Doxycycline and Monocaprin In Situ Hydrogel: Effect on Stability, Mucoadhesion and Texture Analysis and In Vitro Release
Abstract
1. Introduction
2. Results
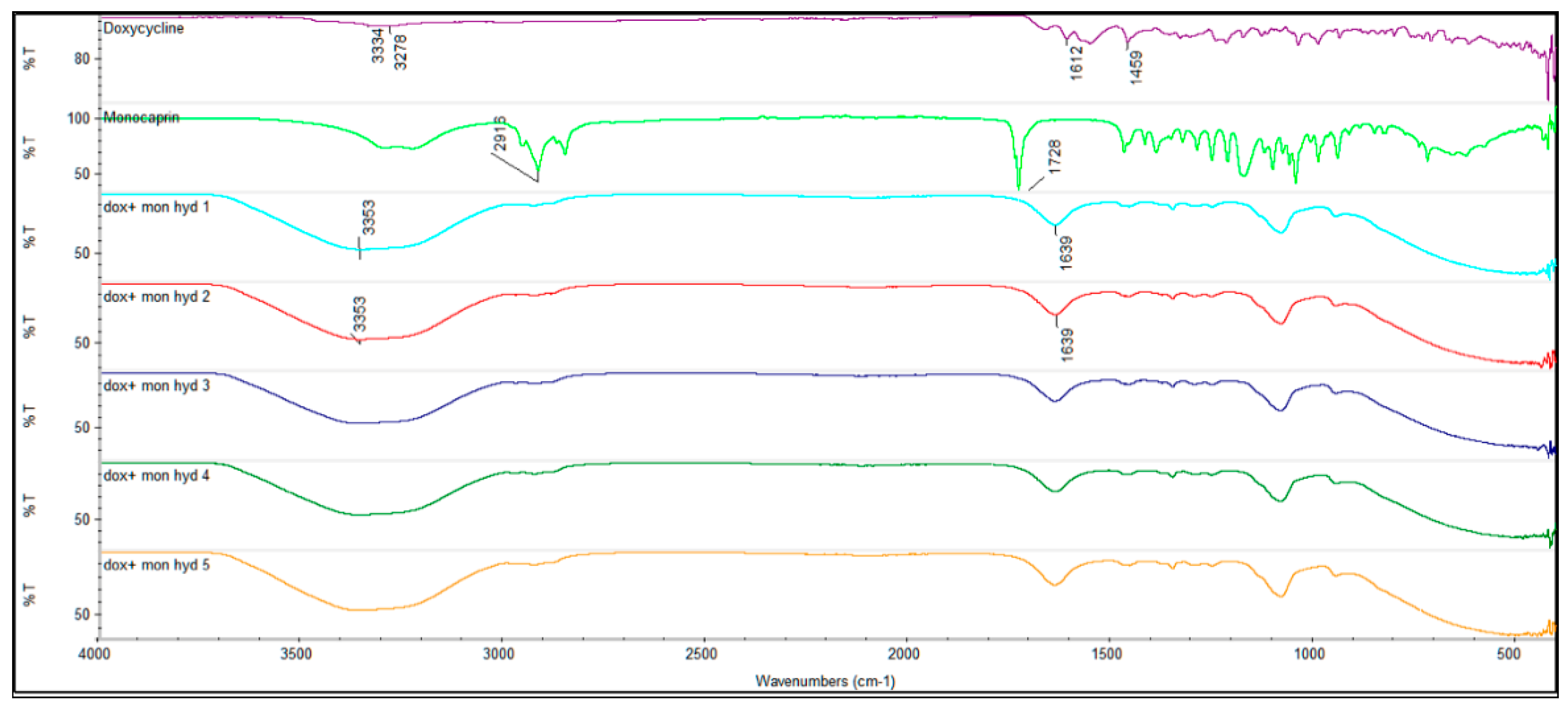
2.1. Drug Interaction
2.1.1. FTIR
2.1.2. Anti-Microbial Studies
2.2. Monocaprin UHPLC-CAD Method Validation
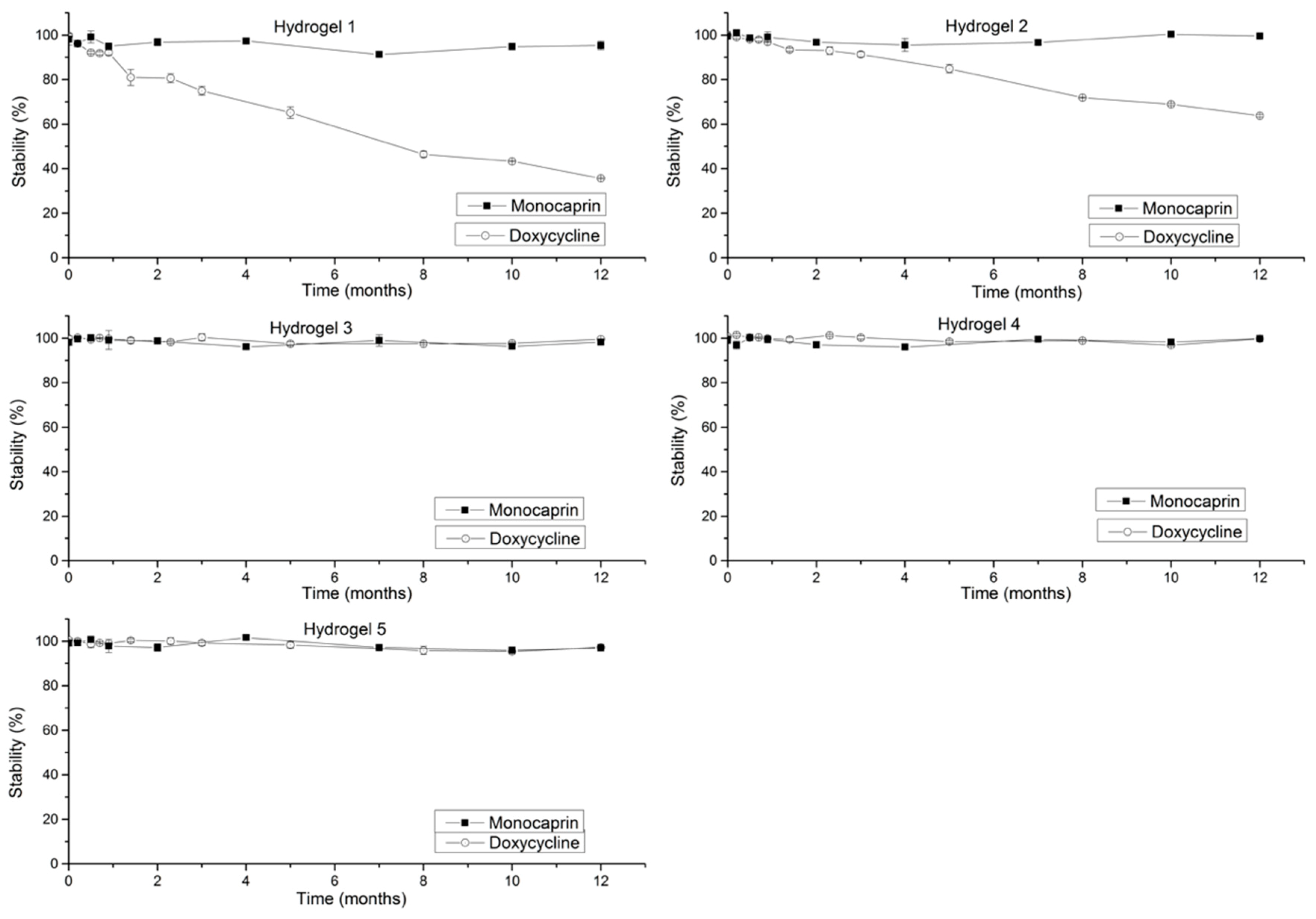
2.3. Stability Studies
2.3.1. Doxycycline Stability (Effect of Increase in Concentration on Stability)
2.3.2. Monocaprin Stability (Effect of Increase in Concentration on Stability)
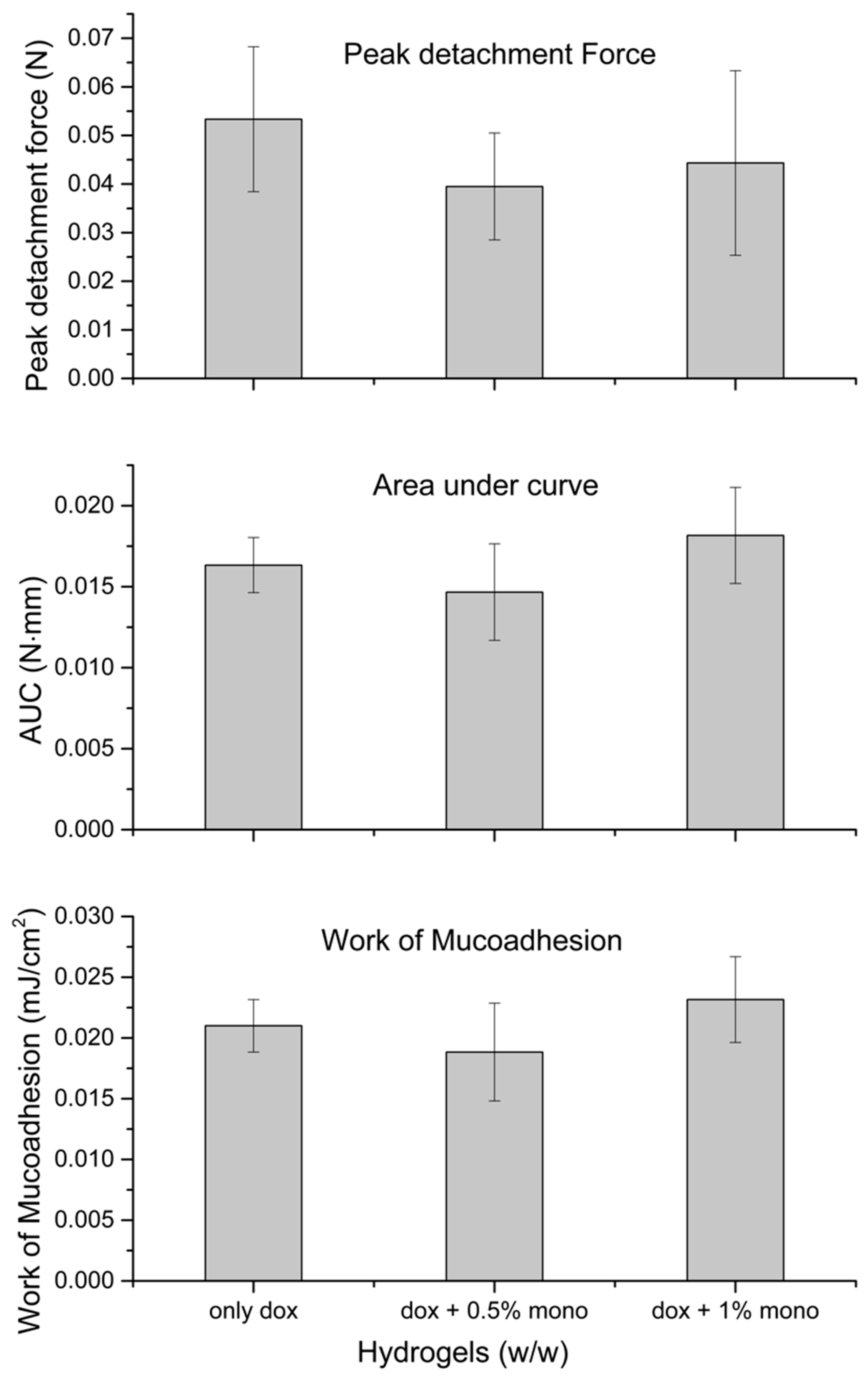
2.4. Mucoadhesion
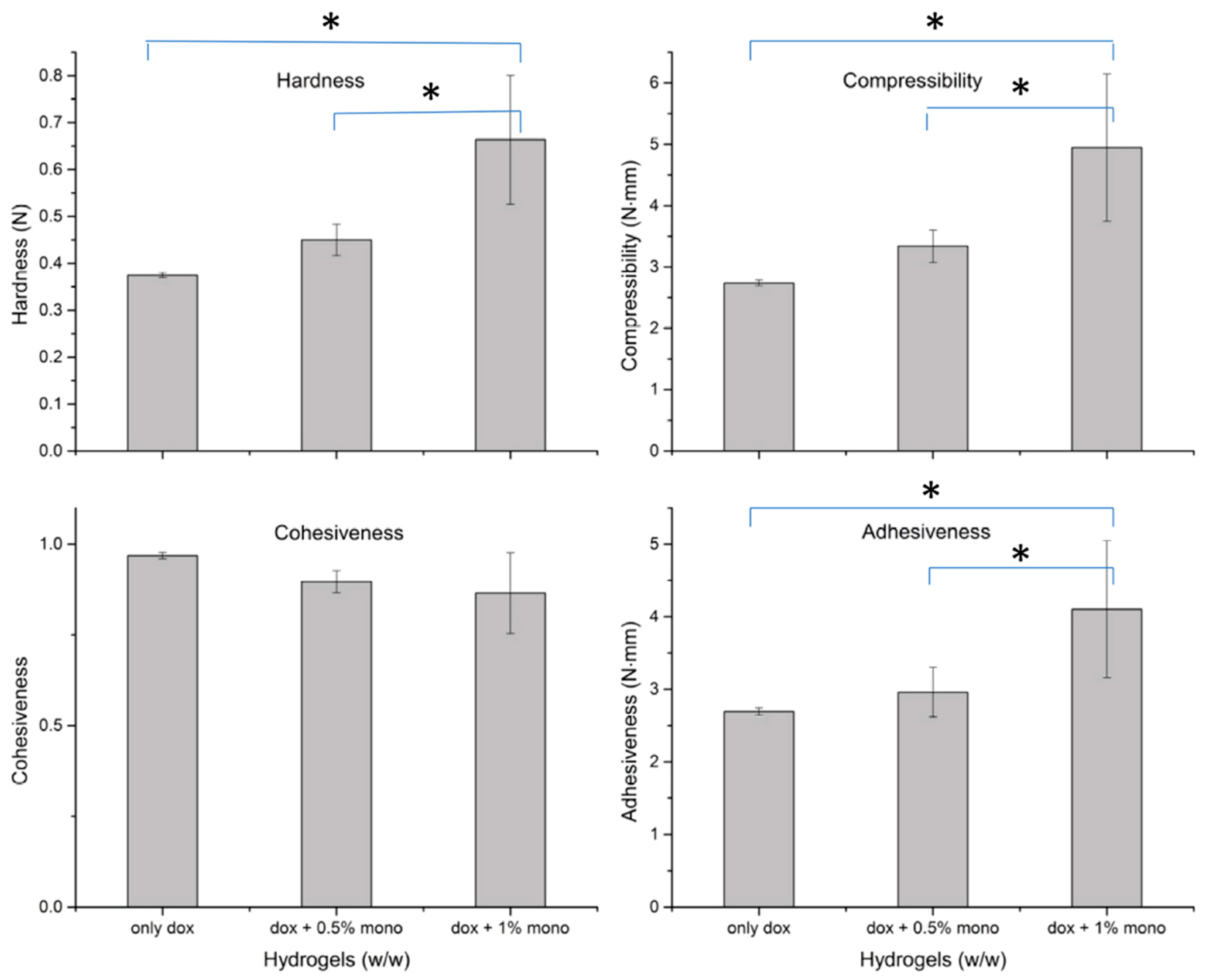
2.5. Texture Analysis
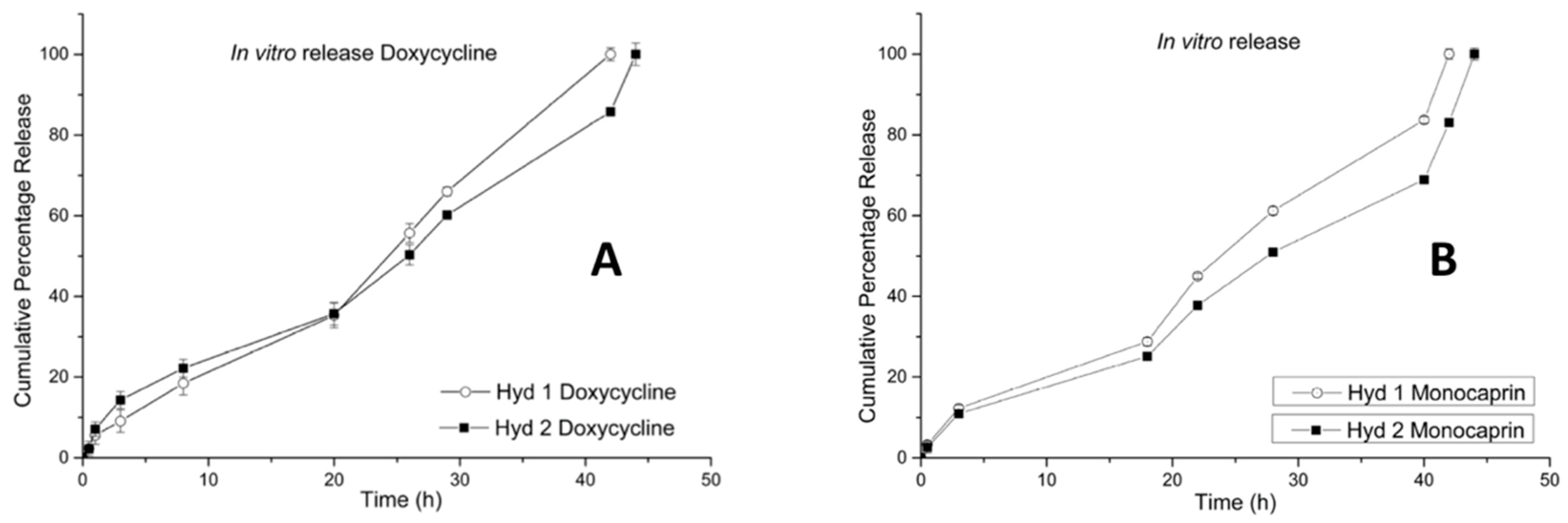
2.6. In Vitro Release (Doxycycline and Monocaprin) Release Mechanism
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Manufacture of Hydrogels
5.3. Gelation Temperature
5.4. Fourier-Transform Infrared Spectroscopy (FTIR)
5.5. Stability Studies
5.6. Analysis of Doxycycline
5.7. Analysis of Monocaprin
- Linearity: A calibration curve was made with concentration ranging from 3 µg/mL to 60 µg/mL. All the samples were injected 3 times.
- Precision: The intraday and inter-day precision was measured for 3 different concentrations of monocaprin ranging from 5 µg/mL to 25 µg/mL and each injected for 5 times. The area under curve (AUC) was obtained for all the injections and precision is expressed as relative standard deviation (%RSD).
- Accuracy: Accuracy was measured as the percentage recovery. Three different concentrations of monocaprin (0.1, 0.15 and 0.2% w/v) in 5 % w/v HPBCD solutions, were injected (n = 3) after suitable dilution and the average percentage recovery was calculated.
- Limit of detection (LOD) and limit of quantification (LOQ): The LOD and LOQ were calculated from linearity obtained from 5 dilutions, 1.1, 2.2, 3.2, 4.3 and 5.4 µg/mL solutions, by using following Equations (1) and (2) [36],
5.8. In Vitro Mucoadhesion
5.9. Texture Profile Analysis (TPA)
- Cohesiveness: was measured as internal structural strength, which sustains strong interconnections with certain level of resistance to rupture. Cohesiveness was calculated from the ratio of area under curve (AUC) under force-time curve for second compression cycle to the first compression cycle (A2/A1) [23,39,42,43].
- Adhesiveness: was measured as, work required to overcome the adhesive forces between the entire surface of probe and hydrogel surface which comes in contact with the probe, while the probe is in retraction mode after the compressibility cycle or also can be defined as negative region of force-time AUC after the first cycle of compression [23,39,42,43].
5.10. In Vitro Antimicrobial Study
5.11. In Vitro Release
5.12. Statistics
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Galey, W.R.; Lonsdale, H.K.; Nacht, S. The in vitro permeability of skin and buccal mucosa to selected drugs and tritiated water. J. Invest. Dermatol. 1976, 67, 713–717. [Google Scholar] [CrossRef]
- Bala, R.; Pawar, P.; Khanna, S.; Arora, S. Orally dissolving strips: A new approach to oral drug delivery system. Int. J. Pharm. Investig. 2013, 3, 67–76. [Google Scholar] [CrossRef]
- Boddé, H.E.; De Vries, M.E.; Junginger, H.E. Mucoadhesive polymers for the buccal delivery of peptides, structure-adhesiveness relationships. J. Control. Release 1990, 13, 225–231. [Google Scholar] [CrossRef]
- Arakaki, K.; Kitamura, N.; Fujiki, H.; Kurokawa, T.; Iwamoto, M.; Ueno, M.; Kanaya, F.; Osada, Y.; Gong, J.P.; Yasuda, K. Artificial cartilage made from a novel double-network hydrogel: In vivo effects on the normal cartilage and ex vivo evaluation of the friction property. J. Biomed. Mater. Res. A 2010, 93, 1160–1168. [Google Scholar] [CrossRef]
- Li, J.; Illeperuma, W.R.K.; Suo, Z.; Vlassak, J.J. Hybrid Hydrogels with Extremely High Stiffness and Toughness. ACS Macro Lett. 2014, 3, 520–523. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. Hydrogels as mucoadhesive and bioadhesive materials: A review. Biomaterials 1996, 17, 1553–1561. [Google Scholar] [CrossRef]
- Alexandridis, P.; Alan Hatton, T. Poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: Thermodynamics, structure, dynamics, and modeling. Colloids Surf. A Physicochem. Eng. Asp. 1995, 96, 1–46. [Google Scholar] [CrossRef]
- Skulason, S.; Holbrook, W.P.; Thormar, H.; Gunnarsson, G.B.; Kristmundsdottir, T. A study of the clinical activity of a gel combining monocaprin and doxycycline: A novel treatment for herpes labialis. J. Oral Pathol. Med. 2012, 41, 61–67. [Google Scholar] [CrossRef]
- Skulason, S.; Holbrook, W.P.; Kristmundsdottir, T. Clinical assessment of the effect of a matrix metalloproteinase inhibitor on aphthous ulcers. Acta Odontol. Scand. 2009, 67, 25–29. [Google Scholar] [CrossRef]
- Patlolla, V.G.R.; Holbrook, W.P.; Gizurarson, S.; Kristmundsdottir, T. Long-term stabilization of aqueous doxycycline formulations, in mucoadhesive hydrogels for treatment of oral mucosal conditions. Curr. Drug Discov. Technol. 2019, in press. [Google Scholar] [CrossRef]
- Thormar, H.; Isaacs, C.E.; Brown, H.R.; Barshatzky, M.R.; Pessolano, T. Inactivation of enveloped viruses and killing of cells by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 1987, 31, 27–31. [Google Scholar] [CrossRef]
- Bergsson, G.; Arnfinnsson, J.; Steingrimsson, O.; Thormar, H. Killing of Gram-positive cocci by fatty acids and monoglycerides. APMIS 2001, 109, 670–678. [Google Scholar] [CrossRef]
- Bergsson, G.; Steingrimsson, O.; Thormar, H. Bactericidal effects of fatty acids and monoglycerides on Helicobacter pylori. Int. J. Antimicrob. Agents 2002, 20, 258–262. [Google Scholar] [CrossRef]
- Thormar, H.; Bergsson, G.; Gunnarsson, E.; Georgsson, G.; Witvrouw, M.; Steingrimsson, O.; De Clercq, E.; Kristmundsdottir, T. Hydrogels containing monocaprin have potent microbicidal activities against sexually transmitted viruses and bacteria in vitro. Sex. Transm. Infect. 1999, 75, 181–185. [Google Scholar] [CrossRef]
- Kristmundsdottir, T.; Arnadottir, S.G.; Bergsson, G.; Thormar, H. Development and evaluation of microbicidal hydrogels containing monoglyceride as the active ingredient. J. Pharm. Sci. 1999, 88, 1011–1015. [Google Scholar] [CrossRef]
- Bergsson, G.; Arnfinnsson, J.; Steingrimsson, O.; Thormar, H. In vitro killing of Candida albicans by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 2001, 45, 3209–3212. [Google Scholar] [CrossRef]
- Thorgeirsdottir, T.O.; Kristmundsdottir, T.; Thormar, H.; Axelsdottir, I.; Holbrook, W.P. Antimicrobial activity of monocaprin: A monoglyceride with potential use as a denture disinfectant. Acta Odontol. Scand. 2006, 64, 21–26. [Google Scholar] [CrossRef]
- Benjamin, M.M.; Khalil, R.A. Matrix metalloproteinase inhibitors as investigative tools in the pathogenesis and management of vascular disease. Exp. Suppl. 2012, 103, 209–279. [Google Scholar] [CrossRef]
- Liu, J.; Xiong, W.; Baca-Regen, L.; Nagase, H.; Baxter, B.T. Mechanism of inhibition of matrix metalloproteinase-2 expression by doxycycline in human aortic smooth muscle cells. J. Vasc. Surg. 2003, 38, 1376–1383. [Google Scholar] [CrossRef]
- Goktolga, U.; Cavkaytar, S.; Altinbas, S.K.; Tapisiz, O.L.; Tapisiz, A.; Erdem, O. Effect of the non-specific matrix metalloproteinase inhibitor Doxycycline on endometriotic implants in an experimental rat model. Exp. Ther. Med. 2015, 9, 1813–1818. [Google Scholar] [CrossRef]
- Thorgeirsdottir, T.Ó.; Thormar, H.; Kristmundsdottir, T. The influence of formulation variables on stability and microbicidal activity of monoglyceride monocaprin. J. Drug Deliv. Sci. Technol. 2005, 15, 233–236. [Google Scholar] [CrossRef]
- Patlolla, V.G.R.; Holbrook, W.P.; Gizurarson, S.; Kristmundsdottir, T. Evaluation of in vitro mucoadhesiveness and Texture Profile Analysis of doxycycline in situ hydrogels. Pharmazie 2019, in press. [Google Scholar]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Peter, G.A. Tetracycline Formulations Stabilized by Bisulfites. U.S. Patent 3,132,993, 12 May 1964. [Google Scholar]
- Power, D.F.; Fieldson, G.; Chang, Y. Tetracycline Stabilizing Formulations. U.S. Patent 13/119,547, 25 March 2010. [Google Scholar]
- Zhang, H.; Chen, M.; He, Z.; Wang, Z.; Zhang, M.; Wan, Q.; Liang, D.; Repka, M.A.; Wu, C. Molecular modeling-based inclusion mechanism and stability studies of doxycycline and hydroxypropyl-beta-cyclodextrin complex for ophthalmic delivery. AAPS PharmSciTech 2013, 14, 10–18. [Google Scholar] [CrossRef][Green Version]
- Nozawa, S.; Akazawa, Y.; Yasui, S.; Yagyu, M. Aqueous Doxycycline Compositions. U.S. Patent 3,846,548, 5 November 1974. [Google Scholar]
- Skidmore, R.; Kovach, R.; Walker, C.; Thomas, J.; Bradshaw, M.; Leyden, J.; Powala, C.; Ashley, R. Effects of Subantimicrobial-Dose Doxycycline in the Treatment of Moderate Acne. Arch. Dermatol. 2003, 139, 459–464. [Google Scholar] [CrossRef]
- Bostanci, N.; Belibasakis, G.N. Doxycycline inhibits TREM-1 induction by Porphyromonas gingivalis. FEMS Immunol. Med. Microbiol. 2012, 66, 37–44. [Google Scholar] [CrossRef]
- Thomas, J.; Walker, C.; Bradshaw, M. Long-term use of subantimicrobial dose doxycycline does not lead to changes in antimicrobial susceptibility. J. Periodontol. 2000, 71, 1472–1483. [Google Scholar] [CrossRef]
- Walker, C.; Preshaw, P.M.; Novak, J.; Hefti, A.F.; Bradshaw, M.; Powala, C. Long-term treatment with sub-antimicrobial dose doxycycline has no antibacterial effect on intestinal flora. J. Clin. Periodontol. 2005, 32, 1163–1169. [Google Scholar] [CrossRef]
- Holbrook, W.P.; Kristmundsdóttir, T.; Thormar, H.; Jónsdóttir, S.Á.; Helgadótir, H. OI0259 A denture adhesive containing monocaprin for reducing contamination with Candida. Oral. Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, e355. [Google Scholar] [CrossRef]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Mucosal Applications of Poloxamer 407-Based Hydrogels: An Overview. Pharmaceutics 2018, 10, 159. [Google Scholar] [CrossRef]
- Skulason, S. Bioadhesive Drug Deivery Systems in the Treatment of Oral Conditions Including Cold Sores and Aphthous Ulcers. Ph.D. Thesis, University of Iceland, Reykjavik, Iceland, June 2009. [Google Scholar]
- ICH Harmonised Tripartite Guideline. Validation of Analytical Procedures: Text and Methodology Q2(R1). Available online: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf (accessed on 26 September 2019).
- Skulason, S.; Asgeirsdottir, M.S.; Magnusson, J.P.; Kristmundsdottir, T. Evaluation of polymeric films for buccal drug delivery. Pharmazie 2009, 64, 197–201. [Google Scholar]
- Amasya, G.; Karavana, S.Y.; Sen, T.; Baloglu, E.; Tarimci, N. Bioadhesive and mechanical properties of triamcinolone acetonide buccal gels. Turk. J. Pharm. Sci. 2012, 9, 1–12. [Google Scholar]
- Jones, D.S.; Woolfson, A.D.; Brown, A.F. Textural analysis and flow rheometry of novel, bioadhesive antimicrobial oral gels. Pharm. Res. 1997, 14, 450–457. [Google Scholar] [CrossRef]
- Oliveira Cardoso, V.M.; Stringhetti Ferreira Cury, B.; Evangelista, R.C.; Daflon Gremiao, M.P. Development and characterization of cross-linked gellan gum and retrograded starch blend hydrogels for drug delivery applications. J. Mech. Behav. Biomed. Mater. 2017, 65, 317–333. [Google Scholar] [CrossRef]
- Sezer, A.D.; Cevher, E.; Hatipoglu, F.; Ogurtan, Z.; Bas, A.L.; Akbuga, J. Preparation of fucoidan-chitosan hydrogel and its application as burn healing accelerator on rabbits. Biol. Pharm. Bull. 2008, 31, 2326–2333. [Google Scholar] [CrossRef]
- Bourne, M.C. Texture Profile Analysis. Food Technol. 1978, 32, 62–66. [Google Scholar]
- Szczesniak, A.S. General foods texture profile revisited—Ten years perspective. J. Texture Stud. 1975, 6, 5–17. [Google Scholar] [CrossRef]
- Zhang, L.; Parsons, D.L.; Navarre, C.; Kompella, U.B. Development and in-vitro evaluation of sustained release poloxamer 407 (P407) gel formulations of ceftiofur. J. Control Release 2002, 85, 73–81. [Google Scholar] [CrossRef]
- Abdelbary, G.A.; Aburahma, M.H. Oro-dental mucoadhesive proniosomal gel formulation loaded with lornoxicam for management of dental pain. J. Liposome Res. 2015, 25, 107–121. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patlolla, V.G.R.; Peter Holbrook, W.; Gizurarson, S.; Kristmundsdottir, T. Doxycycline and Monocaprin In Situ Hydrogel: Effect on Stability, Mucoadhesion and Texture Analysis and In Vitro Release. Gels 2019, 5, 47. https://doi.org/10.3390/gels5040047
Patlolla VGR, Peter Holbrook W, Gizurarson S, Kristmundsdottir T. Doxycycline and Monocaprin In Situ Hydrogel: Effect on Stability, Mucoadhesion and Texture Analysis and In Vitro Release. Gels. 2019; 5(4):47. https://doi.org/10.3390/gels5040047
Chicago/Turabian StylePatlolla, Venu Gopal Reddy, William Peter Holbrook, Sveinbjorn Gizurarson, and Thordis Kristmundsdottir. 2019. "Doxycycline and Monocaprin In Situ Hydrogel: Effect on Stability, Mucoadhesion and Texture Analysis and In Vitro Release" Gels 5, no. 4: 47. https://doi.org/10.3390/gels5040047
APA StylePatlolla, V. G. R., Peter Holbrook, W., Gizurarson, S., & Kristmundsdottir, T. (2019). Doxycycline and Monocaprin In Situ Hydrogel: Effect on Stability, Mucoadhesion and Texture Analysis and In Vitro Release. Gels, 5(4), 47. https://doi.org/10.3390/gels5040047





