Supermacroporous Composite Cryogels in Biomedical Applications
Abstract
1. Introduction
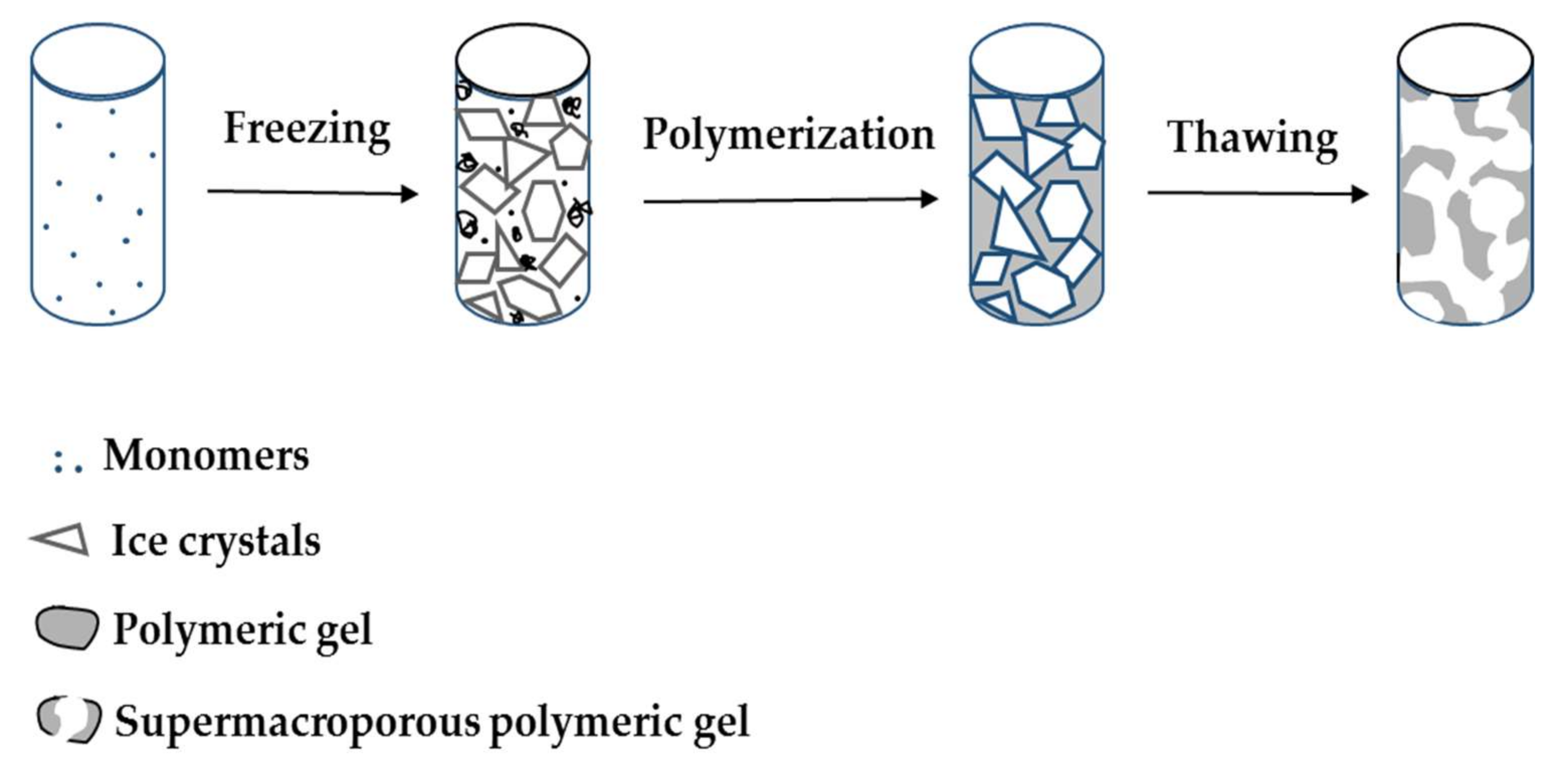
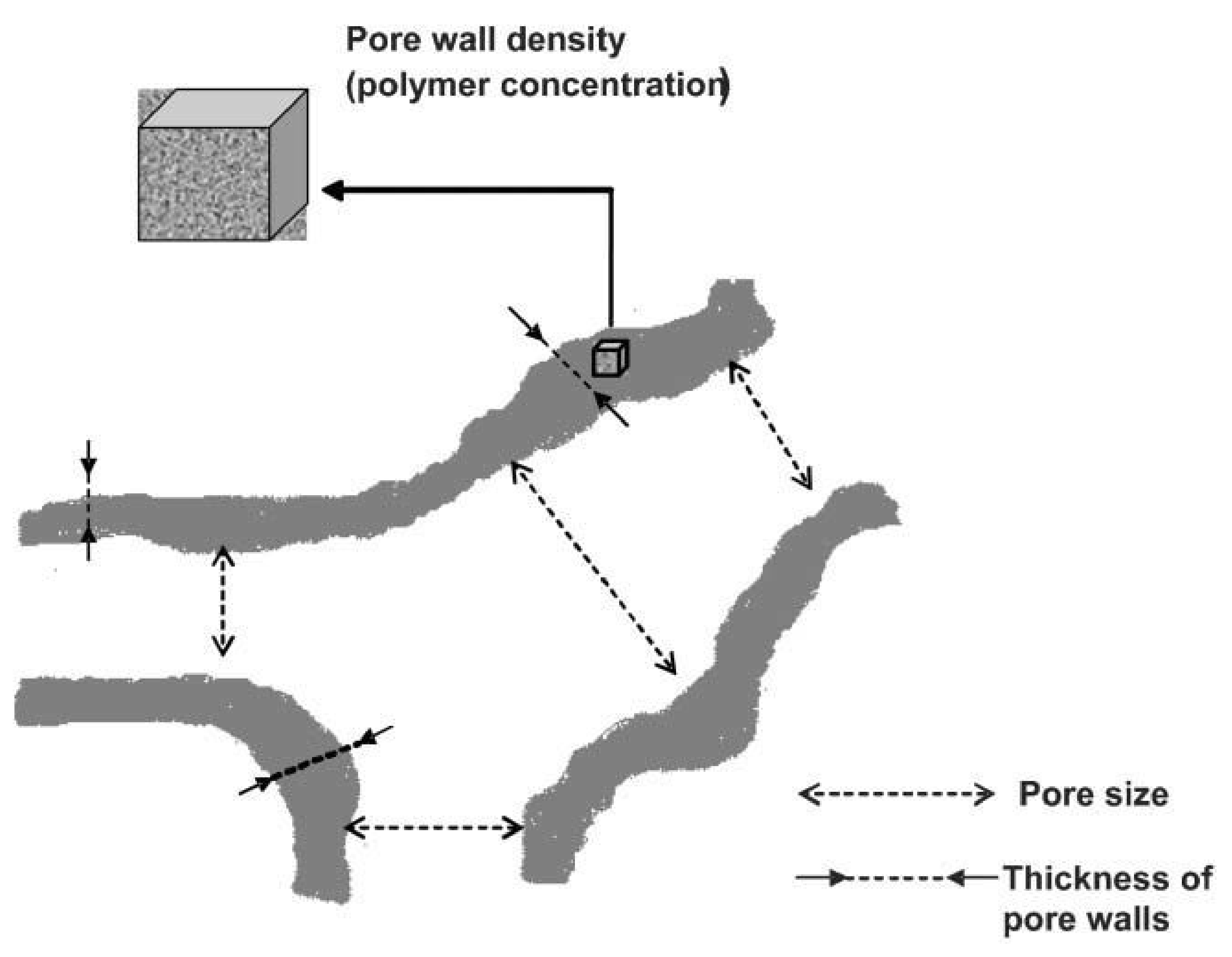
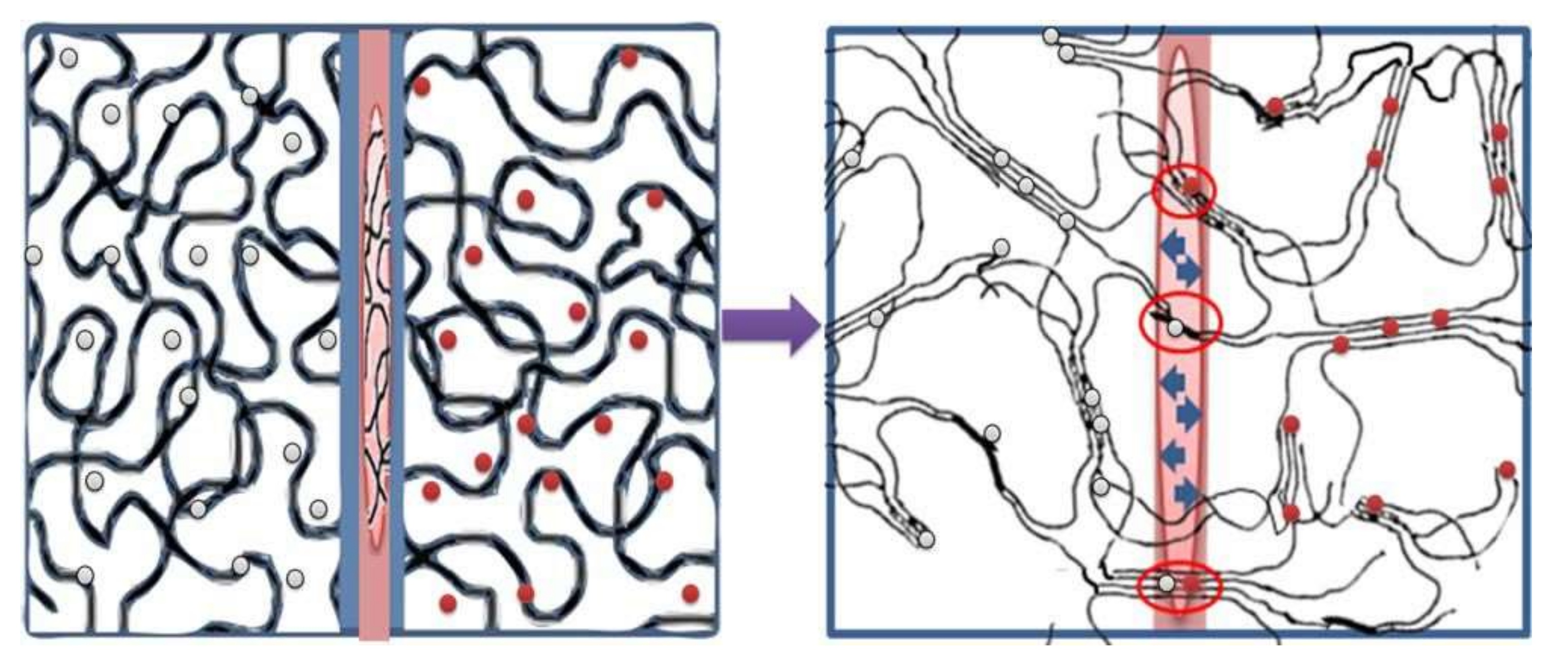
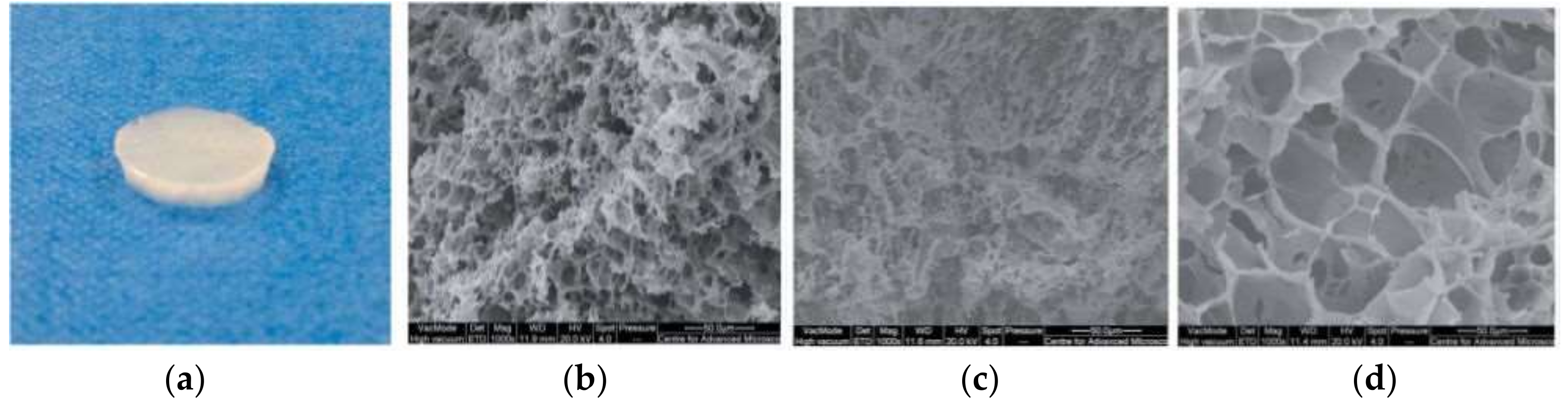
2. Supermacroporous Cryogels and Composite Cryogels
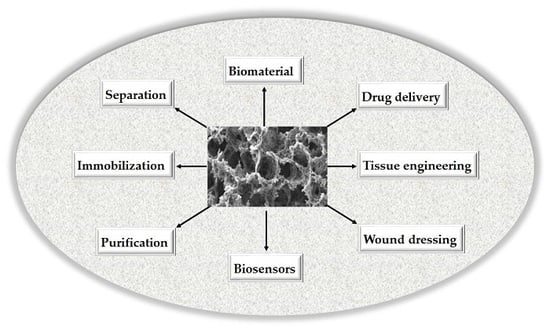
3. Composite Cryogels as Versatile Tools for Biomedical Applications
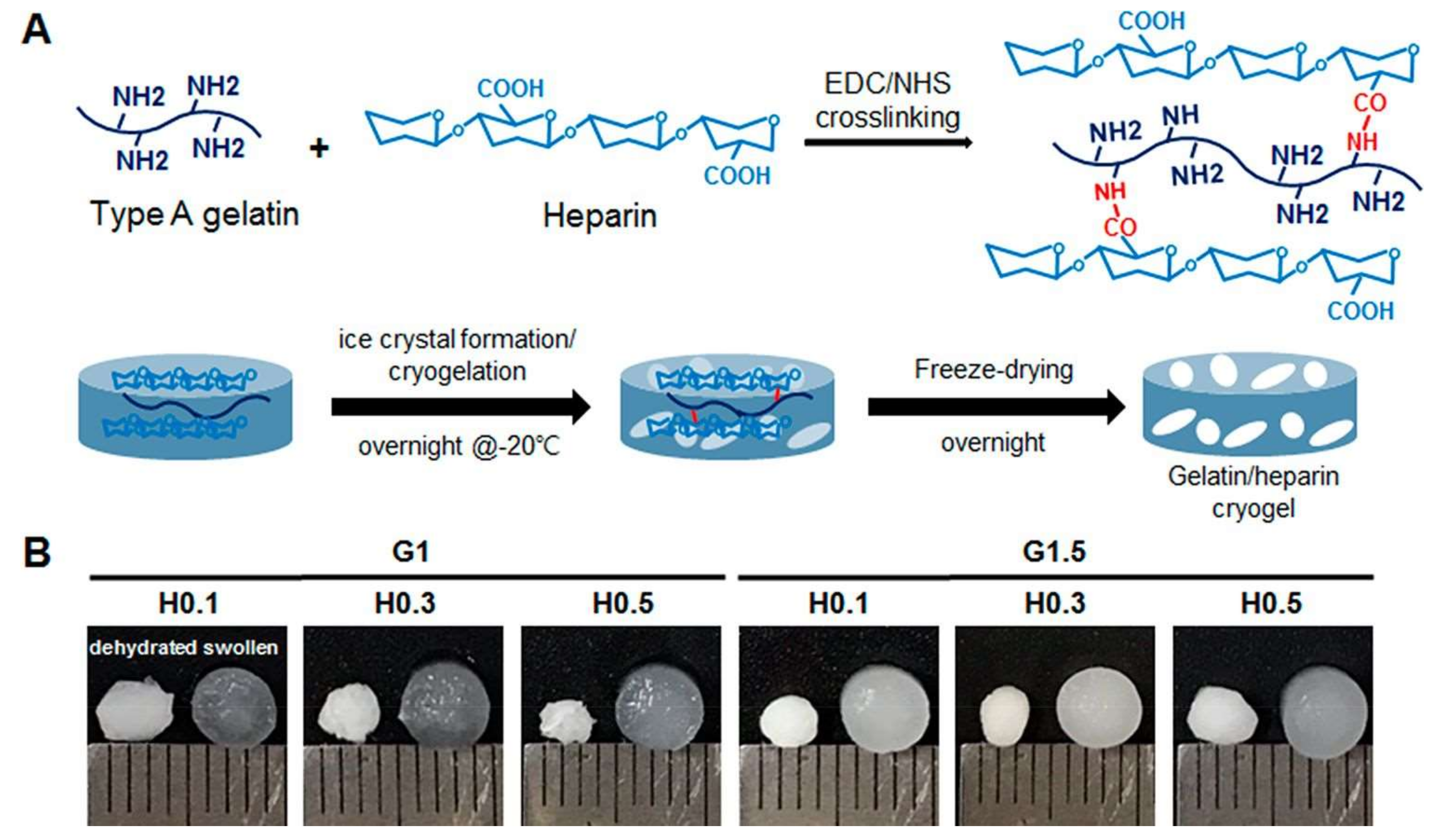
3.1. Purification and Separation Applications
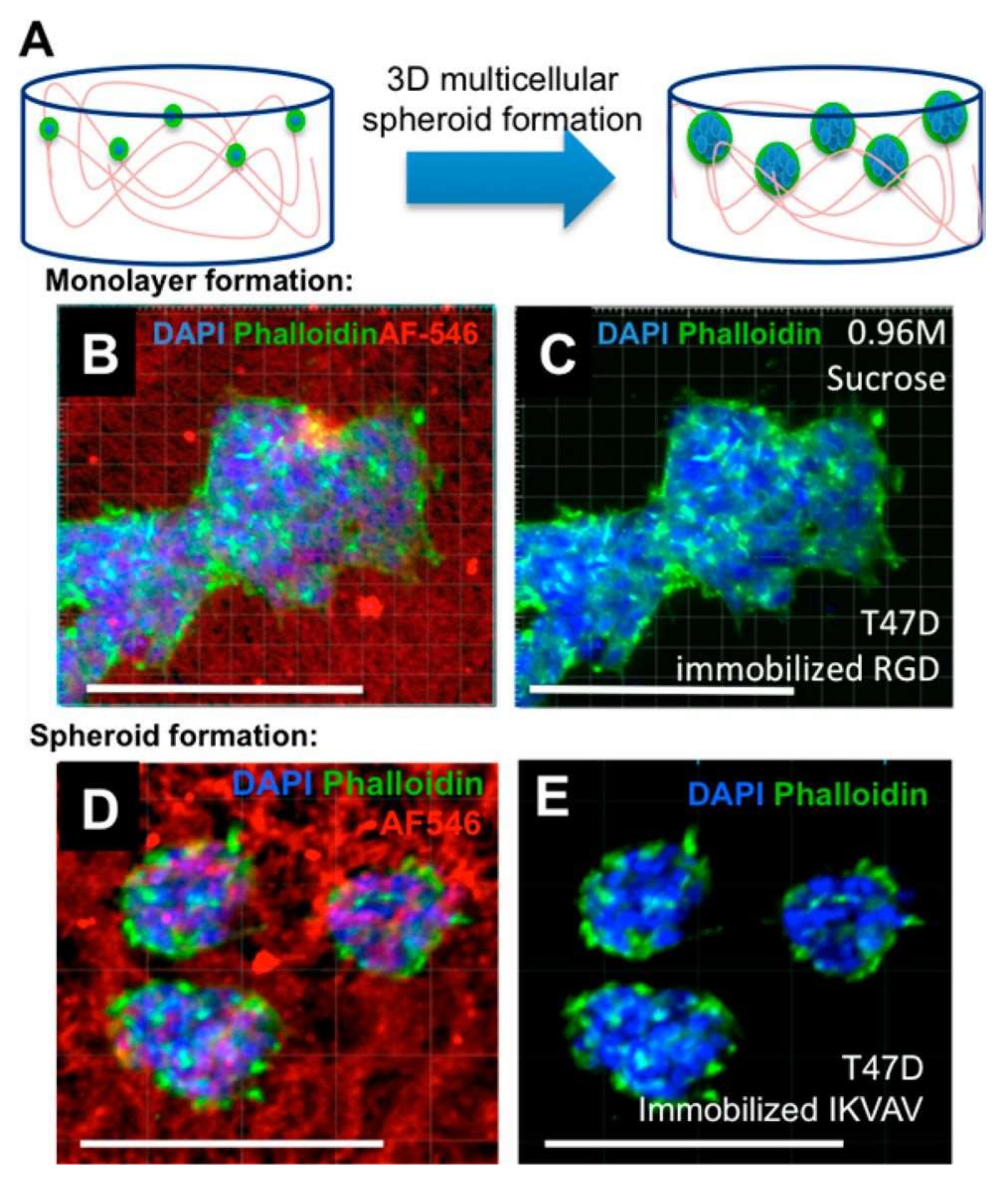
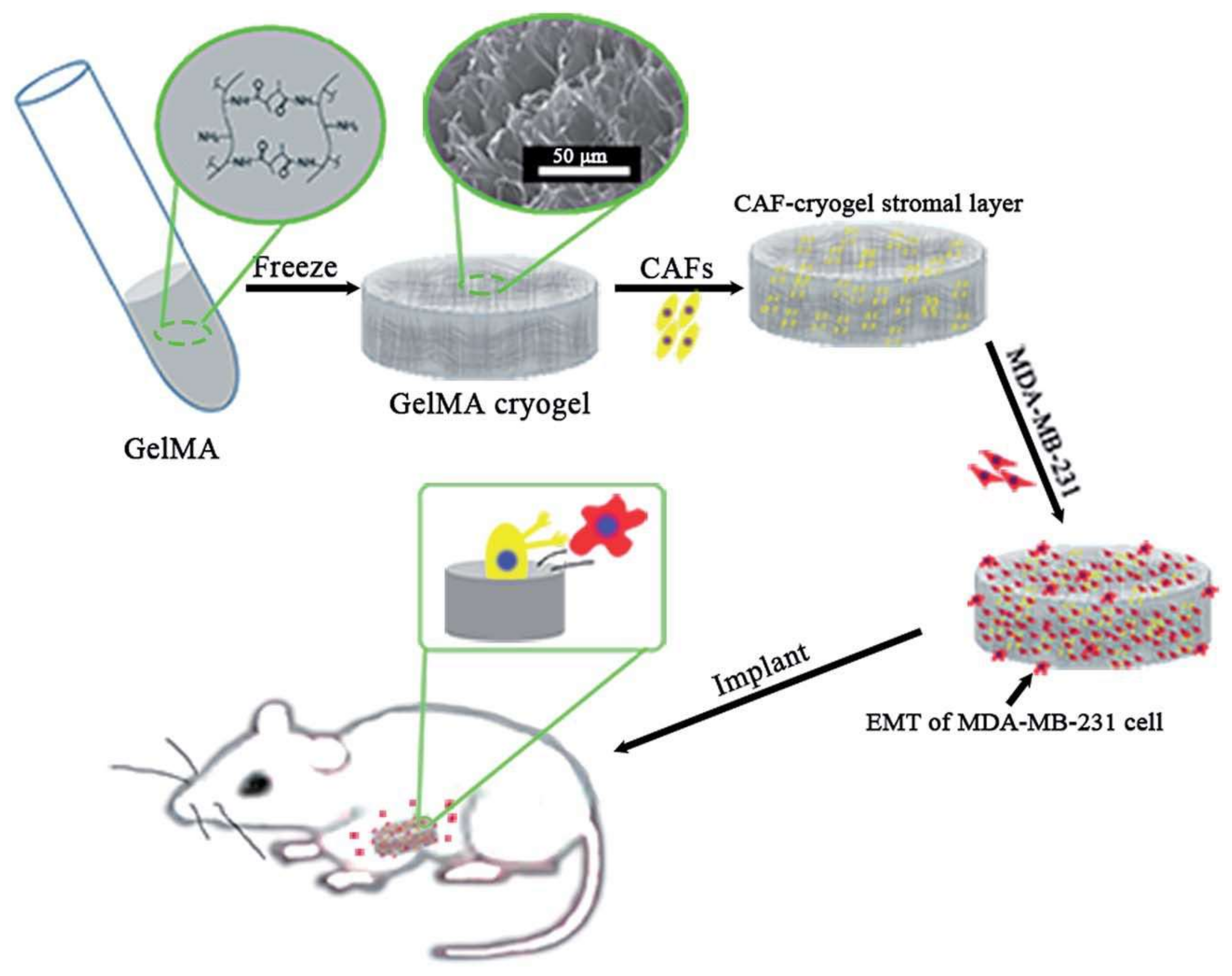
3.2. Tissue Engineering Applications
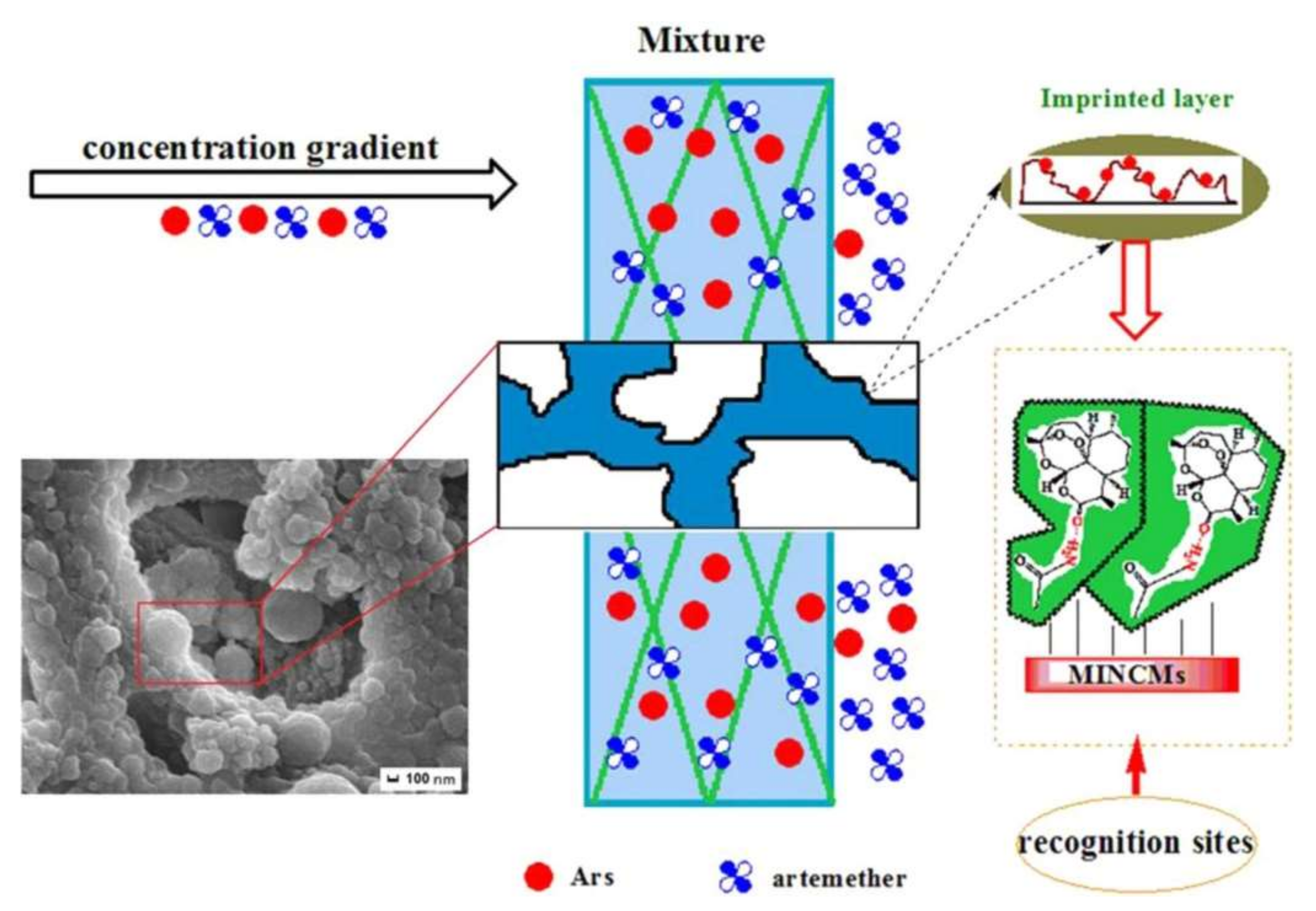
3.3. Drug Delivery Applications
3.4. Other Applications
4. Conclusions and Outlooks
Author Contributions
Funding
Conflicts of Interest
References
- Tan, S.C.; Yiap, B.B. DNA, RNA, and protein extraction: The past and the present. J. Biomed. Biotechnol. 2009, 574398, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hage, D.S.; Anguizola, J.A.; Bi, C.; Li, R.; Matsuda, R.; Papastavros, E.; Pfaunmiller, E.; Vargas, J.; Zheng, X. Pharmaceutical and biomedical applications of affinity chromatography: Recent trends and developments. J. Pharm. Biomed. Anal. 2012, 69, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Shih, T.Y.; Blacklow, S.O.; Li, A.W.; Freedman, B.R.; Bencherif, S.; Koshy, S.T.; Darnell, M.C.; Mooney, D.J. Injectable, tough alginate cryogels as cancer vaccines. Adv. Healthcare Mater. 2018, 7, 1701469–1701482. [Google Scholar] [CrossRef] [PubMed]
- Andaç, M.; Galaev, I.Y.; Denizli, A. Affinity based and molecularly imprinted cryogels: Applications in biomacromolecule purification. J. Chromatogr. B 2016, 1021, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Bereli, N.; Türkmen, D.; Yavuz, H.; Galaev, I.Y.; Denizli, A. Cryogels for Affinity Chromatography. Advanced Separations by Specialized Sorbents; Chromatographic Science Series; CRC Press: Boca Raton, FL, USA, 2015; pp. 39–67. [Google Scholar]
- Lozinsky, V.I. Cryostructuring of polymeric systems. 50.† cryogels and cryotropic gel-formation: Terms and definitions. Gels 2018, 4, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, H.; Denizli, A. A new affinity separation medium: Supermacroporous cryogels. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Reedijk, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–12. [Google Scholar]
- Akgönüllü, S.; Yavuz, H.; Denizli, A. Preparation of imprinted cryogel cartridge for chiral separation of l-phenylalanine. Artif. Cell. Nanomed. Biotechnol. 2017, 45, 800–807. [Google Scholar] [CrossRef]
- Ingavle, G.C.; Baillie, L.W.J.; Zheng, Y.; Lis, E.K.; Savina, I.N.; Howell, C.A.; Mikhalovsky, S.V.; Sandeman, S.R. Affinity binding of antibodies to supermacroporous cryogel adsorbents with immobilized protein A for removal of anthrax toxin protective antigen. Biomaterials 2015, 50, 140–153. [Google Scholar] [CrossRef]
- Çetin, K.; Denizli, A. 5-Fluorouracil delivery from metal-ion mediated molecularly imprinted cryogel discs. Coll. Surf. B 2015, 126, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.Y.; Chen, C.H.; Hsiao, C.Y.; Chen, J.P. Incorporation of chitosan in biomimetic gelatin/chondroitin-6-sulfate/hyaluronan cryogel for cartilage tissue engineering. Carbohydr. Polym. 2015, 117, 722–730. [Google Scholar] [CrossRef]
- Singh, N.K.; Dsouza, R.N.; Grasselli, M.; Fernández-Lahore, M. High capacity cryogel-type adsorbents for protein purification. J. Chromatogr. A 2014, 1355, 143–148. [Google Scholar] [CrossRef]
- Inanan, T.; Tüzmen, N.; Karipcin, F. Oxime-functionalized cryogel disks for catalase immobilization. Int. J. Biol. Macromol. 2018, 114, 812–820. [Google Scholar] [CrossRef]
- Savina, I.N.; Gun’ko, V.M.; Turov, V.V.; Dainiak, M.; Phillips, G.J.; Galaev, I.Y.; Mikhalovsky, S.V. Porous structure and water state in cross-linked polymer and protein cryo-hydrogels. Soft Matter 2011, 7, 4276–4283. [Google Scholar] [CrossRef]
- Grant, N.H.; Clark, D.E.; Alburn, H.E. Imidazole- and base-catalyzed hydrolysis of penicillin in frozen systems. J. Am. Chem. Soc. 1961, 83, 4476–4477. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Simenel, I.A.; Kurskaya, E.A.; Kulakova, V.K.; Galaev, I.Y.; Mattiasson, B. Synthesis of N-vinylcaprolactam polymers in water-containing media. Polymer 2000, 41, 6507–6518. [Google Scholar] [CrossRef]
- Plieva, F.M.; De Seta, E.; Yu, I.; Mattiasson, B. Macroporous elastic polyacrylamide monolith columns: Processing under compression and scale-up. Sep. Purif. Technol. 2009, 65, 110–116. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Galaev, I.Y.; Plieva, F.M.; Savina, I.N.; Jungvid, H.; Mattiasson, B. Polymeric cryogels as promising materials of biotechnological interest. Trends Biotechnol. 2003, 21, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Plieva, F.M.; Karlsson, M.; Aguilar, M.R.; Gomez, D.; Mikhalovsky, S.; Galaev, I.Y. Pore structure in supermacroporous polyacrylamide based cryogels. Soft Matter. 2005, 1, 303–309. [Google Scholar] [CrossRef]
- Buchtova, N.; Budtova, T. Cellulose aero-, cryo- and xerogels: Towards understanding of morphology control. Cellulose 2016, 23, 2585–2595. [Google Scholar] [CrossRef]
- Henderson, T.M.A.; Ladewig, K.; Haylock, D.N.; McLean, K.M.; O’Connor, A.J. Cryogels for biomedical applications. J. Mater. Chem. B 2013, 1, 2682–2695. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Savina, I.N.; Mikhalovsky, S.V. Cryogels: Morphological, structural and adsorption characterisation. Adv. Coll. Int. Sci. 2013, 187, 1–46. [Google Scholar] [CrossRef]
- Plieva, F.M.; Savina, I.N.; Deraz, S.; Andersson, J.; Galaev, I.G.; Mattiasson, B. Characterization of supermacroporous monolithic polyacrylamide based matrices designed for chromatography of bioparticles. J. Chromatogr. B 2004, 807, 129–137. [Google Scholar] [CrossRef]
- Yılmaz, F.; Bereli, N.; Yavuz, H.; Denizli, A. Supermacroporous hydrophobic affinity cryogels for protein chromatography. Biochem. Eng. J. 2009, 43, 272–279. [Google Scholar] [CrossRef]
- Babaç, C.; Yavuz, H.; Galaev, I.Y.; Pişkin, E.; Denizli, A. Binding of antibodies to concanavalin A-modified monolithic cryogel. React. Func. Polym. 2006, 66, 1263–1271. [Google Scholar] [CrossRef]
- Arvidsson, P.; Plieva, F.M.; Savina, I.N.; Lozinsky, V.I.; Fexby, S.; Bülow, L.; Galaev, I.Y.; Matiasson, B. Chromatography of microbial cells using continuous supermacroporous affinity and ion-exchange columns. J. Chromatogr. A 2002, 977, 27–38. [Google Scholar] [CrossRef]
- Reichelt, S.; Abe, C.; Hainich, S.; Knolle, W.; Decker, U.; Prager, A.; Konieczny, R. Electron-beam derived polymeric cryogels. Soft Matter 2013, 9, 2484–2492. [Google Scholar] [CrossRef]
- Bereli, N.; Şener, G.; Altıntaş, E.B.; Yavuz, H.; Denizli, A. Poly(glycidyl methacrylate) beads embedded cryogels for pseudo-spesific affinity depletion of albumin and immunoglobulin G. Mater. Sci. Eng. C 2010, 30, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Perçin, I.; Sağlar, E.; Yavuz, H.; Aksöz, E.; Denizli, A. Poly(hydroxyethyl methacrylate) based affinity cryogel for plasmid DNA purification. Int. J. Biol. Macromol. 2011, 48, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Özgür, E.; Bereli, N.; Türkmen, D.; Ünal, S.; Denizli, A. PHEMA cryogel for in-vitro removal of anti-dsDNA antibodies from SLE plasma. Mater. Sci. Eng. C 2011, 31, 915–920. [Google Scholar]
- Mahmood, K.; Zia, K.M.; Zuber, M.; Salman, M.; Anjum, M.N. Recent developments in curcumin and curcumin based polymeric materials for biomedical applications: A review. Int. J. Biol. Macromol. 2015, 81, 877–890. [Google Scholar] [CrossRef]
- Ye, J.; Yun, J.; Lin, D.Q.; Xu, L.; Kirsebom, H.; Shen, S.; Yang, G.; Yao, K.; Guan, Y.X.; Yao, S.J. Poly(hydroxyethyl methacrylate)-based composite cryogel with embedded macroporous cellulose beads for the separation of human serum immunoglobulin and albumin. J. Sep. Sci. 2013, 36, 3813–3820. [Google Scholar] [CrossRef]
- Zhao, F.; Yao, D.; Guo, R.; Deng, L.; Dong, A.; Zhang, J. Composites of polymer hydrogels and nanoparticulate systems for biomedical and pharmaceutical applications. Nanomaterials 2015, 5, 2054–2130. [Google Scholar] [CrossRef]
- Derazshamshir, A.; Baydemir, G.; Andaç, M.; Say, R.; Galaev, I.Y.; Denizli, A. Molecularly imprinted PHEMA based cryogels for depletion of hemoglobin from human blood. Macromol. Chem. Phys. 2010, 211, 657–668. [Google Scholar] [CrossRef]
- Kumar, A.; Srivastava, A. Cell separation using cryogel-based affinity chromatography. Nat. Protoc. 2010, 5, 1737–1747. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh, S.; Xu, C.; Kirsebom, H.; Ye, L.; Mattiasson, B. Cryogelation of molecularly imprinted nanoparticles: A macroporous structure as affinity chromatography column for removal of β-blockers from complex samples. J. Chromatogr. A 2013, 1274, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Memic, A.; Colombani, T.; Eggermont, L.J.; Rezaeeyazdi, M.; Steingold, J.; Rogers, Z.J.; Navare, K.J.; Mohammed, H.S.; Bencherif, S.A. Latest advances in cryogel technology for biomedical applications. Adv. Therap. 2019, 1800114, 1–45. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Andaç, M.; Galaev, I.Y.; Yavuz, H.; Denizli, A. Molecularly imprinted cryogels for human serum albumin depletion. In Affinity Chromatography: Methods and Protocols; Zachariou, M., Ed.; Methods in Molecular Biology Series; Springer Protocols: Berlin, Germany, 2015; Volume 1286, pp. 233–237. [Google Scholar]
- Yavuz, H.; Bereli, N.; Yılmaz, F.; Armutçu, C.; Denizli, A. Antibody purification from human plasma by metal chelated affinity membranes. In Affinity Chromatography: Methods and Protocols; Zachariou, M., Ed.; Methods in Molecular Biology Series; Springer Protocols: Berlin, Germany, 2015; Volume 1286, pp. 43–46. [Google Scholar]
- Yavuz, H.; Bereli, N.; Baydemir, G.; Andaç, M.; Türkmen, D.; Denizli, A. Cryogels: Applications in Extracorporeal Affinity Therapy. In Supermacroporous Cryogels: Biomedical and Biotechnological Applications; CRC Press: Boca Raton, FL, USA, 2016; pp. 387–416. [Google Scholar]
- Andaç, M.; Galaev, I.Y.; Denizli, A. Molecularly imprinted cryogels for protein purification. In Biomaterials from Nature for Advanced Devices and Therapies; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 403–428. [Google Scholar]
- Andaç, M.; Denizli, A. Affinity recognition-based polymeric cryogels for protein depletion studies. RSC Adv. 2014, 4, 31130–31141. [Google Scholar] [CrossRef]
- Noppe, W.; Plieva, F.M.; Vanhoorelbeke, K.; Deckmyn, H.; Tuncel, M.; Tuncel, A.; Galaev, I.Y.; Mattiasson, B. Macroporous monolithic gels, cryogels, with immobilized phages from phage-display library as a new platform for fast development of affinity adsorbent capable of target capture from crude feeds. J. Biotechnol. 2007, 131, 293–299. [Google Scholar] [CrossRef]
- Sun, S.; Tang, Y.; Fu, Q.; Liu, X.; Du, W.; Guo, L.; Zhao, Y. Preparation of agarose/chitosan composite supermacroporous monolithic cryogels for affinity purification of glycoproteins. J. Sep. Sci. 2012, 35, 893–900. [Google Scholar] [CrossRef]
- Uygun, M.; Uygun, D.A.; Özçalışkan, E.; Akgöl, S.; Denizli, A. Concanavalin A immobilized poly(ethylene glycol dimethacrylate) based affinity cryogel matrix and usability of invertase immobilization. J. Chromatogr. B 2012, 887–888, 73–78. [Google Scholar] [CrossRef]
- Alkan, H.; Bereli, N.; Baysal, Z.; Denizli, A. Antibody purification with protein A attached supermacroporous poly(hydroxyethyl methacrylate) cryogel. Biochem. Eng. J. 2009, 45, 201–208. [Google Scholar] [CrossRef]
- Saylan, Y.; Bereli, N.; Uzun, L.; Denizli, A. Monolithic boronate affinity columns for IgG separation. Sep. Sci. Technol. 2014, 49, 1555–1565. [Google Scholar] [CrossRef]
- Asliyuce, S.; Uzun, L.; Rad, A.Y.; Unal, S.; Say, R.; Denizli, R. Molecular imprinting based composite cryogel membranes for purification of anti-hepatitis B surface antibody by fast protein liquid chromatography. J. Chromatogr. B 2012, 889–890, 95–102. [Google Scholar] [CrossRef]
- Bereli, N.; Saylan, Y.; Uzun, L.; Say, R.; Denizli, A. L-Histidine imprinted supermacroporous cryogels for protein recognition. Sep. Purif. Technol. 2011, 82, 28–35. [Google Scholar] [CrossRef]
- Baydemir, G.; Türkoğlu, E. A.; Andaç, M.; Perçin, I.; Denizli, A. Composite cryogels for lysozyme purification. Biotechnol. Appl. Biochem. 2014, 200–207. [Google Scholar] [CrossRef]
- Dragan, E.S.; Cocarta, A.I.; Gierszewska, M. Designing novel macroporous composite hydrogels based on methacrylic acid copolymers and chitosan and in vitro assessment of lysozyme controlled delivery. Coll. Surf. B 2016, 139, 33–41. [Google Scholar] [CrossRef]
- Baydemir, G.; Andaç, M.; Perçin, I.; Derazshamshir, A.; Denizli, A. Molecularly imprinted composite cryogels for hemoglobin depletion from human blood. J. Mol. Recognit. 2014, 27, 528–536. [Google Scholar] [CrossRef]
- Göktürk, I.; Derazshamshir, A.; Yılmaz, F.; Denizli, A. Poly(vinyl alcohol)/polyethyleneimine (PVA/PEI) blended monolithic cryogel columns for the depletion of haemoglobin from human blood. Sep. Sci. Technol. 2016, 51, 1787–1797. [Google Scholar] [CrossRef]
- Baydemir, G.; Bereli, N.; Andaç, M.; Say, R.; Galaev, I.Y.; Denizli, A. Supermacroporous poly(hydroxyethyl methacrylate) based cryogel with embedded bilirubin imprinted particles. React. Funct. Polym. 2009, 69, 36–42. [Google Scholar] [CrossRef]
- Perçin, I.; Baydemir, G.; Ergün, B.; Denizli, A. Macroporous PHEMA based cryogel discs for bilirubin removal. Artif. Cell. Nanomed. Biotechnol. 2013, 41, 172–177. [Google Scholar] [CrossRef]
- Li, Z.; Song, X.; Cui, S.; Jiao, Y.; Zhou, C. Fabrication of macroporous reduced graphene oxide composite aerogels reinforced with chitosan for high bilirubin adsorption. RSC Adv. 2018, 8, 8338–8348. [Google Scholar] [CrossRef]
- Kavoshchian, M.; Üzek, R.; Uyanık, S.A.; Şenel, S.; Denizli, A. HSA immobilized novel polymeric matrix as an alternative sorbent in hemoperfusion columns for bilirubin removal. React. Funct. Poly. 2015, 96, 25–31. [Google Scholar] [CrossRef]
- La Spina, R.; Tripisciano, C.; Mecca, T.; Cunsolo, F.; Weber, V.; Mattiasson, B. Chemically modified poly(2-hydroxyethyl methacrylate) cryogel for the adsorption of heparin. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1121–1362. [Google Scholar] [CrossRef]
- Tijink, M.; Janssen, J.; Timmer, M.; Austen, J.; Aldenhoff, Y.; Kooman, J.; Koole, L.; Damoiseaux, J.; van Oerle, R.; Henskensh, Y.; Stamatialis, D. Development of novel membranes for blood purification therapies based on copolymers of N-vinylpyrrolidone and n-butylmethacrylate. J. Mater. Chem. B 2013, 1, 6066–6077. [Google Scholar] [CrossRef][Green Version]
- Zhao, S.; Wang, D.; Zhu, S.; Liu, X.; Zhang, H. 3D cryogel composites as adsorbent for isolation of protein and small molecules. Talanta 2019, 191, 229–234. [Google Scholar] [CrossRef]
- Hixon, K.R.; Lu, T.; Sell, S.A. A comprehensive review of cryogels and their roles in tissue engineering applications. Acta Biomater. 2017, 62, 29–41. [Google Scholar] [CrossRef]
- Hacker, M.C.; Nawaz, H.A. Multi-functional macromers for hydrogel design in biomedical engineering and regenerative medicine. Int. J. Mol. Sci. 2015, 16, 27677–27706. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Biçen Ünlüer, Ö; Ersöz, A.; Denizli, A.; Demirel, R.; Say, R. Separation and purification of hyaluronic acid by embedded glucuronic acid imprinted polymers into cryogel. J. Chromatogr. B 2013, 934, 46–52. [Google Scholar]
- Tam, R.Y.; Fisher, S.A.; Baker, A.E.G.; Shoichet, M.S. Transparent porous polysaccharide cryogels provide biochemically defined, biomimetic matrices for tunable 3D cell culture. Chem. Mater. 2016, 28, 3762–3770. [Google Scholar] [CrossRef]
- Petrov, P.; Mokreva, P.; Kostov, I.; Uzunova, L.; Tzoneva, R. Novel electrically conducting 2-hydroxyethylcellulose/polyaniline nanocomposite cryogels: Synthesis and application in tissue engineering. Carbohyd. Polym. 2016, 140, 349–355. [Google Scholar] [CrossRef]
- Von der Ehe, C.; Bus, T.; Weber, C.; Stumpf, S.; Bellstedt, P.; Hartlieb, M.; Schubert, U.S.; Gottschaldt, M. Glycopolymer-functionalized cryogels as catch and release devices for the pre-enrichment of pathogens. ACS Macro Lett. 2016, 5, 326–331. [Google Scholar] [CrossRef]
- Erdem, A.; Ngwabebhoh, F.A.; Yildiz, U. Fabrication and characterization of soft macroporous Jeffamine cryogels as potential materials for tissue applications. RSC Adv. 2016, 6, 111872–111881. [Google Scholar] [CrossRef]
- Zhang, G.; Song, X.; Mei, J.; Ye, G.; Wang, L.; Yu, L.; Xing, M.M.Q.; Qiu, X. A simple 3D cryogel co-culture system used to study the role of CAFs in EMT of MDA-MB-231 cells. RSC Adv. 2017, 7, 17208–17216. [Google Scholar] [CrossRef]
- Konovalova, M.V.; Markov, P.A.; Durnev, E.A.; Kurek, D.V.; Popov, S.V.; Varlamov, V.P. Preparation and biocompatibility evaluation of pectin and chitosan cryogels for biomedical application. J. Biomed. Mater. Res. 2017, 105, 547–556. [Google Scholar] [CrossRef]
- Zou, X.; Deng, P.; Zhou, C.; Hou, Y.; Chen, R.; Liang, F.; Liao, L. Preparation of a novel antibacterial chitosan-poly(ethylene glycol) cryogel/silver nanoparticles composites. J. Biomater. Sci. Polym. Edi. 2017, 28, 1324–1337. [Google Scholar] [CrossRef]
- Kim, I.; Lee, S.S.; Bae, S.; Lee, H.; Hwang, N.S. Heparin functionalized injectable cryogel with rapid shape-recovery property for neovascularization. Biomacromolecules 2018, 19, 2257–2269. [Google Scholar] [CrossRef]
- Sedlacık, T.; Acar, O.K.; Studenovska, H.; Kotelnikov, I.; Kucka, J.; Konecna, Z.; Zikmund, T.; Kaiser, J.; Kose, G.T.; Rypacek, F. Chondrogenic potential of macroporous biodegradable cryogels based on synthetic poly(a-amino acids). Soft Matter. 2018, 14, 228–239. [Google Scholar] [CrossRef]
- Dinu, M.V.; Cocarta, A.I.; Dragan, E.S. Synthesis, characterization and drug release properties of 3D chitosan/clinoptilolite biocomposite cryogels. Carbo. Polym. 2016, 153, 203–211. [Google Scholar] [CrossRef]
- Koshy, S.T.; Zhang, D.K.Y.; Grolman, J.M.; Stafford, A.G.; Mooney, D.J. Injectable nanocomposite cryogels for versatile protein drug delivery. Acta Biomater. 2018, 65, 36–43. [Google Scholar] [CrossRef]
- Günter, E.A.; Popeyko, O.V. Calcium pectinate gel beads obtained from callus cultures pectins as promising systems for colontargeted drug delivery. Carbohydr. Polym. 2016, 147, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Bakhshpour, M.; Yavuz, H.; Denizli, A. Controlled release of mitomycin C from PHEMAH–Cu(II) cryogel membranes. Artif. Cell. Nanomed. Biotechnol. 2018, 46, 946–954. [Google Scholar] [CrossRef]
- Öncel, P.; Çetin, K.; Topçu, A.A.; Yavuz, H.; Denizli, A. Molecularly imprinted cryogel membranes for mitomycin C delivery. J. Biomater. Sci. Polym. Edi. 2017, 28, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, X.; Meng, M.; Lv, P.; Yan, M.; Wei, X.; Li, H.; Yan, Y.; Li, C. Bio-inspired adhesion: Fabrication of molecularly imprinted nanocomposite membranes by developing a hybrid organic–inorganic nanoparticles composite structure. J. Membr. Sci. 2015, 490, 169–178. [Google Scholar] [CrossRef]
- Wu, Y.; Yan, M.; Lu, J.; Wang, C.; Zhao, J.; Cui, J.; Li, C.; Yan, Y. Facile bio-functionalized design of thermally responsive molecularly imprinted composite membrane for temperature-dependent recognition and separation applications. Chem. Eng. J. 2017, 309, 98–107. [Google Scholar] [CrossRef]
- Lima, G.G.; Traon, F.; Moal, E.; Canillas, M.; Rodriguez, M.A.; McCarthy, H.O.; Dunne, N.; Devine, D.M.; Nugent, M.J.D. Composite cryogels for dual drug delivery and enhanced mechanical properties. Polym. Comp. 2018, 39, E210–E220. [Google Scholar] [CrossRef]
- Hajizadeh, S.; Kettisen, K.; Gram, M.; Bülow, L.; Ye, L. Composite imprinted macroporous hydrogels for haemoglobin purification from cell homogenate. J. Chromatogr. A 2018, 1534, 22–31. [Google Scholar] [CrossRef]
- Pencheva, V.; Margaritova, E.; Borinarova, M.; Slavkova, M.; Momekova, D.; Petrov, P.D. A novel approach for fabricating nanocomposite materials by embedding stabilized core-shell micelles into polysaccharide cryogel matrix. Carbohydr. Polym. 2018, 183, 165–172. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Ergün, B.; Baydemir, G.; Andaç, M.; Yavuz, H.; Denizli, A. Ion imprinted beads embedded cryogels or in-vitro removal of iron from-thalassemic human plasma. J. Appl. Polym. Sci. 2012, 125, 254–262. [Google Scholar] [CrossRef]
- Berezhna, L.G.; Ivanov, A.E.; Leistner, A.; Lehmann, A.; Viloria-Cols, M.; Jungvid, H. Structure and biocompatibility of poly(vinyl alcohol)-based and agarose-based monolithic composites with embedded divinylbenzenestyrene polymeric particles. Prog. Biomater. 2013, 2, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Akande, W.; Mikhalovska, L.; James, S.; Mikhalovsky, S. Poly (2-hydroxyethyl methacrylate) macroporous cryogel for extracorporeal medical devices. Int. J. Biomed. Mater. Res. 2015, 3, 46–55. [Google Scholar] [CrossRef]
- Savina, I.N.; Ingavle, G.C.; Cundy, A.B.; Mikhalovsky, S.V. A simple method for the production of large volume 3D macroporous hydrogels for advanced biotechnological, medical and environmental applications. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Calo, E.; Barros, J.; Ballamy, L.; Khutoryanskiy, V.V. Poly(vinyl alcohol)–Gantrez® AN cryogels for wound care applications. RSC Adv. 2016, 6, 105487–105495. [Google Scholar] [CrossRef]
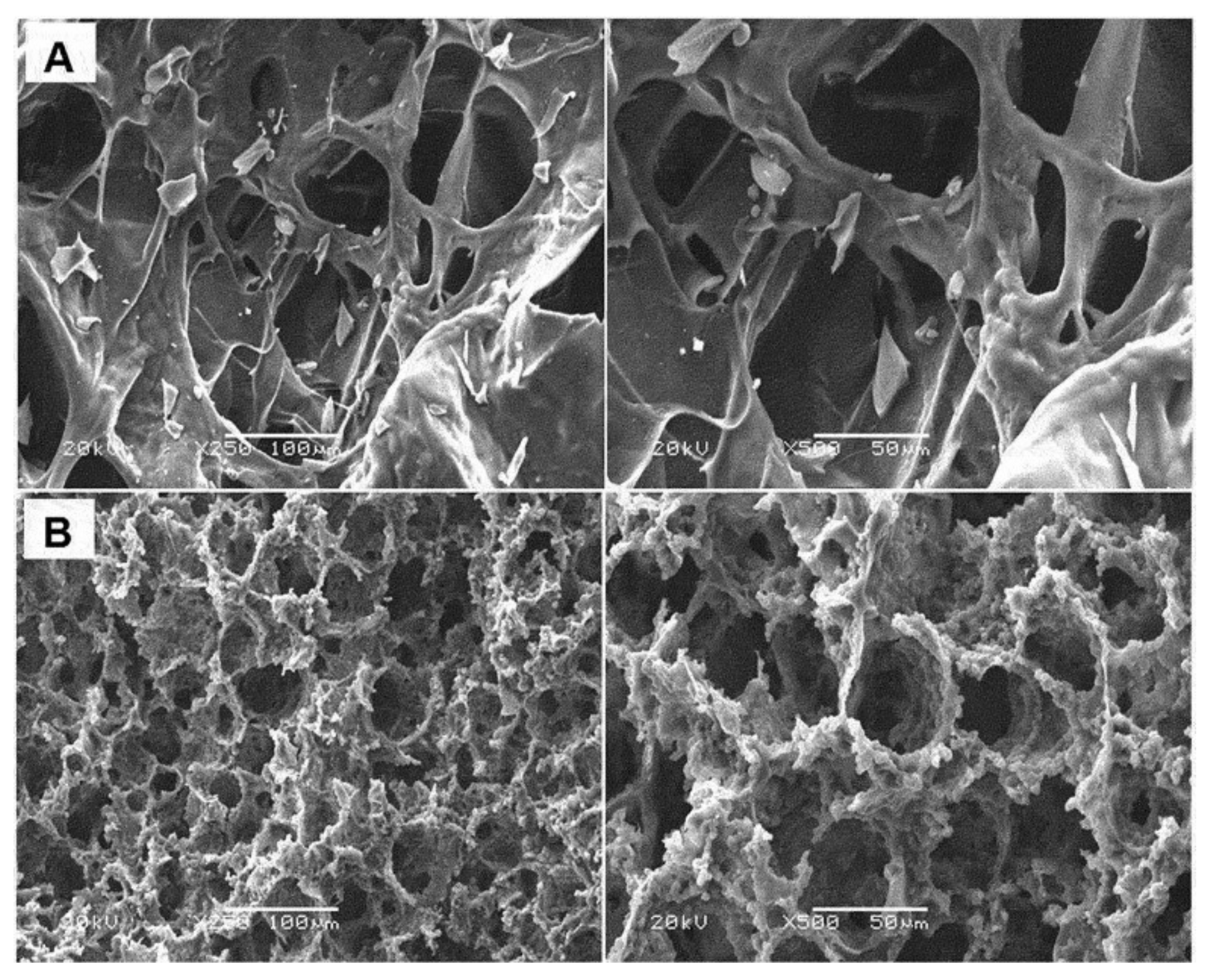

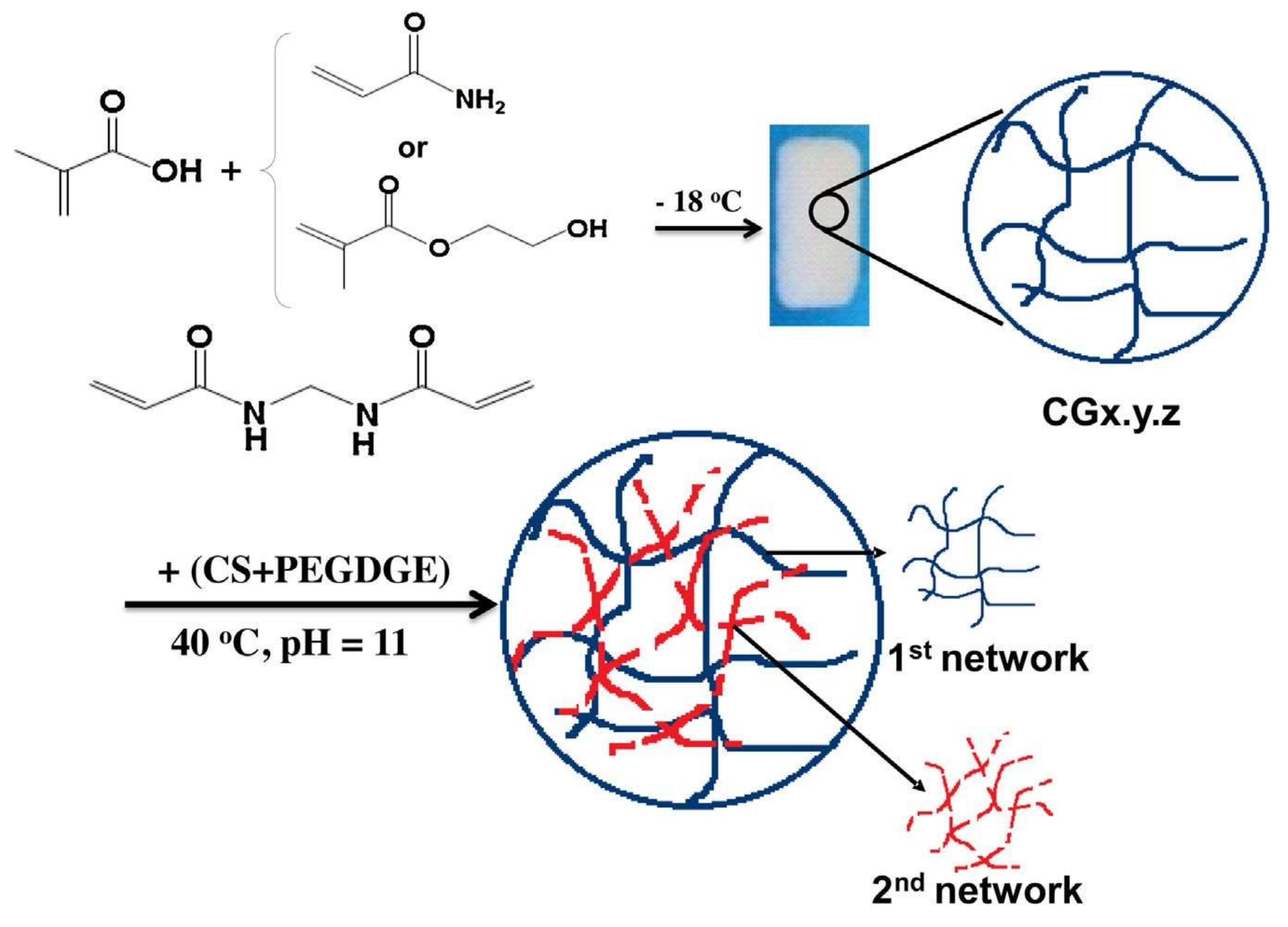
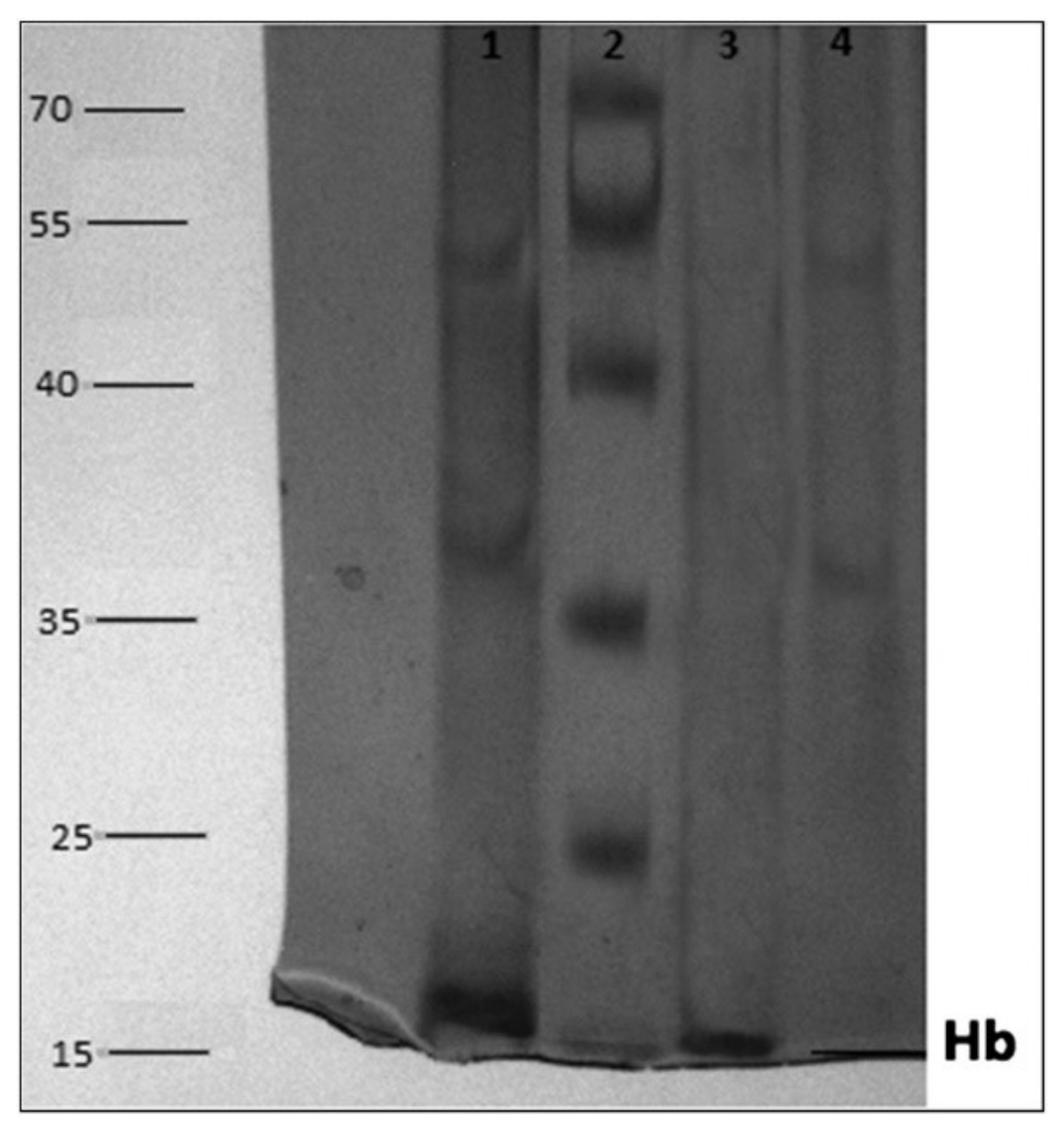

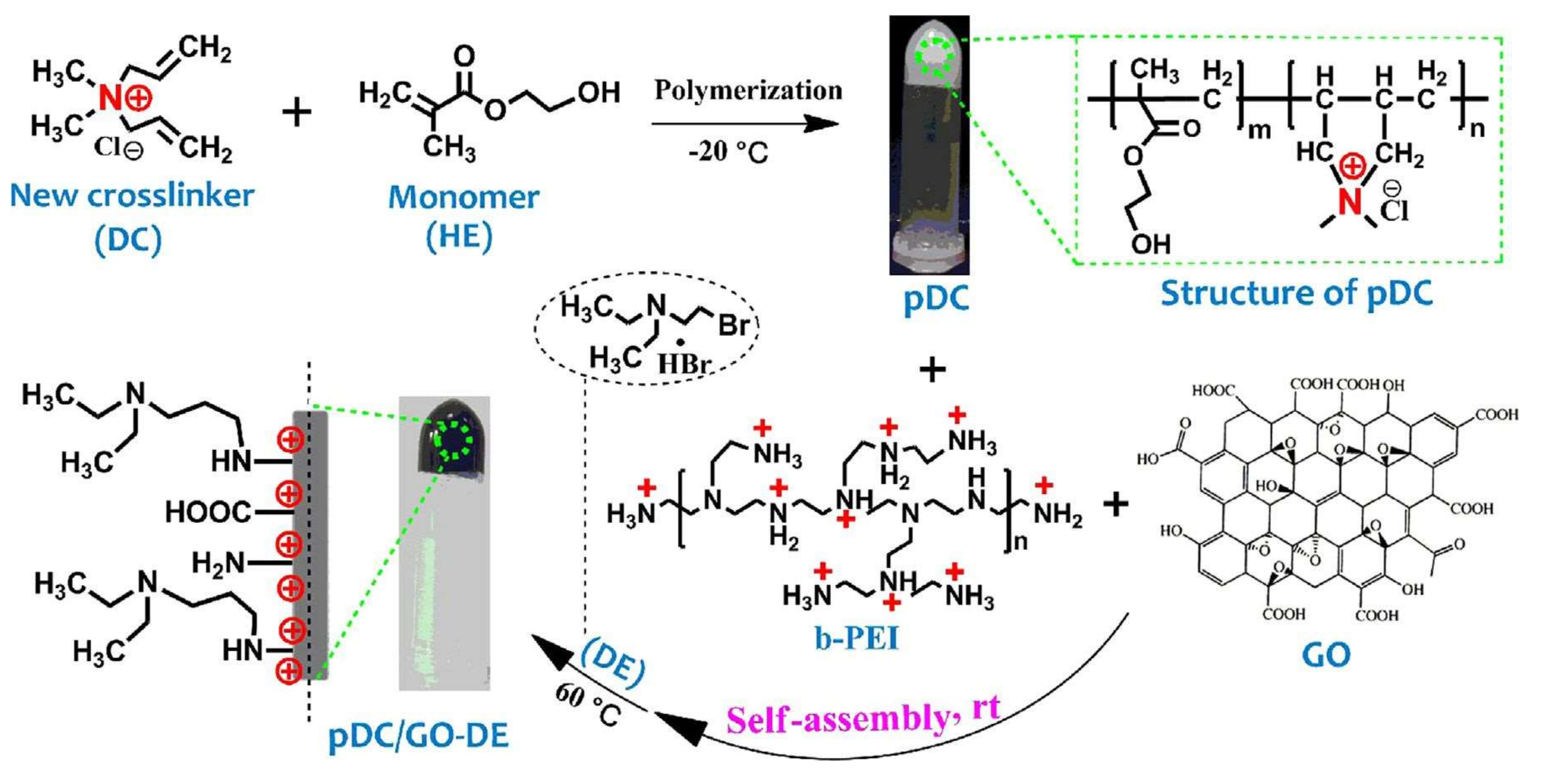

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saylan, Y.; Denizli, A. Supermacroporous Composite Cryogels in Biomedical Applications. Gels 2019, 5, 20. https://doi.org/10.3390/gels5020020
Saylan Y, Denizli A. Supermacroporous Composite Cryogels in Biomedical Applications. Gels. 2019; 5(2):20. https://doi.org/10.3390/gels5020020
Chicago/Turabian StyleSaylan, Yeşeren, and Adil Denizli. 2019. "Supermacroporous Composite Cryogels in Biomedical Applications" Gels 5, no. 2: 20. https://doi.org/10.3390/gels5020020
APA StyleSaylan, Y., & Denizli, A. (2019). Supermacroporous Composite Cryogels in Biomedical Applications. Gels, 5(2), 20. https://doi.org/10.3390/gels5020020