Interaction between Negatively Charged Fish Gelatin and Cyclodextrin in Aqueous Solution: Characteristics and Formation Mechanism
Abstract
:1. Introduction
2. Results and Discussion
2.1. Gel Strength
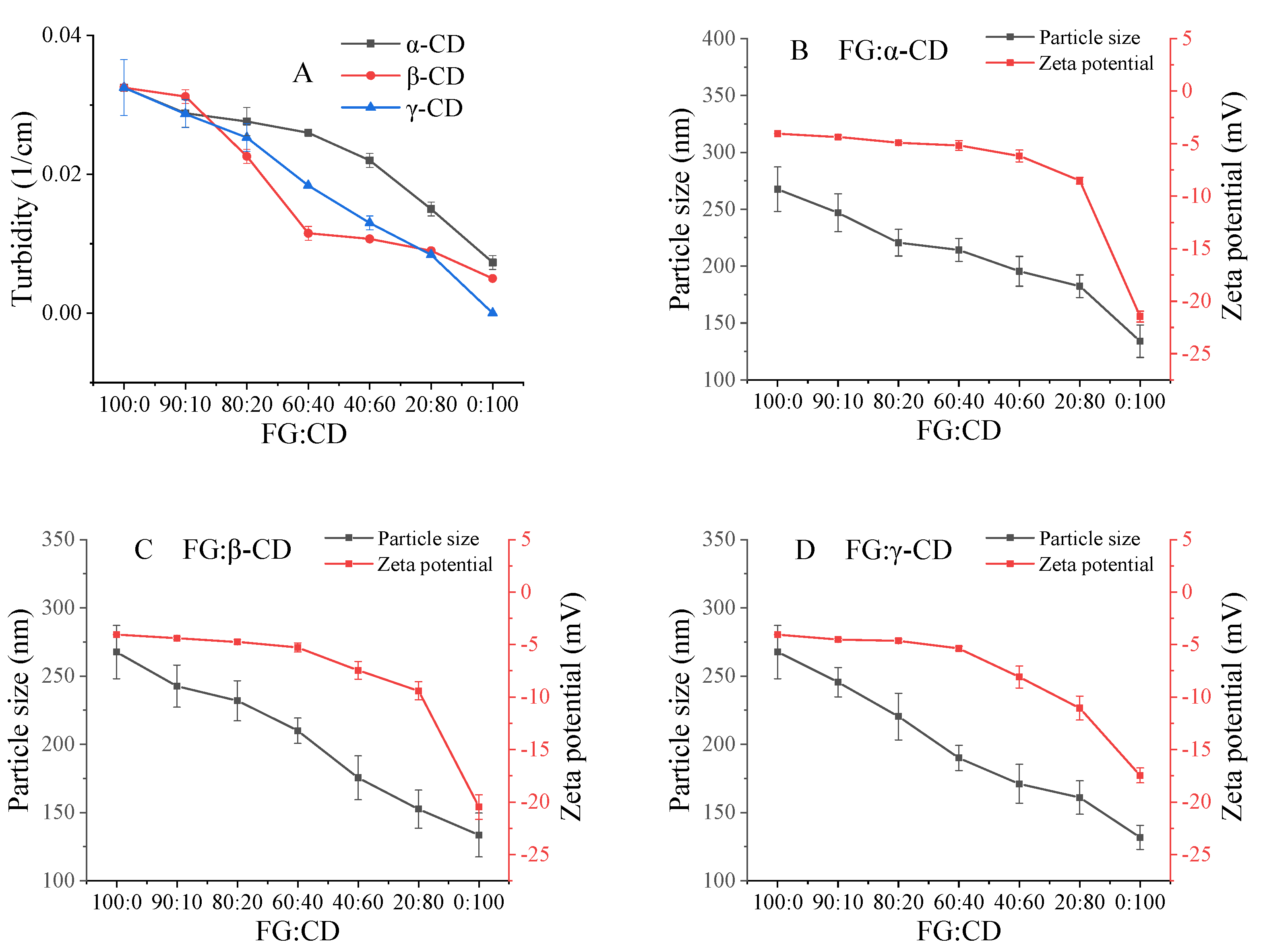
2.2. Turbidity, Particle Size, and Zeta Potential
2.3. Flow Behaviors
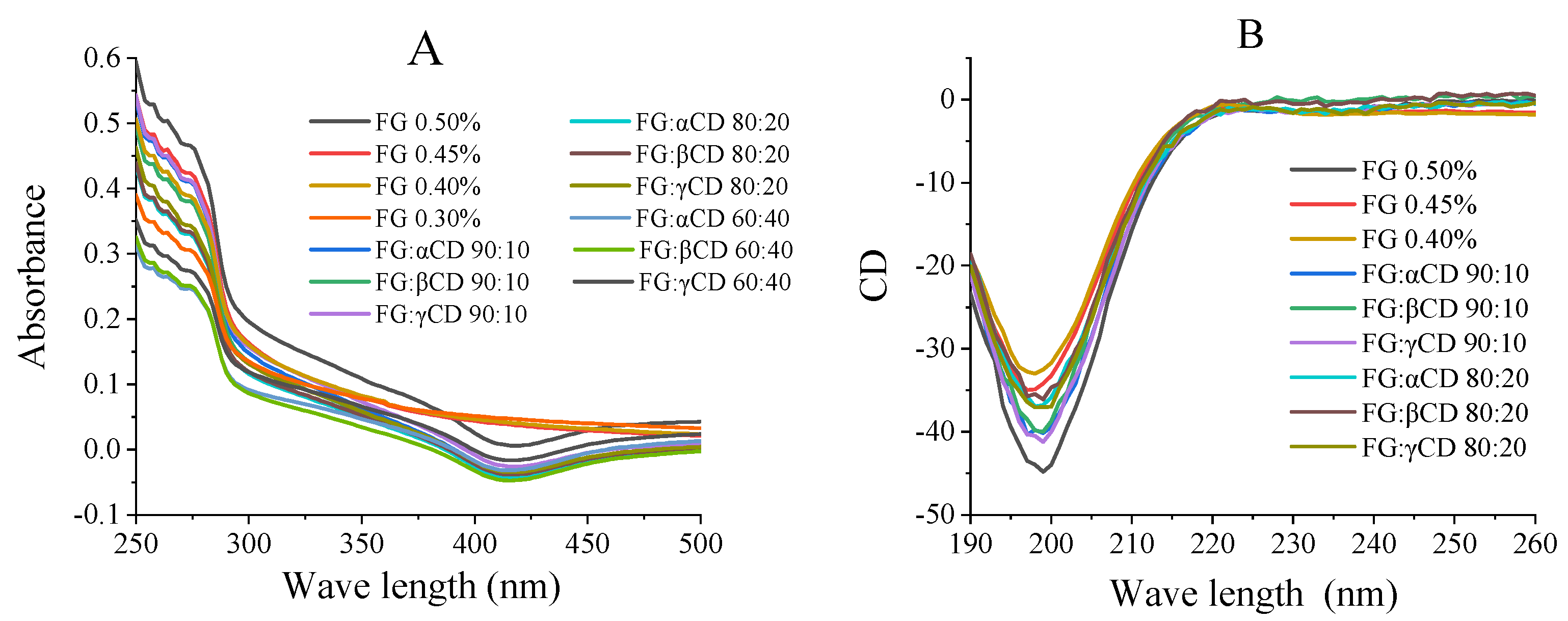
2.4. UV Absorption Spectrum Measurements
2.5. Far-UV Circular Dichroism Measurements
2.6. Fluorescence Spectroscopy
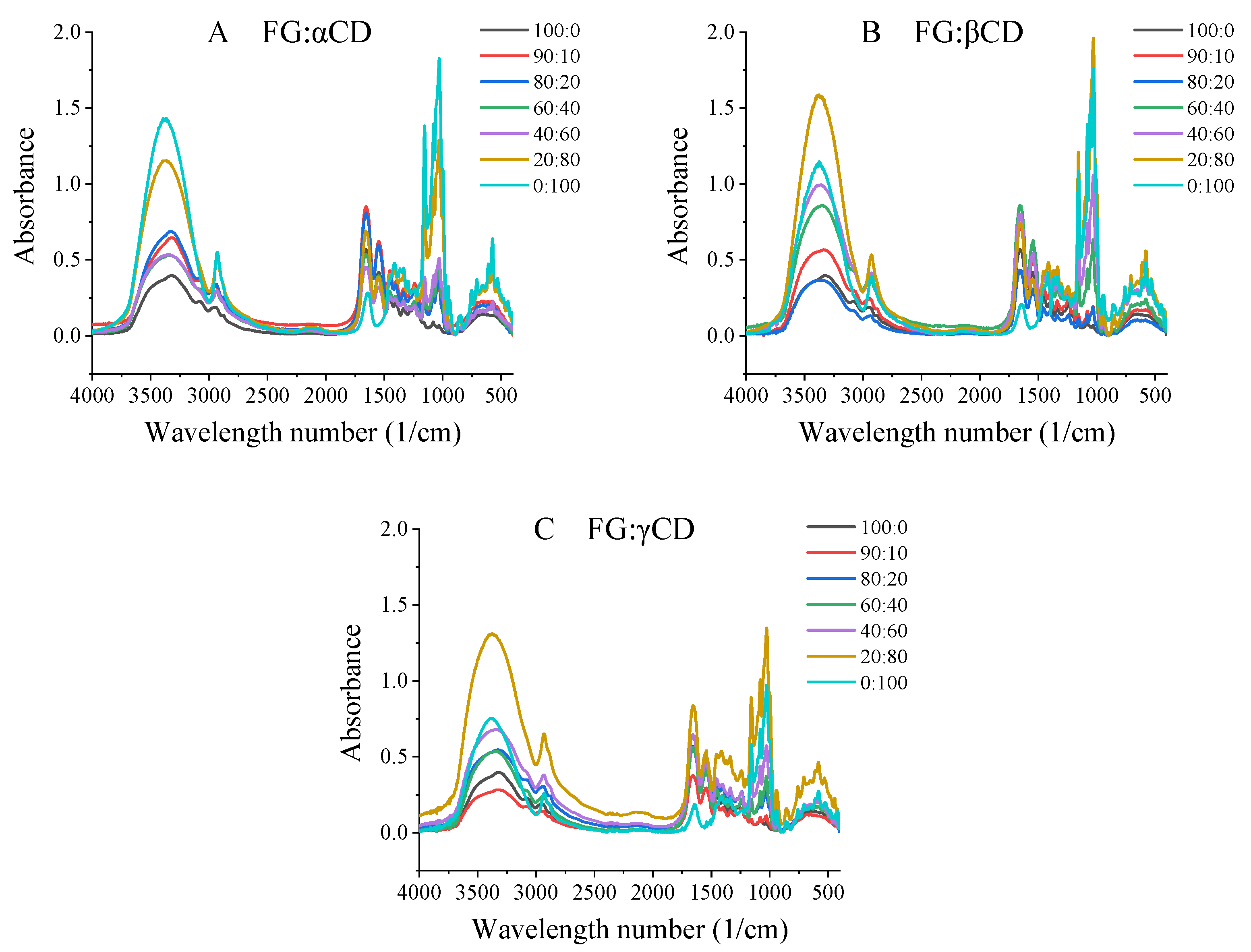
2.7. Fourier Transform Infrared (FTIR) Spectroscopy
3. Conclusions
4. Methods
4.1. Materials
4.2. Sample Preparation
4.3. Gel Strength
4.4. Turbidity
4.5. Zeta Potential and Particle Size Measurements
4.6. Flow Behaviors
4.7. UV Absorption Spectrum Measurements
4.8. Far-UV Circular Dichroism Measurements
4.9. Fluorescence Spectroscopy
4.10. Fourier Transform Infrared (FTIR) Spectroscopy
4.11. Statistics Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patino, J.M.R.; Pilosof, A.M. Protein-polysaccharide interactions at fluid interfaces. Food Hydrocoll. 2011, 25, 1925–1937. [Google Scholar] [CrossRef]
- Souza CJ, F.; Garcia-Rojas, E.E. Interpolymeric complexing between egg white proteins and xanthan gum: Effect of salt and protein/polysaccharide ratio. Food Hydrocoll. 2016, 66, 268–275. [Google Scholar] [CrossRef]
- Razzak, M.A.; Kim, M.; Chung, D. Elucidation of aqueous interactions between fish gelatin and sodium alginate. Carbohydr. Polym. 2016, 148, 181–188. [Google Scholar] [CrossRef] [PubMed]
- You, G.; Niu, G.; Long, H.; Zhang, C.; Liu, X. Elucidation of interactions between gelatin aggregates and hsian-tsao gum in aqueous solutions. Food Chem. 2020, 319, 126532. [Google Scholar] [CrossRef]
- Zou, W.; Mourad, F.K.; Zhang, X.; Ahn, D.U.; Cai, Z.; Jin, Y. Phase separation behavior and characterization of ovalbumin and propylene glycol alginate complex coacervates. Food Hydrocoll. 2020, 108, 105978. [Google Scholar] [CrossRef]
- Lu, X.; Xie, S.; Wang, L.; Xie, H.; Fang, W. Electrostatic-driven structural transformation in the complexation of lysozyme and κ-carrageenan. Chem. Phys. 2020, 538, 110910. [Google Scholar] [CrossRef]
- Razavi, M.S.; Golmohammadi, A.; Nematollahzadeh, A.; Fiori, F.; Farris, S. Preparation of cinnamon essential oil emulsion by bacterial cellulose nanocrystals and fish gelatin. Food Hydrocoll. 2020, 109, 106111. [Google Scholar] [CrossRef]
- Sow, L.C.; Toh NZ, Y.; Wong, C.W.; Yang, H. Combination of sodium alginate with tilapia fish gelatin for improved texture properties and nanostructure modification. Food Hydrocoll. 2019, 94, 459–467. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.; Shangguan, X.; Wang, H.; Zhang, L.; Bansal, N. Characteristics of fish gelatin-anionic polysaccharide complexes and their applications in yoghurt: Rheology and tribology. Food Chem. 2020, 343, 128413. [Google Scholar] [CrossRef]
- Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J.M. Applications of cyclodextrins in food science. A review. Trends Food Sci. Technol. 2020, 104, 132–143. [Google Scholar] [CrossRef]
- Sivakumar, K.; Parinamachivayam, G.; Murali Krishnan, M.; Chakravarty, S.; Bharathi, A. Preparation, characterization and molecular modeling studies of the beta-cyclodextrin inclusion complex with benzoguanamine and its analytical application as chemosensor for the selective sensing of Ce4+. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 200, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Kimmel, J.; Zhou, L.; Rao, J.; Chen, B. Combining solid dispersion-based spray drying with cyclodextrin to improve the functionality and mitigate the beany odor of pea protein isolate. Carbohydr. Polym. 2020, 245, 116546. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.F.; Hui, J.; Shang, M.; Qi, L.; Chang, X.; Yao, W.; Hao, W.; Dong, X. Effects of deodorization by powdered activated carbon, β-cyclodextrin and yeast on odor and functional properties of tiger puffer (Takifugu rubripes) skin gelatin. Int. J. Biol. Macromol. 2018, 118, 116–123. [Google Scholar] [CrossRef]
- Huang, T.; Zhao, H.; Fang, Y.; Lu, J.; Yang, W. Comparison of gelling properties and flow behaviors of microbial transglutaminase (MTGase) and pectin modified fish gelatin. J. Texture Stud. 2019, 50, 400–409. [Google Scholar] [CrossRef]
- Liu, J.; Shim, Y.Y.; Wang, Y.; Reaney MJ, T. Intermolecular interaction and complex coacervation between bovine serum albumin and gum from whole flaxseed (Linum usitatissimum L.). Food Hydrocoll. 2015, 49, 95–103. [Google Scholar] [CrossRef]
- Park, J.M.; Muhoberac, B.B.; Dubin, P.L.; Xia, J. Effects of protein charge heterogeneity in protein-polyelectrolyte complexation. Macromolecules 1992, 25, 290–295. [Google Scholar] [CrossRef]
- Zhan, F.; Ding, S.; Xie, W.; Zhu, X.; Chen, Y. Towards understanding the interaction of β-lactoglobulin with capsaicin: Multi-spectroscopic, thermodynamic, molecular docking and molecular dynamics simulation approaches. Food Hydrocoll. 2020, 105, 105767. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.; Wang, H.; Shangguan, X.; Zhang, L.; Zhang, N.-H.; Bansal, N. Pectin and enzyme complex modified fish scales gelatin: Rheological behavior, gel properties and nanostructure. Carbohydr. Polym. 2017, 156, 294–302. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; Tu, Z.; Zou, Z.; Shangguan, X.; Bansal, N. Glycosylated fish gelatin emulsion: Rheological, tribological properties and its application as model coffee creamers. Food Hydrocoll. 2020, 102, 105552. [Google Scholar] [CrossRef]
- Yang, H.; Yang, S.; Kong, J.; Dong, A.; Yu, S. Obtaining information about protein secondary structures in aqueous solution using Fourier transform IR spectroscopy. Nat. Protoc. 2015, 10, 382–396. [Google Scholar] [CrossRef]
- Chen, L.F.; Shen, Q.; Shen, J.P.; Shi, D.T.; Chen, T.; Yu, H.R. Studies and comparison of the liquid adsorption and surface properties of α-, β- and γ-cyclodextrins by FTIR and capillary rise method. Colloids Surf. A Physicochem. Eng. Asp. 2012, 411, 69–73. [Google Scholar] [CrossRef]
- Gu, Z.J.; Zhang, Q.C.; Shen, Q. Synthesis and comparison of polyaniline nanofibers templated by α-, β- and γ-cyclodextrin. J. Polym. Res. 2015, 22, 7. [Google Scholar] [CrossRef]
- Fan, L.; Du, Y.; Huang, R.; Wang, Q.; Wang, X.; Zhang, L. Preparation and characterization of alginate/gelatin blend fibers. J. Appl. Polym. Sci. 2005, 96, 1625–1629. [Google Scholar] [CrossRef]
- Huang, S.; Tu, Z.C.; Sha, X.M.; Wang, H.; Hu, Z.Z. Gelling properties and interaction analysis of fish gelatin–low-methoxyl pectin system with different concentrations of Ca2+. LWT-Food Sci. Technol. 2020, 132, 109826. [Google Scholar] [CrossRef]
- Bonenfant, D.; Niquette, P.; Mimeault, M.; Furtos-Matei, A.; Hausler, R. UV-VIS and FTIR spectroscopic analyses of inclusion complexes of nonylphenol and nonylphenol ethoxylate with β-cyclodextrin. Water Res. 2009, 43, 3575–3581. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.; Shangguan, X.; Wang, H.; Sha, X. Gelation kinetics and characterization of enzymatically enhanced fish scale gelatin–pectin coacervate. J. Sci. Food Agric. 2018, 98, 1024–1032. [Google Scholar] [CrossRef] [PubMed]


| Pure FG (w/v) | 5% | 4.50% | 4% | 3% | 2% | 1% |
|---|---|---|---|---|---|---|
| 299.63 ± 13.31 Af | 229.02 ± 12.31 Ae | 154.16 ± 1.82 Ad | 100.63 ± 2.81 Ac | 51.6 ± 1.24 Bb | 3.04 ± 0.00 Aa | |
| FG–CD | 100:00:00 | 90:10:00 | 80:20:00 | 60:40:00 | 40:60 | 0.888889 |
| FG–αCD | 299.63 ± 13.31 Af | 267.14 ± 11.01 Be | 184.43 ± 23.00 Bd | 104.49 ± 12.51 ABc | 46.05 ± 3.80 Ab | 7.44 ± 0.88 Ca |
| FG–βCD | 299.63 ± 13.31 Abc | 283.93 ± 12.83 BCb | 194.29 ± 10.77 Ba | - | - | - |
| FG–γCD | 299.63 ± 13.31 Af | 292.81 ± 10.18 CDe | 223.44 ± 10.46 Cd | 101.84 ± 14.32 ABc | 49.21 ± 4.30 ABb | 5.03 ± 1.35 Ba |
| Content | Various Ratios | β-Sheet (%) | Random Coil (%) | α-Helix (%) | β-Turn (%) |
|---|---|---|---|---|---|
| FG | 0.50% | 27.37 ± 0.21 Bbc | 65.60 ± 0.44 Aab | 0 ± 0 Aa | 7.07 ± 0.29 D |
| 0.45% | 26.30 ± 0.10 Ab | 69.73 ± 0.06 Bc | 0 ± 0 Aa | 3.97 ± 0.12 BCa | |
| 0.40% | 26.37 ± 0.25 A | 69.87 ± 0.25 BC | 0 ± 0 Aa | 3.77 ± 0.55 Ba | |
| 0.30% | 27.73 ± 0.06 Cb | 71.10 ± 0.52 Dd | 0 ± 0 Aa | 1.20 ± 0.52 Aa | |
| FG–αCD | 90:10:00 | 27.20 ± 0.10 Ab | 65.77 ± 0.29 Ab | 0 ± 0 Aa | 7.00 ± 0.36 BCbc |
| 80:20:00 | 27.30 ± 0.10 ABc | 66.10 ± 0.27 ABab | 0 ± 0 Aa | 6.60 ± 0.17 ABbc | |
| 60:40:00 | 27.83 ± 0.21 Cbc | 65.80 ± 0.25 Ab | 0 ± 0 Aa | 6.37 ± 0.12 Ac | |
| FG–βCD | 90:10:00 | 26.17 ± 0.35 Ba | 65.77 ± 0.60 ABabc | 0 ± 0 Aa | 9.17 ± 0.51 BCd |
| 80:20:00 | 25.17 ± 0.40 Aa | 66.57 ± 0.35 ABb | 0 ± 0 Aa | 8.07 ± 0.83 Ad | |
| 60:40:00 | 26.47 ± 0.31 BCa | 65.13 ± 0.35 Aa | 0 ± 0 Aa | 8.43 ± 0.64 ABd | |
| FG–γCD | 90:10:00 | 28.13 ± 0.13 ABd | 65.10 ± 0.10 Aa | 0 ± 0 Aa | 6.80 ± 0.17 BCb |
| 80:20:00 | 28.07 ± 0.08 Ad | 65.60 ± 0.40 ABa | 0 ± 0 Aa | 6.33 ± 0.31 Bb | |
| 60:40:00 | 28.03 ± 0.12 Ac | 66.17 ± 0.29 BCbc | 0 ± 0 Aa | 5.80 ± 0.17 Ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Q.; Ma, N.; Ding, K.; Zhan, S.; Lou, Q.; Huang, T. Interaction between Negatively Charged Fish Gelatin and Cyclodextrin in Aqueous Solution: Characteristics and Formation Mechanism. Gels 2021, 7, 260. https://doi.org/10.3390/gels7040260
Fang Q, Ma N, Ding K, Zhan S, Lou Q, Huang T. Interaction between Negatively Charged Fish Gelatin and Cyclodextrin in Aqueous Solution: Characteristics and Formation Mechanism. Gels. 2021; 7(4):260. https://doi.org/10.3390/gels7040260
Chicago/Turabian StyleFang, Qi, Nao Ma, Keying Ding, Shengnan Zhan, Qiaoming Lou, and Tao Huang. 2021. "Interaction between Negatively Charged Fish Gelatin and Cyclodextrin in Aqueous Solution: Characteristics and Formation Mechanism" Gels 7, no. 4: 260. https://doi.org/10.3390/gels7040260
APA StyleFang, Q., Ma, N., Ding, K., Zhan, S., Lou, Q., & Huang, T. (2021). Interaction between Negatively Charged Fish Gelatin and Cyclodextrin in Aqueous Solution: Characteristics and Formation Mechanism. Gels, 7(4), 260. https://doi.org/10.3390/gels7040260







