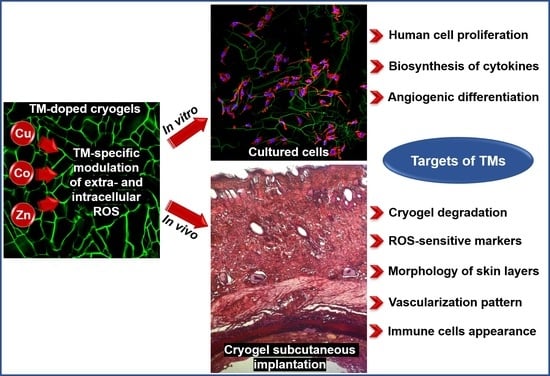
Regenerative Activities of ROS-Modulating Trace Metals in Subcutaneously Implanted Biodegradable Cryogel
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Cryogels
2.3. Cell Maintenance and Seeding
2.4. Cell Detection in Cryogels
2.5. Immunocytochemistry
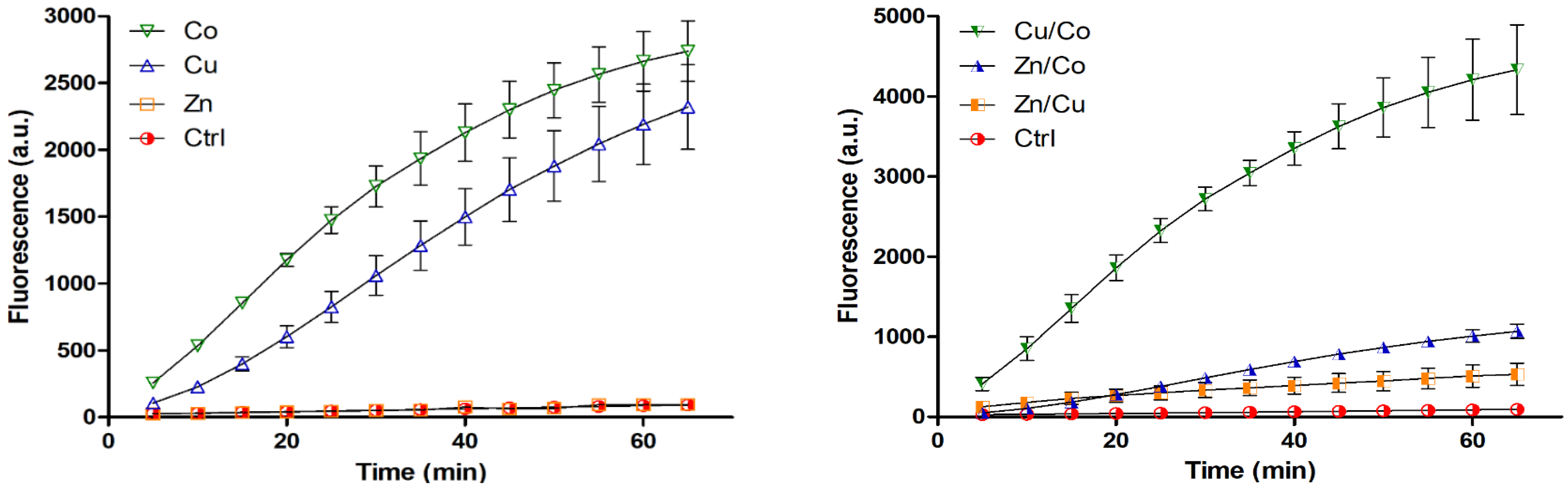
2.6. Detection of ROS and Glutathione
2.7. Multiplexed Fluorescent Bead-Based Immunoassay
2.8. In Vivo Study
2.8.1. Animals
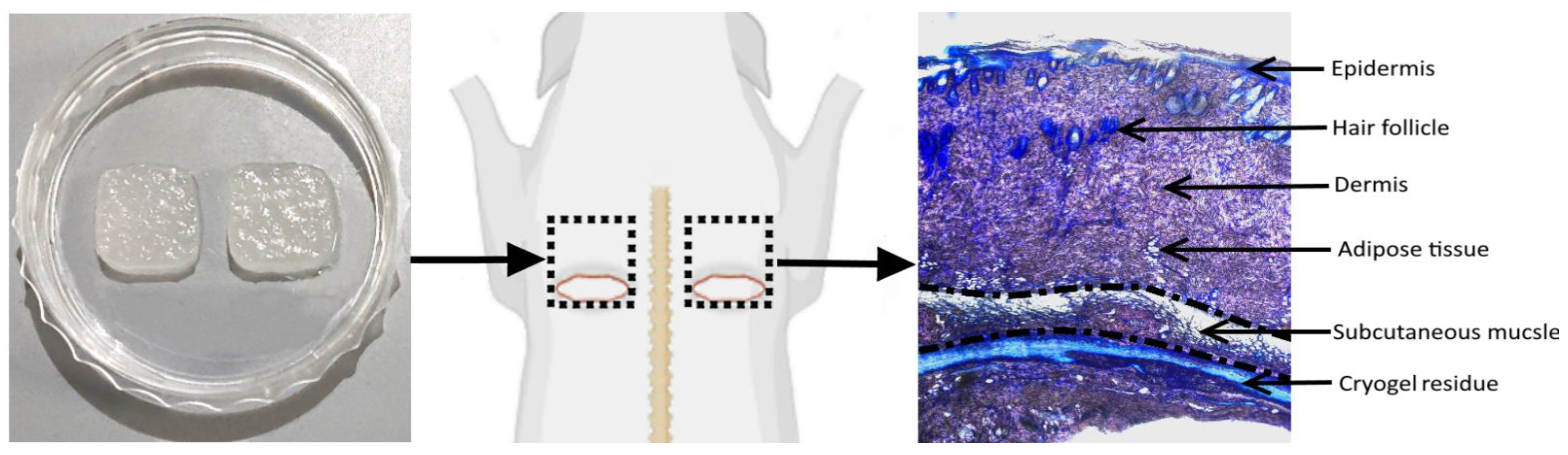
2.8.2. Subcutaneous Implantation Model
2.9. Histological Evaluation
2.10. Statistical Analysis
3. Results
3.1. Characterization of Cryogels
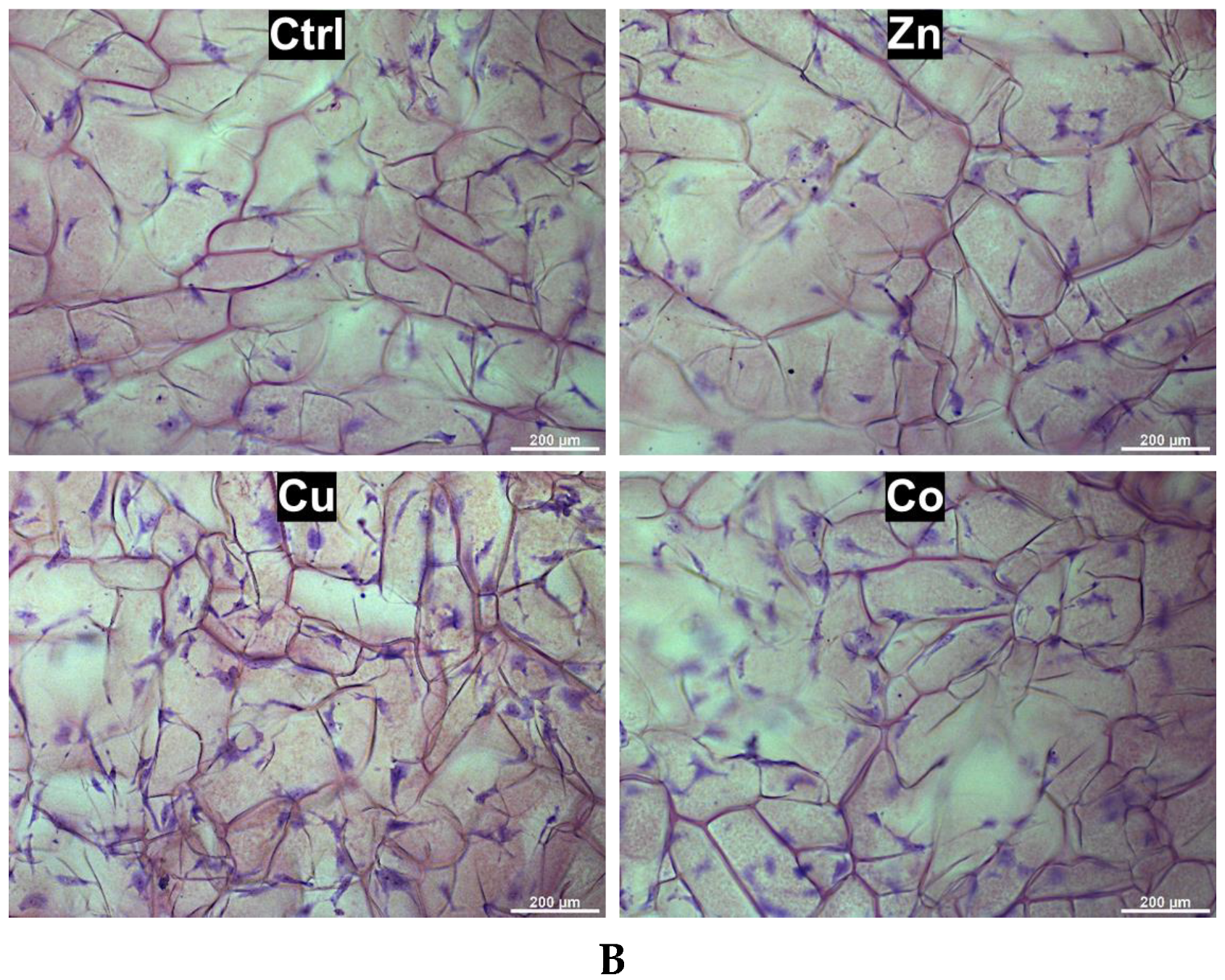
3.2. Behavior of Fibroblasts in Metal-Doped Cryogels
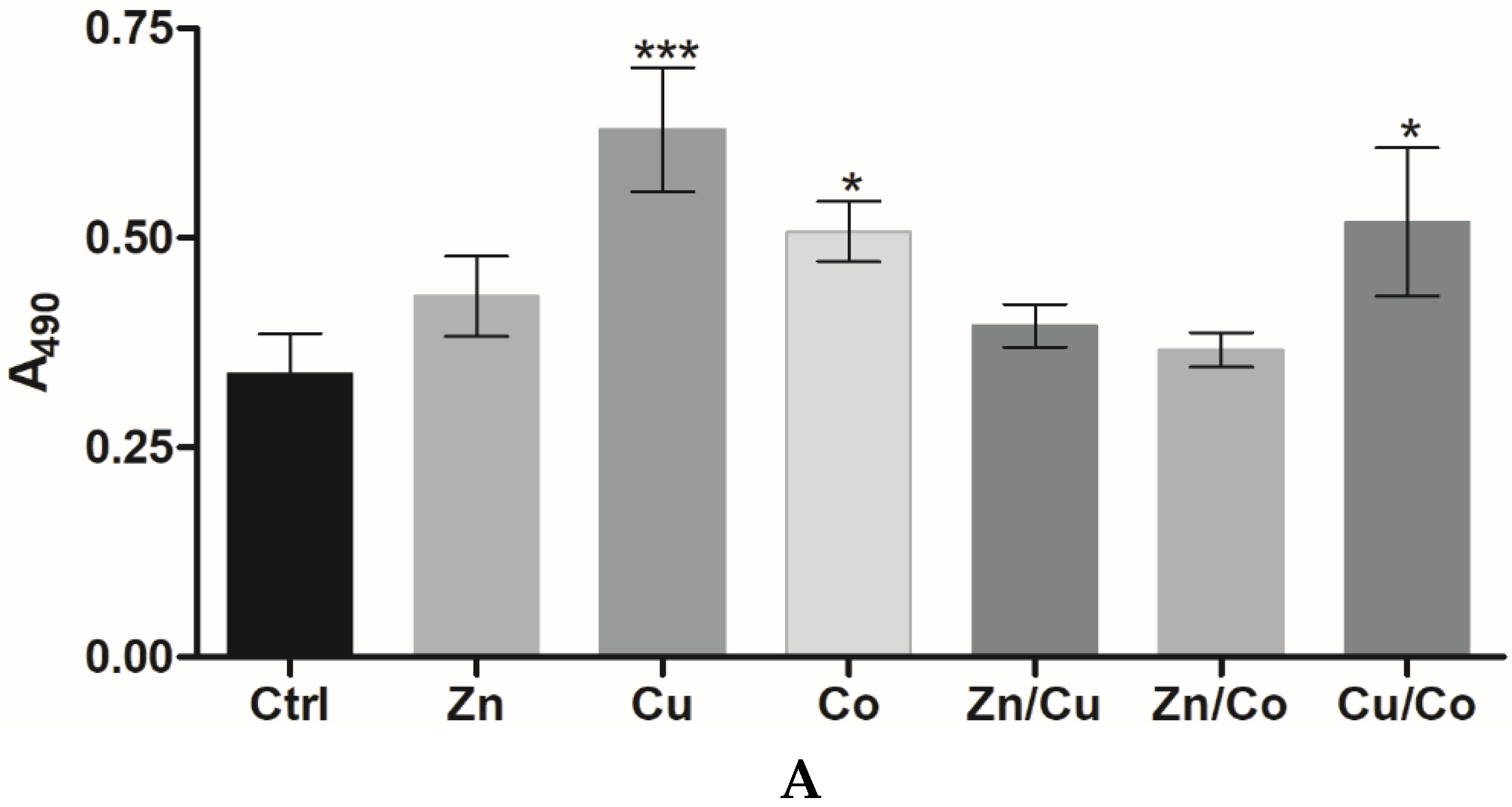
3.2.1. Effect of Cryogel Composition on Cell Proliferation
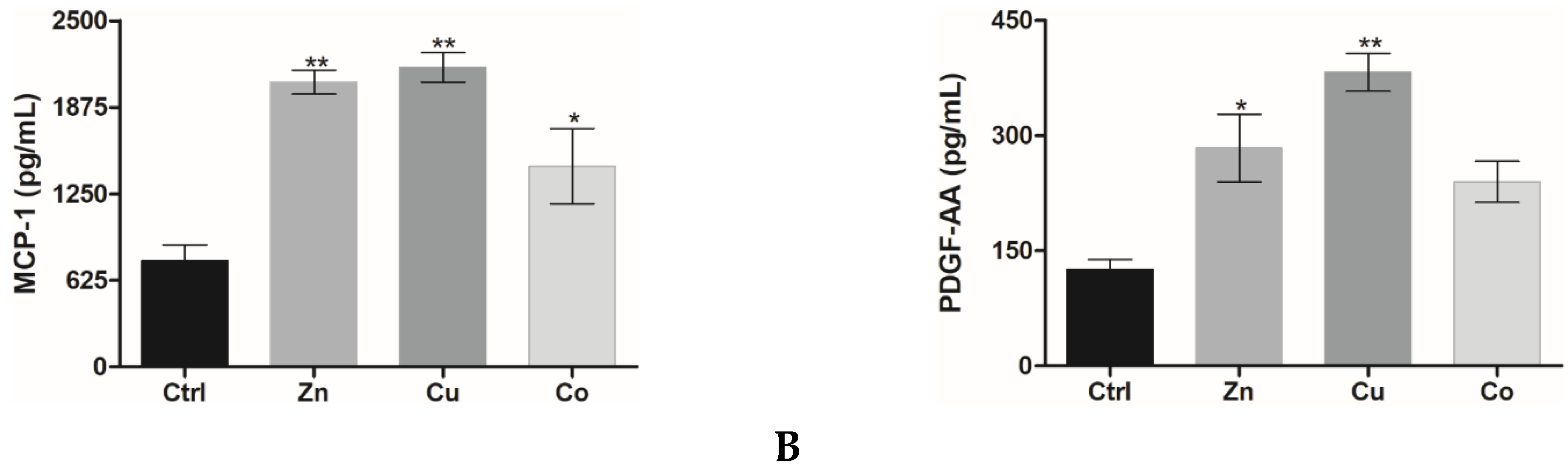
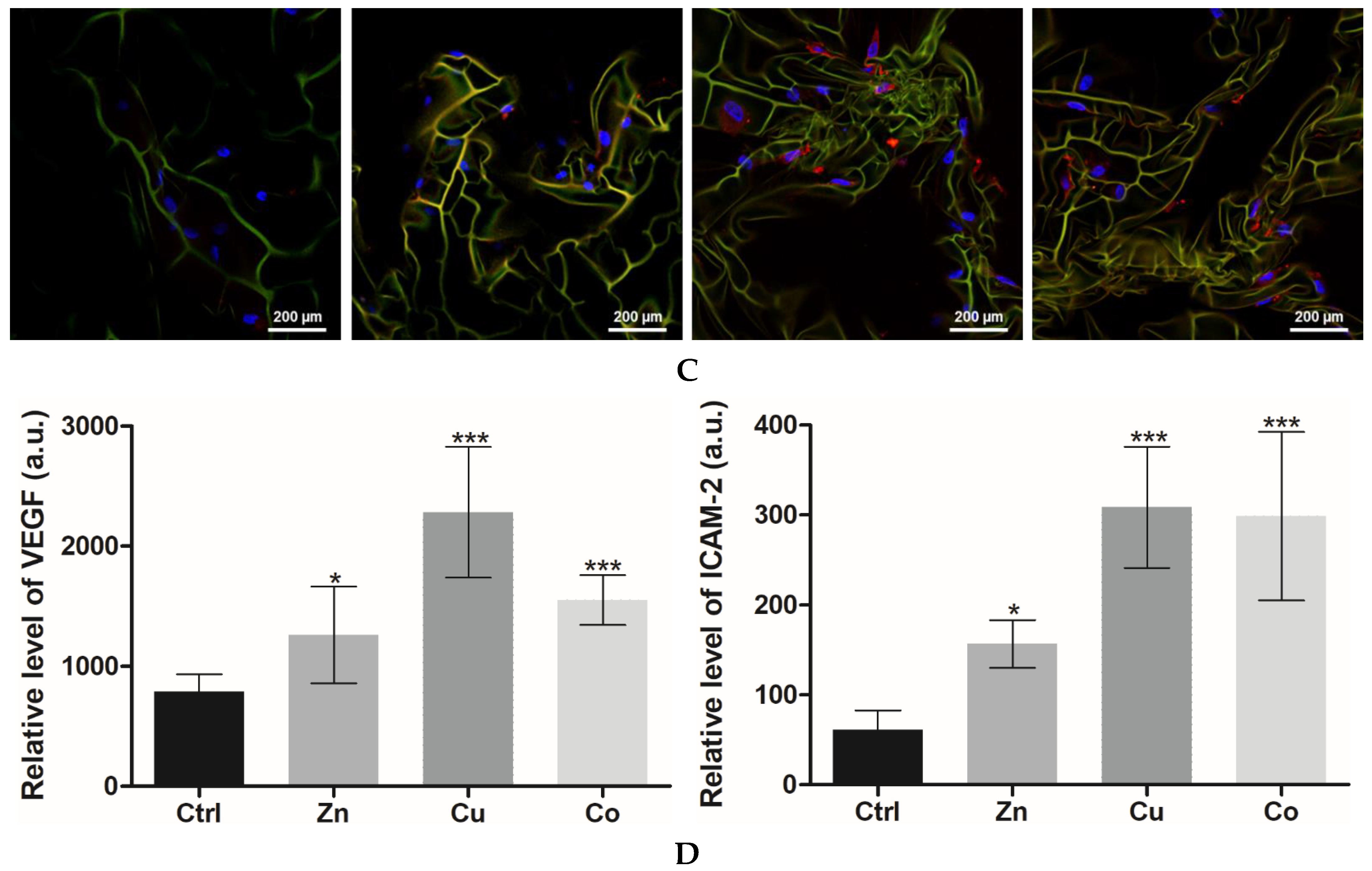
3.2.2. Cytokine and Growth Factor Profile
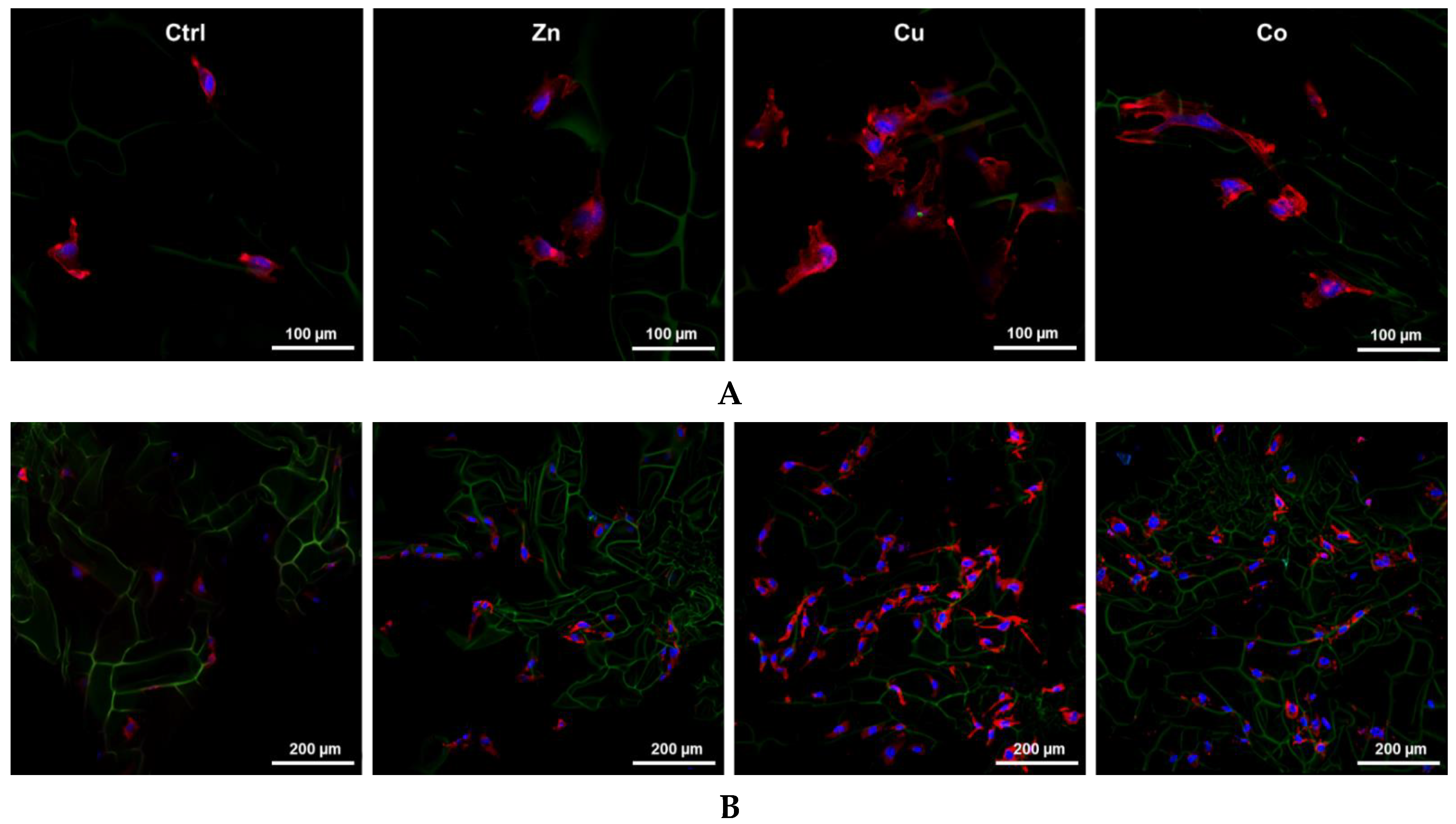
3.3. Behavior of HUVECs in Metal-Doped Cryogels
3.3.1. Proliferation and Spreading
3.3.2. Angiogenic Differentiation
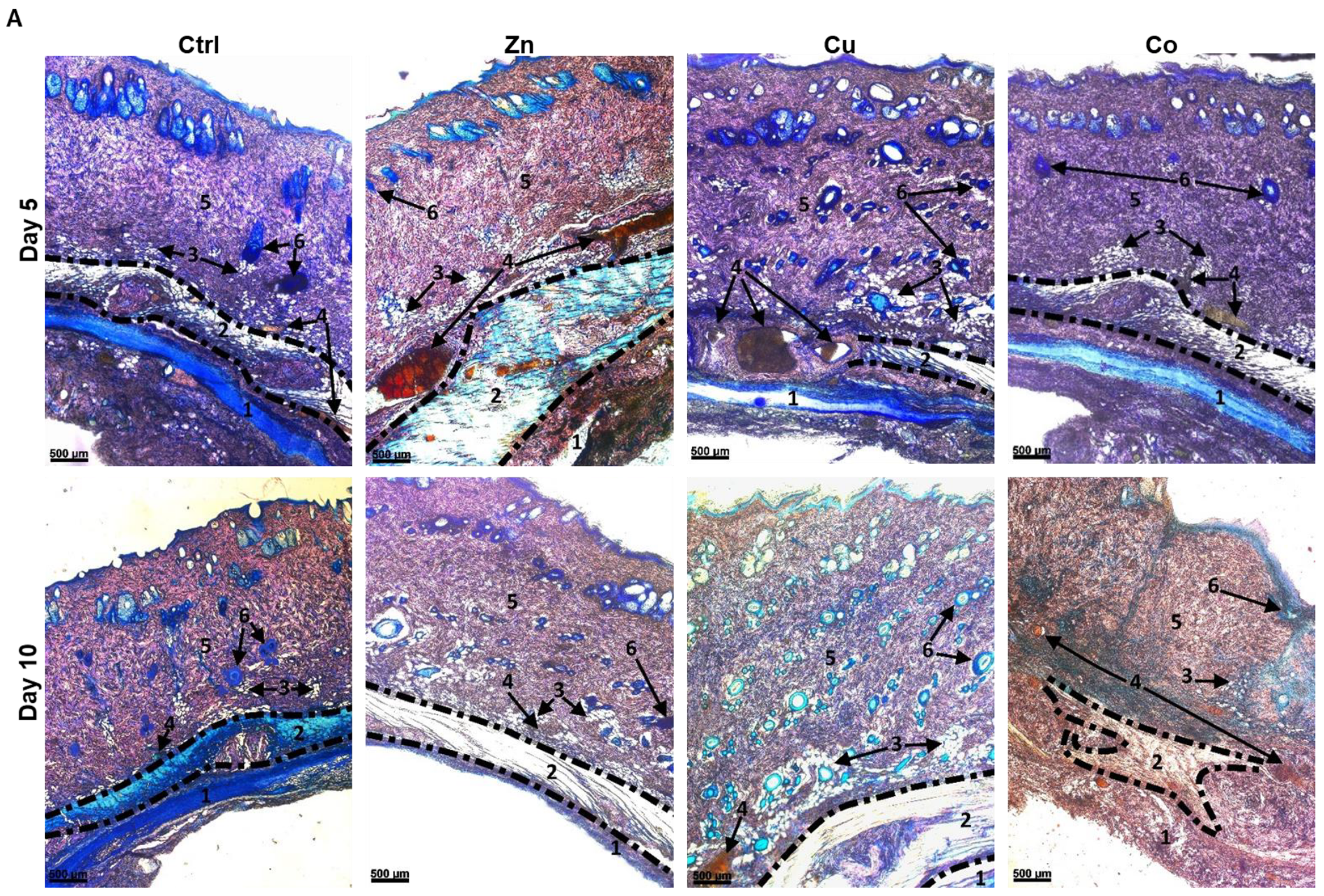
3.4. Effects of Metal-Doped Cryogels upon Subcutaneous Implantation
3.4.1. In Vivo Model Overview
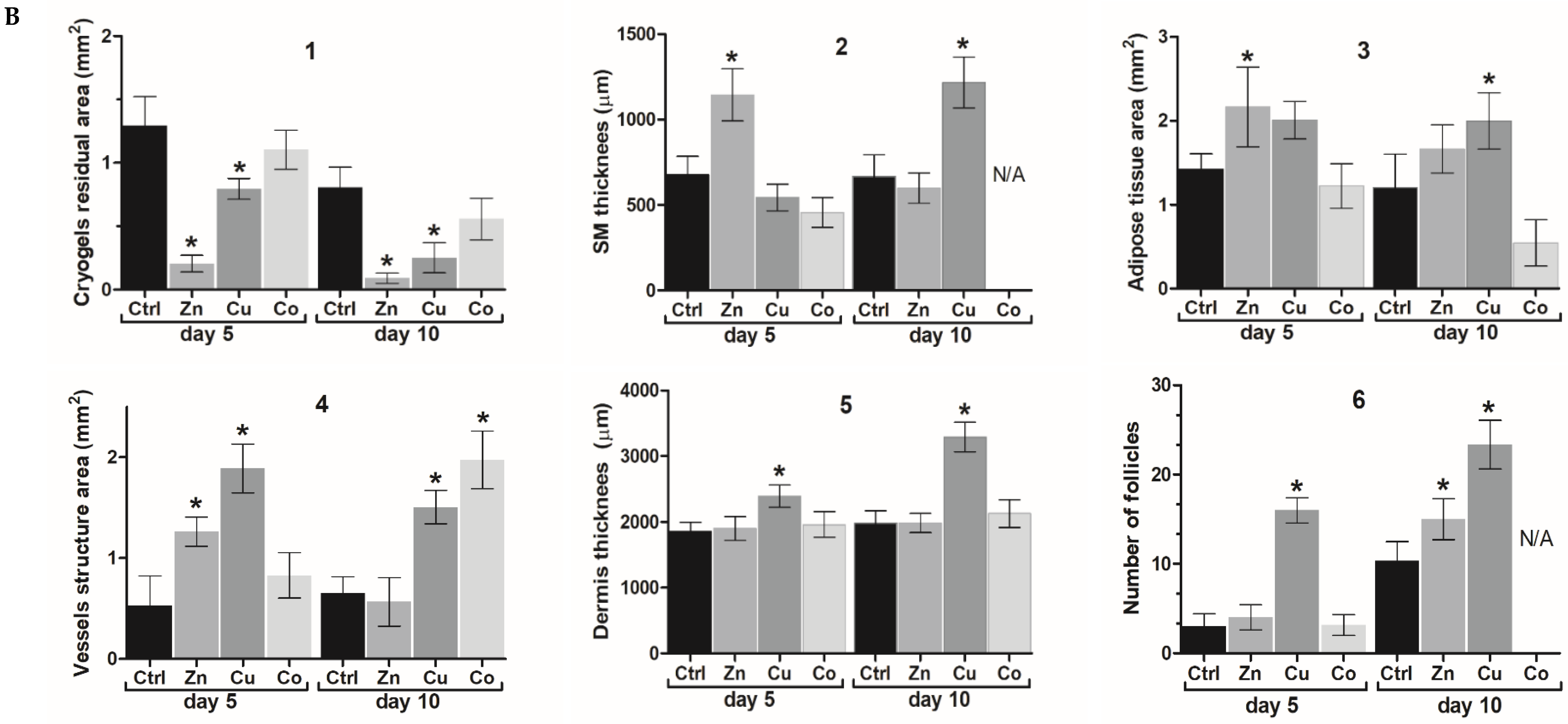
3.4.2. Biodegradation of Cryogels
3.4.3. Subcutaneous Muscle and Adipose Tissue
3.4.4. Vascular System
3.4.5. Dermis
3.4.6. Hair Follicles and Epidermis
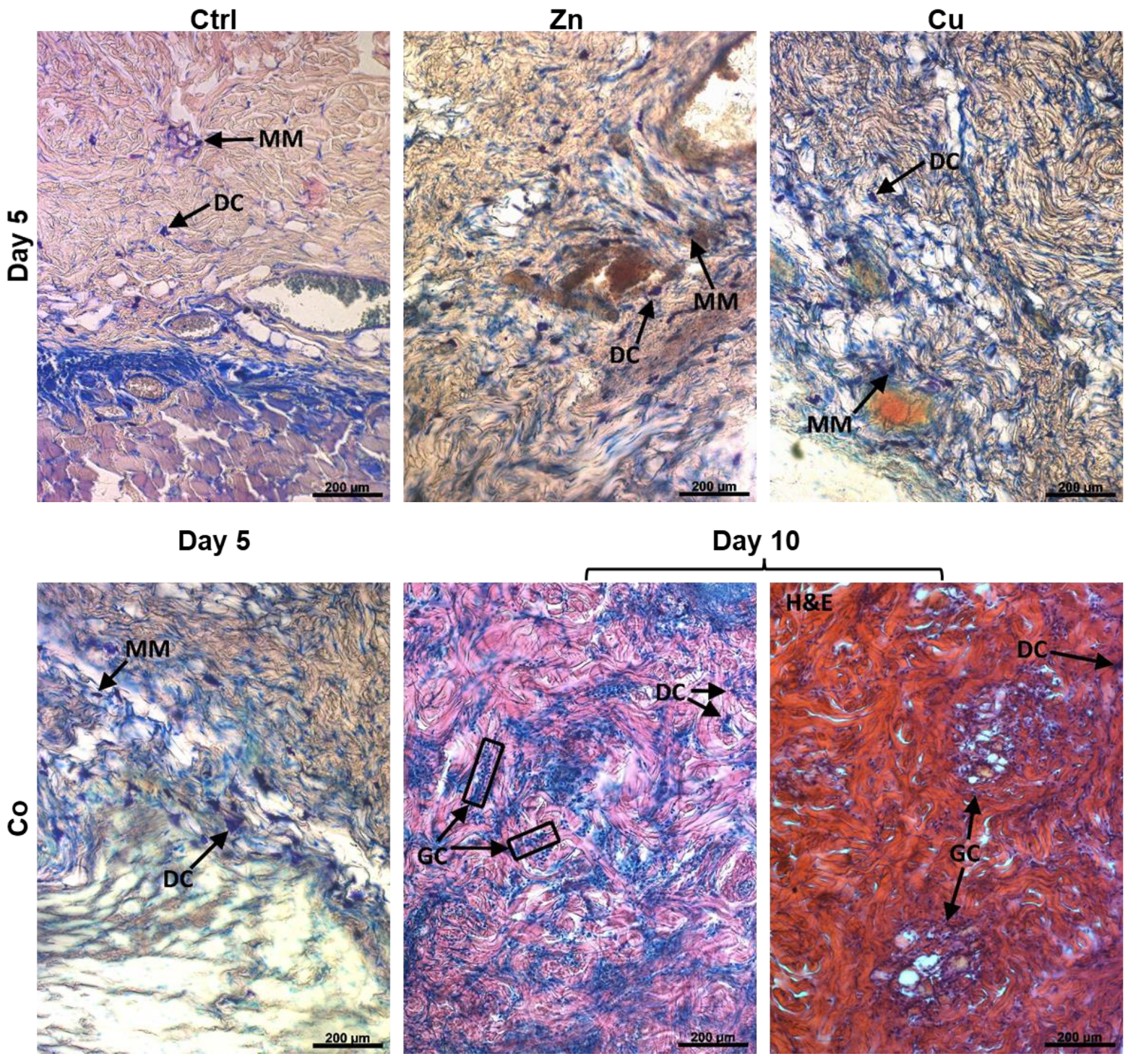
3.4.7. Immune Cells Appearance
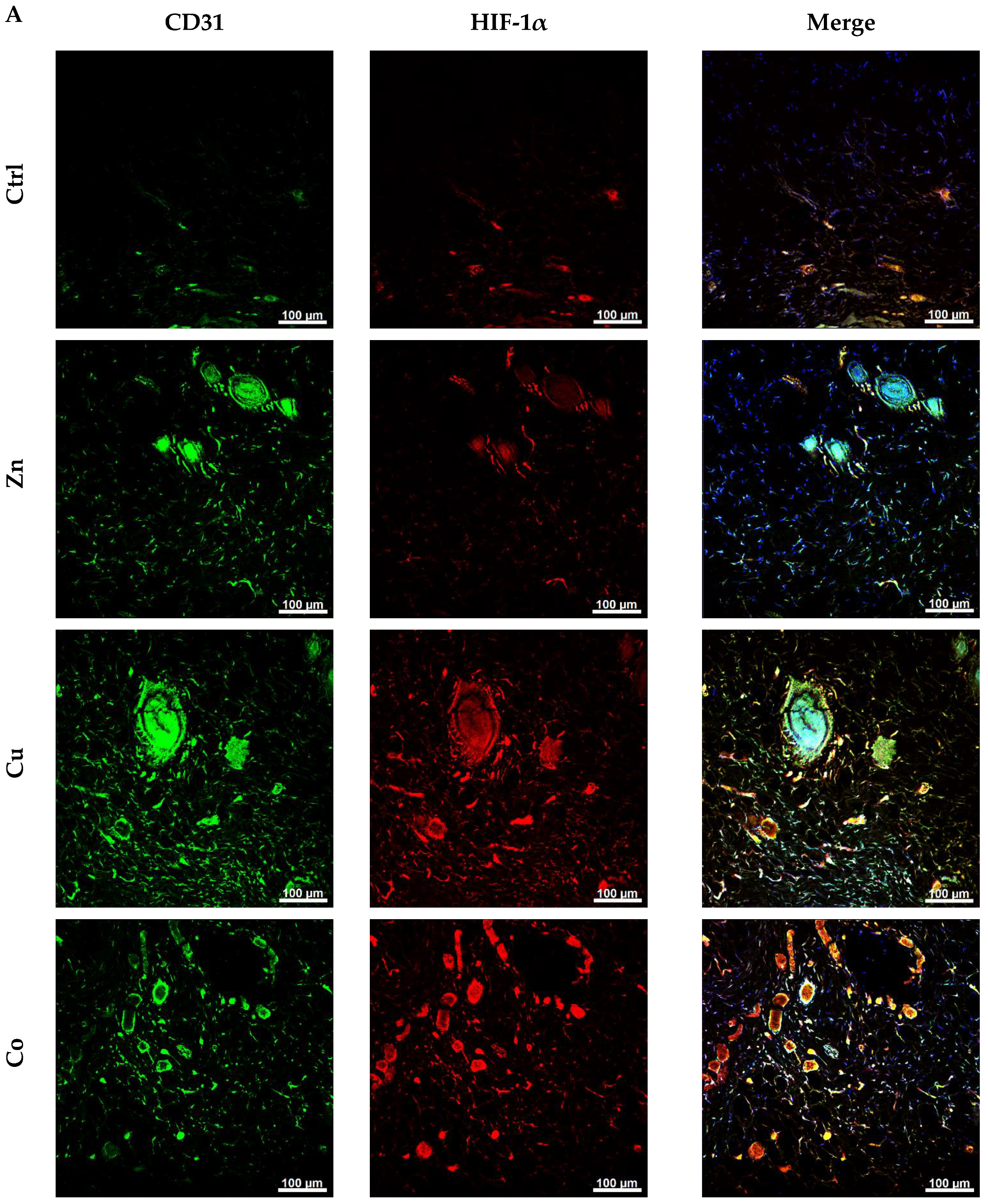
3.4.8. Immunohistochemical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbasian, M.; Massoumi, B.; Mohammad-Rezaei, R.; Samadian, H.; Jaymand, M. Scaffolding polymeric biomaterials: Are naturally occurring biological macromolecules more appropriate for tissue engineering? Int. J. Biol. Macromol. 2019, 134, 673–694. [Google Scholar] [CrossRef] [PubMed]
- Savina, I.N.; Zoughaib, M.; Yergeshov, A.A. Design and Assessment of Biodegradable Macroporous Cryogels as Advanced Tissue Engineering and Drug Carrying Materials. Gels 2021, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Rustad, K.C.; Wong, V.W.; Sorkin, M.; Glotzbach, J.P.; Major, M.R.; Rajadas, J.; Longaker, M.T.; Gurtner, G.C. Enhancement of mesenchymal stem cell angiogenic capacity and stemness by a biomimetic hydrogel scaffold. Biomaterials 2012, 33, 80–90. [Google Scholar] [CrossRef]
- Yergeshov, A.A.; Siraeva, Z.Y.; Kazakova, R.R.; Mullin, R.I.; Davliev, D.M.; Zakirova, A.A.; Salikhova, T.I.; Kuznetsova, E.V.; Luong, D.T.; Savina, I.N.; et al. Effect of gelatin cryogel on proliferation and synthetic activity of fibroblasts in excision wound model. Genes Cells 2015, 10, 29–33. [Google Scholar]
- Kamalov, M.I.; Lavrov, I.A.; Yergeshov, A.A.; Siraeva, Z.Y.; Baltin, M.E.; Rizvanov, A.A.; Kuznetcova, S.V.; Petrova, N.V.; Savina, I.N.; Abdullin, T.I. Non-invasive topical drug delivery to spinal cord with carboxyl-modified trifunctional copolymer of ethylene oxide and propylene oxide. Colloids Surf. B Biointerfaces 2016, 140, 196–203. [Google Scholar] [CrossRef]
- Etulain, J. Platelets in wound healing and regenerative medicine. Platelets 2018, 29, 556–568. [Google Scholar] [CrossRef]
- Zoughaib, M.; Pavlov, R.V.; Gaynanova, G.A.; Garifullin, R.; Evtugyn, V.G.; Abdullin, T.I. Amphiphilic RGD and GHK peptides synergistically enhance liposomal delivery into cancer and endothelial cells. Mater. Adv. 2021, 2, 7715–7730. [Google Scholar] [CrossRef]
- Brochhausen, C.; Lehmann, M.; Halstenberg, S.; Meurer, A.; Klaus, G.; Kirkpatrick, C.J. Signalling molecules and growth factors for tissue engineering of cartilage—what can we learn from the growth plate? J. Tissue Eng. Regen. Med. 2009, 3, 416–429. [Google Scholar] [CrossRef]
- Su, Y.; Cockerill, I.; Wang, Y.; Qin, Y.-X.; Chang, L.; Zheng, Y.; Zhu, D. Zinc-Based Biomaterials for Regeneration and Therapy. Trends Biotechnol. 2019, 37, 428–441. [Google Scholar] [CrossRef]
- Mourino, V.; Cattalini, J.P.; Boccaccini, A.R. Metallic ions as therapeutic agents in tissue engineering scaffolds: An overview of their biological applications and strategies for new developments. J. R. Soc. Interface 2012, 9, 401–419. [Google Scholar] [CrossRef]
- Ryan, E.J.; Ryan, A.J.; Gonzalez-Vazquez, A.; Philippart, A.; Ciraldo, F.E.; Hobbs, C.; Nicolosi, V.; Boccaccini, A.R.; Kearney, C.J.; O’Brien, F.J. Collagen scaffolds functionalised with copper-eluting bioactive glass reduce infection and enhance osteogenesis and angiogenesis both in vitro and in vivo. Biomaterials 2019, 197, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Lin, B.; Jiang, Z.; Huang, W.; Li, J.; Zeng, X.; Wang, H.; Wang, D.; Zhang, Y. Hypoxia-Mimicking Cobalt-Doped Borosilicate Bioactive Glass Scaffolds with Enhanced Angiogenic and Osteogenic Capacity for Bone Regeneration. Int. J. Biol. Sci. 2019, 15, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhou, M.; Gu, W.; Shen, Z.; Ma, X.; Lu, F.; Yang, X.; Zheng, Y.; Gou, Z. Zinc-/copper-substituted dicalcium silicate cement: Advanced biomaterials with enhanced osteogenesis and long-term antibacterial properties. J. Mater. Chem. B 2020, 8, 1060–1070. [Google Scholar] [CrossRef] [PubMed]
- Flegeau, K.; Pace, R.; Gautier, H.; Rethore, G.; Guicheux, J.; Le Visage, C.; Weiss, P. Toward the development of biomimetic injectable and macroporous biohydrogels for regenerative medicine. Adv. Colloid Interface Sci. 2017, 247, 589–609. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, H.W. Emerging properties of hydrogels in tissue engineering. J. Tissue Eng. 2018, 9, 2041731418768285. [Google Scholar] [CrossRef]
- Tao, B.; Lin, C.; Deng, Y.; Yuan, Z.; Shen, X.; Chen, M.; He, Y.; Peng, Z.; Hu, Y.; Cai, K. Copper-nanoparticle-embedded hydrogel for killing bacteria and promoting wound healing with photothermal therapy. J. Mater. Chem. B 2019, 7, 2534–2548. [Google Scholar] [CrossRef]
- Zhou, Q.; Kang, H.; Bielec, M.; Wu, X.; Cheng, Q.; Wei, W.; Dai, H. Influence of different divalent ions cross-linking sodium alginate-polyacrylamide hydrogels on antibacterial properties and wound healing. Carbohydr. Polym. 2018, 197, 292–304. [Google Scholar] [CrossRef]
- Shi, Q.; Luo, X.; Huang, Z.; Midgley, A.C.; Wang, B.; Liu, R.; Zhi, D.; Wei, T.; Zhou, X.; Qiao, M.; et al. Cobalt-mediated multi-functional dressings promote bacteria-infected wound healing. Acta Biomater. 2019, 86, 465–479. [Google Scholar] [CrossRef]
- Luong, D.; Yergeshov, A.A.; Zoughaib, M.; Sadykova, F.R.; Gareev, B.I.; Savina, I.N.; Abdullin, T.I. Transition metal-doped cryogels as bioactive materials for wound healing applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109759. [Google Scholar] [CrossRef]
- Zoughaib, M.; Luong, D.; Garifullin, R.; Gatina, D.Z.; Fedosimova, S.V.; Abdullin, T.I. Enhanced angiogenic effects of RGD, GHK peptides and copper (II) compositions in synthetic cryogel ECM model. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111660. [Google Scholar] [CrossRef]
- Luong, T.D.; Zoughaib, M.; Garifullin, R.; Kuznetsova, S.; Guler, M.O.; Abdullin, T.I. In Situ functionalization of Poly(hydroxyethyl methacrylate) Cryogels with Oligopeptides via β-Cyclodextrin–Adamantane Complexation for Studying Cell-Instructive Peptide Environment. ACS Appl. Bio Mater. 2020, 3, 1116–1128. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhou, Y.; Fan, W.; Han, P.; Chang, J.; Yuen, J.; Zhang, M.; Xiao, Y. Hypoxia-mimicking mesoporous bioactive glass scaffolds with controllable cobalt ion release for bone tissue engineering. Biomaterials 2012, 33, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Glushchenko, N.N.; Bogoslovskaya, O.A.; Shagdarova, B.T.; Il’ina, A.V.; Olkhovskaya, I.P.; Varlamov, V.P. Searching for synergistic effects of low-molecular weight chitosan derivatives, chitosan and copper nanoparticles for wound healing ointment. Adv. Nat. Sci. Nanosci. Nanotechnol. 2021, 12, 035016. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.; Xu, M.; Han, P.; Chen, L.; Chang, J.; Xiao, Y. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials 2013, 34, 422–433. [Google Scholar] [CrossRef]
- Khan, S.A.; Kanwal, S.; Rizwan, K.; Shahid, S. Enhanced antimicrobial, antioxidant, in vivo antitumor and in vitro anticancer effects against breast cancer cell line by green synthesized un-doped SnO2 and Co-doped SnO2 nanoparticles from Clerodendrum inerme. Microb. Pathog. 2018, 125, 366–384. [Google Scholar] [CrossRef]
- Abdollahi, Z.; Zare, E.N.; Salimi, F.; Goudarzi, I.; Tay, F.R.; Makvandi, P. Bioactive Carboxymethyl Starch-Based Hydrogels Decorated with CuO Nanoparticles: Antioxidant and Antimicrobial Properties and Accelerated Wound Healing In Vivo. Int. J. Mol. Sci. 2021, 22, 2531. [Google Scholar] [CrossRef]
- Szabo, R.; Bodolea, C.; Mocan, T. Iron, Copper, and Zinc Homeostasis: Physiology, Physiopathology, and Nanomediated Applications. Nanomaterials 2021, 11, 2958. [Google Scholar] [CrossRef]
- Powell, S.R. The antioxidant properties of zinc. J. Nutr. 2000, 130, 1447S–1454S. [Google Scholar] [CrossRef]
- Valko, M.; Jomova, K.; Rhodes, C.J.; Kuca, K.; Musilek, K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch Toxicol. 2016, 90, 1–37. [Google Scholar] [CrossRef]
- Modulevsky, D.J.; Cuerrier, C.M.; Pelling, A.E. Biocompatibility of Subcutaneously Implanted Plant-Derived Cellulose Biomaterials. PLoS ONE 2016, 11, e0157894. [Google Scholar] [CrossRef]
- Khorramirouz, R.; Go, J.L.; Noble, C.; Jana, S.; Maxson, E.; Lerman, A.; Young, M.D. A novel surgical technique for a rat subcutaneous implantation of a tissue engineered scaffold. Acta Histochem. 2018, 120, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Ahn, H.H.; Shin, Y.N.; Cho, M.H.; Khang, G.; Lee, H.B. An in vivo study of the host tissue response to subcutaneous implantation of PLGA- and/or porcine small intestinal submucosa-based scaffolds. Biomaterials 2007, 28, 5137–5143. [Google Scholar] [CrossRef] [PubMed]
- Tsepaeva, O.V.; Nemtarev, A.V.; Abdullin, T.I.; Grigor’Eva, L.R.; Kuznetsova, E.V.; Akhmadishina, R.A.; Ziganshina, L.E.; Cong, H.H.; Mironov, V.F. Design, Synthesis, and Cancer Cell Growth Inhibitory Activity of Triphenylphosphonium Derivatives of the Triterpenoid Betulin. J. Nat. Prod. 2017, 80, 2232–2239. [Google Scholar] [CrossRef] [PubMed]
- Zoughaib, M.H.; Luong, D.T.; Siraeva, Z.Y.; Yergeshov, A.A.; Salikhova, T.I.; Kuznetsova, S.V.; Kiyamova, R.G.; Abdullin, T.I. Tumor Cell Behavior in Porous Hydrogels: Effect of Application Technique and Doxorubicin Treatment. Bull. Exp. Biol. Med. 2019, 167, 590–598. [Google Scholar] [CrossRef]
- Akhmadishina, R.A.; Kuznetsova, E.V.; Sadrieva, G.R.; Sabirzyanova, L.R.; Nizamov, I.S.; Akhmedova, G.R.; Nizamov, I.D.; Abdullin, T.I. Glutathione salts of O, O-diorganyl dithiophosphoric acids: Synthesis and study as redox modulating and antiproliferative compounds. Peptides 2018, 99, 179–188. [Google Scholar] [CrossRef]
- Huang, M.T.; Mason, J.C.; Birdsey, G.M.; Amsellem, V.; Gerwin, N.; Haskard, D.O.; Ridley, A.J.; Randi, A.M. Endothelial intercellular adhesion molecule (ICAM)-2 regulates angiogenesis. Blood 2005, 106, 1636–1643. [Google Scholar] [CrossRef]
- Gustafsson, A.; Jonasson, S.; Sandstrom, T.; Lorentzen, J.C.; Bucht, A. Genetic variation influences immune responses in sensitive rats following exposure to TiO2 nanoparticles. Toxicology 2014, 326, 74–85. [Google Scholar] [CrossRef]
- Philips, N.; Hwang, H.; Chauhan, S.; Leonardi, D.; Gonzalez, S. Stimulation of cell proliferation and expression of matrixmetalloproteinase-1 and interluekin-8 genes in dermal fibroblasts by copper. Connect. Tissue Res. 2010, 51, 224–229. [Google Scholar] [CrossRef]
- Hyldig, K.; Riis, S.; Pennisi, C.P.; Zachar, V.; Fink, T. Implications of Extracellular Matrix Production by Adipose Tissue-Derived Stem Cells for Development of Wound Healing Therapies. Int. J. Mol. Sci. 2017, 18, 1167. [Google Scholar] [CrossRef]
- Naldaiz-Gastesi, N.; Goicoechea, M.; Alonso-Martin, S.; Aiastui, A.; Lopez-Mayorga, M.; Garcia-Belda, P.; Lacalle, J.; San Jose, C.; Arauzo-Bravo, M.J.; Trouilh, L.; et al. Identification and Characterization of the Dermal Panniculus Carnosus Muscle Stem Cells. Stem Cell Rep. 2016, 7, 411–424. [Google Scholar] [CrossRef]
- Perni, S.; Alotaibi, H.F.; Yergeshov, A.A.; Dang, T.; Abdullin, T.I.; Prokopovich, P. Long acting anti-infection constructs on titanium. J. Control. Release 2020, 326, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Meruane, M.A.; Rojas, M.; Marcelain, K. The use of adipose tissue-derived stem cells within a dermal substitute improves skin regeneration by increasing neoangiogenesis and collagen synthesis. Plast. Reconstr. Surg. 2012, 130, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Muller-Rover, S.; Handjiski, B.; van der Veen, C.; Eichmuller, S.; Foitzik, K.; McKay, I.A.; Stenn, K.S.; Paus, R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Investig. Dermatol. 2001, 117, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.W.; Kloepper, J.; Langan, E.A.; Kim, Y.; Yeo, J.; Kim, M.J.; Hsi, T.C.; Rose, C.; Yoon, G.S.; Lee, S.J.; et al. A Guide to Studying Human Hair Follicle Cycling In Vivo. J. Investig. Dermatol. 2016, 136, 34–44. [Google Scholar] [CrossRef]
- Jidigam, V.K.; Srinivasan, R.C.; Patthey, C.; Gunhaga, L. Apical constriction and epithelial invagination are regulated by BMP activity. Biol. Open 2015, 4, 1782–1791. [Google Scholar] [CrossRef]
- Lugo-Villarino, G.; Balla, K.M.; Stachura, D.L.; Banuelos, K.; Werneck, M.B.; Traver, D. Identification of dendritic antigen-presenting cells in the zebrafish. Proc. Natl. Acad. Sci. USA 2010, 107, 15850–15855. [Google Scholar] [CrossRef]
- Al-Maawi, S.; Orlowska, A.; Sader, R.; James Kirkpatrick, C.; Ghanaati, S. In vivo cellular reactions to different biomaterials-Physiological and pathological aspects and their consequences. Semin. Immunol. 2017, 29, 49–61. [Google Scholar] [CrossRef]
- Spadaro, J.A.; Becker, R.O.; Bachman, C.H. Size-specific metal complexing sites in native collagen. Nature 1970, 225, 1134–1136. [Google Scholar] [CrossRef]
- Hoppe, A.; Mourino, V.; Boccaccini, A.R. Therapeutic inorganic ions in bioactive glasses to enhance bone formation and beyond. Biomater. Sci. 2013, 1, 254–256. [Google Scholar] [CrossRef]
- Zhong, J.; Yang, Y.; Liao, L.; Zhang, C. Matrix stiffness-regulated cellular functions under different dimensionalities. Biomater. Sci. 2020, 8, 2734–2755. [Google Scholar] [CrossRef]
- Galaup, C.; Picard, C.; Couderc, F.; Gilard, V.; Collin, F. Luminescent lanthanide complexes for reactive oxygen species biosensing and possible application in Alzheimer’s diseases. FEBS J. 2021, 15859. [Google Scholar] [CrossRef] [PubMed]
- Alhayaza, R.; Haque, E.; Karbasiafshar, C.; Sellke, F.W.; Abid, M.R. The Relationship Between Reactive Oxygen Species and Endothelial Cell Metabolism. Front Chem. 2020, 8, 592688. [Google Scholar] [CrossRef] [PubMed]
- van der Vliet, A.; Janssen-Heininger, Y.M.W. Hydrogen Peroxide as a Damage Signal in Tissue Injury and Inflammation: Murderer, Mediator, or Messenger? J. Cell. Biochem. 2014, 115, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Niethammer, P.; Grabher, C.; Look, A.T.; Mitchison, T.J. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 2009, 459, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R. Critical Role of Zinc as Either an Antioxidant or a Prooxidant in Cellular Systems. Oxidative Med. Cell. Longev. 2018, 2018, 9156285. [Google Scholar] [CrossRef]
- Maret, W. Metallothionein redox biology in the cytoprotective and cytotoxic functions of zinc. Exp. Gerontol. 2008, 43, 363–369. [Google Scholar] [CrossRef]
- Rodriguez-Menendez, S.; Garcia, M.; Fernandez, B.; Alvarez, L.; Fernandez-Vega-Cueto, A.; Coca-Prados, M.; Pereiro, R.; Gonzalez-Iglesias, H. The Zinc-Metallothionein Redox System Reduces Oxidative Stress in Retinal Pigment Epithelial Cells. Nutrients 2018, 10, 1874. [Google Scholar] [CrossRef]
- Andrews, G.K. Cellular zinc sensors: MTF-1 regulation of gene expression. Biometals 2001, 14, 223–237. [Google Scholar] [CrossRef]
- Tsepaeva, O.V.; Salikhova, T.I.; Grigor’Eva, L.R.; Ponomaryov, D.V.; Dang, T.; Ishkaeva, R.A.; Abdullin, T.I.; Nemtarev, A.V.; Mironov, V.F. Synthesis and in vitro evaluation of triphenylphosphonium derivatives of acetylsalicylic and salicylic acids: Structure-dependent interactions with cancer cells, bacteria, and mitochondria. Med. Chem. Res. 2021, 30, 925–939. [Google Scholar] [CrossRef]
- Sabbioni, E.; Fortaner, S.; Farina, M.; Del Torchio, R.; Olivato, I.; Petrarca, C.; Bernardini, G.; Mariani-Costantini, R.; Perconti, S.; Di Giampaolo, L.; et al. Cytotoxicity and morphological transforming potential of cobalt nanoparticles, microparticles and ions in Balb/3T3 mouse fibroblasts: An in vitro model. Nanotoxicology 2014, 8, 455–464. [Google Scholar] [CrossRef]
- Lovejoy, D.B.; Richardson, D.R. Iron chelators as anti-neoplastic agents: Current developments and promise of the PIH class of chelators. Curr. Med. Chem. 2003, 10, 1035–1049. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Yu, D.; Yan, X.; Wang, B.; Yu, Z.; Song, Y.; Sheng, L. Hypoxia destroys the microstructure of microtubules and causes dysfunction of endothelial cells via the PI3K/Stathmin1 pathway. Cell Biosci. 2019, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Nardinocchi, L.; Pantisano, V.; Puca, R.; Porru, M.; Aiello, A.; Grasselli, A.; Leonetti, C.; Safran, M.; Rechavi, G.; Givol, D.; et al. Zinc downregulates HIF-1alpha and inhibits its activity in tumor cells in vitro and in vivo. PLoS ONE 2010, 5, e15048. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Bai, L.; Zhou, J.; Chen, H.; Zhang, L. Sequential delivery of VEGF, FGF-2 and PDGF from the polymeric system enhance HUVECs angiogenesis in vitro and CAM angiogenesis. Cell. Immunol. 2018, 323, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Wang, K.; Zhelyabovska, O.; Saad, Y.; Kolattukudy, P.E. MCP-1-induced protein promotes endothelial-like and angiogenic properties in human bone marrow monocytic cells. J. Pharmacol. Exp. Ther. 2013, 347, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Sanberg, P.R.; Park, D.H.; Kuzmin-Nichols, N.; Cruz, E.; Hossne, N.A., Jr.; Buffolo, E.; Willing, A.E. Monocyte transplantation for neural and cardiovascular ischemia repair. J. Cell. Mol. Med. 2010, 14, 553–563. [Google Scholar] [CrossRef]
- Ozturk, B.Y.; Inci, I.; Egri, S.; Ozturk, A.M.; Yetkin, H.; Goktas, G.; Elmas, C.; Piskin, E.; Erdogan, D. The treatment of segmental bone defects in rabbit tibiae with vascular endothelial growth factor (VEGF)-loaded gelatin/hydroxyapatite "cryogel" scaffold. Eur. J. Orthop. Surg. Traumatol. Orthop. Traumatol. 2013, 23, 767–774. [Google Scholar] [CrossRef]
- Matsui, M.; Tabata, Y. Enhanced angiogenesis by multiple release of platelet-rich plasma contents and basic fibroblast growth factor from gelatin hydrogels. Acta Biomater. 2012, 8, 1792–1801. [Google Scholar] [CrossRef]
- Nillesen, S.T.; Geutjes, P.J.; Wismans, R.; Schalkwijk, J.; Daamen, W.F.; van Kuppevelt, T.H. Increased angiogenesis and blood vessel maturation in acellular collagen-heparin scaffolds containing both FGF2 and VEGF. Biomaterials 2007, 28, 1123–1131. [Google Scholar] [CrossRef]
- Joseph, B.; Augustine, R.; Kalarikkal, N.; Thomas, S.; Seantier, B.; Grohens, Y. Recent advances in electrospun polycaprolactone based scaffolds for wound healing and skin bioengineering applications. Mater. Today Commun. 2019, 19, 319–335. [Google Scholar] [CrossRef]
- Rennert, R.C.; Sorkin, M.; Garg, R.K.; Januszyk, M.; Gurtner, G.C. Cellular response to a novel fetal acellular collagen matrix: Implications for tissue regeneration. Int. J. Biomater. 2013, 2013, 527957. [Google Scholar] [CrossRef]
- Giavaresi, G.; Torricelli, P.; Fornasari, P.M.; Giardino, R.; Barbucci, R.; Leone, G. Blood vessel formation after soft-tissue implantation of hyaluronan-based hydrogel supplemented with copper ions. Biomaterials 2005, 26, 3001–3008. [Google Scholar] [CrossRef] [PubMed]
- Siebert, L.; Luna-Cerón, E.; García-Rivera, L.E.; Oh, J.; Jang, J.; Rosas-Gómez, D.A.; Pérez-Gómez, M.D.; Maschkowitz, G.; Fickenscher, H.; Oceguera-Cuevas, D.; et al. Light-Controlled Growth Factors Release on Tetrapodal ZnO-Incorporated 3D-Printed Hydrogels for Developing Smart Wound Scaffold. Adv. Funct. Mater. 2021, 31, 2007555. [Google Scholar] [CrossRef]
- Nosrati, R.; Kheirouri, S.; Ghodsi, R.; Ojaghi, H. The effects of zinc treatment on matrix metalloproteinases: A systematic review. J. Trace Elem. Med. Biol. 2019, 56, 107–115. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L. Effects of CoCl2-simulated hypoxia on the expression levels of matrix metalloproteinases in renal adenocarcinoma cells and renal tubular epithelial cells. Exp. Ther. Med. 2018, 16, 1454–1460. [Google Scholar] [CrossRef]
- Luo, L.; Petit, A.; Antoniou, J.; Zukor, D.J.; Huk, O.L.; Liu, R.C.; Winnik, F.M.; Mwale, F. Effect of cobalt and chromium ions on MMP-1, TIMP-1, and TNF-alpha gene expression in human U937 macrophages: A role for tyrosine kinases. Biomaterials 2005, 26, 5587–5593. [Google Scholar] [CrossRef]
- Pyo, H.K.; Yoo, H.G.; Won, C.H.; Lee, S.H.; Kang, Y.J.; Eun, H.C.; Cho, K.H.; Kim, K.H. The effect of tripeptide-copper complex on human hair growth in vitro. Arch. Pharmacal Res. 2007, 30, 834–839. [Google Scholar] [CrossRef]
- Zhao, S.; Li, L.; Wang, H.; Zhang, Y.; Cheng, X.; Zhou, N.; Rahaman, M.N.; Liu, Z.; Huang, W.; Zhang, C. Wound dressings composed of copper-doped borate bioactive glass microfibers stimulate angiogenesis and heal full-thickness skin defects in a rodent model. Biomaterials 2015, 53, 379–391. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, Y.; Chen, J.; Duan, X.; Guo, B. Mussel-inspired adhesive antioxidant antibacterial hemostatic composite hydrogel wound dressing via photo-polymerization for infected skin wound healing. Bioact. Mater. 2022, 8, 341–354. [Google Scholar] [CrossRef]
- Gerard, C.; Bordeleau, L.-J.; Barralet, J.; Doillon, C.J. The stimulation of angiogenesis and collagen deposition by copper. Biomaterials 2010, 31, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Yoon, J.; Park, B. Pomolic acid suppresses HIF1 α/VEGF-mediated angiogenesis by targeting p38-MAPK and mTOR signaling cascades. Phytomedicine 2016, 23, 1716–1726. [Google Scholar] [CrossRef] [PubMed]
- Ahtzaz, S.; Nasir, M.; Shahzadi, L.; Amir, W.; Anjum, A.; Arshad, R.; Iqbal, F.; Chaudhry, A.A.; Yar, M.; ur Rehman, I. A study on the effect of zinc oxide and zinc peroxide nanoparticles to enhance angiogenesis-pro-angiogenic grafts for tissue regeneration applications. Mater. Des. 2017, 132, 409–418. [Google Scholar] [CrossRef]
- Lin, Y.; Brown, R.F.; Jung, S.B.; Day, D.E. Angiogenic effects of borate glass microfibers in a rodent model. J. Biomed. Mater. Res. Part A 2014, 102, 4491–4499. [Google Scholar] [CrossRef]
- Perez, R.A.; Kim, J.H.; Buitrago, J.O.; Wall, I.B.; Kim, H.W. Novel therapeutic core-shell hydrogel scaffolds with sequential delivery of cobalt and bone morphogenetic protein-2 for synergistic bone regeneration. Acta Biomater. 2015, 23, 295–308. [Google Scholar] [CrossRef]
- Barrioni, B.R.; de Laia, A.G.S.; Valverde, T.M.; Martins, T.M.D.; Caliari, M.V.; de Sa, M.A.; de Goes, A.M.; Pereira, M.D. Evaluation of in vitro and in vivo biocompatibility and structure of cobalt releasing sol-gel bioactive glass. Ceram. Int. 2018, 44, 20337–20347. [Google Scholar] [CrossRef]
- Lin, P.-H.; Sermersheim, M.; Li, H.; Lee, P.H.U.; Steinberg, S.M.; Ma, J. Zinc in Wound Healing Modulation. Nutrients 2017, 10, 16. [Google Scholar] [CrossRef]
- Dierichs, L.; Kloubert, V.; Rink, L. Cellular zinc homeostasis modulates polarization of THP-1-derived macrophages. Eur. J. Nutr. 2018, 57, 2161–2169. [Google Scholar] [CrossRef]
- Song, Y.; Wu, H.; Gao, Y.; Li, J.; Lin, K.; Liu, B.; Lei, X.; Cheng, P.; Zhang, S.; Wang, Y.; et al. Zinc Silicate/Nano-Hydroxyapatite/Collagen Scaffolds Promote Angiogenesis and Bone Regeneration via the p38 MAPK Pathway in Activated Monocytes. ACS Appl. Mater. Interfaces 2020, 12, 16058–16075. [Google Scholar] [CrossRef]
- Gammoh, N.Z.; Rink, L. Zinc in Infection and Inflammation. Nutrients 2017, 9, 624. [Google Scholar] [CrossRef]
- Landgraeber, S.; Jäger, M.; Jacobs, J.J.; Hallab, N.J. The Pathology of Orthopedic Implant Failure Is Mediated by Innate Immune System Cytokines. Mediat. Inflamm. 2014, 2014, 185150. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yergeshov, A.A.; Zoughaib, M.; Ishkaeva, R.A.; Savina, I.N.; Abdullin, T.I. Regenerative Activities of ROS-Modulating Trace Metals in Subcutaneously Implanted Biodegradable Cryogel. Gels 2022, 8, 118. https://doi.org/10.3390/gels8020118
Yergeshov AA, Zoughaib M, Ishkaeva RA, Savina IN, Abdullin TI. Regenerative Activities of ROS-Modulating Trace Metals in Subcutaneously Implanted Biodegradable Cryogel. Gels. 2022; 8(2):118. https://doi.org/10.3390/gels8020118
Chicago/Turabian StyleYergeshov, Abdulla A., Mohamed Zoughaib, Rezeda A. Ishkaeva, Irina N. Savina, and Timur I. Abdullin. 2022. "Regenerative Activities of ROS-Modulating Trace Metals in Subcutaneously Implanted Biodegradable Cryogel" Gels 8, no. 2: 118. https://doi.org/10.3390/gels8020118
APA StyleYergeshov, A. A., Zoughaib, M., Ishkaeva, R. A., Savina, I. N., & Abdullin, T. I. (2022). Regenerative Activities of ROS-Modulating Trace Metals in Subcutaneously Implanted Biodegradable Cryogel. Gels, 8(2), 118. https://doi.org/10.3390/gels8020118