Clinical Application of Antibacterial Hydrogel and Coating in Orthopaedic and Traumatology Surgery
Abstract
:1. Introduction
2. Periprosthetic Joint Infections
2.1. Prophylactic Use in Joint Replacement
2.2. Therapeutic Use in PJI
3. Fracture Related Infections
3.1. Prophylactic Use in Fracture Fixations
3.2. Therapeutic Use in FRI
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kärrholm, J.; Rogmark, C.; Naucler, E.; Nåtman, J.; Vinblad, J.; Mohaddes, M.; Rolfson, O. Swedish Hip Arthroplasty Register Annual Report 2019; Rolfson O.: Gothenburg, Sweden, 2021. [Google Scholar] [CrossRef]
- Torre, M.; Carrani, E.; Luzi, I.; Laricchiuta, P.; Ceccarelli, S. (Eds.) Progetto Registro Italiano Artroprotesi. In Quarto Report. Potenziare la Qualità dei Dati per Migliorare la Sicurezza dei Pazienti; Il Pensiero Scientifico Editore: Rome, Italy, 2017. [Google Scholar]
- Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR). Hip, Knee & Shoulder Arthroplasty: 2020 Annual Report. Available online: https://aoanjrr.sahmri.com/annual-reports-2020 (accessed on 15 June 2021).
- Kenney, C.; Dick, S.; Lea, J.; Liu, J.; Ebraheim, N.A. A systematic review of the causes of failure of Revision Total Hip Arthroplasty. J. Orthop. 2019, 16, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Schmidmaier, G.; Lucke, M.; Wildemann, B.; Haas, N.P.; Raschke, M. Prophylaxis and treatment of implant-related infections by antibiotic-coated implants: A review. Injury 2006, 37, S105–S112. [Google Scholar] [CrossRef] [PubMed]
- Metsemakers, W.J.; Smeets, B.; Nijs, S.; Hoekstra, H. Infection after fracture fixation of the tibia: Analysis of healthcare utilization and related costs. Injury 2017, 48, 1204–1210. [Google Scholar] [CrossRef]
- Zahid, M.; Lodhi, M.; Afzal, A.; Rehan, Z.A.; Mehmood, M.; Javed, T.; Shabbir, R.; Siuta, D.; Althobaiti, F.; Dessok, E.S. Development of Hydrogels with the Incorporation of Raphanus sativus L. Seed Extract in Sodium Alginate for Wound-Healing Application. Gels 2021, 7, 107. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Maleki, H.; Larrañeta, E.; Fajardo, A.; Bakhshian, N.A.; Shavandi, A.; Sheikhi, A.; Ghorbanpour, M.; Farokhi, M.; Praveen, G.; et al. Status and future scope of plant-based green hydrogels in biomedical engineering. Appl. Mater. Today 2019, 16, 213–246. [Google Scholar] [CrossRef] [Green Version]
- Pitarresi, G.; Palumbo, F.S.; Calascibetta, F.; Fiorica, C.; Di Stefano, M.; Giammona, G. Medicated hydrogels of hyaluronic acid derivatives for use in orthopedic field. Int. J. Pharm. 2013, 449, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Overstreet, D.; McLaren, A.; Calara, F.; Vernon, B.L.; McLemore, R. Local Gentamicin Delivery from Resorbable Viscous Hydrogels Is Therapeutically Effective. Clin. Orthop. Relat. Res. 2014, 473, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Drago, L.; Boot, W.; Dimas, K.; Malizos, K.; Hänsch, G.M.; Stuyck, J.; Gawlitta, D.; Romano, C.L. Does implant coating with antibacterial-loaded hydrogel reduce bacterial colonization and biofilm formation in vitro? Clin. Orthop. Relat. Res. 2014, 472, 3311–3323. [Google Scholar] [CrossRef] [Green Version]
- Giavaresi, G.; Meani, E.; Sartori, M.; Ferrari, A.; Bellini, D.; Sacchetta, A.C.; Meraner, J.; Sambri, A.; Vocale, C.; Sambri, V.; et al. Efficacy of antibacterial-loaded coating in an in vivo model of acutely highly contaminated implant. Int. Orthop. 2013, 38, 1505–1512. [Google Scholar] [CrossRef] [Green Version]
- Schmidmaier, G.; Wildemann, B.; Stemberger, A.; Haas, N.P.; Raschke, M. Biodegradable poly (D,L-lactide) coating of implants for continuous release of growth factors. J. Biomed. Mater. Res. 2001, 58, 449–455. [Google Scholar] [CrossRef]
- Strobel, C.; Schmidmaier, G.; Wildemann, B. Changing the release kinetics of gentamicin from poly (D,L-lactide) implant coatings using only one polymer. Int. J. Artif. Organs 2011, 34, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Nast, S.; Fassbender, M.; Bormann, N.; Beck, S.; Montali, A.; Lucke, M.; Schmidmaier, G.; Wildemann, B. In Vivo quantification of gentamicin released from an implant coating. J. Biomater. Appl. 2016, 31, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Graeser, V.; Moghaddam-Alvandi, A.; Westhauser, F.; Dapunt, U.; Kamradt, T.; Woerner, S.M.; Schmidmaier, G. Patients’ safety: Is there a systemic release of gentamicin by gentamicin-coated tibia nails in clinical use? Ther. Clin. Risk Manag. 2016, 12, 1387–1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romano, C.L.; Malizos, K.; Capuano, N.; Mezzoprete, R.; D’Arienzo, M.; Van Der Straeten, C.; Scarponi, S.; Drago, L. Does an Antibiotic-Loaded Hydrogel Coating Reduce Early Post-Surgical Infection After Joint Arthroplasty? J. Bone Jt. Infect. 2016, 1, 34–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Meo, D.; Calogero, V.; Are, L.; Cavallo, A.U.; Persiani, P.; Villani, C. Antibiotic-Loaded Hydrogel Coating to Reduce Early Postsurgical Infections in Aseptic Hip Revision Surgery: A Retrospective, Matched Case-Control Study. Microorganisms 2020, 8, 571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoccali, C.; Scoccianti, G.; Biagini, R.; Daolio, P.A.; Giardina, F.L.; Campanacci, D.A. Antibacterial hydrogel coating in joint mega-prosthesis: Results of a comparative series. Eur. J. Orthop. Surg. Traumatol. 2021, 1–9. [Google Scholar] [CrossRef]
- Trentinaglia, M.T.; Van Der Straeten, C.; Morelli, I.; Logoluso, N.; Drago, L.; Romanò, C.L. Economic Evaluation of Antibacterial Coatings on Healthcare Costs in First Year Following Total Joint Arthroplasty. J. Arthroplast. 2018, 33, 1656–1662. [Google Scholar] [CrossRef]
- Iannotti, F.; Prati, P.; Fidanza, A.; Iorio, R.; Ferretti, A.; Pèrez Prieto, D.; Kort, N.; Violante, B.; Pipino, G.; Schiavone Panni, A. Prevention of Periprosthetic Joint Infection (PJI): A Clinical Practice Protocol in High-Risk Patients. Trop. Med. Infect. Dis. 2020, 5, 186. [Google Scholar] [CrossRef]
- Abosala, A.; Ali, M. The Use of Calcium Sulphate beads in Periprosthetic Joint Infection, a systematic review. J. Bone Jt. Infect. 2020, 5, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Zagra, L.; Gallazzi, E.; Romanò, D.; Scarponi, S.; Romanò, C. Two-stage cementless hip revision for peri-prosthetic infection with an antibacterial hydrogel coating: Results of a comparative series. Int. Orthop. 2019, 43, 111–115. [Google Scholar] [CrossRef]
- Capuano, N.; Logoluso, N.; Gallazzi, E.; Drago, L.; Romanò, C.L. One-stage exchange with antibacterial hydrogel coated implants provides similar results to two-stage revision, without the coating, for the treatment of peri-prosthetic infection. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 3362–3367. [Google Scholar] [CrossRef]
- Pellegrini, A.; Legnani, C. High rate of infection eradication following cementless one-stage revision hip arthroplasty with an antibacterial hydrogel coating. Int. J. Artif. Organs 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Ghirardelli, S.; Fidanza, A.; Prati, P.; Iannotti, F.; Indelli, P.F. Debridement, antibiotic pearls, and retention of the implant in the treatment of infected total hip arthroplasty. Hip Int. 2020, 30, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Ferry, T.; Batailler, C.; Petitjean, C.; Chateau, J.; Fevre, C.; Forestier, E.; Brosset, S.; Leboucher, G.; Kolenda, C.; Laurent, F.; et al. The Potential Innovative Use of Bacteriophages Within the DAC® Hydrogel to Treat Patients with Knee Megaprosthesis Infection Requiring “Debridement Antibiotics and Implant Retention” and Soft Tissue Coverage as Salvage Therapy. Front. Med. 2020, 7, 342. [Google Scholar] [CrossRef] [PubMed]
- Malizos, K.; Blauth, M.; Danita, A.; Capuano, N.; Mezzoprete, R.; Logoluso, N.; Drago, L.; Romanò, C.L. Fast-resorbable antibiotic-loaded hydrogel coating to reduce post-surgical infection after internal osteosynthesis: A multicenter randomized controlled trial. J. Orthop. Traumatol. 2017, in press. [Google Scholar] [CrossRef] [Green Version]
- De Meo, D.; Cannari, F.M.; Petriello, L.; Persiani, P.; Villani, C. Gentamicin-Coated Tibia Nail in Fractures and Nonunion to Reduce Fracture-Related Infections: A Systematic Review. Molecules 2020, 25, 5471. [Google Scholar] [CrossRef]
- Pinto, D.; Manjunatha, K.; Savur, A.D.; Ahmed, N.R.; Mallya, S.; Ramya, V. Comparative study of the efficacy of gentamicin-coated intramedullary interlocking nail versus regular intramedullary interlocking nail in Gustilo type I and II open tibia fractures. Chin. J. Traumatol. 2019, 22, 270–273. [Google Scholar] [CrossRef]
- Metsemakers, W.-J.; Reul, M.; Nijs, S. The use of gentamicin-coated nails in complex open tibia fracture and revision cases: A retrospective analysis of a single centre case series and review of the literature. Injury 2015, 46, 2433–2437. [Google Scholar] [CrossRef]
- Moghaddam, A.; Weis, J.; Haubruck, P.; Raven, T.F.; Addali, A.; Schmidmaier, G. Evaluation of the clinical use of the ETN PROtect® in non-union therapy. Injury 2019, 50, 32–39. [Google Scholar] [CrossRef]
- Corona, P.S.; Altayó, M.; Amat, C.; Vicente, M.; Velez, R. Reconstruction of infected post-traumatic bone defects of the distal femur with the Compress® implant. Preliminary results of a staged non-biological strategy. Injury 2021, in press. [Google Scholar] [CrossRef]
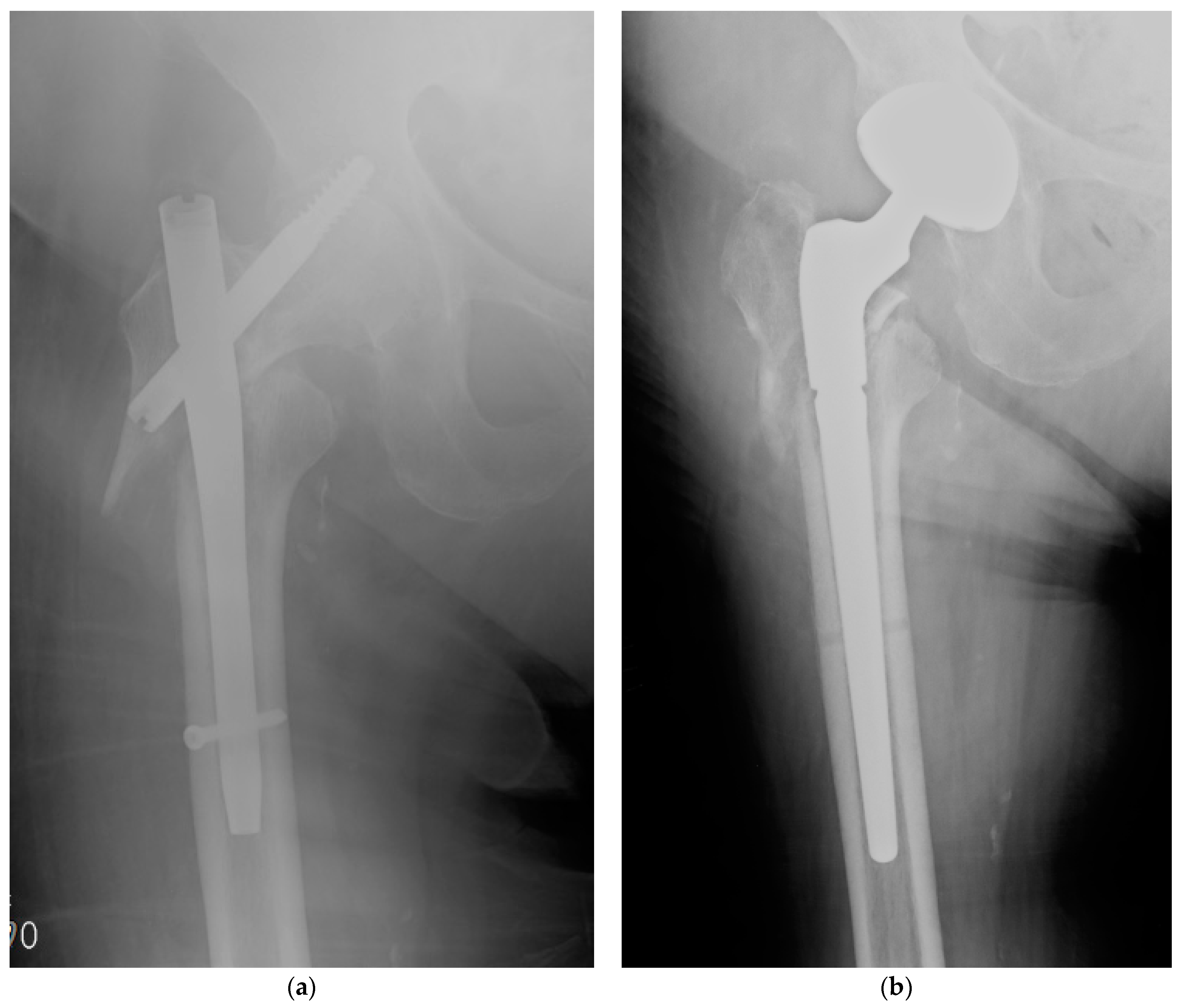
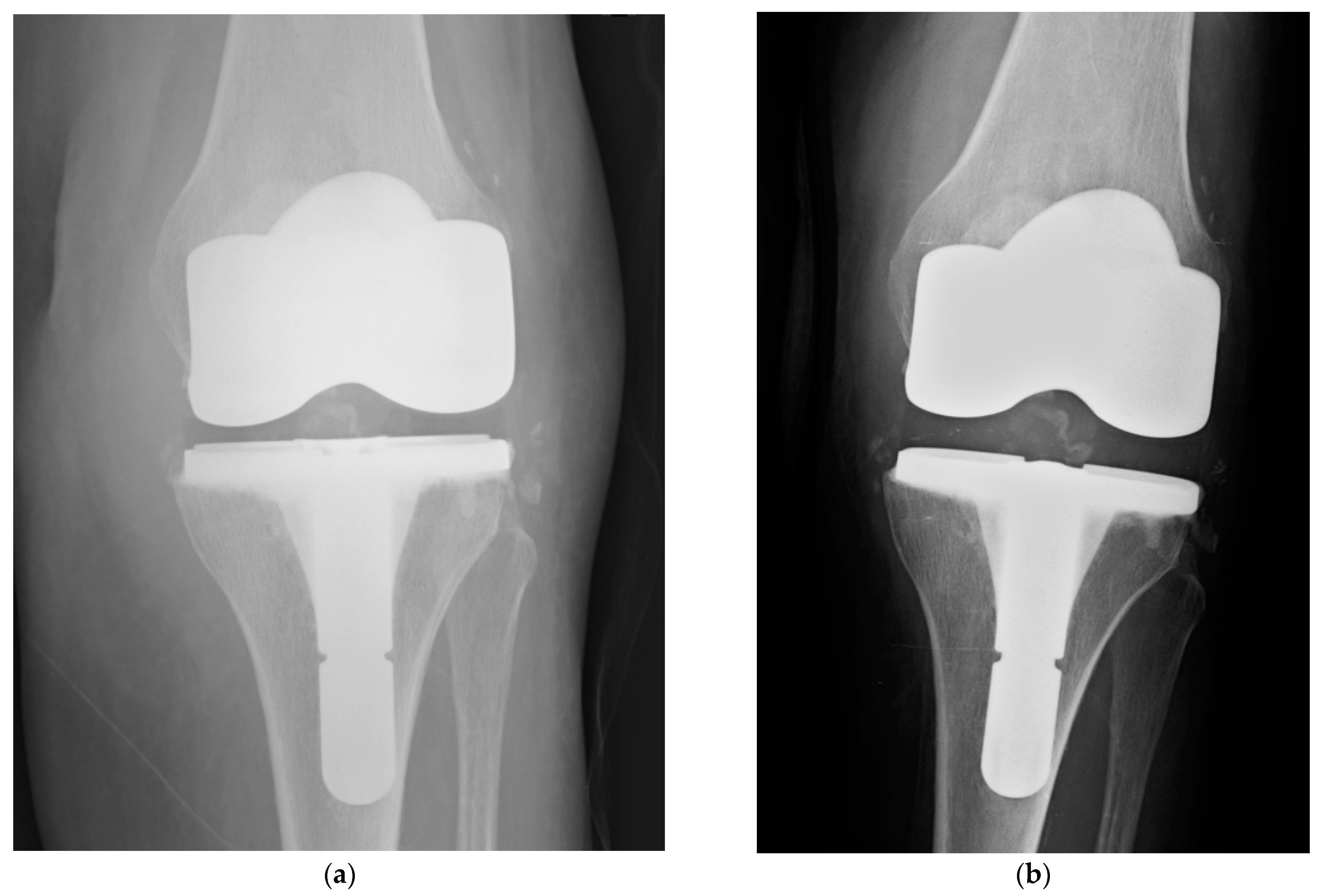

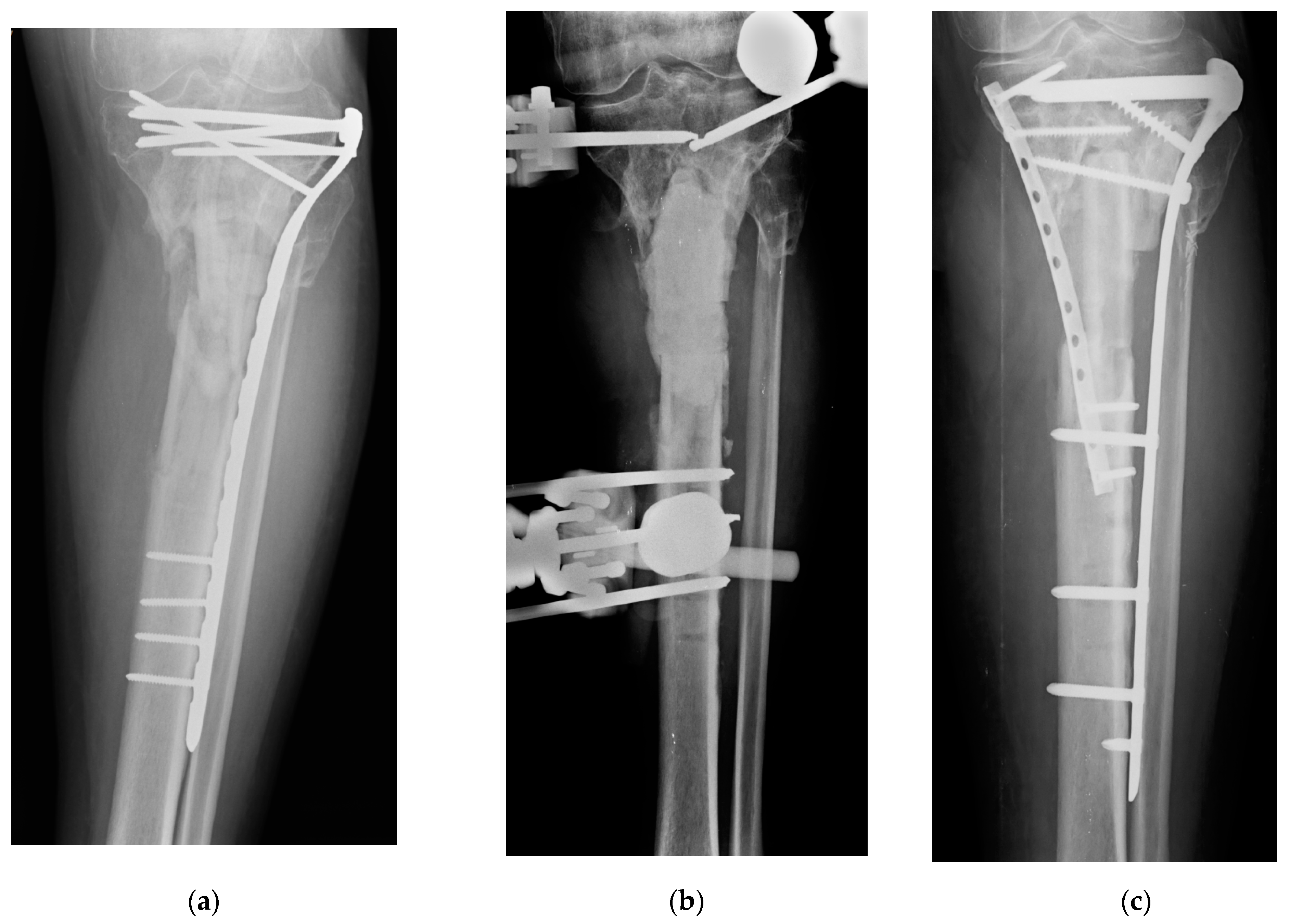
| Type of Antimicrobial Coating | DAC (Hydrogel) | ETN PROtect (Coating) |
|---|---|---|
| Type of implants on which is applicable |
| Only available for Expert Tibial Nail (De Puy Synthes companies) |
| Mode of application | Directly in the operating room | Applied to tibial nail during production |
| Type of antimicrobials available | Antibiotics are added, upon surgeon’s responsibility, by a list of mixable antibiotics | Gentamicin |
| Intended use by manufacturer | Prevention of infection in fractures and joint replacement | Prevention of infection in fractures |
| Described use by literature |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Meo, D.; Ceccarelli, G.; Iaiani, G.; Lo Torto, F.; Ribuffo, D.; Persiani, P.; Villani, C. Clinical Application of Antibacterial Hydrogel and Coating in Orthopaedic and Traumatology Surgery. Gels 2021, 7, 126. https://doi.org/10.3390/gels7030126
De Meo D, Ceccarelli G, Iaiani G, Lo Torto F, Ribuffo D, Persiani P, Villani C. Clinical Application of Antibacterial Hydrogel and Coating in Orthopaedic and Traumatology Surgery. Gels. 2021; 7(3):126. https://doi.org/10.3390/gels7030126
Chicago/Turabian StyleDe Meo, Daniele, Giancarlo Ceccarelli, Giancarlo Iaiani, Federico Lo Torto, Diego Ribuffo, Pietro Persiani, and Ciro Villani. 2021. "Clinical Application of Antibacterial Hydrogel and Coating in Orthopaedic and Traumatology Surgery" Gels 7, no. 3: 126. https://doi.org/10.3390/gels7030126
APA StyleDe Meo, D., Ceccarelli, G., Iaiani, G., Lo Torto, F., Ribuffo, D., Persiani, P., & Villani, C. (2021). Clinical Application of Antibacterial Hydrogel and Coating in Orthopaedic and Traumatology Surgery. Gels, 7(3), 126. https://doi.org/10.3390/gels7030126







