Polyolefin-Supported Hydrogels for Selective Cleaning Treatments of Paintings
Abstract
1. Introduction
2. Results and Discussion
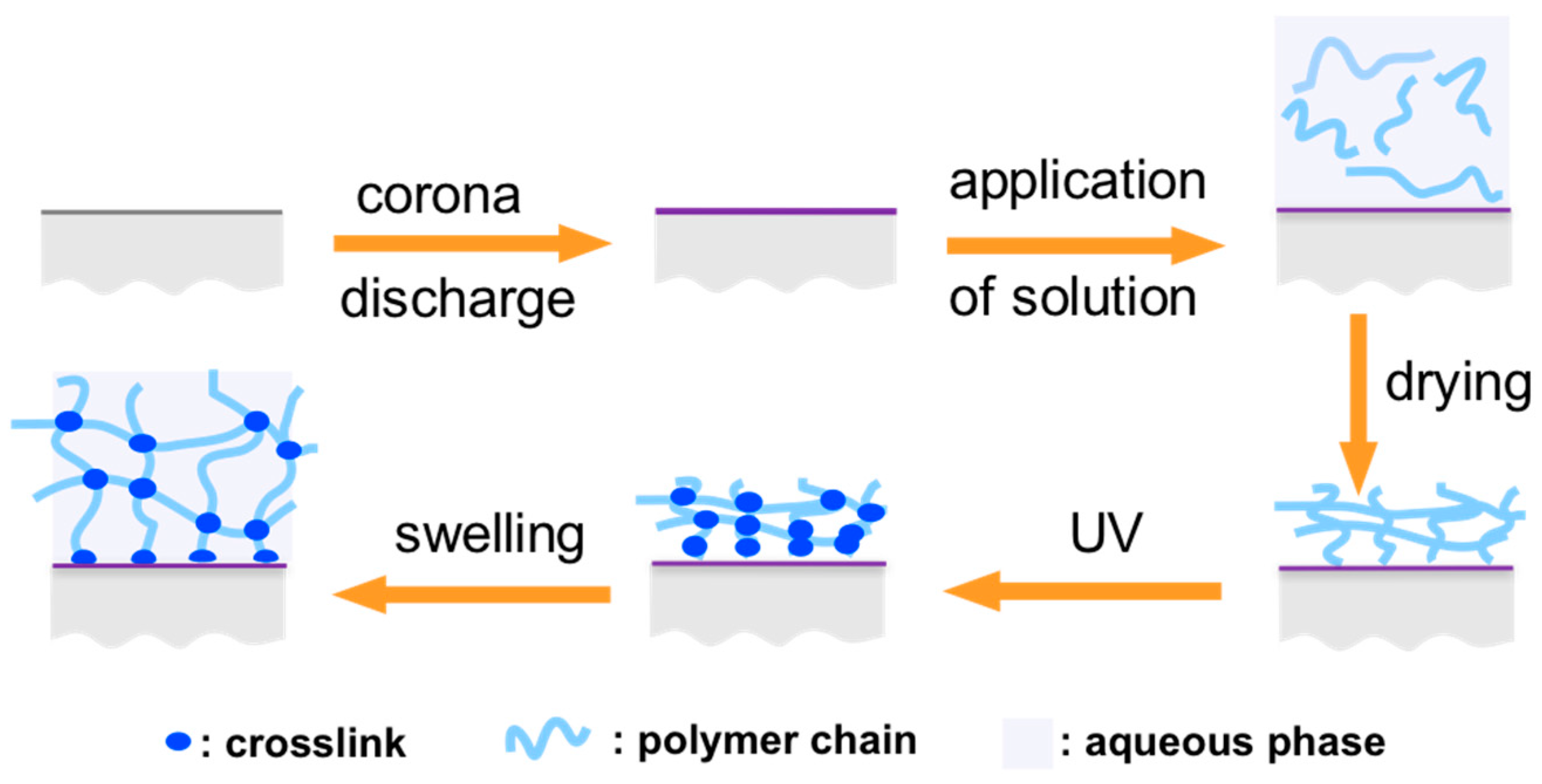
2.1. Concept of Polyolefin-Supported Hydrogels
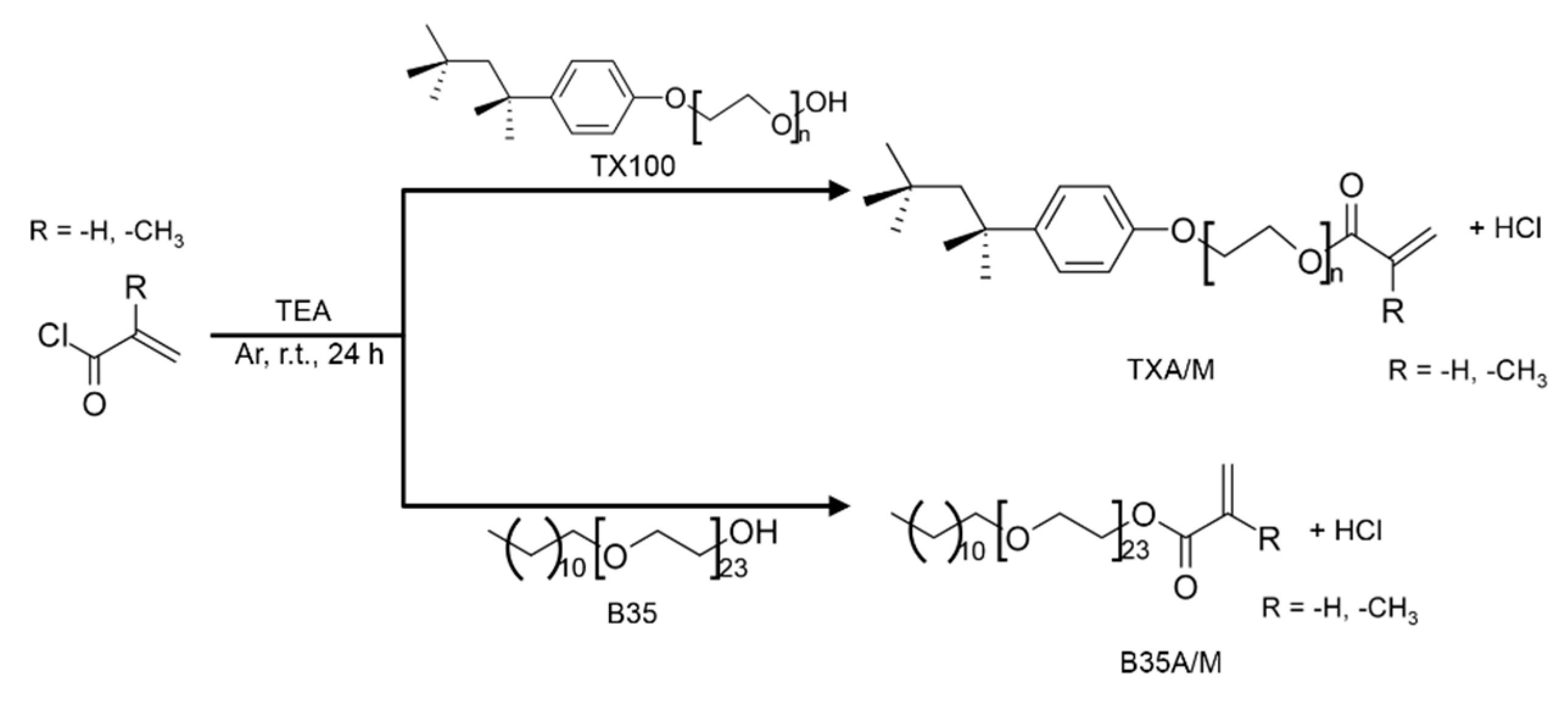
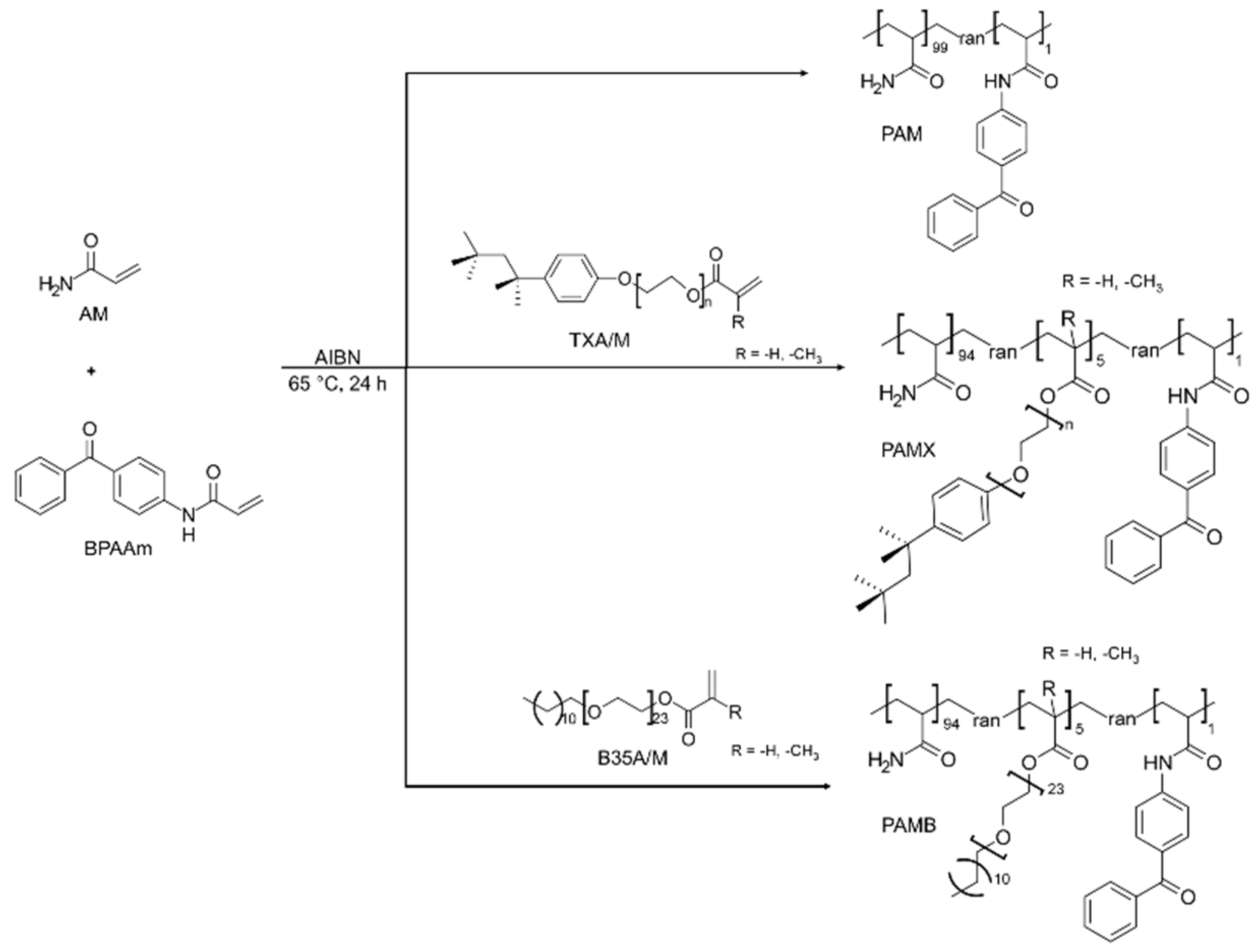
2.2. Monomer and Polymer Synthesis
2.3. Preparation Procedure for the PE-PAM Sheets
2.4. Stability Tests of PE-PAM Sheets
2.5. Water Loading Tests
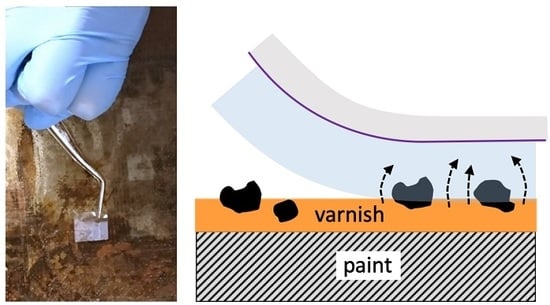
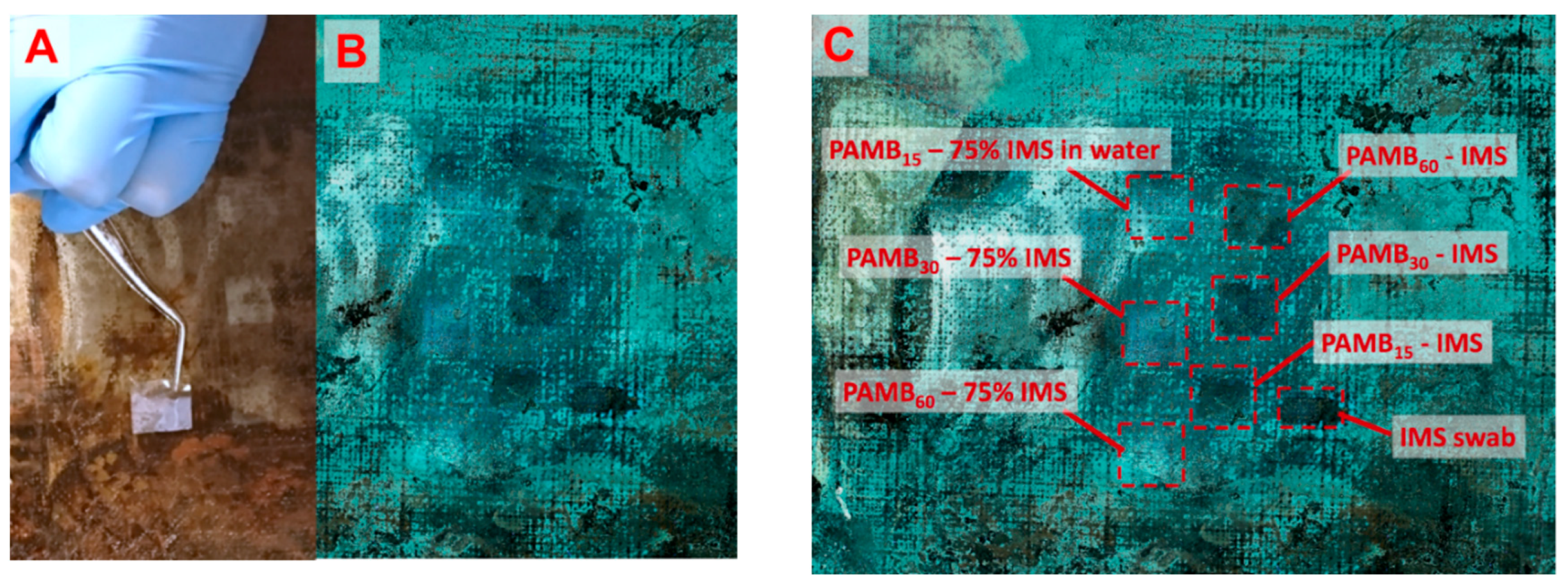
2.5.1. Test 1—Soil Removal from Aged Varnish
2.5.2. Test 2—Removal of Mastic Varnish from Egg Tempera Paint
2.5.3. Test 3—Surface Solubilization of Dammar Varnish
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Syntheses
4.2.1. Monomers
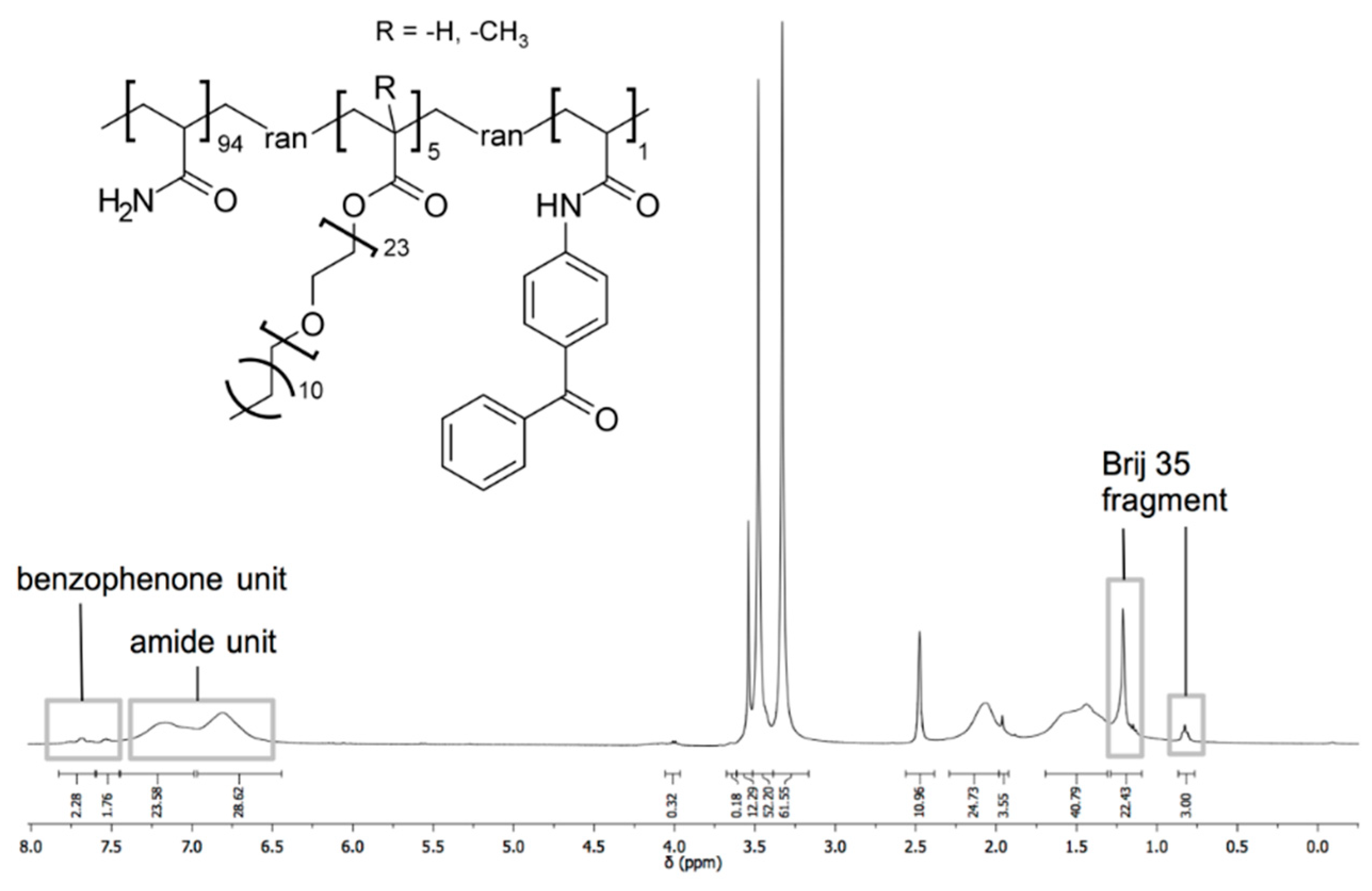
4.2.2. Polymers
PAMX
PAMB
PAM-EO3 and PAM-EO9:
PE-PAM Sheet Preparation
4.3. Stability Tests of PE-PAM Sheets
4.4. Water Loading Test
4.5. Preliminary Cleaning Tests of Painting Surfaces with PE-PAM Sheets
4.5.1. Soil Removal from Mock-Up Laropal K80 Varnish
4.5.2. Removal of Mastic Varnish from Egg Tempera Paint
4.5.3. Surface Solubilization of Dammar Varnish
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | atomic force microscopy |
| AIBN | azobisisobutyronitrile |
| B35A | Brij 35 acrylate |
| B35M | Brij 35 methacrylate |
| BPAAm | benzophenone acrylamide |
| DI | deionized |
| DMSO-D6 | fully deuterated dimethyl sulfoxide |
| DMSO | dimethyl sulfoxide |
| EA | ethyl acetate |
| EO3A/EO9A | Ecosurf EH-3 or EH-9 acrylate |
| EtOH | ethanol |
| 1H-NMR | proton nuclear magnetic resonance |
| IMS | industrial methylated spirit (denatured alcohol) |
| MeOH | methanol |
| PAM | polyacrylamide |
| PAM15 | “15” indicates 15 min photocrosslinking time for polyacrylamide layer |
| PAMB | copolymer of AM, B35A and BPAAm |
| PAM-EO3/EO9 | copolymer of AM, EO3 or EO9, respectively, and BPAAm |
| PAMX | copolymer of AM, TXA and BPAAm |
| PE | polyethylene |
| PE-PAM | polyethylene -supported polyacrylamide |
| TEA | triethylamine |
| TXA | 4-(1,1,3,3-tetramethylbutyl)phenyl-polyethylene glycol (Triton X-100) acrylate |
| TXM | Triton X-100 methacrylate |
| UV | ultraviolet light |
References
- Kreeft, D.; Arkenbout, E.; Henselmans, P.; van Furth, W.; Breedveld, P. Review of Techniques to Achieve Optical Surface Cleanliness and Their Potential Application to Surgical Endoscopes. Surg. Innov. 2017, 24, 509–527. [Google Scholar] [CrossRef]
- Singh, B. Sprayable Gel Cleaner for Optical and Electronic Surfaces. US patent US8993501B2, 31 March 2015. [Google Scholar]
- Tardif, F.; Danel, A.; Raccurt, O. Understanding of wet and alternative particle removal processes in microelectronics: Theoretical capabilities and limitations. J. Telecommun. Inf. Technol. 2005, 1, 11–19. [Google Scholar]
- Ling, M.L.; Apisarnthanarak, A.; Thu, L.T.A.; Villanueva, V.; Pandjaitan, C.; Yusof, M.Y. APSIC Guidelines for environmental cleaning and decontamination. Antimicrob. Resist. Infect. Control 2015, 4, 2–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leas, B.F.; Sullivan, N.; Han, J.H.; Pegues, D.A.P.; Kaczmarek, J.L.; Umscheid, C.A. Environmental Cleaning for the Prevention of Healthcare-Associated Infections; AHRQ Publication No. 15-EHC020-EF August 2015, Effective Health Care Programme, Technical Brief Number 22; Agency for Healthcare Research and Quality U.S. Department of Health and Human Services: Rockville, MD, USA, 2015.
- Angelova, L.; Ormsby, B.; Townsend, J.H.; Wolbers, R. Gels in the Conservation of Art, London 16–18 October 2017; Archetype: London, UK, 2017; ISBN 978-1-909492-50-9. [Google Scholar]
- Giorgi, R.; Baglioni, M.; Berti, D.; Baglioni, P. New Methodologies for the Conservation of Cultural Heritage: Micellar Solutions, Microemulsions, and Hydroxide Nanoparticles. Acc. Chem. Res. 2010, 43, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Siano, S.; Salimbeni, R. Advances in Laser Cleaning of Artwork and Objects of Historical Interest: The Optimized Pulse Duration Approach. Acc. Chem. Res. 2010, 43, 739–750. [Google Scholar] [CrossRef]
- Carretti, E.; Bonini, M.; Dei, L.; Berrie, B.H.; Angelova, L.V.; Baglioni, P.; Weiss, R.G. New Frontiers in Materials Science for Art Conservation: Responsive Gels and Beyond. Acc. Chem. Res. 2010, 43, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Gorel, F. Assessment of Agar Gel Loaded with Micro-Emulsion for the Cleaning of Porous Surfaces, CeROArt Web J. Available online: https://ceroart.revues.org/1827 (accessed on 17 November 2010).
- Gulotta, D.; Saviello, D.; Gherardi, F.; Toniolo, L.; Anzani, M.; Rabbolini, A.; Goidanich, S. Setup of a sustainable indoor cleaning methodology for the sculpted stone surfaces of the Duomo of Milan. Herit. Sci. 2014, 2, 1–13. [Google Scholar] [CrossRef]
- Mazzuca, C.; Micheli, L.; Cervelli, E.; Basoli, F.; Cencetti, C.; Coviello, T.; Iannuccelli, S.; Sotgiu, S.; Palleschi, A. Cleaning of paper artworks: Development of an efficient gelbased material able to remove starch paste. Appl. Mater. Interfaces 2014, 6, 16519–16528. [Google Scholar] [CrossRef]
- Angelova, L.V.; Berrie, B.H.; de Ghetaldi, K.; Kerr, A.; Weiss, R.G. Partially hydrolyzed poly (vinyl acetate)-borax-based gel-like materials for conservation of art: Characterization and applications. Stud. Conserv. 2015, 60, 227–244. [Google Scholar] [CrossRef]
- Natali, I.; Carretti, E.; Angelova, L.; Baglioni, P.; Weiss, R.G.; Dei, L. Structural and mechanical properties of “peelable” organoaqueous dispersions with partially hydrolyzed poly(vinyl acetate)-borate networks: Applications to cleaning painted surfaces. Langmuir 2011, 27, 13226–13235. [Google Scholar] [CrossRef]
- Pizzorusso, G.; Fratini, E.; Eiblmeier, J.; Giorgi, R.; Chelazzi, D.; Chevalier, A.; Baglioni, P. Physicochemical characterization of acrylamide/bisacrylamide hydrogels and their application for the conservation of easel paintings. Langmuir 2012, 28, 3952–3961. [Google Scholar] [CrossRef] [PubMed]
- Domingues, J.A.L.; Bonelli, N.; Giorgi, R.; Fratini, E.; Gorel, F.; Baglioni, P. Innovative hydrogels based on semi-interpenetrating p(hema)/pvp networks for the cleaning of water-sensitive cultural heritage artifacts. Langmuir 2013, 29, 2746–2755. [Google Scholar] [CrossRef] [PubMed]
- Domingues, J.; Bonelli, N.; Giorgi, R.; Baglioni, P. Chemical semi-IPN hydrogels for the removal of adhesives from canvas paintings. Appl. Phys. A Mater. 2014, 114, 705–710. [Google Scholar] [CrossRef]
- Wolbers, R.C. Cleaning Painted Surfaces: Aqueous Methods; Archetype Publications: London, UK, 2000. [Google Scholar]
- Feller, R.L.; Stolow, N.; Jones, E.H. On Picture Varnishes and Their Solvents; National Gallery of Art: Washington, DC, USA, 1985. [Google Scholar]
- Baglioni, P.; Carretti, E.; Chelazzi, D. Nanomaterials in art conservation. Nat. Nanotechnol. 2015, 10, 287–290. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Brazel, C.S.; Peppas, N.A. Dimensionless analysis of swelling of hydrophilic glassy polymers with subsequent drug release from relaxing structures. Biomaterials 1999, 20, 721–732. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Peppas, N.A. Effect of the morphology of hydrophilic polymeric matrices on the diffusion and release of water soluble drugs. J. Membr. Sci. 1981, 9, 211–227. [Google Scholar] [CrossRef]
- Myung, D.; Duhamel, P.E.; Cochran, J.R.; Noolandi, J.; Ta, C.N.; Frank, C.W. Development of hydrogel-based keratoprostheses: A materials perspective. Biotechnol. Prog. 2008, 24, 735–741. [Google Scholar] [CrossRef]
- Krysmann, M.J.; Castelletto, V.; Kelarakis, A.; Hamley, I.W.; Hule, R.A.; Pochan, D. Self-Assembly and Hydrogelation of an Amyloid Peptide Fragment. J. Biochem. 2008, 47, 4597–4605. [Google Scholar] [CrossRef]
- Carrigan, S.D.; Scott, G.; Tabrizian, M. Real-Time QCM-D Immunoassay through Oriented Antibody Immobilization Using Cross-Linked Hydrogel Biointerfaces. Langmuir 2005, 21, 5966–5973. [Google Scholar] [CrossRef]
- Ferruti, P.; Bianchi, S.; Ranucci, E.; Chiellini, F.; Piras, A.M. Novel agmatine-containing poly (amidoamine) hydrogels as scaffolds for tissue engineering. Biomacromolecules 2005, 6, 2229–2235. [Google Scholar] [CrossRef] [PubMed]
- Shome, A.; Debnath, S.; Das, P.K. Head group modulated pH-responsive hydrogel of amino acid-based amphiphiles: Entrapment and release of cytochrome c and vitamin B12. Langmuir 2008, 24, 4280–4288. [Google Scholar] [CrossRef] [PubMed]
- Stoner, J.H.; Rushfield, R.A. Conservation of Easel Paintings: Principles and Practice; Routledge: New York, NY, USA, 2012. [Google Scholar]
- de la Rie, E.R. Photochemical and Thermal Degradation of Films of Dammar Resin. Stud. Conserv. 1988, 33, 53–70. [Google Scholar]
- Noble, P.; van Loon, A.; Boon, J.J. Chemical changes in Old Master paintings II: Darkening due to increased transparency as a result of metal soap formation. In Proceedings of the ICOM Committee for Conservation, 14th Triennial Meeting, The Hague, The Netherlands, 12–16 September 2005; Volume 1, pp. 496–503. [Google Scholar]
- Noble, P.; van Loon, A.; Boon, J.J. Selective darkening of ground layers and paint layers associated with the wood structure in seventeenth-century panel paintings. In Preparation for Painting: The Artists’s Choice and Its Consequences; Townsend, J.H., Doherty, T., Heydenreich, G., Ridge, J., Eds.; Archetype Publications: London, UK, 2008; pp. 68–78. [Google Scholar]
- Kampasakali, E.; Ormsby, B.; Cosentino, A.; Miliani, C.; Learner, T.A. Preliminary evaluation of the surfaces of acrylic emulsion paint films and the effects of wet-cleaning treatment by atomic force microscopy (AFM). Stud. Conserv. 2011, 56, 216–230. [Google Scholar] [CrossRef]
- Burnstock, A.; van den Berg, K.-J. Twentieth Century Oil Paint; The Interface Between Science and Conservation and the Challenges for Modern Oil Paint Research; van den Berg, K.J., Burnstock, A., de Keijzer, M., Krueger, J., Learner, T., de Tagle, A., Heydenreich, G., Eds.; Issues in Contemporary Oil Paint Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–19. [Google Scholar]
- van Loon, A. Color Changes and Chemical Reactivity in Seventeenth-Century Oil Paintings. Ph.D. Thesis, University of Amsterdam, Amsterdam, The Netherlands, 2008. [MOLART Report 14, available from Archetype Publications.]. [Google Scholar]
- Theodorakopoulos, C.; Zafiropoulos, V.; Boon, J.J.; Boyatzis, S.C. Spectroscopic Investigations on the Depth-Dependent Degradation Gradients of Aged Triterpenoid Varnishes. Appl. Spectrosc. 2007, 61, 1045–1051. [Google Scholar] [CrossRef]
- Theodorakopoulos, C.; Boon, J.J.; Zafiropulos, V. Direct temperature mass spectrometric study on the depth-dependent compositional gradients of aged triterpenoid varnishes. Int. J. Mass Spectrom. 2009, 284, 98–107. [Google Scholar] [CrossRef]
- Theodorakopoulos, C.; Zafiropulos, V. Uncovering of scalar oxidation within naturally aged varnish layers. J. Cult. Herit. 2003, 4, 216–222. [Google Scholar] [CrossRef]
- Stolow, N. Part II: Solvent Action. In On Picture Varnishes and Their Solvents; Revised and enlarged edition 1985; Feller, R.L., Stolow, N., Jones, E.H., Eds.; National Gallery of Art: Washington, DC, USA, 1985. [Google Scholar]
- Burnstock, A.; White, R. The effects of selected solvents and soaps on a simulated canvas painting. In Cleaning, Retouching and Coatings: Technology and Practice for Easel Paintings and Polychrome Sculpture: Preprints of the Contributions to the Brussel Congress; Mills, J.S., Smith, P., Eds.; International Institute for Conservation of Historic and Artistic Works: London, UK, 1990; pp. 111–118. [Google Scholar]
- Ormsby, B.; Kampasakali, E.; Miliani, C.; Learner, T. An FTIR-based exploration of the effects of wet cleaning treatments on artists’ acrylic emulsion paint films. e-Preserv. Sci. 2009, 6, 186–195. [Google Scholar]
- Angelova, L.V.; Ormsby, B.; Richardson, E. Diffusion of water from a range of conservation treatment gels into paint films studied by unilateral NMR. Part I: Acrylic emulsion paint. Microchem. J. 2016, 124, 311–320. [Google Scholar] [CrossRef]
- Dorge, V. (Ed.) Solvent Gels for the Cleaning of Works of Art: The Residue Question; Getty Conservation Institute: Los Angeles, CA, USA, 2004. [Google Scholar]
- Casoli, A.; Di Diego, Z.; Isca, C. Cleaning painted surfaces: Evaluation of leaching phenomenon induced by solvents applied for the removal of gel residues. Environ. Sci. Pollut. Res. 2014, 21, 13252–13263. [Google Scholar] [CrossRef]
- Maltesh, C.; Xu, Q.; Somasundaran, P.; Benton, W.J.; Hung, N. Aggregation behavior of and surface tension reduction by comblike amphiphilic polymers. Langmuir 1992, 8, 1511–1513. [Google Scholar] [CrossRef]
- Yu, A.; Shashkina, Y.D.; Zaroslov, V.A.; Smirnov, O.E.; Philippova, A.R.; Khokhlov, T.A.; Pryakhina, N.A. Churochkina. Hydrophobic aggregation in aqueous solutions of hydrophobically modified polyacrylamide in the vicinity of overlap concentration. Polymer 2003, 44, 2289–2293. [Google Scholar] [CrossRef]
- Ida, S.; Kawahara, T.; Kawabata, H.; Ishikawa, T.; Hirokawa, Y. Effect of Monomer Sequence along Network Chains on Thermoresponsive Properties of Polymer Gels. Gels 2018, 4, 22. [Google Scholar] [CrossRef]
- Voorhaar, L.; Hoogenboom, R. Supramolecular polymer networks: Hydrogels and bulk materials. Chem. Soc. Rev. 2016, 45, 4013–4031. [Google Scholar] [CrossRef]
- Jonas, U.; van den Brom, C.R.; Brunsen, A.; Roskamp, R.F. Surface attached polymeric hydrogel films. In Handbook of Biofunctional Surfaces; Knoll, W., Ed.; Pan Stanford Publishing: Singapore, Singapore, 2012; p. 277. [Google Scholar]
- Harmon, M.E.; Kuckling, D.; Pareek, P.; Frank, C.W. Photo-cross-linkable PNIPAAm copolymers. 4. Effects of copolymerization and cross-linking on the volume-phase transition in constrained hydrogel layers. Langmuir 2003, 19, 10947–10956. [Google Scholar] [CrossRef]
- Toomey, R.; Freidank, D.; Ruhe, J. Swelling Behavior of Thin, Surface-Attached Polymer Networks. Macromolecules 2004, 37, 882–887. [Google Scholar] [CrossRef]
- Anac, I.; Aulasevich, A.; Junk, M.J.N.; Jakubowicz, P.; Roskamp, R.F.; Menges, B.; Jonas, U.; Knoll, W. Temperature dependent swelling behavior of thin poly(N-isopropylacrylamide) copolymer gel layers in ethanol-water mixtures. Macromol. Chem. Phys. 2010, 211, 1018–1025. [Google Scholar] [CrossRef]
- Junk, M.; Berger, R.; Jonas, U. Atomic Force Spectroscopy of Thermoresponsive Photo-Cross-Linked Hydrogel Films. Langmuir 2010, 26, 7262–7269. [Google Scholar] [CrossRef]
- Mateescu, A.; Freese, S.; Frank, P.; Jonas, U.; Theodorakopoulos, C. Novel surface-attached gels from photo-crosslinkable polyacrylamides for the cleaning of works of art. In Gels in the Conservation of Art, London 16–18 October 2017; Archetype: London, UK, 2017; pp. 237–244. [Google Scholar]
- Larraz, E.; Elvira, C.; San Román, J. Novel acrylic macromonomer with amphiphilic character derived from Triton X-100: Radical copolymerization with methyl methacrylate and thermal properties. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 1641–1649. [Google Scholar] [CrossRef]
- Fadida, T.; Lellouche, J.-P. Poly-N-(4-benzoylphenyl) methacrylamide nanoparticles: Preparation, characterization, and photoreactivity features. J. Polym. Res. 2012, 19, 1–12. [Google Scholar] [CrossRef]
- Beines, P.W.; Klosterkamp, I.; Menges, B.; Jonas, U.; Knoll, W. Responsive Thin Hydrogel Layers from Photocrosslinkable Poly(N-isopropylacrylamide) Terpolymers. Langmuir 2007, 23, 2231–2238. [Google Scholar] [CrossRef] [PubMed]
- Galatis, P.; Boyatzis, S.; Theodorakopoulos, C. Removal of Soiling from Resin Coatings Using Hydrogels. e-Preserv. Sci. 2012, 9, 72–83. [Google Scholar]



| Copolymer Composition | H2O | EtOH | H2O: EtOH (1:1) | MeOH | Acetone | Diethyl Ether | EA | DMSO |
|---|---|---|---|---|---|---|---|---|
| AM94/B35A5/BPAAm1 | o | - | o+ | - | o - | - | - | + |
| AM94/TX100A5/BPAAm1 | o+ | / | o+ | - | o- | - | - | / |
| AM94/B35M5/BPAAm1 | - | - | o- | - | o- | - | - | o+ |
| AM94/TX100M5/BPAAm1 | o- | - | o+ | - | o- | - | - | o+ |
| AM89/B35A5/MAA5/BPAAm1 | - | - | o+ | - | o | - | - | o+ |
| AM94/EO-3A5/BPAAm1 | o+ | - | + | - | o | - | - | + |
| AM94/EO-9A5/BPAAm1 | o+ | - | + | - | o | - | - | + |
| Abbreviation | Polymer Composition | tcr/min |
|---|---|---|
| PE-PAM15 | AM99/BPAAm1 | 15 |
| PE-PAM30 | AM99/BPAAm1 | 30 |
| PE-PAM60 | AM99/BPAAm1 | 60 |
| PE-PAMB10 | AM94/B35A5/BPAAm1 | 10 |
| PE-PAMB15 | AM94/B35A5/BPAAm1 | 15 |
| PE-PAMB20 | AM94/B35A5/BPAAm1 | 20 |
| PE-PAMB30 | AM94/B35A5/BPAAm1 | 30 |
| PE-PAMB60 | AM94/B35A5/BPAAm1 | 60 |
| PE-PAMX15 | AM94/TXA5/BPAAm1 | 15 |
| PE-PAMX30 | AM94/TXA5/BPAAm1 | 30 |
| PE-PAMX60 | AM94/TXA5/BPAAm1 | 60 |
| PE-PAM-EO315 | AM94/EO-3A5/BPAAm1 | 15 |
| PE-PAM-EO915 | AM94/EO-9A5/BPAAm1 | 15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freese, S.; Diraoui, S.; Mateescu, A.; Frank, P.; Theodorakopoulos, C.; Jonas, U. Polyolefin-Supported Hydrogels for Selective Cleaning Treatments of Paintings. Gels 2020, 6, 1. https://doi.org/10.3390/gels6010001
Freese S, Diraoui S, Mateescu A, Frank P, Theodorakopoulos C, Jonas U. Polyolefin-Supported Hydrogels for Selective Cleaning Treatments of Paintings. Gels. 2020; 6(1):1. https://doi.org/10.3390/gels6010001
Chicago/Turabian StyleFreese, Silvia, Samar Diraoui, Anca Mateescu, Petra Frank, Charis Theodorakopoulos, and Ulrich Jonas. 2020. "Polyolefin-Supported Hydrogels for Selective Cleaning Treatments of Paintings" Gels 6, no. 1: 1. https://doi.org/10.3390/gels6010001
APA StyleFreese, S., Diraoui, S., Mateescu, A., Frank, P., Theodorakopoulos, C., & Jonas, U. (2020). Polyolefin-Supported Hydrogels for Selective Cleaning Treatments of Paintings. Gels, 6(1), 1. https://doi.org/10.3390/gels6010001