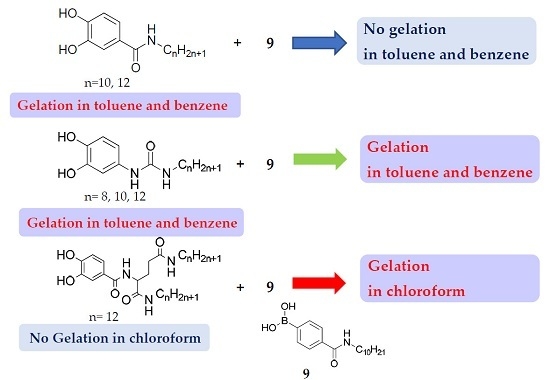
Gelating Abilities of Two-Component System of Catecholic Derivatives and a Boronic Acid
Abstract
1. Introduction
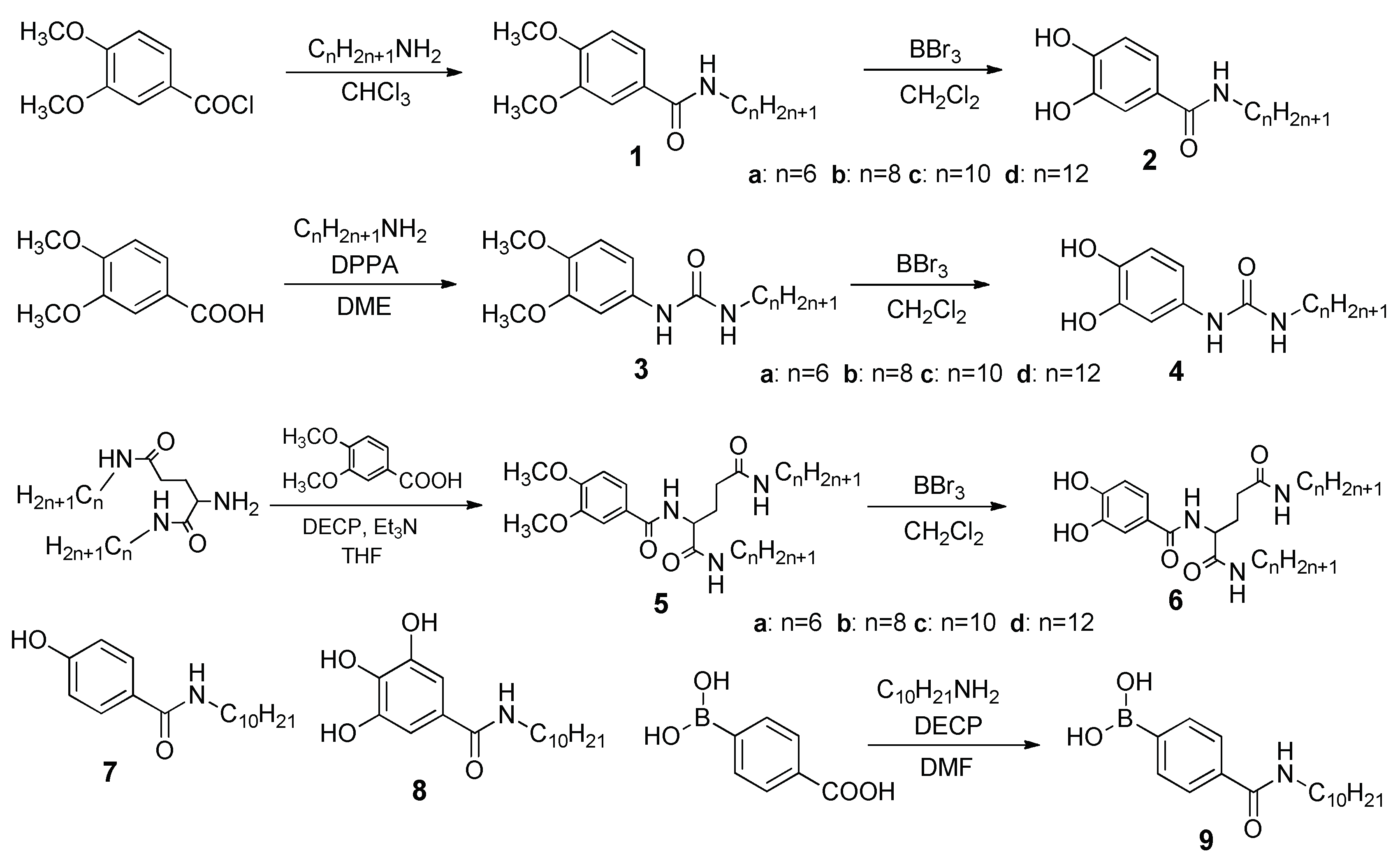
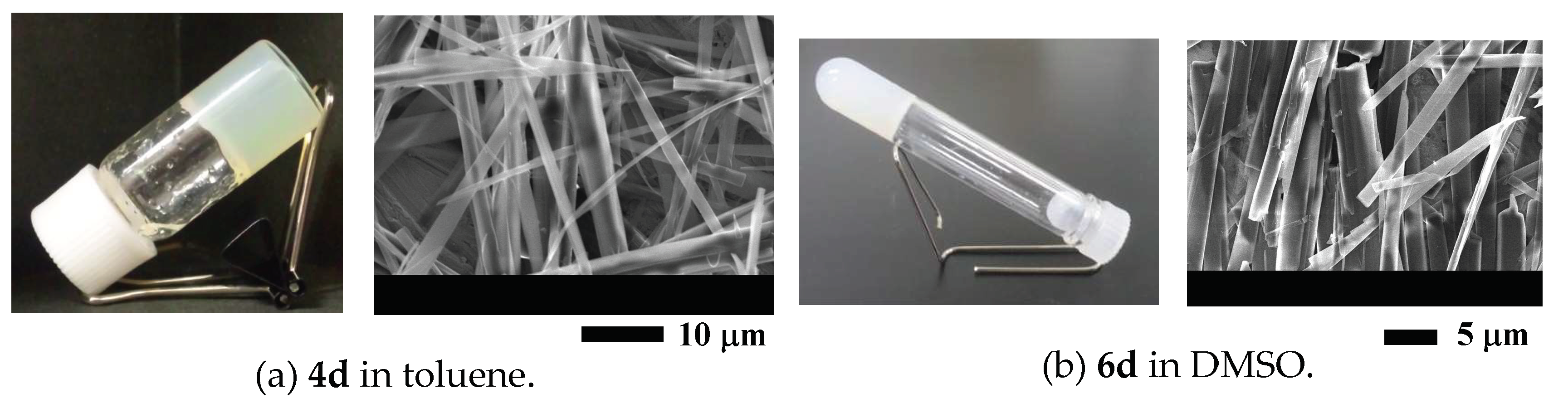
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. General
4.2. Typical Procedure for Syntheses of 2a–d
4.3. Typical Procedure for Syntheses of 4a–d
4.4. Typical Procedure for Syntheses of 6a–d
4.5. Procedure for Synthesis of 9
Author Contributions
Funding
Conflicts of Interest
References
- Boncheva, M.; Whitesides, G.M. Making Things by Self-Assembly. MRS Bull. 2005, 30, 736–742. [Google Scholar] [CrossRef]
- Terech, P.; Weiss, R.G. Low Molecular Mass Gelators of Organic Liquids and the Properties of Their Gels. Chem. Rev. 1997, 97, 3133–3159. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, D.J.; Weiss, R.G. Organogels and Low Molecular Mass Organic Gelators. Adv. Mater. 2000, 12, 1237–1247. [Google Scholar] [CrossRef]
- Estroff, A.L.; Hamilton, A.D. Water Gelation by Small Organic Molecules. Chem. Rev. 2004, 104, 1201–1217. [Google Scholar] [CrossRef]
- Sangeetha, N.M.; Maitra, U. Supramolecular gels: Functions and uses. Chem. Soc. Rev. 2005, 34, 821–836. [Google Scholar] [CrossRef]
- George, M.; Weiss, R.G. Molecular Organogels. Soft Matter Comprised of Low-Molecular-Mass Organic Gelators and Organic Liquids. Acc. Chem. Res. 2006, 39, 489–497. [Google Scholar] [CrossRef]
- Dastidar, P. Supramolecular gelling agents: Can they be designed? Chem. Soc. Rev. 2008, 37, 2699–2715. [Google Scholar] [CrossRef]
- Piepenbrock, M.-O.M.; Lloyd, G.O.; Clarke, N.; Steed, J.W. Metal- and Anion-Binding Supramolecular Gels. Chem. Rev. 2010, 110, 1960–2004. [Google Scholar] [CrossRef]
- Smith, D.K. Lost in translation? Chirality effects in the self-assembly of nanostructured gel-phase materials. Chem. Soc. Rev. 2009, 38, 684–694. [Google Scholar] [CrossRef]
- Chivers, P.R.A.; Smith, D.K. Shaping and structuring supramolecular gels. Nat. Rev. Mat. 2019, 4, 463–478. [Google Scholar] [CrossRef]
- Ruiz-Olles, J.; Slavik, P.; Whitelaw, N.K.; Smith, D.K. Self-Assembled Gels Formed in Deep Eutectic Solvents: Supramolecular Eutectogels with High Ionic Conductivity. Angew. Chem. Int. Ed. 2019, 58, 4173–4178. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Xu, Y.; Fei, J.; Xue, H.; Li, X.; Wang, C.; Fytas, G.; Li, J. The Ultrafast Assembly of a Dipeptide Supramolecular Organogel and Its Phase Transition from Gel to Crystal. Angew. Chem. Int. Ed. 2019, 58, 11072–11077. [Google Scholar] [CrossRef] [PubMed]
- Hatai, J.; Schmuck, C. Diverse Properties of Guanidiniocarbonyl Pyrrole-Based Molecules: Artificial Analogues of Arginine. Acc. Chem. Res. 2019, 52, 1709–1720. [Google Scholar] [CrossRef]
- Sinawang, G.; Kobayashi, Y.; Zheng, Y.; Takashima, Y.; Harada, A.; Yamaguchi, H. Preparation of Supramolecular Ionic Liquid Gels Based on Host–Guest Interactions and Their Swelling and Ionic Conductive Properties. Macromolecules 2019, 52, 2932–2938. [Google Scholar] [CrossRef]
- Tanaka, W.; Shigemitsu, H.; Fujisaku, T.; Kubota, R.; Minami, S.; Urayama, K.; Hamachi, I. Post-assembly Fabrication of a Functional Multicomponent Supramolecular Hydrogel Based on a Self-Sorting Double Network. J. Am. Chem. Soc. 2019, 141, 4997–5004. [Google Scholar] [CrossRef] [PubMed]
- Hirst, A.R.; Escuder, B.; Miravet, J.F.; Smith, D.K. High-Tech Applications of Self-Assembling Supramolecular Nanostructured Gel-Phase Materials: From Regenerative Medicine to Electronic Devices. Angew. Chem. Int. Ed. 2008, 47, 8002–8018. [Google Scholar] [CrossRef]
- Banerjee, S.; Das, R.K.; Maitra, U.J. Supramolecular gels ‘in action’. Mater. Chem. 2009, 19, 6649–6687. [Google Scholar] [CrossRef]
- Zhao, F.; Ma, M.L.; Xu, B. Molecular hydrogels of therapeutic agents. Chem. Soc. Rev. 2009, 38, 883–891. [Google Scholar] [CrossRef]
- Dawn, A.; Shiraki, T.; Haraguchi, S.; Tamura, S.; Shinkai, S. What Kind of “Soft Materials” Can We Design from Molecular Gels? Chem. Asian J. 2011, 6, 266–282. [Google Scholar] [CrossRef]
- Lee, J.; Aida, T. “Bucky gels” for tailoring electroactive materials and devices: The composites of carbon materials with ionic liquids. Chem. Commun. 2011, 47, 6757–6762. [Google Scholar] [CrossRef]
- Babu, S.S.; Prasanthkumar, S.; Ajayaghosh, A. Self-Assembled Gelators for Organic Electronics. Angew. Chem. Int. Ed. 2012, 51, 1766–1776. [Google Scholar] [CrossRef] [PubMed]
- Matson, J.B.; Stupp, S.I. Self-assembling peptide scaffolds for regenerative medicine. Chem. Commun. 2012, 48, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, A.; Matsushita, R.; Sakura, K.; Moriguchi, T.; Araki, K. Organogelators Derived from [3.3]Metacyclophane Skeleton with a Urea Unit. Chem. Lett. 2012, 41, 485–487. [Google Scholar] [CrossRef]
- Tsuge, A.; Fujiwara, T.; Yakeya, D.; Kawasaki, H.; Moriguchi, T.; Araki, K. Organogelators based on metacyclophane skeleton having urea units in the bridge. Tetrahedron 2015, 71, 9429–9432. [Google Scholar] [CrossRef]
- Tsuge, A.; Yakeya, D.; Moriguchi, T.; Kaneko, D.; Kawahara, T.; Araki, K. Formation of Luminescent Organogels from Europium-based Complexes. Chem. Lett. 2013, 42, 263–265. [Google Scholar] [CrossRef]
- Yakeya, D.; Kitou, N.; Kinugawa, S.; Moriguchi, T.; Tsuge, A. Design and properties of glutamic acid-based coumarin derivatives as organogelators. Tetrahedron 2017, 73, 3973–3978. [Google Scholar] [CrossRef]
- Yakeya, D.; Moriguchi, T.; Tsuge, A. On-off switching of gel formation by red-ox reaction. Tetrahedron Lett. 2018, 59, 712–714. [Google Scholar] [CrossRef]
- Jenkins, C.L.; Siebert, H.M.; Wilker, J.J. Integrating Mussel Chemistry into a Bio-Based Polymer to Create Degradable Adhesives. Macromolecules 2017, 50, 561–568. [Google Scholar] [CrossRef]
- Ahn, B.K. Perspectives on Mussel-Inspired Wet Adhesion. J. Am. Chem. Soc. 2017, 139, 10166–10171. [Google Scholar] [CrossRef]
- Saiz-Poseu, J.; Mancebo-Aracil, J.; Nador, F.; Busqué, F.; Ruiz-Molina, D. The Chemistry behind Catechol-Based Adhesion. Angew. Chem. Int. Ed. 2019, 58, 694–714. [Google Scholar] [CrossRef]
- James, T.D.; Sandanayake, K.R.A.S.; Shinkai, S. A Glucose-Selective Molecular Fluorescence Sensor. Angew. Chem. Int. Ed. Engl. 1994, 33, 2207–2209. [Google Scholar] [CrossRef]
- Rowan, S.J.; Cantrill, S.J.; Cousins, G.R.L.; Sanders, J.K.M.; Stoddart, J.F. Dynamic covalent chemistry. Angew. Chem. Int. Ed. Engl. 2002, 41, 899–952. [Google Scholar] [CrossRef]
- Takata, T. Polyrotaxane and Polyrotaxane Network: Supramolecular Architectures Based on the Concept of Dynamic Covalent Bond Chemistry. Polym. J. 2006, 38, 1–20. [Google Scholar] [CrossRef]
- Maeda, H.; Haketa, Y.; Nakanishi, T. Aryl-Substituted C3-Bridged Oligopyrroles as Anion Receptors for Formation of Supramolecular Organogels. J. Am. Chem. Soc. 2007, 129, 13661–13674. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, M.; Nakamura, T.; Nakagawa, T.; Itagaki, H. Reversible sol–gel transition of a tris–urea gelator that responds to chemical stimuli. Tetrahedron Lett. 2007, 48, 8990–8993. [Google Scholar] [CrossRef]
- Kim, T.H.; Choi, M.S.; Sohn, B.-H.; Park, S.-Y.; Lyoo, W.S.; Lee, T.S. Gelation-induced fluorescence enhancement of benzoxazole-based organogel and its naked-eye fluoride detection. Chem. Commun. 2008, 2364–2366. [Google Scholar] [CrossRef]
- Kobayashi, H.; Amaike, M.; Jung, J.H.; Friggeri, A.; Shinkai, S.; Resinhoudt, D.N. Organogel or polymer gel; facilitated gelation of a sugar-based organic gel by the addition of a boronic acid-appended polymerElectronic supplementary information (ESI) available: Experimental details and synthesis of compounds 3 and 4. See http://www.rsc.org/suppdata/cc/b1/b102436c/. Chem. Commun. 2001, 1038–1039. [Google Scholar] [CrossRef]
- Zhou, C.; Gao, W.; Yang, K.; Xu, L.; Ding, J.; Chen, J.; Lin, M.; Huang, X.; Wang, S.; Wu, H. A Novel Glucose/pH Responsive Low-Molecular-Weight Organogel of Easy Recycling. Langmuir 2013, 29, 13568–13575. [Google Scholar] [CrossRef]
- Duncan, T.T.; Weiss, R.G. Influence of length and structure of aryl boronic acid crosslinkers on organogels with partially hydrolyzed poly(vinyl acetate). Colloid Polym. Sci. 2018, 296, 1047–1056. [Google Scholar] [CrossRef]
- Kubo, Y.; Yoshizumi, W.; Minami, T. Development of Chemical Stimuli-responsive Organogel Using Boronate Ester-substituted Cyclotricatechylene. Chem. Lett. 2008, 37, 1238–1239. [Google Scholar] [CrossRef]
- Koumoto, K.; Yamashita, T.; Kimura, T.; Luboradzki., R.; Shinkai, S. TEM and SEM observations of super-structures constructed in organogel systems from a combination of boronic-acid-appended bola-amphiphiles with chiral diols. Nanotechnology 2001, 12, 25–31. [Google Scholar] [CrossRef]

| 2a | 2b | 2c | 2d | 4a | 4b | 4c | 4d | |
|---|---|---|---|---|---|---|---|---|
| Hexane | I | I | I | I | I | I | I | I |
| Cyclohexane | I | I | I | I | I | I | I | I |
| Toluene | I | P | G(7.0) | G(5.5) | S | G(4.5) | G(3.5) | G(2.5) |
| Benzene | S | P | G(7.0) | G(5.0) | S | PG | PG | G(4.0) |
| Chloroform | I | P | P | G(3.0) | I | P | PG | G(3.0) |
| EtOH | S | S | S | S | S | S | S | G(1.0) |
| MeOH | S | S | S | S | S | S | S | S |
| DMF | S | S | S | S | S | S | G(2.0) | G(1.5) |
| DMSO | S | S | S | S | S | S | S | G(3.0) |
| 6a | 6b | 6c | 6d | 7 | 8 | |
|---|---|---|---|---|---|---|
| Hexane | I | I | I | I | I | I |
| Cyclohexane | I | I | I | I | I | I |
| Toluene | I | P | PG | G(4.5) | S | G(0.8) |
| Benzene | I | P | PG | G(4.5) | S | G(0.8) |
| Chloroform | I | S | G(7.0) | S | S | G(1.0) |
| EtOH | S | S | PG | S | S | G(1.5) |
| MeOH | S | S | PG | S | S | S |
| DMF | S | S | S | S | S | S |
| DMSO | S | S | S | G(3.0) | S | S |
| 2c/9 | 2b/9 | 4b/9 | 4c/9 | 4d/9 | 6b/9 | 6c/9 | 6d/9 | |
|---|---|---|---|---|---|---|---|---|
| Toluene | S | S | G(7.0) | G(6.5) | G(4.5) | P | G(3.5) | G(2.5) |
| Benzene | S | S | G(7.5) | G(7.0) | G(5.5) | P | G(3.5) | G(2.5) |
| Chloroform | P | S | P | PG | G(8.0) | S | G(4.0) | G(4.0) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuge, A.; Kamoto, R.; Yakeya, D.; Araki, K. Gelating Abilities of Two-Component System of Catecholic Derivatives and a Boronic Acid. Gels 2019, 5, 45. https://doi.org/10.3390/gels5040045
Tsuge A, Kamoto R, Yakeya D, Araki K. Gelating Abilities of Two-Component System of Catecholic Derivatives and a Boronic Acid. Gels. 2019; 5(4):45. https://doi.org/10.3390/gels5040045
Chicago/Turabian StyleTsuge, Akihiko, Ryota Kamoto, Daisuke Yakeya, and Koji Araki. 2019. "Gelating Abilities of Two-Component System of Catecholic Derivatives and a Boronic Acid" Gels 5, no. 4: 45. https://doi.org/10.3390/gels5040045
APA StyleTsuge, A., Kamoto, R., Yakeya, D., & Araki, K. (2019). Gelating Abilities of Two-Component System of Catecholic Derivatives and a Boronic Acid. Gels, 5(4), 45. https://doi.org/10.3390/gels5040045