Development of Antibacterial and Antifouling Innovative and Eco-Sustainable Sol–Gel Based Materials: From Marine Areas Protection to Healthcare Applications
Abstract
1. Introduction
- (1)
- If a foulant overtakes the surface, the coating layer will reduce the number of possible formations, entropically unfavorable, consequently inducing steric repulsion and inhibiting settlement;
- (2)
- If a tightly bound hydration layer surrounded the coating layer, water would have to be replaced for an adhering fouling particle. The dehydration process is thermodynamically unfavorable, leading to the repulsion of advancing foulants [1].
- Organic fouling: settlement of the organic matter, such as proteins, polysaccharides, lipids, etc.;
- Inorganic fouling: inorganic substances, such as salts and metal oxides, result from crystallization or corrosion processes;
- Particulate fouling: growth of colloidal particles;
- Biofouling: settlement of the biological matter, which grows into biofilm microorganisms and leads to macroscopic biofouling [8].
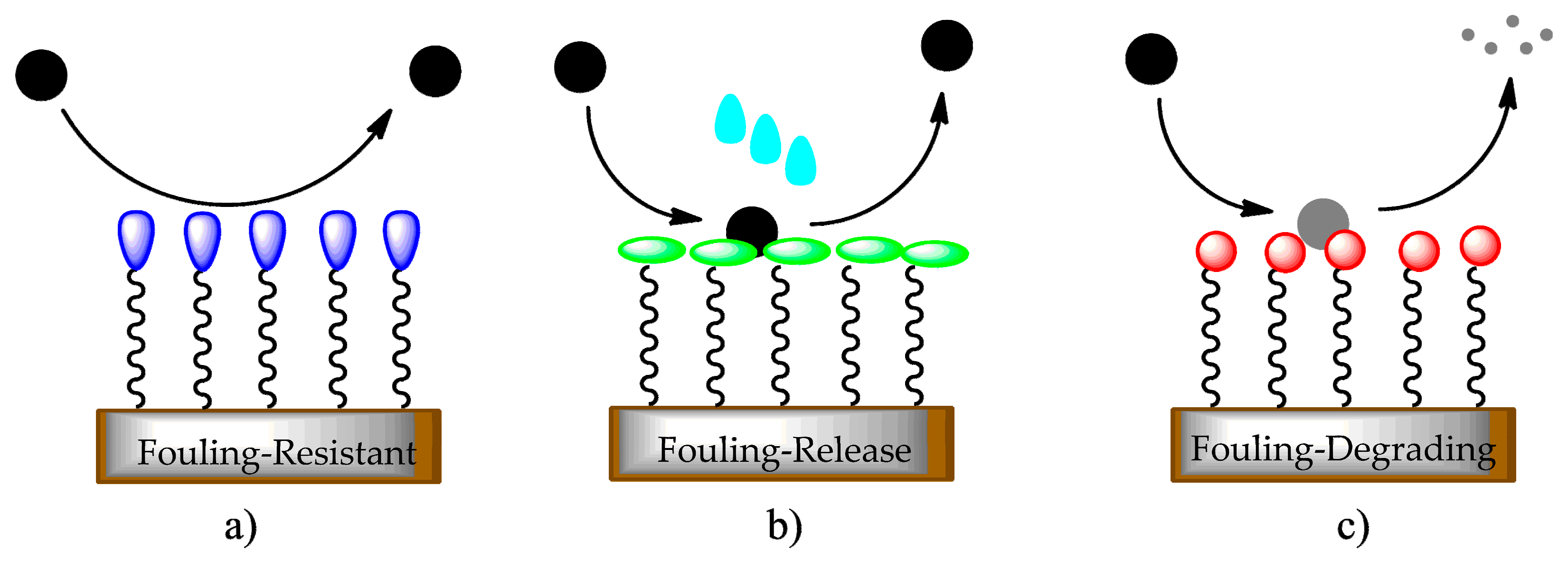
- Fouling-resistant: inhibits adhesion of macromolecules, microorganisms, or bacteria, usually associated with forming an intensely hydrated surface, as this provides a physical barrier to foulants [9];
- Fouling-release: provides limited foulant–surface adhesion but promotes simple removal of foulants by applying a little mechanical force, such as a water jet or an external trigger [9];
- Fouling-degrading: deteriorates adsorbed foulants through oxidizing agents and/or other microorganisms by bactericidal functionalities [10].
- v.
- Primary adsorption: penetrate through and adsorb onto the substrate;
- vi.
- Secondary adsorption: adsorb on top of the substrate;
- vii.
- Tertiary adsorption; adsorb inside the substrate [13].
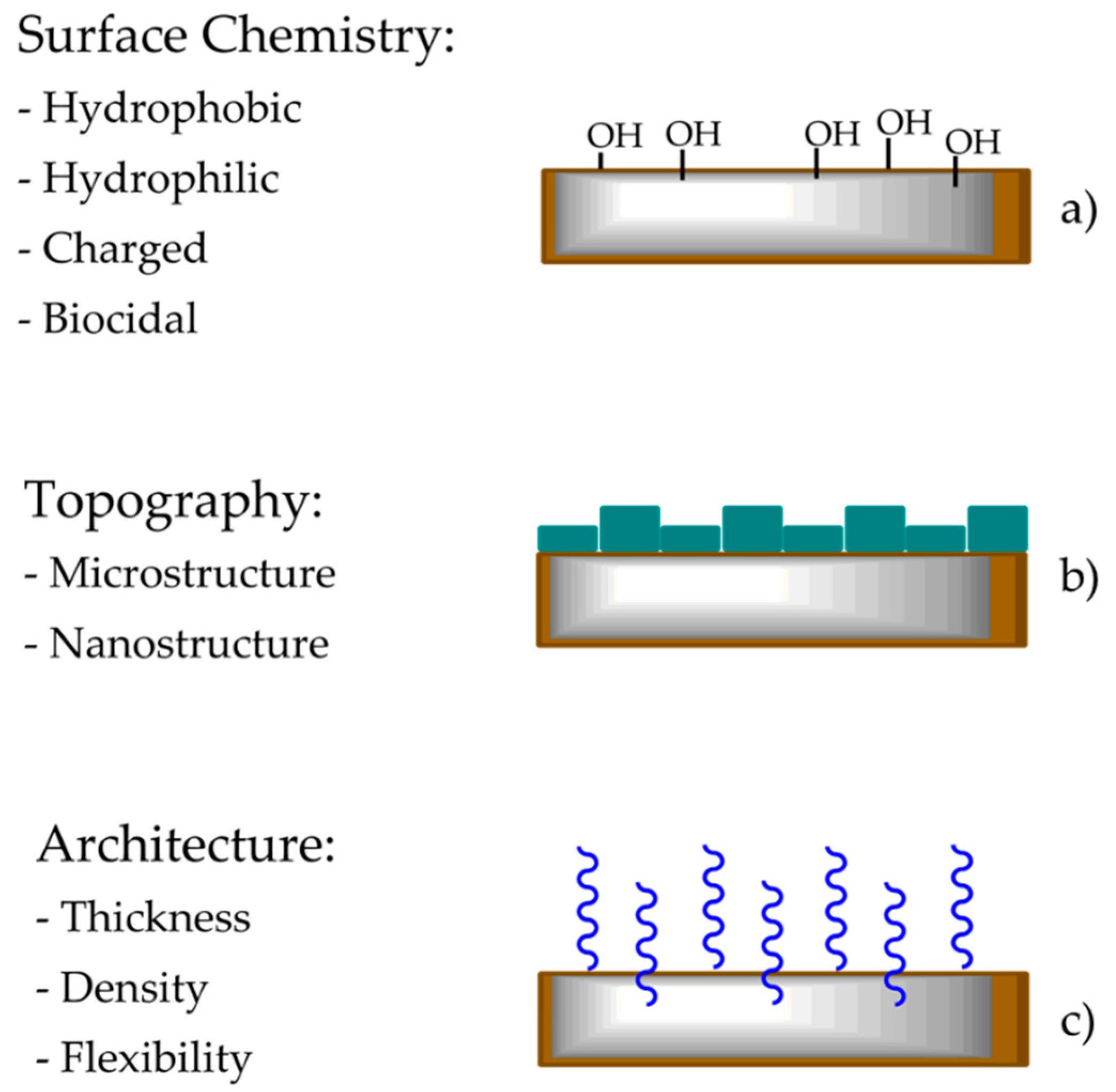
- (a)
- The thin coating ensures excellent adhesion between the substrate and the top layer;
- (b)
- Protection against corrosion;
- (c)
- Simple, economic, and efficient production;
- (d)
- Highly controlled composition.
- (a)
- The contraction of the material that occurs during curing and processing;
- (b)
- Presence of residues of unreacted chemicals;
- (c)
- Use of organic solvents, which can be unhealthy.
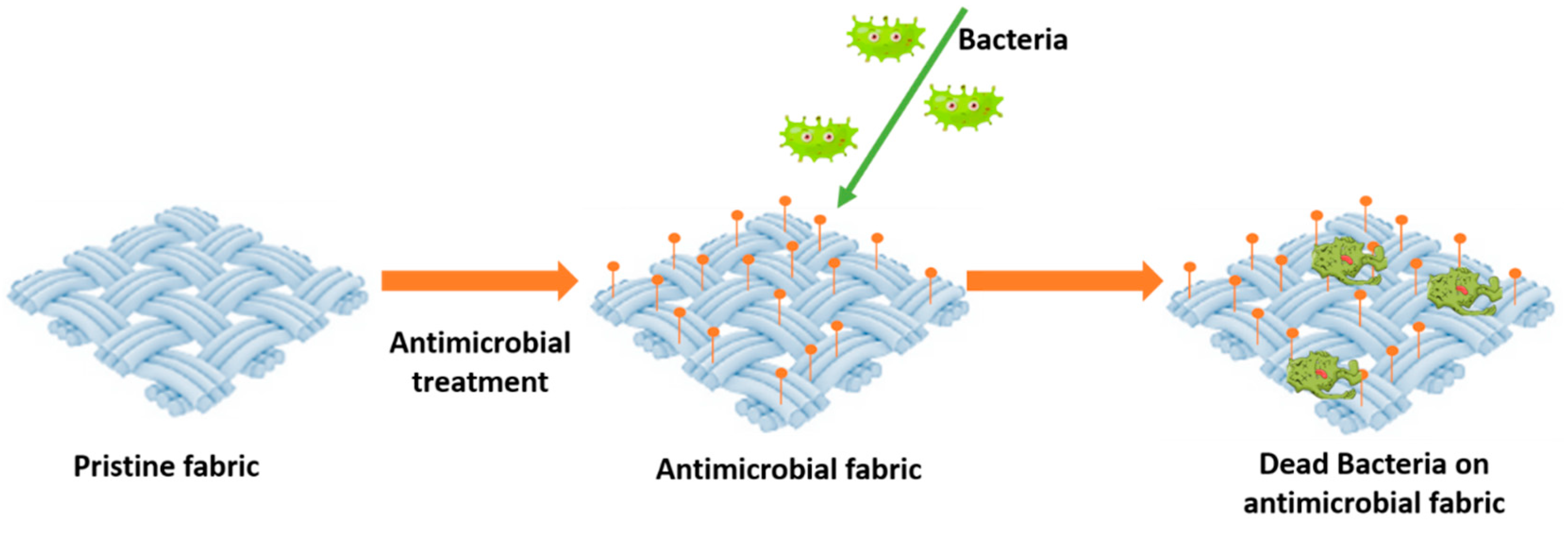
2. Antibacterial Agents for Concrete
2.1. Polymers and Inorganic Biocidal Additives as Antibacterial Agents
2.2. The Use of Nanotechnologies to Prevent Microbial Growth
2.3. Hybrid Geopolymer-Based Materials with Antimicrobial Properties
3. Antifouling and Antibacterial Agents for Cultural Heritage
- In the first case, the simple mechanical removal of the biodeteriorants was carried out, and it was observed how the recolonization of the stone occurs rapidly;
- In the second case, Rocima was used to remove the already existing biofouling followed by microwave treatment. In this case, the biocidal activity lasted for five years;
- In the third case, the stone material was treated with a mixture of Biotin R, in 5% ethyl alcohol, used alone or mixed with Titania nanoparticles and Silver–Titania core-shell nanoparticles (TiO2 and Ag-TiO2). This treatment resulted in a prolonged biocidal action up to 8 months after application.
4. Antifouling Coatings for Filtration Membrane Technology
5. Antifouling and Foul-Release Coatings for Marine Applications
- High fuel consumption because of the increased resistance due to biofouling, making the hull rougher and the ship heavier. It was proven that microfouling could increase fuel consumption by up to 18% and reduce the sailing speed by at least 20% [99];
- High maintenance costs because drydocking operations need to be performed more frequently [100]. More pollution since cleaning processes generate a large number of toxic substances that are discharged into the ocean;
- Increased ship hull corrosion since the protective coating surface deteriorates because of biological processes. The hull surface is more susceptible to corrosion and discoloration [101].
- Biocide-release coatings: based on the dispersion of biocides with different types of polymeric binders released over time in seawater. Currently, these coatings are the most used;
- Fouling resistant coatings: prevent the attachment of “foulers” to the surface;
- Fouling release coatings: reduce the adhesion between marine organisms and the materials of which the submerged surfaces are made;
- Fouling degrading coatings: inhibit/kill “foulers”.
- Contact leaching coatings (insoluble matrix): the polymer matrix is insoluble in water, while the toxic substances or biocides are incorporated into the paint and released gradually, leaving free pores that are freed by the passage of water that dissolves the toxic particles; however, the matrix remains intact. In this case, the biocides are released at a rate that decreases over time, reducing the effect of the protection. They have a short life (12–24 months), and this has greatly limited their use even if, over time, the duration and the release rate were made more durable;
- Controlled depletive polymer (CDP) coatings (matrix soluble by hydration): in this case, the matrix is composed of biocides and a resin-based soluble matrix that, with the passage of water, are dissolved and released on the surface to protect and contrast the “foulers”. The release process is more controlled than in the previous case and is based on the hydration and dissolution mechanism of the soluble binder. It has a constant and controlled release with a duration of about 36 months;
- Self-polishing copolymer coatings: SPC paints use an acrylic or methacrylate polymer matrix. The release mechanism of the biocides is based on the dissolution of the matrix by hydrolysis in seawater, which gives a considerable smoothness to the surface, thus reducing friction and, therefore, the resistance to the ship’s motion. In addition to making the surface smooth, decomposition by hydrolysis also detaches organisms from the hull, releasing biocides. The release rate of substances can be controlled based on the degree of polymerization and the polymer chains’ hydrophilic properties. Paints with modern SPC technology have five years and occupy 80% of the antifouling paint market;
- CDP and SPC mixed coatings: I combined the properties of the last two technologies mentioned. The dissolution of the matrix is obtained both by hydrolysis and by hydration with better control of the biocide release rate and a duration of 5 years.
6. Development of Antibacterial and Antifouling Innovative and Eco-Sustainable Solutions from Marine Areas Protection to Healthcare Applications—Textile Materials
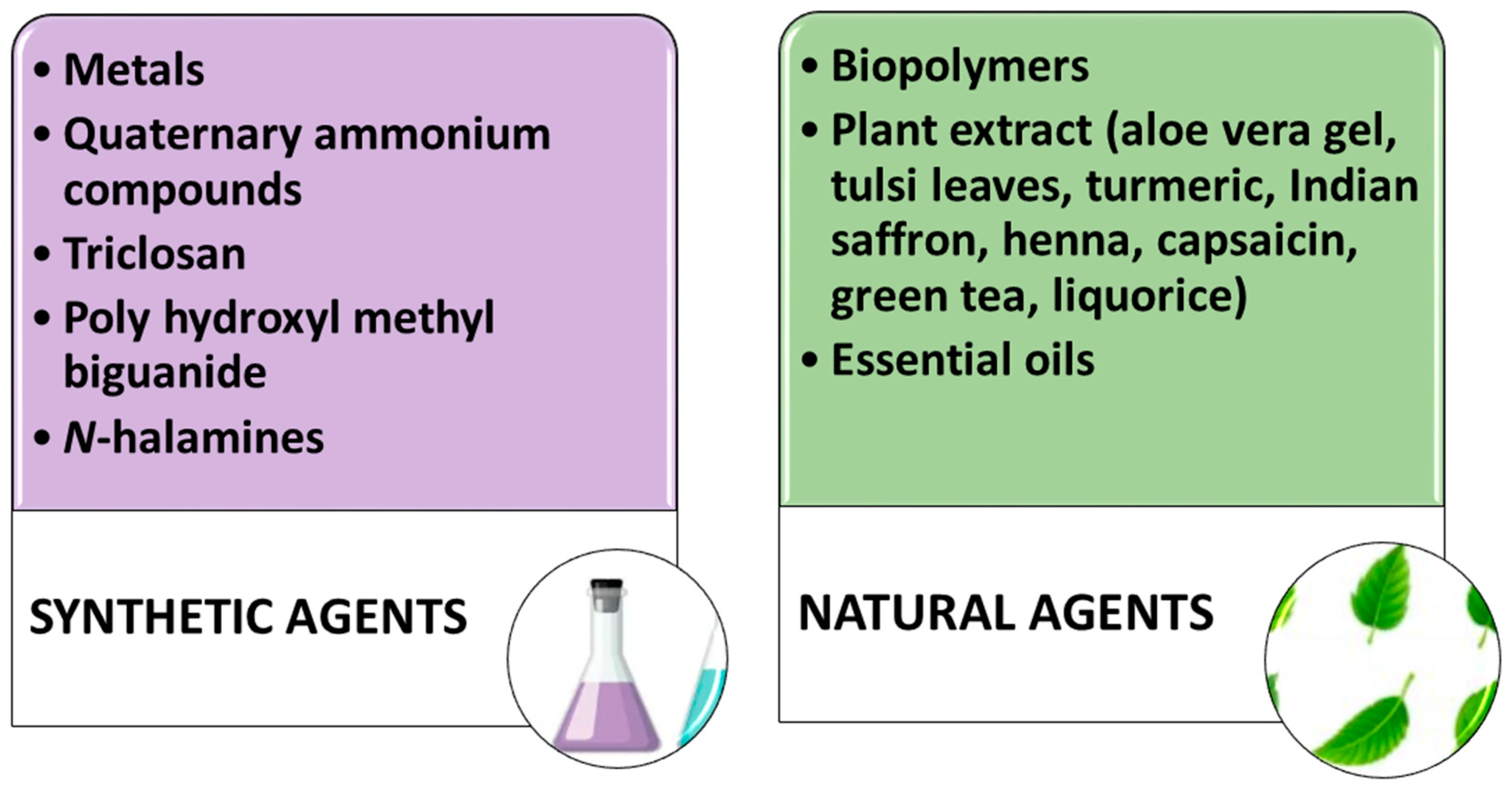
6.1. Sustainability Issues of Commercial Antimicrobial Formulations
6.2. Sol–Gel Technology: Synthesis of Antimicrobial Formulations
6.3. Biopolymers as Antimicrobial Agents
6.4. Plant Extracts Used for Imparting Antimicrobial Activity to Fabrics
6.5. Antimicrobial Properties of Essential Oils
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maan, A.M.C.; Hofman, A.H.; de Vos, W.M.; Kamperman, M. Recent Developments and Practical Feasibility of Polymer-Based Antifouling Coatings. Adv. Funct. Mater. 2020, 30, 3–8. [Google Scholar] [CrossRef]
- Dafforn, K.A.; Lewis, J.A.; Johnston, E.L. Antifouling strategies: History and regulation, ecological impacts and mitigation. Mar. Pollut. Bull. 2011, 62, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Makvandi, P.; Zare, E.N.; Tay, F.R.; Niu, L. Advances in Antimicrobial Organic and Inorganic Nanocompounds in Biomedicine. Adv. Ther. 2020, 3, 2000024. [Google Scholar] [CrossRef]
- Makvandi, P.; Wang, C.; Zare, E.N.; Borzacchiello, A.; Niu, L.; Tay, F.R. Metal-Based Nanomaterials in Biomedical Applications: Antimicrobial Activity and Cytotoxicity Aspects. Adv. Funct. Mater. 2020, 30, 1910021. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, J. Polymers against Microorganisms: On the Race to Efficient Antimicrobial Materials; Springer: Cham, Switzerland, 2016; pp. 1–278. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, H.; Guo, Z.; Feng, D.; Thiyagarajan, V.; Yao, H. Combat biofouling with microscopic ridge-like surface morphology: A bioinspired study. J. R. Soc. Interface 2018, 15, 20170823. [Google Scholar] [CrossRef]
- Chen, W.-L.; Cordero, R.; Tran, H.; Ober, C.K. 50th Anniversary Perspective: Polymer Brushes: Novel Surfaces for Future Materials. Macromolecules 2017, 50, 4089–4113. [Google Scholar] [CrossRef]
- Nurioglu, A.G.; Esteves, A.C.C.; De With, G. Non-toxic, non-biocide-release antifouling coatings based on molecular structure design for marine applications. J. Mater. Chem. B 2015, 3, 6547–6570. [Google Scholar] [CrossRef]
- Sakala, G.P.; Reches, M. Peptide-Based Approaches to Fight Biofouling. Adv. Mater. Interfaces 2018, 5, 1800073. [Google Scholar] [CrossRef]
- Asha, A.B.; Chen, Y.; Zhang, H.; Ghaemi, S.; Ishihara, K.; Liu, Y.; Narain, R. Rapid Mussel-Inspired Surface Zwitteration for Enhanced Antifouling and Antibacterial Properties. Langmuir 2019, 35, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Ostuni, E.; Chapman, R.G.; Holmlin, R.E.; Takayama, S.; Whitesides, G.M. A Survey of Structure−Property Relationships of Surfaces that Resist the Adsorption of Protein. Langmuir 2001, 17, 5605–5620. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef]
- Currie, E.P.K.; Norde, W.; Cohen Stuart, M.A. Tethered polymer chains: Surface chemistry and their impact on colloidal and surface properties. Adv. Colloid Interface Sci. 2003, 100–102, 205–265. [Google Scholar] [CrossRef]
- Brzozowska, A.M.; Hofs, B.; de Keizer, A.; Fokkink, R.; Cohen Stuart, M.A.; Norde, W. Reduction of protein adsorption on silica and polystyrene surfaces due to coating with Complex Coacervate Core Micelles. Colloids Surf. A Physicochem. Eng. Asp. 2009, 347, 146–155. [Google Scholar] [CrossRef]
- Jeon, S.I.; Lee, J.H.; Andrade, J.D.; De Gennes, P.G. Protein-surface interactions in the presence of polyethylene oxide. I. Simplified theory. J. Colloid Interface Sci. 1991, 142, 149–158. [Google Scholar] [CrossRef]
- Ye, Q.; Zhao, W.; Yang, W.; Pei, X.; Zhou, F. Grafting Binary PEG and Fluoropolymer Brushes from Mix-Biomimic Initiator as “Ambiguous” Surfaces for Antibiofouling. Macromol. Chem. Phys. 2017, 218, 1700085. [Google Scholar] [CrossRef]
- Yang, L.; Wu, H.; Liu, Y.; Xia, Q.; Yang, Y.; Chen, N.; Yang, M.; Luo, R.; Liu, G.; Wang, Y. A robust mussel-inspired zwitterionic coating on biodegradable poly(L-lactide) stent with enhanced anticoagulant, anti-inflammatory, and anti-hyperplasia properties. Chem. Eng. J. 2022, 427, 130910. [Google Scholar] [CrossRef]
- Chang, Y.; Shu, S.H.; Shih, Y.J.; Chu, C.W.; Ruaan, R.C.; Chen, W.Y. Hemocompatible mixed-charge copolymer brushes of pseudozwitterionic surfaces resistant to nonspecific plasma protein fouling. Langmuir 2010, 26, 3522–3530. [Google Scholar] [CrossRef]
- Ye, Q.; Gao, T.; Wan, F.; Yu, B.; Pei, X.; Zhou, F.; Xue, Q. Grafting poly(ionic liquid) brushes for anti-bacterial and anti-biofouling applications. J. Mater. Chem. 2012, 22, 13123–13131. [Google Scholar] [CrossRef]
- Schumacher, J.F.; Carman, M.L.; Estes, T.G.; Feinberg, A.W.; Wilson, L.H.; Callow, M.E.; Callow, J.A.; Finlay, J.A.; Brennan, A.B. Engineered antifouling microtopographies—Effect of feature size, geometry, and roughness on settlement of zoospores of the green alga Ulva. Biofouling 2007, 23, 55–62. [Google Scholar] [CrossRef]
- Singh, N.B.; Kalra, M.; Saxena, S.K. Nanoscience of Cement and Concrete. Mater. Today Proc. 2017, 4, 5478–5487. [Google Scholar] [CrossRef]
- Miller, S.A.; Moore, F.C. Climate and health damages from global concrete production. Nat. Clim. Chang. 2020, 10, 439–443. [Google Scholar] [CrossRef]
- Häubner, N.; Schumann, R.; Karsten, U. Aeroterrestrial Microalgae Growing in Biofilms on Facades—Response to Temperature and Water Stress. Microb. Ecol. 2006, 51, 285–293. [Google Scholar] [CrossRef]
- Lors, C.; Aube, J.; Guyoneaud, R.; Vandenbulcke, F.; Damidot, D. Biodeterioration of mortars exposed to sewers in relation to microbial diversity of biofilms formed on the mortars surface. Int. Biodeterior. Biodegrad. 2018, 130, 23–31. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Bhattacharyya, S.; Chaudhuri, P.; Sudarshan, M.; Mukherjee, S. In vitro deterioration study of concrete and marble by Aspergillus tamarii. J. Build. Eng. 2020, 32, 101774. [Google Scholar] [CrossRef]
- Noeiaghaei, T.; Mukherjee, A.; Dhami, N.; Chae, S.-R. Biogenic deterioration of concrete and its mitigation technologies. Constr. Build. Mater. 2017, 149, 575–586. [Google Scholar] [CrossRef]
- Pagaling, E.; Yang, K.; Yan, T. Pyrosequencing reveals correlations between extremely acidophilic bacterial communities with hydrogen sulphide concentrations, pH and inert polymer coatings at concrete sewer crown surfaces. J. Appl. Microbiol. 2014, 117, 50–64. [Google Scholar] [CrossRef]
- Wei, S.; Jiang, Z.; Liu, H.; Zhou, D.; Sanchez-Silva, M. Microbiologically induced deterioration of concrete—A review. Braz. J. Microbiol. 2014, 44, 1001–1007. [Google Scholar] [CrossRef]
- De Muynck, W.; De Belie, N.; Verstraete, W. Effectiveness of admixtures, surface treatments and antimicrobial compounds against biogenic sulfuric acid corrosion of concrete. Cem. Concr. Compos. 2009, 31, 163–170. [Google Scholar] [CrossRef]
- Kong, L.; Fang, J.; Zhang, B. Effectiveness of Surface Coatings Against Intensified Sewage Corrosion of Concrete. J. Wuhan Univ. Technol. Sci. Ed. 2019, 34, 1177–1186. [Google Scholar] [CrossRef]
- Javaherdashti, R.; Alasvand, K. Chapter 3—An Introduction to Microbial Corrosion. In Biological Treatment of Microbial Corrosion; Javaherdashti, R., Alasvand, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 25–70. ISBN 978-0-12-816108-1. [Google Scholar]
- Qiu, L.; Dong, S.; Ashour, A.; Han, B. Antimicrobial concrete for smart and durable infrastructures: A review. Constr. Build. Mater. 2020, 260, 120456. [Google Scholar] [CrossRef]
- Plutino, M.R.; Romeo, A.; Castriciano, M.A.; Scolaro, L.M. 1,1′-bis(Diphenylphosphino)ferrocene platinum(ii) complexes as a route to functionalized multiporphyrin systems. Nanomaterials 2021, 11, 178. [Google Scholar] [CrossRef] [PubMed]
- Sikora, P.; Augustyniak, A.; Cendrowski, K.; Nawrotek, P.; Mijowska, E. Antimicrobial Activity of Al₂O₃, CuO, Fe₃O₄, and ZnO Nanoparticles in Scope of Their Further Application in Cement-Based Building Materials. Nanomaterials 2018, 8, 212. [Google Scholar] [CrossRef]
- Nam, K.Y. Characterization and antimicrobial efficacy of Portland cement impregnated with silver nanoparticles. J. Adv. Prosthodont. 2017, 9, 217–223. [Google Scholar] [CrossRef]
- Dyshlyuk, L.; Babich, O.; Ivanova, S.; Vasilchenco, N.; Atuchin, V.; Korolkov, I.; Russakov, D.; Prosekov, A. Antimicrobial potential of ZnO, TiO2 and SiO2 nanoparticles in protecting building materials from biodegradation. Int. Biodeterior. Biodegrad. 2020, 146, 104821. [Google Scholar] [CrossRef]
- Le, T.T.; Nguyen, T.V.; Nguyen, T.A.; Nguyen, T.T.H.; Thai, H.; Tran, D.L.; Dinh, D.A.; Nguyen, T.M.; Lu, L.T. Thermal, mechanical and antibacterial properties of water-based acrylic Polymer/SiO2–Ag nanocomposite coating. Mater. Chem. Phys. 2019, 232, 362–366. [Google Scholar] [CrossRef]
- Domínguez, M.; Zarzuela, R.; Moreno-Garrido, I.; Carbú, M.; Cantoral, J.M.; Mosquera, M.J.; Gil, M.L.A. Anti-fouling nano-Ag/SiO2 ormosil treatments for building materials: The role of cell-surface interactions on toxicity and bioreceptivity. Prog. Org. Coat. 2021, 153, 106120. [Google Scholar] [CrossRef]
- Gao, Q.; Wu, X.; Zhu, R. Antifouling energy-efficient coatings based on BiOClxBr1−x microflowers: NIR reflective property and superhydrophobicity. Constr. Build. Mater. 2020, 257, 119569. [Google Scholar] [CrossRef]
- Zhu, C.; Lv, J.; Chen, L.; Lin, W.; Zhang, J.; Yang, J.; Feng, J. Dark, heat-reflective, anti-ice rain and superhydrophobic cement concrete surfaces. Constr. Build. Mater. 2019, 220, 21–28. [Google Scholar] [CrossRef]
- Verdier, T.; Bertron, A.; Erable, B.; Roques, C. Bacterial Biofilm Characterization and Microscopic Evaluation of the Antibacterial Properties of a Photocatalytic Coating Protecting Building Material. Coatings 2018, 8, 93. [Google Scholar] [CrossRef]
- Janus, M.; Kusiak-Nejman, E.; Rokicka-Konieczna, P.; Markowska-Szczupak, A.; Zając, K.; Morawski, A.W. Bacterial Inactivation on Concrete Plates Loaded with Modified TiO2 Photocatalysts under Visible Light Irradiation. Molecules 2019, 24, 3026. [Google Scholar] [CrossRef]
- Dehkordi, B.A.; Nilforoushan, M.R.; Talebian, N.; Tayebi, M. A comparative study on the self-cleaning behavior and antibacterial activity of Portland cement by addition of TiO2 and ZnO nanoparticles. Mater. Res. Express 2021, 8, 35403. [Google Scholar] [CrossRef]
- Baalamurugan, J.; Ganesh Kumar, V.; Stalin Dhas, T.; Taran, S.; Nalini, S.; Karthick, V.; Ravi, M.; Govindaraju, K. Utilization of induction furnace steel slag based iron oxide nanocomposites for antibacterial studies. SN Appl. Sci. 2021, 3, 295. [Google Scholar] [CrossRef]
- Rodwihok, C.; Suwannakeaw, M.; Charoensri, K.; Wongratanaphisan, D.; Woon Woo, S.; Kim, H.S. Alkali/zinc-activated fly ash nanocomposites for dye removal and antibacterial applications. Bioresour. Technol. 2021, 331, 125060. [Google Scholar] [CrossRef]
- Rubio-Avalos, J.-C. Antibacterial Metakaolin-Based Geopolymer Cement. In Proceedings of the Calcined Clays for Sustainable Concrete; Martirena, F., Favier, A., Scrivener, K., Eds.; Springer: Dordrecht, The Netherlands, 2018; pp. 398–403. [Google Scholar]
- Dal Poggetto, G.; Catauro, M.; Crescente, G.; Leonelli, C. Efficient Addition of Waste Glass in MK-Based Geopolymers: Microstructure, Antibacterial and Cytotoxicity Investigation. Polymers 2021, 13, 1493. [Google Scholar] [CrossRef]
- Roghanian, N.; Banthia, N. Development of a sustainable coating and repair material to prevent bio-corrosion in concrete sewer and waste-water pipes. Cem. Concr. Compos. 2019, 100, 99–107. [Google Scholar] [CrossRef]
- Justo-Reinoso, I.; Hernandez, M.T. Use of Sustainable Antimicrobial Aggregates for the In-Situ Inhibition of Biogenic Corrosion on Concrete Sewer Pipes. MRS Adv. 2019, 4, 2939–2949. [Google Scholar] [CrossRef]
- Ortega-Morales, B.O.; Reyes-Estebanez, M.M.; Gaylarde, C.C.; Camacho-Chab, J.C.; Sanmartín, P.; Chan-Bacab, M.J.; Granados-Echegoyen, C.A.; Pereañez-Sacarias, J.E. Antimicrobial Properties of Nanomaterials Used to Control Microbial Colonization of Stone Substrata. In Advanced Materials for the Conservation of Stone; Hosseini, M., Karapanagiotis, I., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 277–298. ISBN 978-3-319-72260-3. [Google Scholar]
- Romeo, R.; Carnabuci, S.; Fenech, L.; Plutino, M.R.; Albinati, A. Overcrowded organometallic platinum(II) complexes that behave as molecular gears. Angew. Chem.—Int. Ed. 2006, 45, 4494–4498. [Google Scholar] [CrossRef]
- Sinicropi, M.S.; Iacopetta, D.; Rosano, C.; Randino, R.; Caruso, A.; Saturnino, C.; Muià, N.; Ceramella, J.; Puoci, F.; Rodriquez, M.; et al. N-thioalkylcarbazoles derivatives as new anti-proliferative agents: Synthesis, characterisation and molecular mechanism evaluation. J. Enzym. Inhib. Med. Chem. 2018, 33, 434–444. [Google Scholar] [CrossRef]
- Saturnino, C.; Popolo, A.; Ramunno, A.; Adesso, S.; Pecoraro, M.; Plutino, M.R.; Rizzato, S.; Albinati, A.; Marzocco, S.; Sala, M.; et al. Anti-inflammatory, antioxidant and crystallographic studies of N-Palmitoyl-ethanol amine (PEA) derivatives. Molecules 2017, 22, 616. [Google Scholar] [CrossRef]
- Li, R.; Li, T.; Zhou, Q. Impact of Titanium Dioxide (TiO2) Modification on Its Application to Pollution Treatment—A Review. Catalysts 2020, 10, 804. [Google Scholar] [CrossRef]
- Ielo, I.; Galletta, M.; Rando, G.; Sfameni, S.; Cardiano, P.; Sabatino, G.; Drommi, D.; Rosace, G.; Plutino, M.R. Design, synthesis and characterization of hybrid coatings suitable for geopolymeric-based supports for the restoration of cultural heritage. IOP Conf. Ser. Mater. Sci. Eng. 2020, 777, 012003. [Google Scholar] [CrossRef]
- Saturnino, C.; Caruso, A.; Longo, P.; Capasso, A.; Pingitore, A.; Caroleo, M.C.; Cione, E.; Perri, M.; Nicolo, F.; Nardo, V.M.; et al. Crystallographic Study and Biological Evaluation of 1,4-dimethyl-N-alkylcarbazoles. Curr. Top. Med. Chem. 2015, 15, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Guillitte, O. Bioreceptivity: A new concept for building ecology studies. Sci. Total Environ. 1995, 167, 215–220. [Google Scholar] [CrossRef]
- Allsopp, C.; Allsopp, D. An updated survey of commercial products to protect materials against biodeterioration. Int. Biodeterior. Bull. 1983, 19, 99–145. [Google Scholar] [CrossRef]
- Urzì, C.; De Leo, F. Evaluation of the efficiency of water-repellent and biocide compounds against microbial colonization of mortars. Int. Biodeterior. Biodegrad. 2007, 60, 25–34. [Google Scholar] [CrossRef]
- Liu, X.; Liang, Y.; Zhou, F.; Liu, W. Extreme wettability and tunable adhesion: Biomimicking beyond nature? Soft Matter 2012, 8, 2070–2086. [Google Scholar] [CrossRef]
- Lejars, M.; Margaillan, A.; Bressy, C. Fouling release coatings: A nontoxic alternative to biocidal antifouling coatings. Chem. Rev. 2012, 112, 4347–4390. [Google Scholar] [CrossRef]
- Bruno, L.; Rugnini, L.; Spizzichino, V.; Caneve, L.; Canini, A.; Ellwood, N.T.W. Biodeterioration of Roman hypogea: The case study of the Catacombs of SS. Marcellino and Pietro (Rome, Italy). Ann. Microbiol. 2019, 69, 1023–1032. [Google Scholar] [CrossRef]
- Palla, F.; Bruno, M.; Mercurio, F.; Tantillo, A.; Rotolo, V. Essential oils as natural biocides in conservation of cultural heritage. Molecules 2020, 25, 730. [Google Scholar] [CrossRef] [PubMed]
- Castellano, A.; Colleoni, C.; Iacono, G.; Mezzi, A.; Plutino, M.R.; Malucelli, G.; Rosace, G. Synthesis and characterization of a phosphorous/nitrogen based sol-gel coating as a novel halogen- and formaldehyde-free flame retardant finishing for cotton fabric. Polym. Degrad. Stab. 2019, 162, 148–159. [Google Scholar] [CrossRef]
- Fidanza, M.R.; Caneva, G. Natural biocides for the conservation of stone cultural heritage: A review. J. Cult. Herit. 2019, 38, 271–286. [Google Scholar] [CrossRef]
- Johnson, M. Of Biocolonization of Stone: Middle Plains Control Missouri and Preventive Village Sites; Smithsonian Institution Scholarly Press: Washington, DC, USA, 2011. [Google Scholar]
- Warscheid, T.; Braams, J. Biodeterioration of stone: A review. Int. Biodeterior. Biodegrad. 2000, 46, 343–368. [Google Scholar] [CrossRef]
- Lo Schiavo, S.; De Leo, F.; Urzì, C. Present and future perspectives for biocides and antifouling products for stone-built cultural heritage: Ionic liquids as a challenging alternative. Appl. Sci. 2020, 10, 6568. [Google Scholar] [CrossRef]
- Mascalchi, M.; Orsini, C.; Pinna, D.; Salvadori, B.; Siano, S.; Riminesi, C. Assessment of different methods for the removal of biofilms and lichens on gravestones of the English Cemetery in Florence. Int. Biodeterior. Biodegrad. 2020, 154, 105041. [Google Scholar] [CrossRef]
- Beach, E.S.; Cui, Z.; Anastas, P.T. Green Chemistry: A design framework for sustainability. Energy Environ. Sci. 2009, 2, 1038–1049. [Google Scholar] [CrossRef]
- Sierra-Fernandez, A.; Gomez-Villalba, L.S.; Rabanal, M.E.; Fort, R. New nanomaterials for applications in conservation and restoration of stony materials: A review. Mater. Constr. 2017, 67, e107. [Google Scholar] [CrossRef]
- Ruffolo, S.A.; La Russa, M.F. Nanostructured coatings for stone protection: An overview. Front. Mater. 2019, 6, 147. [Google Scholar] [CrossRef]
- Semenzin, E.; Giubilato, E.; Badetti, E.; Picone, M.; Volpi Ghirardini, A.; Hristozov, D.; Brunelli, A.; Marcomini, A. Guiding the development of sustainable nano-enabled products for the conservation of works of art: Proposal for a framework implementing the Safe by Design concept. Environ. Sci. Pollut. Res. 2019, 26, 26146–26158. [Google Scholar] [CrossRef]
- Yalcinkaya, F.; Boyraz, E.; Maryska, J.; Kucerova, K. A Review on Membrane Technology and Chemical Surface Modification for the Oily Wastewater Treatment. Materials 2020, 13, 493. [Google Scholar] [CrossRef]
- Shahkaramipour, N.; Tran, T.N.; Ramanan, S.; Lin, H. Membranes with Surface-Enhanced Antifouling Properties for Water Purification. Membranes 2017, 7, 13. [Google Scholar] [CrossRef]
- Wang, S.; Fang, L.; Cheng, L.; Jeon, S.; Kato, N. Improved antifouling properties of membranes by simple introduction of zwitterionic copolymers via electrostatic adsorption. J. Memb. Sci. 2018, 564, 672–681. [Google Scholar] [CrossRef]
- Yang, R.; Xu, J.; Ozaydin-ince, G.; Wong, S.Y.; Gleason, K.K. Surface-Tethered Zwitterionic Ultrathin Antifouling Coatings on Reverse Osmosis Membranes by Initiated Chemical Vapor Deposition. Chem. Mater. 2011, 23, 1263–1272. [Google Scholar] [CrossRef]
- Hirsch, U.; Ruehl, M.; Teuscher, N.; Heilmann, A. Applied Surface Science Antifouling coatings via plasma polymerization and atom transfer radical polymerization on thin film composite membranes for reverse osmosis. Appl. Surf. Sci. 2018, 436, 207–216. [Google Scholar] [CrossRef]
- Soltannia, B.; Amirul, M.; Cho, J.; Mohammadtabar, F.; Wang, R.; Piunova, V.A.; Almansoori, Z.; Rastgar, M.; Myles, A.J.; La, Y.; et al. Thermally stable core-shell star-shaped block copolymers for antifouling enhancement of water purification membranes. J. Memb. Sci. 2020, 598, 117686. [Google Scholar] [CrossRef]
- Li, Y.; Su, Y.; Zhao, X.; He, X.; Zhang, R.; Zhao, J.; Fan, X.; Jiang, Z. Antifouling, High-Flux Nanofiltration Membranes Enabled by Dual Functional Polydopamine. ACS Appl. Mater. Interfaces 2014, 6, 5548–5557. [Google Scholar] [CrossRef]
- Wu, H.; Ang, M.; Kong, J.; Zhao, C. RSC Advances One-pot synthesis of polydopamine—Zn complex antifouling coatings on membranes for ultra fi ltration under harsh conditions. RSC Adv. 2016, 6, 103390–103398. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, S.; Zhang, X.; Shi, Z.; Wei, Z.; Bao, J.; Zhao, W.; Zhao, C. Engineering of Tannic Acid Inspired Antifouling and Antibacterial Membranes through Co-deposition of Zwitterionic Polymers and Ag Nanoparticles. Ind. Eng. Chem. Res. 2019, 58, 11689–11697. [Google Scholar] [CrossRef]
- Wang, R.; Song, X.; Xiang, T.; Liu, Q.; Su, B.; Zhao, W.; Zhao, C. Mussel-inspired chitosan-polyurethane coatings for improving the antifouling and antibacterial properties of polyethersulfone membranes. Carbohydr. Polym. 2017, 168, 310–319. [Google Scholar] [CrossRef]
- Mozafari, M.; Seyedpour, S.F.; Khoshhal, S.; Rahimpour, A.; Arabi, A.; Dadashi, M.; Rabbani, M.; Tiraferri, A.; Mohsenian, H.; Sangermano, M.; et al. Facile Cu-BTC surface modi fi cation of thin chitosan fi lm coated polyethersulfone membranes with improved antifouling properties for sustainable removal of manganese. J. Memb. Sci. 2019, 588, 117200. [Google Scholar] [CrossRef]
- Liu, T.; Wang, L.; Liu, X.; Sun, C.; Lv, Y.; Miao, R.; Wang, X. Dynamic photocatalytic membrane coated with ZnIn 2 S 4 for enhanced photocatalytic performance and antifouling property. Chem. Eng. J. 2020, 379, 122379. [Google Scholar] [CrossRef]
- Tammelin, T.; Mathew, A.P. Waterborne nanocellulose coatings for improving the antifouling and antibacterial properties of polyethersulfone membranes. J. Membr. Sci. 2021, 620, 118842. [Google Scholar] [CrossRef]
- Shamaei, L.; Khorshidi, B.; Islam, M.A.; Sadrzadeh, M. Industrial waste lignin as an antifouling coating for the treatment of oily wastewater: Creating wealth from waste. J. Clean. Prod. 2020, 256, 120304. [Google Scholar] [CrossRef]
- Song, W.; Li, Z.; Li, Y.; You, H.; Qi, P.; Liu, F.; Loy, D.A. Facile sol-gel coating process for anti-biofouling modification of poly (vinylidene fluoride) microfiltration membrane based on novel zwitterionic organosilica. J. Memb. Sci. 2018, 550, 266–277. [Google Scholar] [CrossRef]
- Cui, J.; Zhou, Z.; Xie, A.; Meng, M.; Cui, Y.; Liu, S.; Lu, J.; Zhou, S.; Yan, Y.; Dong, H. Bio-inspired fabrication of superhydrophilic nanocomposite membrane based on surface modification of SiO2 anchored by polydopamine towards effective oil-water emulsions separation. Sep. Purif. Technol. 2019, 209, 434–442. [Google Scholar] [CrossRef]
- He, M.; Gao, K.; Zhou, L.; Jiao, Z.; Wu, M.; Cao, J.; You, X.; Cai, Z.; Su, Y.; Jiang, Z. Acta Biomaterialia Zwitterionic materials for antifouling membrane surface construction q. Acta Biomater. 2016, 40, 142–152. [Google Scholar] [CrossRef]
- Romeo, R.; Plutino, M.R.; Scolaro, L.M.; Stoccoro, S. Reactivity of tertiary phosphines toward a cyclometallated platinum(II) complex: Evaluation of steric and electronic contributions. Inorg. Chim. Acta 1997, 265, 225–233. [Google Scholar] [CrossRef]
- Hetrick, E.M.; Schoenfisch, M.H. Reducing implant-related infections: Active release strategies. Chem. Soc. Rev. 2006, 35, 780–789. [Google Scholar] [CrossRef]
- Cloutier, M.; Mantovani, D.; Rosei, F. Antibacterial Coatings: Challenges, Perspectives, and Opportunities. Trends Biotechnol. 2015, 33, 637–652. [Google Scholar] [CrossRef]
- Kenawy, E.R.; Worley, S.D.; Broughton, R. The chemistry and applications of antimicrobial polymers: A state-of-the-art review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef]
- Vasilev, K.; Cook, J.; Griesser, H.J. Antibacterial surfaces for biomedical devices. Expert Rev. Med. Devices 2009, 6, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.M. Controlled Release of Environmentally Friendly Antifouling Agents from Marine Coatings. Ph.D. Thesis, Technical University of Denmark, Kgs. Lyngby, Denmark, April 2009. Available online: https://orbit.dtu.dk/en/publications/controlled-release-of-environmentally-friendly-antifouling-agents (accessed on 30 November 2021).
- Yang, W.J.; Neoh, K.-G.; Kang, E.-T.; Teo, S.L.-M.; Rittschof, D. Polymer brush coatings for combating marine biofouling. Prog. Polym. Sci. 2014, 39, 1017–1042. [Google Scholar] [CrossRef]
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology—past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Lewin, R. Microbial adhesion is a sticky problem. Science 1984, 224, 375–378. [Google Scholar] [CrossRef]
- Abbott, A.; Abel, P.D.; Arnold, D.W.; Milne, A. Cost–benefit analysis of the use of TBT: The case for a treatment approach. Sci. Total Environ. 2000, 258, 5–19. [Google Scholar] [CrossRef]
- Cooney, J.J.; Tang, R.J. [47] Quantifying effects of antifouling paints on microbial biofilm formation. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 310, pp. 637–644. ISBN 0076-6879. [Google Scholar]
- Kim, S.; Kwak, S.; Lee, S.; Cho, W.K.; Lee, J.K.; Kang, S.M. One-step functionalization of zwitterionic poly[(3-(methacryloylamino)propyl)dimethyl(3-sulfopropyl)ammonium hydroxide] surfaces by metal-polyphenol coating. Chem. Commun. 2015, 51, 5340–5342. [Google Scholar] [CrossRef]
- Laschewsky, A. Structures and synthesis of zwitterionic polymers. Polymers 2014, 6, 1544–1601. [Google Scholar] [CrossRef]
- Rahman, M.M.; Chun, H.-H.; Park, H. Waterborne polysiloxane–urethane–urea for potential marine coatings. J. Coat. Technol. Res. 2011, 8, 389–399. [Google Scholar] [CrossRef]
- Majumdar, P.; Webster, D.C. Preparation of siloxane-urethane coatings having spontaneously formed stable biphasic microtopograpical surfaces. Macromolecules 2005, 38, 5857–5859. [Google Scholar] [CrossRef]
- Adkins, J.D.; Mera, A.E.; Roe-Short, M.A.; Pawlikowski, G.T.; Brady, R.F. Novel non-toxic coatings designed to resist marine fouling. Prog. Org. Coat. 1996, 29, 1–5. [Google Scholar] [CrossRef]
- Ahmed, F.; Lalia, B.S.; Kochkodan, V.; Hilal, N.; Hashaikeh, R. Electrically conductive polymeric membranes for fouling prevention and detection: A review. Desalination 2016, 391, 1–15. [Google Scholar] [CrossRef]
- Saffarimiandoab, F.; Yavuzturk, G.B.; Erkoc-Ilter, S.; Guclu, S.; Unal, S.; Tunaboylu, B.; Menceloglu, Y.Z.; Koyuncu, I. Evaluation of biofouling behavior of zwitterionic silane coated reverse osmosis membranes fouled by marine bacteria. Prog. Org. Coat. 2019, 134, 303–311. [Google Scholar] [CrossRef]
- Bodkhe, R.B.; Stafslien, S.J.; Cilz, N.; Daniels, J.; Thompson, S.E.M.; Callow, M.E.; Callow, J.A.; Webster, D.C. Polyurethanes with amphiphilic surfaces made using telechelic functional PDMS having orthogonal acid functional groups. Prog. Org. Coat. 2012, 75, 38–48. [Google Scholar] [CrossRef]
- Sommer, S.; Ekin, A.; Webster, D.C.; Stafslien, S.J.; Daniels, J.; VanderWal, L.J.; Thompson, S.E.M.; Callow, M.E.; Callow, J.A. A preliminary study on the properties and fouling-release performance of siloxane–polyurethane coatings prepared from poly(dimethylsiloxane) (PDMS) macromers. Biofouling 2010, 26, 961–972. [Google Scholar] [CrossRef]
- Ekin, A.; Webster, D.C. Combinatorial and high-throughput screening of the effect of siloxane composition on the surface properties of crosslinked siloxane-polyurethane coatings. J. Comb. Chem. 2007, 9, 178–188. [Google Scholar] [CrossRef]
- Majumdar, P.; Stafslien, S.; Daniels, J.; Webster, D.C. High throughput combinatorial characterization of thermosetting siloxane-urethane coatings having spontaneously formed microtopographical surfaces. J. Coat. Technol. Res. 2007, 4, 131–138. [Google Scholar] [CrossRef]
- Majumdar, P.; Webster, D.C. Surface microtopography in siloxane–polyurethane thermosets: The influence of siloxane and extent of reaction. Polymer 2007, 48, 7499–7509. [Google Scholar] [CrossRef]
- Mostafaei, A.; Nasirpouri, F. Preparation and characterization of a novel conducting nanocomposite blended with epoxy coating for antifouling and antibacterial applications. J. Coat. Technol. Res. 2013, 10, 679–694. [Google Scholar] [CrossRef]
- Saha, J.; Mondal, M.I.H. Antimicrobial Textiles from Natural Resources; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9780128214855. [Google Scholar]
- Gupta, S.K.; Goswami, K.K.; Majumdar, A. Thickness Loss of Handmade Carpets after Dynamic Loading BT—Functional Textiles and Clothing; Majumdar, A., Gupta, D., Gupta, S., Eds.; Springer: Singapore, 2019; pp. 321–335. [Google Scholar]
- Terzioglu, F.; Grethe, T.; Both, C.; Joßen, A.; Mahltig, B.; Rabe, M. Coating technologies for antimicrobial textile surfaces: State of the art and future prospects for textile finishing. In Handbook of Antimicrobial Coatings; Elsevier: Amsterdam, The Netherlands, 2017; pp. 123–135. ISBN 9780128119822. [Google Scholar]
- Eid, B.M.; Ibrahim, N.A. Recent developments in sustainable finishing of cellulosic textiles employing biotechnology. J. Clean. Prod. 2021, 284, 124701. [Google Scholar] [CrossRef]
- Foxhall, L. The fabric of society: Recognising the importance of textiles and their manufacture in the ancient past. Antiquity 2017, 91, 808–811. [Google Scholar] [CrossRef][Green Version]
- Liu, Q.; Huang, J.; Zhang, J.; Hong, Y.; Wan, Y.; Wang, Q.; Gong, M.; Wu, Z.; Guo, C.F. Thermal, Waterproof, Breathable, and Antibacterial Cloth with a Nanoporous Structure. ACS Appl. Mater. Interfaces 2018, 10, 2026–2032. [Google Scholar] [CrossRef]
- Timma, L.M.; Lewald, L.; Gier, F.; Homey, L.; Neyer, C.; Nickisch-Hartfiel, A.; Gutmann, J.S.; Oberthür, M. Nonfouling textiles with tunable antimicrobial activity based on a zwitterionic polyamine finish. RSC Adv. 2019, 9, 9783–9791. [Google Scholar] [CrossRef]
- Joshi, M.; Purwar, R. Developments in new processes for colour removal from effluent. Rev. Prog. Color. Relat. Top. 2008, 34, 58–71. [Google Scholar] [CrossRef]
- Hassan, M.M. Antimicrobial coatings for textiles. In Handbook of Antimicrobial Coatings; Elsevier: Amsterdam, The Netherlands, 2017; pp. 321–355. ISBN 9780128119822. [Google Scholar]
- Ramaratnam, K.; Iyer, S.K.; Kinnan, M.K.; Chumanov, G.; Brown, P.J.; Luzinov, I. Ultrahydrophobic Textiles Using Nanoparticles: Lotus Approach. J. Eng. Fiber. Fabr. 2008, 3, 155892500800300. [Google Scholar] [CrossRef]
- Somasundaram, S.; Kumaravel, V. Application of Nanoparticles for Self-Cleaning Surfaces. In Emerging Nanostructured Materials for Energy and Environmental Science. Environmental Chemistry for a Sustainable World; Rajendran, S., Naushad, M., Raju, K., Boukherroub, R., Eds.; Springer: Cham, Switzerland, 2019; Volume 23, pp. 471–498. [Google Scholar]
- Holder, K.M.; Smith, R.J.; Grunlan, J.C. A review of flame retardant nanocoatings prepared using layer-by-layer assembly of polyelectrolytes. J. Mater. Sci. 2017, 52, 12923–12959. [Google Scholar] [CrossRef]
- Hardin, I.R.; Kim, Y. Nanotechnology for antimicrobial textiles. In Antimicrobial Textiles; Elsevier: Amsterdam, The Netherlands, 2016; pp. 87–97. [Google Scholar]
- Tsuzuki, T.; Wang, X. Nanoparticle Coatings for UV Protective Textiles. Res. J. Text. Appar. 2010, 14, 9–20. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Trovato, V.; Colleoni, C.; Castellano, A.; Plutino, M.R. The key role of 3-glycidoxypropyltrimethoxysilane sol–gel precursor in the development of wearable sensors for health monitoring. J. Sol.-Gel Sci. Technol. 2018, 87, 27–40. [Google Scholar] [CrossRef]
- Tarimala, S.; Kothari, N.; Abidi, N.; Hequet, E.; Fralick, J.; Dai, L.L. New approach to antibacterial treatment of cotton fabric with silver nanoparticle–doped silica using sol–gel process. J. Appl. Polym. Sci. 2006, 101, 2938–2943. [Google Scholar] [CrossRef]
- Jeon, H.-J.; Yi, S.-C.; Oh, S.-G. Preparation and antibacterial effects of Ag–SiO2 thin films by sol–gel method. Biomaterials 2003, 24, 4921–4928. [Google Scholar] [CrossRef]
- Poli, R.; Colleoni, C.; Calvimontes, A.; Polášková, H.; Dutschk, V.; Rosace, G. Innovative sol–gel route in neutral hydroalcoholic condition to obtain antibacterial cotton finishing by zinc precursor. J. Sol.-Gel Sci. Technol. 2015, 74, 151–160. [Google Scholar] [CrossRef]
- Chattopadhyay, D.P.; Patel, B.H. Nano metal particles: Synthesis, characterization and application to textiles. In Manufacturing Nanostructures; One Central Press: Altrincham Cheshire, UK, 2015; pp. 184–215. [Google Scholar]
- Libertino, S.; Plutino, M.R.; Rosace, G. Design and development of wearable sensing nanomaterials for smart textiles. In AIP Conference Proceedings; AIP Publishing LLC.: Melville, NY, USA, 2018; Volume 1990. [Google Scholar] [CrossRef]
- Klein, L.; Aparicio, M.; Andrei, J. Handbook of Sol.-Gel Science and Technology; Springer: Cham, Switzerland, 2018; ISBN 978-3-319-32099-1. [Google Scholar]
- Berendjchi, A.; Khajavi, R.; Yazdanshenas, M. Fabrication of superhydrophobic and antibacterial surface on cotton fabric by doped silica-based sols with nanoparticles of copper. Nanoscale Res. Lett. 2011, 6, 594. [Google Scholar] [CrossRef] [PubMed]
- Bui, V.; Park, D.; Lee, Y.-C. Chitosan Combined with ZnO, TiO2 and Ag Nanoparticles for Antimicrobial Wound Healing Applications: A Mini Review of the Research Trends. Polymers 2017, 9, 21. [Google Scholar] [CrossRef]
- Shahid-Ul-Islam; Shahid, M.; Mohammad, F. Green chemistry approaches to develop antimicrobial textiles based on sustainable biopolymers—A review. Ind. Eng. Chem. Res. 2013, 52, 5245–5260. [Google Scholar] [CrossRef]
- Ielo, I.; Iacopetta, D.; Saturnino, C.; Longo, P.; Galletta, M.; Drommi, D.; Rosace, G.; Sinicropi, M.S.; Plutino, M.R. Gold Derivatives Development as Prospective Anticancer Drugs for Breast Cancer Treatment. Appl. Sci. 2021, 11, 2089. [Google Scholar] [CrossRef]
- Rosace, G.; Guido, E.; Colleoni, C.; Brucale, M.; Piperopoulos, E.; Milone, C.; Plutino, M.R. Halochromic resorufin-GPTMS hybrid sol-gel: Chemical-physical properties and use as pH sensor fabric coating. Sens. Actuators B Chem. 2017, 241, 85–95. [Google Scholar] [CrossRef]
- Khanzada, H.; Salam, A.; Qadir, M.B.; Phan, D.-N.; Hassan, T.; Munir, M.U.; Pasha, K.; Hassan, N.; Khan, M.Q.; Kim, I.S. Fabrication of Promising Antimicrobial Aloe Vera/PVA Electrospun Nanofibers for Protective Clothing. Materials 2020, 13, 3884. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, F.; Periolatto, M. Antimicrobial Finish of Textiles by Chitosan UV-Curing. J. Nanosci. Nanotechnol. 2012, 12, 4803–4810. [Google Scholar] [CrossRef]
- Cafeo, G.; Carbotti, G.; Cuzzola, A.; Fabbi, M.; Ferrini, S.; Kohnke, F.H.; Papanikolaou, G.; Plutino, M.R.; Rosano, C.; White, A.J.P. Drug delivery with a calixpyrrole-trans -Pt(II) complex. J. Am. Chem. Soc. 2013, 135, 2544–2551. [Google Scholar] [CrossRef] [PubMed]
- Voncina, B. Application of Cyclodextrins in Textile Dyeing. In Textile Dyeing; Hauser, P., Ed.; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar]
- Bajpai, M.; Gupta, P.; Bajpai, S.K. Silver(I) ions loaded cyclodextrin-grafted-cotton fabric with excellent antimicrobial property. Fibers Polym. 2010, 11, 8–13. [Google Scholar] [CrossRef]
- Attarchi, N.; Montazer, M.; Toliyat, T.; Samadi, N.; Harifi, T. Novel cellulose fabric with multifunctional properties through diverse methods of Ag/TiO2/β-cyclodextrin nanocomposites synthesis. Cellulose 2018, 25, 1449–1462. [Google Scholar] [CrossRef]
- Murugesh Babu, K.; Ravindra, K.B. Bioactive antimicrobial agents for finishing of textiles for health care products. J. Text. Inst. 2015, 106, 706–717. [Google Scholar] [CrossRef]
- Tawiah, B.; Tawiah, B.; Badoe, W.; Badoe, W.; Fu, S. Advances in the Development of Antimicrobial Agents for Textiles: The Quest for Natural Products. Review. Fibres Text. East. Eur. 2016, 24, 136–149. [Google Scholar] [CrossRef]
- Ali, S.W.; Purwar, R.; Joshi, M.; Rajendran, S. Antibacterial properties of Aloe vera gel-finished cotton fabric. Cellulose 2014, 21, 2063–2072. [Google Scholar] [CrossRef]
- Khurshid, M.; Ayyoob, M.; Asad, M.; Shah, S. Assessment of Eco-Friendly Natural Antimicrobial Textile Finish Extracted from Aloe Vera and Neem Plants. Fibres Text. East. Eur. 2015, 23, 120–124. [Google Scholar] [CrossRef]
- Thilagavathi, G.; Rajendrakumar, K.; Rajendran, R. Development of ecofriendly antimicrobial textile finishes using herbs. Indian J. Fibre Text. Res. 2005, 30, 431–436. [Google Scholar]
- Sathianarayanan, M.P.; Bhat, N.V.; Kokate, S.S.; Walunj, V.E. Antibacterial finish for cotton fabric from herbal products. Indian J. Fibre Text. Res. 2010, 35, 50–58. [Google Scholar]
- Han, S.; Yang, Y. Antimicrobial activity of wool fabric treated with curcumin. Dye. Pigment. 2005, 64, 157–161. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; El-Gamal, A.R.; Gouda, M.; Mahrous, F. A new approach for natural dyeing and functional finishing of cotton cellulose. Carbohydr. Polym. 2010, 82, 1205–1211. [Google Scholar] [CrossRef]
- Dev, V.R.G.; Venugopal, J.; Sudha, S.; Deepika, G.; Ramakrishna, S. Dyeing and antimicrobial characteristics of chitosan treated wool fabrics with henna dye. Carbohydr. Polym. 2009, 75, 646–650. [Google Scholar] [CrossRef]
- Liu, X.; Lin, T.; Peng, B.; Wang, X. Antibacterial activity of capsaicin-coated wool fabric. Text. Res. J. 2012, 82, 584–590. [Google Scholar] [CrossRef]
- Syamili, E.; Elayarajah, B.; Kulanthaivelu; Rajendran, R.; Venkatraja, B.; Kumar, P.A. Antibacterial Cotton Finish Using Green Tea Leaf Extracts Interacted with Copper. Asian J. Text. 2011, 2, 6–16. [Google Scholar] [CrossRef]
- Shahmoradi Ghaheh, F.; Mortazavi, S.M.; Alihosseini, F.; Fassihi, A.; Shams Nateri, A.; Abedi, D. Assessment of antibacterial activity of wool fabrics dyed with natural dyes. J. Clean. Prod. 2014, 72, 139–145. [Google Scholar] [CrossRef]
- Yilmaz, F. Application of Glycyrrhiza glabra L. Root as a Natural Antibacterial Agent in Finishing of Textile. Ind. Crops Prod. 2020, 157, 112899. [Google Scholar] [CrossRef]
- Ielo, I.; Giacobello, F.; Sfameni, S.; Rando, G.; Galletta, M.; Trovato, V.; Rosace, G.; Plutino, M.R. Nanostructured surface finishing and coatings: Functional properties and applications. Materials 2021, 14, 2733. [Google Scholar] [CrossRef]
- De Luca, G.; Bonaccorsi, P.; Trovato, V.; Mancuso, A.; Papalia, T.; Pistone, A.; Casaletto, M.P.; Mezzi, A.; Brunetti, B.; Minuti, L.; et al. Tripodal tris-disulfides as capping agents for a controlled mixed functionalization of gold nanoparticles. New J. Chem. 2018, 42, 16436–16440. [Google Scholar] [CrossRef]


| Antibacterial Agents | Authors | Ref. |
|---|---|---|
| ZnO and MgO NPs | Singh et al. | [21] |
| Metal zeolites and antibacterial polymeric fibers | De Muynck et al. | [29] |
| Epoxy resins | Kong et al. | [30] |
| Quaternary ammonium compounds | Javaherdashti et al. | [31] |
| Halogenated complex | Qiu et al. | [32] |
| Metal oxide, silver, and tungsten powder | Plutino et al. | [33] |
| CuO, Cu2O, ZnO, TiO2, Al2O3, and Fe3O4 nanoparticles | Sikora et al. | [34] |
| Silver nanoparticles in commercial silica-based coating | Nam, K.Y. | [35] |
| ZnO, TiO2, SiO2 nanoparticles | Dyshlyuk et al. | [36] |
| SiO2–Ag nanohybrid compounds in acrylic coatings | Le et al. | [37] |
| Silver nanoparticles in N-SiO2 nanocarriers | Dominguez et al. | [38] |
| BiOClxBr1−x micro flowers | Gao et al. | [39] |
| TiO2 nanoparticles, fluorine silicon sol | Zhu et al. | [40] |
| TiO2 nanoparticles | Verdier et al. | [41] |
| TiO2 modified with carbon and nitrogen | Janus et al. | [42] |
| TiO2 and ZnO nanoparticles in addition to polyethylene glycol (PEG) | Dehkordi et al. | [43] |
| Fe2O3 contained in steel slag of an industrial induction furnace | Baalamurugan et al. | [44] |
| Fly ashes recycled by alkali activation process supported with Zn | Rodwihok et al. | [45] |
| Metakaolin-based geopolymer cement loaded with 5-chloro-2-(2,4-Dichlorophenoxy) phenol | Rubio-Avalos, J.C. | [46] |
| Metakaolin-based geopolymer cement loaded with glass waste | Dal Poggetto et al. | [47] |
| Zinc particles or zinc doped clay particles | Roghanian et al. | [48] |
| Granular activated carbon and fundamental oxygen furnace steel slag particles, copper, and cobalt as inhibitory metals | Justo-Reinoso et al. | [49] |
| Commercial Product and Active Ingredient | Solvent | Spectrum of Action | Ref. |
|---|---|---|---|
| Biotin T (CTS) di-n-decyl-dimethylammoniumchloride | water | fungi, bacteria, and algae | [67] |
| Biotin T (CTS) 3-iodo-2-propynylbutyl carbammate | ethanol | fungi, bacteria, and algae | [68] |
| Rocima. 103 (CTS) di-n-decyl-dimethylammoniumchloride | water | lichens, fungi, bacteria, and algae | [67,68] |
| Preventol RI80 (CTS) alchyl-dimethyl-benzilammoniumchloride | water | fungi, bacteria, and algae | [67,68] |
| Class of Functional Molecule | Deposition Method | FunctionalMolecules | Coated Membrane | AntifoulingCapabilities | Ref. |
| Zwitterionic copolymer | Dip-coating | SBMA and MTAC | PVC-PAN-PSS | Anti-organic fouling and anti-biofouling | [76] |
| Zwitterionic copolymer | iCVD | PDE and 1,3-propane sultone | RO | Anti-biofouling | [77] |
| Zwitterionic copolymers | si-ATRP | HEMA, MPC and SBMA | RO | Anti-biofouling | [78] |
| Star-shaped block copolymer | Self-assembly | PS core and PEGMA, PDMAEMA, PMAA arms | PSF | Anti-oil fouling | [79] |
| Mussel-inspired | Dip-coating | Fluorinated polyamine on PDA layer | PES | Anti-organic fouling and fouling-release | [80] |
| Hybrid mussel-inspired | In situ polymerization | Polydopamine–Zn complex | PSF | Anti-fouling | [81] |
| Zwitterionic polymer and metal NPs | Dip-coating | TA, AgNPs and zwitterionic PEI | PES | Anti-bio fouling | [82] |
| Mussel-inspired and metal NPs | In situ reduction | Catechol, Ag NPs, chitosan-polyurethane | PES | Anti-bio fouling | [83] |
| Biopolymer and MOF | Dip-coating | Cu-BTC, chitosan | PES | Anti-bio fouling | [84] |
| Photocatalyst | Dead-end filtration | ZnIn2S4 | PVDF | Anti-organic fouling | [85] |
| Biopolymer | LBL | CNC, T-CNF, PAHCl | PES | Anti-bio fouling | [86] |
| Biopolymer | LBL | Kraft lignin, pDAC | PES | Anti-oil fouling | [87] |
| Zwitterionic organosilica polymer | Sol–gel and filtration | Zwitterionic organosilica monomer | PVDF | Antifouling and anti-bioadhesion | [88] |
| Silica NPs and mussel-inspired | Sol–gel | TEOS, polydopamine | PVDF | Anti-oil fouling | [89] |
| Antifouling Technology | Properties | Mechanism of Action | Ref. |
|---|---|---|---|
| Contact leaching coatings | Biocides are incorporated into water-insoluble matrices | Biocidal paint, dissolution of water-soluble biocides that are released gradually | [98] |
| Controlled depletive polymer (CDP) coatings | Biocides are incorporated in a resin-based soluble matrix | Biocidal paint, physical dissolution of the soluble matrix, and release of the biocides | [98] |
| Self-polishing copolymer (SPC) coatings | Biocides are incorporated in an acrylic or methacrylate polymer matrix | Biocidal paint, decomposition by hydrolysis of the matrix detaches organisms from the hull, releasing biocides | [98] |
| CDP and SPC mixed coatings | It combines the properties of the CDP and SPC technologies | Biocidal paint, dissolution by hydrolysis, and hydration of the matrix with control of the biocide release | [98] |
| Zwitterionic polymer coatings | It combines amphiphilic and zwitterionic groups on the surface of the coatings. | Non-biocidal paint, formation of a hydration layer around zwitterionic moiety | [102,103] |
| Silicone or fluorine-based coatings | Combination of polymers with low surface energy and modulus. | Foul-release paint, minimization of the adhesion force between the foulers and the material of which the hull surface is made | [104,105,106] |
| Conductive antifouling coatings | Use of negative charges on the surface of the film. | Foul-release paint, high electrostatic repulsion between the films and the foulants | [107] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ielo, I.; Giacobello, F.; Castellano, A.; Sfameni, S.; Rando, G.; Plutino, M.R. Development of Antibacterial and Antifouling Innovative and Eco-Sustainable Sol–Gel Based Materials: From Marine Areas Protection to Healthcare Applications. Gels 2022, 8, 26. https://doi.org/10.3390/gels8010026
Ielo I, Giacobello F, Castellano A, Sfameni S, Rando G, Plutino MR. Development of Antibacterial and Antifouling Innovative and Eco-Sustainable Sol–Gel Based Materials: From Marine Areas Protection to Healthcare Applications. Gels. 2022; 8(1):26. https://doi.org/10.3390/gels8010026
Chicago/Turabian StyleIelo, Ileana, Fausta Giacobello, Angela Castellano, Silvia Sfameni, Giulia Rando, and Maria Rosaria Plutino. 2022. "Development of Antibacterial and Antifouling Innovative and Eco-Sustainable Sol–Gel Based Materials: From Marine Areas Protection to Healthcare Applications" Gels 8, no. 1: 26. https://doi.org/10.3390/gels8010026
APA StyleIelo, I., Giacobello, F., Castellano, A., Sfameni, S., Rando, G., & Plutino, M. R. (2022). Development of Antibacterial and Antifouling Innovative and Eco-Sustainable Sol–Gel Based Materials: From Marine Areas Protection to Healthcare Applications. Gels, 8(1), 26. https://doi.org/10.3390/gels8010026









