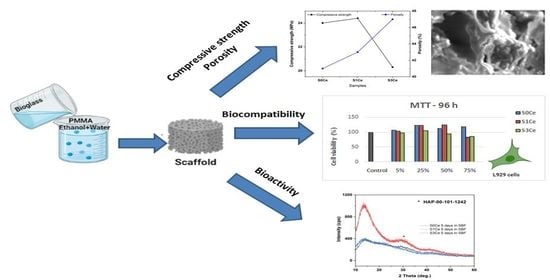
Preparation and Biocompatibility of Poly Methyl Methacrylate (PMMA)-Mesoporous Bioactive Glass (MBG) Composite Scaffolds
Abstract
:1. Introduction
2. Results and Discussion
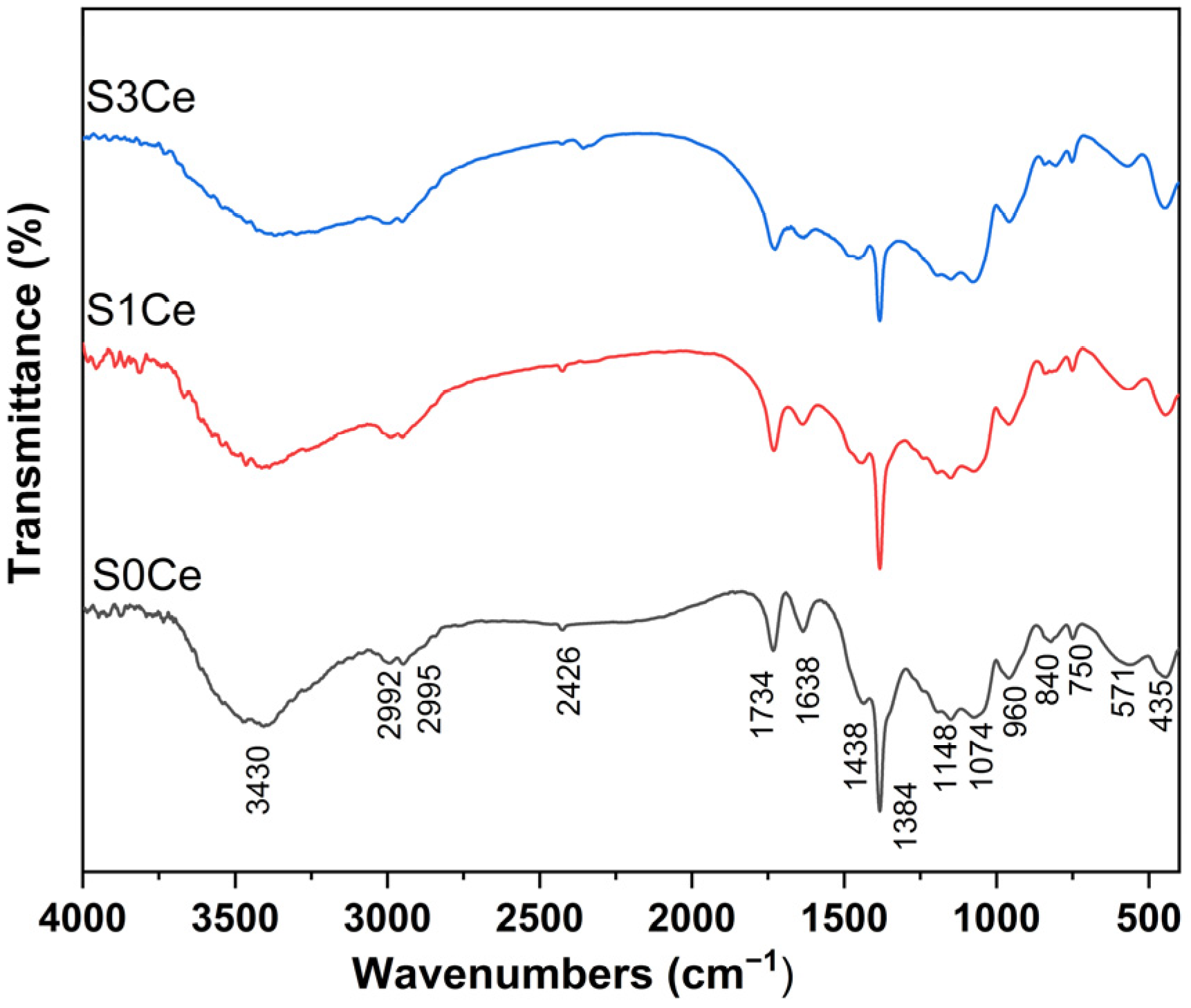
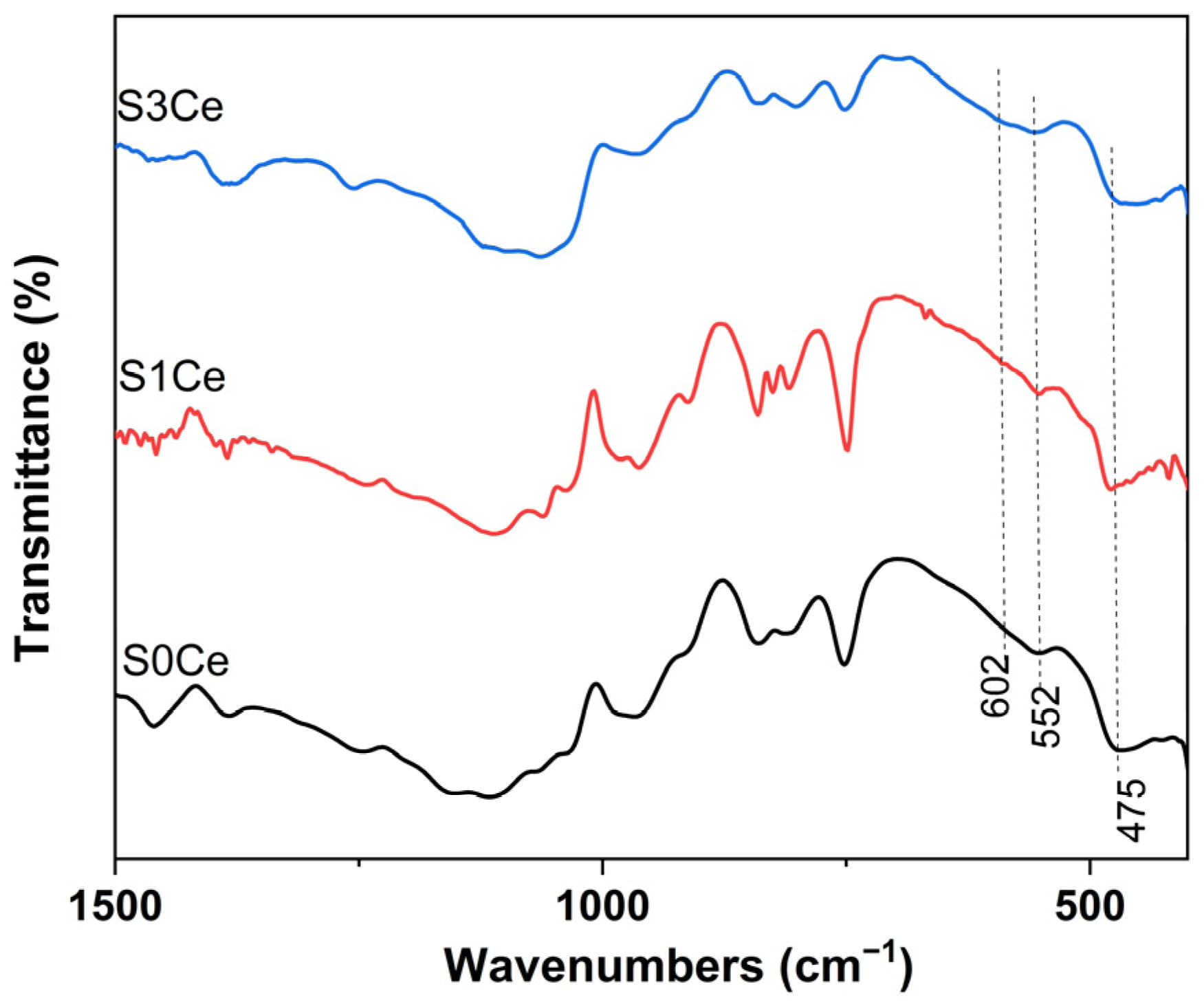
2.1. FTIR
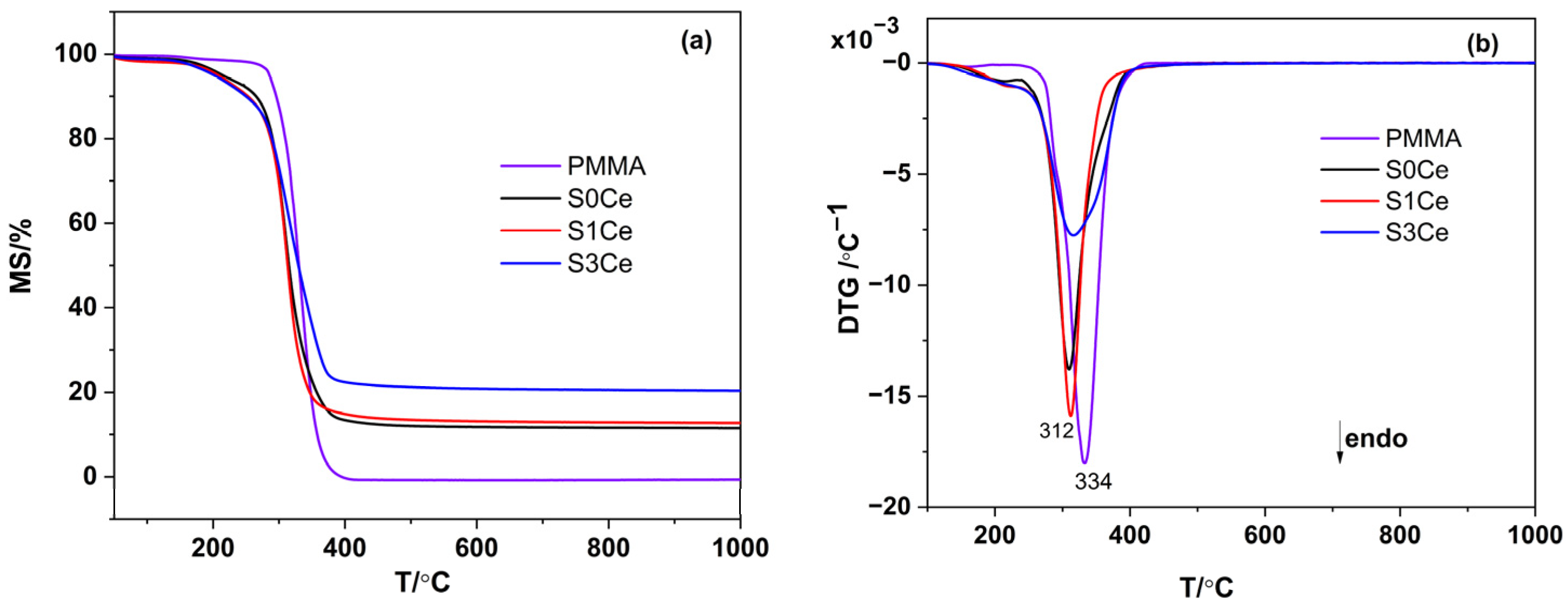
2.2. Thermal Analysis
2.3. UV-Vis
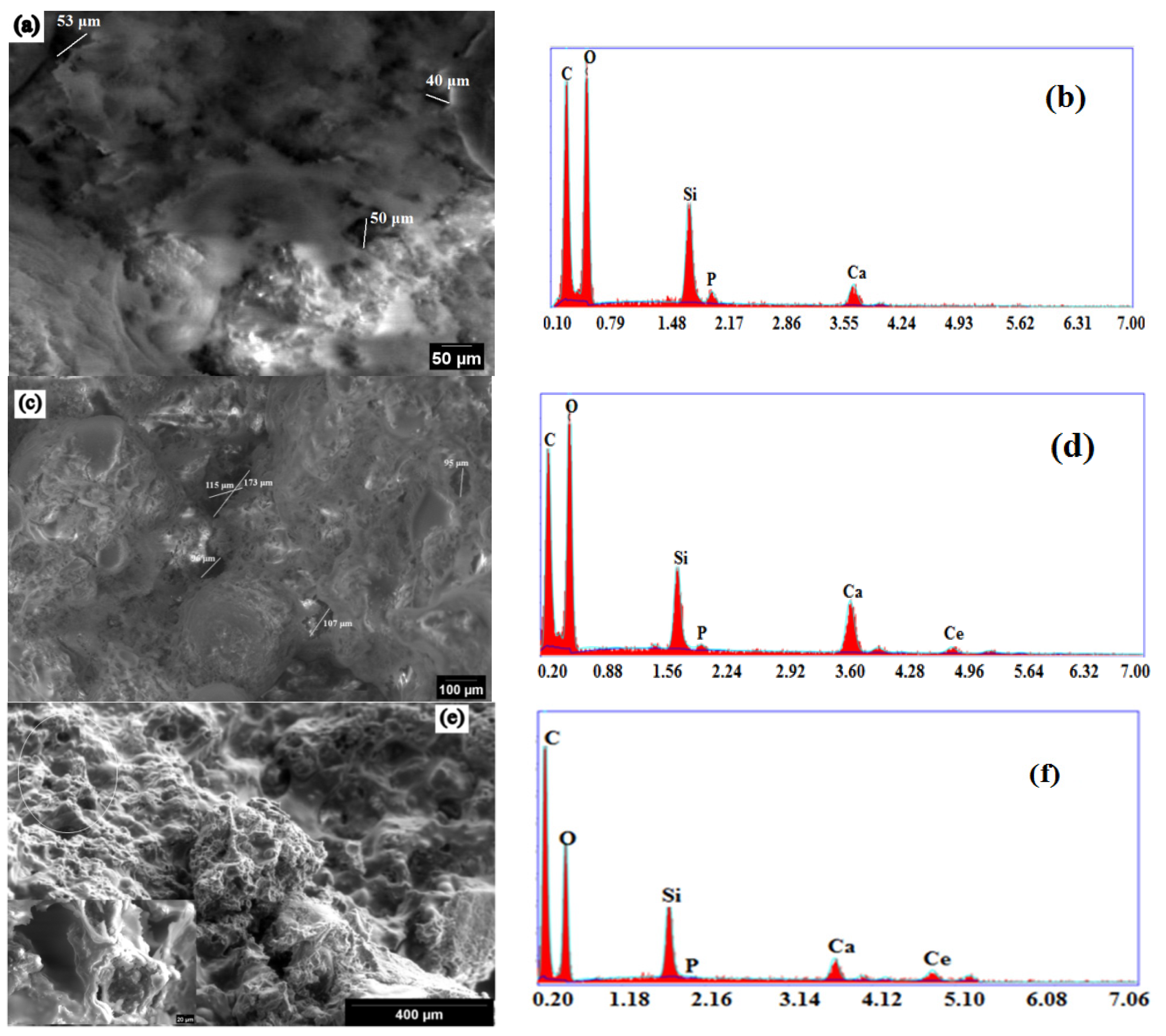
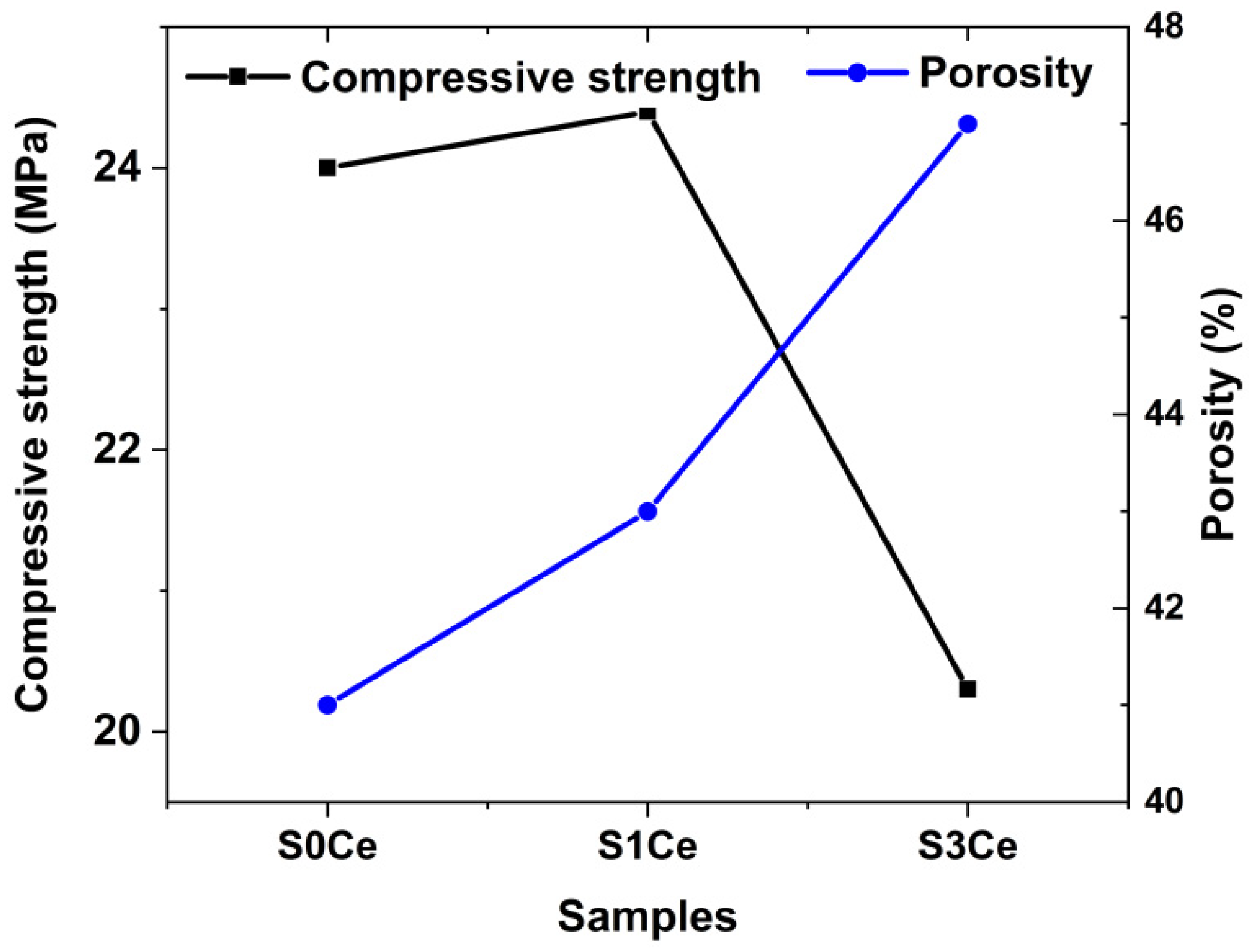
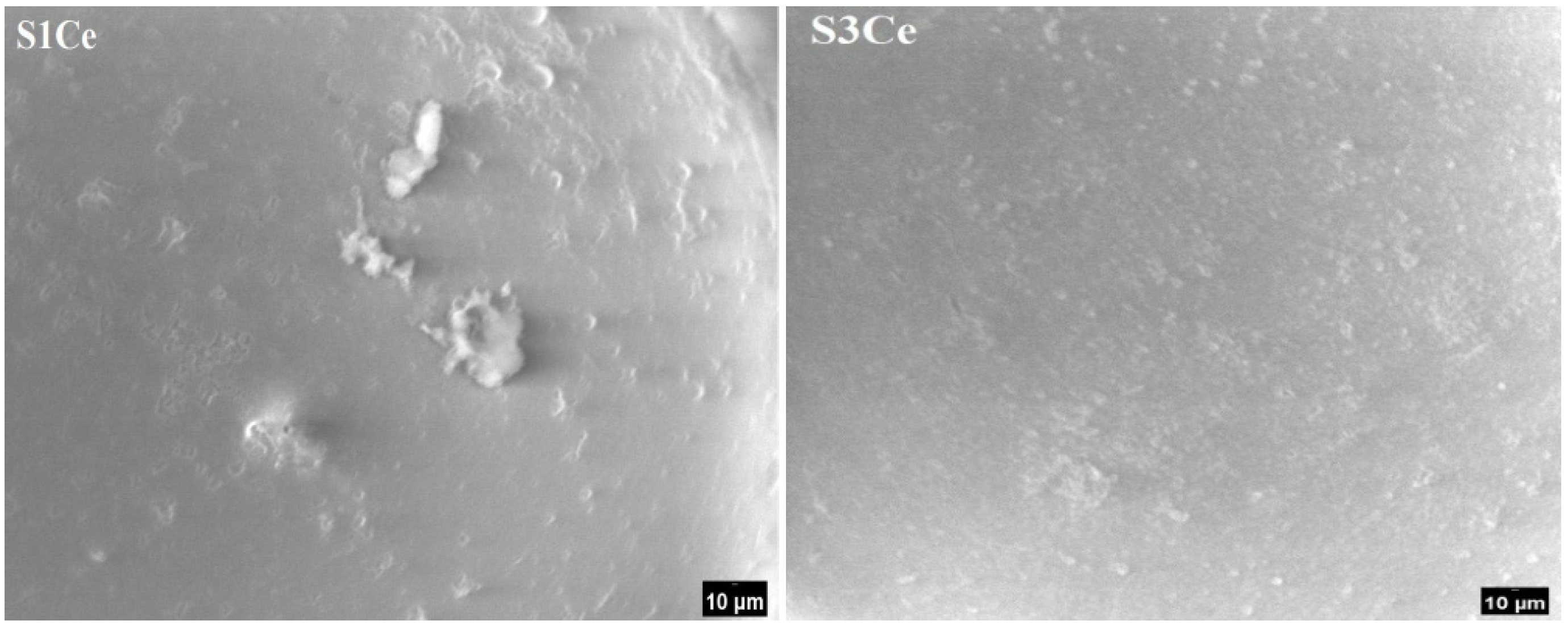
2.4. Morphology and Mechanical Properties Evaluation
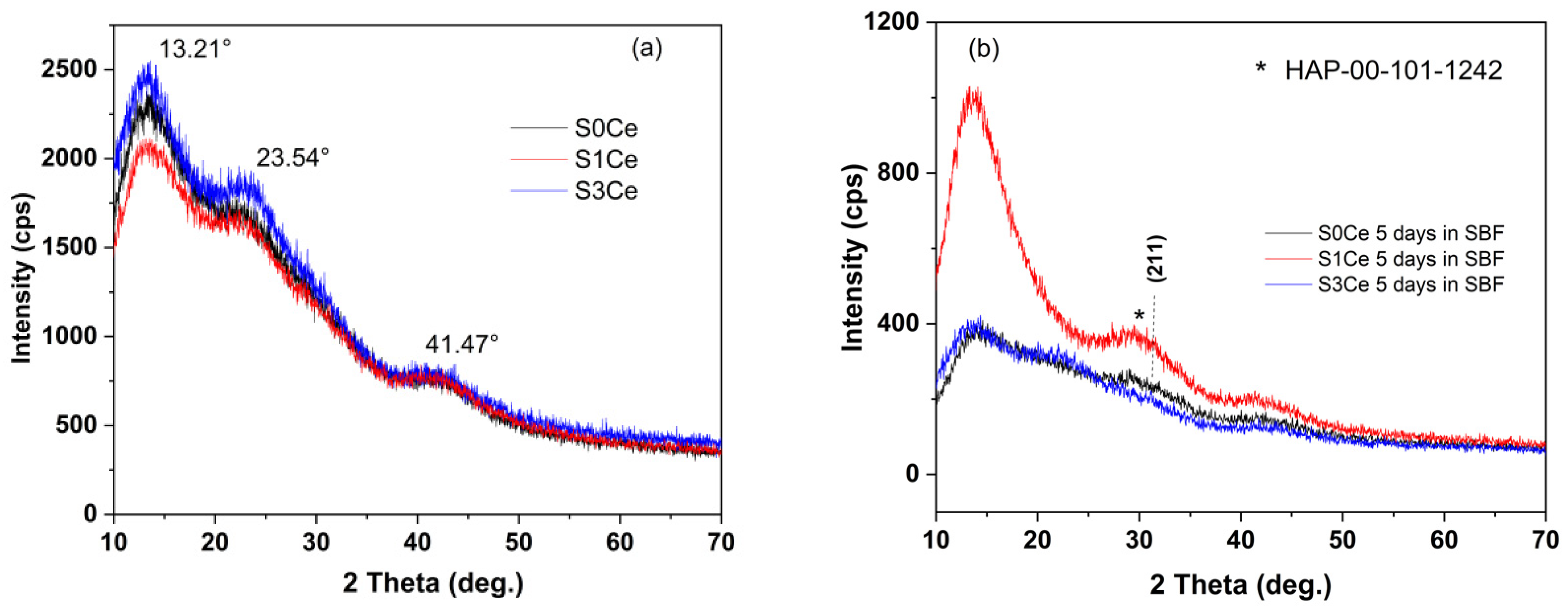
2.5. In Vitro Bioactivity Assessment
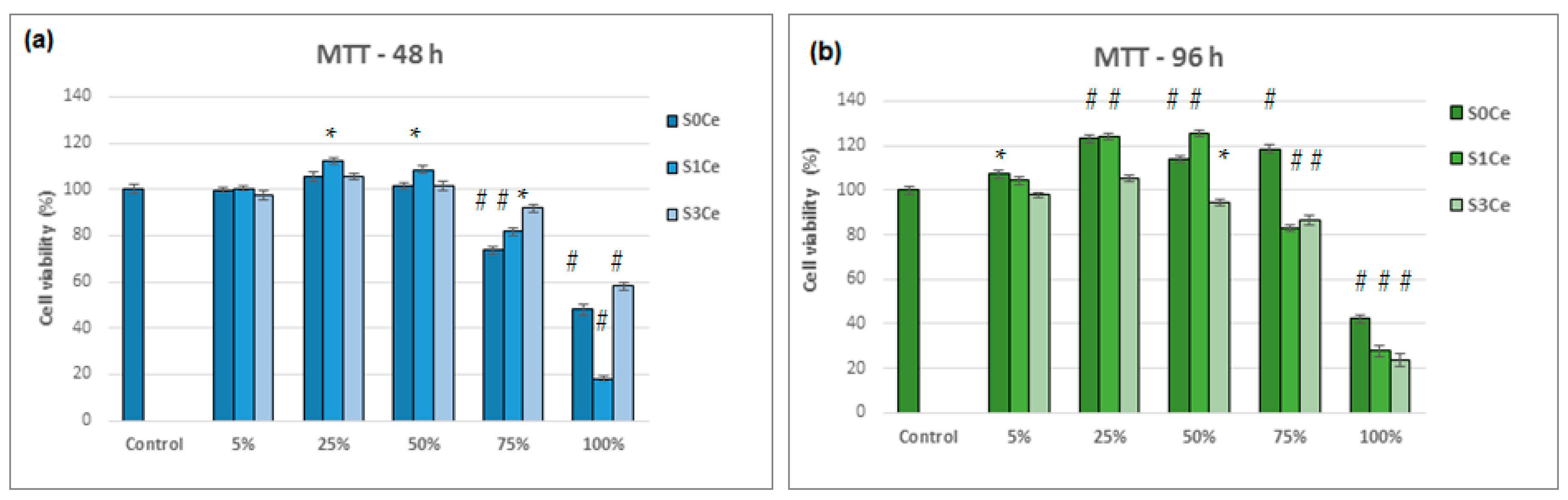
2.6. Biocompatibility Evaluation
3. Conclusions
4. Materials and Methods
4.1. Reagents
4.2. Preparation of MBG Solution
4.3. Preparation of the Polymer-MBG Scaffolds
4.4. Composite Scaffolds Characterization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, Y.; Mei, F.; Duan, Y.L.; Gao, Y.; Xiong, Z.; Zhang, T.; Zhang, H. Bone tissue engineering using bone marrow stromal cells and an injectable sodium alginate/gelatin scaffold. J. Biomed. Mater. Res. A 2012, 100A, 1044–1050. [Google Scholar] [CrossRef]
- Dolcimascolo, A.; Calabrese, G.; Conoci, S.; Parenti, R. Innovative Biomaterials for Tissue Engineering. In Book Biomaterial-Supported Tissue Reconstruction or Regeneration; Barbeck, M., Jung, O., Smeets, R., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Funda, G.; Taschieri, S.; Bruno, G.A.; Grecchi, E.; Paolo, S.; Girolamo, D.; Del Fabbro, M. Nanotechnology Scaffolds for Alveolar Bone Regeneration. Materials 2020, 13, 201. [Google Scholar] [CrossRef] [Green Version]
- Fiume, E.; Serino, G.; Bignardi, C.; Verné, E.; Baino, F. Bread-Derived Bioactive Porous Scaffolds: An Innovative and Sustainable Approach to Bone Tissue Engineering. Molecules 2019, 24, 2954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durgalakshmi, D.; Balakumar, S. Analysis of solvent induced porous PMMA–Bioglass monoliths by the phase separation method-mechanical and in vitro biocompatible studies. Phys. Chem. Chem. Phys. 2015, 17, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Luo, D.; Liu, Y. Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration. Int. J. Appl. Dent. Sci. 2020, 12, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, I.; Anghel, E.M.; Predoana, L.; Mocioiu, O.C.; Jecu, L.; Raut, I.; Munteanu, C.; Culita, D.; Zaharescu, M. Influence of ZnO addition on the structural, in vitro behavior and antimicrobial activity of sol–gel derived CaO–P2O5–SiO2 bioactive glasses. Ceram. Int. 2016, 42, 3033–3045. [Google Scholar] [CrossRef]
- Atkinson, I.; Anghel, E.M.; Petrescu, S.; Seciu, A.M.; Stefan, L.M.; Mocioiu, O.C.; Predoana, L.; Voicescu, M.; Somacescu, S.; Culita, D.; et al. Cerium-containing mesoporous bioactive glasses: Material characterization, in vitro bioactivity, biocompatibility and cytotoxicity evaluation. Micro. Mesop. Mater. 2019, 276, 76–88. [Google Scholar] [CrossRef]
- Charnley, J. Anchorage of the femoral head prosthesis to the shaft of the femur. Br. Vol. 1960, 42, 28–30. [Google Scholar] [CrossRef] [Green Version]
- Shundo, A.; Hori, K.; Penaloza, D.P.; Yoshihiro, K.; Annaka, M.; Tanaka, K. Nonsolvents-induced swelling of poly (methyl methacrylate) nanoparticles. Phys. Chem. Chem. Phys. 2013, 15, 16574–16578. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.S. Prosthodontic Applications of Polymethyl Methacrylate (PMMA): An Update. Polymers 2020, 12, 2299. [Google Scholar] [CrossRef]
- Manoukian, O.S.; Sardashti, N.; Stedman, T.; Gailiunas, K.; Ojha, A.; Penalosa, A.; Mancuso, C.; Hobert, M.; Kumbar, S.G. Biomaterials for Tissue Engineering and Regenerative Medicine. In Book Encyclopedia of Biomedical Engineering; Narayan, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 462–482. [Google Scholar] [CrossRef]
- Radha, G.; Balakumar, S.; Venkatesan, B.; Vellaichamy, E. A novel nano-hydroxyapatite—PMMA hybrid scaffolds adopted by conjugated thermal induced phase separation (TIPS) and wet-chemical approach: Analysis of its mechanical and biological properties. Mater. Sci. Eng. C 2017, 75, 221–228. [Google Scholar] [CrossRef]
- Jagdale, P.; Serino, G.; Oza, G.; Audenino, A.L.; Bignardi, C.; Tagliaferro, A.; Alvarez-Gayosso, C. Physical Characterization of Bismuth Oxide Nanoparticle Based Ceramic Composite for Future Biomedical Application. Materials 2021, 14, 1626. [Google Scholar] [CrossRef]
- Murphy, W.L.; Dennis, R.G.; Kileney, J.L.; Mooney, D.J. Salt fusion: An approach to improve pore interconnectivity within tissue engineering scaffolds. Tissue Eng. 2002, 8, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Matbouei, A.; Fathi, A.; Mahmood Rabiee, S.; Shirzad, M. Layered manufacturing of a three-dimensional polymethyl methacrylate (PMMA) scaffold used for bone regeneration. Mater. Tech. 2019, 34, 167–177. [Google Scholar] [CrossRef]
- Han, X.; Lin, H.; Chen, X.; Li, X.; Guo, G.; Qu, F. One-step method for the preparation of poly(methyl methacrylate) modified titanium bioactive glass three-dimensional scaffolds for bone tissue engineering. IET Nanobiotech. 2016, 10, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Westhauser, F.; Rehder, F.; Decker, S.; Kunisch, E.; Moghaddam, A.; Zheng, K.; Boccaccini, A.R. Ionic dissolution products of Cerium-doped bioactive glass nanoparticles promote cellular osteogenic differentiation and extracellular matrix formation of human bone marrow derived mesenchymal stromal cells. Biomed. Mater. 2020, 16, 035028. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, J.; Calas, S.; Gatti, S.; Kribich, R.K.; Myara, M.; Pille, G.; Ettienne, P.; Moreau, Y. Characterization by IR spectroscopy of an hybrid sol–gel material used for photonic devices fabrication. J. Non-Cryst. Solids 2008, 354, 651–658. [Google Scholar] [CrossRef]
- Chibac-Scutaru, A.L.; Melinte, V.; Coseri, S. Cellulose Acetate Incorporating Organically Functionalized CeO2 NPs: Efficient Materials for UV Filtering Applications. Materials 2020, 13, 2955. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, G.P.; Kaur, S.; Singh, D.P. Modifier role of cerium in lithium aluminium borate glasses. J. Molec. Struct. 2012, 1020, 83–87. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, L. Structural Features and Synthesis of CeO2-Doped Boroaluminosilicate Oxyfluoride Transparent Glass Ceramics. J. Chem. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Mori, Y.; Pinnavaia, T.J. Optimizing organic functionality in mesostructured silica: Direct assembly of mercaptopropyl groups in wormhole framework structures. Chem. Mater. 2001, 13, 2173–2178. [Google Scholar] [CrossRef]
- Nicolini, V.; Gambuzzi, E.; Malavasi, G.; Menabue, L.; Menziani, M.C.; Lusvardi, G.; Pedone, A.; Benedetti, F.; Luches, P.; D’Addato, S.; et al. Evidence of catalase mimetic activity in Ce3+/Ce4+ doped bioactive glasses. J. Phys. Chem. 2015, B119, 4009–4019. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, O.G.; Hussein, A.M.; Ahmed, H.M. From insulating PMMA polymer to conjugated double bond behavior: Green chemistry as a novel approach to fabricate small band gap polymers. Polymers 2017, 9, 626. [Google Scholar] [CrossRef] [Green Version]
- Matamoros-Ambrocio, M.; Sánchez-Mora, E.; Gómez-Barojas, E.; Luna-López, J.A. Synthesis and Study of the Optical Properties of PMMA Microspheres and Opals. Polymers 2021, 13, 2171. [Google Scholar] [CrossRef]
- Paul, A.; Mulholland, M.; Zaman, M.S. Ultraviolet absorption of cerium (IV) in some simple glasses. J. Mater. Sci. 1976, 11, 2082–2086. [Google Scholar] [CrossRef]
- Cicconi, M.R.; Veber, A.; Neuville, D.R.; Baudelet, F.; De Ligny, D. Cerium speciation in silicate glasses: Structure-property relationships. J. Non-Cryst. Solids 2021, 563, 120785. [Google Scholar] [CrossRef]
- Wang, P.; Chen, S.; Gao, S.; Zhang, J.; Wang, H.; Wu, Z. Niobium oxide confined by ceria nanotubes as a novel SCR catalyst with excellent resistance to potassium, phosphorus, and lead. Appl. Catal. B Environ. 2018, 231, 299–309. [Google Scholar] [CrossRef]
- Morgan, E.F.; Unnikrisnan, G.U.; Hussein, A.I. Bone Mechanical Properties in Healthy and Diseased States. Annu. Rev. Biomed. Eng. 2018, 20, 119–143. [Google Scholar] [CrossRef] [PubMed]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaborowska, M.; Bodin, A.; Bäckdahl, H.; Popp, J.; Goldstein, A.; Gatenholm, P. Microporous bacterial cellulose as a potential scaffoldfor bone regeneration. Acta Biomater. 2010, 6, 2540–2547. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Baroud, G.; Bernstein, A.; Döbelin, N.; Galea, L.; Hesse, B.; Heuberger, R.; Meille, S.; Michel, P.; von Rechenberg, B.; et al. Characterization and distribution of mechanically competent mineralized tissue in micropores of beta-tricalcium phosphate bone substitutes. Mater. Today 2017, 20, 106–115. [Google Scholar] [CrossRef]
- Zhou, H.-L.; Feng, K.-Q.; Chen, C.-H.; Yan, D.-L. Influence of CeO2 addition on the preparation of foamed glass-ceramics from high-titanium blast furnace slag. Int. J. Miner. Metall. 2018, 25, 689–695. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering boneand cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Fiume, E.; Serino, G.; Bignardi, C.; Verné, E.; Baino, F. Sintering Behavior of a Six-Oxide Silicate Bioactive Glass for Scaffold Manufacturing. Appl. Sci. 2020, 10, 8279. [Google Scholar] [CrossRef]
- Chowdhury, S.K.R.; Mishra, A.; Pradhan, B.; Saha, D. Wear characteristic and biocompatibility of some polymer composite acetabular cups. Wear 2004, 256, 1026–1036. [Google Scholar] [CrossRef]
- Hashem, M.; Fayez Al Rez, M.; Fouad, H.; Elsarnagawy, T.; Elsharawy, M.A.; Umar, A.; Assery, M.; Ansar, S.G. Influence of Titanium Oxide Nanoparticles on the Physical and Thermomechanical Behavior of Poly Methyl Methacrylate (PMMA): A Denture Base Resin. Sci. Adv. Mater. 2017, 9, 938–944. [Google Scholar] [CrossRef]
- Gopi, D.; Shinyjoy, E.; Karthika, A.; Nithiya, S.; Kavitha, L.; Rajeswari, D.; Tang, T. Single walled carbon nanotubes reinforced mineralized hydroxyapatite composite coatings on titanium for improved biocompatible implant applications. RSC Adv. 2015, 5, 36766. [Google Scholar] [CrossRef]
- Notingher, I.; Jones, J.R.; Verrier, S.; Bisson, I.; Embanga, P.; Edwards, P.; Polak, J.M.; Hench, L.L. Application of FTIR and Raman spectroscopy to characterization obioactive materials and living cells. Spectroscopy 2003, 17, 275–288. [Google Scholar] [CrossRef] [Green Version]
- Naganuma, T.; Traversa, E. The effect of cerium valence states at cerium oxide nanoparticle surfaces on cell proliferation. Biomaterials 2014, 35, 4441–4453. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Li, W.; Zheng, X.; Li, X.; Sun, Y.; Wang, Y.; Li, C.; Wang, L. Cerium and Its Oxidant-Based Nanomaterials for Antibacterial Applications: A State-of-the-Art Review. Front. Mater. 2020, 7, 213. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, J.; Jiang, L.; Zhou, T.; Yan, X.; Yuan, L.; Chen, W. Influence Analysis and Stepwise Regression of Coal Mechanical Parameters on Uniaxial Compressive Strength Based on Orthogonal Testing Method. Energies 2020, 13, 3640. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 277, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Craciunescu, O.; Gaspar, A.; Trif, M.; Moisei, M.; Oancea, A.; Moldovan, L.; Zarnescu, O. Preparation and characterization of a collagen-liposome-chondroitin sulfate matrix with potential application for inflammatory disorders treatment. J. Nanomater. 2014, 903691, 1–9. [Google Scholar] [CrossRef]
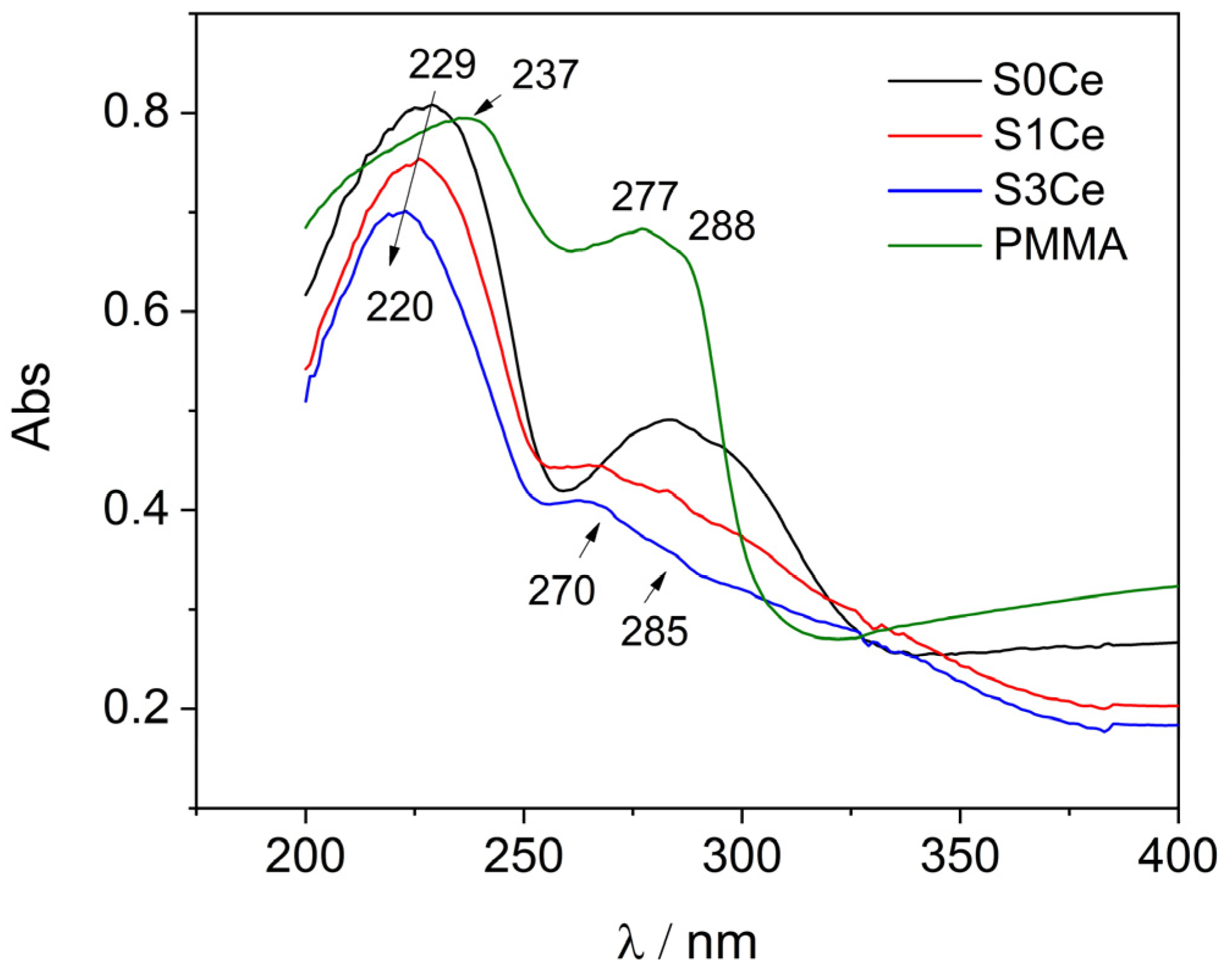


| Sample | PMMA-MBGs | Ce4+ | Ce3+ |
|---|---|---|---|
| S0Ce | 13.40% (210.22 nm) | - | - |
| 51.70% (229.11 nm) | - | - | |
| 5.29% (240.87 nm) | - | - | |
| 3.42% (273.69 nm) | - | - | |
| 12.10% (284.78 nm) | - | - | |
| 12.18% (300.97 nm) | - | - | |
| 1.91% (312.31 nm) | - | - | |
| S1Ce | - | 7.72% (212.77 nm) | 4.59% (269.00 nm) |
| 72.26% (227.75 nm) | - | 4.34% (283.48 nm) | |
| 7.43% (300.10 nm) | 3.66% (239.49 nm) | - | |
| S3Ce | - | 5.26% (214.19 nm) | 3.80% (267.74 nm) |
| 65.32% (220.75 nm) | - | 1.00% (279.62 nm) | |
| - | 24.62% (234.34 nm) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atkinson, I.; Seciu-Grama, A.M.; Mocioiu, O.C.; Mocioiu, A.M.; Predoana, L.; Voicescu, M.; Cusu, J.P.; Grigorescu, R.M.; Ion, R.M.; Craciunescu, O. Preparation and Biocompatibility of Poly Methyl Methacrylate (PMMA)-Mesoporous Bioactive Glass (MBG) Composite Scaffolds. Gels 2021, 7, 180. https://doi.org/10.3390/gels7040180
Atkinson I, Seciu-Grama AM, Mocioiu OC, Mocioiu AM, Predoana L, Voicescu M, Cusu JP, Grigorescu RM, Ion RM, Craciunescu O. Preparation and Biocompatibility of Poly Methyl Methacrylate (PMMA)-Mesoporous Bioactive Glass (MBG) Composite Scaffolds. Gels. 2021; 7(4):180. https://doi.org/10.3390/gels7040180
Chicago/Turabian StyleAtkinson, Irina, Ana Maria Seciu-Grama, Oana Catalina Mocioiu, Ana Maria Mocioiu, Luminita Predoana, Mariana Voicescu, Jeanina Pandele Cusu, Ramona Marina Grigorescu, Rodica Mariana Ion, and Oana Craciunescu. 2021. "Preparation and Biocompatibility of Poly Methyl Methacrylate (PMMA)-Mesoporous Bioactive Glass (MBG) Composite Scaffolds" Gels 7, no. 4: 180. https://doi.org/10.3390/gels7040180
APA StyleAtkinson, I., Seciu-Grama, A. M., Mocioiu, O. C., Mocioiu, A. M., Predoana, L., Voicescu, M., Cusu, J. P., Grigorescu, R. M., Ion, R. M., & Craciunescu, O. (2021). Preparation and Biocompatibility of Poly Methyl Methacrylate (PMMA)-Mesoporous Bioactive Glass (MBG) Composite Scaffolds. Gels, 7(4), 180. https://doi.org/10.3390/gels7040180