Thermosensitive Polyester Hydrogel for Application of Immunosuppressive Drug Delivery System in Skin Allograft
Abstract
1. Introduction
2. Results and Discussion
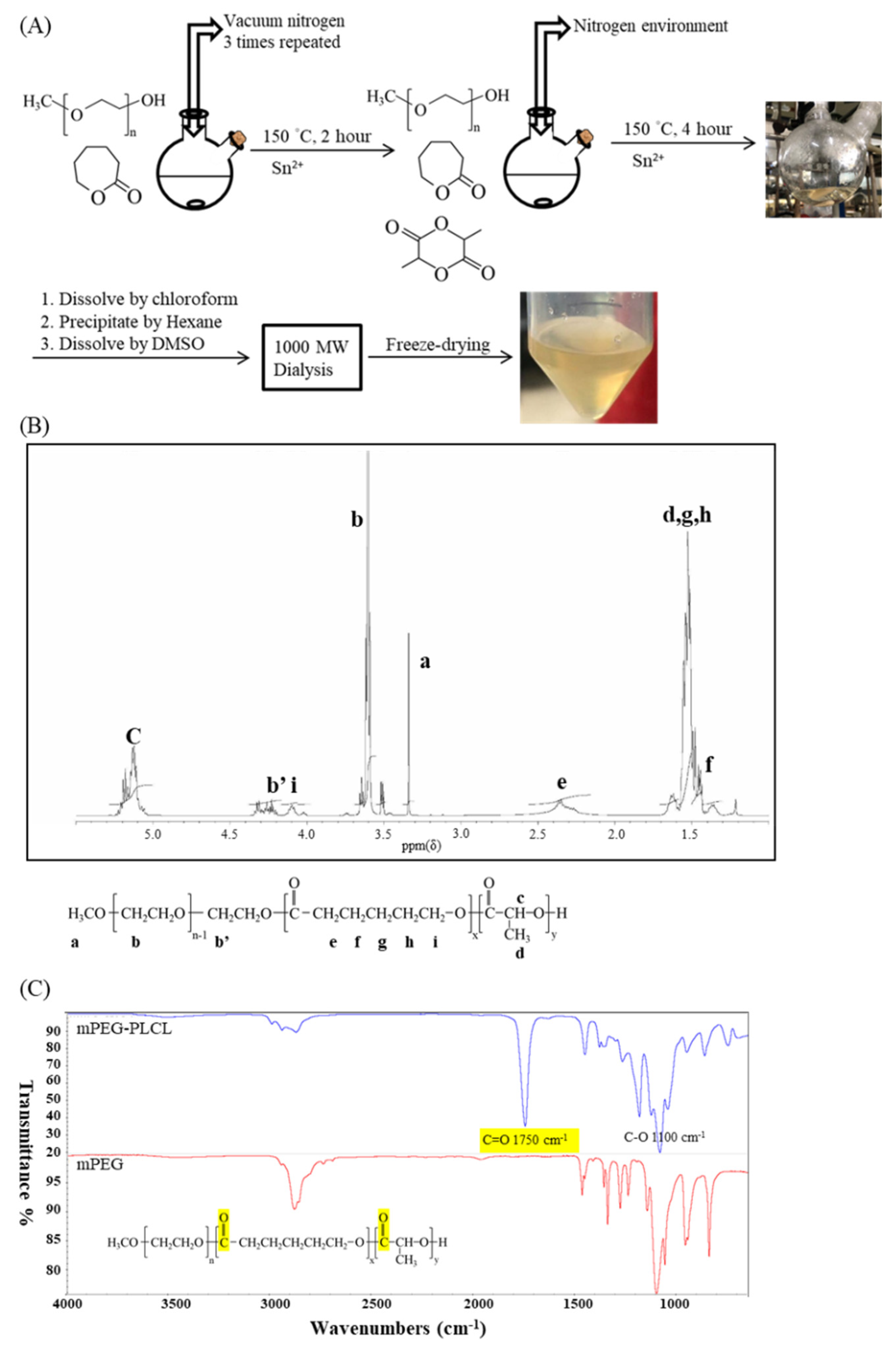
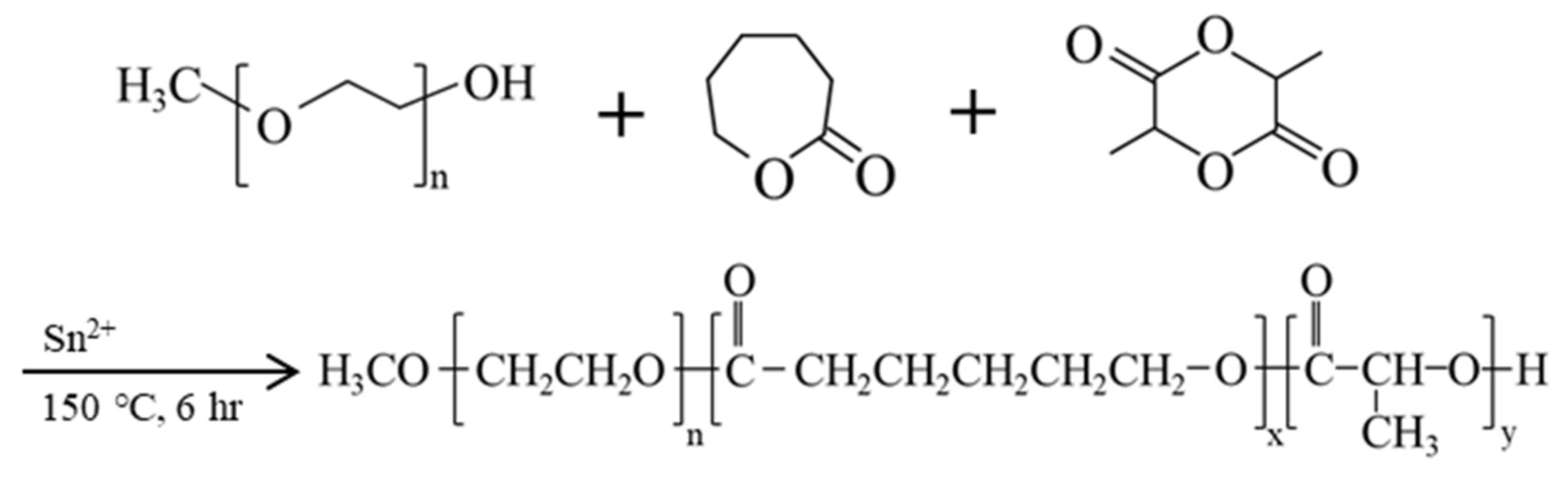
2.1. The Synthesis and Characterization of mPEG-PLCL
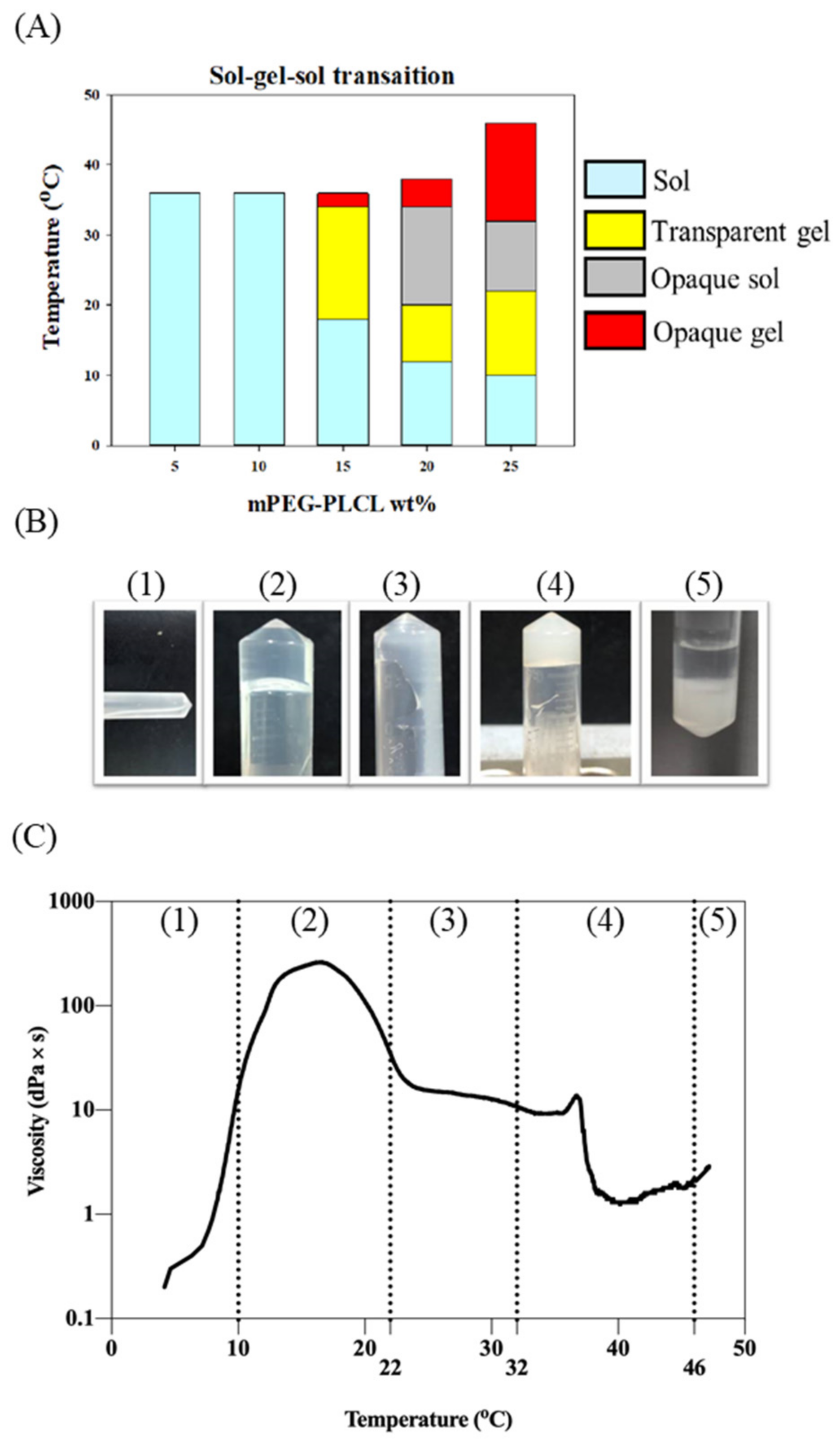
2.2. Sol–Gel–Sol Transition
2.3. Cosolvent Selection and Encapsulation Efficiency
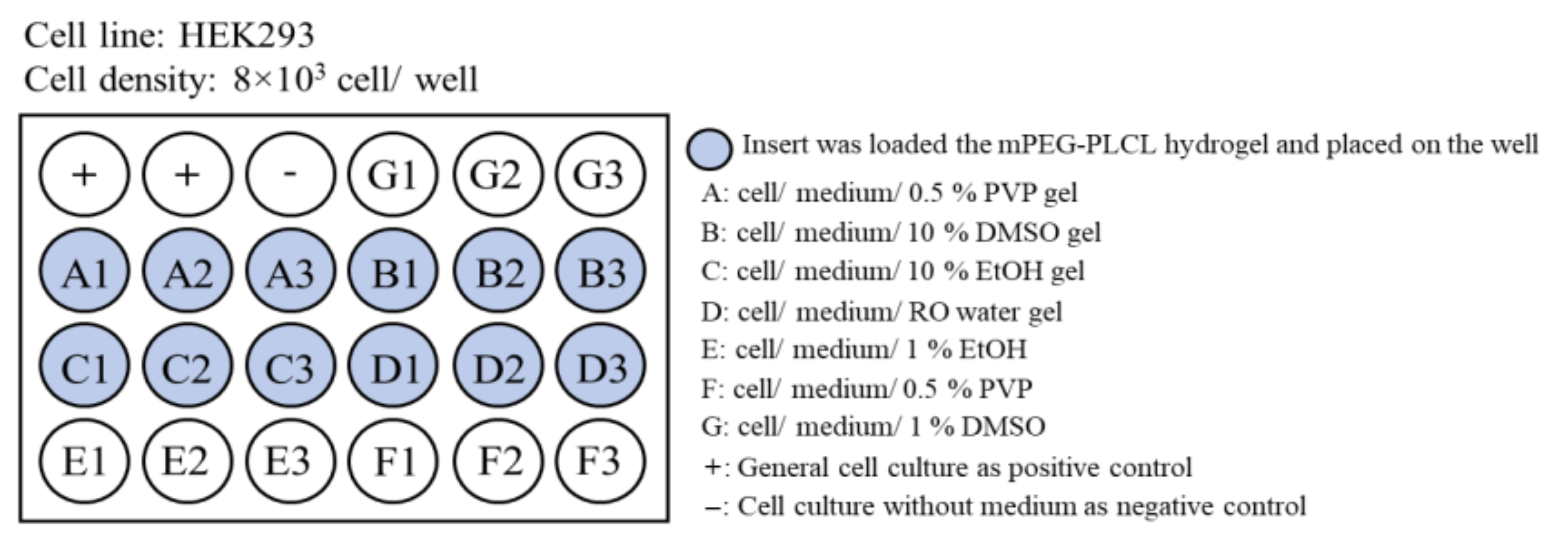
2.4. Biocompatibility Testing
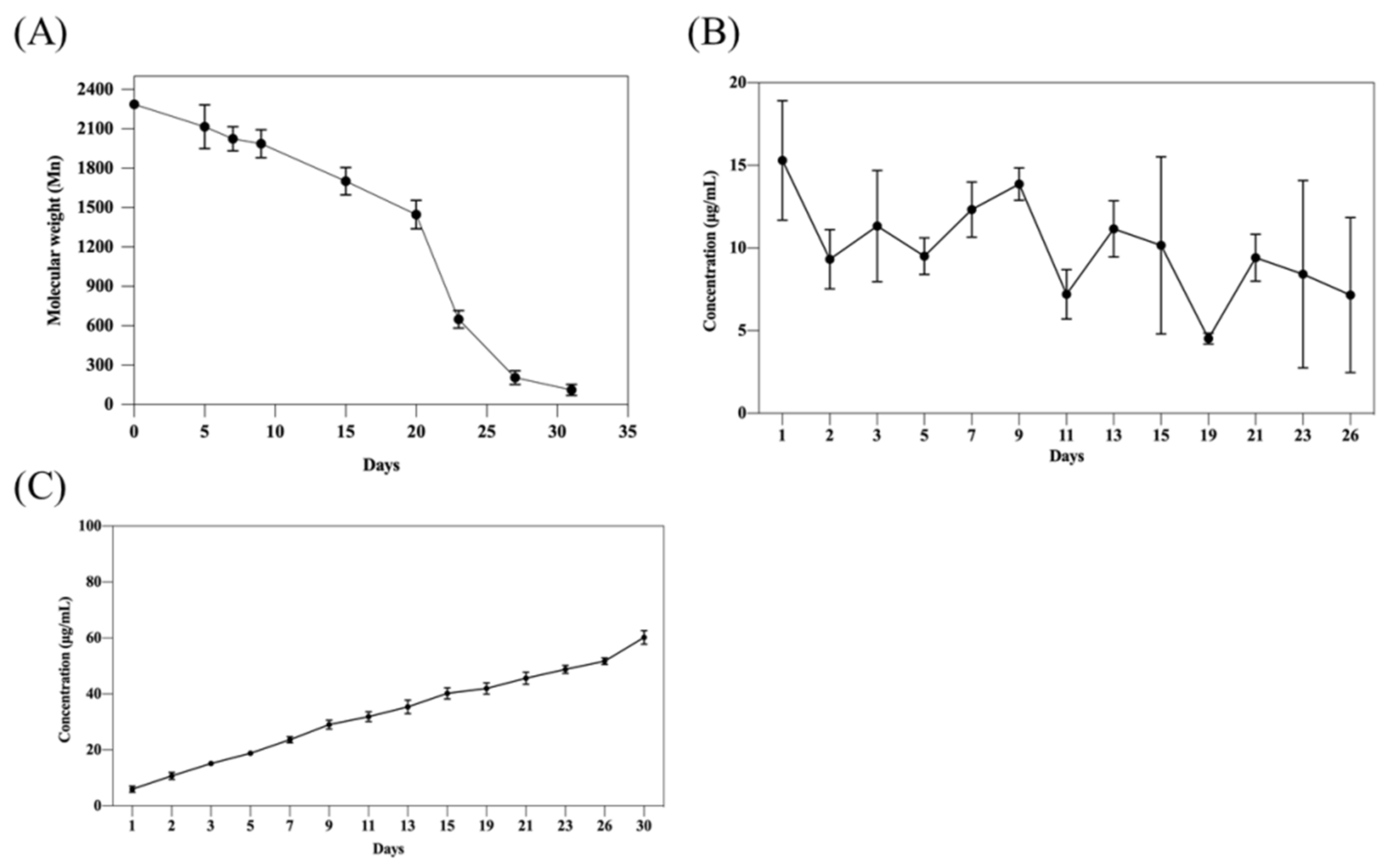
2.5. In Vitro Degradation and Tacrolimus Release
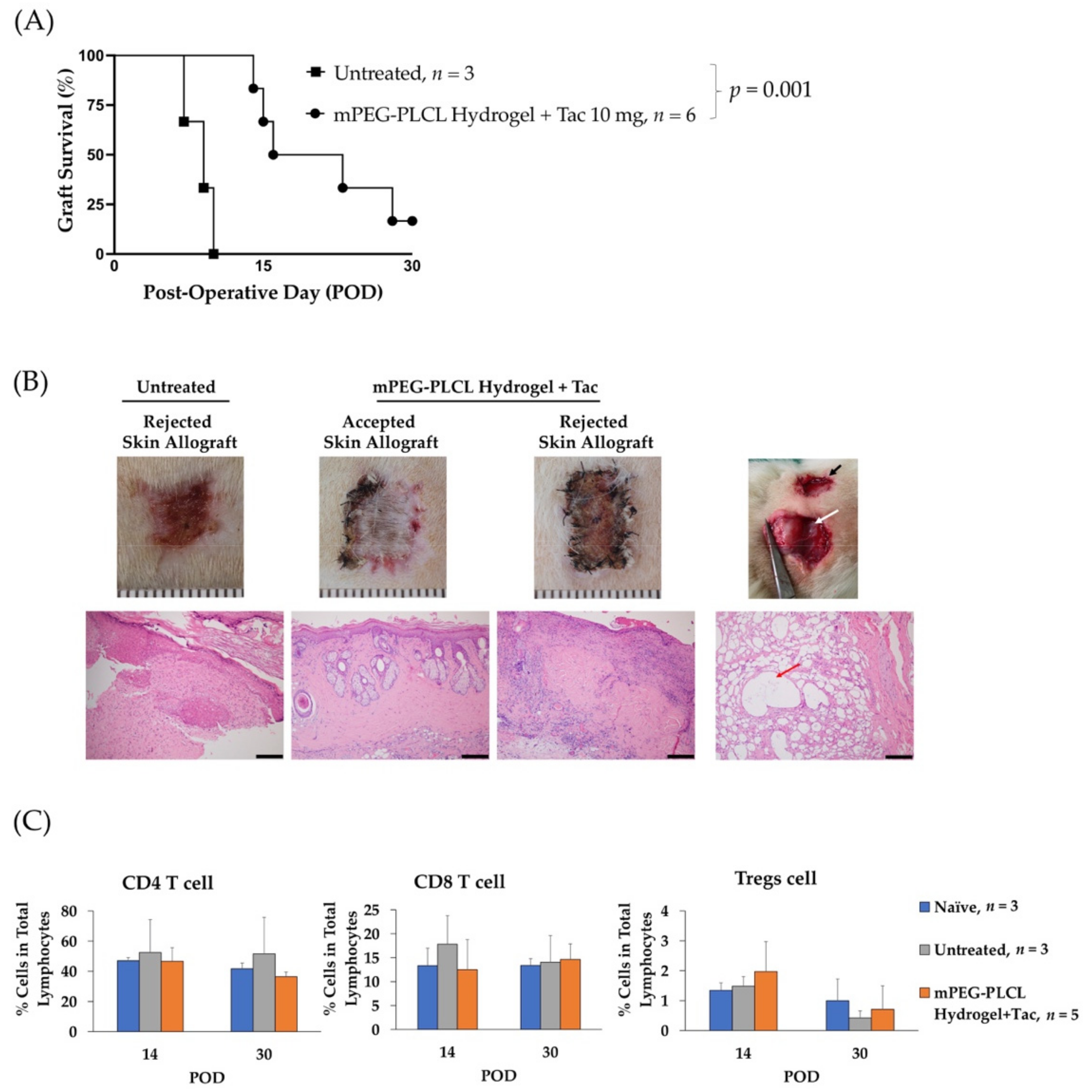
2.6. Allograft Skin Transplantation
3. Conclusions
4. Materials and Methods
4.1. Synthesis of Methoxy Poly(ethylene glycol), D,L-lactide, and ϵ-caprolactone Block Copolymer (mPEG-PLCL)
4.2. 1H Nuclear Magnetic Resonance (1H-NMR) Spectroscopy
4.3. Gel Permeation Chromatography (GPC)
4.4. Fourier-Transformed Infrared Spectroscopy (FT-IR)
4.5. Sol–Gel Transition Phase
4.6. Rheological Property of mPEG-PLCL
4.7. Quantification of Tacrolimus
4.8. Development of Formulation for Higher Encapsulation Efficiency
4.9. In Vitro Degradation Testing of mPEG-PLCL
4.10. In Vitro Drug Release Testing of mPEG-PLCL
4.11. Biocompatibility Testing
4.12. Allograft Skin Transplantation Experiment
4.13. Quantification of Circulating CD4 T Cells, CD8 T Cells, and Regulatory T Cells (Tregs)
4.14. Histology Assessment
4.15. Statistical Significance Analysis
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Ghosh, S. Recent Research and Development in Synthetic Polymer-Based Drug Delivery Systems. J. Chem. Res. 2004, 2004, 241–246. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in Drug Delivery: Progress and Challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Parikh, J.; Raval, A. Review on Hydrolytic Degradation Behavior of Biodegradable Polymers from Controlled Drug Delivery System. Trends Biomater. Artif. Organs 2011, 25, 79–85. [Google Scholar]
- Satish, C.S.; Satish, K.; Shivakumar, H.G. Hydrogels as Controlled Drug Delivery Systems: Synthesis, Crosslinking, Water and Drug Transport Mechanism. Indian J. Pharm. Sci. 2006, 68, 133–140. [Google Scholar]
- Das, D.; Pal, S. Modified Biopolymer-Dextrin Based Crosslinked Hydrogels: Application in Controlled Drug Delivery. RSC Adv. 2015, 5, 25014–25050. [Google Scholar] [CrossRef]
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Yallapu, M.M.; Sadiku, R. A Mini Review on Hydrogels Classification and Recent Developments in Miscellaneous Applications. Mater. Sci. Eng. 2017, 79, 958–971. [Google Scholar] [CrossRef]
- Yannas, I.V.; Lee, E.; Orgill, D.P.; Skrabut, E.M.; Murphy, G.F. Synthesis and Characterization of a Model Extracellular Matrix That Induces Partial Regeneration of Adult Mammalian Skin. Proc. Natl. Acad. Sci. USA 1989, 89, 933–937. [Google Scholar] [CrossRef]
- Wichterle, O.; Lím, D. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Shukla, S.K.; Bhanu, S.; Kankane, S. Responsive Polymers in Controlled Drug Delivery. Prog. Polym. Sci. 2011, 25, 79–85. [Google Scholar] [CrossRef]
- Jeong, B.; Kim, S.W.; Bae, Y.H. Thermosensitive Sol–Gel Reversible Hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 154–162. [Google Scholar] [CrossRef]
- Maeda, T. Structures and Applications of Thermoresponsive Hydrogels and Nanocomposite-Hydrogels Based on Copolymers with Poly (Ethylene Glycol) and Poly (Lactide-Co-Glycolide) Blocks. Bioengineering 2019, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Franklin, L.; Sun, A.M. Microencapsulated Islets as Bioartificial Endocrine Pancreas. Science 1980, 210, 908–910. [Google Scholar]
- Lin, H.-C.; Anggelia, M.R.; Cheng, C.-C.; Ku, K.-L.; Cheng, H.-Y.; Wen, C.-J.; Wang, A.Y.L.; Lin, C.-H.; Chu, I.-M. A Mixed Thermosensitive Hydrogel System for Sustained Delivery of Tacrolimus for Immunosuppressive Therapy. Pharmaceutics 2019, 11, 413. [Google Scholar] [CrossRef] [PubMed]
- Darnell, M.C.; Sun, J.-Y.; Mehta, M.; Johnson, C.; Arany, P.R.; Suo, Z.; Mooney, D.J. Performance and Biocompatibility of Extremely Tough Alginate/Polyacrylamide Hydrogels. Biomaterials 2013, 34, 8042–8048. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Peng, H.-H.; Chen, M.-H.; Sun, J.-S.; Liu, T.-Y.; Chen, M.-H. In Situ Forming Hydrogel Composed of Hyaluronate and Polygalacturonic Acid for Prevention of Peridural Fibrosis. J. Mater. Sci. Mater. Med. 2015, 26, 168. [Google Scholar] [CrossRef]
- Goto, T.; Kino, T.; Hatanaka, H.; Nishiyama, M.; Okuhara, M.; Kohsaka, M.; Aoki, H.; Imanaka, H. Discovery of FK-506, a Novel Immunosuppressant Isolated from Streptomyces Tsukubaensis. Transplant. Proc. 1987, 19 (Suppl. 6), 4–8. [Google Scholar] [PubMed]
- Büttemeyer, R.; Jones, N.F.; Min, Z.; Rao, U. Rejection of the Component Tissues of Limb Allografts in Rats Immunosuppressed with FK-506 and Cyclosporine. Plast. Reconstr. Surg. 1996, 97, 139–151. [Google Scholar] [CrossRef]
- Murase, N.; Starzl, T.E.; Demetris, A.J.; Valdivia, L.; Tanabe, M.; Cramer, D.; Makowka, L. Hamster-to-Rat Heart and Liver Xenotransplantation with FK506 plus Antiproliferative Drugs. Transplantation 1993, 55, 701–708. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thomson, A.W.; Bonham, C.A.; Zeevi, A. Mode of Action of Tacrolimus (FK506): Molecular and Cellular Mechanisms. Ther. Drug Monit. 1995, 17, 584–591. [Google Scholar] [CrossRef]
- Larrañeta, E.; Stewart, S.; Ervine, M.; Al-Kasasbeh, R.; Donnelly, R.F. Hydrogels for Hydropho-bic Drug Delivery. Classification, Synthesis and Applications. J. Funct. Biomater. 2018, 9, 13. [Google Scholar] [CrossRef]
- Djekic, L.; Čalija, B.; Medarević, Ð. Gelation Behavior, Drug Solubilization Capacity and Release Kinetics of Poloxamer 407 Aqueous Solutions: The Combined Effect of Copolymer, Cosolvent and Hydrophobic Drug. J. Mol. Liq. 2020, 303, 112639. [Google Scholar] [CrossRef]
- Franco, P.; de Marco, I. The Use of Poly(N-Vinyl Pyrrolidone) in the Delivery of Drugs: A Review. Polymers 2020, 12, 1114. [Google Scholar] [CrossRef] [PubMed]
- Kurakula, M.; Rao, G.S.N.K. Pharmaceutical Assessment of Polyvinylpyrrolidone (PVP): As Excipient from Conventional to Controlled Delivery Systems with a Spotlight on COVID-19 Inhibition. J. Drug Deliv. Sci. Technol. 2020, 60, 102406. [Google Scholar] [CrossRef]
- Sammour, O.A.; Hammad, M.A.; Megrab, N.A.; Zidan, A.S. Formulation and Optimization of Mouth Dissolve Tablets Containing Rofecoxib Solid Dispersion. AAPS PharmSciTech 2020, 7, E55. [Google Scholar] [CrossRef]
- Han, C.; Guo, Y.; Chen, X.; Yao, M.; Zhang, Y.; Zhang, Q.; Wei, X. Phase behaviour and temperature-responsive properties of a gemini surfactant/Brij-30/water system. Soft Matter 2017, 13, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Shao, K.; Lu, Y.; Wang, J.; Chen, X.; Zhang, Z.; Wang, X.; Wang, X.; Yang, H.; Liu, G. Different Effects of Tacrolimus on Innate and Adaptive Immune Cells in the Allograft Transplantation. Scand. J. Immunol. 2016, 83, 119–127. [Google Scholar] [CrossRef]
- Brandacher, G.; Lee, W.P.; Schneeberger, S. Minimizing immunosuppression in hand transplantation. Expert Rev. Clin. Immunol. 2012, 8, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.R.; Muthukumar, T.; Dadhania, D.; Taur, Y.; Jenq, R.R.; Toussaint, N.C.; Ling, L.; Pamer, E.; Suthanthiran, M. Gut microbiota and tacrolimus dosing in kidney transplantation. PLoS ONE 2015, 10, e0122399. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Anggelia, M.R.; Cheng, H.-Y.; Wang, A.Y.L.; Chuang, W.-Y.; Lin, C.-H.; Lee, W.P.A.; Wei, F.-C.; Brandacher, G. The Intragraft Vascularized Bone Marrow Component Plays a Critical Role in Tolerance Induction after Reconstructive Transplantation. Cell. Mol. Immunol. 2021, 18, 363–373. [Google Scholar] [CrossRef]

| Feed Molar Ratio of mPEG: LA: CL | 1H-NMR | GPC | |||
|---|---|---|---|---|---|
| a Mn | b Mn | b Mw | c PDI | ||
| mPEG-PLCL | 1:8:2 | 2042 | 1980 | 2034 | 1.03 |
| 25 wt% mPEG-PLCL with | 10% EtOH | 10% DMSO | 0.5% PVP |
|---|---|---|---|
| Encapsulation efficiency | 98 ± 0.5% | 95 ± 0.3% | 99 ± 0.1% |
| Time (Days) | ||||
|---|---|---|---|---|
Tacrolimus (ng/mL) | 1 24.7 ± 3.8 | 7 42 ± 7.4 | 14 29.4 ± 5.9 | 21 26.05 ± 3.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, I.-E.; Anggelia, M.R.; Lin, S.-Y.; Chen, C.-Y.; Chu, I.-M.; Lin, C.-H. Thermosensitive Polyester Hydrogel for Application of Immunosuppressive Drug Delivery System in Skin Allograft. Gels 2021, 7, 229. https://doi.org/10.3390/gels7040229
Wu I-E, Anggelia MR, Lin S-Y, Chen C-Y, Chu I-M, Lin C-H. Thermosensitive Polyester Hydrogel for Application of Immunosuppressive Drug Delivery System in Skin Allograft. Gels. 2021; 7(4):229. https://doi.org/10.3390/gels7040229
Chicago/Turabian StyleWu, I-En, Madonna Rica Anggelia, Sih-Yu Lin, Chiao-Yun Chen, I-Ming Chu, and Cheng-Hung Lin. 2021. "Thermosensitive Polyester Hydrogel for Application of Immunosuppressive Drug Delivery System in Skin Allograft" Gels 7, no. 4: 229. https://doi.org/10.3390/gels7040229
APA StyleWu, I.-E., Anggelia, M. R., Lin, S.-Y., Chen, C.-Y., Chu, I.-M., & Lin, C.-H. (2021). Thermosensitive Polyester Hydrogel for Application of Immunosuppressive Drug Delivery System in Skin Allograft. Gels, 7(4), 229. https://doi.org/10.3390/gels7040229






