In Vivo Evaluation of a Pectin-Honey Hydrogel Coating on Polypropylene Mesh in a Rat Model of Acute Hernia
Abstract
1. Introduction
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Preparation of Pectin-Honey Hydrogels (PHHs) and Mesh Coating
5.2. Surgical Procedures
5.3. Macroscopically Examination
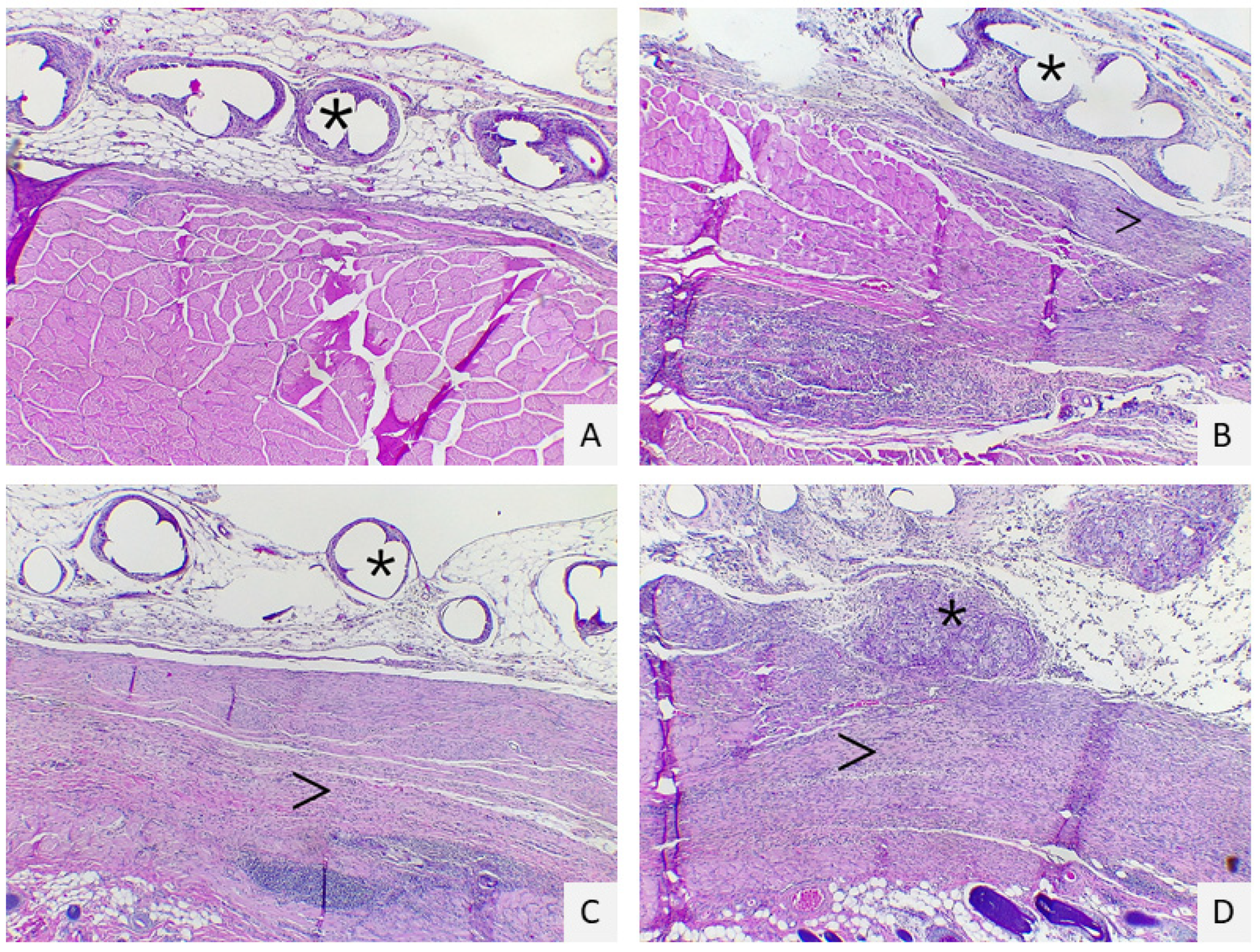
5.4. Histopathological Evaluation
5.5. Immunohistochemical Analysis
5.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Utiyama, E.M.; Rosa, M.B.S.D.F.; Andres, M.D.P.; De Miranda, J.S.; Damous, S.H.B.; Birolini, C.A.V.; Damous, L.L.; Montero, E.F.D.S. Polypropylene and polypropylene/polyglecaprone (Ultrapro(r)) meshes in the repair of incisional hernia in rats. Acta Cir. Bras. 2015, 30, 376–381. [Google Scholar] [CrossRef]
- Nohuz, E.; Alaboud, M.; Darcha, C.; Alloui, A.; Aublet-Cuvelier, B.; Jacquetin, B. Effectiveness of Hyalobarrier and Seprafilm to prevent polypropylene mesh shrinkage: A macroscopic and histological experimental study. Int. Urogynecol. J. 2014, 25, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, D.; Edwards, S.L.; Su, K.; Tan, K.S.; White, J.; Ramshaw, J.A.; Lo, C.; Rosamilia, A.; Werkmeister, J.A.; Gargett, C.E. Human Endometrial Mesenchymal Stem Cells Modulate the Tissue Response and Mechanical Behavior of Polyamide Mesh Implants for Pelvic Organ Prolapse Repair. Tissue Eng. Part A 2013, 20, 785–798. [Google Scholar] [CrossRef]
- Udpa, N.; Iyer, S.R.; Rajoria, R.; Breyer, K.E.; Valentine, H.; Singh, B.; McDonough, S.P.; Brown, B.N.; Bonassar, L.J.; Gao, Y. Effects of Chitosan Coatings on Polypropylene Mesh for Implantation in a Rat Abdominal Wall Model. Tissue Eng. Part A 2013, 19, 2713–2723. [Google Scholar] [CrossRef] [PubMed]
- Van Eps, J.; Fernandez-Moure, J.; Cabrera, F.; Wang, X.; Karim, A.; Corradetti, B.; Chan, P.; Dunkin, B.; Tasciotti, E.; Weiner, B.; et al. Decreased hernia recurrence using autologous platelet-rich plasma (PRP) with Strattice™ mesh in a rodent ventral hernia model. Surg. Endosc. 2016, 30, 3239–3249. [Google Scholar] [CrossRef]
- Di Loreto, F.P.; Mangione, A.; Palmisano, E.; Cerda, J.I.; Dominguez, M.J.; Ponce, G.; Bernaus, M.; Gaffuri, S.; Torresi, G.; Bianco, S. Dried human amniotic membrane as an antiadherent layer for intraperitoneal placing of polypropylene mesh in rats. Surg. Endosc. 2013, 27, 1435–1440. [Google Scholar] [CrossRef]
- Giusto, G.; Vercelli, C.; Iussich, S.; Audisio, A.; Morello, E.; Odore, R.; Gandini, M. A pectin-honey hydrogel prevents postoperative intraperitoneal adhesions in a rat model. BMC Vet. Res. 2016, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Radu, P.; Brătucu, M.; Garofil, D.; Pasnicu, C.; Iorga, C.; Popa, F.; Strâmbu, V. Molecular factors of failure in incisional hernia surgery. Chirurgia 2013, 108, 193–198. [Google Scholar] [PubMed]
- Pereira-Lucena, C.G.; Neto, R.A.; De Rezende, D.T.; Lopes-Filho, G.D.J.; Matos, D.; Linhares, M.M. Early and late postoperative inflammatory and collagen deposition responses in three different meshes: An experimental study in rats. Hernia 2013, 18, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Dubay, D.A.; Wang, X.; Kuhn, M.A.; Robson, M.C.; Franz, M.G. The Prevention of Incisional Hernia Formation Using a Delayed-Release Polymer of Basic Fibroblast Growth Factor. Ann. Surg. 2004, 240, 179–186. [Google Scholar] [CrossRef]
- Burger, J.W.A.; Halm, J.A.; Wijsmuller, A.R.; Raa, S.T.; Jeekel, J. Evaluation of new prosthetic meshes for ventral hernia repair. Surg. Endosc. 2006, 20, 1320–1325. [Google Scholar] [CrossRef]
- Catena, F.; Ansaloni, L.; Gazzotti, F.; Gagliardi, S.; Di Saverio, S.; D’Alessandro, L.; Pinna, A.D. Use of porcine dermal collagen graft (Permacol) for hernia repair in contaminated fields. Hernia 2006, 11, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Naranjo, J.; Turner, N.; Swinehart, I.; Kolich, B.; Shaffiey, S.; Londono, R.; Keane, T.; Reing, J.; Johnson, S.; et al. Mechanical strength vs. degradation of a biologically-derived surgical mesh over time in a rodent full thickness abdominal wall defect. Biomaterials 2016, 108, 81–90. [Google Scholar] [CrossRef]
- Butler, C.E.; Prieto, V.G. Reduction of Adhesions with Composite AlloDerm/Polypropylene Mesh Implants for Abdominal Wall Reconstruction. Plast. Reconstr. Surg. 2004, 114, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, J.; Liu, N.; Zhu, X.; Tang, R. Tailoring electrospun mesh for a compliant remodeling in the repair of full-thickness abdominal wall defect-The role of decellularized human amniotic membrane and silk fibroin. Mater. Sci. Eng. C 2021, 127, 112235. [Google Scholar] [CrossRef] [PubMed]
- Giusto, G.; Vercelli, C.; Comino, F.; Caramello, V.; Tursi, M.; Gandini, M. A new, easy-to-make pectin-honey hydrogel enhances wound healing in rats. BMC Complement. Altern. Med. 2017, 17, 266. [Google Scholar] [CrossRef]
- Pascual, G.; Sotomayor, S.; Rodríguez, M.; Arteaga, V.; Bellón, J.M. Extraperitoneal and intraperitoneal behavior of several biological meshes currently used to repair abdominal wall defects. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 365–372. [Google Scholar] [CrossRef]
- Cornwell, K.G.; Zhang, F.; Lineaweaver, W. Bovine fetal collagen reinforcement in a small animal model of hernia with component repair. J. Surg. Res. 2016, 201, 416–424. [Google Scholar] [CrossRef]
- Chatzoulis, G.; Chatzoulis, K.; Spyridopoulos, P.; Pappas, P.; Ploumis, A. Salvage of an infected titanium mesh in a large incisional ventral hernia using medicinal honey and vacuum-assisted closure: A case report and literature review. Hernia 2012, 16, 475–479. [Google Scholar] [CrossRef]
- Giusto, G.; Beretta, G.; Vercelli, C.; Valle, E.; Iussich, S.; Borghi, R.; Odetti, P.; Monacelli, F.; Tramuta, C.; Grego, E.; et al. Pectin-honey hydrogel: Characterization, antimicrobial activity and biocompatibility. Biomed. Mater. Eng. 2018, 29, 347–356. [Google Scholar] [CrossRef]
- Aysan, E.; Ayar, E.; Aren, A.; Cifter, C. The role of intra-peritoneal honey administration in preventing post-operative peritoneal adhesions. Eur. J. Obstet. Gynecol. Reprod. Biol. 2002, 104, 152–155. [Google Scholar] [CrossRef]
- Emre, A.; Akin, M.; Isikgonul, I.; Yuksel, O.; Anadol, A.Z.; Cifter, C. Comparison of intraperitoneal honey and sodium hyaluronate-carboxymethylcellulose (SeprafilmTM) for the prevention of postoperative intra-abdominal adhesions. Clinics 2009, 64, 363–368. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Udwadia, T.E. Ghee and Honey Dressing for Infected Wounds. Indian J. Surg. 2011, 73, 278–283. [Google Scholar] [CrossRef]
- Klinge, U.; Klosterhalfen, B. Modified classification of surgical meshes for hernia repair based on the analyses of 1,000 explanted meshes. Hernia 2012, 16, 251–258. [Google Scholar] [CrossRef]
- Hwang, H.J.; An, M.S.; Ha, T.K.; Kim, K.H.; Kim, T.H.; Choi, C.S.; Hong, K.H.; Jung, S.J.; Kim, S.-H.; Rho, K.H.; et al. All the commercially available adhesion barriers have the same effect on adhesion prophylaxis?; A comparison of barrier agents using a newly developed, severe intra-abdominal adhesion model. Int. J. Color. Dis. 2013, 28, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Suckow, M.A.; Boynton, F.D.D.; Johnson, C. Use of a Rat Model to Study Ventral Abdominal Hernia Repair. J. Vis. Exp. 2017, 128, 53587. [Google Scholar] [CrossRef]
- Utrabo, C.A.L.; Czeczko, N.G.; Busato, C.R.; Montemór-Netto, M.R.; Lipinski, L.; Malafaia, O. BETWEEN PROLENE®, ULTRAPRO® AND BARD SOFT® MESHES WHICH PRESENTS THE BEST PERFORMANCE IN THE REPAIR OF THE ABDOMINAL WALL? Arq. Bras. Cir. Dig. 2021, 34, e1577. [Google Scholar] [CrossRef]
- Yildiz, T.; Ilce, Z.; Yildirim, M.; Akdoğan, M.; Yürümez, Y.; Varlikli, O.; Dilek, F.H.; Ilĉe, Z.; Yıldız, T.; Varlıkli, O. Antienflamatuar and antiadhesive effect of clioquinol. Int. J. Surg. 2015, 15, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Pascual, G.; Hernández-Gascón, B.; Rodríguez, M.; Sotomayor, S.; Peña, E.; Calvo, B.; Bellón, J.M. The long-term behavior of lightweight and heavyweight meshes used to repair abdominal wall defects is determined by the host tissue repair process provoked by the mesh. Surgery 2012, 152, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Poppas, D.P.; Sung, J.J.; Magro, C.M.; Chen, J.; Toyohara, J.P.; Ramshaw, B.J.; Felsen, D. Hydrogel coated mesh decreases tissue reaction resulting from polypropylene mesh implant: Implication in hernia repair. Hernia 2016, 20, 623–632. [Google Scholar] [CrossRef]
- Deng, Y.; Ren, J.; Chen, G.; Li, G.; Guo, K.; Hu, Q.; Wu, X.; Wang, G.; Gu, G.; Li, J. Evaluation of polypropylene mesh coated with biological hydrogels for temporary closure of open abdomen. J. Biomater. Appl. 2016, 31, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Tonks, A.; Cooper, R.A.; Price, A.J.; Molan, P.C.; Jones, K.P. Stimulation of TNF-α release in monocytes by honey. Cytokine 2001, 14, 240–242. [Google Scholar] [CrossRef]
- Tonks, A.; Cooper, R.; Jones, K.; Blair, S.; Parton, J. Honey stimulates inflammatory cytokine production from monocytes. Cytokine 2003, 21, 242–247. [Google Scholar] [CrossRef]
- Du Toit, D.F.; Page, B.J. An in vitro evaluation of the cell toxicity of honey and silver dressings. J. Wound Care 2009, 18, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Quinn, R. Comparing rat’s to human’s age: How old is my rat in people years? Nutrition 2005, 21, 775–777. [Google Scholar] [CrossRef]
- Vogels, R.R.M.; Kaufmann, R.; Van Den Hil, L.C.L.; Van Steensel, S.; Schreinemacher, M.H.; Lange, J.F.; Kannekens-Bouvy, N. Critical overview of all available animal models for abdominal wall hernia research. Hernia 2017, 21, 667–675. [Google Scholar] [CrossRef]
- Suárez-Grau, J.M.; Chaves, C.R.; Morales-Conde, S.; García, C.M.; Durantez, F.D.; Ruiz, F.J.P. Could we reduce adhesions to the intra-abdominal mesh in the first week? Experimental study with different methods of fixation. Hernia 2020, 24, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.E. Method of Preparing Homogeneous Honey Pectin Composition. U.S. Patent 2,295,274, 8 September 1942. [Google Scholar]
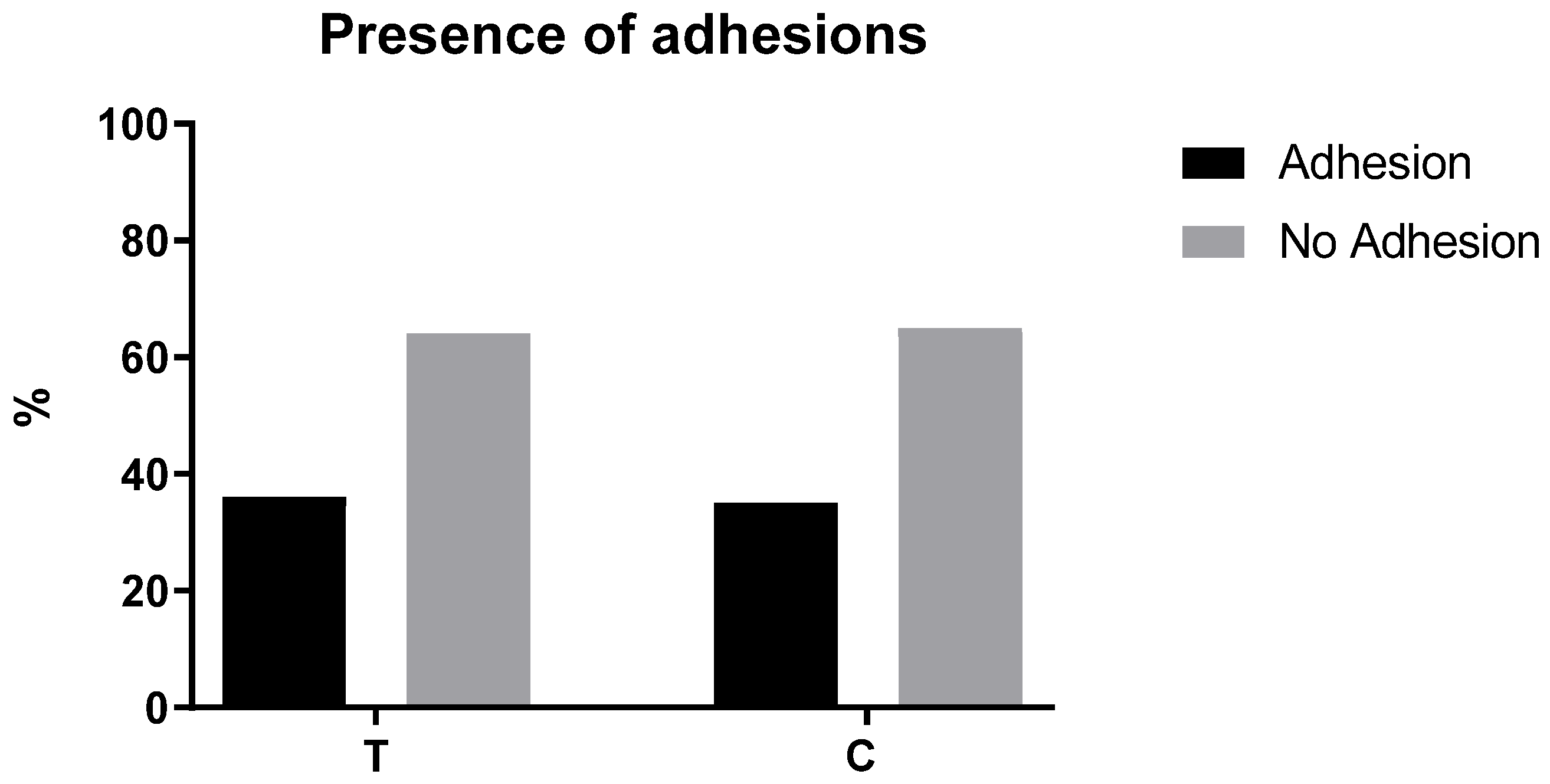

| C (n = 17) | T (n = 14) | p Value (<0.05) | |
|---|---|---|---|
| Cells layers at margins of the granulomas | 2 (1–4) 1.55–2.45 | 3 (1–4) 2.135–3.00 | 0.0488 |
| Inflammatory reaction in the host tissue | 3 (1–4) 1.98–3.19 | 2.5 (1–4) 1.96–3.04 | 0.7869 |
| Inflammatory response on the mesh surface | 2 (2–4) 2.04–2.66 | 4 (2–4) 2.94–3.92 | 0.0009 |
| Tissue maturation | 2(1–3) 1.34–2.19 | 2.5 (1–4) 1.60–2.82 | 0.2538 |
| C (n = 17) | T (n = 14) | p Value (<0.05) | |
|---|---|---|---|
| % cells | 2 (1–3) IQR: 1.45–2.2 | 2 (1–3) 1.9–2.64 | 0.076 |
| Intensity | 1 (1–2) 1.1–1.84 | 2 (1–3) 1.45–2.12 | 0.1153 |
| Score | Cells Layers at the Margins of the Granulomas | Inflammatory Reaction in the Host Tissue | Inflammatory Response on the Mesh Surface | Tissue Maturation |
|---|---|---|---|---|
| 1 | 1–4 layers | Non-dense, mature fibrous tissue | Fibroblast without macrophages or foreign body cells | Dense, mature interstitial tissue, similar to a normal connective or adipose tissue |
| 2 | 5–9 layers | Immature fibrous tissue with fibroblasts and little collagen | Isolated foci or macrophages or foreign body cells | Interstitial tissue with blood vessels, fibroblasts, and a few macrophages |
| 3 | 10–30 layers | Dense granular tissue with fibroblasts and many inflammatory cells | One layer of macrophages foreign body cells | Interstitial tissue with giant inflammatory cells but with permeating connective tissue |
| 4 | >30 layers | Mass of inflammatory cells, with disorganized connective tissue | Multiple layers of macrophages and foreign body cells | Mass of inflammatory cells without permeating connective tissue |
| Score | % Cells | Intensity |
|---|---|---|
| 1 | 0–25 | Weak |
| 2 | 26–50 | Moderate |
| 3 | 51–100 | Strong |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vercelli, C.; Re, G.; Iussich, S.; Odore, R.; Morello, E.M.; Gandini, M.; Giusto, G. In Vivo Evaluation of a Pectin-Honey Hydrogel Coating on Polypropylene Mesh in a Rat Model of Acute Hernia. Gels 2021, 7, 132. https://doi.org/10.3390/gels7030132
Vercelli C, Re G, Iussich S, Odore R, Morello EM, Gandini M, Giusto G. In Vivo Evaluation of a Pectin-Honey Hydrogel Coating on Polypropylene Mesh in a Rat Model of Acute Hernia. Gels. 2021; 7(3):132. https://doi.org/10.3390/gels7030132
Chicago/Turabian StyleVercelli, Cristina, Giovanni Re, Selina Iussich, Rosangela Odore, Emanuela Maria Morello, Marco Gandini, and Gessica Giusto. 2021. "In Vivo Evaluation of a Pectin-Honey Hydrogel Coating on Polypropylene Mesh in a Rat Model of Acute Hernia" Gels 7, no. 3: 132. https://doi.org/10.3390/gels7030132
APA StyleVercelli, C., Re, G., Iussich, S., Odore, R., Morello, E. M., Gandini, M., & Giusto, G. (2021). In Vivo Evaluation of a Pectin-Honey Hydrogel Coating on Polypropylene Mesh in a Rat Model of Acute Hernia. Gels, 7(3), 132. https://doi.org/10.3390/gels7030132







