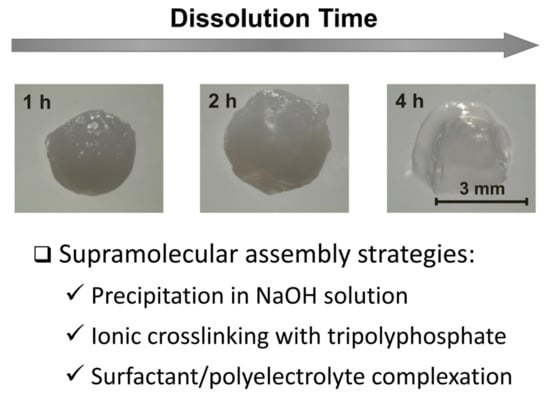
Supramolecular Strategy Effects on Chitosan Bead Stability in Acidic Media: A Comparative Study
Abstract
:1. Introduction
2. Results and Discussion
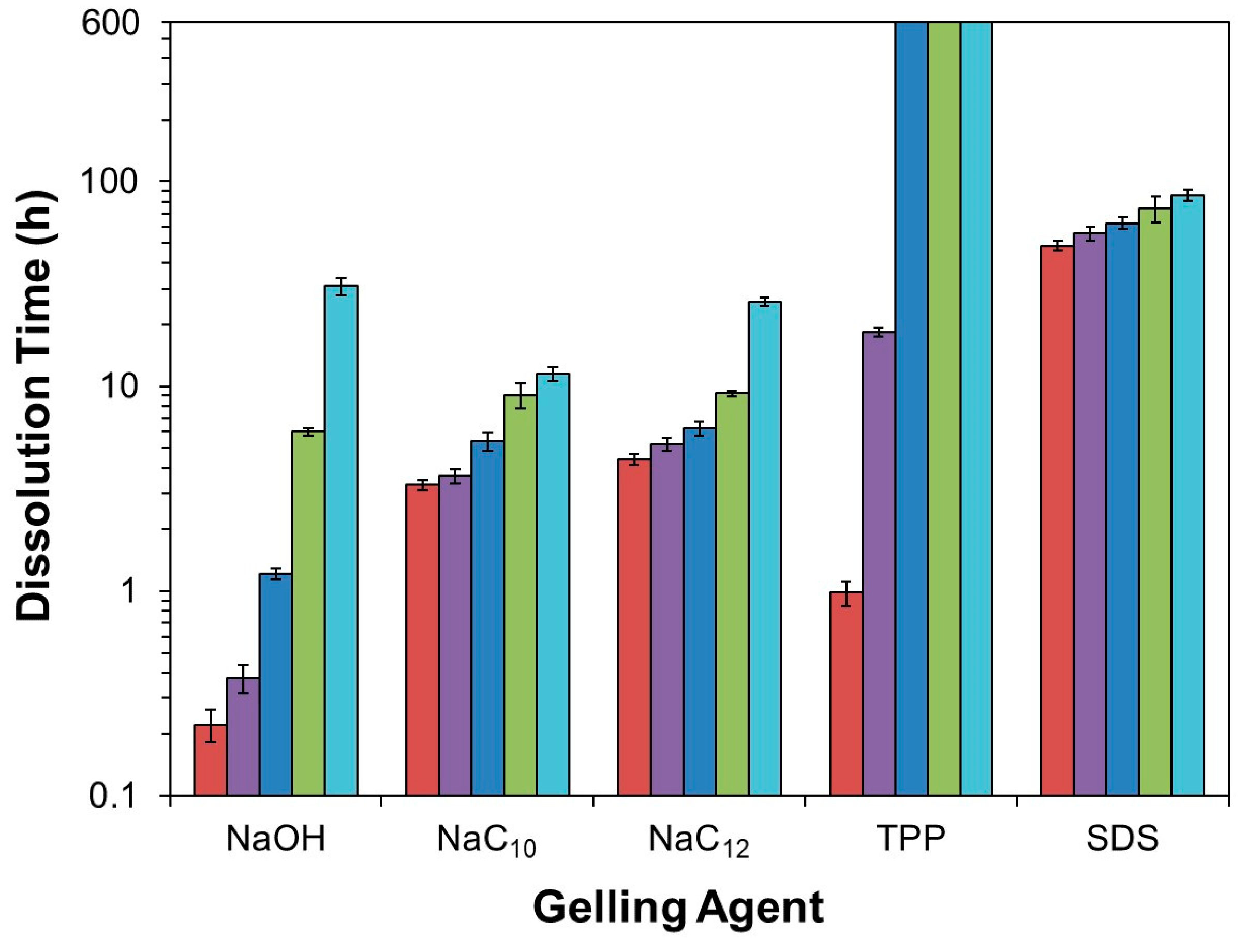
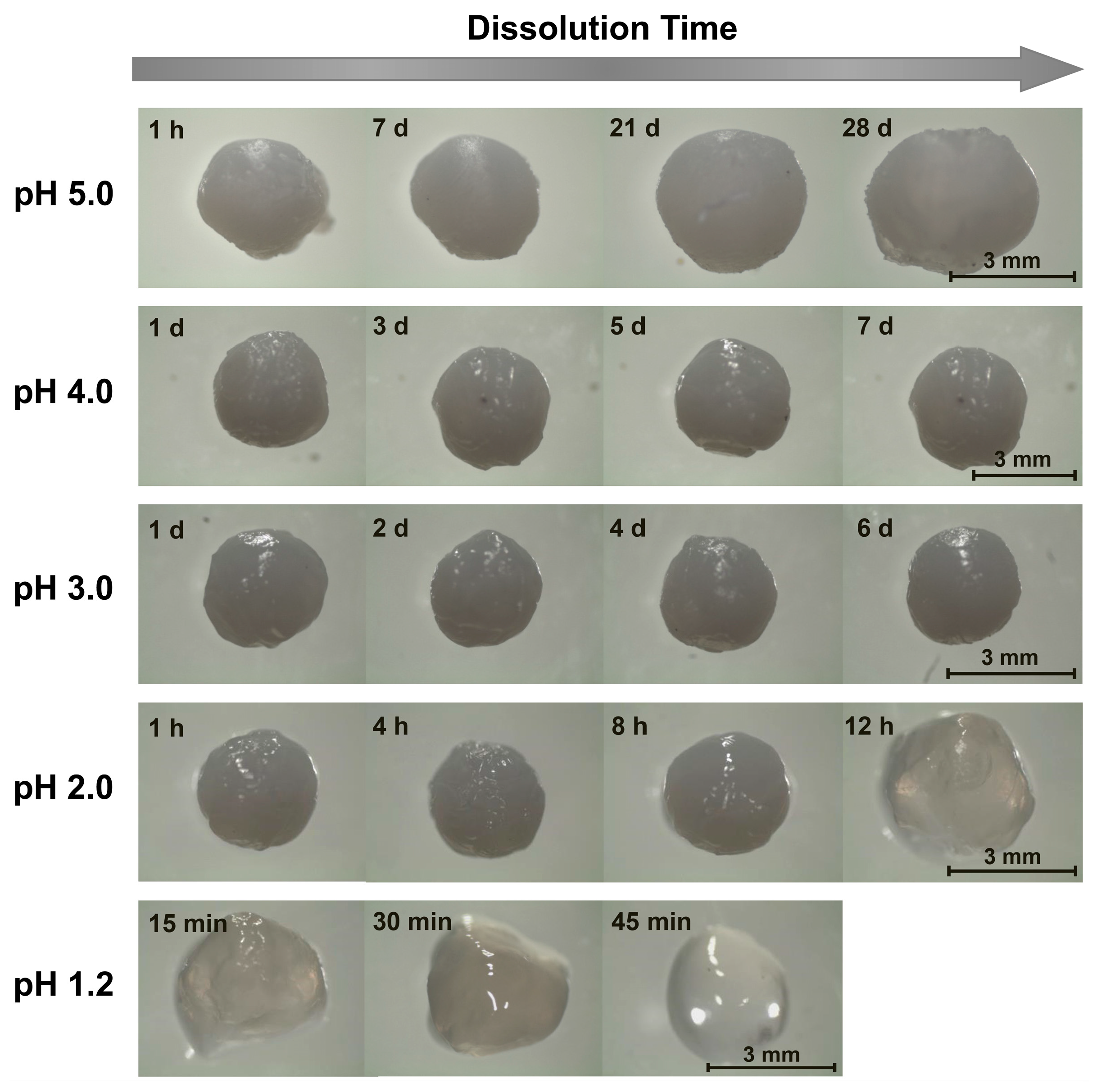
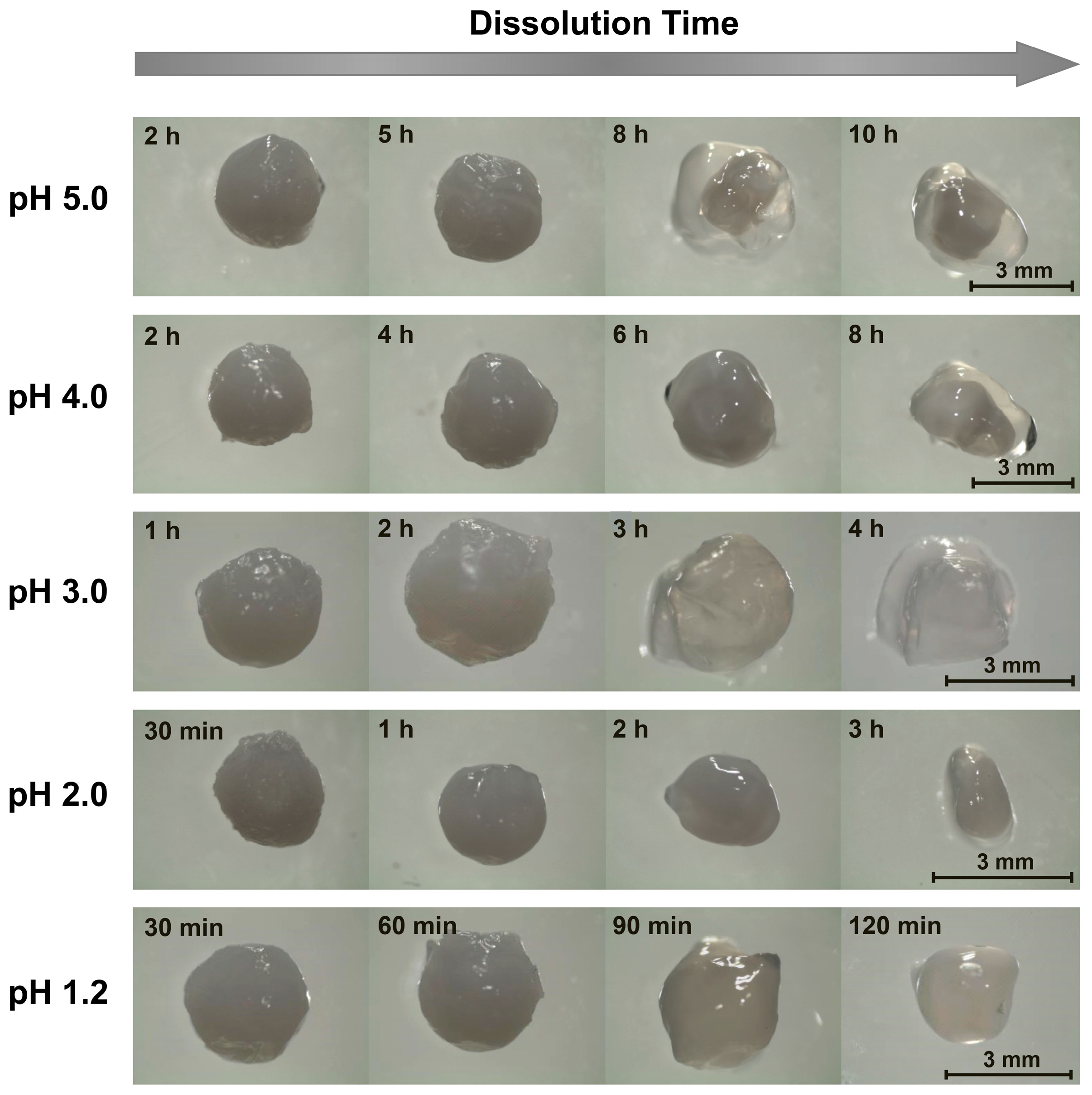
2.1. Alkaline Solution-Derived Beads
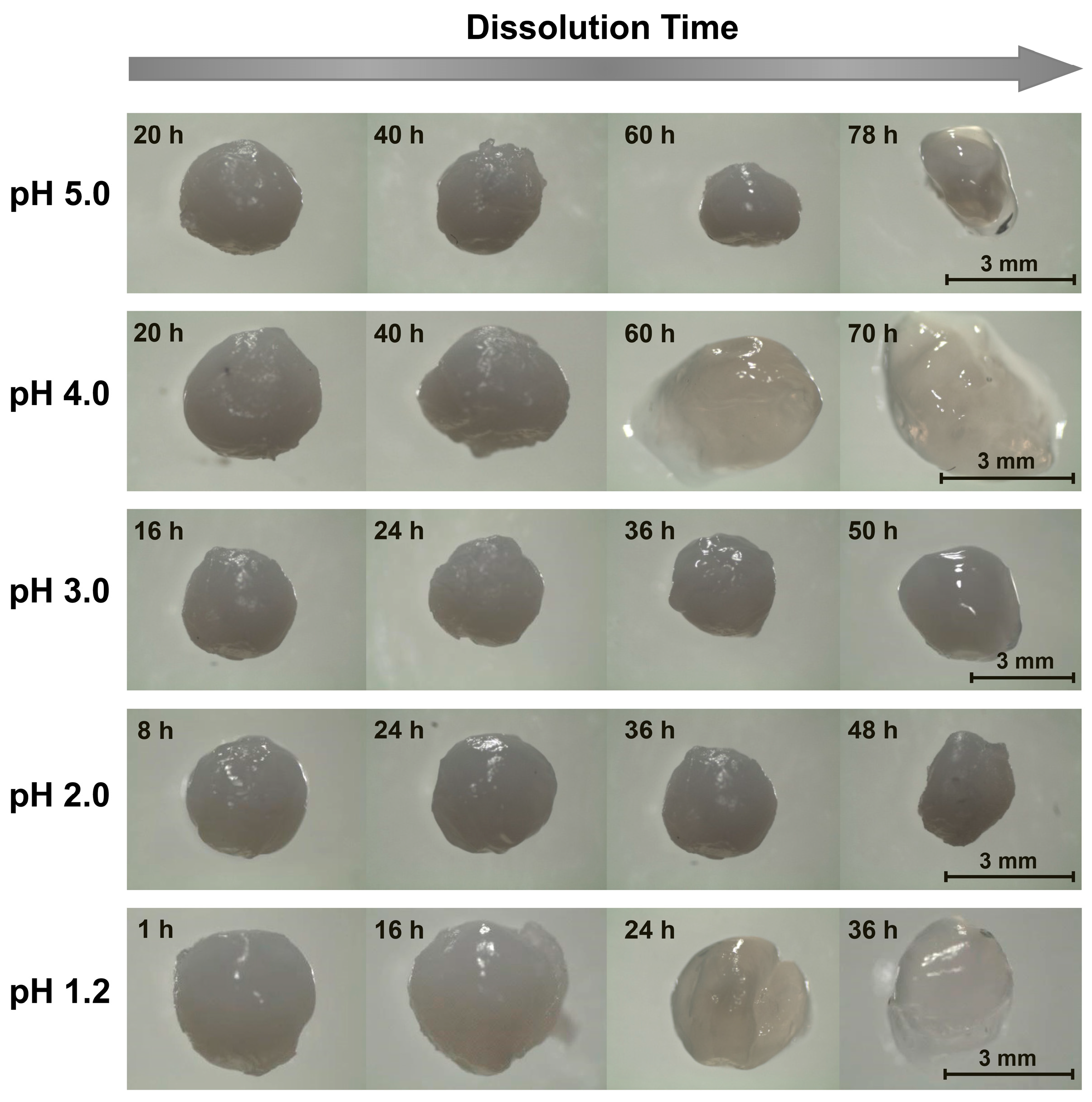
2.2. Tripolyphosphate-Crosslinked Beads
2.3. Surfactant-Complexed Beads
2.4. Further Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Bead Preparation
4.3. Dissolution Experiments
Author Contributions
Funding
Conflicts of Interest
References
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitosan and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Tapola, N.S.; Lyyra, M.L.; Kolehmainen, R.M.; Sarkkinen, E.S.; Schauss, A.G. Safety aspects and cholesterol-lowering efficacy of chitosan tablets. J. Am. Coll. Nutr. 2008, 27, 22–30. [Google Scholar] [CrossRef] [PubMed]
- VandeVord, P.J.; Matthew, H.W.; DeSilva, S.P.; Mayton, L.; Wu, B.; Wooley, P.H. Evaluation of the biocompatibility of a chitosan scaffold in mice. J. Biomed. Mater. Res. 2002, 59, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Chiappisi, L.; Gradzielski, M. Co-assembly in chitosan–surfactant mixtures: thermodynamics, structures, interfacial properties and applications. Adv. Coll. Int. Sci. 2015, 220, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Zhu, K. Controlled drug release properties of ionically cross-linked chitosan beads: The influence of anion structure. Int. J. Pharm. 2002, 233, 217–225. [Google Scholar] [CrossRef]
- Garcia-Fuentes, M.; Alonso, M.J. Chitosan-based drug nanocarriers: Where do we stand? J. Controll. Release 2012, 161, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Madihally, S.V.; Matthew, H.W.T. Porous chitosan scaffolds for tissue engineering. Biomaterials 1999, 20, 1133–1142. [Google Scholar] [CrossRef]
- Krajewska, B. Application of chitin-and chitosan-based materials for enzyme immobilizations: A review. Enzyme Microb. Technol. 2004, 35, 126–139. [Google Scholar] [CrossRef]
- Wahba, M.I. Sodium bicarbonate-gelled chitosan beads as mechanically stable carriers for the covalent immobilization of enzymes. Biotechnol. Prog. 2018, 34, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, C. Chitosan-based biosorbents: Modification and application for biosorption of heavy metals and radionuclides. Bioresour. Technol. 2014, 160, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Lee, D.S.; Lee, M.W.; Woo, S.H. Congo red adsorption from aqueous solutions by using chitosan hydrogel beads impregnated with nonionic or anionic surfactant. Bioresour. Technol. 2009, 100, 3862–3868. [Google Scholar] [CrossRef] [PubMed]
- Aider, M. Chitosan application for active bio-based films production and potential in the food industry. LWT-Food Sci. Technol. 2010, 43, 837–842. [Google Scholar] [CrossRef]
- Coma, V.; Martial-Gros, A.; Garreau, S.; Copinet, A.; Salin, F.; Deschamps, A. Edible antimicrobial films based on chitosan matrix. J. Food Sci. 2002, 67, 1162–1169. [Google Scholar] [CrossRef]
- Sorlier, P.; Denuziere, A.; Viton, C.; Domard, A. Relation between the degree of acetylation and the electrostatic properties of chitin and chitosan. Biomacromolecules 2001, 2, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Liu, Z.; Shen, L.; Guo, Z.; Chou, L.L.; Zhong, W.; Du, Q.; Ge, J. The effect of a layer-by-layer chitosan–heparin coating on the endothelialization and coagulation properties of a coronary stent system. Biomaterials 2009, 30, 2276–2283. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.Z.; Zhu, K.J. The influence of multivalent phosphate structure on the properties of ionically cross-linked chitosan films for controlled drug release. Eur. J. Pharm. Biopharm. 2002, 54, 235–243. [Google Scholar] [CrossRef]
- Mendes, A.C.; Gorzelanny, C.; Halter, N.; Schneider, S.W.; Chronakis, I.S. Hybrid electrospun chitosan-phospholipids nanofibers for transdermal drug delivery. Int. J. Pharm. 2016, 510, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Toivonen, M.S.; Kurki-Suonio, S.; Wagermaier, W.; Hynninen, V.; Hietala, S.; Ikkala, O. Interfacial polyelectrolyte complex spinning of cellulose nanofibrils for advanced bicomponent fibers. Biomacromolecules 2017, 18, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Remunan-Lopez, C.; Vila-Jato, J.L.; Alonso, M.J. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Saether, H.V.; Holme, H.K.; Maurstald, G.; Smidsrod, O.; Stokke, B.T. Polyelectrolyte complex formation using alginate and chitosan. Carbohydr. Polym. 2008, 74, 813–821. [Google Scholar]
- Shchipunov, Y.; Sarin, S.; Kim, I.; Ha, C.-S. Hydrogels formed through regulated self-organization of gradually charging chitosan in solution of xanthan. Green Chem. 2010, 12, 1187–1195. [Google Scholar] [CrossRef]
- Babak, V.G.; Merkovich, E.A.; Desbrieres, J.; Rinaudo, M. Formation of an ordered nanostructure in surfactantpolyelectrolyte complexes formed by interfacial diffusion. Polym. Bull. 2000, 45, 77–81. [Google Scholar] [CrossRef]
- Yu, L.; Liu, X.; Yuan, W.; Brown, L.J.; Wang, D. Confined flocculation of ionic pollutants by poly (L-dopa)-based polyelectrolyte complexes in hydrogel beads for three-dimensional, quantitative, efficient water decontamination. Langmuir 2015, 31, 6351–6366. [Google Scholar] [CrossRef] [PubMed]
- Mi, F.L.; Shyu, S.S.; Lee, S.T.; Wong, T.B. Kinetic study of chitosan-tripolyphosphate complex reaction and acid-resistive properties of the chitosan-tripolyphosphate gel beads prepared by in-liquid curing method. J. Polym. Sci. B Polym. Phys. 1999, 37, 1551–1564. [Google Scholar] [CrossRef]
- Freier, T.; Koh, H.S.; Kazazian, K.; Shoichet, M.S. Controlling cell adhesion and degradation of chitosan films by N-acetylation. Biomaterials 2005, 26, 5872–5878. [Google Scholar] [CrossRef] [PubMed]
- Tomihata, K.; Ikada, Y. In vitro and in vivo degradation of films of chitin and its deacetylated derivatives. Biomaterials 1997, 18, 567–575. [Google Scholar] [CrossRef]
- Vårum, K.; Ottøy, M.; Smidsrød, O. Acid hydrolysis of chitosans. Carbohydr. Polym. 2001, 46, 89–98. [Google Scholar] [CrossRef]
- Huang, Y.; Cai, Y.; Lapitsky, Y. Factors affecting the stability of chitosan/tripolyphosphate micro- and nanogels: Resolving the opposing findings. J. Mater. Chem. B 2015, 3, 5957–5970. [Google Scholar] [CrossRef]
- Mi, F.L.; Shyu, S.S.; Kuan, C.Y.; Lee, S.T.; Lu, K.T.; Jang, S.F. Chitosan–polyelectrolyte complexation for the preparation of gel beads and controlled release of anticancer drug. I. Effect of phosphorous polyelectrolyte complex and enzymatic hydrolysis of polymer. J. Appl. Polym. Sci. 1999, 74, 1868–1879. [Google Scholar] [CrossRef]
- Remunan-Lopez, C.; Bodmeier, R. Mechanical, water uptake and permeability properties of crosslinked chitosan glutamate and alginate films. J. Controll. Release 1997, 44, 215–225. [Google Scholar] [CrossRef]
- Worthen, A.J.; Lapitsky, Y. Stabilization of bioderived surfactant/polyelectrolyte complexes through surfactant conjugation to the biopolymer. Colloid Polym. Sci. 2011, 1589–1596. [Google Scholar] [CrossRef]
- Sashiwa, H.; Saimoto, H.; Shigemasa, Y.; Ogawa, R.; Tokura, S. Lysozyme susceptibility of partially deacetylated chitin. Int. J. Biol. Macromol. 1990, 12, 295–296. [Google Scholar] [CrossRef]
- Kim, H.; Tator, C.H.; Shoichet, M.S. Chitosan implants in the rat spinal cord: biocompatibility and biodegradation. J. Biomed. Mater. Res. A 2011, 97, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Lapitsky, Y.; Zahir, T.; Shoichet, M.S. Modular biodegradable biomaterials from surfactant and polyelectrolyte mixtures. Biomacromolecules 2008, 9, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Rusu-Balaita, L.; Desbrieres, J.; Rinaudo, M. Formation of a biocompatible polyelectrolyte complex: Chitosan-hyaluronan complex stability. Polym. Bull. 2003, 50, 91–98. [Google Scholar] [CrossRef]
- Jóźwiak, T.; Filipkowska, U.; Szymczyk, P.; Rodziewicz, J.; Mielcarek, A. Effect of ionic and covalent crosslinking agents on properties of chitosan beads and sorption effectiveness of Reactive Black 5 dye. React. Funct. Polym. 2017, 114, 58–74. [Google Scholar] [CrossRef]
- Morris, G.A.; Castile, J.; Smith, A.; Adams, G.G.; Harding, S.E. The effect of prolonged storage at different temperatures on the particle size distribution of tripolyphosphate (TPP)—chitosan nanoparticles. Carbohydr. Polym. 2011, 84, 1430–1434. [Google Scholar] [CrossRef]
- Mi, F.L.; Shyu, S.S.; Wong, T.B.; Jang, S.F.; Lee, S.T.; Lu, K.T. Chitosan–polyelectrolyte complexation for the preparation of gel beads and controlled release of anticancer drug. II. Effect of pH-dependent ionic crosslinking or interpolymer complex using tripolyphosphate or polyphosphate as reagent. J. Appl. Polym. Sci. 1999, 74, 1093–1107. [Google Scholar] [CrossRef]
- McBain, J.; Sierichs, W. The solubility of sodium and potassium soaps and the phase diagrams of aqueous potassium soaps. J. Am. Oil Chem. Soc. 1948, 25, 221–225. [Google Scholar] [CrossRef]
- Lipatova, I.; Makarova, L. Effect of hydroacoustic treatment on chitosan dissolution in aqueous acetic acid solutions. Russ. J. Appl. Chem. 2008, 81, 2112–2117. [Google Scholar] [CrossRef]
- Wan Ngah, W.; Endud, C.; Mayanar, R. Removal of copper (II) ions from aqueous solution onto chitosan and cross-linked chitosan beads. React. Funct. Polym. 2002, 50, 181–190. [Google Scholar] [CrossRef]
- Beukenkamp, J.; Rieman, W.; Lindenbaum, S. Behavior of the condensed phosphates in anion-exchange chromatography. Anal. Chem. 1954, 26, 505–512. [Google Scholar] [CrossRef]
- Campbell, A.; Lakshminarayanan, G. Conductances and surface tensions of aqueous solutions of sodium decanoate, sodium laurate, and sodium myristate, at 25 and 35. Can. J. Chem. 1965, 43, 1729–1737. [Google Scholar] [CrossRef]
- Sjostrom, J.; Piculell, L. Interactions between cationically modified hydroxyethyl cellulose and oppositely charged surfactants studied by gel swelling experiments—Effects of surfactant type, hydrophobic modification and added salt. Colloids Surf. A 2001, 183–185, 429. [Google Scholar] [CrossRef]
- Goddard, E.D. Polymer surfactant interaction. 2. Polymer and surfactant of opposite charge. Colloids Surf. 1986, 19, 301–329. [Google Scholar] [CrossRef]
- Malovikova, A.; Hayakawa, K.; Kwak, J.C.T. Surfactant Polyelectrolyte interactions. 4. Surfactant chain-length dependence on the binding of alkylpyidinium cations to dextran sulfate. J. Phys. Chem. 1984, 88, 1930–1933. [Google Scholar] [CrossRef]
- Kanicky, J.R.; Shah, D.O. Effect of premicellar aggregation on the pKa of fatty acid soap solutions. Langmuir 2003, 19, 2034–2038. [Google Scholar] [CrossRef]
- Lapitsky, Y.; Eskuchen, W.J.; Kaler, E.W. Surfactant and polyelectrolyte gel particles that swell reversibly. Langmuir 2006, 22, 6375–6379. [Google Scholar] [CrossRef] [PubMed]
- Lapitsky, Y.; Kaler, E.W. Formation of surfactant and polyelectrolyte gel particles in aqueous solutions. Colloids Surf. A 2004, 250, 179–187. [Google Scholar] [CrossRef]
- Denuziere, A.; Ferrier, D.; Domard, A. Chitosan-chondroitin sulfate and chitosan-hyaluronate polyelectrolyte complexes. Physico-chemical aspects. Carbohydr. Polym. 1996, 29, 317–323. [Google Scholar] [CrossRef]
- Fajardo, A.R.; Piai, J.F.; Rubira, A.F.; Muniz, E.C. Time- and pH-dependent self-rearrangement of a swollen polymer network based on polyelectrolytes complexes of chitosan/chondroitin sulfate. Carbohydr. Polym. 2010, 80, 934–943. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Worthen, A.J.; Irving, K.S.; Lapitsky, Y. Supramolecular Strategy Effects on Chitosan Bead Stability in Acidic Media: A Comparative Study. Gels 2019, 5, 11. https://doi.org/10.3390/gels5010011
Worthen AJ, Irving KS, Lapitsky Y. Supramolecular Strategy Effects on Chitosan Bead Stability in Acidic Media: A Comparative Study. Gels. 2019; 5(1):11. https://doi.org/10.3390/gels5010011
Chicago/Turabian StyleWorthen, Andrew J., Kelly S. Irving, and Yakov Lapitsky. 2019. "Supramolecular Strategy Effects on Chitosan Bead Stability in Acidic Media: A Comparative Study" Gels 5, no. 1: 11. https://doi.org/10.3390/gels5010011
APA StyleWorthen, A. J., Irving, K. S., & Lapitsky, Y. (2019). Supramolecular Strategy Effects on Chitosan Bead Stability in Acidic Media: A Comparative Study. Gels, 5(1), 11. https://doi.org/10.3390/gels5010011