Functional Stimuli-Responsive Gels: Hydrogels and Microgels
Abstract
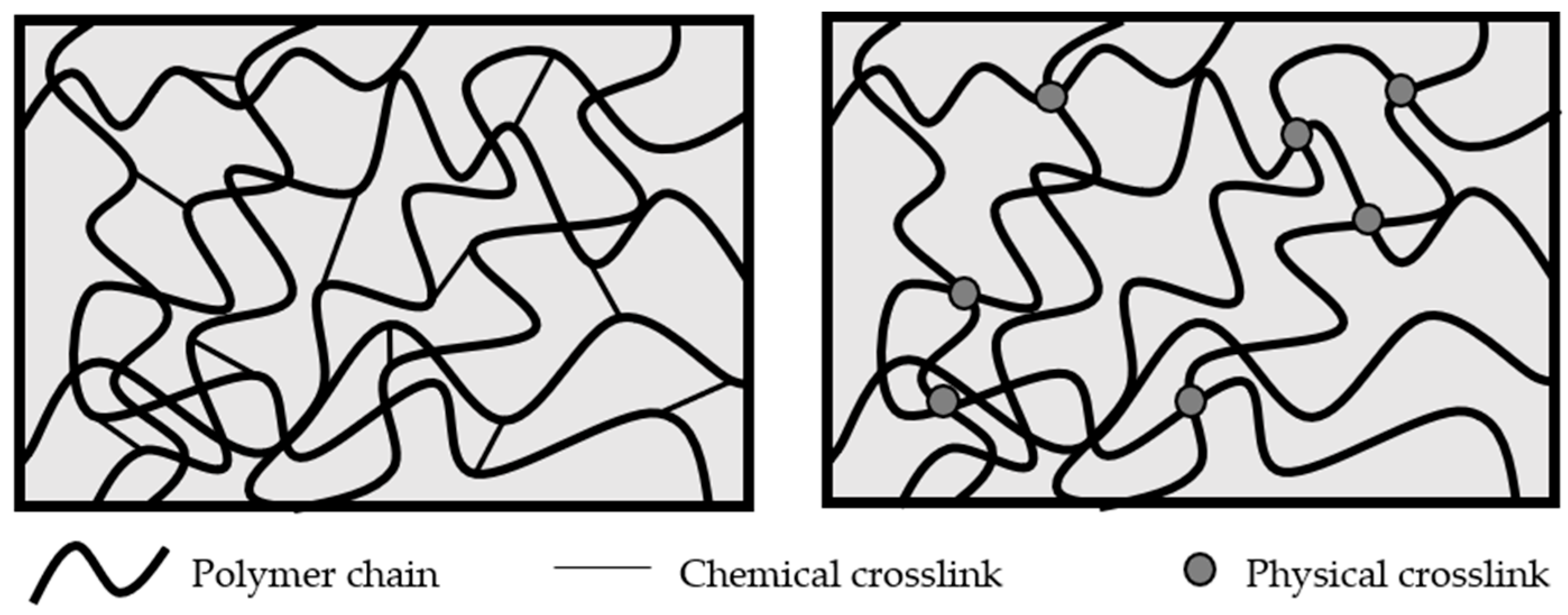
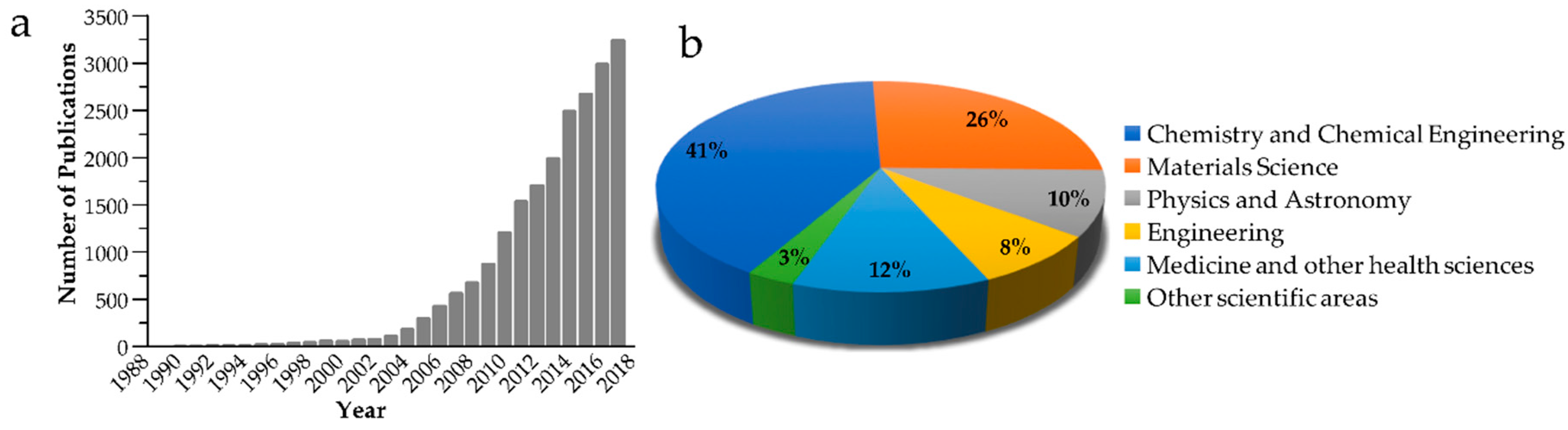
1. Introduction
2. Stimuli-Responsive Functional Hydrogels
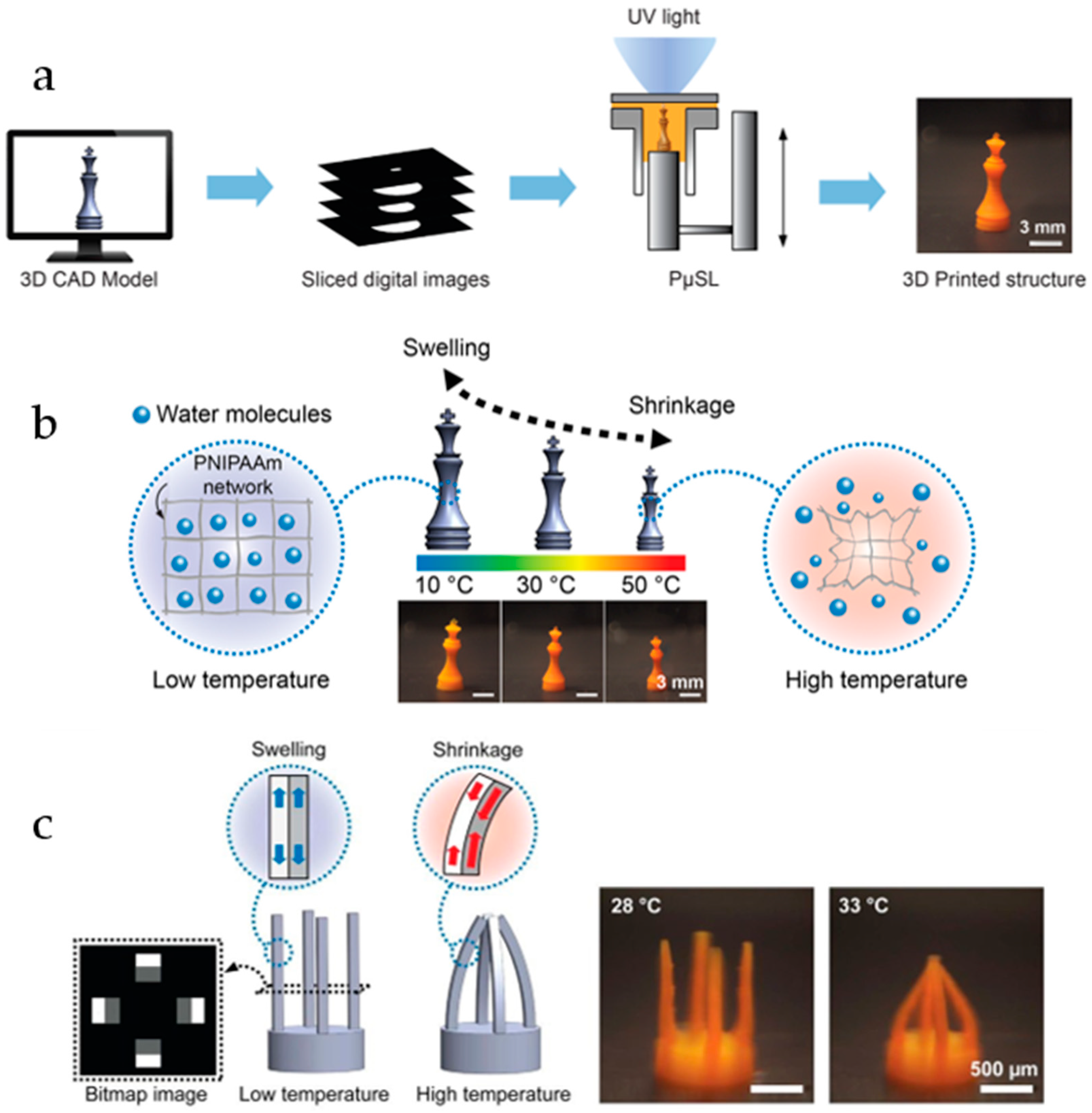
2.1. Thermoresponsive Hydrogels
2.2. pH-Responsive Hydrogels
2.3. Light-Responsive Hydrogels
2.4. Redox-Responsive Hydrogels
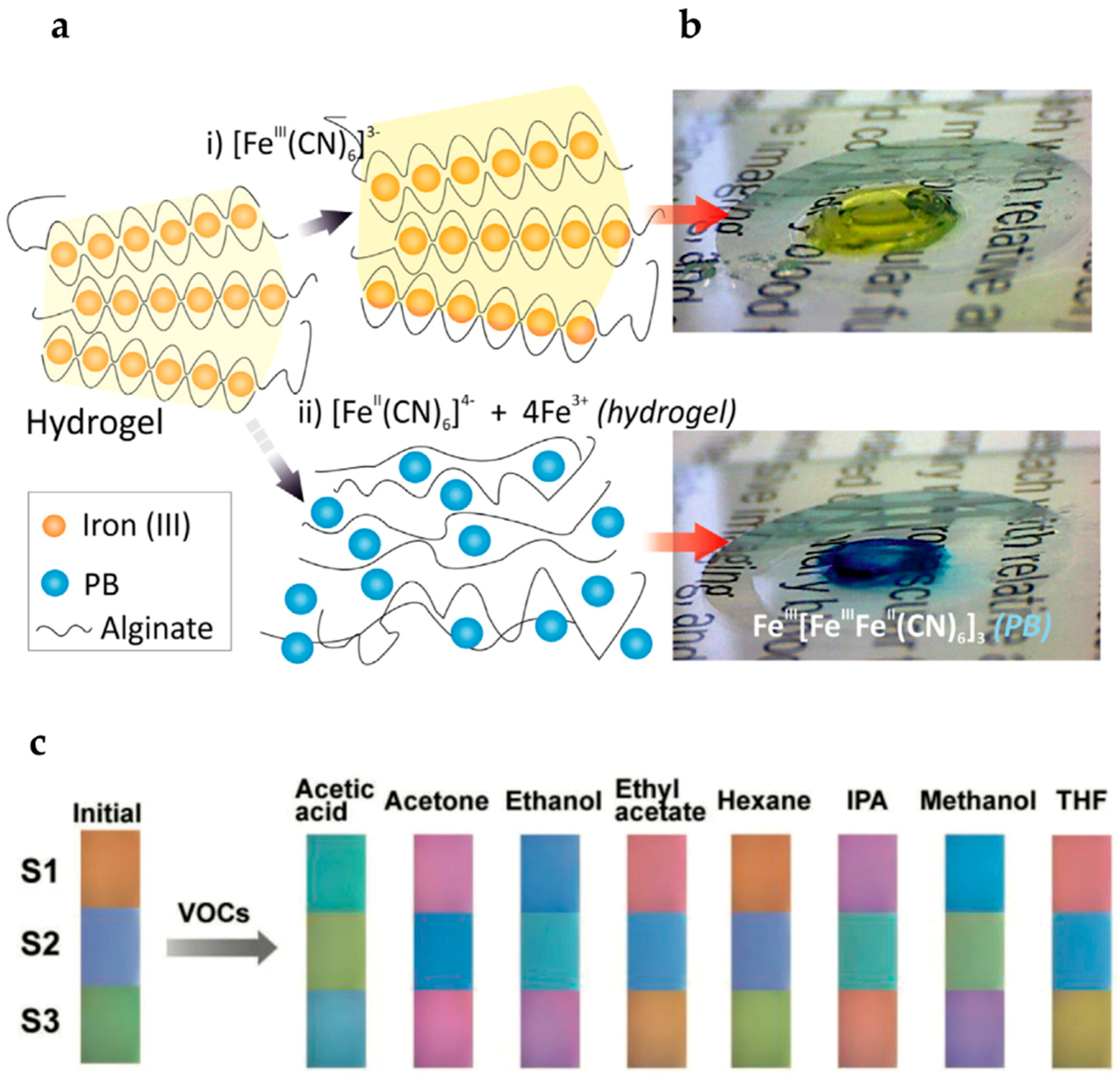
2.5. Analyte-Responsive Hydrogels
2.6. Hydrogels for 3D Printing
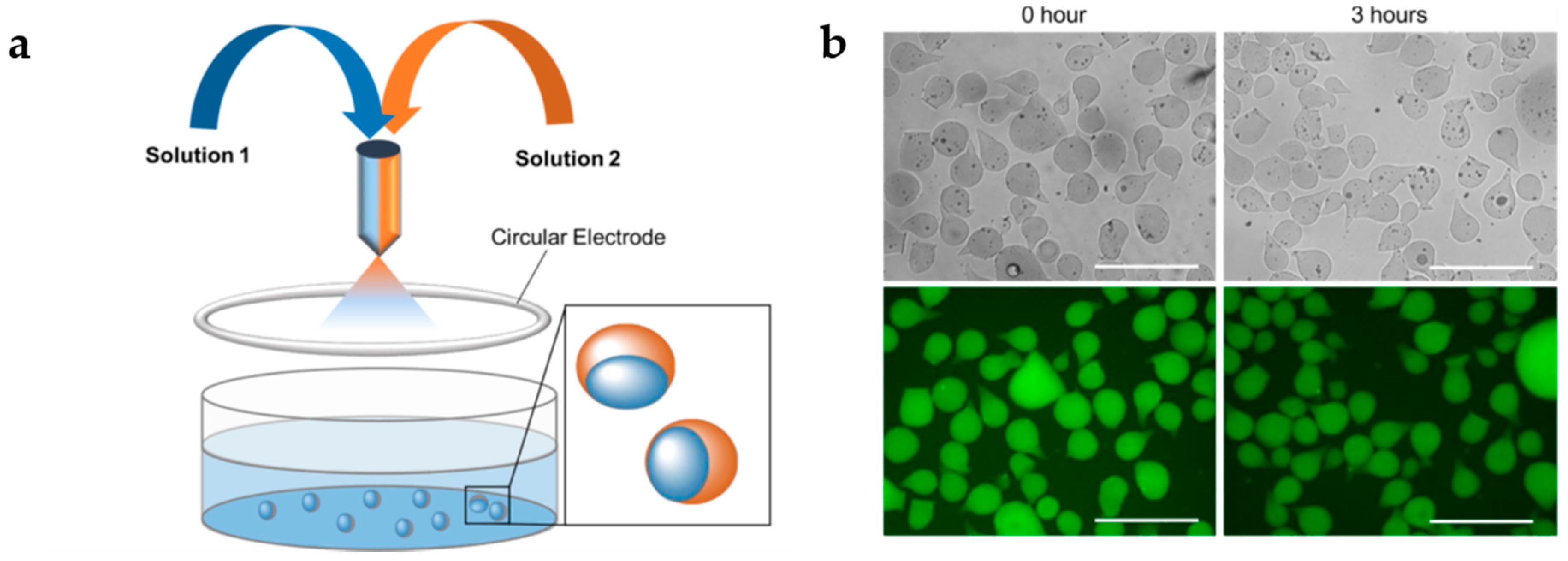
3. Multi-Stimuli-Responsive Hybrid Microgels
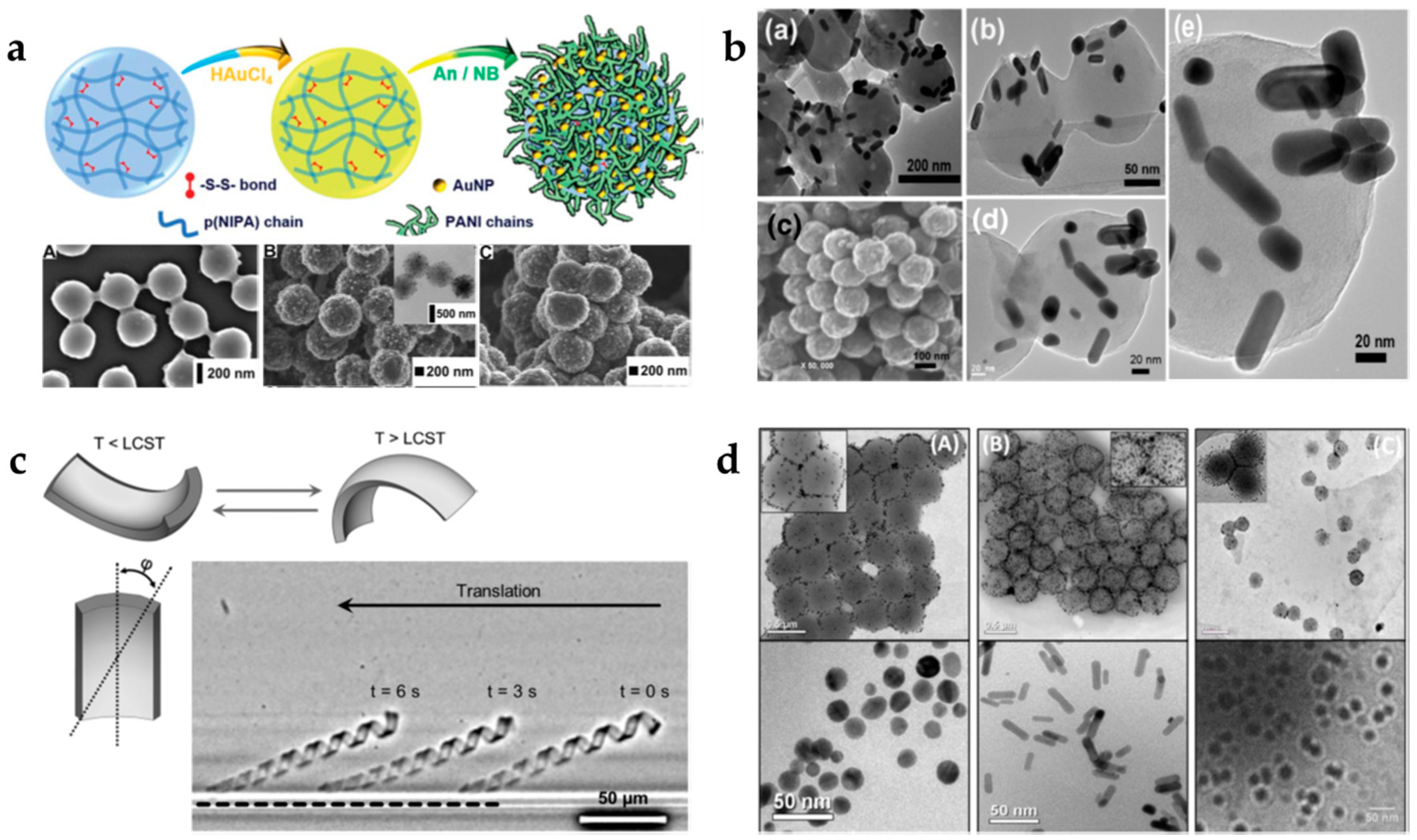
3.1. Metallic Nanoparticles
3.1.1. Gold Nanoparticles
3.1.2. Silver Nanoparticles
3.2. Magnetic Nanoparticles
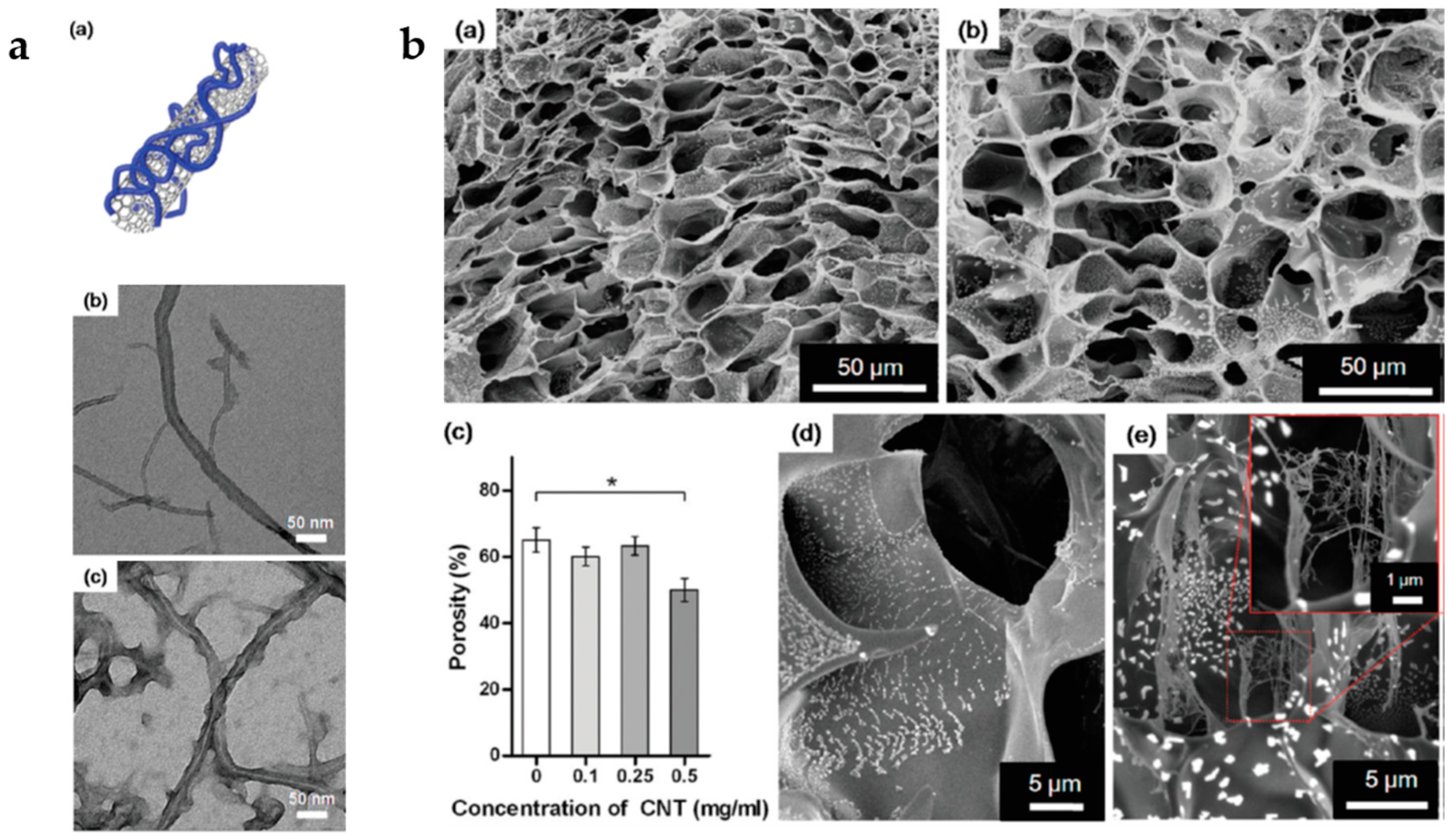
3.3. Carbon-Based Materials
3.4. Other Inorganic Materials
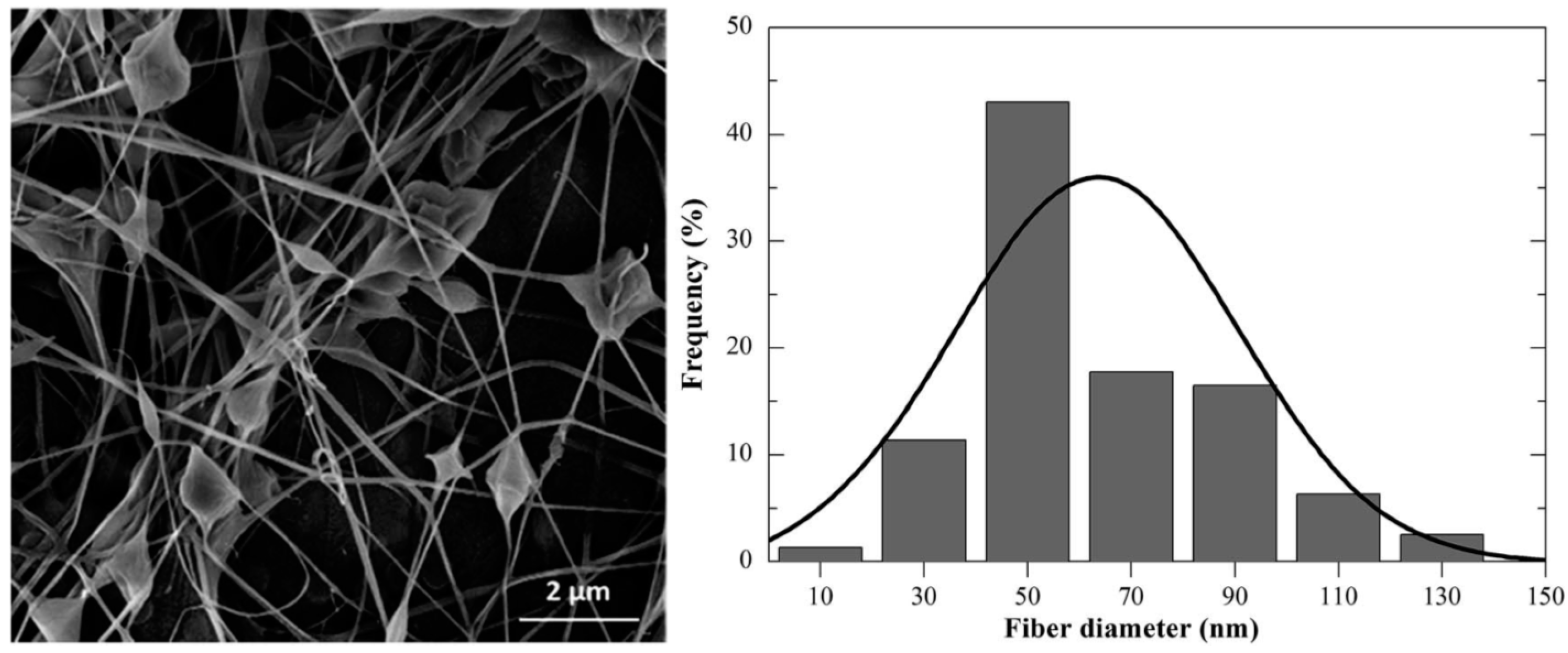
3.5. Multifunctional Hybrid Fibrillary Gels
4. New Generation of Functional Stimuli-Responsive Polymer Hydrogels: Self-Shape (Shape-Memory) and Self-Healable Systems
4.1. Stimuli-Responsive Shape Memory Hydrogels
4.2. Self-Healing Hydrogels
4.3. Stimuli-Responsive Self-Healing Microgels
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Sangeetha, N.M.; Maitra, U. Supramolecular gels: Functions and uses. Chem. Soc. Rev. 2005, 34, 821–836. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.K.; Kasi, R.M.; Kim, S.C.; Sharma, N.; Zhou, Y.X. Stimuli-responsive polymer gels. Soft Matter 2008, 4, 1151–1157. [Google Scholar] [CrossRef]
- Hoffman, A.S.; Stayton, P.S.; Bulmus, V.; Chen, G.H.; Chen, J.P.; Cheung, C.; Chilkoti, A.; Ding, Z.L.; Dong, L.C.; Fong, R.; et al. Really smart bioconjugates of smart polymers and receptor proteins. J. Biomed. Mater. Res. 2000, 52, 577–586. [Google Scholar] [CrossRef]
- Stuart, M.A.; Huck, W.T.; Genzer, J.; Muller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Buwalda, S.J.; Boere, K.W.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control Release 2014, 190, 254–273. [Google Scholar] [CrossRef] [PubMed]
- Bauri, K.; Nandi, M.; De, P. Amino acid-derived stimuli-responsive polymers and their applications. Polym. Chem. 2018, 9, 1257–1287. [Google Scholar] [CrossRef]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, L.; Díaz, A.; Puiggalí, J. Hydrogels for biomedical applications: Cellulose, chitosan, and protein/peptide derivatives. Gels 2017, 3, 27. [Google Scholar] [CrossRef]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of hydrogels and aerogels containing nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Kahn, J.S.; Hu, Y.; Willner, I. Stimuli-responsive DNA-based hydrogels: From basic principles to applications. Acc. Chem. Res. 2017, 50, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Haq, M.A.; Su, Y.; Wang, D. Mechanical properties of PNIPAM based hydrogels: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, A.; Seremeta, K. Polymeric hydrogels as technology platform for drug delivery applications. Gels 2017, 3, 25. [Google Scholar] [CrossRef]
- Li, D.; van Nostrum, C.F.; Mastrobattista, E.; Vermonden, T.; Hennink, W.E. Nanogels for intracellular delivery of biotherapeutics. J. Control Release 2017, 259, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Knipe, J.M.; Peppas, N.A. Multi-responsive hydrogels for drug delivery and tissue engineering applications. Regen. Biomater. 2014, 1, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Lanzalaco, S.; Armelin, E. Poly(N-isopropylacrylamide) and copolymers: A review on recent progresses in biomedical applications. Gels 2017, 3, 36. [Google Scholar] [CrossRef]
- Sharifzadeh, G.; Hosseinkhani, H. Biomolecule-responsive hydrogels in medicine. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Van Blarcom, D.S. Hydrogel-based biosensors and sensing devices for drug delivery. J. Control Release 2016, 240, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Culver, H.R.; Clegg, J.R.; Peppas, N.A. Analyte-responsive hydrogels: Intelligent materials for biosensing and drug delivery. Acc. Chem. Res. 2017, 50, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Battista, E.; Causa, F.; Netti, P. Bioengineering microgels and hydrogel microparticles for sensing biomolecular targets. Gels 2017, 3, 20. [Google Scholar] [CrossRef]
- Li, X.; Gao, Y.; Serpe, M. Stimuli-responsive assemblies for sensing applications. Gels 2016, 2, 8. [Google Scholar] [CrossRef]
- Dhanya, S.; Bahadur, D.; Kundu, G.C.; Srivastava, R. Maleic acid incorporated poly-(N-isopropylacrylamide) polymer nanogels for dual-responsive delivery of doxorubicin hydrochloride. Eur. Polym. J. 2013, 49, 22–32. [Google Scholar] [CrossRef]
- Patenaude, M.; Hoare, T. Injectable, degradable thermoresponsive poly(N-isopropylacrylamide) hydrogels. ACS Macro Lett. 2012, 1, 409–413. [Google Scholar] [CrossRef]
- Tang, S.; Floy, M.; Bhandari, R.; Sunkara, M.; Morris, A.J.; Dziubla, T.D.; Hilt, J.Z. Synthesis and characterization of thermoresponsive hydrogels based on N-isopropylacrylamide crosslinked with 4,4′-dihydroxybiphenyl diacrylate. ACS Omega 2017, 2, 8723–8729. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, K.; Lee, S.J. Nature-inspired thermo-responsive multifunctional membrane adaptively hybridized with PNIPAM and PPY. NPG Asia Mater. 2017, 9, e445. [Google Scholar] [CrossRef]
- D’Eramo, L.; Chollet, B.; Leman, M.; Martwong, E.; Li, M.X.; Geisler, H.; Dupire, J.; Kerdraon, M.; Vergne, C.; Monti, F.; et al. Microfluidic actuators based on temperature-responsive hydrogels. Microsyst. Nanoeng. 2018, 4, 17069. [Google Scholar] [CrossRef]
- Han, D.; Lu, Z.; Chester, S.A.; Lee, H. Micro 3D printing of a temperature-responsive hydrogel using projection micro-stereolithography. Sci. Rep. 2018, 8, 1963. [Google Scholar] [CrossRef] [PubMed]
- Luckanagul, J.A.; Pitakchatwong, C.; Ratnatilaka Na Bhuket, P.; Muangnoi, C.; Rojsitthisak, P.; Chirachanchai, S.; Wang, Q.; Rojsitthisak, P. Chitosan-based polymer hybrids for thermo-responsive nanogel delivery of curcumin. Carbohydr. Polym. 2018, 181, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Tan, J.J.; Lim, S.; Soh, S.L. Rupturing cancer cells by the expansion of functionalized stimuli-responsive hydrogels. NPG Asia Mater. 2018, 10, e465. [Google Scholar] [CrossRef]
- Kouwer, P.H.; Koepf, M.; Le Sage, V.A.; Jaspers, M.; van Buul, A.M.; Eksteen-Akeroyd, Z.H.; Woltinge, T.; Schwartz, E.; Kitto, H.J.; Hoogenboom, R.; et al. Responsive biomimetic networks from polyisocyanopeptide hydrogels. Nature 2013, 493, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Zimoch, J.; Padial, J.S.; Klar, A.S.; Vallmajo-Martin, Q.; Meuli, M.; Biedermann, T.; Wilson, C.J.; Rowan, A.; Reichmann, E. Polyisocyanopeptide hydrogels: A novel thermo-responsive hydrogel supporting pre-vascularization and the development of organotypic structures. Acta Biomater. 2018, 70, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Gil, E.S.; Hudson, S.M. Stimuli-reponsive polymers and their bioconjugates. Prog. Polym. Sci. 2004, 29, 1173–1222. [Google Scholar] [CrossRef]
- Puranik, A.S.; Pao, L.P.; White, V.M.; Peppas, N.A. Synthesis and characterization of pH-responsive nanoscale hydrogels for oral delivery of hydrophobic therapeutics. Eur. J. Pharm. Biopharm. 2016, 108, 196–213. [Google Scholar] [CrossRef] [PubMed]
- Puranik, A.S.; Pao, L.P.; White, V.M.; Peppas, N.A. In vitro evaluation of pH-responsive nanoscale hydrogels for the oral delivery of hydrophobic therapeutics. Ind. Eng. Chem. Res. 2016, 55, 10576–10590. [Google Scholar] [CrossRef]
- Jin, C.; Song, W.J.; Liu, T.; Xin, J.N.; Hiscox, W.C.; Zhang, J.W.; Liu, G.F.; Kong, Z.W. Temperature and pH responsive hydrogels using methacrylated lignosulfonate cross-linker: Synthesis, characterization, and properties. ACS Sustain. Chem. Eng. 2018, 6, 1763–1771. [Google Scholar] [CrossRef]
- Unger, K.; Salzmann, P.; Masciullo, C.; Cecchini, M.; Koller, G.; Coclite, A.M. Novel light-responsive biocompatible hydrogels produced by initiated chemical vapor deposition. ACS Appl. Mater. Interfaces 2017, 9, 17408–17416. [Google Scholar] [CrossRef] [PubMed]
- Fomina, N.; Sankaranarayanan, J.; Almutairi, A. Photochemical mechanisms of light-triggered release from nanocarriers. Adv. Drug Deliv. Rev. 2012, 64, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Tomatsu, I.; Peng, K.; Kros, A. Photoresponsive hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2011, 63, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Mandl, G.A.; Rojas-Gutierrez, P.A.; Capobianco, J.A. A NIR-responsive azobenzene-based supramolecular hydrogel using upconverting nanoparticles. Chem. Commun. 2018, 54, 5847–5850. [Google Scholar] [CrossRef] [PubMed]
- Weis, P.; Wu, S. Light-switchable azobenzene-containing macromolecules: From UV to near infrared. Macromol. Rapid Commun. 2018, 39, 1700220. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Xiao, C.; Hu, C. Facile preparation and dual responsive behaviors of starch-based hydrogel containing azo and carboxylic groups. Int. J. Biol. Macromol. 2018, 115, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.K.; Anderson, H.E.; Lamas, J.; Barret, S.; Cantu, T.; Zauscher, S.; Brittain, W.J.; Betancourt, T. Enhanced release of molecules upon ultraviolet (UV) light irradiation from photoresponsive hydrogels prepared from bifunctional azobenzene and four-arm poly(ethylene glycol). ACS Appl. Mater. Interfaces 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Bonasera, A.; Nochel, U.; Behl, M.; Bleger, D. Reversible modulation of elasticity in fluoroazobenzene-containing hydrogels using green and blue light. Macromol. Rapid Commun. 2018, 39, 1700527. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Takashima, Y.; Yamaguchi, H. Cyclodextrin-based supramolecular polymers. Chem. Soc. Rev. 2009, 38, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Tamesue, S.; Takashima, Y.; Yamaguchi, H.; Shinkai, S.; Harada, A. Photoswitchable supramolecular hydrogels formed by cyclodextrins and azobenzene polymers. Angew. Chem. 2010, 122, 7623–7626. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Yi, S.; Chen, X. Visible-light/temperature dual-responsive hydrogel constructed by α-cyclodextrin and an azobenzene linked surfactant. Soft Matter 2017, 13, 6490–6498. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Tanaka, T. Phase-transition in polymer gels induced by visible-light. Nature 1990, 346, 345–347. [Google Scholar] [CrossRef]
- Suzuki, A.; Ishii, T.; Maruyama, Y. Optical switching in polymer gels. J. Appl. Phys. 1996, 80, 131–136. [Google Scholar] [CrossRef]
- Xu, Y.; Ghag, O.; Reimann, M.; Sitterle, P.; Chatterjee, P.; Nofen, E.; Yu, H.; Jiang, H.; Dai, L.L. Development of visible-light responsive and mechanically enhanced “smart” UCST interpenetrating network hydrogels. Soft Matter 2017, 14, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Ter Schiphorst, J.; Saez, J.; Diamond, D.; Benito-Lopez, F.; Schenning, A. Light-responsive polymers for microfluidic applications. Lab Chip 2018, 18, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Greene, A.F.; Danielson, M.K.; Delawder, A.O.; Liles, K.P.; Li, X.S.; Natraj, A.; Wellen, A.; Barnes, J.C. Redox-responsive artificial molecular muscles: Reversible radical-based self-assembly for actuating hydrogels. Chem. Mater. 2017, 29, 9498–9508. [Google Scholar] [CrossRef]
- Stejskal, J. Conducting polymer hydrogels. Chem. Pap. 2017, 71, 269–291. [Google Scholar] [CrossRef]
- Liu, Y.; Tsao, C.Y.; Kim, E.; Tschirhart, T.; Terrell, J.L.; Bentley, W.E.; Payne, G.F. Using a redox modality to connect synthetic biology to electronics: Hydrogel-based chemo-electro signal transduction for molecular communication. Adv. Healthc. Mater. 2017, 6, 1600908. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wang, A.W.; Lyu, F.C.; Lai, G.S.; Yu, J.H.; Lin, C.T.; Liu, Z.; Yu, A.M.; Su, W.T. A solid-state electrochemical sensing platform based on a supramolecular hydrogel. Sens. Actuators B Chem. 2018, 262, 326–333. [Google Scholar] [CrossRef]
- Wojciechowski, J.P.; Martin, A.D.; Thordarson, P. Kinetically controlled lifetimes in redox-responsive transient supramolecular hydrogels. J. Am. Chem. Soc. 2018, 140, 2869–2874. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Voci, S.; Ravaine, V.; Sojic, N. Tuning electrochemiluminescence in multistimuli responsive hydrogel films. J. Phys. Chem. Lett. 2018, 9, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.F.; Gao, Z.J.; Gao, G.H.; Duan, L.J. Mechanically redox-tunable hydrogels reinforced by hydrophobic association and metal ion coordination. Mater. Chem. Phys. 2018, 207, 175–180. [Google Scholar] [CrossRef]
- Tavakoli, J.; Tang, Y.H. Hydrogel based sensors for biomedical applications: An updated review. Polymers 2017, 9, 364. [Google Scholar] [CrossRef]
- Pujol-Vila, F.; Dietvorst, J.; Gall-Mas, L.; Diaz-Gonzalez, M.; Vigues, N.; Mas, J.; Munoz-Berbel, X. Bioelectrochromic hydrogel for fast antibiotic-susceptibility testing. J. Colloid Interface Sci. 2018, 511, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Sun, M.; Bai, R.; Mao, Y.; Qian, X.; Sikka, D.; Zhao, Y.; Qi, H.J.; Suo, Z.; He, X. Bioinspired hydrogel interferometer for adaptive coloration and chemical sensing. Adv. Mater. 2018, 30, e1800468. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Pramanick, S.; Park, D.; Yeo, J.; Lee, J.; Lee, H.; Kim, W.J. Therapeutic-gas-responsive hydrogel. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.P.C.; Canejo, J.P.; Fernandes, S.N.; Echeverria, C.; Almeida, P.L.; Godinho, M.H. Cellulose-based biomimetics and their applications. Adv. Mater. 2018, 30, e1703655. [Google Scholar] [CrossRef] [PubMed]
- Oechsle, A.L.; Lewis, L.; Hamad, W.Y.; Hatzikiriakos, S.G.; MacLachlan, M.J. CO2-switchable cellulose nanocrystal hydrogels. Chem. Mater. 2018, 30, 376–385. [Google Scholar] [CrossRef]
- Noh, K.-G.; Park, S.-Y. Biosensor array of interpenetrating polymer network with photonic film templated from reactive cholesteric liquid crystal and enzyme-immobilized hydrogel polymer. Adv. Funct. Mater. 2018, 28, 1707562. [Google Scholar] [CrossRef]
- Tan, Z.; Parisi, C.; Di Silvio, L.; Dini, D.; Forte, A.E. Cryogenic 3D printing of super soft hydrogels. Sci. Rep. 2017, 7, 16293. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yang, F.; Zhao, H.; Gao, Q.; Xia, B.; Fu, J. Research on the printability of hydrogels in 3D bioprinting. Sci. Rep. 2016, 6, 29977. [Google Scholar] [CrossRef] [PubMed]
- Karis, D.G.; Ono, R.J.; Zhang, M.S.; Vora, A.; Storti, D.; Ganter, M.A.; Nelson, A. Cross-linkable multi-stimuli responsive hydrogel inks for direct-write 3D printing. Polym. Chem. 2017, 8, 4199–4206. [Google Scholar] [CrossRef]
- Zhou, Y.; Layani, M.; Wang, S.C.; Hu, P.; Ke, Y.J.; Magdassi, S.; Long, Y. Fully printed flexible smart hybrid hydrogels. Adv. Funct. Mater. 2018, 28, 1705365. [Google Scholar] [CrossRef]
- Murray, M.J.; Snowden, M.J. The preparation, characterization and applications of colloidal microgels. Adv. Colloid Interface Sci. 1995, 54, 73–91. [Google Scholar] [CrossRef]
- Pelton, R.H.; Chibante, P. Preparation of aqueous latices with N-isopropylacrylamide. Colloids Surf. 1986, 20, 247–256. [Google Scholar] [CrossRef]
- Echeverria, C.; Lόpez, D.; Mijangos, C. UCST responsive microgels of poly(acrylamide−acrylic acid) copolymers: Structure and viscoelastic properties. Macromolecules 2009, 42, 9118–9123. [Google Scholar] [CrossRef]
- Marques, S.C.S.; Soares, P.I.P.; Echeverria, C.; Godinho, M.H.; Borges, J.P. Confinement of thermoresponsive microgels into fibres via colloidal electrospinning: Experimental and statistical analysis. RSC Adv. 2016, 6, 76370–76380. [Google Scholar] [CrossRef]
- Echeverria, C.; Soares, P.; Robalo, A.; Pereira, L.; Novo, C.M.M.; Ferreira, I.; Borges, J.P. One-pot synthesis of dual-stimuli responsive hybrid PNIPAAm-chitosan microgels. Mater. Des. 2015, 86, 745–751. [Google Scholar] [CrossRef]
- Faria, J.; Echeverria, C.; Borges, J.P.; Godinho, M.H.; Soares, P.I.P. Towards the development of multifunctional hybrid fibrillary gels: Production and optimization by colloidal electrospinning. RSC Adv. 2017, 7, 48972–48979. [Google Scholar] [CrossRef]
- Saunders, B.R. On the structure of poly(N-isopropylacrylamide) microgel particles. Langmuir 2004, 20, 3925–3932. [Google Scholar] [CrossRef] [PubMed]
- Saunders, B.R.; Laajam, N.; Daly, E.; Teow, S.; Hu, X.; Stepto, R. Microgels: From responsive polymer colloids to biomaterials. Adv. Colloid Interface Sci. 2009, 147–148, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Karg, M.; Hellweg, T. New “smart” poly(Nipam) microgels and nanoparticle microgel hybrids: Properties and advances in characterisation. Curr. Opin. Colloid Interface Sci. 2009, 14, 438–450. [Google Scholar] [CrossRef]
- Karg, M.; Wellert, S.; Prevost, S.; Schweins, R.; Dewhurst, C.; Liz-Marzan, L.M.; Hellweg, T. Well defined hybrid PNIPAM core-shell microgels: Size variation of the silica nanoparticle core. Colloid Polym. Sci. 2011, 289, 699–709. [Google Scholar] [CrossRef]
- Plamper, F.A.; Richtering, W. Functional microgels and microgel systems. Acc. Chem. Res. 2017, 50, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Pich, A.; Richtering, W. Chemical Design of Responsive Microgels; Springer: Berlin, Germany, 2011; pp. 4–23. [Google Scholar]
- Kratz, K.; Hellweg, T.; Eimer, W. Influence of charge density on the swelling of colloidal poly(N-isopropylacrylamide-co-acrylic acid) microgels. Colloid Surf. A 2000, 170, 137–149. [Google Scholar] [CrossRef]
- Debord, J.D.; Lyon, L.A. Synthesis and characterization of pH-responsive copolymer microgels with tunable volume phase transition temperatures. Langmuir 2003, 19, 7662–7664. [Google Scholar] [CrossRef]
- Culver, H.R.; Sharma, I.; Wechsler, M.E.; Anslyn, E.V.; Peppas, N.A. Charged poly(N-isopropylacrylamide) nanogels for use as differential protein receptors in a turbidimetric sensor array. Analyst 2017, 142, 3183–3193. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.Q.; Chu, B. Synthesis and volume phase transition of poly(methacrylic acid-co-N-isopropylacrylamide) microgel particles in water. J. Phys. Chem. B 1998, 102, 1364–1371. [Google Scholar] [CrossRef]
- Thorne, J.B.; Vine, G.J.; Snowden, M.J. Microgel applications and commercial considerations. Colloid Polym. Sci. 2011, 289, 625–646. [Google Scholar] [CrossRef]
- Naseem, K.; Begum, R.; Wu, W.; Irfan, A.; Farooqi, Z.H. Advancement in multi-functional poly(styrene)-poly(N-isopropylacrylamide) based core–shell microgels and their applications. Polym. Rev. 2018, 58, 288–325. [Google Scholar] [CrossRef]
- Ballauff, M.; Lu, Y. “Smart” nanoparticles: Preparation, characterization and applications. Polymer 2007, 48, 1815–1823. [Google Scholar] [CrossRef]
- Barth, M.; Wiese, M.; Ogieglo, W.; Go, D.; Kuehne, A.J.C.; Wessling, M. Monolayer microgel composite membranes with tunable permeability. J. Membr. Sci. 2018, 555, 473–482. [Google Scholar] [CrossRef]
- Brugnoni, M.; Scotti, A.; Rudov, A.A.; Gelissen, A.P.H.; Caumanns, T.; Radulescu, A.; Eckert, T.; Pich, A.; Potemkin, I.I.; Richtering, W. Swelling of a responsive network within different constraints in multi-thermosensitive microgels. Macromolecules 2018, 51, 2662–2671. [Google Scholar] [CrossRef]
- Zha, L.S.; Zhang, Y.; Yang, W.L.; Fu, S.K. Monodisperse temperature-sensitive microcontainers. Adv. Mater. 2002, 14, 1090. [Google Scholar] [CrossRef]
- Gandhi, A.; Paul, A.; Sen, S.O.; Sen, K.K. Studies on thermoresponsive polymers: Phase behaviour, drug delivery and biomedical applications. Asian J. Pharm. Sci. 2015, 10, 99–107. [Google Scholar] [CrossRef]
- Pelton, R. Temperature-sensitive aqueous microgels. Adv. Colloid Interface Sci. 2000, 85, 1–33. [Google Scholar] [CrossRef]
- Gracia, L.; Snowden, M.J. Preparation, properties and applications of colloidal microgels. In Handbook of Industrial Water Soluble Polymers; Williams, P.A., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Suzuki, D.; Horigome, K.; Kureha, T.; Matsui, S.; Watanabe, T. Polymeric hydrogel microspheres: Design, synthesis, characterization, assembly and applications. Polym. J. 2017, 49, 695–702. [Google Scholar] [CrossRef]
- Agrawal, G.; Agrawal, R. Stimuli-responsive microgels and microgel-based systems: Advances in the exploitation of microgel colloidal properties and their interfacial activity. Polymers 2018, 10, 418. [Google Scholar] [CrossRef]
- Soares, P.I.P.; Echeverria, C.; Baptista, A.C.; João, C.F.C.; Fernandes, S.N.; Almeida, A.P.C.; Silva, J.C.; Godinho, M.H.; Borges, J.P. 4-hybrid polysaccharide-based systems for biomedical applications. In Hybrid Polymer Composite Materials; Woodhead Publishing: Sawston, UK, 2017; pp. 107–149. [Google Scholar]
- Karg, M. Multifunctional inorganic/organic hybrid microgels. Colloid Polym. Sci. 2012, 290, 673–688. [Google Scholar] [CrossRef]
- Edwards, P.P.; Thomas, J.M. Gold in a metallic divided state—From faraday to present-day nanoscience. Angew. Chem. Int. Ed. 2007, 46, 5480–5486. [Google Scholar] [CrossRef] [PubMed]
- Haes, A.J.; Stuart, D.A.; Nie, S.M.; Van Duyne, R.P. Using solution-phase nanoparticles, surface-confined nanoparticle arrays and single nanoparticles as biological sensing platforms. J. Fluoresc. 2004, 14, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.W. Nanoelectrochemistry: Metal nanoparticles, nanoelectrodes, and nanopores. Chem. Rev. 2008, 108, 2688–2720. [Google Scholar] [CrossRef] [PubMed]
- Vivero-Escoto, J.L.; Huang, Y.T. Inorganic-organic hybrid nanomaterials for therapeutic and diagnostic imaging applications. Int. J. Mol. Sci. 2011, 12, 3888–3927. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Zhang, L.; Wang, T.; Wang, Q.M.; Gao, Y.; Wang, N. Poly(N-isopropylacrylamide)-Au hybrid microgels: Synthesis, characterization, thermally tunable optical and catalytic properties. Soft Matter 2013, 9, 10966–10970. [Google Scholar] [CrossRef]
- Xiao, C.F.; Wu, Q.S.; Chang, A.P.; Peng, Y.H.; Xu, W.T.; Wu, W.T. Responsive au@polymer hybrid microgels for the simultaneous modulation and monitoring of au-catalyzed chemical reaction. J. Mater. Chem. A 2014, 2, 9514–9523. [Google Scholar] [CrossRef]
- Rehman, S.U.; Khan, A.R.; Shah, A.; Badshah, A.; Siddiq, M. Preparation and characterization of poly(N-isoproylacrylamide-co-dimethylaminoethyl methacrylate) microgels and their composites of gold nanoparticles. Colloid Surf. A 2017, 520, 826–833. [Google Scholar] [CrossRef]
- Chen, L.Y.; Ou, C.M.; Chen, W.Y.; Huang, C.C.; Chang, H.T. Synthesis of photoluminescent Au ND-PNIPAM hybrid microgel for the detection of Hg2+. ACS Appl. Mater. Interfaces 2013, 5, 4383–4388. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.C.; Ding, Y.; Wu, T.; Lv, L.Y.; Yan, Z.C. A turn-on fluorescent probe for Hg2+ detection by using gold nanoparticle-based hybrid microgels. Sens. Actuators B Chem. 2016, 228, 767–773. [Google Scholar] [CrossRef]
- Mackiewicz, M.; Karbarz, M.; Romanski, J.; Stojek, Z. An environmentally sensitive three-component hybrid microgel. RSC Adv. 2016, 6, 83493–83500. [Google Scholar] [CrossRef]
- Khan, A.; Alhoshan, M. Preparation and characterization of pH-responsive and thermoresponsive hybrid microgel particles with gold nanorods. J. Polym. Sci. A 2013, 51, 39–46. [Google Scholar] [CrossRef]
- Mourran, A.; Zhang, H.; Vinokur, R.; Moller, M. Soft microrobots employing nonequilibrium actuation via plasmonic heating. Adv. Mater. 2017, 29, 1604825. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, S.S.; Bhol, P.; Chakkarambath, A.; Mohanta, J.; Samantaray, K.; Bhat, S.K.; Panda, S.K.; Mohanty, P.S.; Si, S. Thermo-responsive PNIPAM-metal hybrids: An efficient nanocatalyst for the reduction of 4-nitrophenol. Appl. Surf. Sci. 2017, 420, 753–763. [Google Scholar] [CrossRef]
- Fernandez-Lopez, C.; Polavarapu, L.; Solis, D.M.; Taboada, J.M.; Obelleiro, F.; Contreras-Caceres, R.; Pastoriza-Santos, I.; Perez-Juste, J. Gold nanorod-PNIPAM hybrids with reversible plasmon coupling: Synthesis, modeling, and sers properties. ACS Appl. Mater. Interfaces 2015, 7, 12530–12538. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Caceres, R.; Sanchez-Iglesias, A.; Karg, M.; Pastoriza-Santos, I.; Perez-Juste, J.; Pacifico, J.; Hellweg, T.; Fernandez-Barbero, A.; Liz-Marzan, L.M. Encapsulation and growth of gold nanoparticles in thermoresponsive microgels. Adv. Mater. 2008, 20, 1666–1670. [Google Scholar] [CrossRef]
- Fernandez-Lopez, C.; Perez-Balado, C.; Perez-Juste, J.; Pastoriza-Santos, I.; de Lera, A.R.; Liz-Marzan, L.M. A general LbL strategy for the growth of PNIPAM microgels on Au nanoparticles with arbitrary shapes. Soft Matter 2012, 8, 4165–4170. [Google Scholar] [CrossRef]
- Perez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzan, L.M. Multifunctionality in metal@microgel colloidal nanocomposites. J. Mater. Chem. A 2013, 1, 20–26. [Google Scholar] [CrossRef]
- Carregal-Romero, S.; Buurma, N.J.; Perez-Juste, J.; Liz-Marzan, L.M.; Herves, P. Catalysis by Au@pNIPAM nanocomposites: Effect of the cross-linking density. Chem. Mater. 2010, 22, 3051–3059. [Google Scholar] [CrossRef]
- Shi, S.; Wang, Q.; Wang, T.; Ren, S.; Gao, Y.; Wang, N. Thermo-, pH-, and light-responsive poly(N-isopropylacrylamide-co-methacrylic acid)–Au hybrid microgels prepared by the in situ reduction method based on au-thiol chemistry. J. Phys. Chem. B 2014, 118, 7177–7186. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, G.; Schurings, M.P.; van Rijn, P.; Pich, A. Formation of catalytically active gold-polymer microgel hybrids via a controlled in situ reductive process. J. Mater. Chem. A 2013, 1, 13244–13251. [Google Scholar] [CrossRef]
- Jia, H.; Schmitz, D.; Ott, A.; Pich, A.; Lu, Y. Cyclodextrin modified microgels as “nanoreactor” for the generation of Au nanoparticles with enhanced catalytic activity. J. Mater. Chem. A 2015, 3, 6187–6195. [Google Scholar] [CrossRef]
- Hou, L.; Wu, P.Y. The effect of added gold nanoparticles on the volume phase transition behavior for PVCL-based microgels. RSC Adv. 2014, 4, 39231–39241. [Google Scholar] [CrossRef]
- Shah, L.A.; Haleem, A.; Sayed, M.; Siddiq, M. Synthesis of sensitive hybrid polymer microgels for catalytic reduction of organic pollutants. J. Environ. Chem. Eng. 2016, 4, 3492–3497. [Google Scholar] [CrossRef]
- Khan, S.R.; Farooqi, Z.H.; Waheed-uz-Zaman; Ali, A.; Begum, R.; Kanwal, F.; Siddiq, M. Kinetics and mechanism of reduction of nitrobenzene catalyzed by silver-poly(N-isopropylacryl amide-co-allylacetic acid) hybrid microgels. Mater. Chem. Phys. 2016, 171, 318–327. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wang, X.Q.; Zha, L.S.; Lin, D.L.; Yang, J.M.; Zhou, J.F.; Zhang, L. Temperature- and pH-tunable plasmonic properties and sers efficiency of the silver nanoparticles within the dual stimuli-responsive microgels. J. Mater. Chem. C 2014, 2, 7326–7335. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Liu, X.Y.; Yang, J.M.; Lin, D.L.; Chen, X.; Zha, L.S. Investigation of Ag nanoparticles loading temperature responsive hybrid microgels and their temperature controlled catalytic activity. Colloid Surf. A 2012, 393, 105–110. [Google Scholar] [CrossRef]
- Tang, Y.C.; Wu, T.; Hu, B.T.; Yang, Q.; Liu, L.; Yu, B.; Ding, Y.; Ye, S.Y. Synthesis of thermo- and pH-responsive Ag nanoparticle-embedded hybrid microgels and their catalytic activity in methylene blue reduction. Mater. Chem. Phys. 2015, 149, 460–466. [Google Scholar] [CrossRef]
- Khan, A.; El-Toni, A.M.; Alrokayan, S.; Alsalhi, M.; Alhoshan, M.; Aldwayyan, A.S. Microwave-assisted synthesis of silver nanoparticles using poly-N-isopropylacrylamide/acrylic acid microgel particles. Colloid Surf. A 2011, 377, 356–360. [Google Scholar] [CrossRef]
- Wu, W.; Shen, J.; Li, Y.; Zhu, H.; Banerjee, P.; Zhou, S. Specific glucose-to-SPR signal transduction at physiological pH by molecularly imprinted responsive hybrid microgels. Biomaterials 2012, 33, 7115–7125. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Jiang, X.M.; Xu, W.T.; Zhou, M.M.; Hu, Y.M.; Wu, W.T. Tailoring the glucose-responsive volume phase transition behaviour of Ag@poly(phenylboronic acid) hybrid microgels: From monotonous swelling to monotonous shrinking upon adding glucose at physiological pH. Polym. Chem. 2014, 5, 2352–2362. [Google Scholar] [CrossRef]
- Han, D.M.; Zhang, Q.M.; Serpe, M.J. Poly(N-isopropylacrylamide)-co-(acrylic acid) microgel/Ag nanoparticle hybrids for the colorimetric sensing of H2O2. Nanoscale 2015, 7, 2784–2789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.G.; Xu, S.Q.; Kumacheva, E. Photogeneration of fluorescent silver nanoclusters in polymer microgels. Adv. Mater. 2005, 17, 2336–2340. [Google Scholar] [CrossRef]
- Naeem, H.; Farooqi, Z.H.; Shah, L.A.; Siddiq, M. Synthesis and characterization of p(NIPAM-AA-AAm) microgels for tuning of optical properties of silver nanoparticles. J. Polym. Res. 2012, 19, 1–10. [Google Scholar] [CrossRef]
- Tang, F.; Ma, N.; Tong, L.; He, F.; Li, L. Control of metal-enhanced fluorescence with pH- and thermoresponsive hybrid microgels. Langmuir 2012, 28, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Zhang, C.; Yang, J.M.; Lin, D.L.; Zhang, L.; Chen, X.; Zha, L.S. Silver nanoparticles loading pH responsive hybrid microgels: pH tunable plasmonic coupling demonstrated by surface enhanced raman scattering. RSC Adv. 2013, 3, 3384–3390. [Google Scholar] [CrossRef]
- Soares, P.I.P.; Ferreira, I.M.M.; Igreja, R.A.G.B.N.; Novo, C.M.M.; Borges, J.P.M.R. Application of hyperthermia for cancer treatment: Recent patents review. In Recent Patents on Anti-Cancer Drug Discovery; Bentham Science Publishers: Emirate of Sharjah, UAE, 2012; Volume 7, pp. 64–73. [Google Scholar]
- Soares, P.I.; Alves, A.M.; Pereira, L.C.; Coutinho, J.T.; Ferreira, I.M.; Novo, C.M.; Borges, J.P. Effects of surfactants on the magnetic properties of iron oxide colloids. J. Colloid Interface Sci. 2014, 419, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.I.P.; Laia, C.A.T.; Carvalho, A.; Pereira, L.C.J.; Coutinho, J.T.; Ferreira, I.M.M.; Novo, C.M.M.; Borges, J.P. Iron oxide nanoparticles stabilized with a bilayer of oleic acid for magnetic hyperthermia and mri applications. Appl. Surf. Sci. 2016, 383, 240–247. [Google Scholar] [CrossRef]
- Soares, P.I.; Lochte, F.; Echeverria, C.; Pereira, L.C.; Coutinho, J.T.; Ferreira, I.M.; Novo, C.M.; Borges, J.P. Thermal and magnetic properties of iron oxide colloids: Influence of surfactants. Nanotechnology 2015, 26, 425704. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.I.; Machado, D.; Laia, C.; Pereira, L.C.; Coutinho, J.T.; Ferreira, I.M.; Novo, C.M.; Borges, J.P. Thermal and magnetic properties of chitosan-iron oxide nanoparticles. Carbohydr. Polym. 2016, 149, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Mora, V.; Soares, P.; Echeverria, C.; Hernández, R.; Mijangos, C. Composite chitosan/agarose ferrogels for potential applications in magnetic hyperthermia. Gels 2015, 1, 69–80. [Google Scholar] [CrossRef]
- Chen, T.; Cao, Z.; Guo, X.; Nie, J.; Xu, J.; Fan, Z.; Du, B. Preparation and characterization of thermosensitive organic–inorganic hybrid microgels with functional Fe3O4 nanoparticles as crosslinker. Polymer 2011, 52, 172–179. [Google Scholar] [CrossRef]
- Laurenti, M.; Guardia, P.; Contreras-Caceres, R.; Perez-Juste, J.; Fernandez-Barbero, A.; Lopez-Cabarcos, E.; Rubio-Retama, J. Synthesis of thermosensitive microgels with a tunable magnetic core. Langmuir 2011, 27, 10484–10491. [Google Scholar] [CrossRef] [PubMed]
- Regmi, R.; Bhattarai, S.R.; Sudakar, C.; Wani, A.S.; Cunningham, R.; Vaishnava, P.P.; Naik, R.; Oupicky, D.; Lawes, G. Hyperthermia controlled rapid drug release from thermosensitive magnetic microgels. J. Mater. Chem. 2010, 20, 6158–6163. [Google Scholar] [CrossRef]
- Echeverria, C.; Mijangos, C. UCST-like hybrid PAAm-AA/Fe3O4 microgels. Effect of Fe3O4 nanoparticles on morphology, thermosensitivity and elasticity. Langmuir 2011, 27, 8027–8035. [Google Scholar] [CrossRef] [PubMed]
- Boularas, M.; Gombart, E.; Tranchant, J.F.; Billon, L.; Save, M. Design of smart oligo(ethylene glycol)-based biocompatible hybrid microgels loaded with magnetic nanoparticles. Macromol. Rapid Commun. 2015, 36, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Boularas, M.; Deniau-Lejeune, E.; Alard, V.; Tranchant, J.F.; Billon, L.; Save, M. Dual stimuli-responsive oligo(ethylene glycol)-based microgels: Insight into the role of internal structure in volume phase transitions and loading of magnetic nanoparticles to design stable thermoresponsive hybrid microgels. Polym. Chem. 2016, 7, 350–363. [Google Scholar] [CrossRef]
- Medeiros, S.F.; Filizzola, J.O.C.; Oliveira, P.F.M.; Silva, T.M.; Lara, B.R.; Lopes, M.V.; Rossi-Bergmann, B.; Elaissari, A.; Santos, A.M. Fabrication of biocompatible and stimuli-responsive hybrid microgels with magnetic properties via aqueous precipitation polymerization. Mater. Lett. 2016, 175, 296–299. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, A.J.; Yuan, Z.C.; Chen, D.J. Fabrication and characterization of temperature-, pH- and magnetic-field-sensitive organic/inorganic hybrid poly (ethylene glycol)-based hydrogels. Colloid Surf. A 2012, 415, 68–76. [Google Scholar] [CrossRef]
- Yuan, Z.C.; Wang, Y.; Chen, D.J. Preparation and characterization of thermo-, pH-, and magnetic-field-responsive organic/inorganic hybrid microgels based on poly(ethylene glycol). J. Mater. Sci. 2014, 49, 3287–3296. [Google Scholar] [CrossRef]
- Chengjun, Z.; Qinglin, W. Recent development in applications of cellulose nanocrystals for advanced polymer-based nanocomposites by novel fabrication strategies. In Nanocrystals-Synthesis, Characterization and Applications; Neralla, S., Ed.; Intech: Rijeka, Croatia, 2012; pp. 103–118. [Google Scholar]
- Sengel, S.B.; Sahiner, N. Poly(vinyl phosphonic acid) nanogels with tailored properties and their use for biomedical and environmental applications. Eur. Polym. J. 2016, 75, 264–275. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, W.; Yang, F.K.; Feng, H.L.; Yang, X.L. Facile method for synthesis of Fe3O4@polymer microspheres and their application as magnetic support for loading metal nanoparticles. J. Phys. Chem. C 2011, 115, 15875–15884. [Google Scholar] [CrossRef]
- Nabid, M.R.; Bide, Y.; Aghaghafari, E.; Rezaei, S.J.T. PdNPs@P2VP-Fe3O4 organic–inorganic hybrid microgels as a nanoreactor for selective aerobic oxidation of alcohols. Catal. Lett. 2013, 144, 355–363. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Hampel, S.; Cirillo, G.; Nicoletta, F.P.; Hassan, A.; Vittorio, O.; Picci, N.; Iemma, F. Spherical gelatin/CNTs hybrid microgels as electro-responsive drug delivery systems. Int. J. Pharm. 2013, 448, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.R.; Bae, H.; Cha, J.M.; Mun, J.Y.; Chen, Y.C.; Tekin, H.; Shin, H.; Farshchi, S.; Dokmeci, M.R.; Tang, S.; et al. Carbon nanotube reinforced hybrid microgels as scaffold materials for cell encapsulation. ACS Nano 2012, 6, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Zhou, M.; Greensmith, P.J.; Wang, W.; Hoyland, J.A.; Kinloch, I.A.; Freemont, T.; Saunders, B.R. A study of conductive hydrogel composites of pH-responsive microgels and carbon nanotubes. Soft Matter 2016, 12, 4142–4153. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yi, J.; Velado, D.; Yu, Y.; Zhou, S. Immobilization of carbon dots in molecularly imprinted microgels for optical sensing of glucose at physiological ph. ACS Appl. Mater. Interfaces 2015, 7, 15735–15745. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Milani, A.H.; Greensmith, P.J.; Yan, J.; Adlam, D.J.; Hoyland, J.A.; Kinloch, I.A.; Freemont, A.J.; Saunders, B.R. A study of physical and covalent hydrogels containing pH-responsive microgel particles and graphene oxide. Langmuir 2014, 30, 13384–13393. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Mallela, J.; Garapati, U.S.; Ravi, S.; Chinnasamy, V.; Girard, Y.; Howell, M.; Mohapatra, S. A chitosan-modified graphene nanogel for noninvasive controlled drug release. Nanomedicine 2013, 9, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.Y.; Liu, J.J.; Li, J.L.; Zhang, Z.X.; Weng, Y.Y.; Yuan, B.; Yang, K.; Ma, Y.Q. Tunable dual-stimuli response of a microgel composite consisting of reduced graphene oxide nanoparticles and poly(N-isopropylacrylamide) hydrogel microspheres. J. Mater. Chem. B 2014, 2, 3791–3798. [Google Scholar] [CrossRef]
- Zhou, M.M.; Xie, J.D.; Yan, S.T.; Jiang, X.M.; Ye, T.; Wu, W.T. Graphene@poly(phenylboronic acid)s microgels with selectively glucose-responsive volume phase transition behavior at a physiological pH. Macromolecules 2014, 47, 6055–6066. [Google Scholar] [CrossRef]
- Girard, H.A.; Benayoun, P.; Blin, C.; Trouve, A.; Gesset, C.; Arnault, J.C.; Bergonzo, P. Encapsulated nanodiamonds in smart microgels toward self-assembled diamond nanoarrays. Diam. Relat. Mater. 2013, 33, 32–37. [Google Scholar] [CrossRef]
- Agrawal, G.; Schurings, M.; Zhu, X.M.; Pich, A. Microgel/SiO2 hybrid colloids prepared using a water soluble silica precursor. Polymer 2012, 53, 1189–1197. [Google Scholar] [CrossRef]
- Li, Z.B.; Chen, T.Y.; Nie, J.J.; Xu, J.T.; Fan, Z.Q.; Du, B.Y. P(Nipam-co-Tmspma)/silica hybrid microgels: Structures, swelling properties and applications in fabricating macroporous silica. Mater. Chem. Phys. 2013, 138, 650–657. [Google Scholar] [CrossRef]
- Chai, S.G.; Zhang, J.Z.; Yang, T.T.; Yuan, J.J.; Cheng, S.Y. Thermoresponsive microgel decorated with silica nanoparticles in shell: Biomimetic synthesis and drug release application. Colloid Surf. A 2010, 356, 32–39. [Google Scholar] [CrossRef]
- Dechezelles, J.F.; Malik, V.; Crassous, J.J.; Schurtenberger, P. Hybrid raspberry microgels with tunable thermoresponsive behavior. Soft Matter 2013, 9, 2798–2802. [Google Scholar] [CrossRef]
- Contin, A.; Biffis, A.; Sterchele, S.; Dormbach, K.; Schipmann, S.; Pich, A. Metal nanoparticles inside microgel/clay nanohybrids: Synthesis, characterization and catalytic efficiency in cross-coupling reactions. J. Colloid Interface Sci. 2014, 414, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.S.; Hu, Y.; Gu, X.Y.; Hu, M.; Wang, C.Y. Poly(acrylamide) microgel-reinforced poly(acrylamide)/hectorite nanocomposite hydrogels. Colloid Surf. A 2016, 489, 1–8. [Google Scholar] [CrossRef]
- Wu, W.; Shen, J.; Banerjee, P.; Zhou, S. Chitosan-based responsive hybrid nanogels for integration of optical pH-sensing, tumor cell imaging and controlled drug delivery. Biomaterials 2010, 31, 8371–8381. [Google Scholar] [CrossRef] [PubMed]
- Gui, R.J.; An, X.Q.; Gong, J.; Chen, T. Thermosensitive, reversible luminescence properties and bright fluorescence imaging of water-soluble quantum dots/microgels nanocompounds. Mater. Lett. 2012, 88, 122–125. [Google Scholar] [CrossRef]
- Cai, Y.T.; Du, G.L.; Gao, G.R.; Chen, J.; Fu, J. Colour-tunable quantum dots/poly(Nipam-co-aac) hybrid microgels based on electrostatic interactions. RSC Adv. 2016, 6, 98147–98152. [Google Scholar] [CrossRef]
- Lai, W.F.; Susha, A.S.; Rogach, A.L. Multicompartment microgel beads for co-delivery of multiple drugs at individual release rates. ACS Appl. Mater. Interfaces 2016, 8, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.C.; Gao, N.; Li, J.; Wang, C.; Wang, H.; Zhou, M.M.; Zhang, M.; Li, G.T. Poly(ionic liquid)-based monodisperse microgels as a unique platform for producing functional materials. J. Mater. Chem. C 2015, 3, 623–631. [Google Scholar] [CrossRef]
- Baptista, A.C.; Martins, J.I.; Fortunato, E.; Martins, R.; Borges, J.P.; Ferreira, I. Thin and flexible bio-batteries made of electrospun cellulose-based membranes. Biosens. Bioelectron. 2011, 26, 2742–2745. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Baptista, A.; Soares, P.; Ferreira, I.; Borges, J.P. Nanofibers and nanoparticles in biomedical applications. In Bioengineered Nanomaterials; Tiwari, A., Ed.; CRC Press: New York, NY, USA, 2013; pp. 98–100. [Google Scholar]
- Canejo, J.P.; Borges, J.P.; Godinho, M.H.; Brogueira, P.; Teixeira, P.I.C.; Terentjev, E.M. Helical twisting of electrospun liquid crystalline cellulose micro- and nanofibers. Adv. Mater. 2008, 20, 4821–4825. [Google Scholar] [CrossRef]
- Agarwal, S.; Greiner, A.; Wendorff, J.H. Functional materials by electrospinning of polymers. Prog. Polym. Sci. 2013, 38, 963–991. [Google Scholar] [CrossRef]
- Crespy, D.; Friedemann, K.; Popa, A.M. Colloid-electrospinning: Fabrication of multicompartment nanofibers by the electrospinning of organic or/and inorganic dispersions and emulsions. Macromol. Rapid Commun. 2012, 33, 1978–1995. [Google Scholar] [CrossRef] [PubMed]
- Elahi, M.F.; Lu, W. Core-shell fibers for biomedical applications—A review. J. Bioeng. Biomed. Sci. 2013, 3, 1–14. [Google Scholar] [CrossRef]
- Jiang, S.; He, W.; Landfester, K.; Crespy, D.; Mylon, S.E. The structure of fibers produced by colloid-electrospinning depends on the aggregation state of particles in the electrospinning feed. Polymer 2017, 127, 101–105. [Google Scholar] [CrossRef]
- Diaz, J.E.; Barrero, A.; Marquez, M.; Fernandez-Nieves, A.; Loscertales, I.G. Absorption properties of microgel-pvp composite nanofibers made by electrospinning. Macromol. Rapid Commun. 2010, 31, 183–189. [Google Scholar] [PubMed]
- Gu, S.Y.; Wang, Z.M.; Li, J.B.; Ren, J. Switchable wettability of thermo-responsive biocompatible nanofibrous films created by electrospinning. Macromol. Mater. Eng. 2010, 295, 32–36. [Google Scholar] [CrossRef]
- Liu, C.; Qin, H.; Mather, P.T. Review of progress in shape-memory polymers. J. Mater. Chem. 2007, 17, 1543–1558. [Google Scholar] [CrossRef]
- Hager, M.D.; Bode, S.; Weber, C.; Schubert, U.S. Shape memory polymers: Past, present and future developments. Prog. Polym. Sci. 2015, 49–50, 3–33. [Google Scholar] [CrossRef]
- Wache, H.M.; Tartakowska, D.J.; Hentrich, A.; Wagner, M.H. Development of a polymer stent with shape memory effect as a drug delivery system. J. Mater. Sci. Mater. M 2003, 14, 109–112. [Google Scholar] [CrossRef]
- Lendlein, A.; Behl, M.; Hiebl, B.; Wischke, C. Shape-memory polymers as a technology platform for biomedical applications. Expert Rev. Med. Devices 2010, 7, 357–379. [Google Scholar] [CrossRef] [PubMed]
- Yakacki, C.M.; Gall, K. Shape-memory polymers for biomedical applications. In Shape-Memory Polymers; Lendlein, A., Ed.; Springer: Berlin, Germany, 2010; pp. 147–175. [Google Scholar]
- Du, H.Y.; Zhang, J.H. Solvent induced shape recovery of shape memory polymer based on chemically cross-linked poly(vinyl alcohol). Soft Matter 2010, 6, 3370–3376. [Google Scholar] [CrossRef]
- Cui, Y.; Tan, M.; Zhu, A.; Guo, M. Mechanically strong and stretchable peg-based supramolecular hydrogel with water-responsive shape-memory property. J. Mater. Chem. B 2014, 2, 2978–2982. [Google Scholar] [CrossRef]
- Miyamae, K.; Nakahata, M.; Takashima, Y.; Harada, A. Self-healing, expansion-contraction, and shape-memory properties of a preorganized supramolecular hydrogel through host-guest interactions. Angew. Chem. Int. Ed. 2015, 54, 8984–8987. [Google Scholar] [CrossRef] [PubMed]
- Osada, Y.; Matsuda, A. Shape memory in hydrogels. Nature 1995, 376, 219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Huang, W.M.; Lu, H.B.; Sun, L. Thermo-/chemo-responsive shape memory/change effect in a hydrogel and its composites. Mater. Des. 2014, 53, 1077–1088. [Google Scholar] [CrossRef]
- Zhang, J.L.; Huang, W.M.; Gao, G.R.; Fu, J.; Zhou, Y.; Salvekar, A.V.; Venkatraman, S.S.; Wong, Y.S.; Tay, K.H.; Birch, W.R. Shape memory/change effect in a double network nanocomposite tough hydrogel. Eur. Polym. J. 2014, 58, 41–51. [Google Scholar] [CrossRef]
- Fan, Y.; Zhou, W.; Yasin, A.; Li, H.; Yang, H. Dual-responsive shape memory hydrogels with novel thermoplasticity based on a hydrophobically modified polyampholyte. Soft Matter 2015, 11, 4218–4225. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.W.; Lu, C.H.; Guo, W.W.; Aleman-Garcia, M.A.; Ren, J.T.; Willner, I. A shape memory acrylamide/DNA hydrogel exhibiting switchable dual pH-responsiveness. Adv. Funct. Mater. 2015, 25, 6867–6874. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, W.; Kahn, J.S.; Aleman-Garcia, M.A.; Willner, I. A shape-memory DNA-based hydrogel exhibiting two internal memories. Angew. Chem. Int. Ed. 2016, 55, 4210–4214. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, H.; Fortin, D.; Xia, H.; Zhao, Y. Poly(vinyl alcohol)-poly(ethylene glycol) double-network hydrogel: A general approach to shape memory and self-healing functionalities. Langmuir 2015, 31, 11709–11716. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yan, Q.; Xia, H.; Zhao, Y. Therapeutic-ultrasound-triggered shape memory of a melamine-enhanced poly(vinyl alcohol) physical hydrogel. ACS Appl. Mater. Interfaces 2015, 7, 12067–12073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, M.; Jiang, H.; Shi, J.; Li, F.; Xia, Y.; Zhang, G.; Li, H. Bio-inspired layered chitosan/graphene oxide nanocomposite hydrogels with high strength and pH-driven shape memory effect. Carbohydr. Polym. 2017, 177, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Lu, W.; Yang, X.; He, J.; Le, X.; Wang, L.; Zhang, J.; Serpe, M.J.; Huang, Y.; Chen, T. Bioinspired anisotropic hydrogel actuators with on-off switchable and color-tunable fluorescence behaviors. Adv. Funct. Mater. 2018, 28, 1704568. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, L.; Wang, T.; Sun, W.; Tong, Z. Nir-triggered rapid shape memory pam-go-gelatin hydrogels with high mechanical strength. ACS Appl. Mater. Interfaces 2016, 8, 12384–12392. [Google Scholar] [CrossRef] [PubMed]
- Wool, R.P. Self-healing materials: A review. Soft Matter 2008, 4, 400–418. [Google Scholar] [CrossRef]
- Wu, D.Y.; Meure, S.; Solomon, D. Self-healing polymeric materials: A review of recent developments. Prog. Polym. Sci. 2008, 33, 479–522. [Google Scholar] [CrossRef]
- Toohey, K.S.; Sottos, N.R.; Lewis, J.A.; Moore, J.S.; White, S.R. Self-healing materials with microvascular networks. Nat. Mater. 2007, 6, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Lehn, J.M. From supramolecular chemistry towards constitutional dynamic chemistry and adaptive chemistry. Chem. Soc. Rev. 2007, 36, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Caulder, D.L.; Raymond, K.N. Supermolecules by design. Acc. Chem. Res. 1999, 32, 975–982. [Google Scholar] [CrossRef]
- Amaral, A.J.R.; Pasparakis, G. Stimuli responsive self-healing polymers: Gels, elastomers and membranes. Polym. Chem. 2017, 8, 6464–6484. [Google Scholar] [CrossRef]
- Phadke, A.; Zhang, C.; Arman, B.; Hsu, C.C.; Mashelkar, R.A.; Lele, A.K.; Tauber, M.J.; Arya, G.; Varghese, S. Rapid self-healing hydrogels. Proc. Natl. Acad. Sci. USA 2012, 109, 4383–4388. [Google Scholar] [CrossRef] [PubMed]
- Tuncaboylu, D.C.; Sari, M.; Oppermann, W.; Okay, O. Tough and self-healing hydrogels formed via hydrophobic interactions. Macromolecules 2011, 44, 4997–5005. [Google Scholar] [CrossRef]
- Tuncaboylu, D.C.; Argun, A.; Sahin, M.; Sari, M.; Okay, O. Structure optimization of self-healing hydrogels formed via hydrophobic interactions. Polymer 2012, 53, 5513–5522. [Google Scholar] [CrossRef]
- Tuncaboylu, D.C.; Argun, A.; Algi, M.P.; Okay, O. Autonomic self-healing in covalently crosslinked hydrogels containing hydrophobic domains. Polymer 2013, 54, 6381–6388. [Google Scholar] [CrossRef]
- Gulyuz, U.; Okay, O. Self-healing polyacrylic acid hydrogels. Soft Matter 2013, 9, 10287–10293. [Google Scholar] [CrossRef]
- Zhang, H.J.; Xia, H.S.; Zhao, Y. Poly(vinyl alcohol) hydrogel can autonomously self-heal. ACS Macro Lett. 2012, 1, 1233–1236. [Google Scholar] [CrossRef]
- Gulyuz, U.; Okay, O. Self-healing poly(N-isopropylacrylamide) hydrogels. Eur. Polym. J. 2015, 72, 12–22. [Google Scholar] [CrossRef]
- Cong, H.P.; Wang, P.; Yu, S.H. Highly elastic and superstretchable graphene oxide/polyacrylamide hydrogels. Small 2014, 10, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Cong, H.-P.; Wang, P.; Yu, S.-H. Stretchable and self-healing graphene oxide–polymer composite hydrogels: A dual-network design. Chem. Mater. 2013, 25, 3357–3362. [Google Scholar] [CrossRef]
- Zhang, E.; Wang, T.; Zhao, L.; Sun, W.; Liu, X.; Tong, Z. Fast self-healing of graphene oxide-hectorite clay-poly(N,N-dimethylacrylamide) hybrid hydrogels realized by near-infrared irradiation. ACS Appl. Mater. Interfaces 2014, 6, 22855–22861. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Song, G.; He, C.; Wang, H. Self-healing in tough graphene oxide composite hydrogels. Macromol. Rapid Commun. 2013, 34, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, C.; He, C.; Zhao, J.; Yang, X.; Wang, H. Synthesis of graphene peroxide and its application in fabricating super extensible and highly resilient nanocomposite hydrogels. ACS Nano 2012, 6, 8194–8202. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Smitthipong, W.; Zeng, H.B. Mussel-inspired hydrogels for biomedical and environmental applications. Polym. Chem. 2015, 6, 353–358. [Google Scholar] [CrossRef]
- Li, L.; Yan, B.; Yang, J.; Chen, L.; Zeng, H. Novel mussel-inspired injectable self-healing hydrogel with anti-biofouling property. Adv. Mater. 2015, 27, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yan, B.; Yang, J.; Huang, W.; Chen, L.; Zeng, H. Injectable self-healing hydrogel with antimicrobial and antifouling properties. ACS Appl. Mater. Interfaces 2017, 9, 9221–9225. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Ma, P.X. Stimuli-responsive supramolecular hydrogels with high extensibility and fast self-healing via precoordinated mussel-inspired chemistry. Chem. Mater. 2015, 27, 7627–7635. [Google Scholar] [CrossRef] [PubMed]
- Latnikova, A.; Grigoriev, D.; Schenderlein, M.; Mohwald, H.; Shchukin, D. A new approach towards “active” self-healing coatings: Exploitation of microgels. Soft Matter 2012, 8, 10837–10844. [Google Scholar] [CrossRef]
- Park, C.W.; South, A.B.; Hu, X.B.; Verdes, C.; Kim, J.D.; Lyon, L.A. Gold nanoparticles reinforce self-healing microgel multilayers. Colloid Polym. Sci. 2011, 289, 583–590. [Google Scholar] [CrossRef]
- Serpe, M.J.; Jones, C.D.; Lyon, L.A. Layer-by-layer deposition of thermoresponsive microgel thin films. Langmuir 2003, 19, 8759–8764. [Google Scholar] [CrossRef]
- South, A.B.; Lyon, L.A. Autonomic self-healing of hydrogel thin films. Angew. Chem. Int. Ed. 2010, 49, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Gaulding, J.C.; Spears, M.W.; Lyon, L.A. Plastic deformation, wrinkling, and recovery in microgel multilayers. Polym. Chem. 2013, 4, 4890–4896. [Google Scholar] [CrossRef] [PubMed]
- Spears, M.W., Jr.; Herman, E.S.; Gaulding, J.C.; Lyon, L.A. Dynamic materials from microgel multilayers. Langmuir 2014, 30, 6314–6323. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Echeverria, C.; Fernandes, S.N.; Godinho, M.H.; Borges, J.P.; Soares, P.I.P. Functional Stimuli-Responsive Gels: Hydrogels and Microgels. Gels 2018, 4, 54. https://doi.org/10.3390/gels4020054
Echeverria C, Fernandes SN, Godinho MH, Borges JP, Soares PIP. Functional Stimuli-Responsive Gels: Hydrogels and Microgels. Gels. 2018; 4(2):54. https://doi.org/10.3390/gels4020054
Chicago/Turabian StyleEcheverria, Coro, Susete N. Fernandes, Maria H. Godinho, João Paulo Borges, and Paula I. P. Soares. 2018. "Functional Stimuli-Responsive Gels: Hydrogels and Microgels" Gels 4, no. 2: 54. https://doi.org/10.3390/gels4020054
APA StyleEcheverria, C., Fernandes, S. N., Godinho, M. H., Borges, J. P., & Soares, P. I. P. (2018). Functional Stimuli-Responsive Gels: Hydrogels and Microgels. Gels, 4(2), 54. https://doi.org/10.3390/gels4020054








