Acid-Catalyzed Water Extraction of Two Polysaccharides from Artemisia argyi and Their Physicochemical Properties and Antioxidant Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Yields and Chemical Compositions
2.2. Molecular Weight and Monosaccharide Composition
2.3. FT-IR
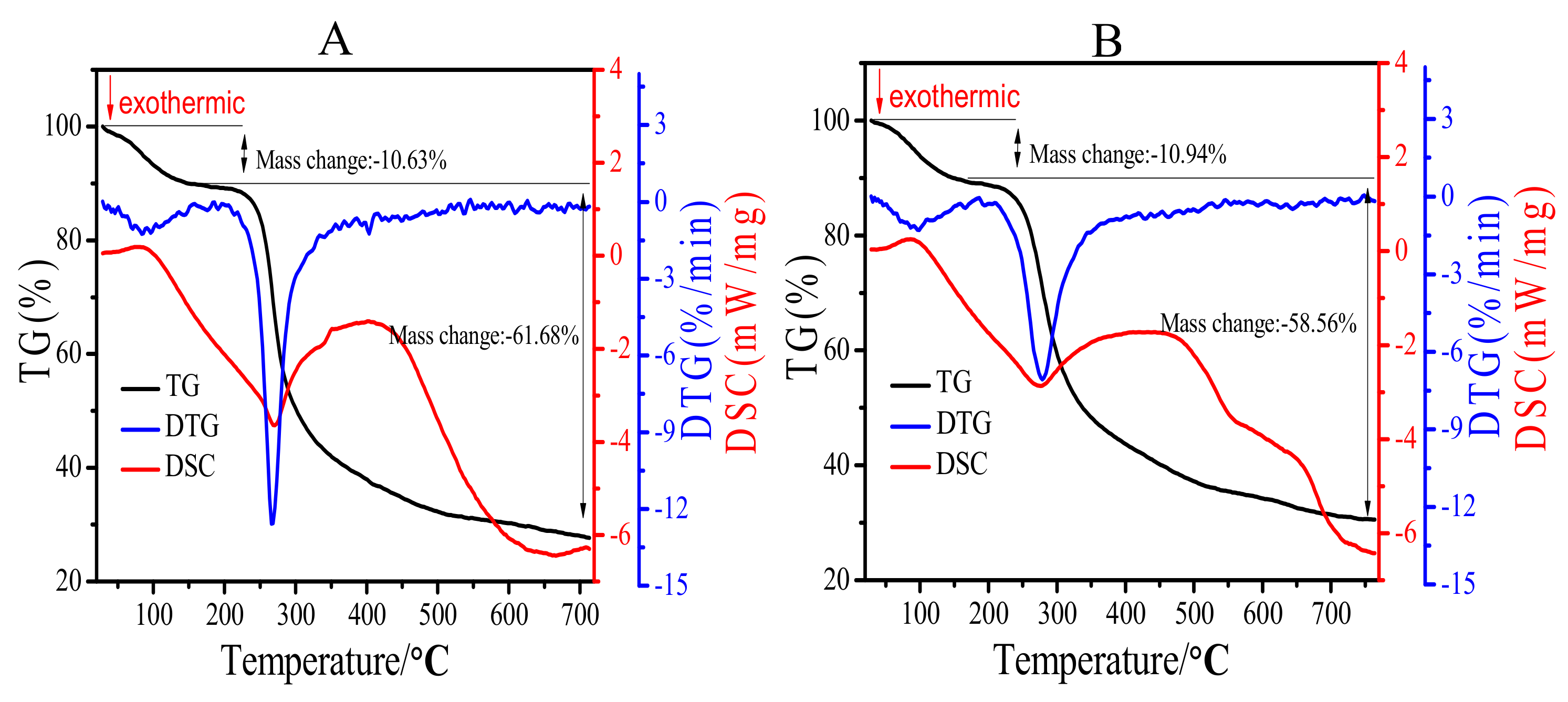
2.4. Thermal Analysis
2.5. Methylation Analysis
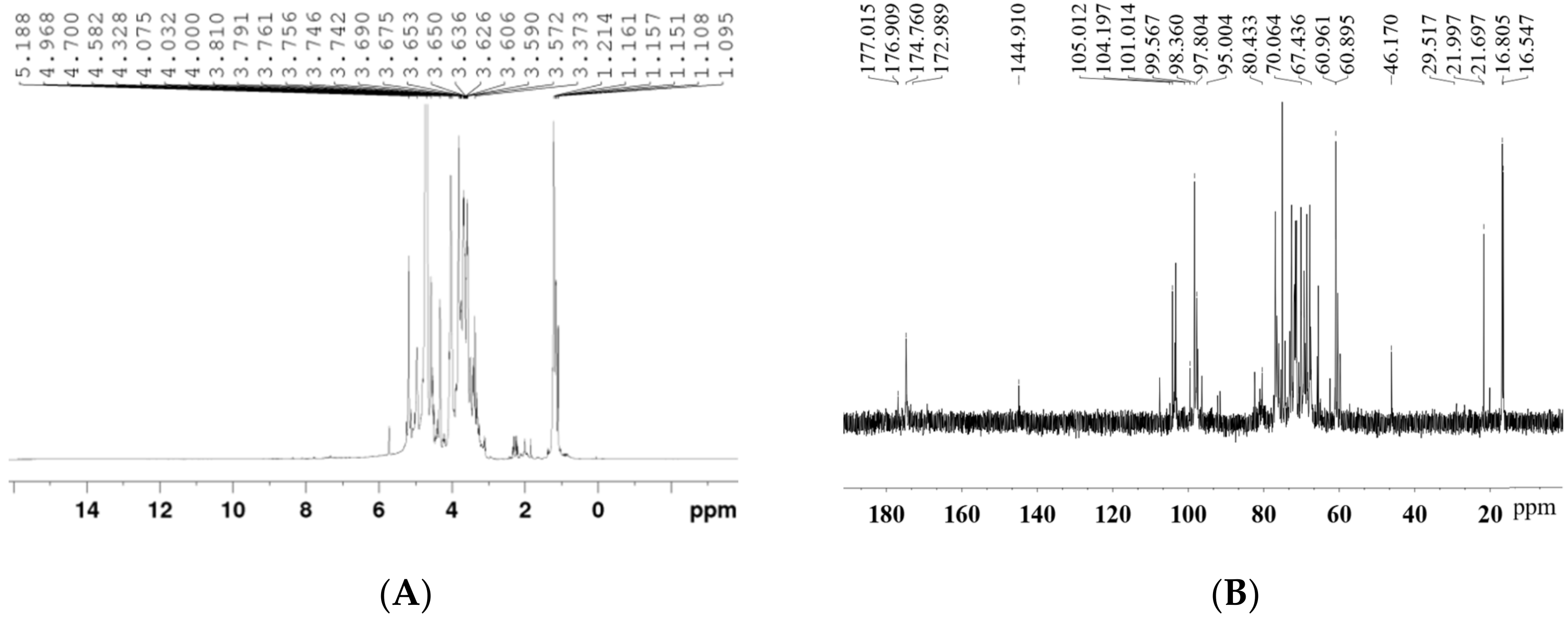
2.6. Nuclear Magnetic Resonance (NMR) Analysis
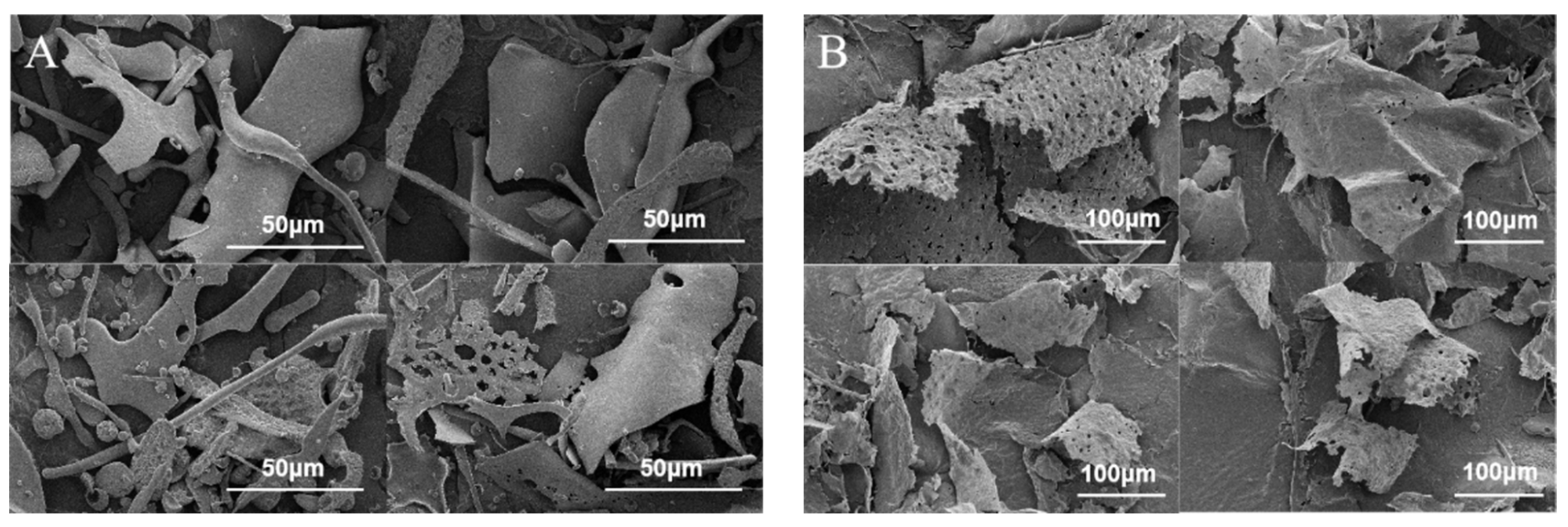
2.7. SEM Analysis
2.8. Antioxidant Activities
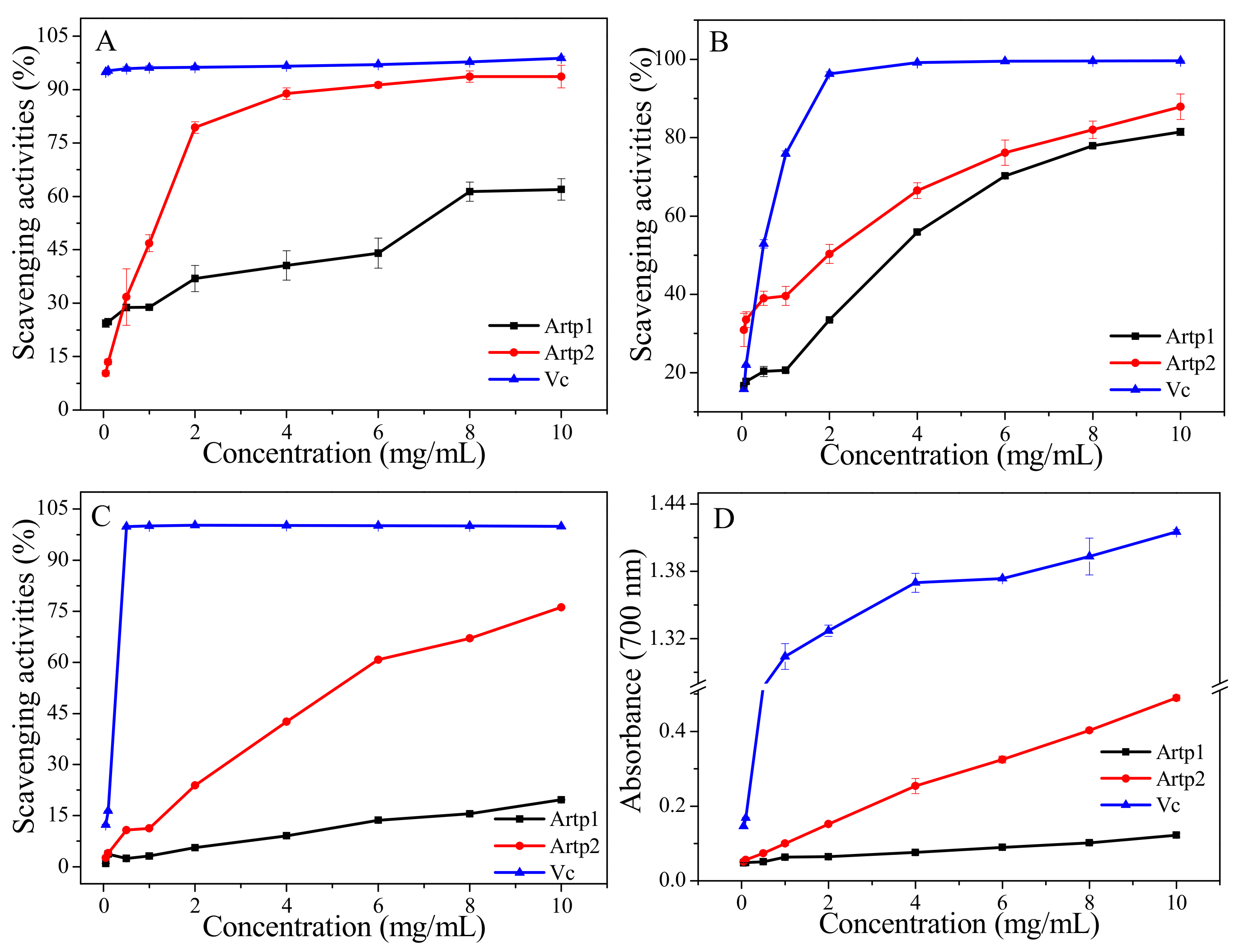
2.8.1. DPPH Radical Scavenging Assay
2.8.2. Hydroxyl Radical Scavenging Assay
2.8.3. ABTS Radical Scavenging Assay
2.8.4. Reducing Power
3. Conclusions
4. Materials and Methods
4.1. Materials and Reagents
4.2. Extraction the Crude Polysaccharides from A. argyi
4.3. Purification of Polysaccharide
4.4. Characterization of Artp1 and Artp2
4.4.1. Chemical Composition Analysis
4.4.2. Molecular Weight Detection and Monosaccharide Composition Analysis
4.4.3. Fourier Transform Infrared Spectroscopy Analysis (FT-IR)
4.4.4. Thermal Analysis
4.4.5. Methylation Analysis
4.4.6. Nuclear Magnetic Resonance (NMR) and Scanning Electron Microscope (SEM) Analyses
4.5. Antioxidant Activities
4.5.1. DPPH Radical Scavenging Assay
4.5.2. Hydroxyl Radical Scavenging Assay
4.5.3. ABTS Radical Scavenging Assay
4.5.4. Reducing Power
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Commission of the Ministry of Health of the People’s Republic of China. Pharmacopoeia of the People’s Republic of China; Chinese Medical Science and Technology Press: Beijing, China, 2020.
- Khan, M.; Bo, Y.; Shawi, A.; Yi, F.; Yang, H.; Ma, T. Jaceosidin induces apoptosis in U87 glioblastoma cells through G2/M phase arrest. Evid. Based Complementary Altern. Med. 2012, 2012, 703034. [Google Scholar] [CrossRef]
- Bao, X.; Yuan, H.; Wang, C.; Liu, J.; Lan, M. Antitumor and immunomodulatory activities of a polysaccharide from Artemisia argyi. Carbohydr. Polym. 2013, 98, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Healthy China · Tangyin Northern Artemisia argyi Genuine Medicinal Materials International Summit Forum Was Held in Beijing. Available online: http://www.rmzxb.com.cn/c/2018-05-08/2047335.shtml (accessed on 8 May 2018).
- Song, X.; Wen, X.; He, J.; Zhao, H.; Li, S.; Wang, M. Phytochemical components and biological activities of Artemisia argyi. J. Funct. Foods. 2019, 52, 648–662. [Google Scholar] [CrossRef]
- Lan, M.B.; Zhang, Y.H.; Zheng, Y.; Yuan, H.H.; Zhao, H.L.; Gao, F. Antioxidant and immunomodulatory activities of polysaccharides from moxa (Artemisia argyi) leaf. Food Sci. Biotechnol. 2010, 19, 1463–1469. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Z.; Zhao, H.; Liu, M.; Lin, C.; Li, L.; Ma, B. Pectin polysaccharide from Flos Magnoliae (Xin Yi, Magnolia biondii Pamp. flower buds): Hot-compressed water extraction, purification and partial structural characterization. Food Hydrocoll. 2022, 122, 107061. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, L.; Li, H.; Huang, X.; Liu, X.; Guo, L.; Huang, L. Characterization of moxa floss combustion by TG/DSC, TG-FTIR and IR. Bioresour. Technol. 2019, 288, 121516. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Hou, C.; Yan, Y.; Shi, M.; Liu, Y. Comparison of structural characterization and antioxidant activity of polysaccharides from jujube (Ziziphus jujuba Mill.) fruit. Int. J. Biol. Macromol. 2020, 149, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.Z.; Li, Y.P.; Zhang, G.; Gao, Y.; Ye, H.; Gao, J.; Wang, P. Effect of extraction techniques on properties of polysaccharides from Enteromorpha prolifera and their applicability in iron chelation. Carbohydr. Polym. 2018, 181, 616–623. [Google Scholar] [CrossRef]
- Filippov, M.P. Practical infrared spectroscopy of pectic substances. Food Hydrocoll. 1992, 6, 115–142. [Google Scholar]
- Zhang, S.; Li, X.Z. Inhibition of α-glucosidase by polysaccharides from the fruit hull of Camellia oleifera Abel. Carbohydr. Polym. 2015, 115, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lei, Z.; Zhao, M.; Wu, C.; Wang, L.; Xu, Y. Microwave-assisted extraction of an acidic polysaccharide from Ribes nigrum L.: Structural characteristics and biological activities. Ind. Crop. Prod. 2020, 147, 112249. [Google Scholar] [CrossRef]
- Yan, J.M.; Zhu, L.; Qu, Y.; Qu, X.; Mu, M.; Zhang, M.; Muneer, G.; Zhou, Y.; Sun, L. Analyses of active antioxidant polysaccharides from four edible mushrooms. Int. J. Biol. Macromol. 2019, 123, 945–956. [Google Scholar] [CrossRef]
- Khemakhem, I.; Abdelhedi, O.; Trigui, I.; Ayadi, M.A.; Bouaziz, M. Structural, antioxidant and antibacterial activities of polysaccharides extracted from olive leaves. Int. J. Biol. Macromol. 2018, 106, 425–432. [Google Scholar] [CrossRef]
- Huang, L.H.; LI, Y.Y. Analysis on contents nutrition in Artemisia argyi. Food Res Dev. 2014, 35, 124–127. [Google Scholar]
- Liu, J.; Zhao, Y.; Wu, Q.; John, A.; Jiang, Y.; Yang, J.; Liu, H.; Yang, B. Structure characterisation of polysaccharides in vegetable “okra” and evaluation of hypoglycemic activity. Food Chem. 2018, 242, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.X.; Gao, Y.Y.; Pan, Y.; Zou, Y.F.; He, M.; He, C.L.; Li, L.X.; Yin, Z.Q.; Lv, C. Purification, chemical characterization and antioxidant activities of polysaccharides isolated from Mycena dendrobii. Carbohydr. Polym. 2019, 203, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, L. Structural characterization and antioxidant activity of polysaccharide from four auriculariales. Carbohydr. Polym. 2020, 229, 115407. [Google Scholar] [CrossRef]
- Ji, X.L.; Liu, F.; Peng, Q.; Wang, M. Purification, structural characterization, and hypolipidemic effects of a neutral polysaccharide from Ziziphus Jujuba cv. Muzao. Food Chem. 2018, 245, 1124–1130. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.M.; Qin, G.Y. Structure characterization and antioxidant activity of polysaccharides from Chinese quince seed meal. Food Chem. 2017, 234, 314–322. [Google Scholar] [CrossRef]
- Ma, F.M.; Wu, J.W.; Li, P.; Tao, D.; Zhao, H.; Zhang, B.; Li, B. Effect of solution plasma process with hydrogen peroxide on the degradation of water-soluble polysaccharide from Auricularia auricula. II: Solution conformation and antioxidant activities in vitro. Carbohydr. Polym. 2018, 198, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, P.Z.; Shen, J.W.; Qian, Y.Y.; Liu, M.; Ruan, Y.; Wang, X.G.; Zhang, S.N.; Ma, B.J. Physicochemical properties and bioactivities of original and Se-enriched polysaccharides with different molecular weights extracted from Pleurotus ostreatus. Int. J. Biol. Macromol. 2019, 141, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Yuanfa Liu, Y.F.; Fan, L.P.; Ai, L.; Shan, L. Antioxidant activities of polysaccharides from the fruiting bodies of Zizyphus Jujuba cv. Jinsixiaozao. Carbohydr. Polym. 2011, 84, 390–394. [Google Scholar] [CrossRef]
- Liu, F.; Ooi, V.E.C.; Chang, S.T. Free radical scavenging activities of mushroom polysaccharide extracts. Life Sci. 1997, 60, 763–771. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, W.; Wen, P.; Shen, M.; Li, H.; Ren, Y.; Xiao, Y.; Song, Q.; Chen, Y.; Yu, Q.; et al. Two water-soluble polysaccharides from mung bean skin: Physicochemical characterization, antioxidant and antibacterial activities. Food Hydrocoll. 2020, 100, 105412. [Google Scholar] [CrossRef]
- Ye, C.; Lai, Y. Optimization of extraction process and antioxidant activity of polysaccharides from leaves of Artemisia argyi Levl. et Vant. J. Food Process. Preserv. 2015, 39, 1309–1317. [Google Scholar] [CrossRef]
- Hajji, M.; Hamdi, M.; Sellimi, S.; Ksouda, G.; Laouer, H.; Li, S.; Nasri, M. Structural characterization, antioxidant and antibacterial activities of a novel polysaccharide from Periploca laevigata root barks. Carbohydr. Polym. 2019, 206, 380–388. [Google Scholar] [CrossRef]

| Samples | Artp1 | Artp2 |
|---|---|---|
| Carbohydrate contents (%) | 57.43 ± 3.25 | 44.82 ± 2.52 |
| Uronic acid content (%) | 37.39 ± 4.46 | 39.10 ± 2.64 |
| Protein contents (%) | 1.07 ± 0.01 | 2.56 ± 0.34 |
| Phenolic contents (%) | 0.02 ± 0.005 | 0.14 ± 0.022 |
| Molecular Weight (kDa) | Artp1 | Artp2 |
|---|---|---|
| Weight-average molecular weight (Mw) | 42.17 | 175.22 |
| Number-average molecular weight (Mn) | 28.19 | 115.95 |
| Polymer dispersity index (PDI) | 1.49 | 1.51 |
| Monosaccharide Composition (Molar Ratio %) | Artp1 | Artp2 |
|---|---|---|
| Fucose (Fuc) | 0.5 | 3.8 |
| Arabinose (Ara) | 1.3 | 1.6 |
| Rhamnose (Rha) | 25.1 | 16.7 |
| Galactose (Gal) | 24.7 | 13.5 |
| Glucose (Glc) | 0.2 | 5.3 |
| Xylose (Xyl) | 5.2 | 12.8 |
| Mannose (Man) | 0.8 | 1.6 |
| Galacturonic acid (GalA) | 40.4 | 38.7 |
| Glucuronic acid (GlcA) | 0.2 | 4.4 |
| Mannuronic acid (ManA) | 1.6 | 1.6 |
| Methylated Sugar | Molar Ratio (%) | Linkage Type |
|---|---|---|
| 3,4-Me2-Rhap | 8.32 | 2-Rhap |
| 3-Me1-Rhap | 17.68 | 2,4-Rhap |
| 2,3,4,6-Me4-Galp | 31.53 | t-Galp |
| 2,4,6-Me3-Galp | 1.51 | 1,3-Galp |
| 2,3,4-Me3-Galp | 1.41 | 1,6-Galp |
| 2,3,6-Me3-GalAp | 28.27 | 1,4-GalAp |
| 2,6-Me2-GalAp | 1.61 | 1,3,4-GalAp |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruan, Y.; Niu, C.; Zhang, P.; Qian, Y.; Li, X.; Wang, L.; Ma, B. Acid-Catalyzed Water Extraction of Two Polysaccharides from Artemisia argyi and Their Physicochemical Properties and Antioxidant Activities. Gels 2022, 8, 5. https://doi.org/10.3390/gels8010005
Ruan Y, Niu C, Zhang P, Qian Y, Li X, Wang L, Ma B. Acid-Catalyzed Water Extraction of Two Polysaccharides from Artemisia argyi and Their Physicochemical Properties and Antioxidant Activities. Gels. 2022; 8(1):5. https://doi.org/10.3390/gels8010005
Chicago/Turabian StyleRuan, Yuan, Chaofei Niu, Pengzhan Zhang, Yanyan Qian, Xinxin Li, Li Wang, and Bingji Ma. 2022. "Acid-Catalyzed Water Extraction of Two Polysaccharides from Artemisia argyi and Their Physicochemical Properties and Antioxidant Activities" Gels 8, no. 1: 5. https://doi.org/10.3390/gels8010005
APA StyleRuan, Y., Niu, C., Zhang, P., Qian, Y., Li, X., Wang, L., & Ma, B. (2022). Acid-Catalyzed Water Extraction of Two Polysaccharides from Artemisia argyi and Their Physicochemical Properties and Antioxidant Activities. Gels, 8(1), 5. https://doi.org/10.3390/gels8010005







