Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials
Abstract
1. Introduction
2. Cross-Linking Strategies to Obtain Physical Hydrogels
2.1. Crystallization
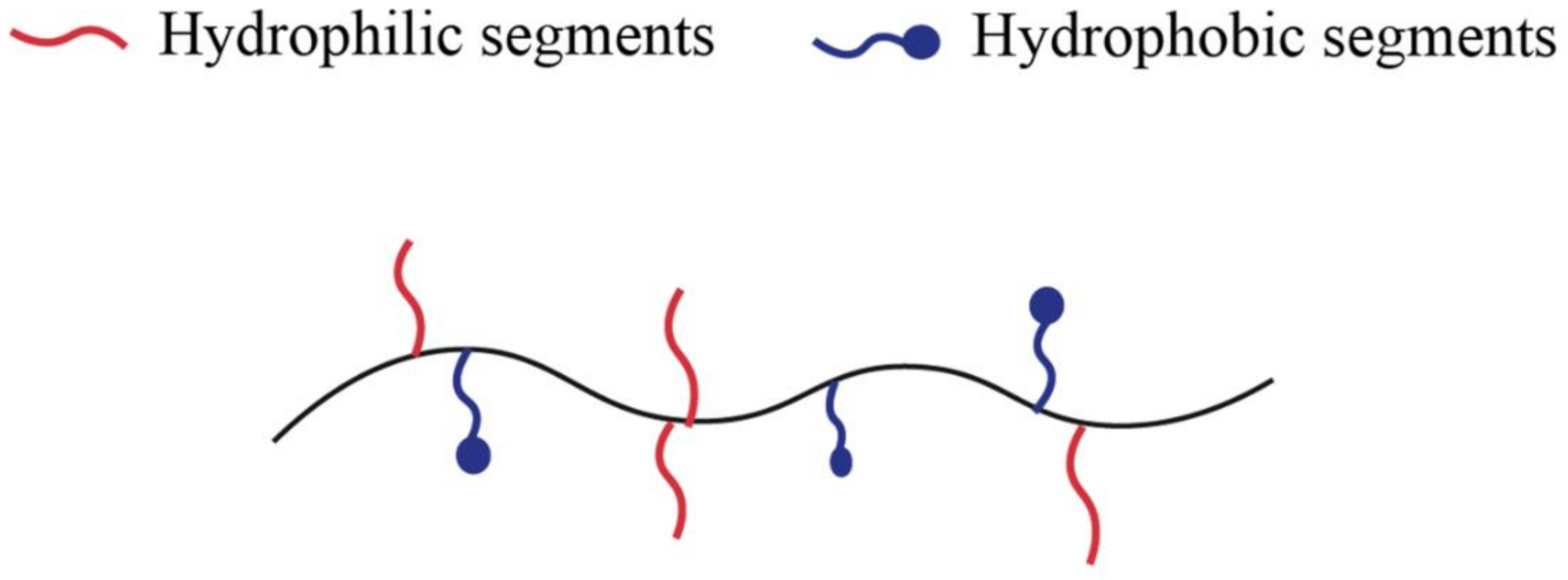
2.2. Amphiphilic Copolymers
2.3. Hydrogel Cross-Linking by Charge Interactions
2.4. Interactions by Hydrogen Bonds
2.5. Stereo-Complexing
2.6. Protein Interactions
3. Cross-Linking Strategies to Obtain Chemical Hydrogels
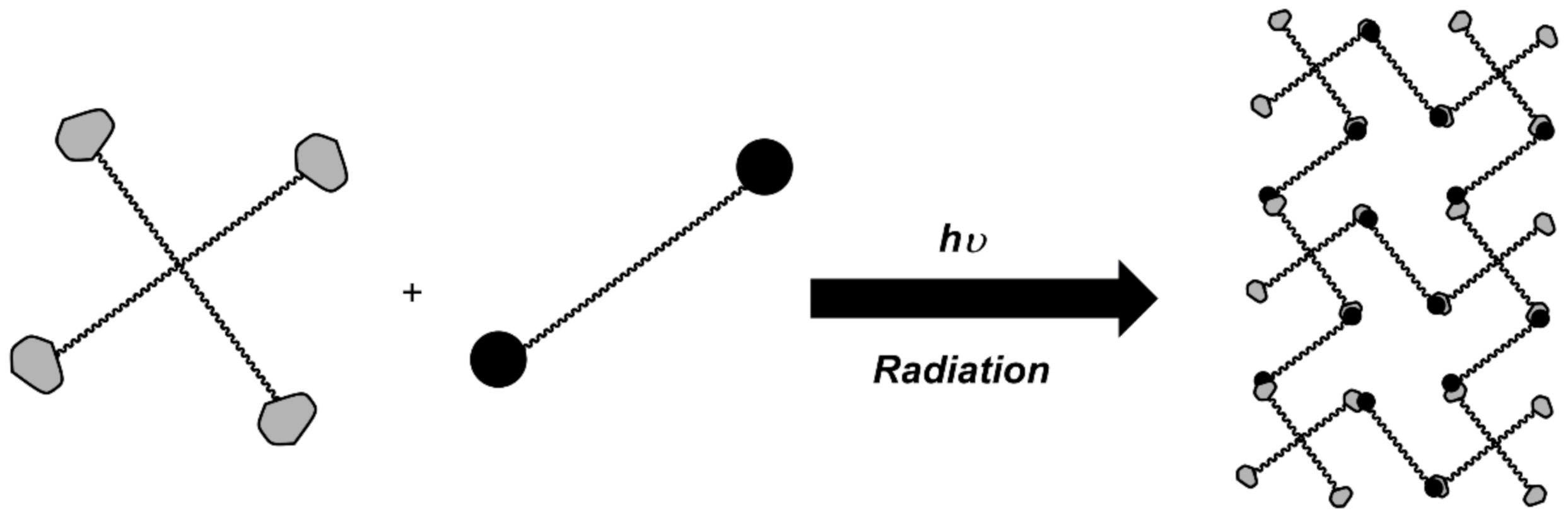
3.1. Graft Copolymerization and Irradiation Crosslinking
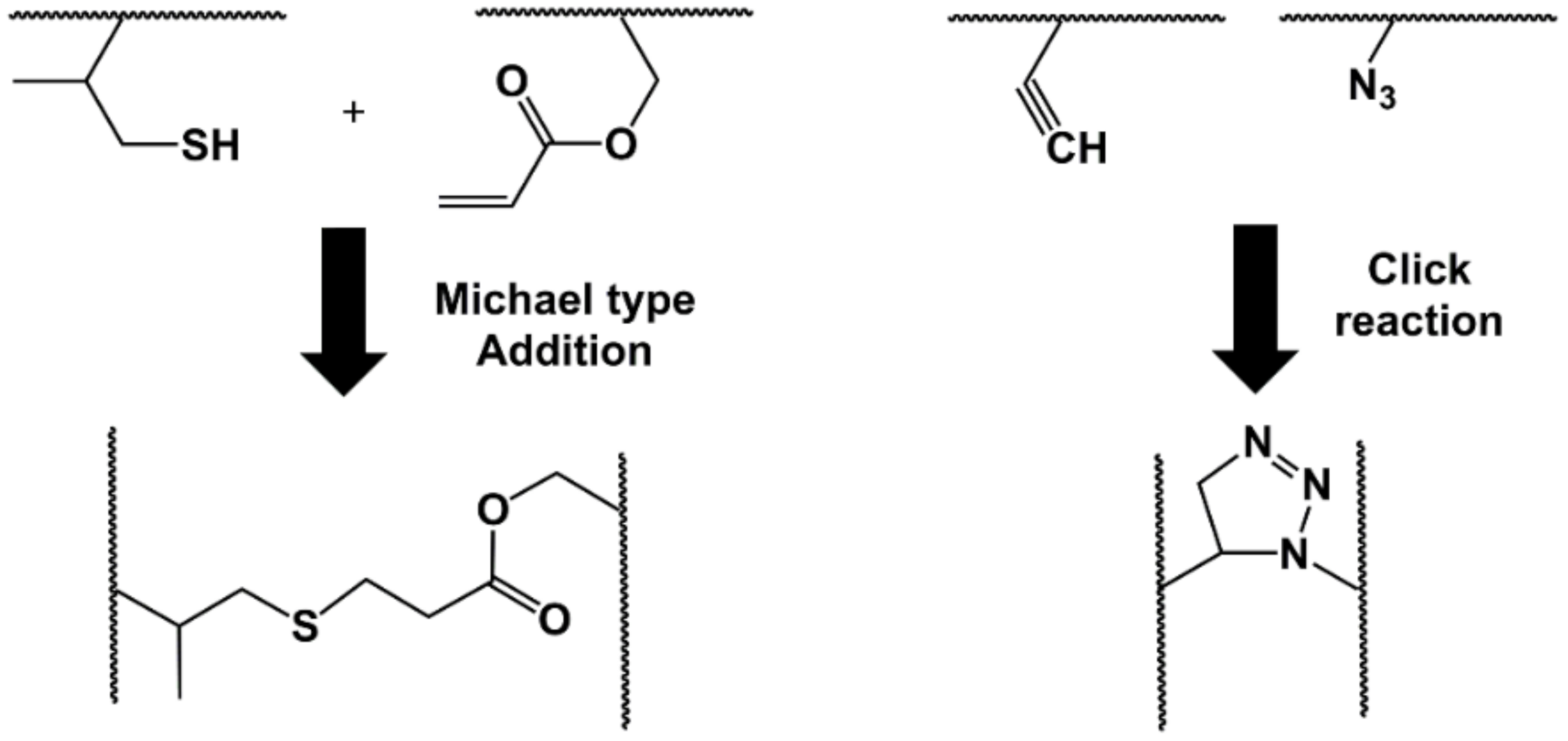
3.2. Reactive Functional Groups
3.3. Enzymatic Method
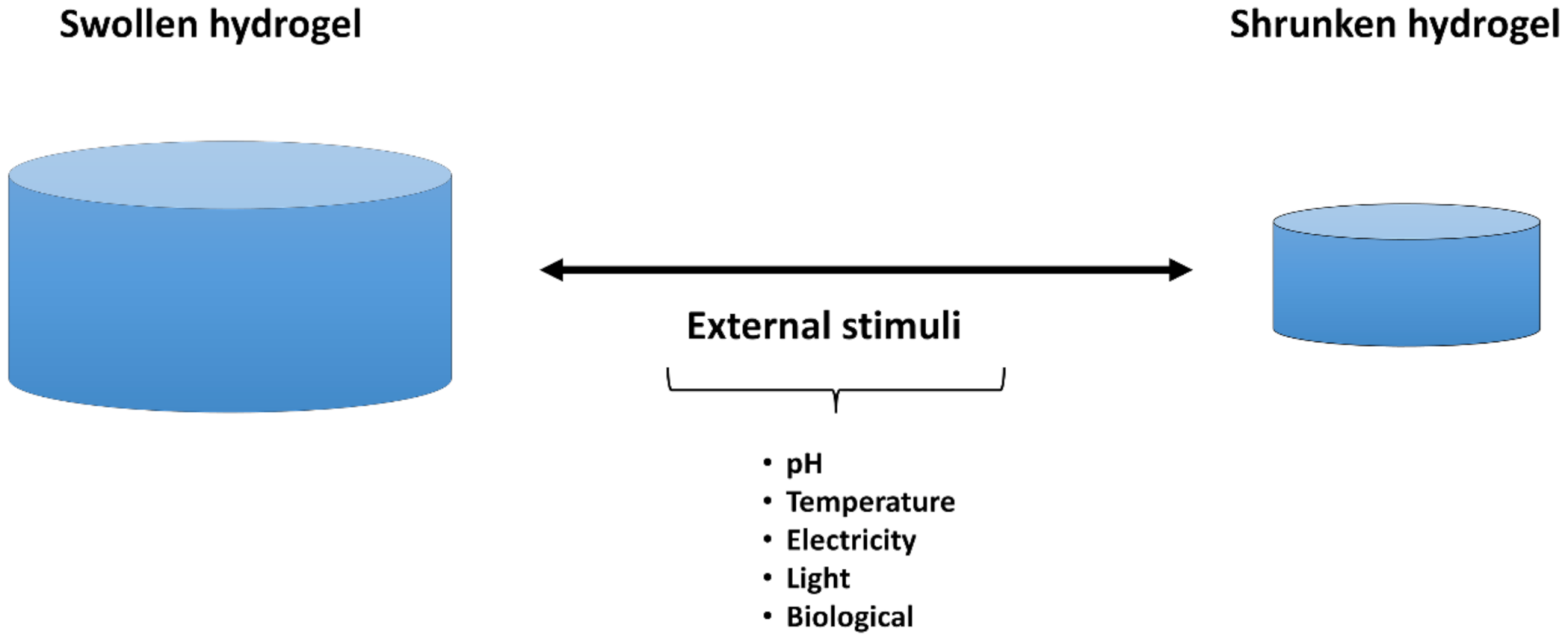
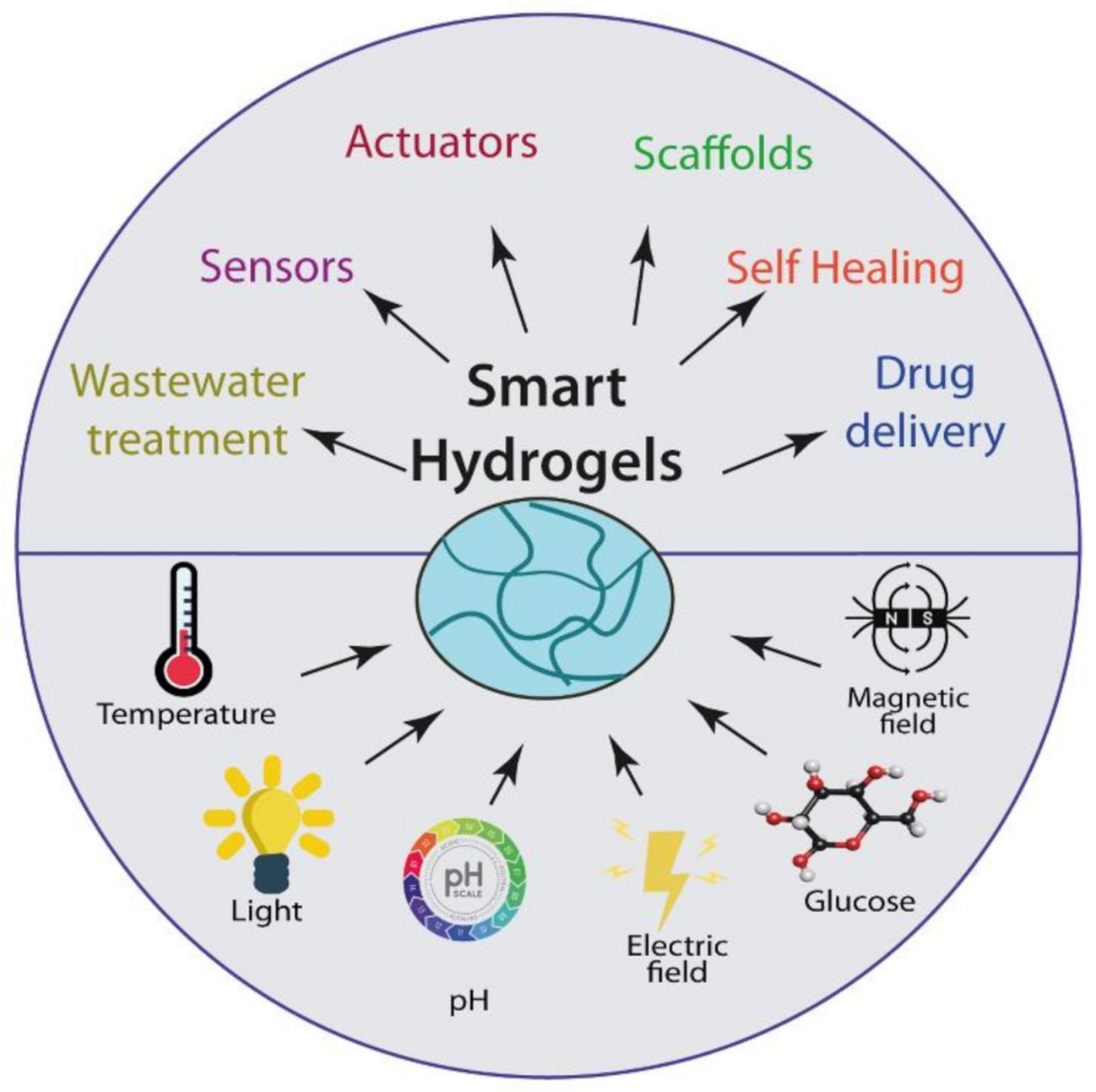
4. Smart Hydrogels
4.1. pH-Sensitive Hydrogels
4.2. Temperature-Sensitive Hydrogels
4.3. Electro-Sensitive Hydrogels
4.4. Photo-Sensitive Polymers
4.5. Enzyme-Sensitive Hydrogel
5. Applications of Smart Hydrogels
| Hydrogel Type | Potential Application | References |
|---|---|---|
| Temperature-responsive | Tissue engineering, drug delivery, imaging, wound dressing, sensors. | [140,160,161] |
| pH-responsive | Drug delivery, sensing, 3D cell culture, antibacterial, Wastewater treatment, drug delivery, tissue engineering. | [162,163,164,165] |
| Light-responsive | Microfluidic devices, drug delivery, soft robotic, actuators. | [166,167,168] |
| Magnetic-responsive | Cancer therapy, regenerative medicine, drug delivery. | [169,170] |
| Electro-responsive | Actuators, drug delivery. | [171,172] |
5.1. Drug Delivery Systems (DDS)
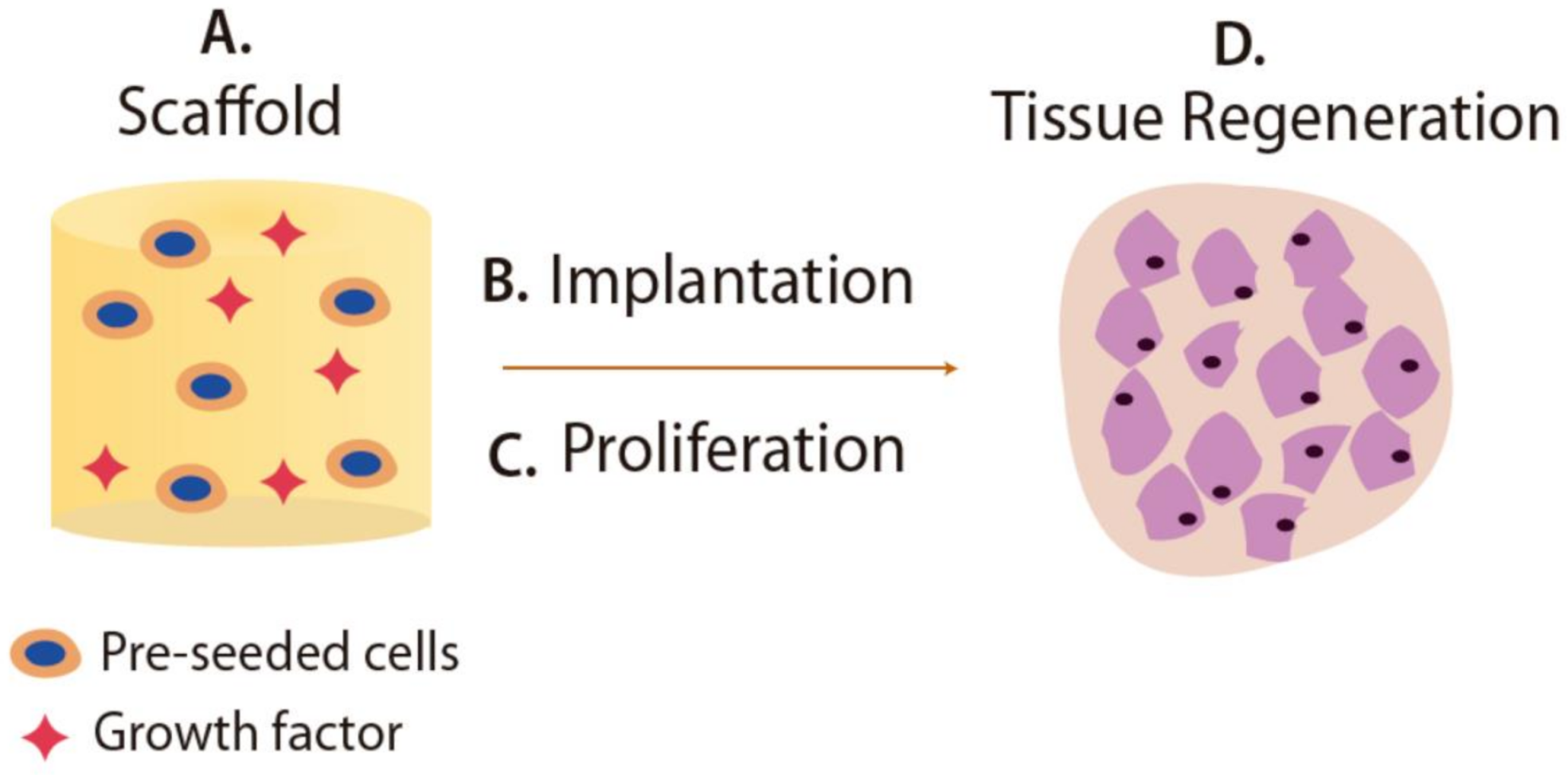
5.2. Scaffolds for Tissue Engineering
5.3. Actuators
5.4. Biosensors
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korah, L.; Anilkumar, G.; Thomas, S. Hydrogels, DNA, and RNA polypeptides for the preparation of biomaterials. In Fundamental Biomaterials: Polymers, 1st ed.; Thomas, S., Balakrishnan, P., Sreekala, M., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 85–104. ISBN 978-008-102-195-8. [Google Scholar]
- Lee, S.; Kwon, I.; Park, K. Hydrogels for delivery of bioactive agents: A historical perspective. Adv. Drug Deliv. Rev. 2013, 65, 17–20. [Google Scholar] [CrossRef]
- Kopeček, J. Hydrogel biomaterials: A smart future? Biomaterials 2007, 28, 5185–5192. [Google Scholar] [CrossRef]
- Yahia, L.; Chirani, N.; Yahia, L.; Gritsch, L.; Motta, F.; Chirani, S.; Fare, S. History and applications of hydrogels. J. Biomed. Sci. 2015, 4. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Ahmed, E. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Ayyala, D.; Blake, D.A.; John, V.T.; Ayyala, R.S. A glaucoma drainage device incorporating a slow-release drug delivery system for the management of fibrosis. In Biomaterials and Regenerative Medicine in Ophtalmology, 2nd ed.; Chirila, T.V., Harkin, D.G., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; pp. 349–367. ISBN 978-0-08-100147-9. [Google Scholar]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Pardo, S.; Bucio, E. Interaction between filler and polymeric matrix in nanocomposites: Magnetic approach and applications. Polymers 2021, 13, 2998. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef]
- Hoffman, A. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Ross-Murphy, S.; McEvoy, H. Fundamentals of hydrogels and gelation. Br. Polym. J. 1986, 18, 2–7. [Google Scholar] [CrossRef]
- Anderson, J.; Brannon, J. Concentration dependence of the distribution coefficient for macromolecules in porous media. J. Polym. Sci. Polym. Phys. Ed. 1981, 19, 405–421. [Google Scholar] [CrossRef]
- Janáček, J.; Stoy, A.; Stoy, V. Mechanical behavior of partially hydrolyzed preswollen polyacrylonitrile networks. J. Polym. Sci. Polym. Symp. 2007, 53, 299–312. [Google Scholar] [CrossRef]
- Sikdar, P.; Uddin, M.; Dip, T.; Islam, S.; Hoque, M.; Dhar, A.; Wu, S. Recent advances in the synthesis of smart hydrogels. Mater. Adv. 2021, 2, 4532–4573. [Google Scholar] [CrossRef]
- Schmidt, B. Hydrophilic polymers. Polymers 2019, 11, 693. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hoffman, A.S. Hydrogel. In Biomaterials Science: An Introduction to Materials in Medicine, 4th ed.; Wagner, W., Sakiyama-Elbert, S., Zhang, G., Yaszemski, M., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2020; pp. 153–166. [Google Scholar]
- Wang, C. Biodegradable polymer microparticles for genetic vaccine delivery. In Molecular Interfacial Phenomena of Polymers and Biopolymers, 1st ed.; Chen, P., Ed.; Woodhead Publishing: Sawston, UK, 2005; pp. 510–537. ISBN 9781855739284. [Google Scholar]
- Finch, C.A. Hydrophilic polymers. In Specialty Polymers; Dyson, R.W., Ed.; Springer: Boston, MA, USA, 1987; pp. 65–82. ISBN 978-1-4615-7894-9. [Google Scholar]
- Deng, F.; Luo, X.B.; Ding, L.; Luo, S. Application of nanomaterials and nanotechnology in the reutilization of metal ion from wastewater. In Nanomaterials for the Removal of Pollutants and Resource Reutilization, 1st ed.; Luo, D., Deng, F., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 149–178. ISBN 9780128148389. [Google Scholar]
- Peppas, N.; Slaughter, B.; Kanzelberger, M. Hydrogels. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier Science, BV: Amsterdam, The Netherlands, 2012; pp. 385–395. ISBN 978-0-08-087862-1. [Google Scholar]
- Liu, S.; Chen, X.; Zhang, Y. Hydrogels and hydrogel composites for 3D and 4D printing applications. In 3D and 4D Printing of Polymer Nanocomposite Materials; Sadasivuni, K.K., Deshmukh, K., Almaaded, M.A., Eds.; Elsevier Inc: Amsterdam, The Netherlands, 2020; pp. 427–465. ISBN 978-0-12-816805-9. [Google Scholar]
- Cyras, V.; D’Amico, D.; Manfredi, L. Crystallization behavior of polymer nanocomposites. In Crystallization in Multiphase Polymer Systems, 1st ed.; Thomas, S., Gowd, E.B., Arif, M., Kalarikkal, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 269–311. ISBN 978-0-12-809-453-2. [Google Scholar]
- Crawford, C.Q.; Quinn, B. Physiochemical properties and degradation. In Microplastic Pollutants; Crawford, C.Q., Quinn, B., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2017; pp. 57–100. ISBN 978-0-12-809406-8. [Google Scholar]
- Schulz, G. Protein crystallization. In Comprehensive Medicinal Chemistry II; Taylor, J., Triggle, D., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2007; pp. 433–446. ISBN 978-0-08-045044-5. [Google Scholar]
- Pan, H.; Jiang, S.; Zhang, T.; Tang, R. In situ solution study of calcium phosphate crystallization kinetics. Res. Methods Biominer. Sci. 2013, 532, 129–144. [Google Scholar] [CrossRef]
- Chan, C.; Li, L. Direct observation of the growth of lamellae and spherulites by AFM. In Intrinsic Molecular Mobility and Toughness of Polymers II, 1st ed.; Henning Kausch, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–41. ISBN 978-3-540-31601-5. [Google Scholar]
- Balani, K.; Verma, V.; Agarwal, A.; Narayan, R. Physical, thermal, and mechanical propeerties of polymers. In Biosurfaces: A Materials Science and Engineering Perspective, 1st ed.; Balani, K., Verma, V., Agarwal, A., Narayan, R., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 329–344. ISBN 978-111-895-062-3. [Google Scholar]
- Giannouli, P.; Morris, E. Cryogelation of xanthan. Food Hydrocoll. 2003, 17, 495–501. [Google Scholar] [CrossRef]
- Yokoyama, F.; Masada, I.; Shimamura, K.; Ikawa, T.; Monobe, K. Morphology and structure of highly elastic poly (vinyl alcohol) hydrogel prepared by repeated freezing-and-melting. Colloid Polym. Sci. 1986, 264, 595–601. [Google Scholar] [CrossRef]
- Takamura, A.; Ishii, F.; Hidaka, H. Drug release from poly (vinyl alcohol) gel prepared by freeze-thaw procedure. J. Control. Release 1992, 20, 21–27. [Google Scholar] [CrossRef]
- Hennink, W.; van Nostrum, C. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 13–36. [Google Scholar] [CrossRef]
- Slager, J.; Domb, A. Biopolymer stereocomplexes. Adv. Drug Deliv. Rev. 2003, 55, 549–583. [Google Scholar] [CrossRef]
- Ravve, A. Physical properties and physical chemistry of polymers. In Principles of Polymer Chemistry, 3rd ed.; Ravve, A., Ed.; Springer: New York, NY, USA, 2012; pp. 17–67. ISBN 978-1-4614-2212-9. [Google Scholar]
- Ikada, Y.; Jamshidi, K.; Tsuji, H.; Hyon, S. Stereocomplex formation between enantiomeric poly (lactides). Macromolecules 1987, 20, 904–906. [Google Scholar] [CrossRef]
- Slager, J.; Domb, A. Heterostereocomplexes prepared from D-PLA and L-PLA and leuprolide. II. Release of leuprolide. Biomacromolecules 2003, 4, 1316–1320. [Google Scholar] [CrossRef]
- Slager, J.; Domb, A. Heterostereocomplexes prepared from d-poly (lactide) and leuprolide. I. Characterization. Biomacromolecules 2003, 4, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Bos, G.; Jacobs, J.; Koten, J.; Van Tomme, S.; Veldhuis, T.; van Nostrum, C.; Den Otter, W.; Hennink, W. In situ crosslinked biodegradable hydrogels loaded with IL-2 are effective tools for local IL-2 therapy. Eur. J. Pharm. Sci. 2004, 21, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Bos, G.; Hennink, W.; Brouwer, L.; den Otter, W.; Veldhuis, T.; van Nostrum, C.; van Luyn, M. Tissue reactions of in situ formed dextran hydrogels crosslinked by stereocomplex formation after subcutaneous implantation in rats. Biomaterials 2005, 26, 3901–3909. [Google Scholar] [CrossRef]
- Chang, H.; Li, C.; Huang, R.; Su, R.; Qi, W.; He, Z. Amphiphilic hydrogels for biomedical applications. J. Mater. Chem. B 2019, 7, 2899–2910. [Google Scholar] [CrossRef]
- Förster, S.; Antonietti, M. Amphiphilic block copolymers in structure-controlled nanomaterial hybrids. Adv. Mater. 1999, 10, 195–217. [Google Scholar] [CrossRef]
- Jeong, B.; Bae, Y.; Kim, S. Thermoreversible gelation of PEG−PLGA−PEG triblock copolymer aqueous solutions. Macromolecules 1999, 32, 7064–7069. [Google Scholar] [CrossRef]
- Rashkov, I.; Manolova, N.; Li, S.; Espartero, J.; Vert, M. Synthesis, characterization, and hydrolytic degradation of PLA/PEO/PLA triblock copolymers with short poly (l-lactic acid) chains. Macromolecules 1996, 29, 50–56. [Google Scholar] [CrossRef]
- Li, S.; Rashkov, I.; Espartero, J.; Manolova, N.; Vert, M. Synthesis, characterization, and hydrolytic degradation of PLA/PEO/PLA triblock copolymers with long poly (l-lactic acid) blocks. Macromolecules 1996, 29, 57–62. [Google Scholar] [CrossRef]
- Molina, I.; Li, S.; Martinez, M.; Vert, M. Protein release from physically crosslinked hydrogels of the PLA/PEO/PLA triblock copolymer-type. Biomaterials 2001, 22, 363–369. [Google Scholar] [CrossRef]
- Bezemer, J.; Radersma, R.; Grijpma, D.; Dijkstra, P.; van Blitterswijk, C.; Feijen, J. Microspheres for protein delivery prepared from amphiphilic multiblock copolymers. 2. Modulation of release rate. J. Control. Release 2000, 67, 249–260. [Google Scholar] [CrossRef]
- Bezemer, J.; Radersma, R.; Grijpma, D.; Dijkstra, P.; van Blitterswijk, C.; Feijen, J. Microspheres for protein delivery prepared from amphiphilic multiblock copolymers. 1. Influence of preparation techniques on particle characteristics and protein delivery. J. Control. Release 2000, 67, 233–248. [Google Scholar] [CrossRef]
- Bezemer, J.; Grijpma, D.; Dijkstra, P.; van Blitterswijk, C.; Feijen, J. Control of protein delivery from amphiphilic poly (ether ester) multiblock copolymers by varying their water content using emulsification techniques. J. Control. Release 2000, 66, 307–320. [Google Scholar] [CrossRef]
- Bezemer, J.; Radersma, R.; Grijpma, D.; Dijkstra, P.; Feijen, J.; van Blitterswijk, C. Zero-order release of lysozyme from poly (ethylene glycol)/poly (butylene terephthalate) matrices. J. Control. Release 2000, 64, 179–192. [Google Scholar] [CrossRef]
- Bezemer, J.; Grijpma, D.; Dijkstra, P.; van Blitterswijk, C.; Feijen, J. A controlled release system for proteins based on poly (ether ester) block-copolymers: Polymer network characterization. J. Control. Release 1999, 62, 393–405. [Google Scholar] [CrossRef]
- García, L.; Aguilar, M.R.; Román, J.S. Biodegradable hydrogels for controlled drug release. In Biomedical Applications of Hydrogels Handbook, 1st ed.; Ottenbrite, R., Park, K., Okano, T., Eds.; Springer: New York, NY, USA, 2010; pp. 147–155. ISBN 978-1-4419-5919-5. [Google Scholar]
- Hoare, T.; Kohane, D. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Frutos, G.; Prior-Cabanillas, A.; París, R.; Quijada-Garrido, I. A system novel controlled drug delivery based on pH-responsive hydrogels included in soft gelatin capsules. Acta Biomater. 2010, 6, 4650–4656. [Google Scholar] [CrossRef]
- Guiseppi-Elie, A. Electroconductive hydrogels: Synthesis, characterization and biomedical applications. Biomaterials 2010, 31, 2701–2716. [Google Scholar] [CrossRef] [PubMed]
- Akiyoshi, K.; Deguchi, S.; Tajima, H.; Nishikawa, T.; Sunamoto, J. Microscopic Structure and thermoresponsiveness of a hydrogel nanoparticle by self-assembly of a hydrophobized polysaccharide. Macromolecules 1997, 30, 857–861. [Google Scholar] [CrossRef]
- Akiyoshi, K.; Kobayashi, S.; Shichibe, S.; Mix, D.; Baudys, M.; Wan Kim, S.; Sunamoto, J. Self-assembled hydrogel nanoparticle of cholesterol-bearing pullulan as a carrier of protein drugs: Complexation and stabilization of insulin. J. Control. Release 1998, 54, 313–320. [Google Scholar] [CrossRef]
- Akiyoshi, K.; Deguchi, S.; Moriguchi, N.; Yamaguchi, S.; Sunamoto, J. Self-aggregates of hydrophobized polysaccharides in water. formation and characteristics of nanoparticles. Macromolecules 1993, 26, 3062–3068. [Google Scholar] [CrossRef]
- Akiyoshi, K.; Taniguchi, I.; Fukui, H.; Sunamoto, J. Hydrogel nanoparticle formed by self-assembly of hydrophobized polysaccharide. Stabilization of adriamycin by complexation. Eur. J. Pharm. Biopharm. 1996, 42, 286–290. [Google Scholar]
- Uchegbu, I.; Schätzlein, A.; Tetley, L.; Gray, A.; Sludden, J.; Siddique, S.; Mosha, E. Polymeric chitosan-based vesicles for drug delivery. J. Pharm. Pharmacol. 2011, 50, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Sludden, J.; Uchegbu, I.; Schätzlein, A. The encapsulation of bleomycin within chitosan based polymeric vesicles does not alter its biodistribution. J. Pharm. Pharmacol. 2010, 52, 377–382. [Google Scholar] [CrossRef]
- Qu, X.; Wirsén, A.; Albertsson, A. Structural change and swelling mechanism of ph-sensitive hydrogels based on chitosan andd, L-lactic acid. J. Appl. Polym. Sci. 1999, 74, 3186–3192. [Google Scholar] [CrossRef]
- Qu, X.; Wirsén, A.; Albertsson, A. Novel Ph-sensitive chitosan hydrogels: Swelling behavior and states of water. Polymer 2000, 41, 4589–4598. [Google Scholar] [CrossRef]
- Yazdani-Pedram, M.; Retuert, J.; Quijada, R. Hydrogels based on modified chitosan, 1. Synthesis and swelling behavior of poly (acrylic acid) grafted chitosan. Macromol. Chem. Phys. 2000, 201, 923–930. [Google Scholar] [CrossRef]
- Kim, S.; Cho, S.; Lee, Y.; Kim, S. Thermo-and ph-responsive behaviors of graft copolymer and blend based on chitosan andn-isopropylacrylamide. J. Appl. Polym. Sci. 2000, 78, 1381–1391. [Google Scholar] [CrossRef]
- Huh, K.; Hashi, J.; Ooya, T.; Yui, N. Synthesis and characterization of dextran grafted with poly (n-isopropylacrylamide-co-n,n-dimethyl-acrylamide). Macromol. Chem. Phys. 2000, 201, 613–619. [Google Scholar] [CrossRef]
- Na, K.; Park, K.; Kim, S.; Bae, Y. Self-assembled hydrogel nanoparticles from curdlan derivatives: Characterization, anti-cancer drug release and interaction with a hepatoma cell line (Hepg2). J. Controlled Release 2000, 69, 225–236. [Google Scholar] [CrossRef]
- Prado, H.; Matulewicz, M.; Bonelli, P.; Cukierman, A. Preparation and characterization of a novel starch-based interpolyelectrolyte complex as matrix for controlled drug release. Carbohydr. Res. 2009, 344, 1325–1331. [Google Scholar] [CrossRef]
- Gao, Y.; Wei, Z.; Li, F.; Mao, Z.; Mei, Y.; Zrinyib, M.; Osada, Y. Synthesis of a morphology controllable Fe3O4 nanoparticle/hydrogel magnetic nanocomposite inspired by magnetotactic bacteria and its application in H2O2 detection. Green Chem. 2014, 16, 1255–1261. [Google Scholar] [CrossRef]
- Katayama, S.; Hirokawa, Y.; Tanaka, T. Reentrant phase transition in acrylamide-derivative copolymer gels. Macromolecules 1984, 17, 2641–2643. [Google Scholar] [CrossRef]
- You, Y.; Yang, J.; Zheng, Q.; Wu, N.; Lv, Z.; Jiang, Z. Ultra-stretchable hydrogels with hierarchical hydrogen bonds. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Yoshimura, R.; Seki, C.; Fujioka, R. Synthesis and characterization of biodegradable hydrogels based on starch and succinic anhydride. Carbohydr. Polym. 2006, 64, 345–349. [Google Scholar] [CrossRef]
- Mehyar, G.; Liu, Z.; Han, J. Dynamics of antimicrobial hydrogels in physiological saline. Carbohydr. Polym. 2008, 74, 92–98. [Google Scholar] [CrossRef]
- Tirrell, J.; Tirrell, D. Synthesis of biopolymers: Proteins, polyesters, polysaccharides and polynucleotides. Curr. Opin. Solid State Mater. Sci. 1996, 1, 407–411. [Google Scholar] [CrossRef]
- McGrath, K.; Fournier, M.; Mason, T.; Tirrell, D. Genetically directed syntheses of new polymeric materials. expression of artificial genes encoding proteins with repeating-(alagly) 3proglugly-elements. J. Am. Chem. Soc. 1992, 114, 727–733. [Google Scholar] [CrossRef]
- Cappello, J.; Crissman, J.; Dorman, M.; Mikolajczak, M.; Textor, G.; Marquet, M.; Ferrari, F. Genetic engineering of structural protein polymers. Biotechnol. Prog. 1990, 6, 198–202. [Google Scholar] [CrossRef]
- Cappello, J.; Crissman, J.; Crissman, M.; Ferrari, F.; Textor, G.; Wallis, O.; Whitledge, J.; Zhou, X.; Burman, D.; Aukerman, L.; et al. In-situ self-assembling protein polymer gel systems for administration, delivery, and release of drugs. J. Controlled Release 1998, 53, 105–117. [Google Scholar] [CrossRef]
- Petka, W.; Harden, J.; Mcgrath, K.; Wirtz, D.; Tirrel, D. Reversible hydrogels from self-assembling artificial proteins. Science 1998, 281, 389–392. [Google Scholar] [CrossRef]
- Miyata, T.; Asami, N.; Uragami, T. A Reversibly antigen-responsive hydrogel. Nature 1999, 399, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Muñoz, F.; Ruiz, J.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Temperature-and Ph-sensitive interpenetrating polymer networks grafted on PP: Cross-linking irradiation dose as a critical variable for the performance as vancomycin-eluting systems. Radiat. Phys. Chem. 2012, 81, 531–540. [Google Scholar] [CrossRef]
- Meléndez-Ortiz, H.; Alvarez-Lorenzo, C.; Concheiro, A.; Jiménez-Páez, V.; Bucio, E. Modification of medical grade PVC with N-vinylimidazole to obtain bactericidal surface. Radiat. Phys. Chem. 2016, 119, 37–43. [Google Scholar] [CrossRef]
- Romero-Fierro, D.; Camacho-Cruz, L.; Bustamante-Torres, M.; Hidalgo-Bonilla, S.; Bucio, E. Modification of cotton gauzes with poly (acrylic acid) and poly (methacrylic acid) using gamma radiation for drug loading studies. Radiat. Phys. Chem. 2022, 190, 109787. [Google Scholar] [CrossRef]
- El-Hag Ali, A.; AlArifi, A. Characterization and in vitro evaluation of starch based hydrogels as carriers for colon specific drug delivery systems. Carbohydr. Polym. 2009, 78, 725–730. [Google Scholar] [CrossRef]
- Eid, M. In vitro release studies of vitamin B12 from poly N-vinyl pyrrolidone/starch hydrogels grafted with acrylic acid synthesized by gamma radiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2008, 266, 5020–5026. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Pino-Ramos, V.H.; Romero-Fierro, D.; Hidalgo-Bonilla, S.P.; Magaña, H.; Bucio, E. Synthesis and antimicrobial properties of highly cross-linked pH-sensitive hydrogels through gamma radiation. Polymers 2021, 13, 2223. [Google Scholar] [CrossRef]
- Wichterle, O.; Lím, D. Hydrophilic gels for biological use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Edman, P.; Ekman, B.; Sjöholm, I. Immobilization of proteins in microspheres of biodegradable polyacryldextran. J. Pharm. Sci. 1980, 69, 838–842. [Google Scholar] [CrossRef]
- Sturesson, C.; Degling Wikingsson, L. Comparison of poly (acryl starch) and poly (lactide-co-glycolide) microspheres as drug delivery system for a rotavirus vaccine. J. Controlled Release 2000, 68, 441–450. [Google Scholar] [CrossRef]
- Heller, J.; Pangburn, S.; Roskos, K. Development of enzymatically degradable protective coatings for use in triggered drug delivery systems: Derivatized starch hydrogels. Biomaterials 1990, 11, 345–350. [Google Scholar] [CrossRef]
- Artursson, P.; Edman, P.; Laakso, T.; Sjöholm, I. Characterization of polyacryl starch microparticles as carriers for proteins and drugs. J. Pharm. Sci. 1984, 73, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Giammona, G.; Pitarresi, G.; Cavallaro, G.; Spadaro, G. New biodegradable hydrogels based on an acryloylated polyaspartamide cross-linked by gamma irradiation. J. Biomater. Sci. Polym. Ed. 1999, 10, 969–987. [Google Scholar] [CrossRef]
- Giammona, G.; Pitarresi, G.; Cavallaro, G.; Buscemi, S.; Saiano, F. New biodegradable hydrogels based on a photocrosslinkable modified polyaspartamide: Synthesis and characterization. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1999, 1428, 29–38. [Google Scholar] [CrossRef]
- Giammona, G.; Tomarchio, V.; Pitarresi, G.; Cavallaro, G. Glycidyl methacrylate derivatization Of A,Β-poly (N-hydroxyethyl)-Dl-aspartamide and A,Β-polyasparthydrazide. Polymer 1997, 38, 3315–3323. [Google Scholar] [CrossRef]
- Martens, P.; Anseth, K. Characterization of hydrogels formed from acrylate modified poly (vinyl alcohol) macromers. Polymer 2000, 41, 7715–7722. [Google Scholar] [CrossRef]
- Jin, Y.; Yamanaka, J.; Sato, S.; Miyata, I.; Yomota, C.; Yonese, M. Characteristics of hyaluronate-hydroxyethyl acrylate blend gel and release of cationic amphiphilic solutes. Chem. Pharm. Bull. 2002, 50, 1341–1348. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Metters, A.; Anseth, K.; Bowman, C. Fundamental studies of a novel, biodegradable PEG-B-PLA hydrogel. Polymer 2000, 41, 3993–4004. [Google Scholar] [CrossRef]
- Kim, S.; Chu, C. Synthesis and characterization of dextran-methacrylate hydrogels and structural study by SEM. J. Biomed. Mater. Res. 2000, 49, 517–527. [Google Scholar] [CrossRef]
- Hubbell, J. Hydrogel systems for barriers and local drug delivery in the control of wound healing. J. Controlled Release 1996, 39, 305–313. [Google Scholar] [CrossRef]
- Andreopoulos, F.; Beckman, E.; Russell, A. Light-induced tailoring of PEG-hydrogel properties. Biomaterials 1998, 19, 1343–1352. [Google Scholar] [CrossRef]
- Ward, J.; Peppas, N. Preparation of controlled release systems by free-radical UV polymerizations in the presence of a drug. J. Controlled Release 2001, 71, 183–192. [Google Scholar] [CrossRef]
- Omidian, H.; Park, K. Introduction to hydrogels. In Biomedical Applications of Hydrogels Handbook; Ottenbrite, R., Park, K., Okano, T., Eds.; Springer: New York, NY, USA, 2010; pp. 1–15. ISBN 978-1-4419-5918-8. [Google Scholar]
- Gulrez, H.S.; Al-Assaf, S.; Phillips, G.O. Hydrogels: Methods of preparation, characterisation and applications. In Progress in Molecular and Environmental Bioengineering—From Analysis and Modeling to Technology Applications; Carpi, A., Ed.; IntechOpen Book Series: New Delhi, India, 2011; ISBN 978-953-307-268-5. [Google Scholar]
- Balakrishnan, B.; Mohanty, M.; Umashankar, P.; Jayakrishnan, A. Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials 2005, 26, 6335–6342. [Google Scholar] [CrossRef]
- Tortora, M.; Cavalieri, F.; Chiessi, E.; Paradossi, G. Michael-type addition reactions for the in situ formation of poly (vinyl alcohol)-based hydrogels. Biomacromolecules 2007, 8, 209–214. [Google Scholar] [CrossRef]
- Jung, J.; Jones, J.; Cronier, S.; Collier, J. Modulating the mechanical properties of self-assembled peptide hydrogels via native chemical ligation. Biomaterials 2008, 29, 2143–2151. [Google Scholar] [CrossRef]
- Koschella, A.; Hartlieb, M.; Heinze, T. A “Click-Chemistry” approach to cellulose-based hydrogels. Carbohydr. Polym. 2011, 86, 154–161. [Google Scholar] [CrossRef]
- Willmott, N.; Cummings, J.; Stuart, J.; Florence, A. Adriamycin-loaded albumin microspheres: Preparation, in vivo distribution and release in the rat. Biopharm. Drug Dispos. 1985, 6, 91–104. [Google Scholar] [CrossRef]
- Olde Damink, L.; Dijkstra, P.; Van Luyn, M.; Van Wachem, P.; Nieuwenhuis, P.; Feijen, J. Glutaraldehyde as a crosslinking agent for collagen-based biomaterials. J. Mater. Sci. Mater. Med. 1995, 6, 460–472. [Google Scholar] [CrossRef]
- Tabata, Y.; Nagano, A.; Muniruzzaman, M.; Ikada, Y. In vitro sorption and desorption of basic fibroblast growth factor from biodegradable hydrogels. Biomaterials 1998, 19, 1781–1789. [Google Scholar] [CrossRef]
- Monteiro, O.; Airoldi, C. Some studies of crosslinking chitosan–glutaraldehyde interaction in a homogeneous system. Int. J. Biol. Macromol. 1999, 26, 119–128. [Google Scholar] [CrossRef]
- Jameela, S.; Jayakrishnan, A. Glutaraldehyde cross-linked chitosan microspheres as a long acting biodegradable drug delivery vehicle: Studies on the in vitro release of mitoxantrone and in vivo degradation of microspheres in rat muscle. Biomaterials 1995, 16, 769–775. [Google Scholar] [CrossRef]
- Cunha, P.; Castro, R.; Rocha, F.; de Paula, R.; Feitosa, J. Low viscosity hydrogel of guar gum: Preparation and physicochemical characterization. Int. J. Biol. Macromol. 2005, 37, 99–104. [Google Scholar] [CrossRef]
- Kulkarni, A.; Soppimath, K.; Aminabhavi, T. Controlled release of diclofenac sodium from sodium alginate beads crosslinked with glutaraldehyde. Pharm. Acta Helv. 1999, 74, 29–36. [Google Scholar] [CrossRef]
- Jin, R.; Dijkstra, P.J. Hydrogels for tissue engineering applications. In Biomedical Applications of Hydrogels Handbook; Ottenbrite, R.M., Park, K., Okano, T., Eds.; Springer: New York, NY, USA, 2010; pp. 247–268. ISBN 978-1-4419-5918-8. [Google Scholar]
- Simonsen, L.; Hovgaard, L.; Mortensen, P.; Brøndsted, H. Dextran hydrogels for colon-specific drug delivery. V. degradation in human intestinal incubation models. Eur. J. Pharm. Sci. 1995, 3, 329–337. [Google Scholar] [CrossRef]
- Gehrke, S.; Uhden, L.; McBride, J. Enhanced loading and activity retention of bioactive proteins in hydrogel delivery systems. J. Controlled Release 1998, 55, 21–33. [Google Scholar] [CrossRef]
- Coviello, T.; Grassi, M.; Rambone, G.; Santucci, E.; Carafa, M.; Murtas, E.; Riccieri, F.; Alhaique, F. Novel hydrogel system from scleroglucan: Synthesis and characterization. J. Controlled Release 1999, 60, 367–378. [Google Scholar] [CrossRef]
- Kuijpers, A.; van Wachem, P.; van Luyn, M.; Engbers, G.; Krijgsveld, J.; Zaat, S.; Dankert, J.; Feijen, J. In vivo and in vitro release of lysozyme from cross-linked gelatin hydrogels: A model system for the delivery of antibacterial proteins from prosthetic heart valves. J. Controlled Release 2000, 67, 323–336. [Google Scholar] [CrossRef]
- de Nooy, A.; Capitani, D.; Masci, G.; Crescenzi, V. Ionic polysaccharide hydrogels via the passerini and ugi multicomponent condensations: Synthesis, behavior and solid-state NMR characterization. Biomacromolecules 2000, 1, 259–267. [Google Scholar] [CrossRef]
- de Nooy, A.; Masci, G.; Crescenzi, V. Versatile synthesis of polysaccharide hydrogels using the passerini and ugi multicomponent condensations. Macromolecules 1999, 32, 1318–1320. [Google Scholar] [CrossRef]
- Chen, T.; Embree, H.; Brown, E.; Taylor, M.; Payne, G. Enzyme-catalyzed gel formation of gelatin and chitosan: Potential for in situ applications. Biomaterials 2003, 24, 2831–2841. [Google Scholar] [CrossRef]
- Jones, A.; Vaughan, D. Hydrogel dressings in the management of a variety of wound types: A review. J. Orthop. Nurs. 2005, 9, 1–11. [Google Scholar] [CrossRef]
- Sperinde, J.; Griffith, L. Control and prediction of gelation kinetics in enzymatically cross-linked poly (ethylene glycol) hydrogels. Macromolecules 2000, 33, 5476–5480. [Google Scholar] [CrossRef]
- Kuhn, W. Reversible Dehnung Und Kontraktion Bei Änderung Der Ionisation Eines Netzwerks Polyvalenter Fadenmolekülionen. Experientia 1949, 5, 318–319. [Google Scholar] [CrossRef] [PubMed]
- Hazra, C.; Kundu, D.; Chatterjee, A. Stimuli-responsive nanocomposites for drug delivery. In Applications of Nanocomposite Materials in Drug Delivery; Inamuddin, A., Asiri, A.M., Mohammad, A., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 827–846. ISBN 978-0-12-813741-3. [Google Scholar]
- Stuart, M.; Huck, W.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.; Szleifer, I.; Tsukruk, V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef]
- Sullad, A.; Manjeshwar, L.; Aminabhavi, T. Novel Ph-sensitive hydrogels prepared from the blends of poly (vinyl alcohol) with acrylic acid-graft-guar gum matrixes for isoniazid delivery. Ind. Eng. Chem. Res. 2010, 49, 7323–7329. [Google Scholar] [CrossRef]
- You, J.; Almeda, D.; Ye, G.; Auguste, D. Bioresponsive matrices in drug delivery. J. Biol. Eng. 2010, 4, 15. [Google Scholar] [CrossRef]
- Peppas, N.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Schoener, C.; Hutson, H.; Peppas, N. Ph-responsive hydrogels with dispersed hydrophobic nanoparticles for the delivery of hydrophobic therapeutic agents. Polym. Int. 2012, 61, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Grainger, S.J.; El-Sayed, M.E. Stimuli-sensitive particles for drug delivery. In Biologically-Responsive Hybrid Biomaterials, 1st ed.; Jabbari, E., Khademhosseini, A., Eds.; World Scientific: Singapore, 2010; pp. 171–190. ISBN 978-981-4295-67-3. [Google Scholar]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Dou, X.; Yang, X.; Li, P.; Zhang, Z.; Schönherr, H.; Zhang, D.; Feng, C. Novel Ph responsive hydrogels for controlled cell adhesion and triggered surface detachment. Soft Matter 2012, 8, 9539–9544. [Google Scholar] [CrossRef]
- Krogsgaard, M.; Behrens, M.; Pedersen, J.; Birkedal, H. Self-healing mussel-inspired multi-Ph-responsive hydrogels. Biomacromolecules 2013, 14, 297–301. [Google Scholar] [CrossRef]
- Van der Linden, H.; Westerweel, J. Temperature-sensitive hydrogels. In Encyclopedia of Microfluidics and Nanofluidics, 2nd ed.; Li, D., Ed.; Springer: New York, NY, USA, 2015; pp. 1–5. ISBN 978-1-4614-5488-5. [Google Scholar]
- Bajpai, A.; Shukla, S.; Bhanu, S.; Kankane, S. Responsive polymers in controlled drug delivery. Prog. Polym. Sci. 2008, 33, 1088–1118. [Google Scholar] [CrossRef]
- Bergueiro, J.; Calderón, M. Thermoresponsive nanodevices in biomedical applications. Macromol. Biosci. 2014, 15, 183–199. [Google Scholar] [CrossRef] [PubMed]
- Matanović, M.; Kristl, J.; Grabnar, P. Thermoresponsive polymers: Insights into decisive hydrogel characteristics, mechanisms of gelation, and promising biomedical applications. Int. J. Pharm. 2014, 472, 262–275. [Google Scholar] [CrossRef]
- Ji, Y.; Zhu, M.; Gong, Y.; Tang, H.; Li, J.; Cao, Y. Thermoresponsive polymers with lower critical solution temperature-or upper critical solution temperature-type phase behaviour do not induce toxicity to human endothelial cells. Basic Clin. Pharmacol. Toxicol. 2016, 120, 79–85. [Google Scholar] [CrossRef]
- Constantin, M.; Cristea, M.; Ascenzi, P.; Fundueanu, G. Lower critical solution temperature versus volume phase transition temperature in thermoresponsive drug delivery systems. Express Polym. Lett. 2011, 5, 839–848. [Google Scholar] [CrossRef]
- Cook, M.; Haddow, P.; Kirton, S.; McAuley, W. Polymers exhibiting lower critical solution temperatures as a route to thermoreversible gelators for healthcare. Adv. Funct. Mater. 2020, 31, 2008123. [Google Scholar] [CrossRef]
- Huang, H.; Qi, X.; Chen, Y.; Wu, Z. Thermo-sensitive hydrogels for delivering biotherapeutic molecules: A review. Saudi Pharm. J. 2019, 27, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Klouda, L.; Mikos, A. Thermoresponsive hydrogels in biomedical applications. Eur. J. Pharm. Biopharm. 2008, 68, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Manouras, T.; Vamvakaki, M. Field responsive materials: Photo-, electro-, magnetic- and ultrasound-sensitive polymers. Polym. Chem. 2017, 8, 74–96. [Google Scholar] [CrossRef]
- Simya, O.; Radhakrishnan, P.; Ashok, A. Engineered nanomaterials for energy applications. In Handbook of Nanomaterials For Industrial Applications, 1st ed.; Hussain, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 751–767. ISBN 9780128133521. [Google Scholar]
- Shahinpoor, M. Muscular biopolymers. In Engineered Biomimicry; Lakhtakia, A., Martín-Palma, R.J., Eds.; Elsevier Inc: Amsterdam, The Netherlands, 2013; pp. 139–160. ISBN 978-0-12-415995-2. [Google Scholar]
- Purkait, M.; Sinha, M.; Mondal, P.; Singh, R. Electric field-responsive membranes. In Interface Science and Technology, 1st ed.; Purkait, M., Sinha, M., Mondal, P., Singh, R., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 173–191. ISBN 9780128139622. [Google Scholar]
- Truong, B.; Ahn, K. Modeling and control of hysteresis for DEAP actuator. Sens. Actuators A Phys. 2013, 201, 193–206. [Google Scholar] [CrossRef]
- Jiang, L.; Wen, L. Photonic sensitive switchable materials. In Biophotonics for Medical Applications; Meglinski, I., Ed.; Woodhead Publishing: Sawston, UK; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; pp. 25–51. ISBN 978-0-85709-662-3. [Google Scholar]
- Xiong, X.; del Campo, A.; Cui, J. Photoresponsive polymers. In Smart Polymers and Their Applications, 2nd ed.; Aguilar, M.R., San Román, J., Eds.; Woodhead Publishing: Sawston, UK; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; pp. 87–153. ISBN 978-0-08-102416-4. [Google Scholar]
- Bastakoti, B.; Liu, Z. Multifunctional polymeric micelles as therapeutic nanostructures: Targeting, imaging, and triggered release. In Nanostructures for Cancer Therapy; Ficai, A., Grumezescu, A.M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 261–283. ISBN 978-0-323-46144-3. [Google Scholar]
- Kuckling, D.; Doering, A.; Krahl, F.; Arndt, K. Stimuli-responsive polymer systems. In Polymer Science: A Comprehensive Reference, 1st ed.; Moeller, M., Matyjaszewski, K., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2012; pp. 377–413. ISBN 978-008-087-862-1. [Google Scholar]
- Upadhyay, K.; Thomas, S.; Tamrakar, R.; Kalarikkal, N. Functionalized photo-responsive polymeric system. In Advanced Functional Polymers for Biomedical Applications; Mozafari, M., Singh, N.P., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 211–233. ISBN 978-0-12-816349-8. [Google Scholar]
- Reduwan, S.M.; Mondal, I.H.; Somoal, S.H.; Pervez, M.; Haque, M. Enzyme-responsibe hydrogels. In Polymers and Polymeric Composites: A Reference Series, 1st ed.; Mondal, I.H., Ed.; Springer: Cham, Switzerland, 2019; pp. 309–330. ISBN 978-3-319-77830-3. [Google Scholar]
- Ulijn, R.; Bibi, N.; Jayawarna, V.; Thornton, P.; Todd, S.; Mart, R.; Smith, A.; Gough, J. Bioresponsive hydrogels. Mater. Today 2007, 10, 40–48. [Google Scholar] [CrossRef]
- Chandrawati, R. Enzyme-responsive polymer hydrogels for therapeutic delivery. Exp. Biol. Med. 2016, 241, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Sannino, A.; Madaghiele, M.; Ambrosio, L. 5 Biocompatibility and other properties of hydrogels in regenerative medicine. Cell. Response Biomater. 2009, 114–135. [Google Scholar] [CrossRef]
- Tang, J.; Javaid, M.U.; Pan, C.; Yu, G.; Berry, R.M.; Tam, K.C. Self-healing stimuli-responsive cellulose nanocrystal hydrogels. Carbohydr. Polym. 2020, 229, 115486. [Google Scholar] [CrossRef]
- Guo, Y.; Bae, J.; Zhao, F.; Yu, G. Functional hydrogels for next-generation batteries and supercapacitors. Trends Chem. 2019, 1, 335–348. [Google Scholar] [CrossRef]
- Pushpamalar, J.; Langford, S.J.; Ahmad, M.B.; Lim, Y.Y.; Hashim, K. Eco-friendly smart hydrogels for soil conditioning and sustain release fertilizer. Int. J. Environ. Sci. Technol. 2017, 15, 2059–2074. [Google Scholar] [CrossRef]
- Shah, L.A.; Khan, S.A. Polymer hydrogels for wastewater treatment. In Environmental Chemistry and Recent Pollution Control Approaches; Saldarriaga-Noreña, H., Ed.; IntechOpen Book Series: London, UK, 2019; ISBN 978-1-83968-064-9. [Google Scholar]
- Zubik, K.; Singhsa, P.; Wang, Y.; Manuspiya, H.; Narain, R. Thermo-responsive poly (N-isopropylacrylamide)-cellulose nanocrystals hybrid hydrogels for wound dressing. Polymers 2017, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Shriky, B.; Kelly, A.; Isreb, M.; Babenko, M.; Mahmoudi, N.; Rogers, S.; Shebanova, O.; Snow, T.; Gough, T. Pluronic F127 thermosensitive injectable smart hydrogels for controlled drug delivery system development. J. Colloid Interface Sci. 2020, 565, 119–130. [Google Scholar] [CrossRef]
- Park, S.H.; Shin, H.S.; Park, S.N. A novel pH-responsive hydrogel based on carboxymethyl cellulose/2-hydroxyethyl acrylate for transdermal delivery of naringenin. Carbohydr. Polym. 2018, 200, 341–352. [Google Scholar] [CrossRef]
- Wan, Y.; Liu, L.; Yuan, S.; Sun, J.; Li, Z. pH-Responsive peptide supramolecular hydrogels with antibacterial activity. Langmuir 2017, 33, 3234–3240. [Google Scholar] [CrossRef] [PubMed]
- Rehman, T.U.; Shah, L.A.; Khan, M.; Irfan, M.; Khattak, N.S. Zwitterionic superabsorbent polymer hydrogels for efficient and selective removal of organic dyes. RSC Adv. 2019, 9, 18565–18577. [Google Scholar] [CrossRef]
- García, M.C. Ionic-strength-responsive polymers for drug delivery applications. In Stimuli Responsive Polymeric Nanocarriers for drug Delivery Application; Woodhead Publishing: Sawston, UK, 2019; pp. 393–409. [Google Scholar] [CrossRef]
- Al-Aribe, K.; Knopf, G.K.; Bassi, A.S. Photo-responsive hydrogel for controlling flow on a microfluidic chip. Int. Soc. Opt. Photonics 2006, 6343, 872–880. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Zhang, Q.; Cheng, Y. Near infrared light-responsive and injectable supramolecular hydrogels for on-demand drug delivery. Chem. Commun. 2016, 52, 978–981. [Google Scholar] [CrossRef]
- Ding, M.; Jing, L.; Yang, H.; Machnicki, C.E.; Fu, X.; Li, K.; Wong, I.Y.; Chen, P.Y. Multifunctional soft machines based on stimuli-responsive hydrogels: From freestanding hydrogels to smart integrated systems. Mater. Today Adv. 2020, 8, 100088. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Chen, C.; Cheng, Y. Magnetic-responsive hydrogels: From strategic design to biomedical applications. J. Control. Release 2021, 335, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, J.; Cui, X.; Wang, X.; Zhang, L.; Tang, P. Recent advances on magnetic sensitive hydrogels in tissue engineering. Front. Chem. 2020, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.H.; Shin, H.H.; Choi, H.W.; Lim, J.H.; Mo, S.J.; Ahrberg, C.D.; Lee, J.M.; Chung, B.G. Electro-responsive hydrogel-based microfluidic actuator platform for photothermal therapy. Lab Chip 2020, 20, 3354–3364. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.V.; Biswanath, D.S. Electrically responsive smart hydrogels in drug delivery: A review. J. Appl. Biomater. Biomech. 2018, 5, 125–139. [Google Scholar] [CrossRef]
- Lin, C.C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Servidio, C.; Curcio, F.; Cassano, R. Strategies for hyaluronic acid-based hydrogel design in drug delivery. Pharmaceutics 2019, 11, 407. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.; Kondiah, P.; Choonara, Y.; du Toit, L.; Ally, N.; Pillay, V. Hydrogel biomaterials for application in ocular drug delivery. Front. Bioeng. Biotechnol. 2020, 8, 228. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 1–17. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, R.; Derakhshankhah, H.; Haghshenas, B.; Massoumi, B.; Abbasian, M.; Jaymand, M. A bio-inspired magnetic natural hydrogel containing gelatin and alginate as a drug delivery system for cancer chemotherapy. Int. J. Biol. Macromol. 2020, 156, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Liang, Y.; Shi, M.; Guo, B.; Gao, Y.; Yin, Z. Biocompatible conductive hydrogels based on dextran and aniline trimer as electro-responsive drug delivery system for localized drug release. Int. J. Biol. Macromol. 2019, 140, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Hilmi, B.; Hamid, Z.A.A.; Akil, H.M.; Yahaya, B.H. The characteristics of the smart polymeras temperature or pH-responsive hydrogel. Procedia Chem. 2016, 19, 406–409. [Google Scholar] [CrossRef]
- Gong, C.; Qi, T.; Wei, X.; Qu, Y.; Wu, Q.; Luo, F.; Qian, Z. Thermosensitive polymeric hydrogels as drug delivery systems. Curr. Med. Chem. 2013, 20, 79–94. [Google Scholar] [CrossRef]
- Derwent, J.J.K.; Mieler, W.F. Thermoresponsive hydrogels as a new ocular drug delivery platform to the posterior segment of the eye. Trans. Am. Ophthalmol. Soc. 2008, 106, 206. [Google Scholar]
- Samal, S.K.; Dash, M.; Dubruel, P.; Van Vlierberghe, S. Smart polymer hydrogels: Properties, synthesis and applications. Smart Polym. Appl. 2014, 237–270. [Google Scholar] [CrossRef]
- Lanzalaco, S.; Armelin, E. Poly(N-isopropylacrylamide) and copolymers: A review on recent progresses in biomedical applications. Gels 2017, 3, 36. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Y.; Fu, W.; Yao, M.; Ding, Z.; Xuan, J.; Li, D.; Wang, S.; Xia, Y.; Cao, M. Poly(N-isopropylacrylamide)-based thermoresponsive composite hydrogels for biomedical applications. Polymers 2020, 12, 580. [Google Scholar] [CrossRef]
- Liu, M.; Song, X.; Wen, Y.; Zhu, J.-L.; Li, J. Injectable thermoresponsive hydrogel formed by alginate-g-poly (N-isopropylacrylamide) releasing doxorubicin-encapsulated micelles as smart drug delivery system. ACS Appl. Mater. Interfaces 2017, 9, 35673–35682. [Google Scholar] [CrossRef] [PubMed]
- Bahram, M.; Mohseni, N.; Moghtader, M. An introduction to hydrogels and some recent applications. In Emerging Concepts in Analisis and Applications of Hydrogels; Majee, B.S., Ed.; IntechOpen Book Series: London, UK, 2016; ISBN 978-953-51-6668-9. [Google Scholar]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. pH sensitive hydrogels in drug delivery: Brief history, properties, swelling, and release mechanism, material selection and applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef]
- Han, Z.; Wang, P.; Mao, G.; Yin, T.; Zhong, D.; Yiming, B.; Hu, X.; Jia, Z.; Nian, G.; Qu, S.; et al. Dual pH-responsive hydrogel actuator for lipophilic drug delivery. ACS Appl. Mater. Interfaces 2020, 12, 12010–12017. [Google Scholar] [CrossRef]
- Webber, M.J.; Anderson, D.G. Smart approaches to glucose-responsive drug delivery. J. Drug Target. 2015, 23, 651–655. [Google Scholar] [CrossRef]
- Hu, D.-N.; Ju, X.-J.; Pu, X.-Q.; Xie, R.; Wang, W.; Liu, Z.; Chu, L.-Y. Injectable temperature/glucose dual-responsive hydrogels for controlled release of insulin. Ind. Eng. Chem. Res. 2021, 60, 8147–8158. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, L.; Li, Y.; Huang, Z.; Luo, S.; He, Y.; Han, H.; Raza, F.; Wu, J.; Ge, L. Injectable pH and redox dual responsive hydrogels based on self-assembled peptides for anti-tumor drug delivery. Biomater. Sci. 2020, 8, 5415–5426. [Google Scholar] [CrossRef]
- Hoang, H.T.; Jo, S.H.; Phan, Q.T.; Park, H.; Park, S.H.; Oh, C.W.; Lim, K.T. Dual pH-/thermo-responsive chitosan-based hydrogels prepared using “click” chemistry for colon-targeted drug delivery applications. Carbohydr. Polym. 2021, 260, 117812. [Google Scholar] [CrossRef]
- Askari, E.; Seyfoori, A.; Amereh, M.; Gharaie, S.; Ghazali, H.; Ghazali, Z.; Khunjush, B.; Akbari, M. Stimuli-responsive hydrogels for local post-surgical drug delivery. Gels 2020, 6, 14. [Google Scholar] [CrossRef]
- Singh, I.; Sharma, A.; Park, B.D. Drug-delivery applications of cellulose nanofibrils. In Nanoarchitectonics for Smart Delivery and Drug Targeting, 1st ed.; Holban, A.M., Grumezescu, A., Eds.; Willian Andrew: Norwich, NY, USA; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 95–117. ISBN 9780323477222. [Google Scholar]
- Mandal, A.; Clegg, J.R.; Anselmo, A.C.; Mitragotri, S. Hydrogels in the clinic. Bioeng. Transl. Med. 2020, 5, e10158. [Google Scholar] [CrossRef]
- Aldana, A.; Abraham, G. Current advances in electrospun gelatin-based scaffolds for tissue engineering applications. Int. J. Pharm. 2017, 523, 441–453. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart hydrogels in tissue engineering and regenerative medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 38. [Google Scholar] [CrossRef]
- Ghanbari, M.; Salavati-Niasari, M.; Mohandes, F.; Dolatyar, B.; Zeynali, B. In vitro study of alginate–gelatin scaffolds incorporated with silica Nps as injectable, biodegradable hydrogels. RSC Adv. 2021, 11, 16688–16697. [Google Scholar] [CrossRef]
- García, C.; Gallardo, A.; López, D.; Elvira, C.; Azzahti, A.; López-Martínez, E.; Cortajarena, A.L.; González-Henríquez, C.M.; Sarabia-Vallejos, M.A.; Rodriguez-Hernandez, J. Smart pH-responsive antimicrobial hydrogel scaffolds prepared by Additive Manufacturing. ACS Appl. Bio Mater. 2018, 1, 1337–1347. [Google Scholar] [CrossRef]
- Banerjee, H.; Suhail, M.; Ren, H. Hydrogel actuators and sensors for biomedical soft robots: Brief overview with impending challenges. Biomimetics 2018, 3, 15. [Google Scholar] [CrossRef]
- Shi, Q.; Liu, H.; Tang, D.; Li, Y.; Li, X.J.; Xu, F. Bioactuators based on stimulus-responsive hydrogels and their emerging biomedical applications. NPG Asia Mater. 2019, 11, 1–21. [Google Scholar] [CrossRef]
- Chen, J.-P.; Cheng, T.-H. Thermo-responsive chitosan-graft-poly (N-isopropylacrylamide) Injectable hydrogel for cultivation of chondrocytes and meniscus cells. Macromol. Biosci. 2006, 6, 1026–1039. [Google Scholar] [CrossRef]
- Stoychev, G.; Puretskiy, N.; Ionov, L. Self-folding all-polymer thermoresponsive microcapsules. Soft Matter 2011, 7, 3277–3279. [Google Scholar] [CrossRef]
- Ramanujan, R.V.; Lao, L.L. The mechanical behavior of smart magnet–hydrogel composites. Smart Mater. Struct. 2006, 15, 952–956. [Google Scholar] [CrossRef]
- Zhang, X.; Pint, C.; Lee, M.; Schubert, B.; Jamshidi, A.; Takei, K.; Ko, H.; Gillies, A.; Bardhan, R.; Urban, J.; et al. Optically-and thermally-responsive programmable materials based on carbon nanotube-hydrogel polymer composites. Nano Lett. 2011, 11, 3239–3244. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Zhu, W.; Sun, S.; Wu, P. MoS2-based dual-responsive flexible anisotropic actuators. Nanoscale 2016, 8, 18800–18807. [Google Scholar] [CrossRef] [PubMed]
- Ionov, L. Hydrogel-based actuators: Possibilities and limitations. Mater. Today 2014, 17, 494–503. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.; Song, J.; Yang, J.; Du, Z.; Zhao, W.; Guo, H.; Wen, C.; Li, Q.; Sui, X.; et al. A multifunctional pro-healing zwitterionic hydrogel for simultaneous optical monitoring of pH and glucose in diabetic wound treatment. Adv. Funct. Mater. 2020, 30, 1905493. [Google Scholar] [CrossRef]
- Zhai, D.; Liu, B.; Shi, Y.; Pan, L.; Wang, Y.; Li, W.; Zhang, R.; Yu, G. Highly sensitive glucose sensor based on Pt nanoparticle/polyaniline hydrogel heterostructures. ACS Nano 2013, 7, 3540–3546. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ren, B.; Zhang, Y.; Xu, L.; Huang, Q.; He, Y.; Li, X.; Wu, J.; Yang, J.; Chen, Q.; et al. From design to applications of stimuli-responsive hydrogel strain sensors. J. Mater. Chem. B 2020, 8, 3171–3191. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels 2021, 7, 182. https://doi.org/10.3390/gels7040182
Bustamante-Torres M, Romero-Fierro D, Arcentales-Vera B, Palomino K, Magaña H, Bucio E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels. 2021; 7(4):182. https://doi.org/10.3390/gels7040182
Chicago/Turabian StyleBustamante-Torres, Moises, David Romero-Fierro, Belén Arcentales-Vera, Kenia Palomino, Héctor Magaña, and Emilio Bucio. 2021. "Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials" Gels 7, no. 4: 182. https://doi.org/10.3390/gels7040182
APA StyleBustamante-Torres, M., Romero-Fierro, D., Arcentales-Vera, B., Palomino, K., Magaña, H., & Bucio, E. (2021). Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels, 7(4), 182. https://doi.org/10.3390/gels7040182







