Fabrication and Characterization of Xanthan Gum-cl-poly(acrylamide-co-alginic acid) Hydrogel for Adsorption of Cadmium Ions from Aqueous Medium
Abstract
:1. Introduction
2. Results and Discussion
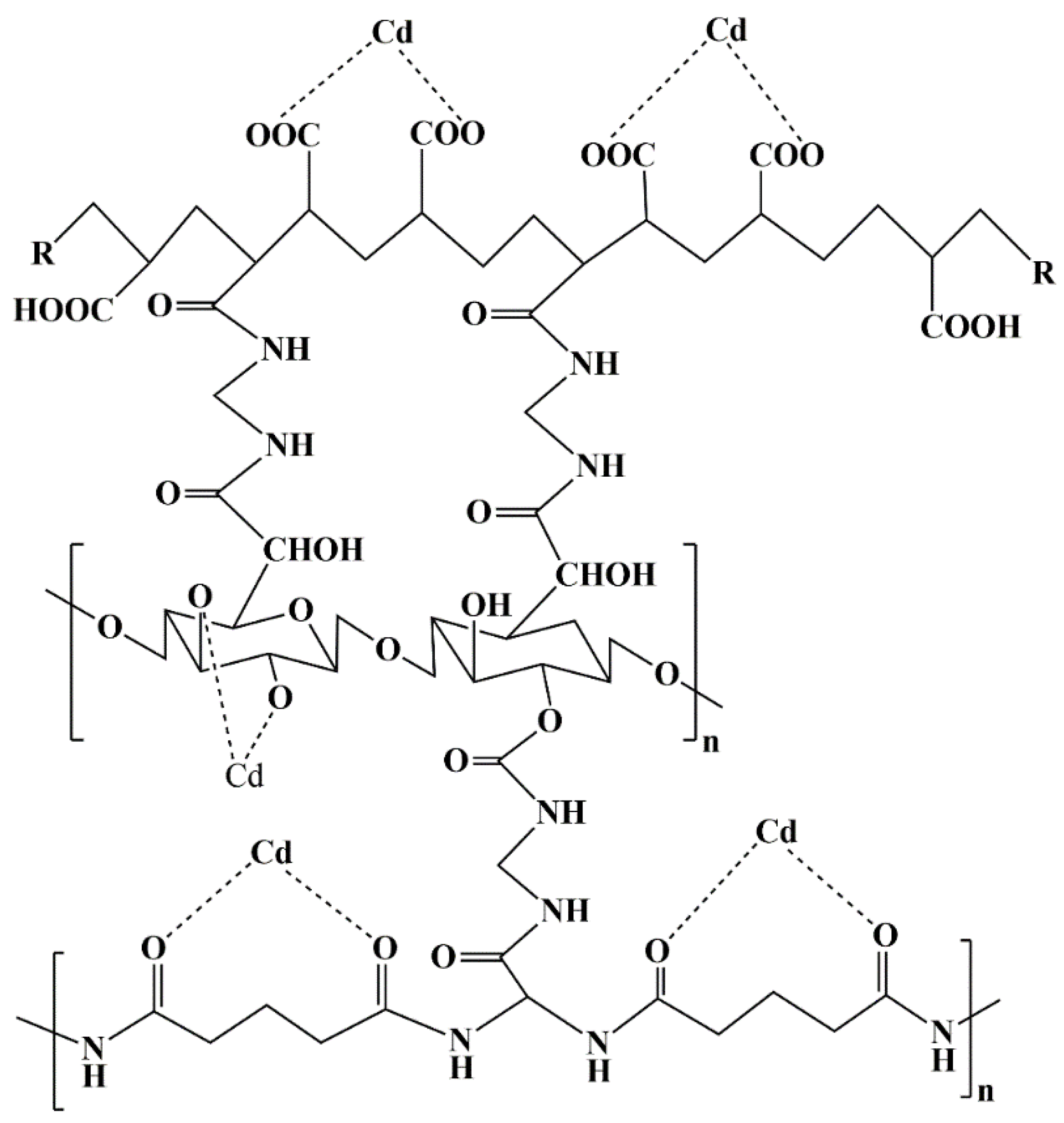
2.1. Adsorption of Cadmium Ions by XG-cl-Poly(AAm-co-AA) Hydrogel
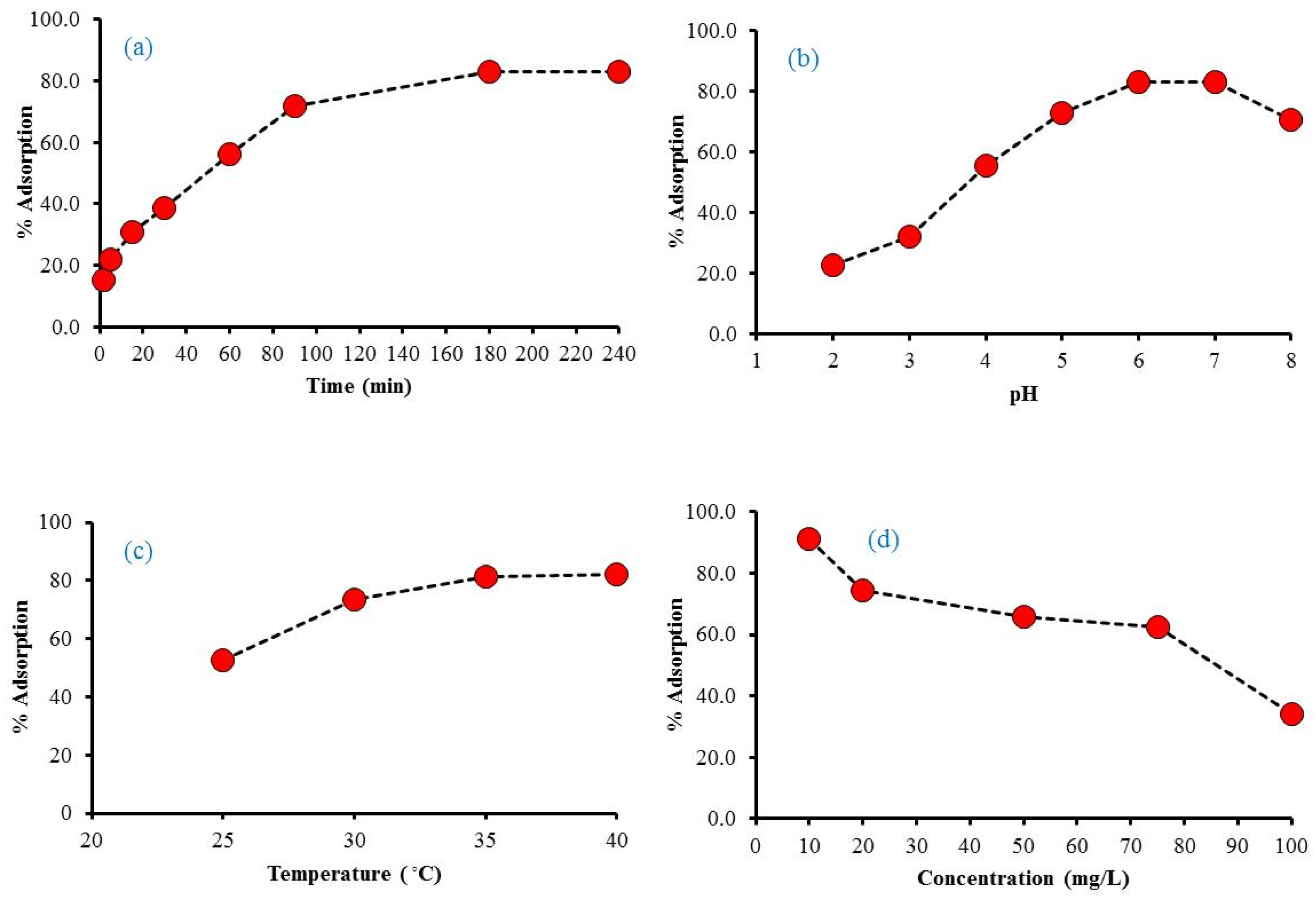
2.1.1. Effect of Various Factors
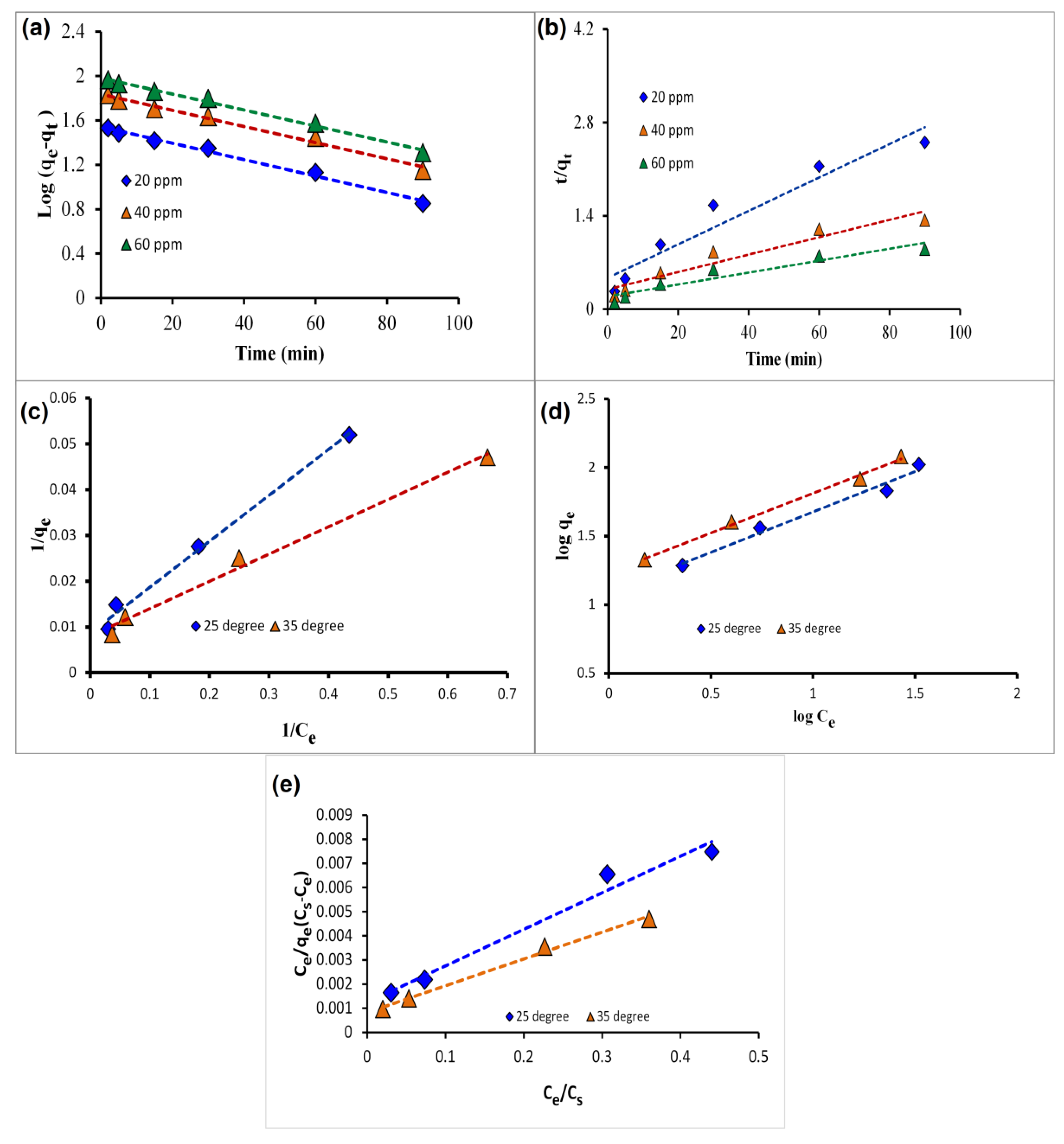
2.1.2. Adsorption Kinetics
2.1.3. Adsorption Isotherms
2.1.4. Thermodynamic Analysis
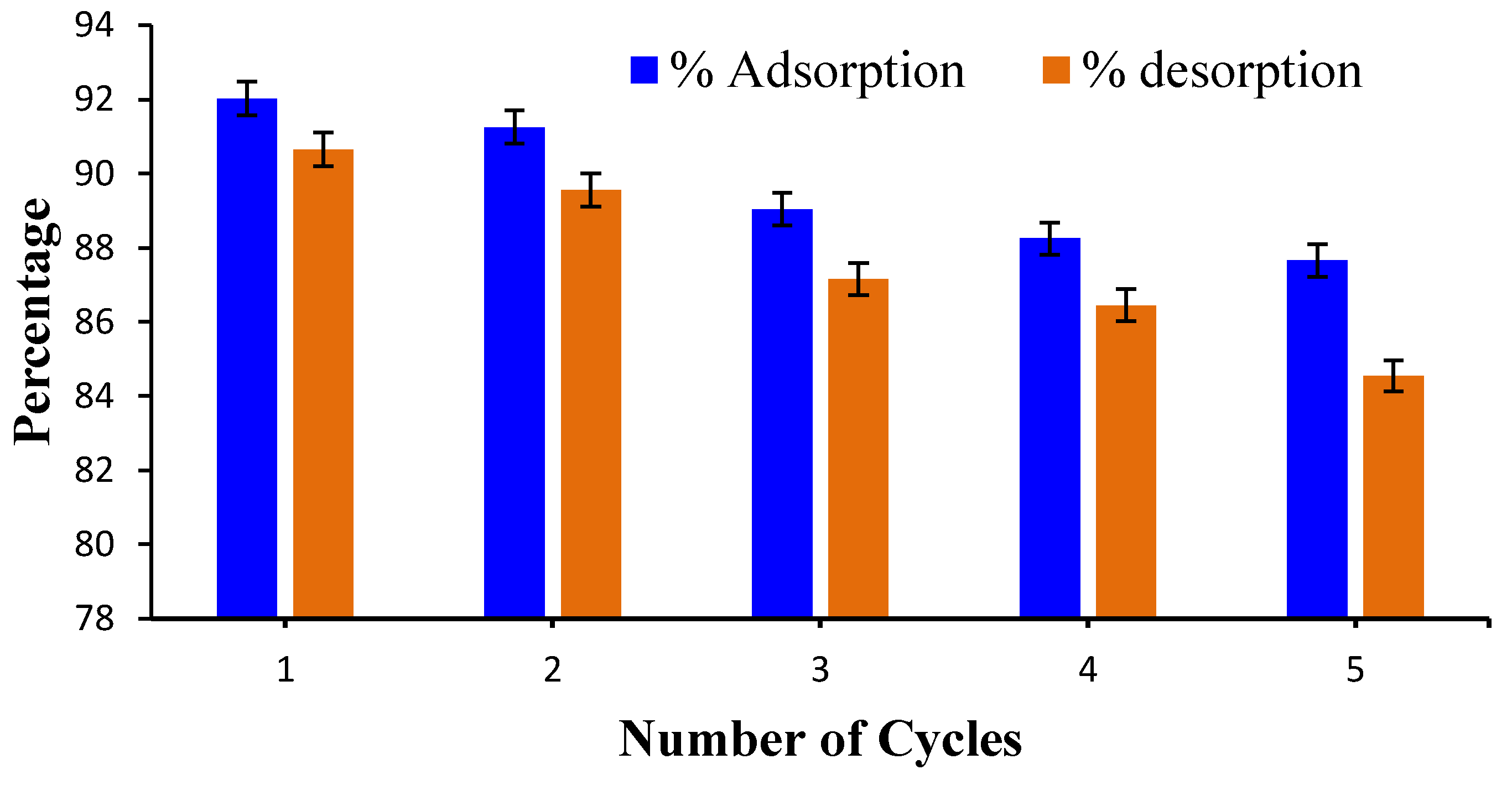
2.1.5. Reusability of Hydrogel
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Xanthan Gum-cl-Poly(Acrylamide-co-Alginic Acid)
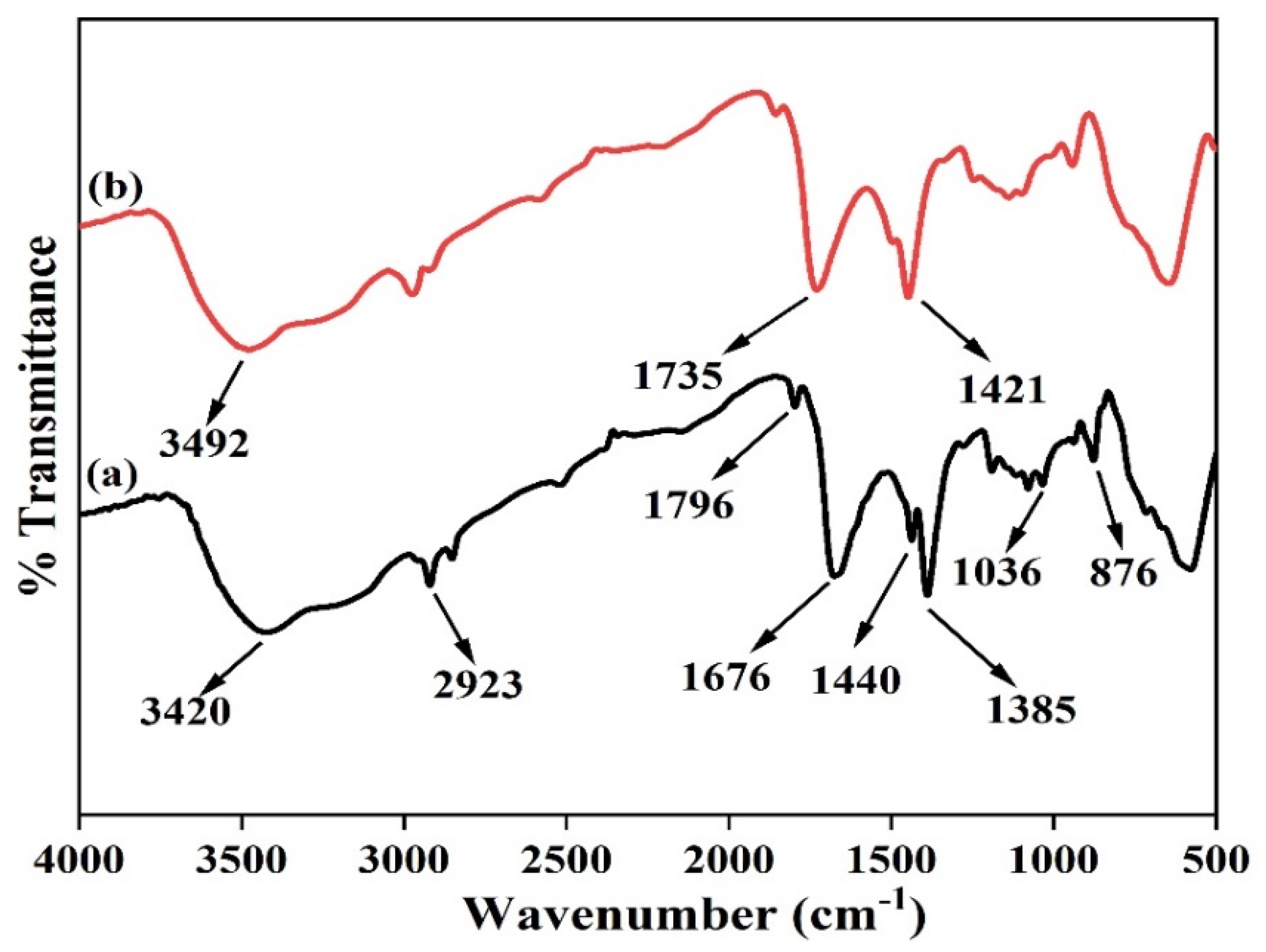
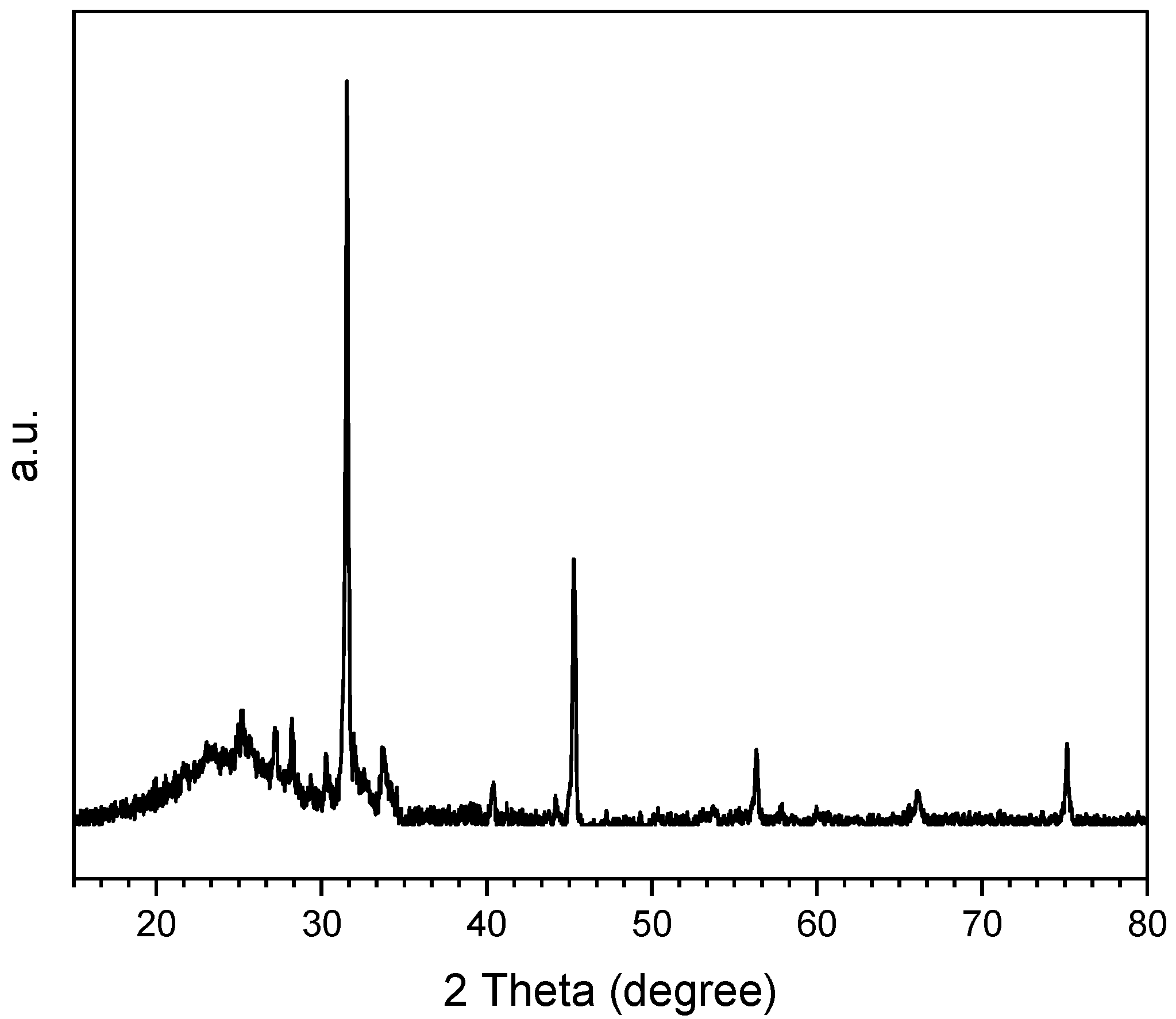
4.3. Characterization
4.4. Adsorption Experiments
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology; Springer: Basel, Switzerland, 2012; pp. 133–164. [Google Scholar]
- Rajendran, S.; Priya, T.; Khoo, K.S.; Hoang, T.K.; Ng, H.-S.; Munawaroh, H.S.H.; Karaman, C.; Orooji, Y.; Show, P.L. A critical review on various remediation approaches for heavy metal contaminants removal from contaminated soils. Chemosphere 2022, 287, 132369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, J.; Zhang, Y.; Ma, J.; Huang, L.; Yu, S.; Chen, L.; Song, G.; Qiu, M.; Wang, X. Applications of water-stable metal-organic frameworks in the removal of water pollutants: A review. Environ. Pollut. 2021, 291, 118076. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Weekly Epidemiological Record: Relevé Épidémiologique Hebdomadaire; World Health Organization: Geneva, Switzerland, 1948. [Google Scholar]
- Bradl, H. Sources and origins of heavy metals. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2005; pp. 1–27. [Google Scholar]
- Roy, A.; Bharadvaja, N. Efficient removal of heavy metals from artificial wastewater using biochar. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100602. [Google Scholar] [CrossRef]
- Kong, Q.; Shi, X.; Ma, W.; Zhang, F.; Yu, T.; Zhao, F.; Zhao, D.; Wei, C. Strategies to improve the adsorption properties of graphene-based adsorbent towards heavy metal ions and their compound pollutants: A review. J. Hazard. Mater. 2021, 415, 125690. [Google Scholar] [CrossRef] [PubMed]
- Talaiekhozani, A.; Rezania, S. Application of photosynthetic bacteria for removal of heavy metals, macro-pollutants and dye from wastewater: A review. J. Water Process Eng. 2017, 19, 312–321. [Google Scholar] [CrossRef]
- Shaker, M.A. Dynamics and thermodynamics of toxic metals adsorption onto soil-extracted humic acid. Chemosphere 2014, 111, 587–595. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, J.; Yang, Y.; Ding, J.; Zhang, A. Experimental and theoretical studies of cadmium adsorption over Fe2O3 sorbent in incineration flue gas. Chem. Eng. J. 2021, 425, 131647. [Google Scholar] [CrossRef]
- Panuccio, M.R.; Sorgonà, A.; Rizzo, M.; Cacco, G. Cadmium adsorption on vermiculite, zeolite and pumice: Batch experimental studies. J. Environ. Manag. 2009, 90, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Petrović, M.; Kaštelan-Macan, M.; Horvat, A. Interactive sorption of metal ions and humic acids onto mineral particles. Water Air Soil Pollut. 1999, 111, 41–56. [Google Scholar] [CrossRef]
- Sajid, M.; Nazal, M.K.; Baig, N.; Osman, A.M. Removal of heavy metals and organic pollutants from water using dendritic polymers based adsorbents: A critical review. Sep. Purif. Technol. 2018, 191, 400–423. [Google Scholar] [CrossRef]
- Razmi, B.; Ghasemi-Fasaei, R. Investigation of Taguchi optimization, equilibrium isotherms, and kinetic modeling for phosphorus adsorption onto natural zeolite of clinoptilolite type. Adsorpt. Sci. Technol. 2018, 36, 1470–1483. [Google Scholar] [CrossRef] [Green Version]
- Es-said, A.; Nafai, H.; Lamzougui, G.; Bouhaouss, A.; Bchitou, R. Comparative adsorption studies of cadmium ions on phosphogypsum and natural clay. Sci. Afr. 2021, 13, e00960. [Google Scholar] [CrossRef]
- Sharma, G.; AlGarni, T.S.; Kumar, P.S.; Bhogal, S.; Kumar, A.; Sharma, S.; Naushad, M.; Othman, Z.A.A.L.; Stadler, F.J. Utilization of Ag2O–Al2O3–ZrO2 decorated onto rGO as adsorbent for the removal of Congo red from aqueous solution. Environ. Res. 2021, 197, 111179. [Google Scholar] [CrossRef] [PubMed]
- Qing, Z.; Wang, L.; Liu, X.; Song, Z.; Qian, F.; Song, Y. Simply synthesized sodium alginate/zirconium hydrogel as adsorbent for phosphate adsorption from aqueous solution: Performance and mechanisms. Chemosphere 2021, 133103. [Google Scholar] [CrossRef]
- Song, Y.; Gotoh, T.; Nakai, S. Synthesis of Oxidant Functionalised Cationic Polymer Hydrogel for Enhanced Removal of Arsenic (III). Gels 2021, 7, 197. [Google Scholar] [CrossRef]
- Yilmaz, M.S. Graphene oxide/hollow mesoporous silica composite for selective adsorption of methylene blue. Microporous Mesoporous Mater. 2022, 330, 111570. [Google Scholar] [CrossRef]
- Madduma-Bandarage, U.S.; Madihally, S.V. Synthetic hydrogels: Synthesis, novel trends, and applications. J. Appl. Polym. Sci. 2021, 138, 50376. [Google Scholar] [CrossRef]
- Sharma, G.; Thakur, B.; Naushad, M.; Kumar, A.; Stadler, F.J.; Alfadul, S.M.; Mola, G.T. Applications of nanocomposite hydrogels for biomedical engineering and environmental protection. Environ. Chem. Lett. 2018, 16, 113–146. [Google Scholar] [CrossRef]
- Ghauri, Z.H.; Islam, A.; Qadir, M.A.; Ghaffar, A.; Gull, N.; Azam, M.; Mehmood, A.; Ghauri, A.A.; Khan, R.U. Novel pH-responsive chitosan/sodium alginate/PEG based hydrogels for release of sodium ceftriaxone. Mater. Chem. Phys. 2022, 277, 125456. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Chauhan, C.; Okram, A.; Sharma, S.; Pathania, D.; Kalia, S. Pectin-crosslinked-guar gum/SPION nanocomposite hydrogel for adsorption of m-cresol and o-chlorophenol. Sustain. Chem. Pharm. 2017, 6, 96–106. [Google Scholar] [CrossRef]
- Sharma, G.; Khosla, A.; Kumar, A.; Kaushal, N.; Sharma, S.; Naushad, M.; Vo, D.-V.N.; Iqbal, J.; Stadler, F.J. A comprehensive review on the removal of noxious pollutants using carrageenan based advanced adsorbents. Chemosphere 2021, 289, 133100. [Google Scholar] [CrossRef]
- Pervaiz, F.; Mushtaq, R.; Noreen, S. Formulation and optimization of terbinafine HCl loaded chitosan/xanthan gum nanoparticles containing gel: Ex-vivo permeation and in-vivo antifungal studies. J. Drug Deliv. Sci. Technol. 2021, 66, 102935. [Google Scholar] [CrossRef]
- Hua, D.; Gao, S.; Zhang, M.; Ma, W.; Huang, C. A novel xanthan gum-based conductive hydrogel with excellent mechanical, biocompatible, and self-healing performances. Carbohydr. Polym. 2020, 247, 116743. [Google Scholar] [CrossRef]
- Amaral, C.N.; Oliveira, P.F.; Pedroni, L.G.; Mansur, C.R. Viscoelastic behavior of hydrogel-based xanthan gum/aluminum lactate with potential applicability for conformance control. J. Appl. Polym. Sci. 2021, 138, 50640. [Google Scholar] [CrossRef]
- Özbaş, F.; Tüzün, E.; Yıldız, A.; Karakuş, S. Sonosynthesis and characterization of konjac gum/xanthan gum supported ironoxide nanoparticles. Int. J. Biol. Macromol. 2021, 183, 1047–1057. [Google Scholar] [CrossRef]
- Sharma, G.; Naushad, M.; Kumar, A.; Rana, S.; Sharma, S.; Bhatnagar, A.; Stadler, F.J.; Ghfar, A.A.; Khan, M.R. Efficient removal of coomassie brilliant blue R-250 dye using starch/poly(alginic acid-cl-acrylamide) nanohydrogel. Process. Saf. Environ. Prot. 2017, 109, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Vilela, P.B.; Matias, C.A.; Dalalibera, A.; Becegato, V.A.; Paulino, A.T. Polyacrylic acid-based and chitosan-based hydrogels for adsorption of cadmium: Equilibrium isotherm, kinetic and thermodynamic studies. J. Environ. Chem. Eng. 2019, 7, 103327. [Google Scholar] [CrossRef]
- Elella, M.H.A.; Goda, E.S.; Gamal, H.; El-Bahy, S.M.; Nour, M.A.; Yoon, K.R. Green antimicrobial adsorbent containing grafted xanthan gum/SiO2 nanocomposites for malachite green dye. Int. J. Biol. Macromol. 2021, 191, 385–395. [Google Scholar] [CrossRef]
- Anjum, F.; Bukhari, S.A.; Siddique, M.; Shahid, M.; Potgieter, J.H.; Jaafar, H.Z.; Ercisli, S.; Zia-Ul-Haq, M. Microwave irradiated copolymerization of xanthan gum with acrylamide for colonic drug delivery. BioResources 2015, 10, 1434–1451. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.; Singh, S.; Pandey, S.; Sanghi, R. Synthesis and characterization of guar gum templated hybrid nano silica. Int. J. Biol. Macromol. 2011, 49, 233–240. [Google Scholar] [CrossRef]
- Sharma, R.K. Synthesis and characterization of graft copolymers of N-Vinyl-2-Pyrrolidone onto guar gum for sorption of Fe2+ and Cr6+ ions. Carbohydr. Polym. 2011, 83, 1929–1936. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, G.; Kumar, A.; AlGarni, T.S.; Naushad, M.; Othman, Z.A.A.L.; Stadler, F.J. Adsorption of cationic dyes onto carrageenan and itaconic acid-based superabsorbent hydrogel: Synthesis, characterization and isotherm analysis. J. Hazard. Mater. 2022, 421, 126729. [Google Scholar] [CrossRef]
- Sharma, G.; Thakur, B.; Kumar, A.; Sharma, S.; Naushad, M.; Stadler, F.J. Atrazine removal using chitin-cl-poly (acrylamide-co-itaconic acid) nanohydrogel: Isotherms and pH responsive nature. Carbohydr. Polym. 2020, 241, 116258. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, G.; Kumar, A.; Dhiman, P.; AlGarni, T.S.; Naushad, M.; Othman, Z.A.A.L.; Stadler, F.J. Controlled synthesis of porous Zn/Fe based layered double hydroxides: Synthesis mechanism, and ciprofloxacin adsorption. Sep. Purif. Technol. 2022, 278, 119481. [Google Scholar] [CrossRef]
- Ebadi, A.; Mohammadzadeh, J.S.S.; Khudiev, A. What is the correct form of BET isotherm for modeling liquid phase adsorption? Adsorption 2009, 15, 65–73. [Google Scholar] [CrossRef]
- Asuquo, E.; Martin, A.; Nzerem, P.; Siperstein, F.; Fan, X. Adsorption of Cd (II) and Pb (II) ions from aqueous solutions using mesoporous activated carbon adsorbent: Equilibrium, kinetics and characterisation studies. J. Environ. Chem. Eng. 2017, 5, 679–698. [Google Scholar] [CrossRef] [Green Version]
- Jain, M.; Yadav, M.; Kohout, T.; Lahtinen, M.; Garg, V.K.; Sillanpää, M. Development of iron oxide/activated carbon nanoparticle composite for the removal of Cr (VI), Cu (II) and Cd (II) ions from aqueous solution. Water Resour. Ind. 2018, 20, 54–74. [Google Scholar] [CrossRef]
- Mohan, D.; Kumar, H.; Sarswat, A.; Alexandre-Franco, M.; Pittman, C.U., Jr. Cadmium and lead remediation using magnetic oak wood and oak bark fast pyrolysis bio-chars. Chem. Eng. J. 2014, 236, 513–528. [Google Scholar] [CrossRef]
- Tu, Y.-J.; You, C.-F.; Chang, C.-K. Kinetics and thermodynamics of adsorption for Cd on green manufactured nano-particles. J. Hazard. Mater. 2012, 235, 116–122. [Google Scholar] [CrossRef]
- Kataria, N.; Garg, V. Green synthesis of Fe3O4 nanoparticles loaded sawdust carbon for cadmium (II) removal from water: Regeneration and mechanism. Chemosphere 2018, 208, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yang, T.; Yang, Y.; Yi, X.; Hao, X.; Xie, T.; Liao, C.J. The behavior and mechanism of the adsorption of Pb(II) and Cd(II) by a porous double network porous hydrogel derived from peanut shells. Mater. Today Commun. 2021, 27, 102449. [Google Scholar] [CrossRef]
- Wang, F.; Li, J.; Su, Y.; Li, Q.; Gao, B.; Yue, Q.; Zhou, W. Adsorption and recycling of Cd(II) from wastewater using straw cellulose hydrogel beads. J. Ind. Eng. Chem. 2019, 80, 361–369. [Google Scholar] [CrossRef]
- Tao, X.; Wang, S.; Li, Z.; Zhou, S. Green synthesis of network nanostructured calcium alginate hydrogel and its removal performance of Cd2+ and Cu2+ ions. Mater. Chem. Phys. 2021, 258, 123931. [Google Scholar] [CrossRef]
- Ayouch, I.; Barrak, I.; Kassab, Z.; el Achaby, M.; Barhoun, A.; Draoui, K. Improved recovery of cadmium from aqueous medium by alginate composite beads filled by bentonite and phosphate washing sludge. Colloids Surf. A Physicochem. Eng. Asp. 2020, 604, 125305. [Google Scholar] [CrossRef]
- Bagheri, S.; Amini, M.M.; Behbahani, M.; Rabiee, G. Low cost thiol-functionalized mesoporous silica, KIT-6-SH, as a useful adsorbent for cadmium ions removal: A study on the adsorption isotherms and kinetics of KIT-6-SH. Microchem. J. 2019, 145, 460–469. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, S.; Zhang, Y.; Miao, L.; Zhang, Y.; Wu, A. Preparation of modified sodium alginate aerogel and its application in removing lead and cadmium ions in wastewater. Int. J. Biol. Macromol. 2020, 157, 687–694. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, Q.; Liu, S.; Liu, T.; Zhang, B. A novel biodegradable β-cyclodextrin-based hydrogel for the removal of heavy metal ions. Carbohydr. Polym. 2013, 97, 496–501. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, Z. Hydrogel-supported nanosized hydrous manganese dioxide: Synthesis, characterization, and adsorption behavior study for Pb2+, Cu2+, Cd2+ and Ni2+ removal from water. Chem. Eng. J. 2015, 281, 69–80. [Google Scholar] [CrossRef]
- Sharma, G.; Naushad, M. Adsorptive removal of noxious cadmium ions from aqueous medium using activated carbon/zirconium oxide composite: Isotherm and kinetic modelling. J. Mol. Liq. 2020, 310, 113025. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, J.; Wang, S.; Zhang, L. Facile preparation of a remarkable MOF adsorbent for Au (III) selective separation from wastewater: Adsorption, Regeneration and Mechanism. J. Mol. Liq. 2021, 118137. [Google Scholar] [CrossRef]
- Hao, C.; Li, G.; Wang, G.; Chen, W.; Wang, S. Preparation of acrylic acid modified alkalized MXene adsorbent and study on its dye adsorption performance. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127730. [Google Scholar] [CrossRef]
- Sharma, G.; Thakur, B.; Kumar, A.; Sharma, S.; Naushad, M.; Stadler, F.J. Gum acacia-cl-poly (acrylamide)@ carbon nitride nanocomposite hydrogel for adsorption of ciprofloxacin and its sustained release in artificial ocular solution. Macromol. Mater. Eng. 2020, 305, 2000274. [Google Scholar] [CrossRef]

| Kinetic Models | Parameters | 10 mg·L−1 | 20 mg·L−1 | 60 mg·L−1 |
|---|---|---|---|---|
| Pseudo-first-order | qe (mg·g−1) | 34.8 | 68.5 | 95.9 |
| k1 (min−1) | 1.70 × 10−2 | 1.68 × 10−2 | 1.65 × 10−2 | |
| R2 | 0.990 | 0.986 | 0.992 | |
| Pseudo-second-order | qe (mg·g−1) | 39.8 | 76.9 | 111.1 |
| k2 (g·mg−1·min−1) | 13.4 × 10−3 | 5.6 × 10−4 | 4.2 × 10−4 | |
| R2 | 0.929 | 0.923 | 0.904 |
| Equilibrium Model | Parameters | 25 °C | 35 °C |
|---|---|---|---|
| Langmuir isotherm | qm (mg·g−1) | 114.9 | 125 |
| b (L·mg−1) | 8.3 × 10−2 | 9.1 × 10−2 | |
| RL | 0.37 | 0.35 | |
| R2 | 0.981 | 0.990 | |
| Freundlich isotherm | KF (L·mg−1) | 12.21 | 17.14 |
| n | 1.69 | 1.72 | |
| R2 | 0.990 | 0.995 | |
| BET | Qs (mg·g−1) | 75.75 | 84.74 |
| CBET (L·mg−1) | 13.225 | 14.755 | |
| R2 | 0.963 | 0.988 |
| Adsorbent | Adsorption Capacity (qm, mg·g−1) | References |
|---|---|---|
| S(H)-PAA hydrogel | 109.8 | [44] |
| SCHBs | 95.6 | [45] |
| NNCA hydrogel | 9.54 | [46] |
| Bentonite/alginate composite beads | 53.2 | [47] |
| Thiol-functionalized mesoporous silica | 78 | [48] |
| sodium alginate-meso-2,3-dimercaptosuccinic acid hybrid aerogel | 91.2 | [49] |
| β-cyclodextrin-based hydrogel | 98.8 | [50] |
| HMO-P(HMAm/HEA) hydrogel | 93.86 | [51] |
| XG-cl-poly(AAm-co-AA) hydrogel | 125 | Present work |
| Co (mg·L−1) | ΔH0 (J·mol−1) | ΔS0 (J·mol−1·K−1) | −ΔG0 (J·mol−1) | |||
|---|---|---|---|---|---|---|
| 298 K | 303 K | 308 K | 313 K | |||
| 20 | 24.5 | 0.09 | 2.32 | 2.77 | 3.22 | 3.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, G.; Kumar, A.; Ghfar, A.A.; García-Peñas, A.; Naushad, M.; Stadler, F.J. Fabrication and Characterization of Xanthan Gum-cl-poly(acrylamide-co-alginic acid) Hydrogel for Adsorption of Cadmium Ions from Aqueous Medium. Gels 2022, 8, 23. https://doi.org/10.3390/gels8010023
Sharma G, Kumar A, Ghfar AA, García-Peñas A, Naushad M, Stadler FJ. Fabrication and Characterization of Xanthan Gum-cl-poly(acrylamide-co-alginic acid) Hydrogel for Adsorption of Cadmium Ions from Aqueous Medium. Gels. 2022; 8(1):23. https://doi.org/10.3390/gels8010023
Chicago/Turabian StyleSharma, Gaurav, Amit Kumar, Ayman A. Ghfar, Alberto García-Peñas, Mu. Naushad, and Florian J. Stadler. 2022. "Fabrication and Characterization of Xanthan Gum-cl-poly(acrylamide-co-alginic acid) Hydrogel for Adsorption of Cadmium Ions from Aqueous Medium" Gels 8, no. 1: 23. https://doi.org/10.3390/gels8010023
APA StyleSharma, G., Kumar, A., Ghfar, A. A., García-Peñas, A., Naushad, M., & Stadler, F. J. (2022). Fabrication and Characterization of Xanthan Gum-cl-poly(acrylamide-co-alginic acid) Hydrogel for Adsorption of Cadmium Ions from Aqueous Medium. Gels, 8(1), 23. https://doi.org/10.3390/gels8010023










