Polyelectrolyte Gels: Fundamentals, Fabrication and Applications
Abstract
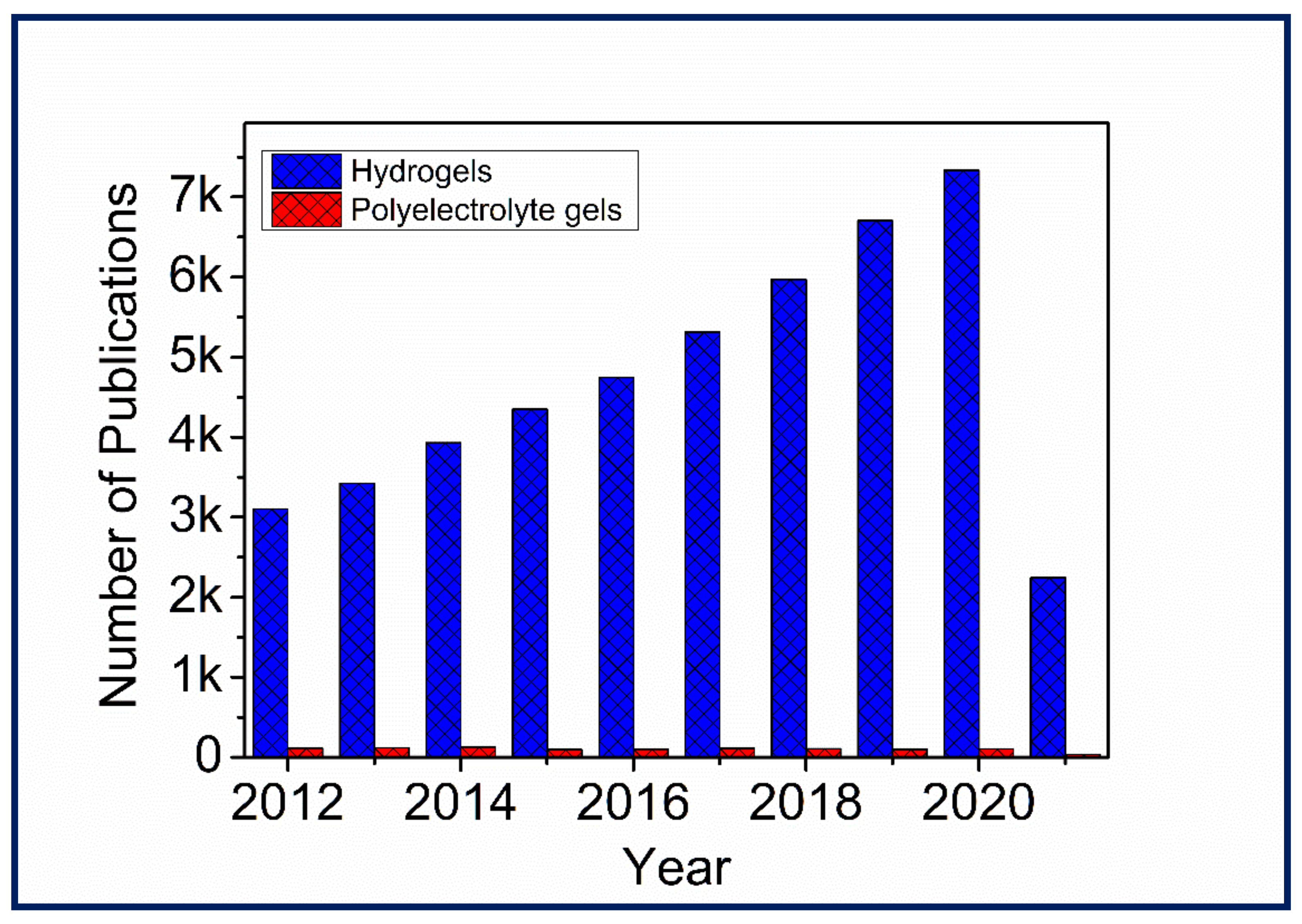
1. Introduction
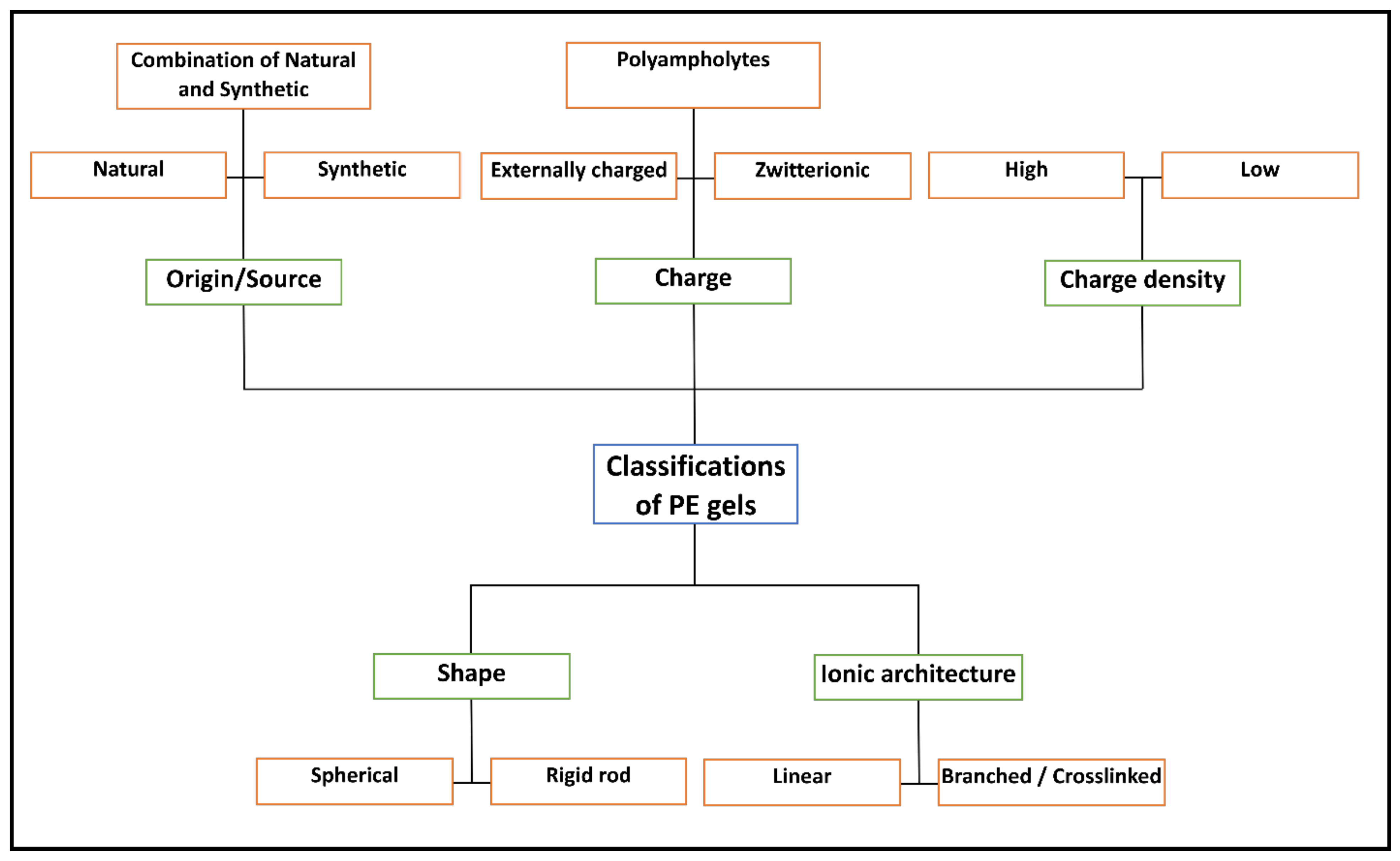
2. Fundamentals of PE Gels
2.1. Classification of PE Gels
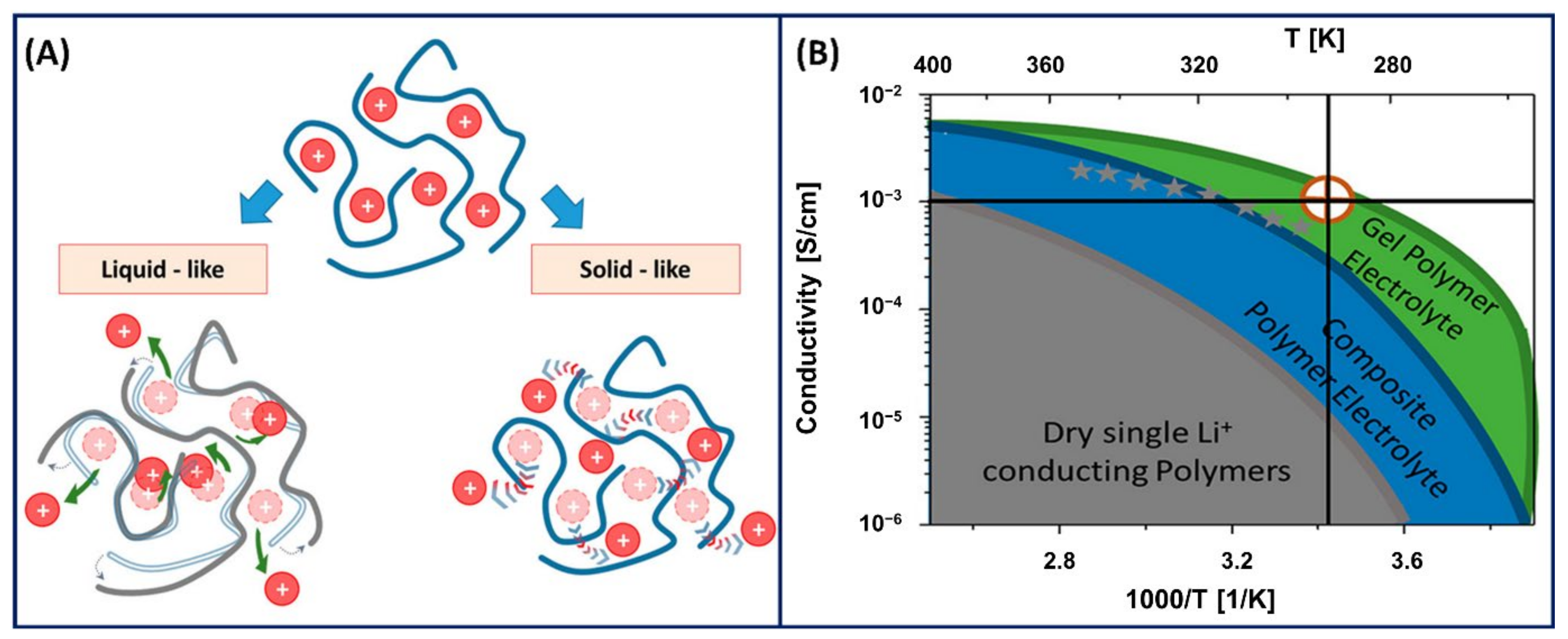
2.2. Important Aspects in PE Gels
2.2.1. Conductivity
2.2.2. Stimuli Responsiveness
2.2.3. Crosslink Density of PE Gels
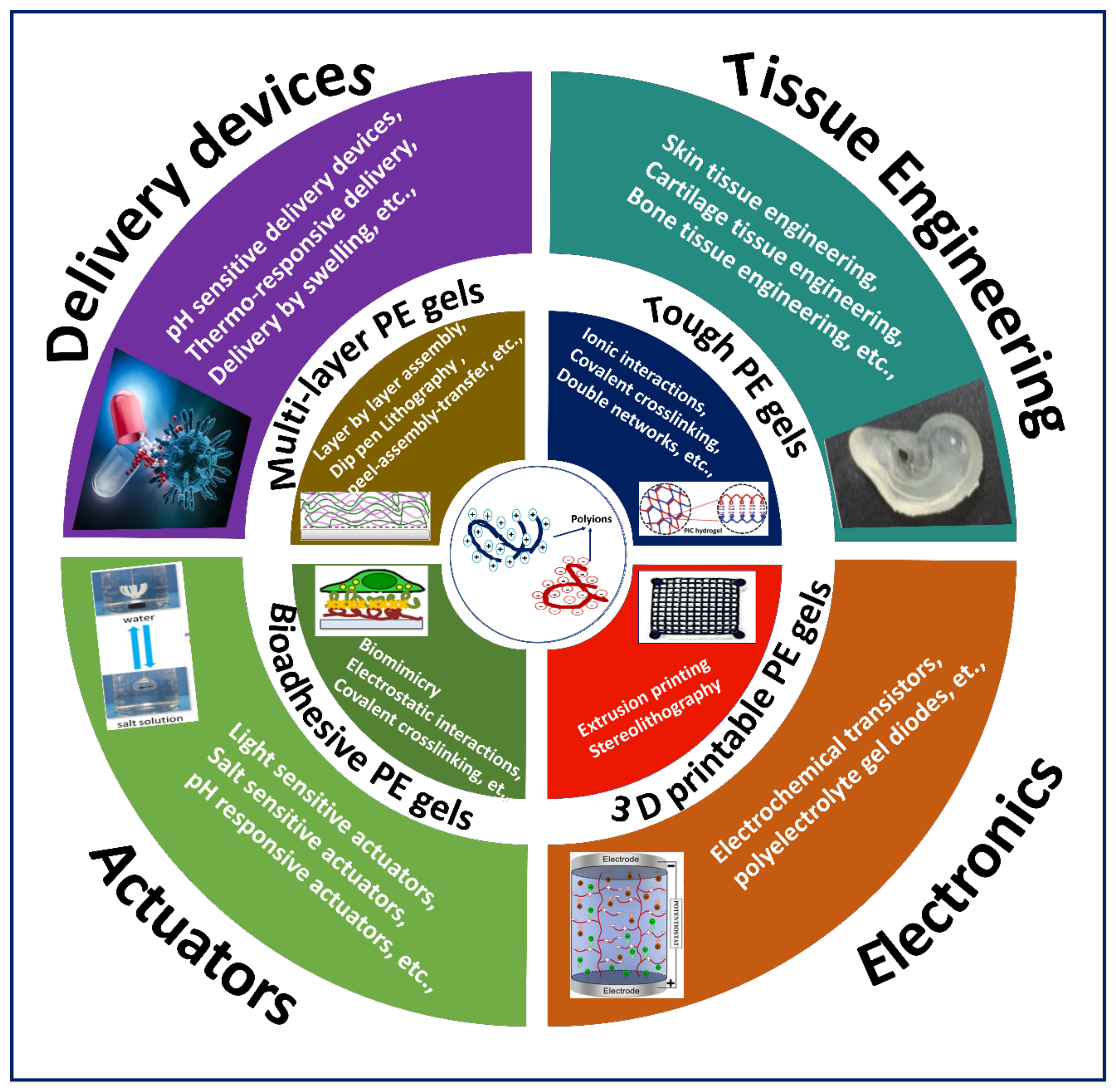
3. Fabrication of Different Types of PE Gels
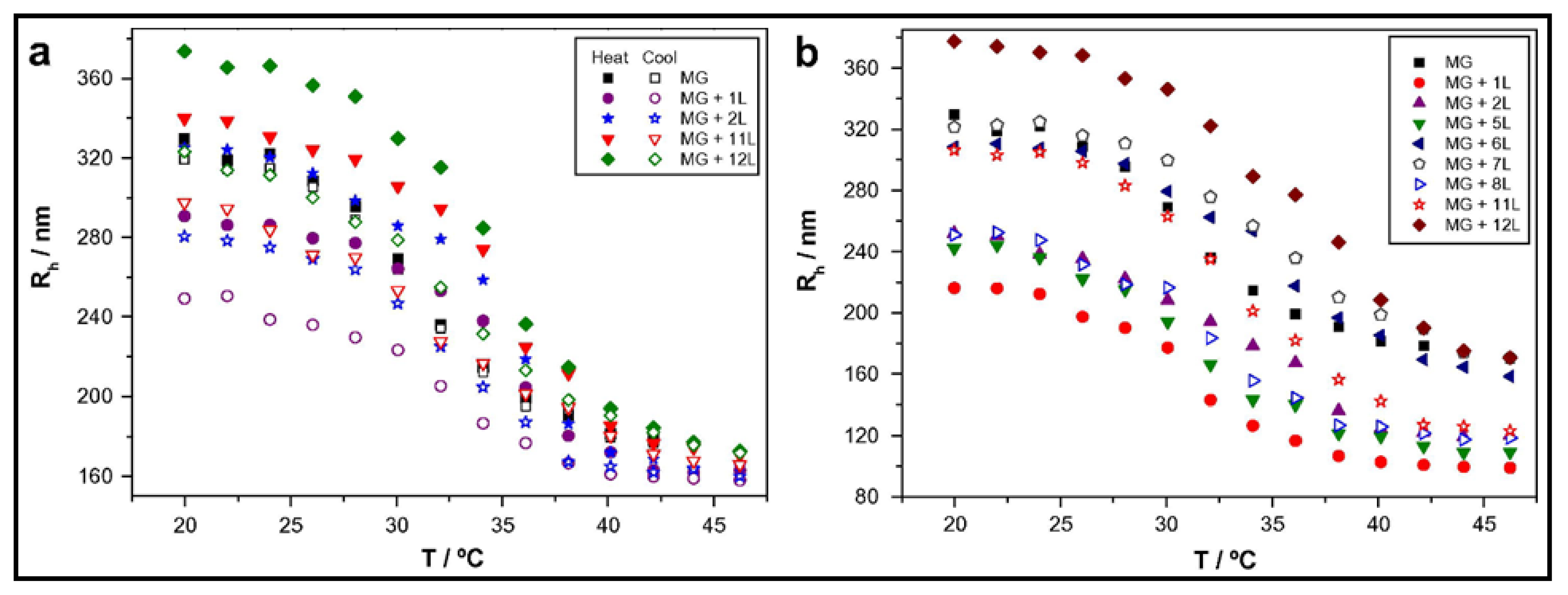
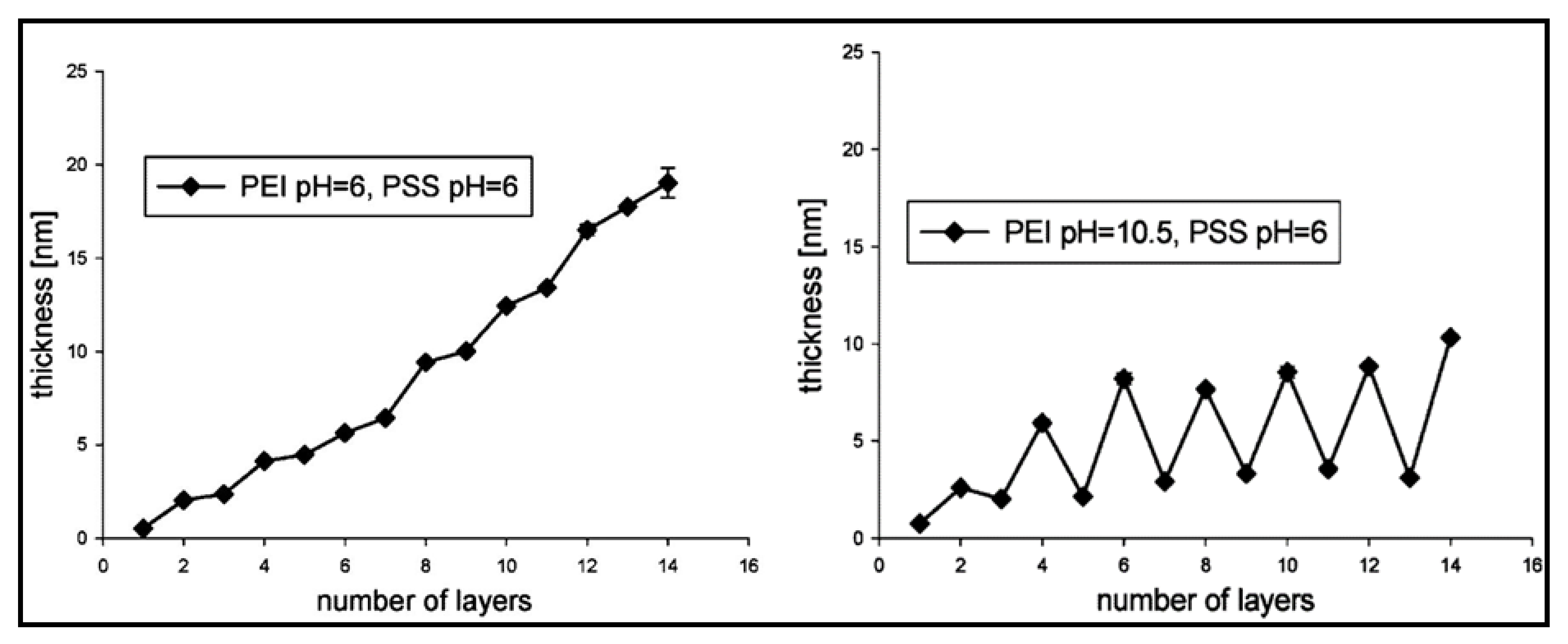
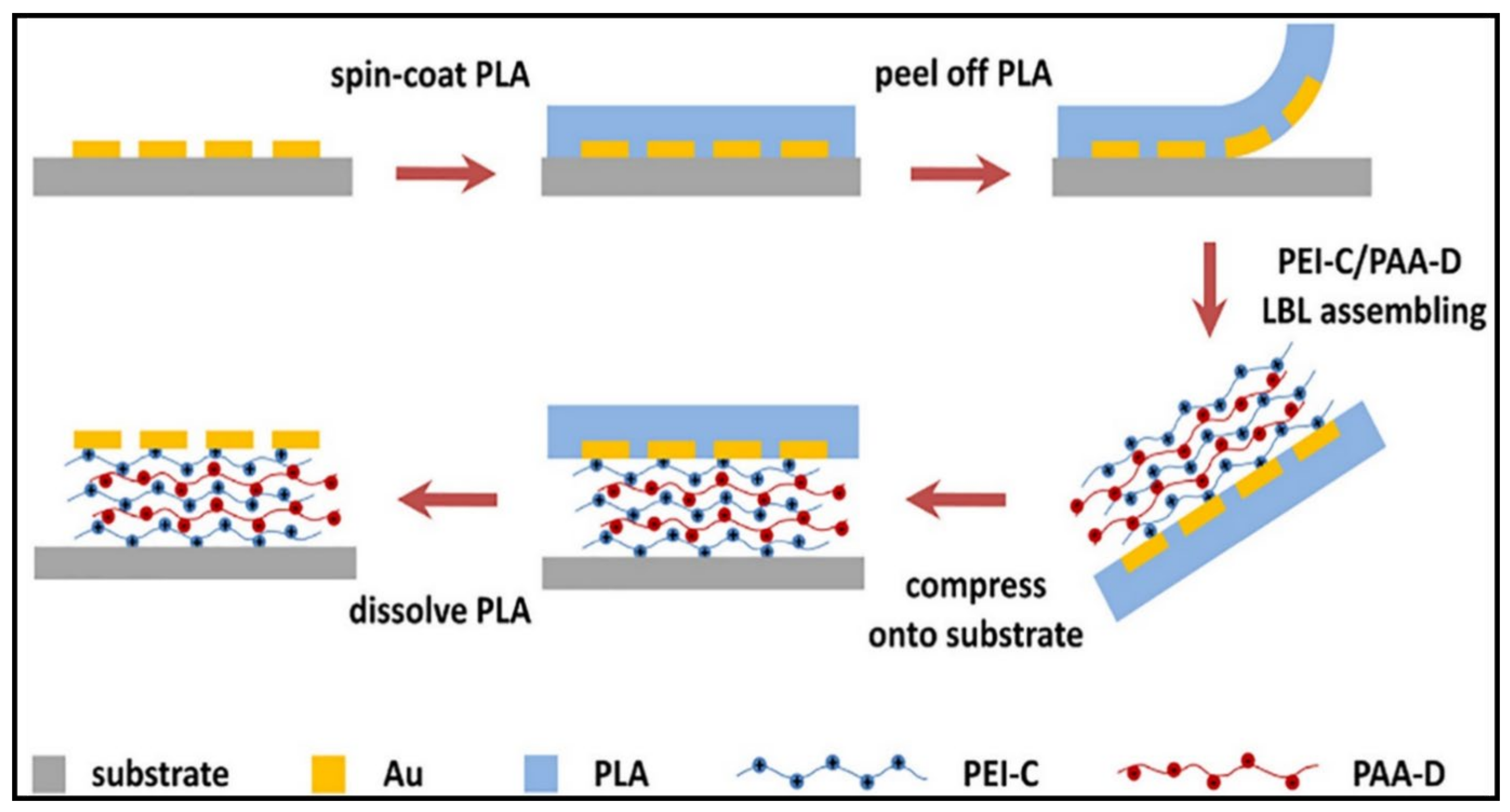
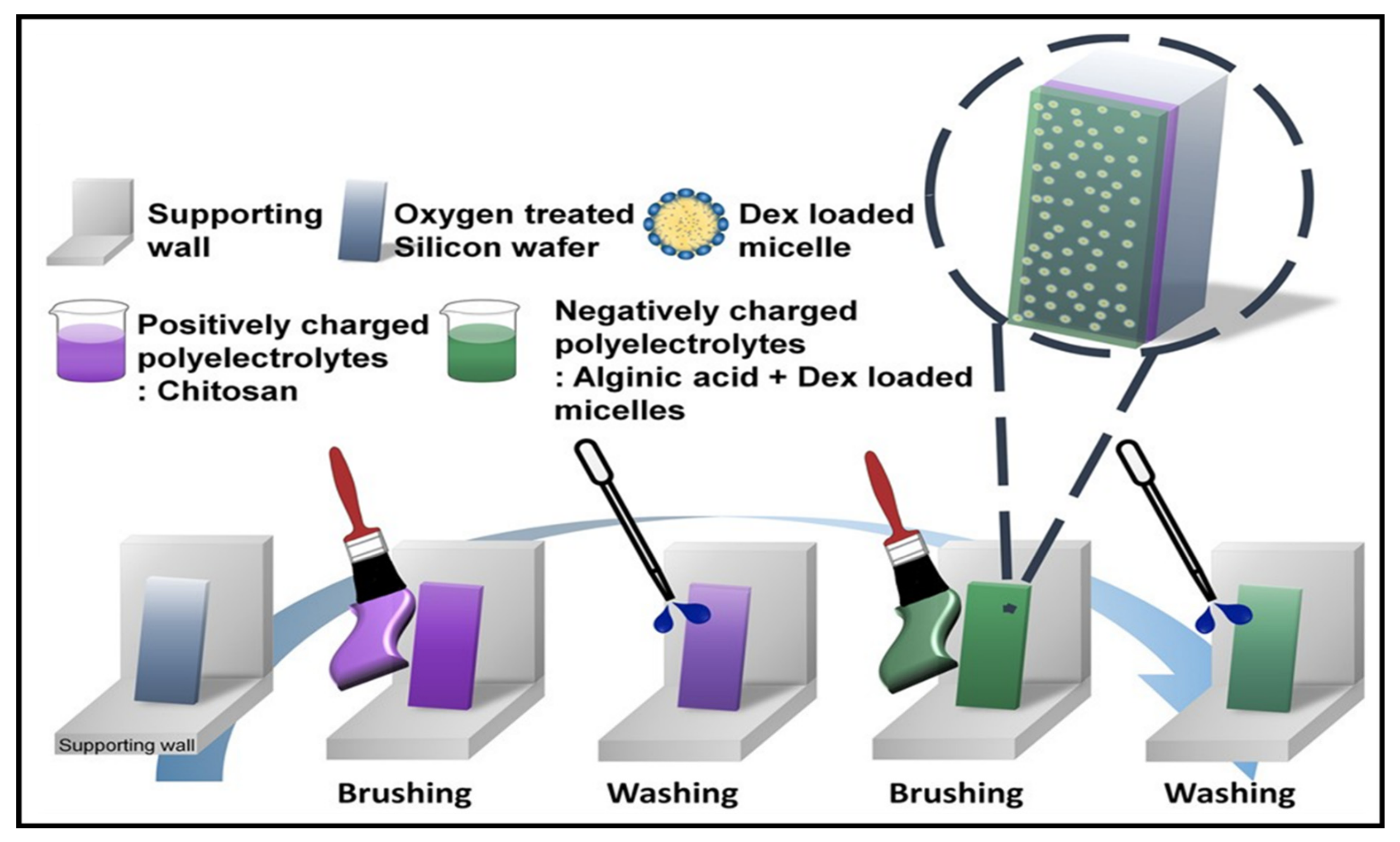
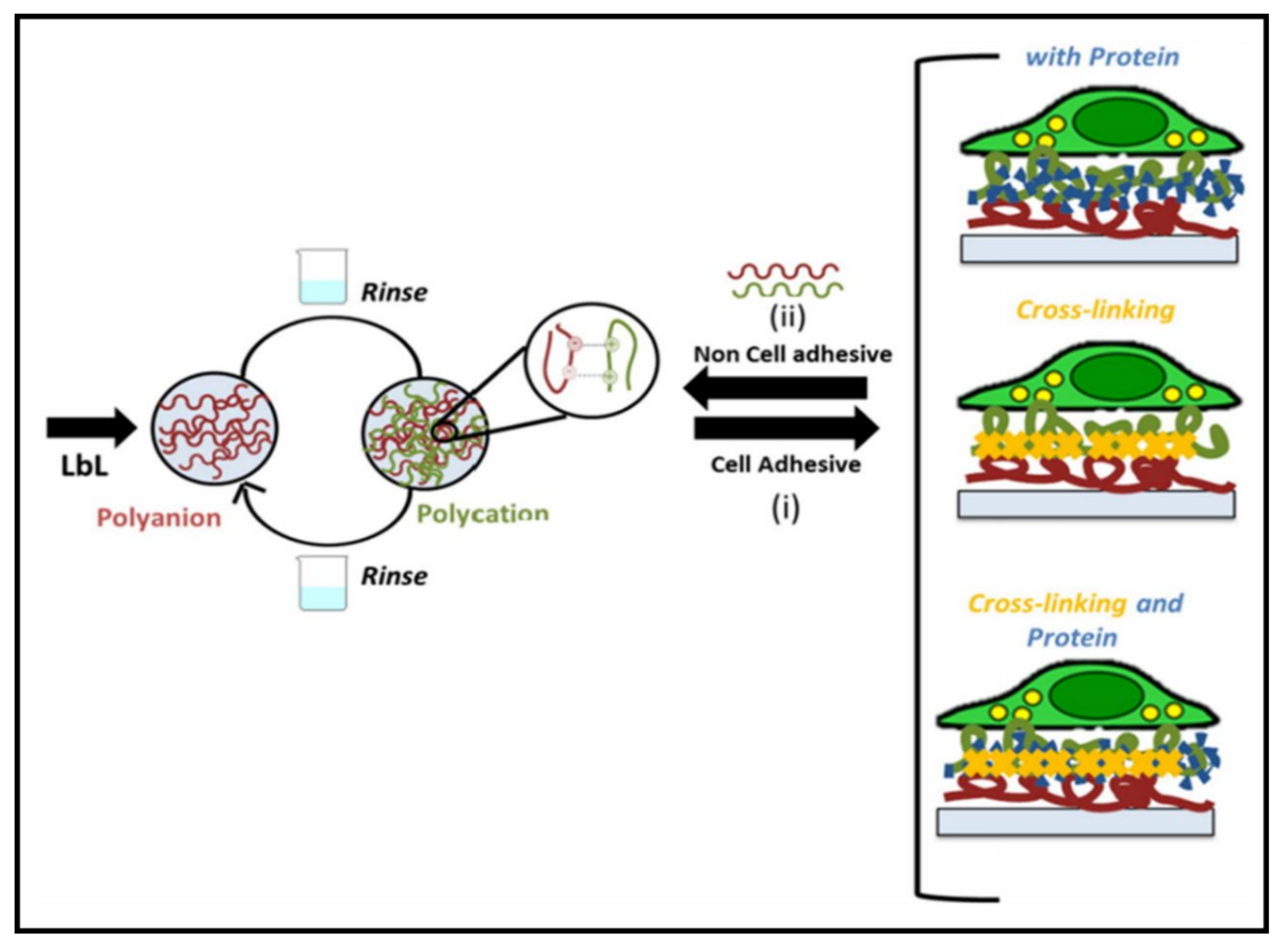
3.1. Multilayered PE Gels
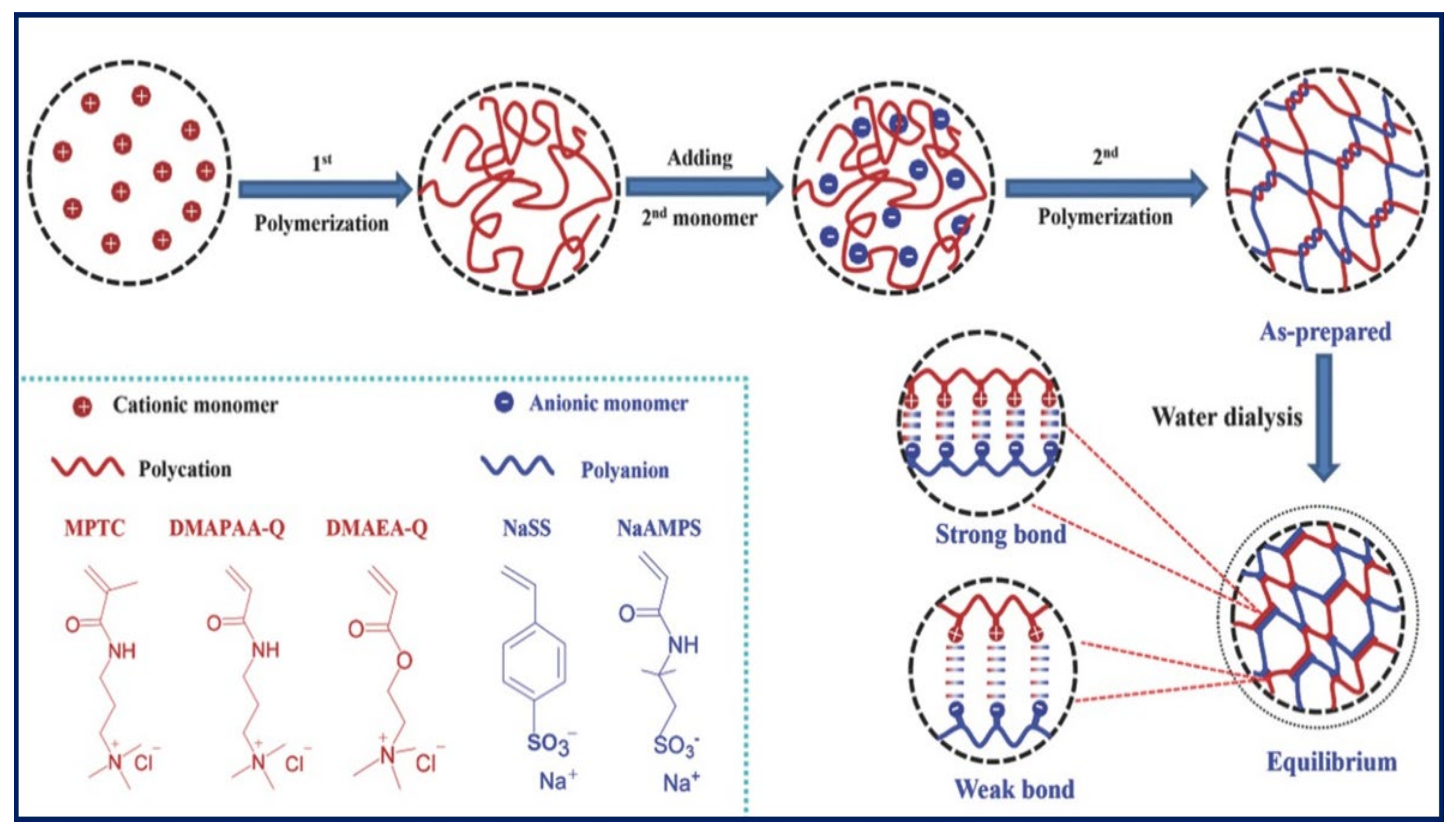
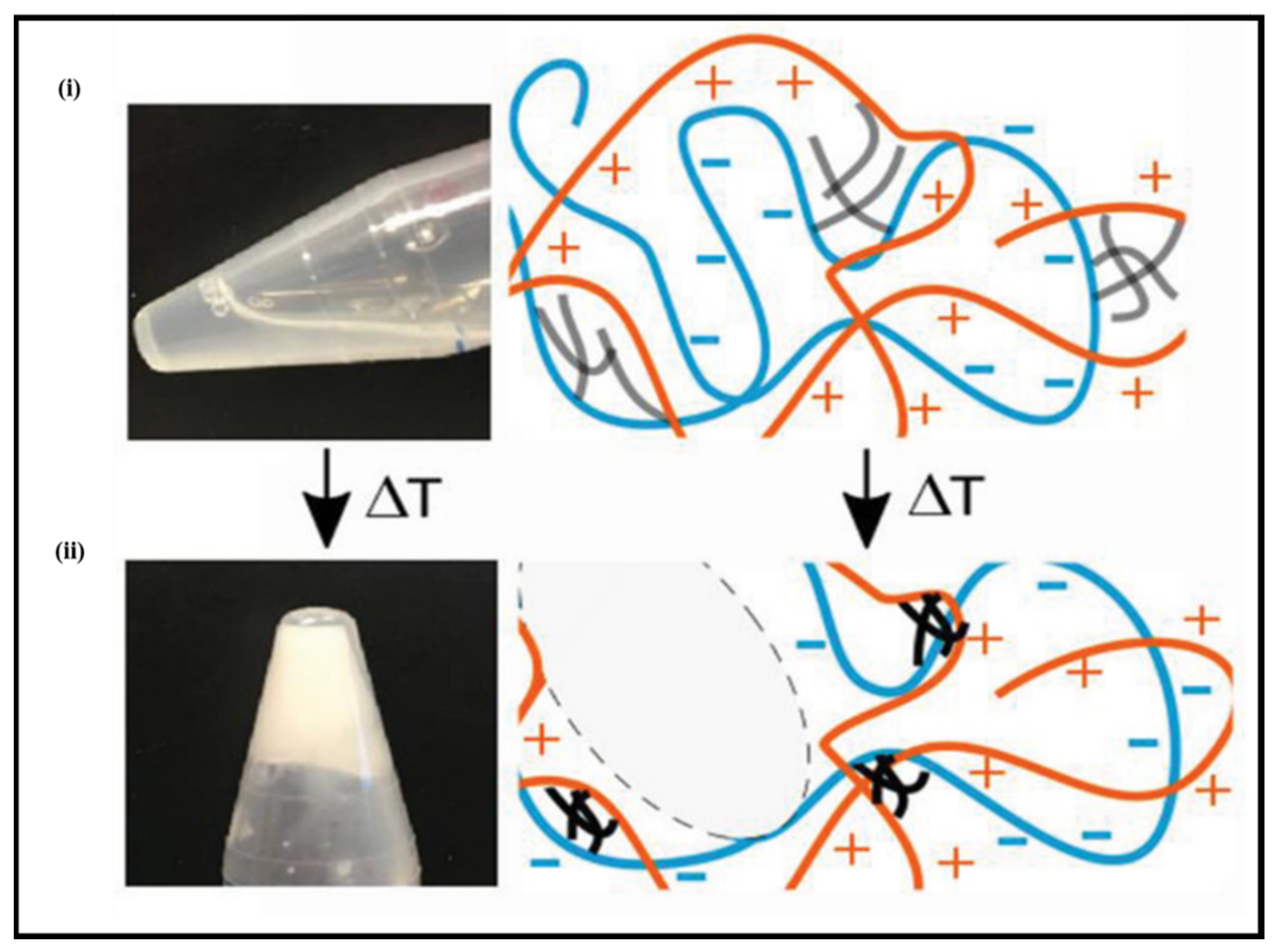
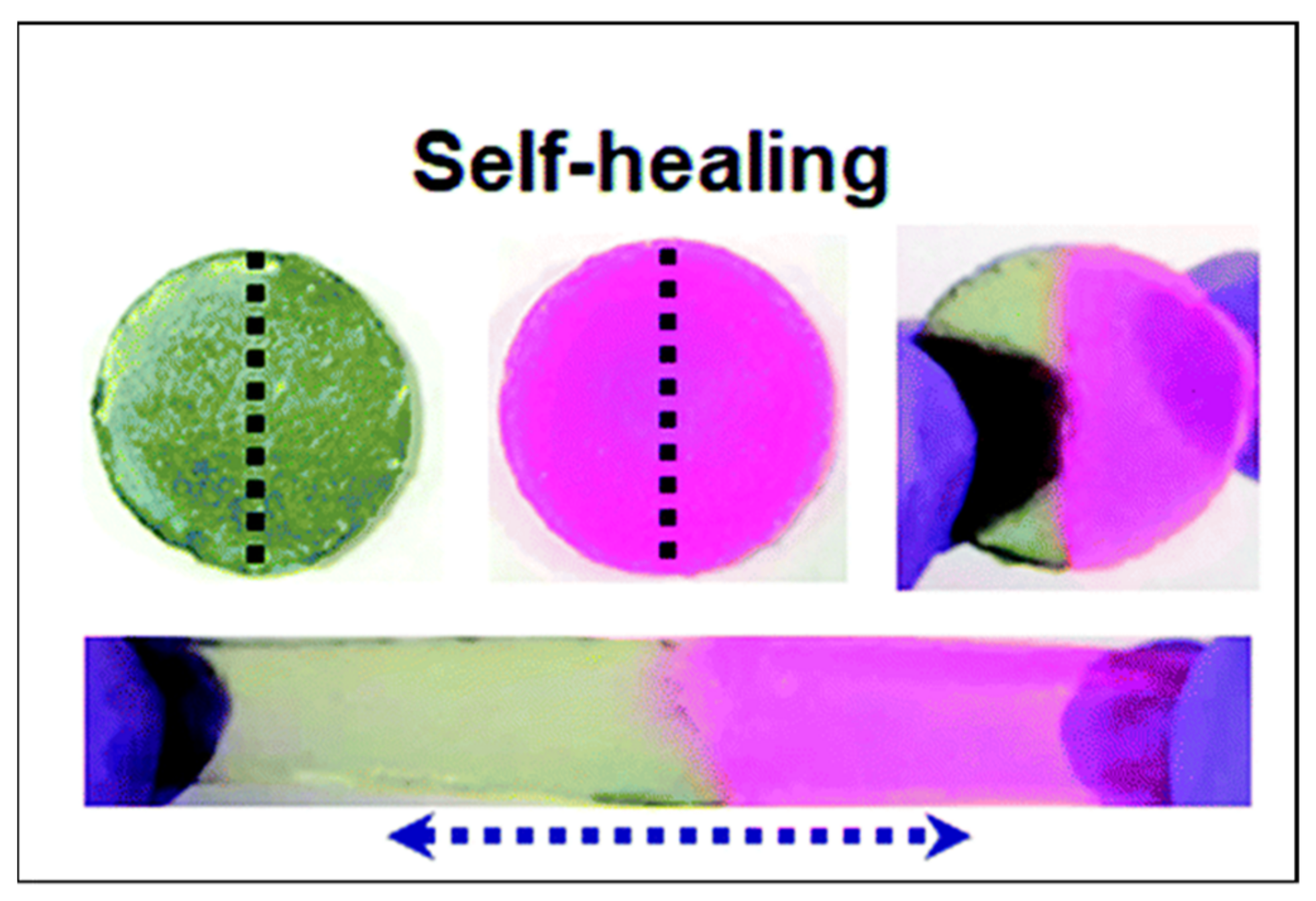
3.2. Tough PE Gels
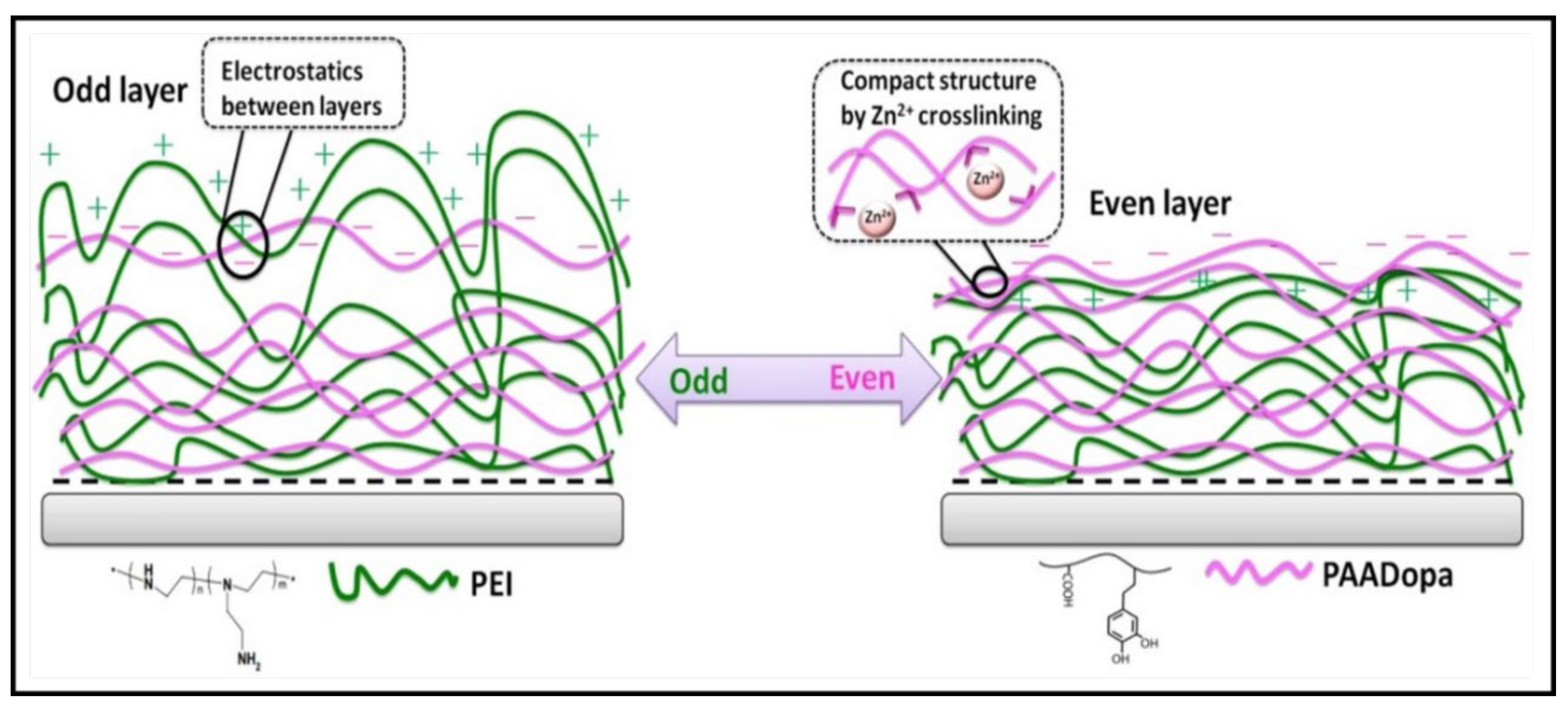
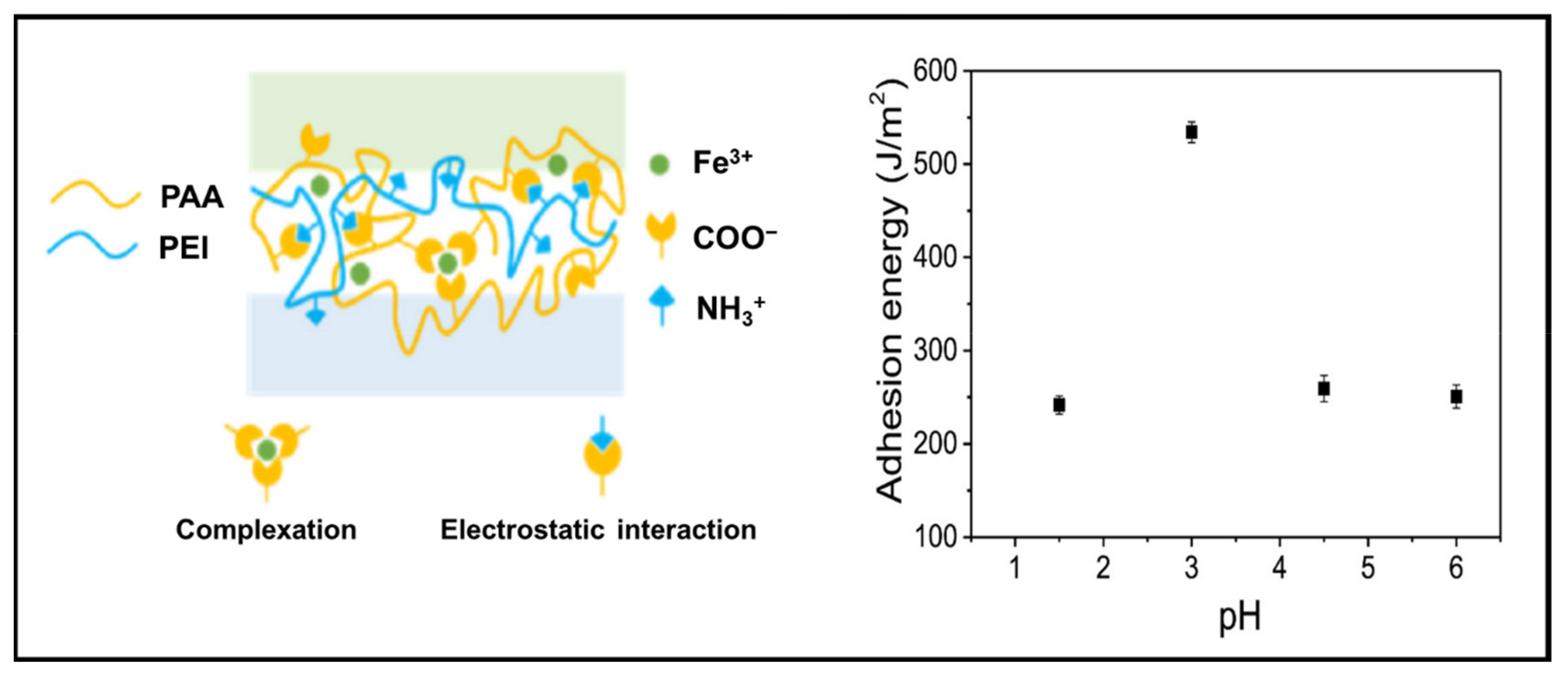
3.3. Bioadhesive PE Gels
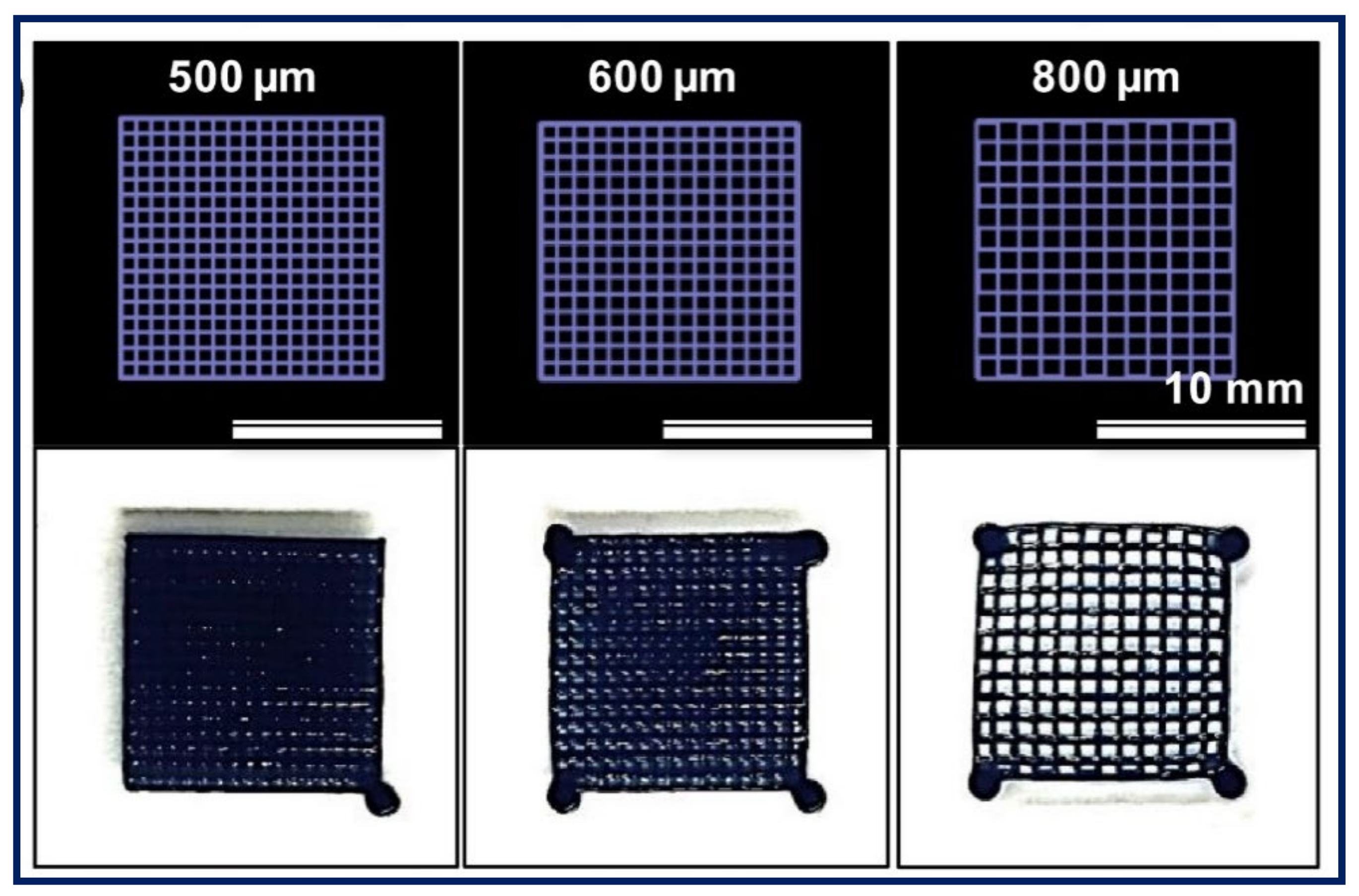
3.4. 3D Printable PE Gels
| PE Type | PE Gel Composition | Application | Ref |
|---|---|---|---|
| Multilayered gels | PNIPAM-co-MAA nanogel coated with PLL and PGA or CHT and DS | Storage and release of biomolecules | [73] |
| PNIPAM-co-AA core and PNIPAM shell microgel coated with PDADMAC and PSS | Drug encapsulation and delivery | [53] | |
| PNIPAM-co-MAA microgels coated with PLL and PGA or PAH and PAA | Biosensing and controlled release | [75] | |
| PEI and PSS | Drug encapsulation, microreactors, sensors | [78] | |
| PMAA nanogels with CS/ALG | Drug encapsulation and delivery | [11] | |
| CHT/CS on polyurethane discs | Tissue engineering | [84] | |
| PNIPAM grafted onto CHT/ALG | Drug encapsulation and delivery | [79] | |
| Polyetherimide and PAA | Plasmonic microstructures and tissue engineering | [95] | |
| Tough gels | ALG/collagen ionically crosslinked using calcium ions | Wound healing | [155] |
| CHT and ALG | Drug delivery and tissue engineering | [106] | |
| DMAEA-Q and NaSS | Fundamental study | [107] | |
| ALG and polyacrylamide | Fundamental study | [103] | |
| PAMPS and PAAm | Fundamental study | [110] | |
| PCDME and PAMPS | Fundamental study | [111] | |
| PAMPS, PAAm and PEDOT | Load-bearing tissue engineering applications | [112] | |
| PVA and P(AM-co-SBMA) | Stretchable electronics and energy storage | [32] | |
| Bioadhesive gels | PAA-g-PNIPAM and PDMAPAA-g-PNIPAM | Tissue engineering | [120] |
| P(SBMA-co-AMPS) and P(SBMA-co-DAC) | Tissue engineering and bioelectronics | [122] | |
| PVP-PAA and PVA-PAAM gels coated with PEI and PAA | Tissue engineering | [133] | |
| PMAA and PDMAEMA | Tissue engineering | [118] | |
| C-HA and C-CHT | Orthopaedic applications | [22] | |
| DOPA-PAA and PEI crosslinked with zinc | Drug delivery, biosensing, tissue engineering | [126] | |
| Poly(TMAEMA-co-SBMA) and polyphosphate | Tissue engineering, drug delivery and biosensing | [131] | |
| Chitooligosaccharide and poly(N-acryloyl 2-glycine) | Tissue engineering | [9] | |
| PEI and PAA complexed with iron ions | Tissue engineering | [133] | |
| 3D printable gels | ALG and calcium ions | Tissue engineering | [139] |
| ALG and PEI | Bone tissue engineering | [140] | |
| ALG and acrylamide | Fundamental study | [144] | |
| Chitosan and sodium hydroxide | Soft actuator | [146] | |
| Gelatin and chitosan | Skin tissue engineering | [149] | |
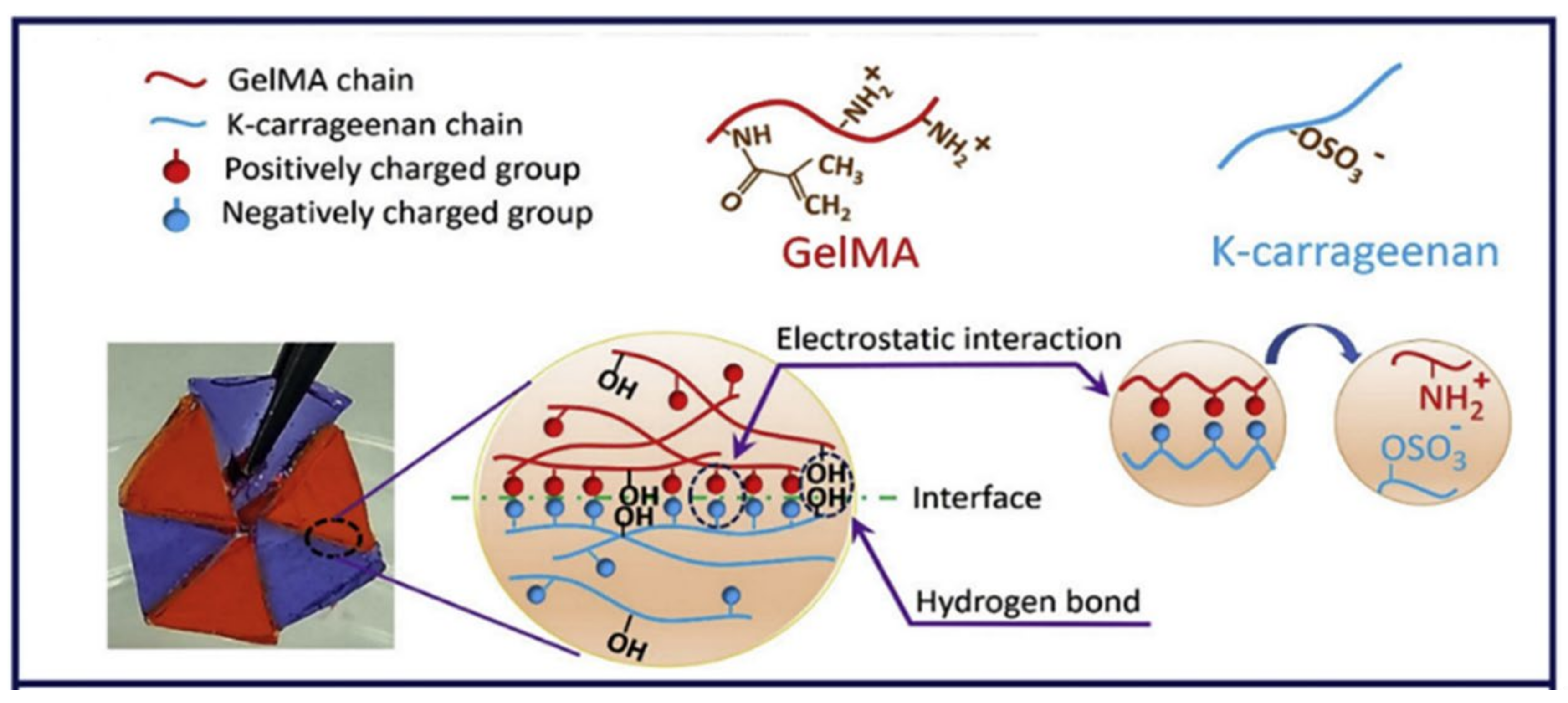
| GelMA and κ-carrageenan | Tissue engineering | [150] | |
| HA and ALG | Tissue engineering | [151] | |
| PEDOT: PSS and PEGDA | Neural tissue engineering | [152] | |
| PAAm, AETA and silica nanoparticles | Sensors and robotics | [153] | |
| PVDF and PDMAAm | Electrochemical devices | [154] |
4. Applications
4.1. Tissue Engineering
4.2. Drug Delivery
4.3. Actuators
4.4. Bioelectronics
5. Conclusions and Future Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Nayak, A.K.; Das, B. 1—Introduction to polymeric gels. In Polymeric Gels; Pal, K., Banerjee, I., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 3–27. [Google Scholar] [CrossRef]
- Kwon, H.J.; Osada, Y.; Gong, J.P. Polyelectrolyte gels-fundamentals and applications. Polym. J. 2006, 38, 1211–1219. [Google Scholar] [CrossRef]
- Osada, Y.; Gong, J.P. Soft and wet materials: Polymer gels. Adv. Mater. 1998, 10, 827–837. [Google Scholar] [CrossRef]
- Rubinstein, M.; Colby, R.H.; Dobrynin, A.V.; Joanny, J.-F. Elastic Modulus and Equilibrium Swelling of Polyelectrolyte Gels. Macromolecules 1996, 29, 398–406. [Google Scholar] [CrossRef]
- Chandran, P.L.; Horkay, F. Aggrecan, an unusual polyelectrolyte: Review of solution behavior and physiological implications. Acta Biomater. 2012, 8, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, Q.; Yuan, J. Porous polyelectrolytes: The interplay of charge and pores for new functionalities. Angew. Chem. Int. Ed. 2018, 57, 6754–6773. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Chu, B. Assembled Materials: Polyelectrolyte–Surfactant Complexes. Adv. Mater. 2000, 12, 545–556. [Google Scholar] [CrossRef]
- Farshbaf, M.; Davaran, S.; Zarebkohan, A.; Annabi, N.; Akbarzadeh, A.; Salehi, R. Significant role of cationic polymers in drug delivery systems. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1872–1891. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Wu, T.; Chen, X.; Liu, Y.; Li, Y.; Xu, Z.; Fan, C.; Liu, W. A Janus Hydrogel Wet Adhesive for Internal Tissue Repair and Anti-Postoperative Adhesion. Adv. Funct. Mater. 2020, 30, 2005689. [Google Scholar] [CrossRef]
- Pérez-Anes, A.; Gargouri, M.; Laure, W.; Van Den Berghe, H.; Courcot, E.; Sobocinski, J.; Tabary, N.; Chai, F.; Blach, J.-F.; Addad, A.; et al. Bioinspired Titanium Drug Eluting Platforms Based on a Poly-β-cyclodextrin-Chitosan Layer-by-Layer Self-Assembly Targeting Infections. ACS Appl. Mater. Interfaces 2015, 7, 12882–12893. [Google Scholar] [CrossRef]
- Li, X.; Du, P.; Liu, P. Layer-by-layer polyelectrolyte complex coated poly(methacrylic acid) nanogels as a drug delivery system for controlled release: Structural effects. RSC Adv. 2014, 4, 56323–56331. [Google Scholar] [CrossRef]
- Paulraj, T.; Feoktistova, N.; Velk, N.; Uhlig, K.; Duschl, C.; Volodkin, D. Microporous Polymeric 3D Scaffolds Templated by the Layer-by-Layer Self-Assembly. Macromol. Rapid Commun. 2014, 35, 1408–1413. [Google Scholar] [CrossRef]
- Macdonald, M.L.; Samuel, R.E.; Shah, N.J.; Padera, R.F.; Beben, Y.M.; Hammond, P.T. Tissue integration of growth factor-eluting layer-by-layer polyelectrolyte multilayer coated implants. Biomaterials 2011, 32, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Pappa, A.-M.; Inal, S.; Roy, K.; Zhang, Y.; Pitsalidis, C.; Hama, A.; Pas, J.; Malliaras, G.G.; Owens, R.M. Polyelectrolyte Layer-by-Layer Assembly on Organic Electrochemical Transistors. ACS Appl. Mater. Interfaces 2017, 9, 10427–10434. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Seo, J.H.; Woo, H.Y. Conjugated polyelectrolytes: A new class of semiconducting material for organic electronic devices. Polymer 2013, 54, 5104–5121. [Google Scholar] [CrossRef]
- Liu, S.; Gao, G.; Xiao, Y.; Fu, J. Tough and responsive oppositely charged nanocomposite hydrogels for use as bilayer actuators assembled through interfacial electrostatic attraction. J. Mater. Chem. B 2016, 4, 3239–3246. [Google Scholar] [CrossRef]
- Meka, V.S.; Sing, M.K.G.; Pichika, M.R.; Nali, S.R.; Kolapalli, V.R.M.; Kesharwani, P. A comprehensive review on polyelectrolyte complexes. Drug Discov. Today 2017, 22, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Holkar, A.; Srivastava, S. Protein–Polyelectrolyte Complexes and Micellar Assemblies. Polymers 2019, 11, 1097. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, Y.; Bhattarai, A. A Review on Polyelectrolytes (PES) and Polyelectrolyte Complexes (PECs). Int. J. Eng. Res. Sci. Technol. 2020, 9, 876–889. [Google Scholar]
- Bhatia, S.R.; Khattak, S.F.; Roberts, S.C. Polyelectrolytes for cell encapsulation. Curr. Opin. Colloid Interface Sci. 2005, 10, 45–51. [Google Scholar] [CrossRef]
- Kwon, H.J.; Gong, J.P. Negatively charged polyelectrolyte gels as bio-tissue model system and for biomedical application. Curr. Opin. Colloid Interface Sci. 2006, 11, 345–350. [Google Scholar] [CrossRef]
- Almeida, A.C.; Vale, A.C.; Pires, R.A.; Reis, R.L.; Alves, N.M. Layer-by-layer films based on catechol-modified polysaccharides produced by dip- and spin-coating onto different substrates. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 1412–1427. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-L.; Xu, J.; Shen, B.-X.; Zhang, S.-S.; Jin, B.-H.; Zhu, Q.-Y.; ZhuGe, D.-L.; Wu, X.-Q.; Xiao, J.; Zhao, Y.-Z. Dual Regulations of Thermosensitive Heparin–Poloxamer Hydrogel Using ε-Polylysine: Bioadhesivity and Controlled KGF Release for Enhancing Wound Healing of Endometrial Injury. ACS Appl. Mater. Interfaces 2017, 9, 29580–29594. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Romero, R.; Sorokina, L.V.; Ballinger, K.R.; Place, L.W.; Kipper, M.J.; Khetani, S.R. A polyelectrolyte multilayer platform for investigating growth factor delivery modes in human liver cultures. J. Biomed. Mater. Res. A 2018, 106, 971–984. [Google Scholar] [CrossRef]
- DeVolder, R.; Kong, H.J. Hydrogels for in vivo-like three-dimensional cellular studies. Wiley Interdiscip. Rev. Syst. Biol. Med. 2012, 4, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Chiappisi, L.; Hoffmann, I.; Gradzielski, M. Complexes of oppositely charged polyelectrolytes and surfactants—Recent developments in the field of biologically derived polyelectrolytes. Soft Matter 2013, 9, 3896–3909. [Google Scholar] [CrossRef]
- Gardel, M.L. Synthetic polymers with biological rigidity. Nature 2013, 493, 618. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.L.; Henzler, K.; Welsch, N.; Ballauff, M.; Borisov, O. Proteins and polyelectrolytes: A charged relationship. Curr. Opin. Colloid Interface Sci. 2012, 17, 90–96. [Google Scholar] [CrossRef]
- Achazi, K.; Haag, R.; Ballauff, M.; Dernedde, J.; Kizhakkedathu, J.N.; Maysinger, D.; Multhaup, G. Understanding the Interaction of Polyelectrolyte Architectures with Proteins and Biosystems. Angew. Chem. Int. Ed. 2021, 60, 3882–3904. [Google Scholar] [CrossRef] [PubMed]
- Haag, S.L.; Bernards, M.T. Polyampholyte Hydrogels in Biomedical Applications. Gels 2017, 3, 41. [Google Scholar] [CrossRef]
- Ramos, D.P.; Sarjinsky, S.; Alizadehgiashi, M.; Möbus, J.; Kumacheva, E. Polyelectrolyte vs Polyampholyte Behavior of Composite Chitosan/Gelatin Films. ACS Omega 2019, 4, 8795–8803. [Google Scholar] [CrossRef]
- Diao, W.; Wu, L.; Ma, X.; Zhuang, Z.; Li, S.; Bu, X.; Fang, Y. Highly stretchable, ionic conductive and self-recoverable zwitterionic polyelectrolyte-based hydrogels by introducing multiple supramolecular sacrificial bonds in double network. J. Appl. Polym. Sci. 2019, 136, 47783. [Google Scholar] [CrossRef]
- Tiyapiboonchaiya, C.; Pringle, J.M.; Sun, J.; Byrne, N.; Howlett, P.C.; MacFarlane, D.R.; Forsyth, M. The zwitterion effect in high-conductivity polyelectrolyte materials. Nat. Mater. 2004, 3, 29–32. [Google Scholar] [CrossRef]
- Schönhoff, M. Self-assembled polyelectrolyte multilayers. Curr. Opin. Colloid Interface Sci. 2003, 8, 86–95. [Google Scholar] [CrossRef]
- Yuan, W.; Weng, G.-M.; Lipton, J.; Li, C.M.; Van Tassel, P.R.; Taylor, A.D. Weak polyelectrolyte-based multilayers via layer-by-layer assembly: Approaches, properties, and applications. Adv. Colloid Interface Sci. 2020, 282, 102200. [Google Scholar] [CrossRef] [PubMed]
- Scheepers, D.; Chatillon, B.; Borneman, Z.; Nijmeijer, K. Influence of charge density and ionic strength on diallyldimethylammonium chloride (DADMAC)-based polyelectrolyte multilayer membrane formation. J. Membr. Sci. 2021, 617, 118619. [Google Scholar] [CrossRef]
- Rojas, O.J.; Ernstsson, M.; Neuman, R.D.; Claesson, P.M. Effect of Polyelectrolyte Charge Density on the Adsorption and Desorption Behavior on Mica. Langmuir 2002, 18, 1604–1612. [Google Scholar] [CrossRef]
- Volodkin, D.; von Klitzing, R. Competing mechanisms in polyelectrolyte multilayer formation and swelling: Polycation–polyanion pairing vs. polyelectrolyte–ion pairing. Curr. Opin. Colloid Interface Sci. 2014, 19, 25–31. [Google Scholar] [CrossRef]
- Yu, Z.; He, Y.; Wang, Y.; Madsen, L.A.; Qiao, R. Molecular Structure and Dynamics of Ionic Liquids in a Rigid-Rod Polyanion-Based Ion Gel. Langmuir 2017, 33, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Kayitmazer, A.B.; Seeman, D.; Minsky, B.B.; Dubin, P.L.; Xu, Y. Protein–polyelectrolyte interactions. Soft Matter 2013, 9, 2553–2583. [Google Scholar] [CrossRef]
- Rud, O.; Richter, T.; Borisov, O.; Holm, C.; Košovan, P. A self-consistent mean-field model for polyelectrolyte gels. Soft Matter 2017, 13, 3264–3274. [Google Scholar] [CrossRef] [PubMed]
- Koetz, J.; Kosmella, S. Polyelectrolytes and Nanoparticles; Springer Science & Business Media: Berlin, Germany, 2007. [Google Scholar]
- Kremer, F.; Schönhals, A. Broadband Dielectric Spectroscopy; Springer Science & Business Media: Berlin, Germany, 2002. [Google Scholar]
- Bocharova, V.; Sokolov, A.P. Perspectives for Polymer Electrolytes: A View from Fundamentals of Ionic Conductivity. Macromolecules 2020, 53, 4141–4157. [Google Scholar] [CrossRef]
- Ngai, K.S.; Ramesh, S.; Ramesh, K.; Juan, J.C. A review of polymer electrolytes: Fundamental, approaches and applications. Ionics 2016, 22, 1259–1279. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, F.; Agapov, A.L.; Saito, T.; Yang, J.; Yu, X.; Hong, K.; Mays, J.; Sokolov, A.P. Examination of the fundamental relation between ionic transport and segmental relaxation in polymer electrolytes. Polymer 2014, 55, 4067–4076. [Google Scholar] [CrossRef]
- Sangoro, J.R.; Kremer, F. Charge Transport and Glassy Dynamics in Ionic Liquids. Acc. Chem. Res. 2012, 45, 525–532. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Forsyth, M.; Izgorodina, E.I.; Abbott, A.P.; Annat, G.; Fraser, K. On the concept of ionicity in ionic liquids. Phys. Chem. Chem. Phys. 2009, 11, 4962–4967. [Google Scholar] [CrossRef]
- Gong, J.; Komatsu, N.; Nitta, T.; Osada, Y. Electrical conductance of polyelectrolyte gels. J. Phys. Chem. B 1997, 101, 740–745. [Google Scholar] [CrossRef]
- Li, H.; Erbaş, A.; Zwanikken, J.; de la Cruz, M.O. Ionic Conductivity in Polyelectrolyte Hydrogels. Macromolecules 2016, 49, 9239–9246. [Google Scholar] [CrossRef]
- Abu-Thabit, N.Y.; Hamdy, A.S. Stimuli-responsive Polyelectrolyte Multilayers for fabrication of self-healing coatings—A review. Surf. Coat. Technol. 2016, 303, 406–424. [Google Scholar] [CrossRef]
- Van Hees, I.A.; Hofman, A.H.; Dompé, M.; van der Gucht, J.; Kamperman, M. Temperature-responsive polyelectrolyte complexes for bio-inspired underwater adhesives. Eur. Polym. J. 2020, 141, 110034. [Google Scholar] [CrossRef]
- Wong, J.E.; Richtering, W. Surface modification of thermoresponsive microgels via layer-by-layer assembly of polyelectrolyte multilayers. In Smart Colloidal Materials; Kremer, F., Richtering, W., Eds.; Springer: Berlin, Germany, 2006; Volume 133, pp. 45–51. [Google Scholar]
- Nakajima, T.; Furukawa, H.; Tanaka, Y.; Kurokawa, T.; Gong, J.P. Effect of void structure on the toughness of double network hydrogels. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1246–1254. [Google Scholar] [CrossRef]
- Sargent, J.L.; Chen, X.; Brezina, M.C.; Aldwin, S.; Howarter, J.A.; Erk, K.A. Behavior of Poly electrolyte Gels in Concentrated Solutions of Highly Soluble Salts. MRS Adv. 2020, 5, 907–915. [Google Scholar] [CrossRef]
- Truong, M.Y.; Dutta, N.K.; Choudhury, N.R.; Kim, M.; Elvin, C.M.; Nairn, K.M.; Hill, A.J. The effect of hydration on molecular chain mobility and the viscoelastic behavior of resilin-mimetic protein-based hydrogels. Biomaterials 2011, 32, 8462–8473. [Google Scholar] [CrossRef]
- Wang, Y.; Ameer, G.A.; Sheppard, B.J.; Langer, R. A tough biodegradable elastomer. Nat. Biotechnol. 2002, 20, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Muthukumar, M. Theory of Charged Gels: Swelling, Elasticity, and Dynamics. Gels 2021, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, J.L.; Balu, R.; Knott, R.; de Campo, L.; Mata, J.P.; Rehm, C.; Hill, A.J.; Dutta, N.K.; Roy Choudhury, N. Structural evolution of photocrosslinked silk fibroin and silk fibroin-based hybrid hydrogels: A small angle and ultra-small angle scattering investigation. Int. J. Biol. Macromol. 2018, 114, 998–1007. [Google Scholar] [CrossRef]
- Horkay, F.; Magda, J.; Alcoutlabi, M.; Atzet, S.; Zarembinski, T. Structural, mechanical and osmotic properties of injectable hyaluronan-based composite hydrogels. Polymer 2010, 51, 4424–4430. [Google Scholar] [CrossRef][Green Version]
- Wanasingha, N.; Dutta, N.K.; Choudhury, N.R. Emerging bioadhesives: From traditional bioactive and bioinert to a new biomimetic protein-based approach. Adv. Colloid Interface Sci. 2021, 296, 102521. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.D. Buildup of Ultrathin Multilayer Films by a Self-Assembly Process: II. Consecutive Adsorption of Anionic and Cationic Bipolar Amphiphiles and Polyelectrolytes on Charged Surfaces. Ber. Der Bunsenges. Für Phys. Chem. 1991, 95, 1430–1434. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.D.; Schmitt, J. Buildup of ultrathin multilayer films by a self-assembly process: III. Consecutively alternating adsorption of anionic and cationic polyelectrolytes on charged surfaces. Thin Solid Film 1992, 210, 831–835. [Google Scholar] [CrossRef]
- Campbell, J.; Vikulina, A.S. Layer-By-Layer Assemblies of Biopolymers: Build-Up, Mechanical Stability and Molecular Dynamics. Polymers 2020, 12, 1949. [Google Scholar] [CrossRef] [PubMed]
- Detzel, C.J.; Larkin, A.L.; Rajagopalan, P. Polyelectrolyte multilayers in tissue engineering. Tissue Eng. Part B Rev. 2011, 17, 101–113. [Google Scholar] [CrossRef]
- Selin, V.; Ankner, J.F.; Sukhishvili, S.A. Ionically Paired Layer-by-Layer Hydrogels: Water and Polyelectrolyte Uptake Controlled by Deposition Time. Gels 2018, 4, 7. [Google Scholar] [CrossRef]
- Tang, K.; Besseling, N.A. Formation of polyelectrolyte multilayers: Ionic strengths and growth regimes. Soft Matter 2016, 12, 1032–1040. [Google Scholar] [CrossRef]
- Bieker, P.; Schönhoff, M. Linear and Exponential Growth Regimes of Multilayers of Weak Polyelectrolytes in Dependence on pH. Macromolecules 2010, 43, 5052–5059. [Google Scholar] [CrossRef]
- Mutschler, A.; Betscha, C.; Ball, V.; Senger, B.; Vrana, N.E.; Boulmedais, F.; Schroder, A.; Schaaf, P.; Lavalle, P. Nature of the Polyanion Governs the Antimicrobial Properties of Poly(arginine)/Polyanion Multilayer Films. Chem. Mater. 2017, 29, 3195–3201. [Google Scholar] [CrossRef]
- Büscher, K.; Graf, K.; Ahrens, H.; Helm, C.A. Influence of Adsorption Conditions on the Structure of Polyelectrolyte Multilayers. Langmuir 2002, 18, 3585–3591. [Google Scholar] [CrossRef]
- Vikulina, A.S.; Anissimov, Y.G.; Singh, P.; Prokopović, V.Z.; Uhlig, K.; Jaeger, M.S.; von Klitzing, R.; Duschl, C.; Volodkin, D. Temperature effect on the build-up of exponentially growing polyelectrolyte multilayers. An exponential-to-linear transition point. Phys. Chem. Chem. Phys. 2016, 18, 7866–7874. [Google Scholar] [CrossRef]
- Gelissen, A.P.H.; Schmid, A.J.; Plamper, F.A.; Pergushov, D.V.; Richtering, W. Quaternized microgels as soft templates for polyelectrolyte layer-by-layer assemblies. Polymer 2014, 55, 1991–1999. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Wong, J.E. Effect of layer-by-layer confinement of polypeptides and polysaccharides onto thermoresponsive microgels: A comparative study. J. Colloid Interface Sci. 2010, 347, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Decher, G.; Schmitt, J. Fine-tuning of the film thickness of ultrathin multilayer films composed of consecutively alternating layers of anionic and cationic polyelectrolytes. In Trends in Colloid and Interface Science V; Helm, C., Lösche, M., Möhwald, H., Eds.; Steinkopff: Darmstadt, Germany, 1992; Volume 89, pp. 160–164. [Google Scholar]
- Costa, E.; Lloyd, M.M.; Chopko, C.; Aguiar-Ricardo, A.; Hammond, P.T. Tuning Smart Microgel Swelling and Responsive Behavior through Strong and Weak Polyelectrolyte Pair Assembly. Langmuir 2012, 28, 10082–10090. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.M.; Caridade, S.G.; Costa, R.R.; Alves, N.l.M.; Groth, T.; Picart, C.; Reis, R.L.; Mano, J.O.F. pH Responsiveness of Multilayered Films and Membranes Made of Polysaccharides. Langmuir 2015, 31, 11318–11328. [Google Scholar] [CrossRef]
- Han, U.; Seo, Y.; Hong, J. Effect of pH on the structure and drug release profiles of layer-by-layer assembled films containing polyelectrolyte, micelles, and graphene oxide. Sci. Rep. 2016, 6, 24158. [Google Scholar] [CrossRef]
- Elzbïeciak, M.; Zapotoczny, S.; Nowak, P.; Krastev, R.; Nowakowska, M.; Warszyński, P. Influence of pH on the Structure of Multilayer Films Composed of Strong and Weak Polyelectrolytes. Langmuir 2009, 25, 3255–3259. [Google Scholar] [CrossRef]
- Mu, B.; Liu, P.; Li, X.; Du, P.; Dong, Y.; Wang, Y. Fabrication of Flocculation-Resistant pH/Ionic Strength/Temperature Multiresponsive Hollow Microspheres and Their Controlled Release. Mol. Pharm. 2012, 9, 91–101. [Google Scholar] [CrossRef]
- Park, S.; Han, U.; Choi, D.; Hong, J. Layer-by-layer assembled polymeric thin films as prospective drug delivery carriers: Design and applications. Biomater. Res. 2018, 22, 29. [Google Scholar] [CrossRef]
- Silva, J.M.; Duarte, A.R.C.; Custódio, C.A.; Sher, P.; Neto, A.I.; Pinho, A.C.M.; Fonseca, J.; Reis, R.L.; Mano, J.F. Nanostructured Hollow Tubes Based on Chitosan and Alginate Multilayers. Adv. Healthc. Mater. 2014, 3, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Madaboosi, N.; Uhlig, K.; Schmidt, S.; Vikulina, A.S.; Möhwald, H.; Duschl, C.; Volodkin, D. A “Cell-Friendly” Window for the Interaction of Cells with Hyaluronic Acid/Poly-l-Lysine Multilayers. Macromol. Biosci. 2018, 18, 1700319. [Google Scholar] [CrossRef]
- Ju, J.A.; Lee, C.J.; Thompson, K.N.; Ory, E.C.; Lee, R.M.; Mathias, T.J.; Pratt, S.J.P.; Vitolo, M.I.; Jewell, C.M.; Martin, S.S. Partial thermal imidization of polyelectrolyte multilayer cell tethering surfaces (TetherChip) enables efficient cell capture and microtentacle fixation for circulating tumor cell analysis. Lab Chip 2020, 20, 2872–2888. [Google Scholar] [CrossRef]
- Imbir, G.; Mzyk, A.; Trembecka-Wójciga, K.; Jasek-Gajda, E.; Plutecka, H.; Schirhagl, R.; Major, R. Polyelectrolyte Multilayer Films Modification with Ag and rGO Influences Platelets Activation and Aggregate Formation under In Vitro Blood Flow. Nanomaterials 2020, 10, 859. [Google Scholar] [CrossRef]
- Schneider, A.; Picart, C.; Senger, B.; Schaaf, P.; Voegel, J.-C.; Frisch, B. Layer-by-Layer Films from Hyaluronan and Amine-Modified Hyaluronan. Langmuir 2007, 23, 2655–2662. [Google Scholar] [CrossRef][Green Version]
- Gaudière, F.; Morin-Grognet, S.; Bidault, L.; Lembré, P.; Pauthe, E.; Vannier, J.-P.; Atmani, H.; Ladam, G.; Labat, B.A. Genipin-Cross-Linked Layer-by-Layer Assemblies: Biocompatible Microenvironments To Direct Bone Cell Fate. Biomacromolecules 2014, 15, 1602–1611. [Google Scholar] [CrossRef]
- Yamanlar, S.; Sant, S.; Boudou, T.; Picart, C.; Khademhosseini, A. Surface functionalization of hyaluronic acid hydrogels by polyelectrolyte multilayer films. Biomaterials 2011, 32, 5590–5599. [Google Scholar] [CrossRef] [PubMed]
- Lengert, E.V.; Koltsov, S.I.; Li, J.; Ermakov, A.V.; Parakhonskiy, B.V.; Skorb, E.V.; Skirtach, A.G. Nanoparticles in Polyelectrolyte Multilayer Layer-by-Layer Films and Capsules—Key Enabling Components of Hybrid Coatings. Coatings 2020, 10, 1131. [Google Scholar] [CrossRef]
- Xu, H.; Schönhoff, M.; Zhang, X. Unconventional Layer-by-Layer Assembly: Surface Molecular Imprinting and Its Applications. Small 2012, 8, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.U.; Lee, S.; Kim, T.-I. Recent Advances in Unconventional Lithography for Challenging 3D Hierarchical Structures and Their Applications. J. Nanomater. 2016, 2016, 7602395. [Google Scholar] [CrossRef]
- Huo, F.; Zheng, Z.; Zheng, G.; Giam, L.R.; Zhang, H.; Mirkin, C.A. Polymer Pen Lithography. Science 2008, 321, 1658. [Google Scholar] [CrossRef] [PubMed]
- Huo, F.; Zheng, G.; Liao, X.; Giam, L.R.; Chai, J.; Chen, X.; Shim, W.; Mirkin, C.A. Beam pen lithography. Nat. Nanotechnol. 2010, 5, 637–640. [Google Scholar] [CrossRef]
- Zhao, J.; Swartz, L.A.; Lin, W.-f.; Schlenoff, P.S.; Frommer, J.; Schlenoff, J.B.; Liu, G.-Y. Three-Dimensional Nanoprinting via Scanning Probe Lithography-Delivered Layer-by-Layer Deposition. ACS Nano 2016, 10, 5656–5662. [Google Scholar] [CrossRef]
- Wang, T.; Song, G.; Liu, F.; Qi, Y.; Luo, C.; Zhang, X.; Li, Y.; Han, E.; Fu, Y.; Jiao, Y. Mechanical stabilization of metallic microstructures by insertion of an adhesive polymer underlayer for further optical and electrical applications. J. Mater. Chem. C Mater. Opt. Electron. Devices 2016, 4, 3231–3237. [Google Scholar] [CrossRef]
- Jiao, Y.; Luo, C.; Chen, D.; Zhang, X.; Li, Y.; Wang, T.; Fu, Y. Mussel-inspired polyelectrolyte multilayer for reinforcing the interfacial mechanical stability of plasmonic microstructures. Surf. Coat. Technol. 2018, 334, 305–311. [Google Scholar] [CrossRef]
- Park, K.; Choi, D.; Hong, J. Nanostructured Polymer Thin Films Fabricated with Brush-based Layer-by-Layer Self-assembly for Site-selective Construction and Drug release. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Franz, T.; Hasler, E.M.; Hagg, R.; Weiler, C.; Jakob, R.P.; Mainil-Varlet, P. In situ compressive stiffness, biochemical composition, and structural integrity of articular cartilage of the human knee joint. Osteoarthr. Cartil. 2001, 9, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Mow, V.C.; Koob, T.J.; Eyre, D.R. Viscoelastic shear properties of articular cartilage and the effects of glycosidase treatments. J. Orthop. Res. 1993, 11, 771–781. [Google Scholar] [CrossRef]
- Zhao, X. Multi-scale multi-mechanism design of tough hydrogels: Building dissipation into stretchy networks. Soft Matter 2014, 10, 672–687. [Google Scholar] [CrossRef]
- Israelachvili, J.N. 21—Interactions of Biological Membranes and Structures. In Intermolecular and Surface Forces, 3rd ed.; Israelachvili, J.N., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 577–616. [Google Scholar] [CrossRef]
- Meot-Ner, M. The Ionic Hydrogen Bond. Chem. Rev. 2005, 105, 213–284. [Google Scholar] [CrossRef]
- Ma, G.; Fang, D.; Liu, Y.; Zhu, X.; Nie, J. Electrospun sodium alginate/poly(ethylene oxide) core–shell nanofibers scaffolds potential for tissue engineering applications. Carbohydr. Polym. 2012, 87, 737–743. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Zhao, X.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef]
- Kuo, C.K.; Ma, P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef]
- Stevens, M.M.; Qanadilo, H.F.; Langer, R.; Prasad Shastri, V. A rapid-curing alginate gel system: Utility in periosteum-derived cartilage tissue engineering. Biomaterials 2004, 25, 887–894. [Google Scholar] [CrossRef]
- Komoto, D.; Furuike, T.; Tamura, H. Preparation of polyelectrolyte complex gel of sodium alginate with chitosan using basic solution of chitosan. Int. J. Biol. Macromol. 2019, 126, 54–59. [Google Scholar] [CrossRef]
- Luo, F.; Sun, T.L.; Nakajima, T.; Kurokawa, T.; Zhao, Y.; Sato, K.; Ihsan, A.B.; Li, X.; Guo, H.; Gong, J.P. Oppositely Charged Polyelectrolytes Form Tough, Self-Healing, and Rebuildable Hydrogels. Adv. Mater. 2015, 27, 2722–2727. [Google Scholar] [CrossRef]
- Tonsomboon, K.; Butcher, A.L.; Oyen, M.L. Strong and tough nanofibrous hydrogel composites based on biomimetic principles. Mater. Sci. Eng. C 2017, 72, 220–227. [Google Scholar] [CrossRef]
- Gong, J.P. Why are double network hydrogels so tough? Soft Matter 2010, 6, 2583–2590. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Yin, H.; King, D.R.; Sun, T.L.; Saruwatari, Y.; Nakajima, T.; Kurokawa, T.; Gong, J.P. Polyzwitterions as a Versatile Building Block of Tough Hydrogels: From Polyelectrolyte Complex Gels to Double-Network Gels. ACS Appl. Mater. Interfaces 2020, 12, 50068–50076. [Google Scholar] [CrossRef]
- Du, G.; Gao, G.; Hou, R.; Cheng, Y.; Chen, T.; Fu, J.; Fei, B. Tough and Fatigue Resistant Biomimetic Hydrogels of Interlaced Self-Assembled Conjugated Polymer Belts with a Polyelectrolyte Network. Chem. Mater. 2014, 26, 3522–3529. [Google Scholar] [CrossRef]
- Chen, Q.; Yan, X.; Zhu, L.; Chen, H.; Jiang, B.; Wei, D.; Huang, L.; Yang, J.; Liu, B.; Zheng, J. Improvement of Mechanical Strength and Fatigue Resistance of Double Network Hydrogels by Ionic Coordination Interactions. Chem. Mater. 2016, 28, 5710–5720. [Google Scholar] [CrossRef]
- Scognamiglio, F.; Travan, A.; Rustighi, I.; Tarchi, P.; Palmisano, S.; Marsich, E.; Borgogna, M.; Donati, I.; de Manzini, N.; Paoletti, S. Adhesive and sealant interfaces for general surgery applications. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 626–639. [Google Scholar] [CrossRef]
- Pei, X.; Wang, J.; Cong, Y.; Fu, J. Recent progress in polymer hydrogel bioadhesives. J. Polym. Sci. 2021, 59, 1312–1337. [Google Scholar] [CrossRef]
- Taboada, G.M.; Yang, K.; Pereira, M.J.N.; Liu, S.S.; Hu, Y.; Karp, J.M.; Artzi, N.; Lee, Y. Overcoming the translational barriers of tissue adhesives. Nat. Rev. 2020, 5, 310–329. [Google Scholar] [CrossRef]
- Alfhaid, L.; Williams, N.H.; Geoghegan, M. Adhesion between oppositely charged polyelectrolytes in salt solution. J. Appl. Polym. Sci. 2020, 137, 49130. [Google Scholar] [CrossRef]
- Alfhaid, L.; La Spina, R.; Tomlinson, M.R.; Hall, A.R.; Seddon, W.D.; Williams, N.H.; Cousin, F.; Gorb, S.; Geoghegan, M. Adhesion between oppositely charged polyelectrolytes. J. Adhes. 2018, 94, 58–76. [Google Scholar] [CrossRef]
- Annabi, N.; Yue, K.; Tamayol, A.; Khademhosseini, A. Elastic sealants for surgical applications. Eur. J. Pharm. Biopharm. 2015, 95, 27–39. [Google Scholar] [CrossRef]
- Dompé, M.; Cedano-Serrano, F.J.; Heckert, O.; van den Heuvel, N.; van der Gucht, J.; Tran, Y.; Hourdet, D.; Creton, C.; Kamperman, M. Thermoresponsive Complex Coacervate-Based Underwater Adhesive. Adv. Mater. 2019, 31, 1808179. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Wang, J.; Tao, Z.; Huang, J.; Rao, P.; Kurokawa, T.; Gong, J.P. Adjacent cationic–aromatic sequences yield strong electrostatic adhesion of hydrogels in seawater. Nat. Commun. 2019, 10, 5127. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Wu, C.; Pei, X.; Cong, Y.; Zhang, R.; Fu, J. Antibacterial Zwitterionic Polyelectrolyte Hydrogel Adhesives with Adhesion Strength Mediated by Electrostatic Mismatch. ACS Appl. Mater. Interfaces 2020, 12, 46816–46826. [Google Scholar] [CrossRef]
- Kord Forooshani, P.; Lee, B.P. Recent approaches in designing bioadhesive materials inspired by mussel adhesive protein. Polym. Sci. Part A Polym. Chem. 2017, 55, 9–33. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Chen, J.; Wang, J.; Zeng, H.; Yu, J. Recent progress in synthesis and application of mussel-inspired adhesives. Nanoscale 2020, 12, 1307–1324. [Google Scholar] [CrossRef] [PubMed]
- Moulay, S. Dopa/Catechol-Tethered Polymers: Bioadhesives and Biomimetic Adhesive Materials. Polym. Rev. 2014, 54, 436–513. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Han, H.; Micciulla, S.; Backes, S.; Li, A.; Xu, J.; Shen, W.; von Klitzing, R.; Guo, X. Odd-even effect during layer-by-layer assembly of polyelectrolytes inspired by marine mussel. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 245–255. [Google Scholar] [CrossRef]
- Lindborg, B.A.; Brekke, J.H.; Scott, C.M.; Chai, Y.W.; Ulrich, C.; Sandquist, L.; Kokkoli, E.; O’Brien, T.D. A chitosan-hyaluronan-based hydrogel-hydrocolloid supports in vitro culture and differentiation of human mesenchymal stem/stromal cells. Tissue Eng. Part A 2015, 21, 1952–1962. [Google Scholar] [CrossRef]
- Da Câmara, P.C.F.; Balaban, R.C.; Hedayati, M.; Popat, K.C.; Martins, A.F.; Kipper, M.J. Novel cationic tannin/glycosaminoglycan-based polyelectrolyte multilayers promote stem cells adhesion and proliferation. RSC Adv. 2019, 9, 25836–25846. [Google Scholar] [CrossRef]
- Silva, J.M.; García, J.R.; Reis, R.L.; García, A.J.; Mano, J.F. Tuning cell adhesive properties via layer-by-layer assembly of chitosan and alginate. Acta Biomater. 2017, 51, 279–293. [Google Scholar] [CrossRef]
- Li, A.; Jia, Y.; Sun, S.; Xu, Y.; Minsky, B.B.; Stuart, M.A.C.; Cölfen, H.; von Klitzing, R.; Guo, X. Mineral-Enhanced Polyacrylic Acid Hydrogel as an Oyster-Inspired Organic–Inorganic Hybrid Adhesive. ACS Appl. Mater. Interfaces 2018, 10, 10471–10479. [Google Scholar] [CrossRef]
- Jhiang, J.-S.; Wu, T.-H.; Chou, C.-J.; Chang, Y.; Huang, C.-J. Gel-like ionic complexes for antimicrobial, hemostatic and adhesive properties. J. Mater. Chem. B 2019, 7, 2878–2887. [Google Scholar] [CrossRef]
- Sangsik, K.; Jun, H.; Yongjin, L.; Sandipan, D.; Hee Young, Y.; Young Mee, J.; YongSeok, J.; Hongbo, Z.; Dong Soo, H. Complexation and coacervation of like-charged polyelectrolytes inspired by mussels. Proc. Natl. Acad. Sci. USA 2016, 113, E847–E853. [Google Scholar] [CrossRef]
- Zhang, J.-N.; Zhu, H.; Liu, T.; Chen, Y.; Jiao, C.; He, C.; Wang, H. Strong adhesion of hydrogels by polyelectrolyte adhesives. Polymer 2020, 206, 122845. [Google Scholar] [CrossRef]
- Almodóvar, J.; Place, L.W.; Gogolski, J.; Erickson, K.; Kipper, M.J. Layer-by-Layer Assembly of Polysaccharide-Based Polyelectrolyte Multilayers: A Spectroscopic Study of Hydrophilicity, Composition, and Ion Pairing. Biomacromolecules 2011, 12, 2755–2765. [Google Scholar] [CrossRef] [PubMed]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed]
- Dorishetty, P.; Dutta, N.K.; Choudhury, N.R. Bioprintable tough hydrogels for tissue engineering applications. Adv. Colloid Interface Sci. 2020, 281, 102163. [Google Scholar] [CrossRef]
- Kyle, S.; Jessop, Z.M.; Al-Sabah, A.; Whitaker, I.S. ‘Printability’ of Candidate Biomaterials for Extrusion Based 3D Printing: State-of-the-Art. Adv. Healthc. Mater. 2017, 6, 1700264. [Google Scholar] [CrossRef]
- Jia, J.; Richards, D.J.; Pollard, S.; Tan, Y.; Rodriguez, J.; Visconti, R.P.; Trusk, T.C.; Yost, M.J.; Yao, H.; Markwald, R.R.; et al. Engineering alginate as bioink for bioprinting. Acta Biomater. 2014, 10, 4323–4331. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, Y.; Martin, J.A.; Ozbolat, I.T. Evaluation of Cell Viability and Functionality in Vessel-like Bioprintable Cell-Laden Tubular Channels. J. Biomech. Eng. 2013, 135. [Google Scholar] [CrossRef]
- Sithole, M.N.; Kumar, P.; du Toit, L.C.; Marimuthu, T.; Choonara, Y.E.; Pillay, V. A 3D bioprinted in situ conjugated-co-fabricated scaffold for potential bone tissue engineering applications. J. Biomed. Mater. Res. Part A 2018, 106, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose–Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, S.; Lin, L. Rheological study on 3D printability of alginate hydrogel and effect of graphene oxide. Int. J. Bioprint. 2016, 2, 13. [Google Scholar] [CrossRef]
- Jeon, O.; Lee, Y.B.; Hinton, T.J.; Feinberg, A.W.; Alsberg, E. Cryopreserved cell-laden alginate microgel bioink for 3D bioprinting of living tissues. Mater. Today Chem. 2019, 12, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Bakarich, S.E.; Panhuis, M.i.h.; Beirne, S.; Wallace, G.G.; Spinks, G.M. Extrusion printing of ionic-covalent entanglement hydrogels with high toughness. J. Mater. Chem. B 2013, 1, 4939–4946. [Google Scholar] [CrossRef] [PubMed]
- Axpe, E.; Oyen, M.L. Applications of Alginate-Based Bioinks in 3D Bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef]
- Zolfagharian, A.; Kouzani, A.Z.; Khoo, S.Y.; Nasri-Nasrabadi, B.; Kaynak, A. Development and analysis of a 3D printed hydrogel soft actuator. Sens. Actuators A Phys. 2017, 265, 94–101. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef]
- Poldervaart, M.T.; Goversen, B.; De Ruijter, M.; Abbadessa, A.; Melchels, F.P.; Öner, F.C.; Dhert, W.J.; Vermonden, T.; Alblas, J.J.P.O. 3D bioprinting of methacrylated hyaluronic acid (MeHA) hydrogel with intrinsic osteogenicity. PLoS ONE 2017, 12, e0177628. [Google Scholar] [CrossRef]
- Ng, W.L.; Yeong, W.Y.; Naing, M.W.J.I.J.O.B. Polyelectrolyte gelatin-chitosan hydrogel optimized for 3D bioprinting in skin tissue engineering. Int. J. Bioprinting 2016, 2, 53–62. [Google Scholar] [CrossRef]
- Li, H.; Tan, Y.J.; Liu, S.; Li, L. Three-Dimensional Bioprinting of Oppositely Charged Hydrogels with Super Strong Interface Bonding. ACS Appl. Mater. Interfaces 2018, 10, 11164–11174. [Google Scholar] [CrossRef]
- Antich, C.; de Vicente, J.; Jiménez, G.; Chocarro, C.; Carrillo, E.; Montañez, E.; Gálvez-Martín, P.; Marchal, J.A. Bio-inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3D bioprinting of articular cartilage engineering constructs. Acta Biomater. 2020, 106, 114–123. [Google Scholar] [CrossRef]
- Heo, D.N.; Lee, S.-J.; Timsina, R.; Qiu, X.; Castro, N.J.; Zhang, L.G. Development of 3D printable conductive hydrogel with crystallized PEDOT:PSS for neural tissue engineering. Mater. Sci. Eng. C 2019, 99, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Odent, J.; Wallin, T.J.; Pan, W.; Kruemplestaedter, K.; Shepherd, R.F.; Giannelis, E.P. Highly Elastic, Transparent, and Conductive 3D-Printed Ionic Composite Hydrogels. Adv. Funct. Mater. 2017, 27, 1701807. [Google Scholar] [CrossRef]
- Rahman, M.S.; Shiblee, M.N.I.; Ahmed, K.; Khosla, A.; Ogawa, J.; Kawakami, M.; Furukawa, H. Flexible and Conductive 3D Printable Polyvinylidene Fluoride and Poly(N,N-dimethylacrylamide) Based Gel Polymer Electrolytes. Macromol. Mater. Eng. 2020, 305, 2000262. [Google Scholar] [CrossRef]
- Branco da Cunha, C.; Klumpers, D.D.; Li, W.A.; Koshy, S.T.; Weaver, J.C.; Chaudhuri, O.; Granja, P.L.; Mooney, D.J. Influence of the stiffness of three-dimensional alginate/collagen-I interpenetrating networks on fibroblast biology. Biomaterials 2014, 35, 8927–8936. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Qin, S.; Gerringer, J.; Grunlan, J.C. Unusually fast and large actuation from multilayer polyelectrolyte thin films. Soft Matter 2019, 15, 2311–2314. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guan, L.; Li, X.; Gao, Z.; Ren, X.; Gao, G. Conductive Organohydrogels with Ultrastretchability, Antifreezing, Self-Healing, and Adhesive Properties for Motion Detection and Signal Transmission. ACS Appl. Mater. Interfaces 2019, 11, 3428–3437. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Kim, K.B.; Kim, Y.-R.; Kim, S.; Kim, H.C.; Lee, J.; Kim, J.; Chung, T.D. Electrokinetic concentration on a microfluidic chip using polyelectrolytic gel plugs for small molecule immunoassay. Electrochim. Acta 2013, 110, 164–171. [Google Scholar] [CrossRef]
- Mithun, U.; Vishalakshi, B.; Karthika, J.S. Preparation and characterization of polyelectrolyte complex of N,N,N-trimethyl chitosan/gellan gum: Evaluation for controlled release of ketoprofen. Iran. Polym. J. 2016, 25, 339–348. [Google Scholar] [CrossRef]
- O’Bryan, C.S.; Kabb, C.P.; Sumerlin, B.S.; Angelini, T.E. Jammed Polyelectrolyte Microgels for 3D Cell Culture Applications: Rheological Behavior with Added Salts. ACS Appl. Bio Mater. 2019, 2, 1509–1517. [Google Scholar] [CrossRef]
- Li, W.; Feng, R.; Wang, R.; Li, D.; Jiang, W.; Liu, H.; Guo, Z.; Serpe, M.J.; Hu, L. Polyelectrolyte-based physical adhesive hydrogels with excellent mechanical properties for biomedical applications. J. Mater. Chem. B 2018, 6, 4799–4807. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, H.-W. Emerging properties of hydrogels in tissue engineering. J. Tissue Eng. 2018, 9, 2041731418768285. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef]
- Claus, J.; Sommer, F.O.; Kragl, U. Ionic liquids in biotechnology and beyond. Solid State Ion. 2018, 314, 119–128. [Google Scholar] [CrossRef]
- Ahn, S.-k.; Kasi, R.M.; Kim, S.-C.; Sharma, N.; Zhou, Y. Stimuli-responsive polymer gels. Soft Matter 2008, 4, 1151–1157. [Google Scholar] [CrossRef]
- Zheng, J.; Xiao, P.; Liu, W.; Zhang, J.; Huang, Y.; Chen, T. Mechanical Robust and Self-Healable Supramolecular Hydrogel. Macromol. Rapid Commun. 2016, 37, 265–270. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Li, A.; Li, T.; Liu, M.; Von Klitzing, R.; Ober, C.K.; Kayitmazer, A.B.; Li, L.; Guo, X. Zinc induced polyelectrolyte coacervate bioadhesive and its transition to a self-healing hydrogel. RSC Adv. 2015, 5, 66871–66878. [Google Scholar] [CrossRef]
- Wang, S.; Urban, M.W. Self-healing polymers. Nat. Rev. Mater. 2020, 5, 562–583. [Google Scholar] [CrossRef]
- Hsieh, F.-Y.; Tao, L.; Wei, Y.; Hsu, S.-H. A novel biodegradable self-healing hydrogel to induce blood capillary formation. NPG Asia Mater. 2017, 9, e363. [Google Scholar] [CrossRef]
- Ye, J.; Fu, S.; Zhou, S.; Li, M.; Li, K.; Sun, W.; Zhai, Y. Advances in hydrogels based on dynamic covalent bonding and prospects for its biomedical application. Eur. Polym. J. 2020, 139, 110024. [Google Scholar] [CrossRef]
- Appel, E.A.; Tibbitt, M.W.; Webber, M.J.; Mattix, B.A.; Veiseh, O.; Langer, R. Self-assembled hydrogels utilizing polymer-nanoparticle interactions. Nat. Commun. 2015, 6, 6295. [Google Scholar] [CrossRef]
- Yang, Y.; Urban, M.W. Self-Healing of Polymers via Supramolecular Chemistry. Adv. Mater. Interfaces 2018, 5, 1800384. [Google Scholar] [CrossRef]
- Wu, S.; Zhu, M.; Lu, D.; Milani, A.H.; Lian, Q.; Fielding, L.A.; Saunders, B.R.; Derry, M.J.; Armes, S.P.; Adlam, D.; et al. Self-curing super-stretchable polymer/microgel complex coacervate gels without covalent bond formation. Chem. Sci. 2019, 10, 8832–8839. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Gong, J.P. Double-Network Hydrogels: Soft and Tough IPN. In Encyclopedia of Polymeric Nanomaterials; Kobayashi, S., Müllen, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Fan, C.; Liao, L.; Zhang, C.; Liu, L. A tough double network hydrogel for cartilage tissue engineering. J. Mater. Chem. B 2013, 1, 4251–4258. [Google Scholar] [CrossRef] [PubMed]
- Bahram, M.; Mohseni, N.; Moghtader, M. An introduction to hydrogels and some recent applications. In Emerging Concepts in Analysis and Applications of Hydrogels; Majee, S.B., Ed.; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Luo, S.-C.; Yu, J.-S.; Chen, T.-C.; Su, W.-F. Peptide-Based Polyelectrolyte Promotes Directional and Long Neurite Outgrowth. ACS Appl. Bio Mater. 2019, 2, 518–526. [Google Scholar] [CrossRef]
- Siyawamwaya, M.; Choonara, Y.E.; Bijukumar, D.; Kumar, P.; Du Toit, L.C.; Pillay, V. A Review: Overview of Novel Polyelectrolyte Complexes as Prospective Drug Bioavailability Enhancers. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 955–968. [Google Scholar] [CrossRef]
- Vehlow, D.; Schmidt, R.; Gebert, A.; Siebert, M.; Lips, K.S.; Müller, M. Polyelectrolyte complex based interfacial drug delivery system with controlled loading and improved release performance for bone therapeutics. Nanomaterials 2016, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Sonje, A.G.; Mahajan, H.S. Nasal inserts containing ondansetron hydrochloride based on Chitosan–gellan gum polyelectrolyte complex: In vitro–in vivo studies. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 64, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Umerska, A.; Tajber, L. Polyelectrolyte complexes as nanoparticulate drug delivery systems. Eur. Pharm. Rev. 2015, 20, 36–40. [Google Scholar]
- García, M.C.; Cuggino, J.C.; Rosset, C.I.; Páez, P.L.; Strumia, M.C.; Manzo, R.H.; Alovero, F.L.; Alvarez Igarzabal, C.I.; Jimenez-Kairuz, A.F. A novel gel based on an ionic complex from a dendronized polymer and ciprofloxacin: Evaluation of its use for controlled topical drug release. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 236–246. [Google Scholar] [CrossRef]
- Quiñones, J.P.; Peniche, H.; Peniche, C. Chitosan Based Self-Assembled Nanoparticles in Drug Delivery. Polymers 2018, 10, 235. [Google Scholar] [CrossRef]
- Müller, M.; Torger, B.; Wehrum, D.; Vehlow, D.; Urban, B.; Woltmann, B.; Hempel, U. Drug delivery and cell interaction of adhesive poly(ethyleneimine)/sulfated polysaccharide complex particle films. Biointerphases 2015, 10, 011001. [Google Scholar] [CrossRef]
- Kilicarslan, M.; Ilhan, M.; Inal, O.; Orhan, K. Preparation and evaluation of clindamycin phosphate loaded chitosan/alginate polyelectrolyte complex film as mucoadhesive drug delivery system for periodontal therapy. Eur. J. Pharm. Sci. 2018, 123, 441–451. [Google Scholar] [CrossRef]
- Mansourpour, M.; Mahjub, R.; Amini, M.; Ostad, S.N.; Shamsa, E.S.; Rafiee-Tehrani, M.; Dorkoosh, F.A. Development of Acid-Resistant Alginate/Trimethyl Chitosan Nanoparticles Containing Cationic β-Cyclodextrin Polymers for Insulin Oral Delivery. AAPS Pharm. Sci. Tech. 2015, 16, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Vehlow, D.; Wong, J.P.H.; Urban, B.; Weisspflog, J.; Gebert, A.; Schumacher, M.; Gelinsky, M.; Stamm, M.; Mueller, M. Catechol Containing Polyelectrolyte Complex Nanoparticles as Local Drug Delivery System for Bortezomib at Bone Substitute Materials. Pharmaceutics 2020, 12, 799. [Google Scholar] [CrossRef]
- Nalam, P.C.; Lee, H.-S.; Bhatt, N.; Carpick, R.W.; Eckmann, D.M.; Composto, R.J. Nanomechanics of pH-Responsive, Drug-Loaded, Bilayered Polymer Grafts. ACS Appl. Mater. Interfaces 2017, 9, 12936–12948. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Vehlow, D.; Torger, B.; Urban, B. Adhesive Drug Delivery Systems Based on Polyelectrolyte Complex Nanoparticles (PEC NP) for Bone Healing. Curr. Pharm. Des. 2018, 24, 1341–1348. [Google Scholar] [CrossRef]
- Woltmann, B.; Torger, B.; Müller, M.; Hempel, U. Interaction between immobilized polyelectrolyte complex nanoparticles and human mesenchymal stromal cells. Int. J. Nanomed. 2014, 9, 2205–2215. [Google Scholar] [CrossRef][Green Version]
- Reis, B.; Vehlow, D.; Rust, T.; Kuckling, D.; Müller, M. Thermoresponsive Catechol Based-Polyelectrolyte Complex Coatings for Controlled Release of Bortezomib. Int. J. Mol. Sci. 2019, 20, 6081. [Google Scholar] [CrossRef] [PubMed]
- Morton, S.W.; Poon, Z.; Hammond, P.T. The architecture and biological performance of drug-loaded LbL nanoparticles. Biomaterials 2013, 34, 5328–5335. [Google Scholar] [CrossRef] [PubMed]
- Hines, L.; Petersen, K.; Lum, G.Z.; Sitti, M. Soft Actuators for Small-Scale Robotics. Adv. Mater. 2017, 29, 1603483. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, H.; Suhail, M.; Ren, H. Hydrogel actuators and sensors for biomedical soft robots: Brief overview with impending challenges. Biomimetics 2018, 3, 15. [Google Scholar] [CrossRef]
- Xiao, S.; Yang, Y.; Zhong, M.; Chen, H.; Zhang, Y.; Yang, J.; Zheng, J. Salt-Responsive Bilayer Hydrogels with Pseudo-Double-Network Structure Actuated by Polyelectrolyte and Antipolyelectrolyte Effects. ACS Appl. Mater. Interfaces 2017, 9, 20843–20851. [Google Scholar] [CrossRef] [PubMed]
- Techawanitchai, P.; Ebara, M.; Idota, N.; Asoh, T.-A.; Kikuchi, A.; Aoyagi, T. Photo-switchable control of pH-responsive actuators via pH jump reaction. Soft Matter 2012, 8, 2844–2851. [Google Scholar] [CrossRef]
- Feron, K.; Lim, R.; Sherwood, C.; Keynes, A.; Brichta, A.; Dastoor, P.C. Organic Bioelectronics: Materials and Biocompatibility. Int. J. Mol. Sci. 2018, 19, 2382. [Google Scholar] [CrossRef]
- Lee, C.-J.; Wu, H.; Hu, Y.; Young, M.; Wang, H.; Lynch, D.; Xu, F.; Cong, H.; Cheng, G. Ionic Conductivity of Polyelectrolyte Hydrogels. ACS Appl. Mater. Interfaces 2018, 10, 5845–5852. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.S.; Burlaka, A.; Paz, J.; Salavagione, H.; Carretero-Gonzalez, J.; Hernandez, R. Compact polyelectrolyte hydrogels of gelatin and chondroitin sulfate as ion’s mobile media in sustainable all-solid state electrochemical devices. Mater. Adv. 2020, 1, 2526–2535. [Google Scholar] [CrossRef]
- Tran-Van, F.; Carrier, M.; Chevrot, C. Sulfonated polythiophene and poly(3,4-ethylenedioxythiophene) derivatives with cations exchange properties. Synth. Met. 2004, 142, 251–258. [Google Scholar] [CrossRef]
- Persson, K.M.; Karlsson, R.; Svennersten, K.; Löffler, S.; Jager, E.W.H.; Richter-Dahlfors, A.; Konradsson, P.; Berggren, M. Electronic Control of Cell Detachment Using a Self-Doped Conducting Polymer. Adv. Mater. 2011, 23, 4403–4408. [Google Scholar] [CrossRef]
- Inal, S.; Rivnay, J.; Leleux, P.; Ferro, M.; Ramuz, M.; Brendel, J.C.; Schmidt, M.M.; Thelakkat, M.; Malliaras, G.G. A High Transconductance Accumulation Mode Electrochemical Transistor. Adv. Mater. 2014, 26, 7450–7455. [Google Scholar] [CrossRef] [PubMed]
- Zeglio, E.; Vagin, M.; Musumeci, C.; Ajjan, F.N.; Gabrielsson, R.; Trinh, X.T.; Son, N.T.; Maziz, A.; Solin, N.; Inganäs, O. Conjugated Polyelectrolyte Blends for Electrochromic and Electrochemical Transistor Devices. Chem. Mater. 2015, 27, 6385–6393. [Google Scholar] [CrossRef]
- Nyamayaro, K.; Triandafilidi, V.; Keyvani, P.; Rottler, J.; Mehrkhodavandi, P.; Hatzikiriakos, S.G. The rectification mechanism in polyelectrolyte gel diodes. Phys. Fluids 2021, 33, 032010. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Su, Z.; Cai, S. Stretchable and transparent ionic diode and logic gates. Extrem. Mech. Lett. 2019, 28, 81–86. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Lu, C.; Wang, Y.; Deng, Y. Flexible and Transparent Paper-Based Ionic Diode Fabricated from Oppositely Charged Microfibrillated Cellulose. J. Phys. Chem. C 2012, 116, 9227–9234. [Google Scholar] [CrossRef]
- So, J.-H.; Koo, H.-J.; Dickey, M.D.; Velev, O.D. Ionic Current Rectification in Soft-Matter Diodes with Liquid-Metal Electrodes. Adv. Funct. Mater. 2012, 22, 625–631. [Google Scholar] [CrossRef]
- Yamamoto, T.; Doi, M. Electrochemical mechanism of ion current rectification of polyelectrolyte gel diodes. Nat. Commun. 2014, 5, 4162. [Google Scholar] [CrossRef]
- Triandafilidi, V.; Hatzikiriakos, S.G.; Rottler, J. Molecular simulations of the piezoionic effect. Soft Matter 2018, 14, 6222–6229. [Google Scholar] [CrossRef]
- Balu, R.; Dutta, N.K.; Choudhury, N.R. Resilin-mimetics as a smart biomaterial platform for biomedical applications. Nat. Commun. 2021, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, J.L.; Dutta, N.K.; Elvin, C.M.; Choudhury, N.R. Fabrication of highly elastic resilin/silk fibroin based hydrogel by rapid photo-crosslinking reaction. J. Mater. Chem. B 2015, 3, 6576–6579. [Google Scholar] [CrossRef] [PubMed]
- Athukoralalage, S.S.; Tran, S.; Balu, R.; Truong, V.K.; Chapman, J.; Dutta, N.; Choudhury, N.R. 3D printable electrically conductive hydrogel scaffolds for biomedical applications: A review. Polymers 2021, 13, 474. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wanasingha, N.; Dorishetty, P.; Dutta, N.K.; Choudhury, N.R. Polyelectrolyte Gels: Fundamentals, Fabrication and Applications. Gels 2021, 7, 148. https://doi.org/10.3390/gels7030148
Wanasingha N, Dorishetty P, Dutta NK, Choudhury NR. Polyelectrolyte Gels: Fundamentals, Fabrication and Applications. Gels. 2021; 7(3):148. https://doi.org/10.3390/gels7030148
Chicago/Turabian StyleWanasingha, Nisal, Pramod Dorishetty, Naba K. Dutta, and Namita Roy Choudhury. 2021. "Polyelectrolyte Gels: Fundamentals, Fabrication and Applications" Gels 7, no. 3: 148. https://doi.org/10.3390/gels7030148
APA StyleWanasingha, N., Dorishetty, P., Dutta, N. K., & Choudhury, N. R. (2021). Polyelectrolyte Gels: Fundamentals, Fabrication and Applications. Gels, 7(3), 148. https://doi.org/10.3390/gels7030148







