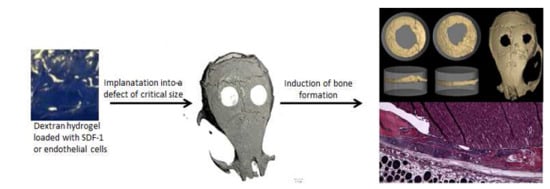
Photocrosslinked Dextran-Based Hydrogels as Carrier System for the Cells and Cytokines Induce Bone Regeneration in Critical Size Defects in Mice
Abstract
1. Introduction
- The material aspect—the choice of classical implant materials like steel or titanium versus new materials like polymers or composites.
- The best molecular cue to induce vascularization, as well as bone growth.
- Loading or immobilization of cytokines or growth factors in the material versus prevascularization of the implant by cell-based tissue engineering.
2. Results and Discussion
2.1. Synthesis of Dextran-Based Hydrogels and Immobilization of Growth Factor
2.2. Endotoxin Assay
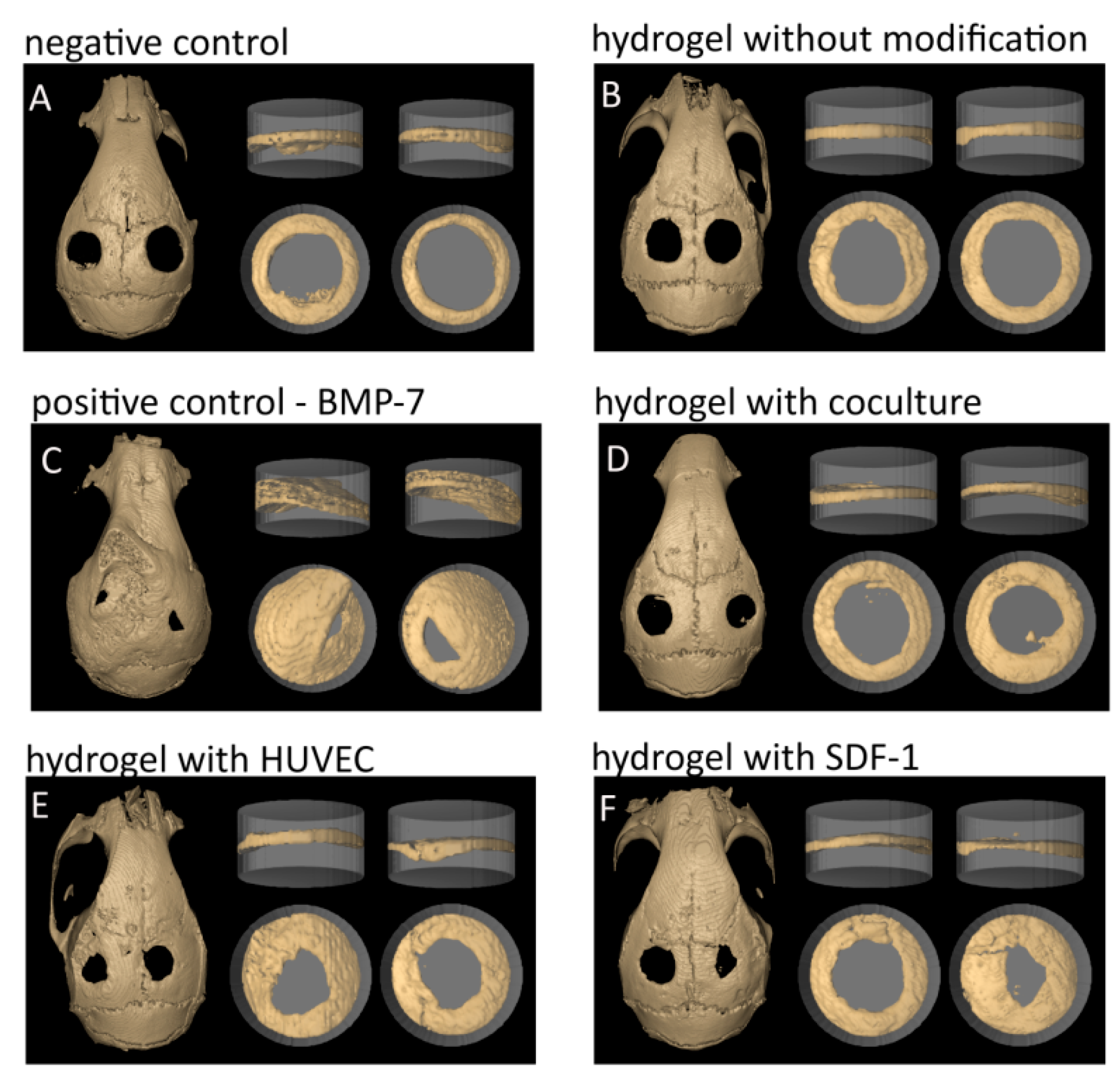
2.3. Calvarial Critical Size Defect (CSD) Model in Mouse
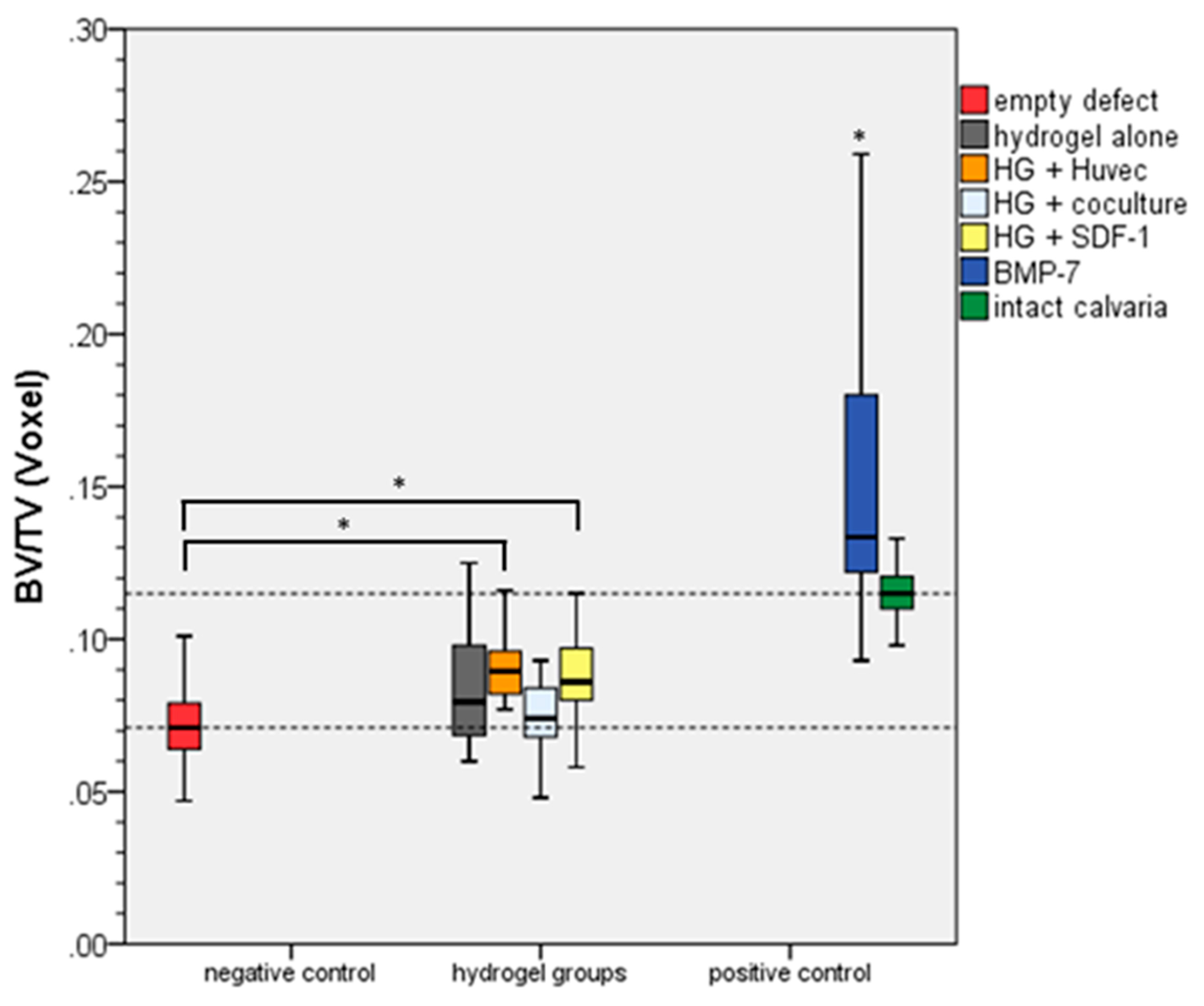
2.4. Bone Volume/Total Volume
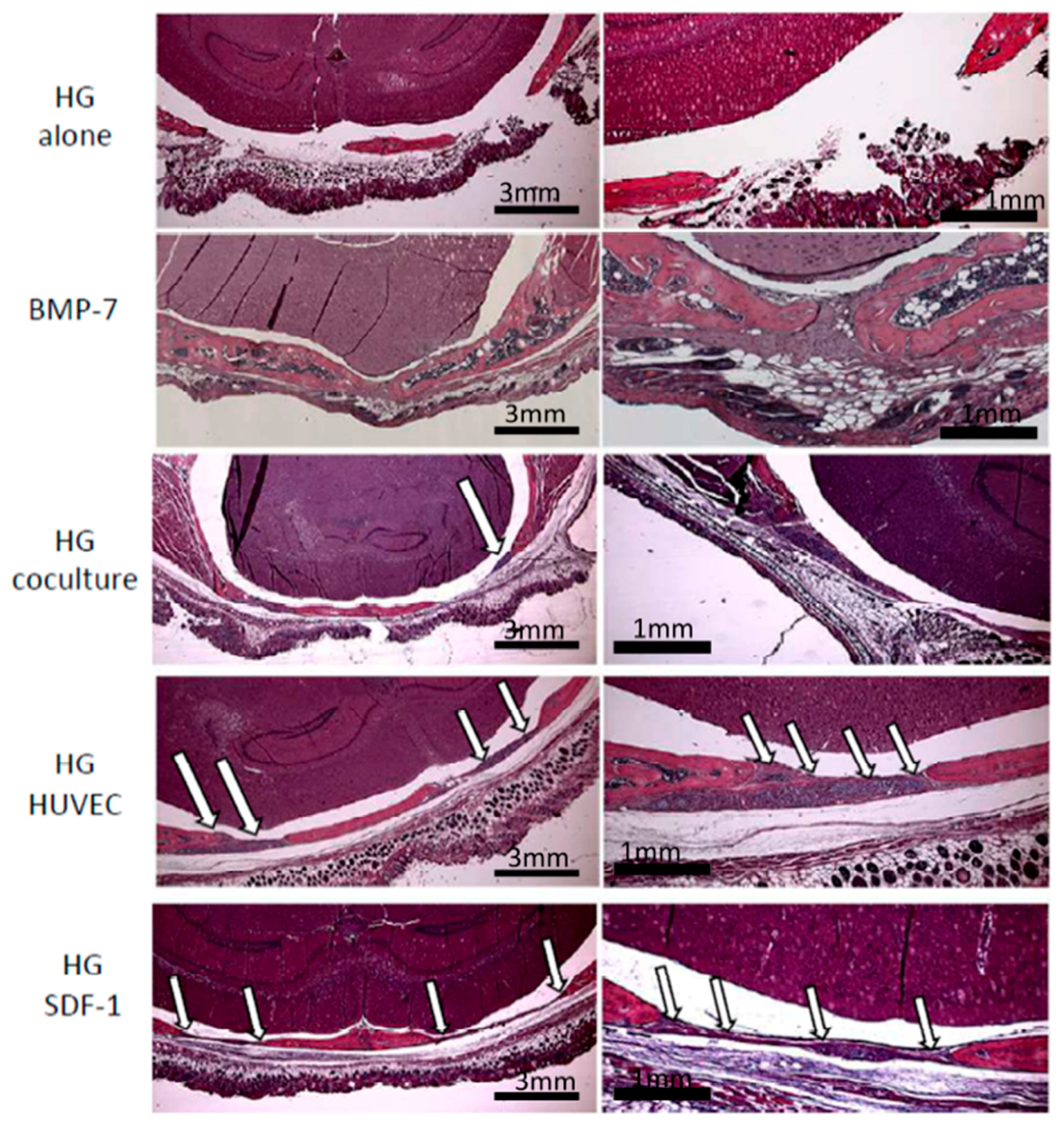
2.5. Histology
3. Conclusions
4. Experimental
4.1. Synthesis of Dextran-Based Hydrogels
4.2. Endotoxin Testing
4.3. Immobilization of SDF-1 or BMP-7
4.4. Cell Seeding
4.5. Human Umbilical Vein Endothelial Cells (HUVEC)
4.6. Mono- and Co-Culture on Dextran Hydrogels
4.7. Animal Model
4.8. Hydrogel Implantation
4.9. Radiographic Analysis
4.10. Histological Analysis
4.11. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pape, H.C.; Evans, A.; Kobbe, P. Autologous bone graft: Properties and techniques. J. Orthop. Trauma 2010, 24, S36–S40. [Google Scholar] [CrossRef] [PubMed]
- Buza, J.A., 3rd; Einhorn, T. Bone healing in 2016. Clin. Cases Miner. Bone Metab. 2016, 13, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Garcia, A.J. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Deliv. Rev. 2015, 94, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.R.; Garcia, A.J. Biomaterial-mediated strategies targeting vascularization for bone repair. Drug Deliv. Transl. Res. 2016, 6, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Ratner, B.D.; Bryant, S.J. Biomaterials: Where we have been and where we are going. Annu. Rev. Biomed. Eng. 2004, 6, 41–75. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Saha, N.; Kitano, T.; Saha, P. Novel hydrogels of pvp-cmc and their swelling effect on viscoelastic properties. J. Appl. Polym. Sci. 2010, 117, 1703–1710. [Google Scholar] [CrossRef]
- Roy, N.; Saha, N.; Kitano, T.; Saha, P. Development and characterization of novel medicated hydrogels for wound dressing. Soft Mater. 2010, 8, 130–148. [Google Scholar] [CrossRef]
- Kopecek, J.; Yang, J.Y. Revie—Hydrogels as smart biomaterials. Polym. Int. 2007, 56, 1078–1098. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Calo, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliver. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef]
- Song, H.H.; Park, K.M.; Gerecht, S. Hydrogels to model 3D in vitro microenvironment of tumor vascularization. Adv. Drug Deliv. Rev. 2014, 79–80, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Jeon, O.; Bouhadir, K.H.; Mansour, J.M.; Alsberg, E. Photocrosslinked alginate hydrogels with tunable biodegradation rates and mechanical properties. Biomaterials 2009, 30, 2724–2734. [Google Scholar] [CrossRef] [PubMed]
- Goonoo, N.; Khanbabaee, B.; Steuber, M.; Bhaw-Luximon, A.; Jonas, U.; Pietsch, U.; Jhurry, D.; Schonherr, H. Kappa-carrageenan enhances the biomineralization and osteogenic differentiation of electrospun polyhydroxybutyrate and polyhydroxybutyrate valerate fibers. Biomacromolecules 2017, 18, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef] [PubMed]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Chung, C.; Jia, X.Q.; Randolph, M.A.; Langer, R. Controlled degradation and mechanical behaviour of photopolymerized hyaluronic acid networks. Biomacromolecules 2005, 6, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Kuss, M.A.; Wu, S.; Wang, Y.; Untrauer, J.B.; Li, W.; Lim, J.Y.; Duan, B. Prevascularization of 3D printed bone scaffolds by bioactive hydrogels and cell co-culture. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 1788–1798. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, I.; Kumar, A.; Joddar, B. A bioactive hydrogel and 3d printed polycaprolactone system for bone tissue engineering. Gels 2017, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Nikpour, P.; Salimi-Kenari, H.; Fahimipour, F.; Rabiee, S.M.; Imani, M.; Dashtimoghadam, E.; Tayebi, L. Dextran hydrogels incorporated with bioactive glass-ceramic: Nanocomposite scaffolds for bone tissue engineering. Carbohydr. Polym. 2018, 190, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Ritz, U.; Kogler, P.; Hofer, I.; Frank, P.; Klees, S.; Gebhard, S.; Brendel, C.; Kaufmann, K.; Hofmann, A.; Rommens, P.M.; et al. Photocrosslinkable polysaccharide hydrogel composites based on dextran or pullulan-amylose blends with cytokines for a human co-culture model of human osteoblasts and endothelial cells. J. Mater. Chem. B 2016, 4, 6552–6564. [Google Scholar] [CrossRef]
- Holloway, J.L.; Ma, H.; Rai, R.; Hankenson, K.D.; Burdick, J.A. Synergistic effects of sdf-1α and bmp-2 delivery from proteolytically degradable hyaluronic acid hydrogels for bone repair. Macromol. Biosci. 2015, 15, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Cebral, R.; Civantos, A.; Ramos, V.; Seijo, B.; Lopez-Lacomba, J.L.; Sanz-Casado, J.V.; Sanchez, A. Gellan gum based physical hydrogels incorporating highly valuable endogen molecules and associating bmp-2 as bone formation platforms. Carbohydr. Polym. 2017, 167, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Brunsen, A.; Ritz, U.; Mateescu, A.; Hofer, I.; Frank, P.; Menges, B.; Hofmann, A.; Rommens, P.M.; Knoll, W.; Jonas, U. Photocrosslinkable dextran hydrogel films as substrates for osteoblast and endothelial cell growth. J. Mater. Chem. 2012, 22, 19590–19604. [Google Scholar] [CrossRef]
- Ronco, C. Endotoxin removal: History of a mission. Blood Purif. 2014, 37, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Gilliam, E.A.; Li, L.W. Innate immune programing by endotoxin and its pathological consequences. Front. Immunol. 2015, 5, 680. [Google Scholar] [CrossRef] [PubMed]
- Unger, R.E.; Peters, K.; Sartoris, A.; Freese, C.; Kirkpatrick, C.J. Human endothelial cell-based assay for endotoxin as sensitive as the conventional limulus amebocyte lysate assay. Biomaterials 2014, 35, 3180–3187. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.M.; Shen, Y.I.; Kusuma, S.; Fox-Talbot, K.; Steenbergen, C.J.; Gerecht, S. Functional neovascularization of biodegradable dextran hydrogels with multiple angiogenic growth factors. Biomaterials 2011, 32, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.M.; Zhang, X.J.; Shen, Y.I.; Sebastian, R.; Dickinson, L.E.; Fox-Talbot, K.; Reinblatt, M.; Steenbergen, C.; Harmon, J.W.; Gerecht, S. Dextran hydrogel scaffolds enhance angiogenic responses and promote complete skin regeneration during burn wound healing. Proc. Natl. Acad. Sci. USA 2011, 108, 20976–20981. [Google Scholar] [CrossRef] [PubMed]
- Van Tomme, S.R.; Hennink, W.E. Biodegradable dextran hydrogels for protein delivery applications. Exp. Rev. Med. Devices 2007, 4, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Van den Brom, C.R.; Anac, I.; Roskamp, R.F.; Retsch, M.; Jonas, U.; Menges, B.; Preece, J.A. The swelling behaviour of thermoresponsive hydrogel/silica nanoparticle composites. J. Mater. Chem. 2010, 20, 4827–4839. [Google Scholar] [CrossRef]
- Eman, R.M.; Hoorntje, E.T.; Oner, F.C.; Kruyt, M.C.; Dhert, W.J.A.; Alblas, J. Cxcl12/stromal-cell-derived factor-1 effectively replaces endothelial progenitor cells to induce vascularized ectopic bone. Stem Cells Dev. 2014, 23, 2950–2958. [Google Scholar] [CrossRef] [PubMed]
- Eman, R.M.; Oner, F.C.; Kruyt, M.C.; Dhert, W.J.A.; Alblas, J. Stromal cell-derived factor-1 stimulates cell recruitment, vascularization and osteogenic differentiation. Tissue Eng. A 2014, 20, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Krieger, J.R.; Ogle, M.E.; McFaline-Figueroa, J.; Segar, C.E.; Temenoff, J.S.; Botchwey, E.A. Spatially localized recruitment of anti-inflammatory monocytes by SDF-1 alpha-releasing hydrogels enhances microvascular network remodeling. Biomaterials 2016, 77, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Tabata, Y. Controlled release of stromal-cell-derived factor-1 from gelatin hydrogels enhances angiogenesis. J. Biomater. Sci. Polym. Ed. 2010, 21, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Mantripragada, V.P.; Jayasuriya, A.C. Bone regeneration using injectable BMP-7 loaded chitosan microparticles in rat femoral defect. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 63, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, N.; Miron, R.; Shi, B.; Cheng, X. Delivery of PDGF-b and BMP-7 by mesoporous bioglass/silk fibrin scaffolds for the repair of osteoporotic defects. Biomaterials 2012, 33, 6698–6708. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Mao, J.J. Engineering dextran-based scaffolds for drug delivery and tissue repair. Nanomedicine 2012, 7, 1771–1784. [Google Scholar] [CrossRef] [PubMed]
- Lieder, R.; Petersen, P.H.; Sigurjonsson, O.E. Endotoxins-the invisible companion in biomaterials research. Tissue Eng. B Rev. 2013, 19, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Young, N.S.; Levin, J.; Prendergast, R.A. An invertebrate coagulation system activated by endotoxin: Evidence for enzymatic mediation. J. Clin. Investig. 1972, 51, 1790–1797. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.S.; Fernandes, M.H. Rodent models in bone-related research: The relevance of calvarial defects in the assessment of bone regeneration strategies. Lab. Anim. 2011, 45, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Hertweck, J.; Ritz, U.; Gotz, H.; Schottel, P.C.; Rommens, P.M.; Hofmann, A. Cd34+ cells seeded in collagen scaffolds promote bone formation in a mouse calvarial defect model. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 1505–1516. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.P.; Leonor, I.B.; Smith, B.J.; Dias, I.R.; Reis, R.L.; Gimble, J.M.; Gomes, M.E. Undifferentiated human adipose-derived stromal/stem cells loaded onto wet-spun starch-polycaprolactone scaffolds enhance bone regeneration: Nude mice calvarial defect in vivo study. J. Biomed. Mater. Res. A 2014, 102, 3102–3111. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Toraldo, G.; Li, A.; Yang, X.; Zhang, H.; Qian, W.P.; Weitzmann, M.N. B cells and t cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood 2007, 109, 3839–3848. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Woo, K.M.; Ko, S.H.; Lee, Y.J.; Park, S.J.; Kim, H.M.; Kwon, B.S. Osteoclastogenesis is enhanced by activated b cells but suppressed by activated cd8(+) t cells. Eur. J. Immunol. 2001, 31, 2179–2188. [Google Scholar] [CrossRef]
- Croes, M.; Oner, F.C.; van Neerven, D.; Sabir, E.; Kruyt, M.C.; Blokhuis, T.J.; Dhert, W.J.; Alblas, J. Proinflammatory t cells and il-17 stimulate osteoblast differentiation. Bone 2016, 84, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Hollinger, J.O.; Kleinschmidt, J.C. The critical size defect as an experimental model to test bone repair materials. J. Craniofac. Surg. 1990, 1, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Liebschner, M.A. Biomechanical considerations of animal models used in tissue engineering of bone. Biomaterials 2004, 25, 1697–1714. [Google Scholar] [CrossRef]
- Krishnan, L.; Priddy, L.B.; Esancy, C.; Klosterhoff, B.S.; Stevens, H.Y.; Tran, L.; Guldberg, R.E. Delivery vehicle effects on bone regeneration and heterotopic ossification induced by high dose bmp-2. Acta Biomater. 2017, 49, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Brannan, P.S.; Gaston, R.G.; Loeffler, B.J.; Lewis, D.R. Complications with the use of bmp-2 in scaphoid nonunion surgery. J. Hand Surg. 2016, 41, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Papanagiotou, M.; Dailiana, Z.H.; Karachalios, T.; Varitimidis, S.; Hantes, M.; Dimakopoulos, G.; Vlychou, M.; Malizos, K.N. Heterotopic ossification after the use of recombinant human bone morphogenetic protein-7. World J. Orthop. 2017, 8, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Maia, F.R.; Lourenco, A.H.; Granja, P.L.; Goncalves, R.M.; Barrias, C.C. Effect of cell density on mesenchymal stem cells aggregation in rgd-alginate 3d matrices under osteoinductive conditions. Macromol. Biosci. 2014, 14, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Kusuma, S.; Gerecht, S. Development of a biodegradable, temperature-sensitive dextran-based polymer as a cell-detaching substrate. Macromol. Biosci. 2012, 12, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Randi, A.M.; Laffan, M.A. Von willebrand factor and angiogenesis: Basic and applied issues. J. Thromb. Haemost. 2017, 15, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wang, J.; He, G.; Qu, F.; Zheng, M. Mobilization of endothelial progenitor cell in patients with acute ischemic stroke. Neurol. Sci. 2018, 39, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.; Ritz, U.; Verrier, S.; Eglin, D.; Alini, M.; Fuchs, S.; Kirkpatrick, C.J.; Rommens, P.M. The effect of human osteoblasts on proliferation and neo-vessel formation of human umbilical vein endothelial cells in a long-term 3d co-culture on polyurethane scaffolds. Biomaterials 2008, 29, 4217–4226. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.; Ritz, U.; Hessmann, M.H.; Schmid, C.; Tresch, A.; Rompe, J.D.; Meurer, A.; Rommens, P.M. Cell viability, osteoblast differentiation and gene expression are altered in human osteoblasts from hypertrophic fracture non-unions. Bone 2008, 42, 894–906. [Google Scholar] [CrossRef] [PubMed]
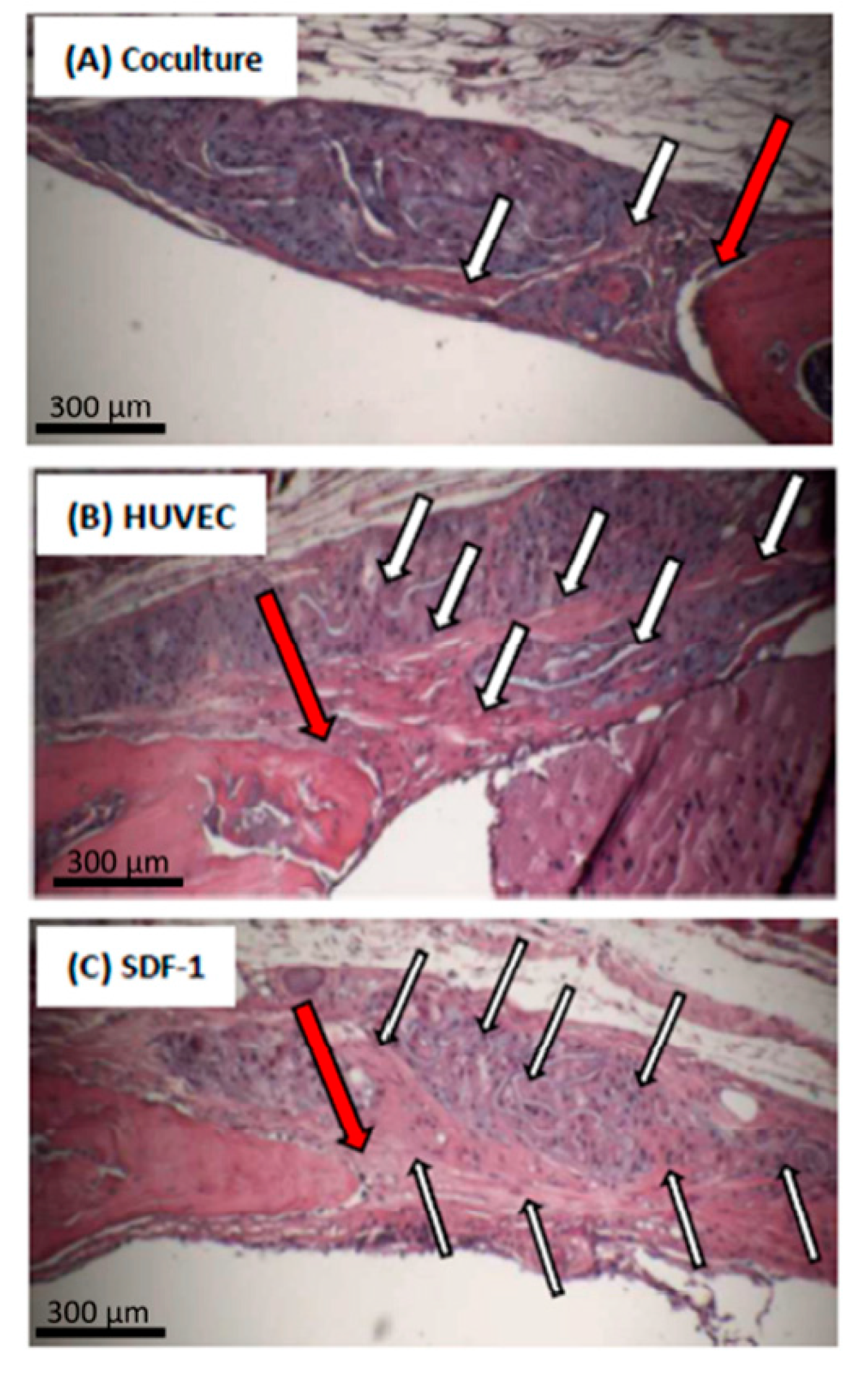
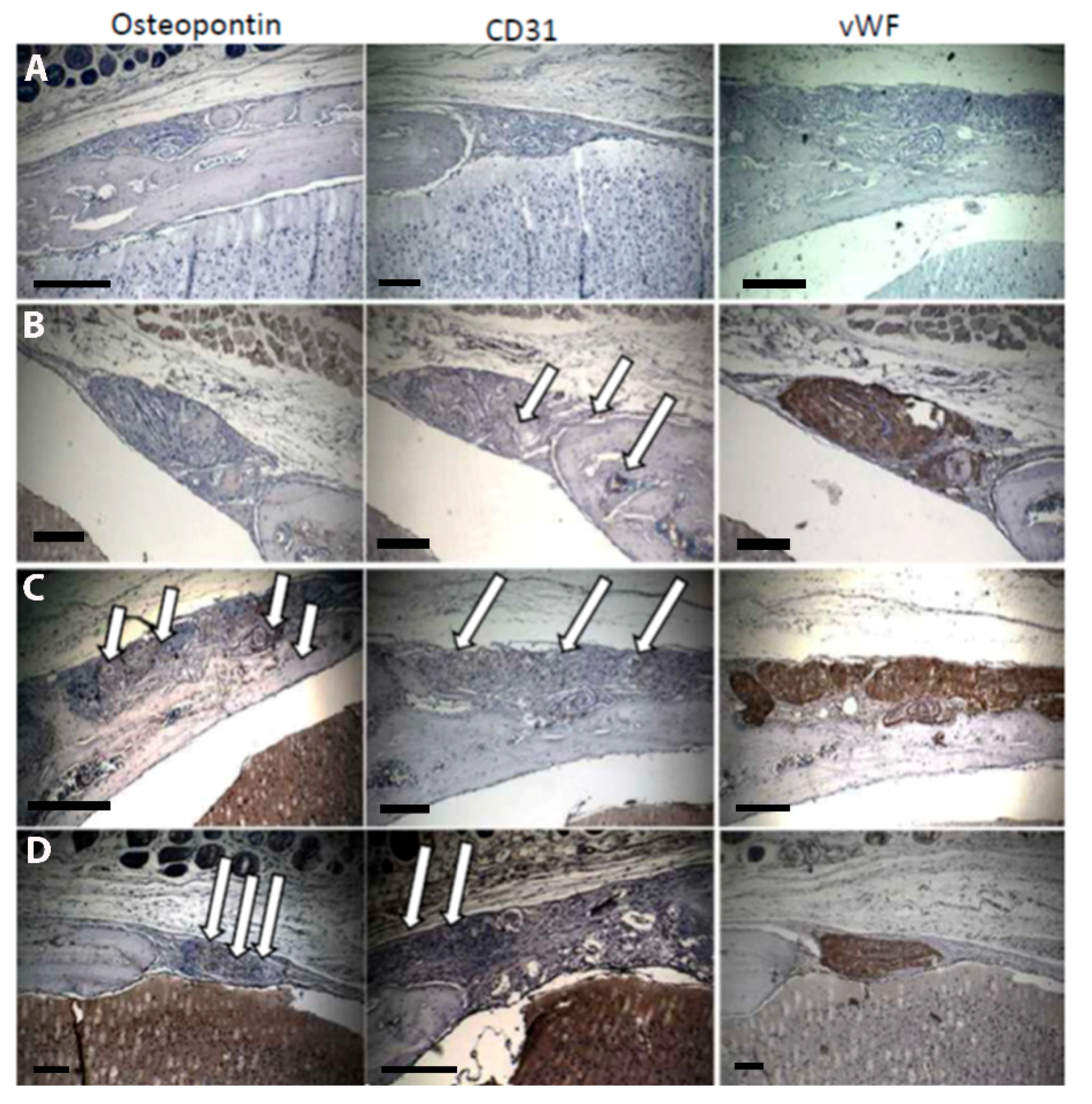
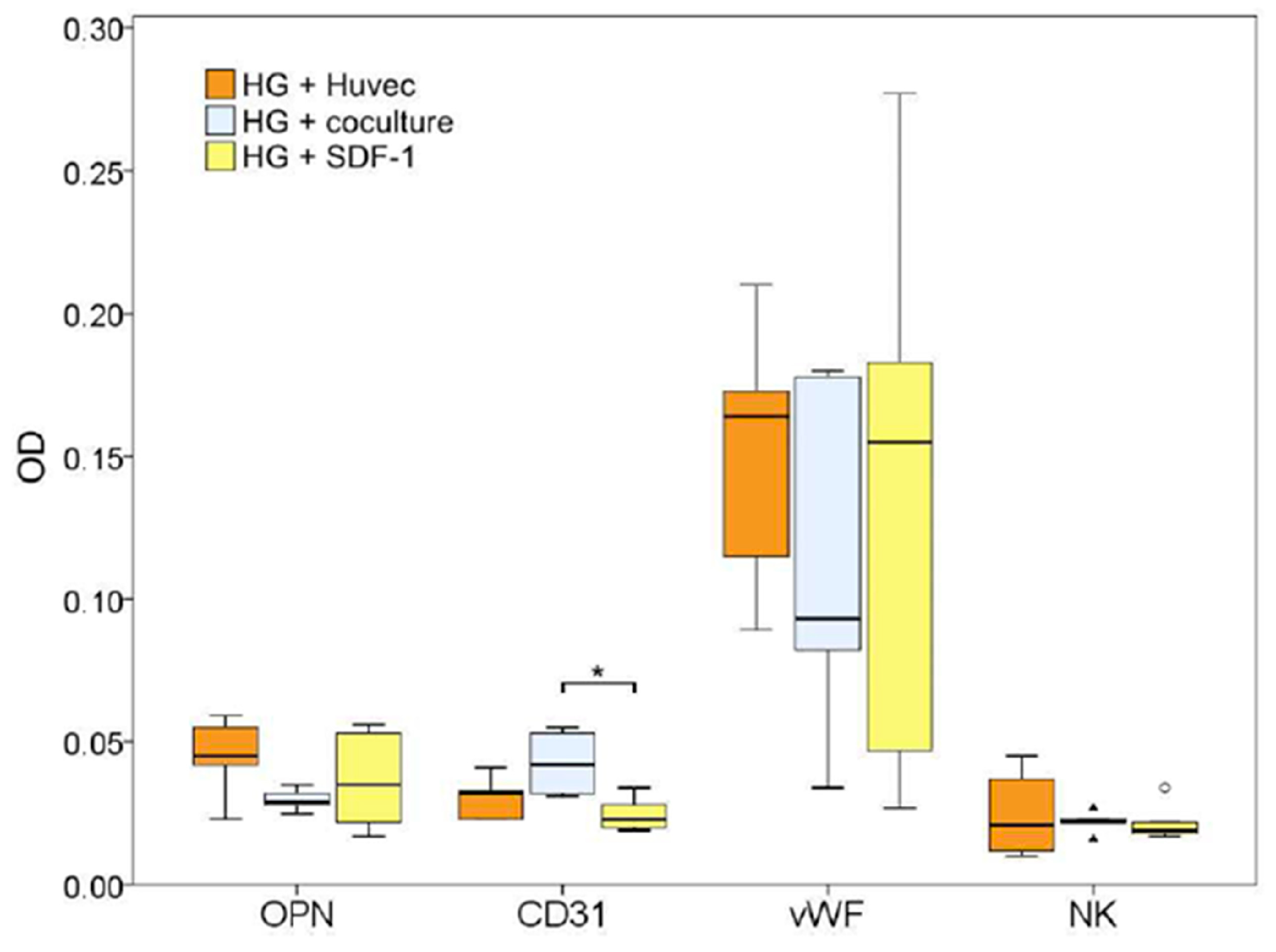
| Sample for Endotoxin Testing | Endotoxin Concentration (EU/mL) |
|---|---|
| Dextran-based hydrogel “non-optimized procedure” | 2.62 |
| H2O before filter exchange | 0.71 |
| H2O after filter exchange | 0.37 |
| Dextran-based hydrogel after filter exchange and usage of newly purchased chemicals with optimized handling procedure | 0.45 |
| Group Name | Number Mice/Defects |
|---|---|
| Control—pure dextran-based hydrogels | 8/16 |
| HUVEC—dextran-based hydrogels with HUVEC monoculture | 8/16 |
| Co-culture—dextran-based hydrogels with co-culture of HUVEC and hOB | 7/14 |
| SDF-1—dextran-based hydrogels with immobilized SDF-1 | 8/16 |
| BMP-7—dextran-based hydrogels with immobilized BMP-7 | 8/16 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ritz, U.; Eberhardt, M.; Klein, A.; Frank, P.; Götz, H.; Hofmann, A.; Rommens, P.M.; Jonas, U. Photocrosslinked Dextran-Based Hydrogels as Carrier System for the Cells and Cytokines Induce Bone Regeneration in Critical Size Defects in Mice. Gels 2018, 4, 63. https://doi.org/10.3390/gels4030063
Ritz U, Eberhardt M, Klein A, Frank P, Götz H, Hofmann A, Rommens PM, Jonas U. Photocrosslinked Dextran-Based Hydrogels as Carrier System for the Cells and Cytokines Induce Bone Regeneration in Critical Size Defects in Mice. Gels. 2018; 4(3):63. https://doi.org/10.3390/gels4030063
Chicago/Turabian StyleRitz, Ulrike, Marc Eberhardt, Anja Klein, Petra Frank, Hermann Götz, Alexander Hofmann, Pol Maria Rommens, and Ulrich Jonas. 2018. "Photocrosslinked Dextran-Based Hydrogels as Carrier System for the Cells and Cytokines Induce Bone Regeneration in Critical Size Defects in Mice" Gels 4, no. 3: 63. https://doi.org/10.3390/gels4030063
APA StyleRitz, U., Eberhardt, M., Klein, A., Frank, P., Götz, H., Hofmann, A., Rommens, P. M., & Jonas, U. (2018). Photocrosslinked Dextran-Based Hydrogels as Carrier System for the Cells and Cytokines Induce Bone Regeneration in Critical Size Defects in Mice. Gels, 4(3), 63. https://doi.org/10.3390/gels4030063