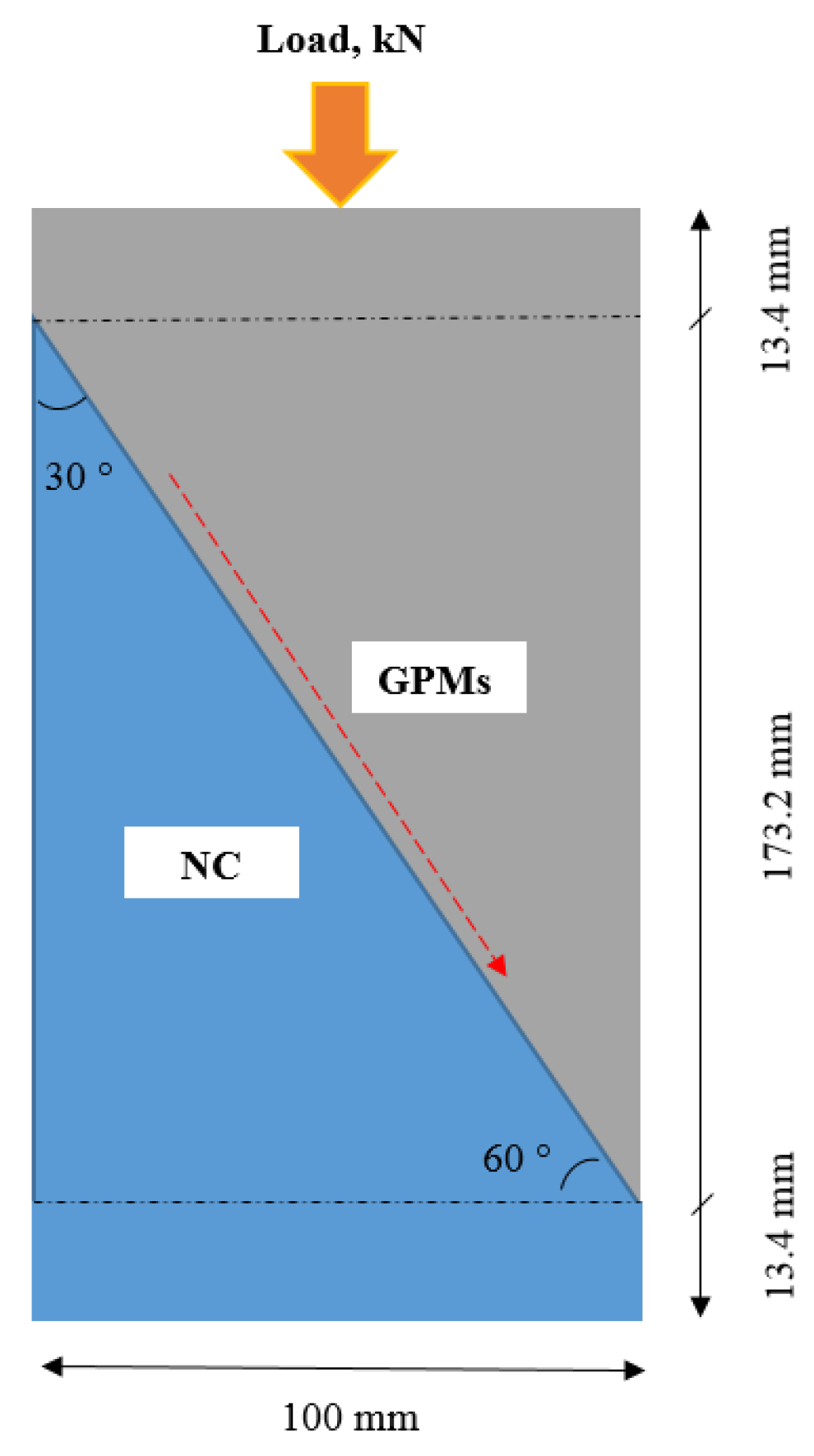
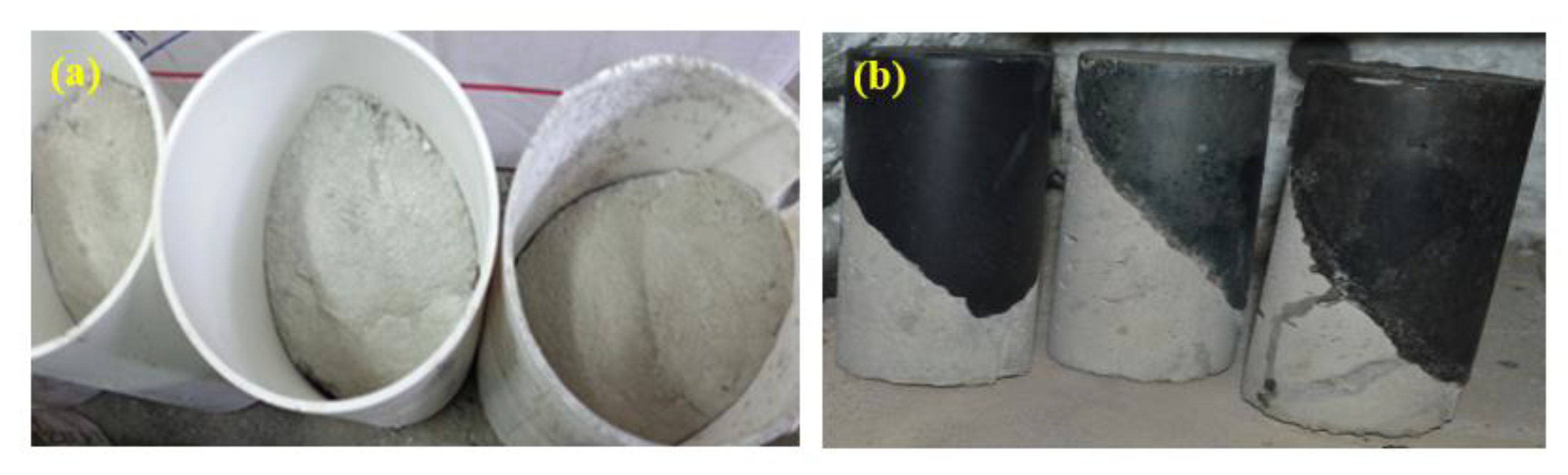
- The obtained GPMs showed a high degree of suitability for repair works in terms of very excellent GPM–concrete substrate compatibility levels, as shown by the SSBS, CTE, and FS of four-point loading GPMs–NC composite beam results.
- vii.
- Based on the CS and SSBS resistance against aggressive environmental conditions (sulfate and sulfuric acid exposure), thermal expansion coefficient, four-point flexural strength of composite beam, and drying shrinkage results, GPMs composed of 50% WCT, 40% GBFS, and 10% FA can be highly recommended as a potential concrete repair material in the construction sector worldwide.
4. Materials and Methods
4.1. Materials Characterization
4.2. Design of GPMs Mixes
4.3. Compressive and Bond Strength Tests
4.4. Resistance to Sulfuric Acid Attack
4.5. Resistance to Sulfate Attack
4.6. Compatibility between GPM and NC Substrate
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, Z.; Lin, X.; Vollpracht, A. Pervious concrete made of alkali activated slag and geopolymers. Constr. Build. Mater. 2018, 189, 797–803. [Google Scholar] [CrossRef]
- Huseien, G.F.; Mirza, J.; Ismail, M.; Hussin, M.W. Influence of different curing temperatures and alkali activators on properties of GBFS geopolymer mortars containing fly ash and palm-oil fuel ash. Constr. Build. Mater. 2016, 125, 1229–1240. [Google Scholar] [CrossRef]
- Huseien, G.F.; Mirza, J.; Hussin, M.W.; Ariffin, M.A.M. Potential use coconut milk as alternative to alkali solution for geopolymer production. J. Teknol. 2016, 78, 133–139. [Google Scholar] [CrossRef][Green Version]
- Faridmehr, I.; Nehdi, M.; Nikoo, M.; Huseien, G.; Ozbakkaloglu, T. Life-Cycle Assessment of Alkali-Activated Materials Incorporating Industrial Byproducts. Materials 2021, 14, 2401. [Google Scholar] [CrossRef] [PubMed]
- Huseien, G.F.; Ismail, M.; Khalid, N.H.A.; Hussin, M.W.; Mirza, J. Compressive strength and microstructure of assorted wastes incorporated geopolymer mortars: Effect of solution molarity. Alex. Eng. J. 2018, 57, 3375–3386. [Google Scholar] [CrossRef]
- Kubba, Z.; Huseien, G.F.; Sam, A.R.M.; Shah, K.W.; Asaad, M.A.; Ismail, M.; Tahir, M.M.; Mirza, J. Impact of curing temperatures and alkaline activators on compressive strength and porosity of ternary blended geopolymer mortars. Case Stud. Constr. Mater. 2018, 9, e00205. [Google Scholar] [CrossRef]
- Shah, K.W.; Huseien, G.F. Bond strength performance of ceramic, fly ash and GBFS ternary wastes combined alkali-activated mortars exposed to aggressive environments. Constr. Build. Mater. 2020, 251, 119088. [Google Scholar] [CrossRef]
- Huseien, G.; Ismail, M.; Tahir, M.; Mirza, J.; Hussein, A.; Khalid, N.; Sarbini, N. Performance of Sustainable Alkali Activated Mortars Containing Solid Waste Ceramic Powder. Chem. Eng. 2018, 63, 673–678. [Google Scholar]
- Ahmad, M.R.; Chen, B.; Shah, S.F.A. Influence of different admixtures on the mechanical and durability properties of one-part alkali-activated mortars. Constr. Build. Mater. 2020, 265, 120320. [Google Scholar] [CrossRef]
- Huseien, G.F.; Mirza, J.; Ismail, M.; Ghoshal, S.; Ariffin, M.A.M. Effect of metakaolin replaced granulated blast furnace slag on fresh and early strength properties of geopolymer mortar. Ain Shams Eng. J. 2018, 9, 1557–1566. [Google Scholar] [CrossRef]
- Aly, A.M.; El-Feky, M.; Kohail, M.; Nasr, E.-S.A. Performance of geopolymer concrete containing recycled rubber. Constr. Build. Mater. 2019, 207, 136–144. [Google Scholar] [CrossRef]
- Huseien, G.F.; Tahir, M.M.; Mirza, J.; Ismail, M.; Shah, K.W.; Asaad, M.A. Effects of POFA replaced with FA on durability properties of GBFS included alkali activated mortars. Constr. Build. Mater. 2018, 175, 174–186. [Google Scholar] [CrossRef]
- Assi, L.; Carter, K.; Deaver, E.; Anay, R.; Ziehl, P. Sustainable concrete: Building a greener future. J. Clean. Prod. 2018, 198, 1641–1651. [Google Scholar] [CrossRef]
- Urner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Vasconcelos, E.; Fernandes, S.; de Aguiar, B.; Pacheco-Torgal, F. Concrete retrofitting using CFRP and geopolymer mortars. in Materials Science Forum. Mater. Sci. Forum 2012, 730, 427–432. [Google Scholar] [CrossRef]
- Huseien, G.; Ismail, M.; Tahir, M.; Mirza, J.; Hussein, A.; Khalid, N.; Sarbini, N. Effect of binder to fine aggregate content on performance of sustainable alkali activated mortars incorporating solid waste materials. Chem. Eng. Trans. 2018, 63, 667–672. [Google Scholar]
- Hamzah, H.K.; Georgescu, D.P.; Khalid NH, A.; Hussein, G.F. Strength performance of free cement mortars incorporating fly ash and slag: Effects of alkaline activator solution dosage. Open J. Sci. Technol. 2020, 3, 87–98. [Google Scholar]
- Zamanabadi, S.N.; Zareei, S.A.; Shoaei, P.; Ameri, F. Ambient-cured alkali-activated slag paste incorporating micro-silica as repair material: Effects of alkali activator solution on physical and mechanical properties. Constr. Build. Mater. 2019, 229, 116911. [Google Scholar] [CrossRef]
- Nunes, V.A.; Borges, P.H.; Zanotti, C. Mechanical compatibility and adhesion between alkali-activated repair mortars and Portland cement concrete substrate. Constr. Build. Mater. 2019, 215, 569–581. [Google Scholar] [CrossRef]
- Alanazi, H.; Yang, M.; Zhang, D.; Gao, Z. Bond strength of PCC pavement repairs using metakaolin-based geopolymer mortar. Cem. Concr. Compos. 2016, 65, 75–82. [Google Scholar] [CrossRef]
- Salazar, R.A.R.; Jesús, C.; De Gutiérrez, R.M.; Pacheco-Torgal, F. Alkali-activated binary mortar based on natural volcanic pozzolan for repair applications. J. Build. Eng. 2019, 25, 100785. [Google Scholar] [CrossRef]
- Geraldo, R.H.; Teixeira, O.G.; Matos, S.; Silva, F.G.; Gonçalves, J.P.; Camarini, G. Study of alkali-activated mortar used as conventional repair in reinforced concrete. Constr. Build. Mater. 2018, 165, 914–919. [Google Scholar] [CrossRef]
- Phoo-Ngernkham, T.; Sata, V.; Hanjitsuwan, S.; Ridtirud, C.; Hatanaka, S.; Chindaprasirt, P. High calcium fly ash geopolymer mortar containing Portland cement for use as repair material. Constr. Build. Mater. 2015, 98, 482–488. [Google Scholar] [CrossRef]
- Huseien, G.F.; Sam, A.R.M.; Shah, K.W.; Mirza, J.; Tahir, M.M. Evaluation of alkali-activated mortars containing high volume waste ceramic powder and fly ash replacing GBFS. Constr. Build. Mater. 2019, 210, 78–92. [Google Scholar] [CrossRef]
- Wang, J.; Huang, T.; Cheng, G.; Liu, Z.; Li, S.; Wang, D. Effects of fly ash on the properties and microstructure of alkali-activated FA/BFS repairing mortar. Fuel 2019, 256, 115919. [Google Scholar] [CrossRef]
- Huseien, G.F.; Shah, K.W. Performance evaluation of alkali-activated mortars containing industrial wastes as surface repair materials. J. Build. Eng. 2020, 30, 101234. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Jalali, S. Reusing ceramic wastes in concrete. Constr. Build. Mater. 2010, 24, 832–838. [Google Scholar] [CrossRef]
- Senthamarai, R.; Manoharan, P.D.; Gobinath, D. Concrete made from ceramic industry waste: Durability properties. Constr. Build. Mater. 2011, 25, 2413–2419. [Google Scholar] [CrossRef]
- Daniyal, M.; Ahmad, S. Application of Waste Ceramic Tile Aggregates in Concrete. Int. J. Innov. Res. Sci. Eng. Technol. 2015, 4, 12808–12815. [Google Scholar]
- Senthamarai, R.; Manoharan, P.D. Manoharan, Concrete with ceramic waste aggregate. Cem. Concr. Compos. 2005, 27, 910–913. [Google Scholar] [CrossRef]
- Huang, B.; Dong, Q.; Burdette, E.G. Laboratory evaluation of incorporating waste ceramic materials into Portland cement and asphaltic concrete. Constr. Build. Mater. 2009, 23, 3451–3456. [Google Scholar] [CrossRef]
- Hussein, A.A.; Jaya, R.P.; Hassan, N.A.; Yaacob, H.; Huseien, G.F.; Ibrahim, M.H.W. Performance of nanoceramic powder on the chemical and physical properties of bitumen. Constr. Build. Mater. 2017, 156, 496–505. [Google Scholar] [CrossRef]
- Samadi, M.; Hussin, M.W.; Lee, H.S.; Sam, A.R.M.; Ismail, M.; Lim, N.H.A.S.; Ariffin, N.F.; Khalid, N.H.A. Properties of mortar containing ceramic powder waste as cement replacement. J. Teknol. 2015, 77, 93–97. [Google Scholar] [CrossRef]
- Huseien, G.F.; Sam, A.R.M.; Mirza, J.; Tahir, M.M.; Asaad, M.A.; Ismail, M.; Shah, K.W. Waste ceramic powder incorporated alkali activated mortars exposed to elevated Temperatures: Performance evaluation. Constr. Build. Mater. 2018, 187, 307–317. [Google Scholar] [CrossRef]
- Fernandes, M.I.; Sousa, A.V.; Dias, A.M. Environmental Impacts and Emissions Trading-Ceramic Industry: A Case Study; Technological Centre of Ceramics and Glass, Portuguese Association of Ceramic Industry: Coimbra, Portugal, 2004. (In Portuguese) [Google Scholar]
- Samadi, M.; Huseien, G.F.; Mohammadhosseini, H.; Lee, H.S.; Lim, N.H.A.S.; Tahir, M.M.; Alyousef, R. Waste ceramic as low cost and eco-friendly materials in the production of sustainable mortars. J. Clean. Prod. 2020, 266, 121825. [Google Scholar] [CrossRef]
- Limbachiya, M.; Meddah, M.S.; Ouchagour, Y. Use of recycled concrete aggregate in fly-ash concrete. Constr. Build. Mater. 2012, 27, 439–449. [Google Scholar] [CrossRef]
- Heidari, A.; Tavakoli, D. A study of the mechanical properties of ground ceramic powder concrete incorporating nano-SiO2 particles. Constr. Build. Mater. 2013, 38, 255–264. [Google Scholar] [CrossRef]
- Huseien, G.F.; Mirza, J.; Ismail, M.; Hussin, M.W.; Arrifin, M.A.M.; Hussein, A.A. The Effect of Sodium Hydroxide Molarity and Other Parameters on Water Absorption of Geopolymer Mortars. Indian J. Sci. Technol. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, F.; Hu, S.; Liu, M. Compatibility of repair materials with substrate low-modulus cement and asphalt mortar (CA mortar). Constr. Build. Mater. 2016, 126, 304–312. [Google Scholar] [CrossRef]
- Pattnaik, R.R.; Rangaraju, P.R. Analysis of compatibility between repair material and substrate concrete using simple beam with third point loading. J. Mater. Civ. Eng. 2007, 19, 1060–1069. [Google Scholar] [CrossRef]
- Czarnecki, L.; Garbacz, A.; Lukowski, P.; Clifton, J.R. Polymer Composites for Repairing of Portland Cement Concrete: Compatibility Project; NIST: Gaithersburg, MD, USA, 1999. [Google Scholar]
- Huseien, G.F.; Ismail, M.; Tahir, M.M.; Mirza, J.; Khalid, N.H.A.; Asaad, M.A.; Husein, A.A.; Sarbini, N.N. Synergism between palm oil fuel ash and slag: Production of environmental-friendly alkali activated mortars with enhanced properties. Constr. Build. Mater. 2018, 170, 235–244. [Google Scholar] [CrossRef]
- Huseien, G.F.; Mirza, J.; Ismail, M. Effects of High Volume Ceramic Binders on Flexural Strength of Self-Compacting Geopolymer Concrete. Adv. Sci. Lett. 2018, 24, 4097–4101. [Google Scholar] [CrossRef]
- Rashad, A.M. Properties of alkali-activated fly ash concrete blended with slag. Iran. J. Mater. Sci. Eng. 2013, 10, 57–64. [Google Scholar]
- Puertas, F.; Martinez-Ramirez, S.; Alonso, S.; Vázquez, T. Alkali-activated fly ash/slag cements: Strength behaviour and hydration products. Cem. Concr. Res. 2000, 30, 1625–1632. [Google Scholar] [CrossRef]
- Dombrowski, K.; Buchwald, A.; Weil, M. Influence of calcium content on the structure and thermal performance of fly ash based geopolymers. J. Mater. Sci. 2007, 42, 3033–3043. [Google Scholar] [CrossRef]
- Phoo-Ngernkham, T.; Hanjitsuwan, S.; Suksiripattanapong, C.; Thumrongvut, J.; Suebsuk, J.; Sookasem, S. Flexural strength of notched concrete beam filled with alkali-activated binders under different types of alkali solutions. Constr. Build. Mater. 2016, 127, 673–678. [Google Scholar] [CrossRef]
- Budiea, A.; Hussin, M.W.; Muthusamy, K.; Ismail, M.E. Performance of high strength POFA concrete in acidic environment. Concr. Res. Lett. 2010, 1, 14–18. [Google Scholar]
- Ariffin, M.; Bhutta, M.; Hussin, M.; Tahir, M.M.; Aziah, N. Sulfuric acid resistance of blended ash geopolymer concrete. Constr. Build. Mater. 2013, 43, 80–86. [Google Scholar] [CrossRef]
- Bamaga, S.O.; Ismail, M.A.; Majid, Z.A.; Ismail, M.; Hussin, M.W. Evaluation of sulfate resistance of mortar containing palm oil fuel ash from different sources. Arab. J. Sci. Eng. 2013, 38, 2293–2301. [Google Scholar] [CrossRef]
- Noruzman, A.H.; Ismail, M.; Bhutta, M.A.R.; Yusuf, T.O.; Shehu, I.A.; Hassan, I.O. Strength and durability characteristics of polymer modified concrete incorporating Vinyl acetate effluent. in Advanced Materials Research. Adv. Mater. Res. 2013, 693, 1053–1056. [Google Scholar] [CrossRef]
- Matschei, T.; Bellmann, F.; Stark, J. Hydration behaviour of sulphate-activated slag cements. Adv. Cem. Res. 2005, 17, 167–178. [Google Scholar] [CrossRef]
- Huseien, G.F.; Shah, K.W. Durability and life cycle evaluation of self-compacting concrete containing fly ash as GBFS replacement with alkali activation. Constr. Build. Mater. 2020, 235, 117458. [Google Scholar] [CrossRef]
- Alabduljabbar, H.; Huseien, G.F.; Sam, A.R.; Alyouef, R.; Algaifi, H.A.; Alaskar, A. Engineering Properties of Waste Sawdust-Based Lightweight Alkali-Activated Concrete: Experimental Assessment and Numerical Prediction. Materials 2020, 13, 5490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhu, H.; Cheng, Y.; Huseien, G.F.; Shah, K.W. Shrinkage mechanisms and shrinkage-mitigating strategies of alkali-activated slag composites: A critical review. Constr. Build. Mater. 2022, 318, 125993. [Google Scholar] [CrossRef]
- Hamzah, H.K.; Huseien, G.F.; Asaad, M.A.; Georgescu, D.P.; Ghoshal, S.K.; Alrshoudi, F. Effect of waste glass bottles-derived nanopowder as slag replacement on mortars with alkali activation: Durability characteristics. Case Stud. Constr. Mater. 2021, 15, e00775. [Google Scholar] [CrossRef]
- Huseien, G.F.; Asaad, M.A.; Abadel, A.A.; Ghoshal, S.K.; Hamzah, H.K.; Benjeddou, O.; Mirza, J. Drying Shrinkage, Sulphuric Acid and Sulphate Resistance of High-Volume Palm Oil Fuel Ash-Included Alkali-Activated Mortars. Sustainability 2022, 14, 498. [Google Scholar] [CrossRef]
- Humad, A.M.; Kothari, A.; Provis, J.L.; Cwirzen, A. The effect of blast furnace slag/fly ash ratio on setting, strength, and shrinkage of alkali-activated pastes and concretes. Front. Mater. 2019, 6, 1–10. [Google Scholar] [CrossRef]
- Huseien, G.F.; Sam, A.R.M.; Shah, K.W.; Budiea, A.M.A.; Mirza, J. Utilizing spend garnets as sand replacement in alkali-activated mortars containing fly ash and GBFS. Constr. Build. Mater 2019, 225, 132–145. [Google Scholar] [CrossRef]
- Joudah, Z.H.; Huseien, G.F.; Samadi, M.; Lim, N.H.A.S. Sustainability evaluation of alkali-activated mortars incorporating industrial wastes. Mater. Today Proc. 2021, 46, 1971–1977. [Google Scholar] [CrossRef]
- Yusuf, M.O.; Johari, M.A.M.; Ahmad, Z.A.; Maslehuddin, M. Evolution of alkaline activated ground blast furnace slag–ultrafine palm oil fuel ash based concrete. Mater. Des. 2014, 55, 387–393. [Google Scholar] [CrossRef]
- Temuujin, J.; van Riessen, A.; MacKenzie, K. Preparation and characterisation of fly ash based geopolymer mortars. Constr. Build. Mater. 2010, 24, 1906–1910. [Google Scholar] [CrossRef]
- Rickard, W.D.; Williams, R.; Temuujin, J.; Van Riessen, A. Assessing the suitability of three Australian fly ashes as an aluminosilicate source for geopolymers in high temperature applications. Mater. Sci. Eng. A 2011, 528, 3390–3397. [Google Scholar] [CrossRef]
- American Society for Testing and Materials. Standard Test Method for Materials Finer than 75-μm (No. 200) Sieve in Mineral Aggregates by Washing. In Annual Book of ASTM StandardsAnnual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2003. [Google Scholar]
- American Society for Testing and Materials. Standard specification for concrete aggregates. In Annual Book of Standards; ASTM International: West Conshohocken, PA, USA, 1994. [Google Scholar]
- Samadi, M.; Shah, K.W.; Huseien, G.F. and Lim, N.H.A.S. Influence of glass silica waste nano powder on the mechanical and microstructure properties of alkali-activated mortars. Nanomaterials 2020, 10, 324. [Google Scholar] [CrossRef] [PubMed]
- Huseien, G.F.; Hamzah, H.K.; Sam, A.R.M.; Khalid, N.H.A.; Shah, K.W.; Deogrescu, D.P.; Mirza, J. Alkali-activated mortars blended with glass bottle waste nano powder: Environmental benefit and sustainability. J. Clean. Prod. 2019, 243, 118636. [Google Scholar] [CrossRef]
- American Society for Testing and Materials. Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (using 2-in. Or [50-mm] Cube Specimens). In Annual Book of ASTM Standards Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- American Society for Testing and Materials. Standard Test Methods for Chemical Resistance of Mortars, Grouts, and Monolithic Surfacing and Polymer Concretes; American Society for Testing and Materials: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Mirza, J.; Durand, B.; Bhutta, A.R.; Tahir, M.M. Preferred test methods to select suitable surface repair materials in severe climates. Constr. Build. Mater. 2014, 50, 692–698. [Google Scholar] [CrossRef]





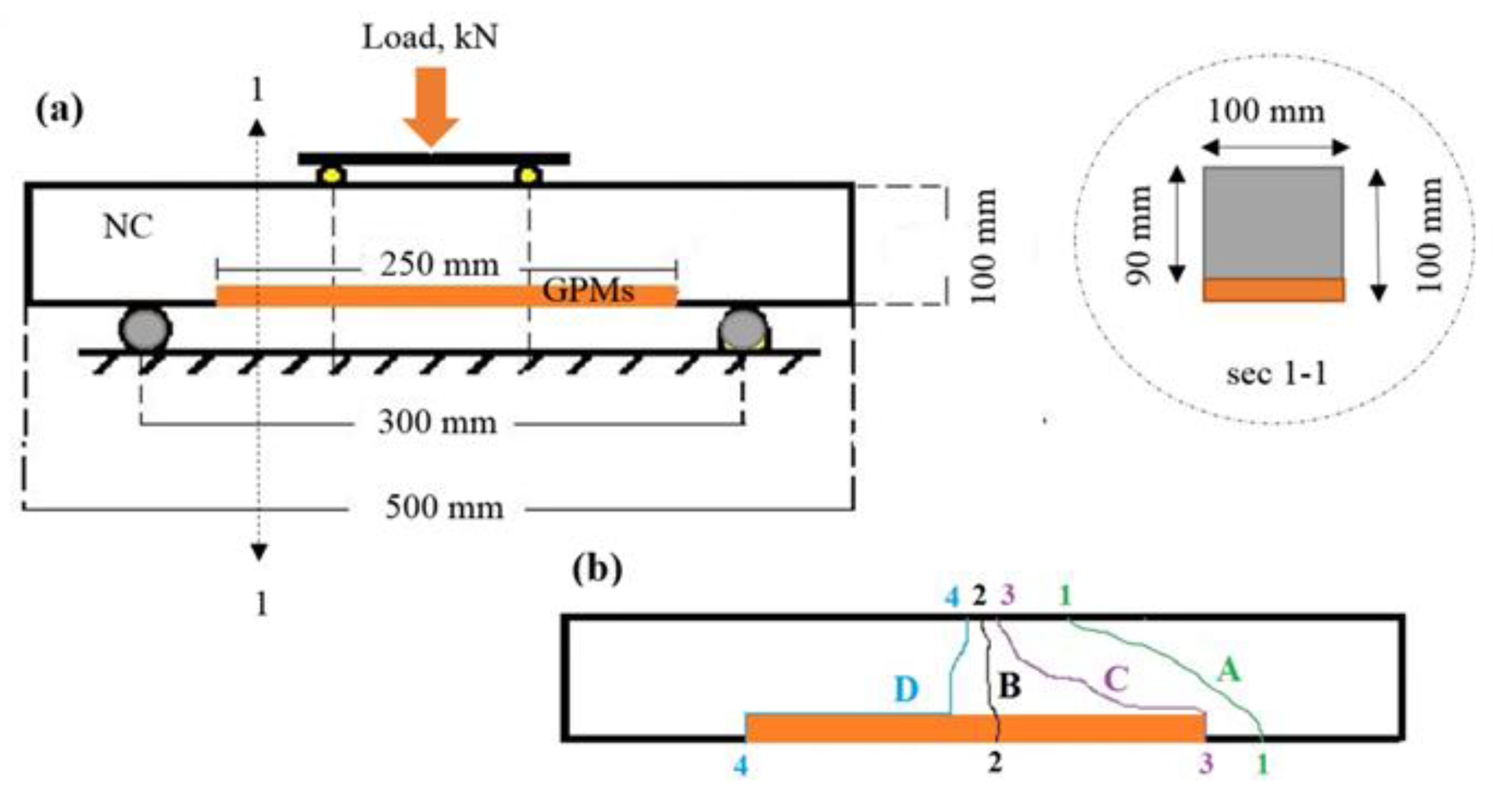
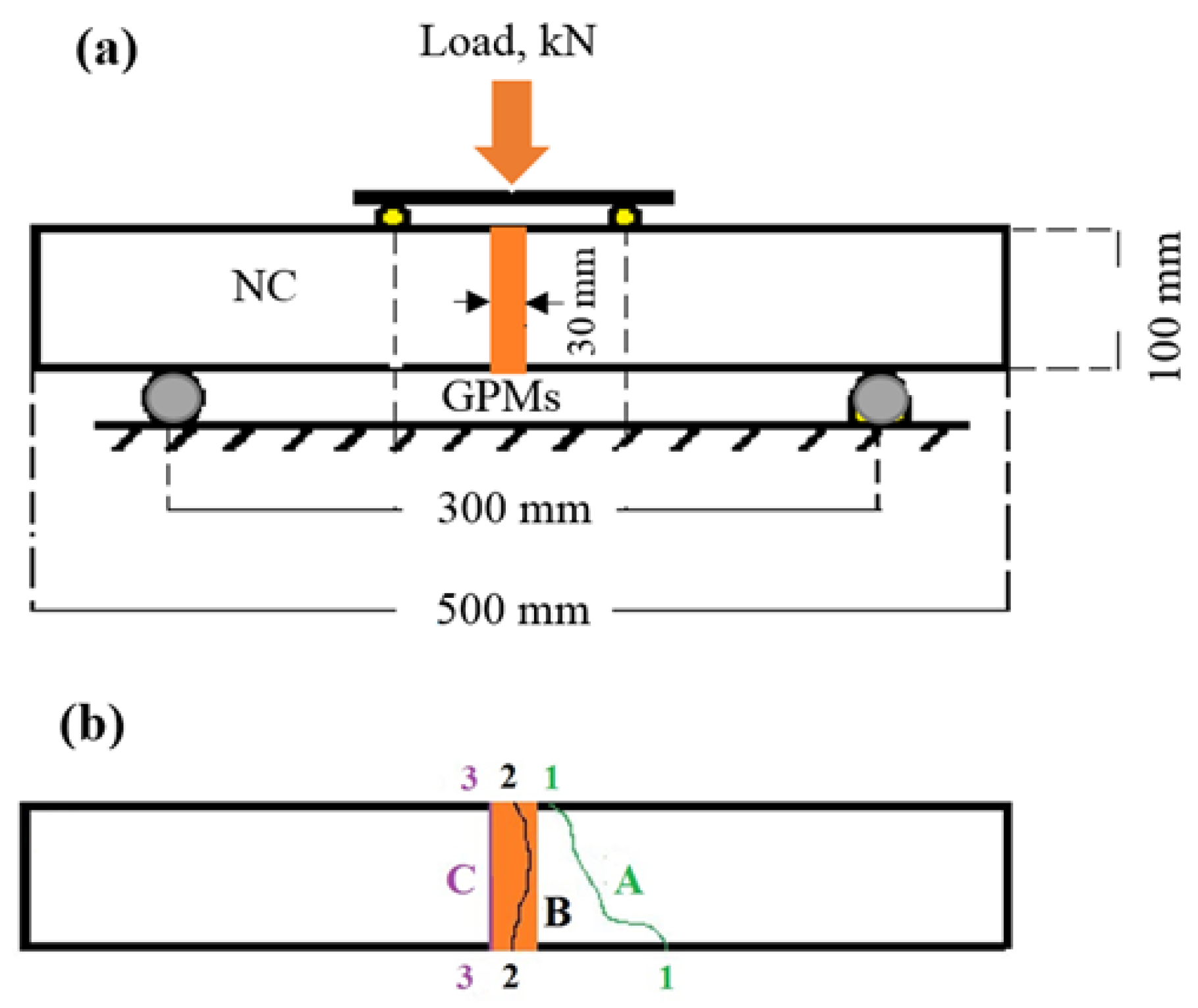
| Mix | FS (MPa) | Failure Zone | ||
|---|---|---|---|---|
| Base Line (NC) | NC | GPMs-NC Beam | ||
| OPC | 0.63 | 1.67 | 1.64 | D |
| GPMs1 | 0.63 | 1.67 | 1.76 | B |
| GPMs2 | 0.63 | 1.67 | 1.73 | B |
| GPMs3 | 0.63 | 1.67 | 1.69 | B |
| GPMs4 | 0.63 | 1.67 | 1.67 | C |
| GPMs5 | 0.63 | 1.67 | 1.64 | D |
| GPMs6 | 0.63 | 1.67 | 1.66 | C |
| GPMs7 | 0.63 | 1.67 | 1.65 | D |
| GPMs8 | 0.63 | 1.67 | 1.62 | D |
| GPMs9 | 0.63 | 1.67 | 1.58 | D |
| GPMs10 | 0.63 | 1.67 | 1.54 | D |
| Chemical Composition | |||
|---|---|---|---|
| Oxides/Material | WCT | FA | GBFS |
| SiO2 | 72.6 | 57.20 | 30.8 |
| Al2O3 | 12.2 | 28.81 | 10.9 |
| Fe2O3 | 0.56 | 3.67 | 0.64 |
| CaO | 0.02 | 5.16 | 51.8 |
| MgO | 0.99 | 1.48 | 4.57 |
| K2O | 0.03 | 0.94 | 0.36 |
| Na2O | 13.46 | 0.07 | 0.46 |
| SO3 | 0.01 | 0.10 | 0.06 |
| LOI | 0.13 | 0.12 | 0.22 |
| Materials’ physical traits | |||
| Specific gravity | 2.61 | 2.2 | 2.9 |
| Surface area-BET (m2/g) | 12.2 | 18.1 | 13.6 |
| Medium diameter (µm) | 35 | 10 | 12.8 |
| Materials (Mass%) | Geopolymer Mortars of the Designed Mixtures | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GPMs1 | GPMs2 | GPMs3 | GPMs4 | GPMs5 | GPMs6 | GPMs7 | GPMs8 | GPMs9 | GPMs10 | |||
| Binder (B) | WCT | 0 | 50 | 50 | 50 | 50 | 60 | 60 | 60 | 70 | 70 | |
| GBFS | 50 | 50 | 40 | 30 | 20 | 40 | 30 | 20 | 30 | 20 | ||
| FA | 50 | 0 | 10 | 20 | 30 | 0 | 10 | 20 | 0 | 10 | ||
| SiO2:Al2O3 | 2.82 | 4.48 | 4.08 | 3.77 | 3.53 | 4.79 | 4.35 | 4.01 | 5.09 | 4.62 | ||
| CaO:SiO2 | 1.68 | 0.50 | 0.39 | 0.29 | 0.20 | 0.37 | 0.27 | 0.18 | 0.26 | 0.17 | ||
| CaO:Al2O3 | 4.75 | 2.24 | 1.59 | 1.09 | 0.70 | 1.77 | 1.19 | 0.74 | 1.31 | 0.79 | ||
| S:B | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | ||
| B:A | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||
| Na2SiO3:NaOH (NS:NH) | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | ||
| NaOH (NH) | H2O | 92.6 | 92.6 | 92.6 | 92.6 | 92.6 | 92.6 | 92.6 | 92.6 | 92.6 | 92.6 | |
| Molarity, M | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | ||
| Na2O | 7.4 | 7.4 | 7.4 | 7.4 | 7.4 | 7.4 | 7.4 | 7.4 | 7.4 | 7.4 | ||
| Na2SiO3 (NS) | Na2O | 14.7 | 14.7 | 14.7 | 14.7 | 14.7 | 14.7 | 14.7 | 14.7 | 14.7 | 14.7 | |
| SiO2 | 29.5 | 29.5 | 29.5 | 29.5 | 29.5 | 29.5 | 29.5 | 29.5 | 29.5 | 29.5 | ||
| H2O | 55.8 | 55.8 | 55.8 | 55.8 | 55.8 | 55.8 | 55.8 | 55.8 | 55.8 | 55.8 | ||
| Total H2O in alkaline solution | 76.8 | 76.8 | 76.8 | 76.8 | 76.8 | 76.8 | 76.8 | 76.8 | 76.8 | 76.8 | ||
| Ratio of SiO2:Na2O (Ms) | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alyousef, R.; Ebid, A.A.K.; Huseien, G.F.; Mohammadhosseini, H.; Alabduljabbar, H.; Poi Ngian, S.; Mohamed, A.M. Effects of Sulfate and Sulfuric Acid on Efficiency of Geopolymers as Concrete Repair Materials. Gels 2022, 8, 53. https://doi.org/10.3390/gels8010053
Alyousef R, Ebid AAK, Huseien GF, Mohammadhosseini H, Alabduljabbar H, Poi Ngian S, Mohamed AM. Effects of Sulfate and Sulfuric Acid on Efficiency of Geopolymers as Concrete Repair Materials. Gels. 2022; 8(1):53. https://doi.org/10.3390/gels8010053
Chicago/Turabian StyleAlyousef, Rayed, Ahmed Abdel Khalek Ebid, Ghasan Fahim Huseien, Hossein Mohammadhosseini, Hisham Alabduljabbar, Shek Poi Ngian, and Abdeliazim Mustafa Mohamed. 2022. "Effects of Sulfate and Sulfuric Acid on Efficiency of Geopolymers as Concrete Repair Materials" Gels 8, no. 1: 53. https://doi.org/10.3390/gels8010053
APA StyleAlyousef, R., Ebid, A. A. K., Huseien, G. F., Mohammadhosseini, H., Alabduljabbar, H., Poi Ngian, S., & Mohamed, A. M. (2022). Effects of Sulfate and Sulfuric Acid on Efficiency of Geopolymers as Concrete Repair Materials. Gels, 8(1), 53. https://doi.org/10.3390/gels8010053








