Tuning the Size of Thermoresponsive Poly(N-Isopropyl Acrylamide) Grafted Silica Microgels
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Silica Core
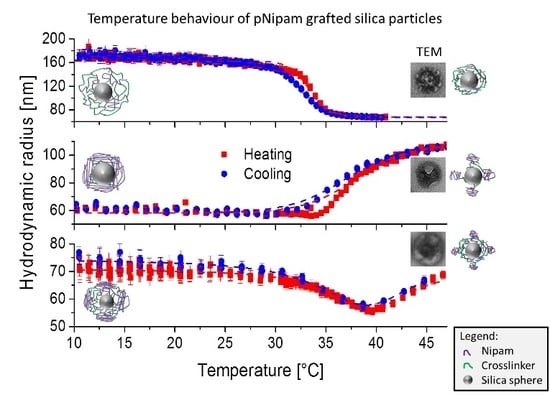
2.2. Temperature Behaviour of the Hybrid Hydrogel
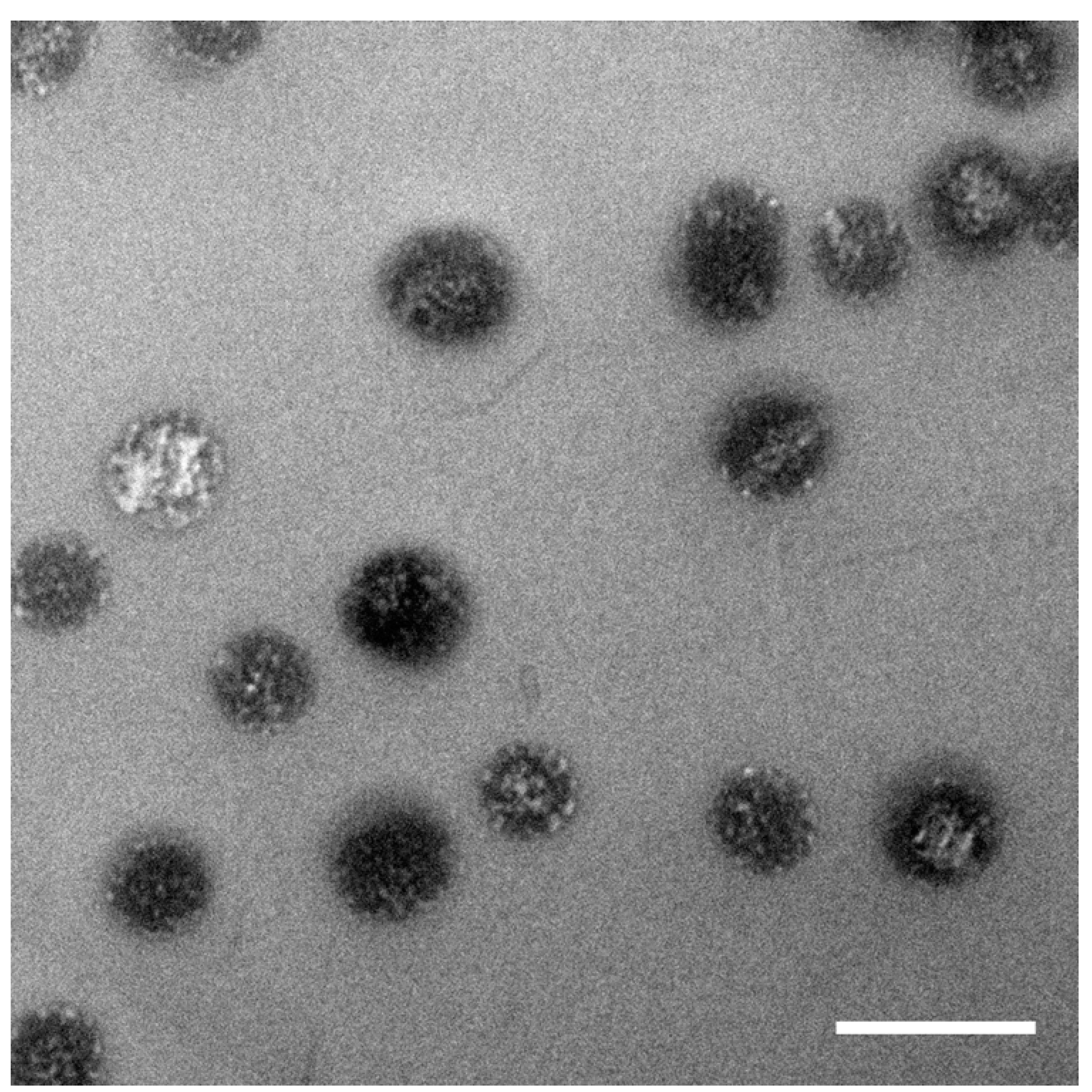
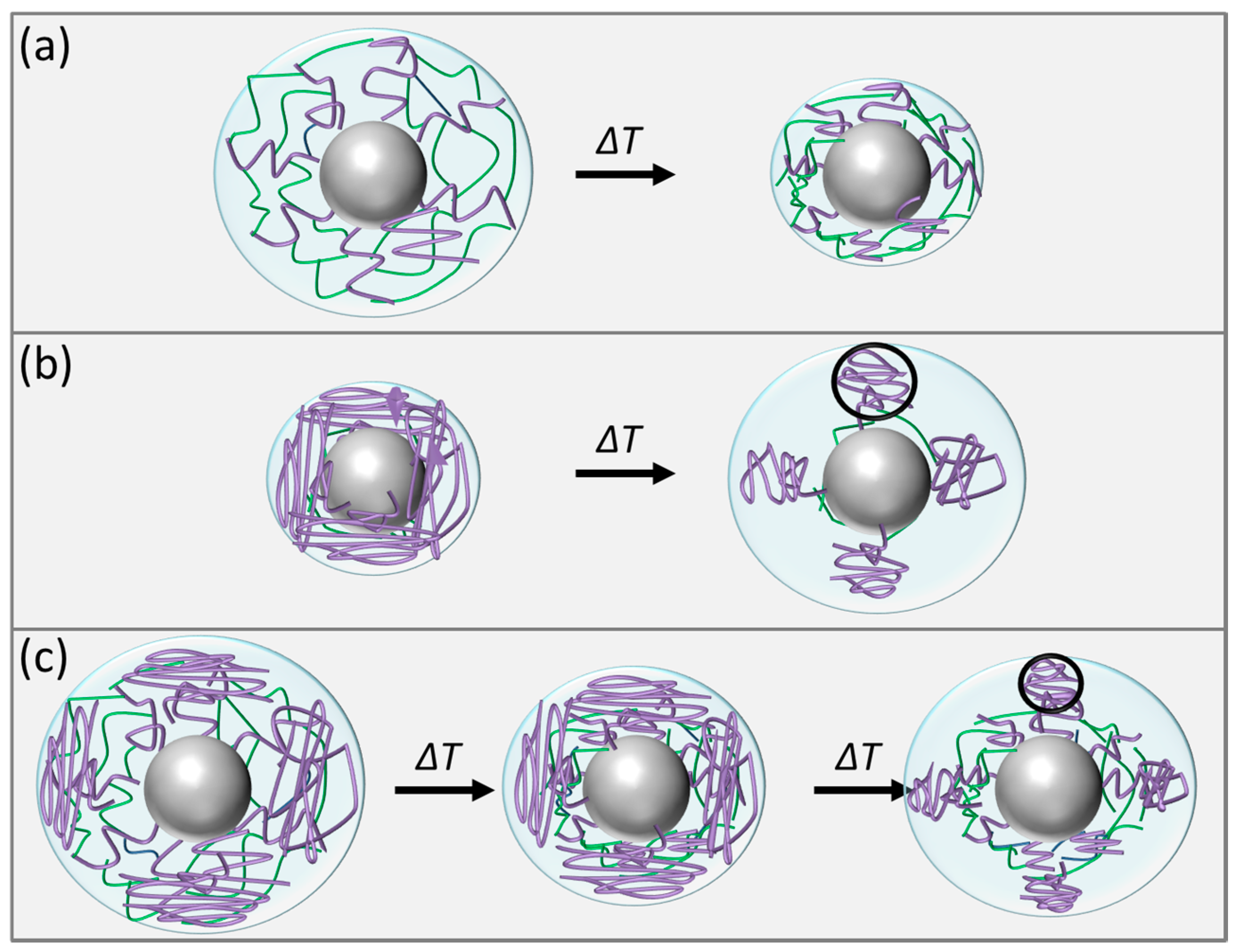
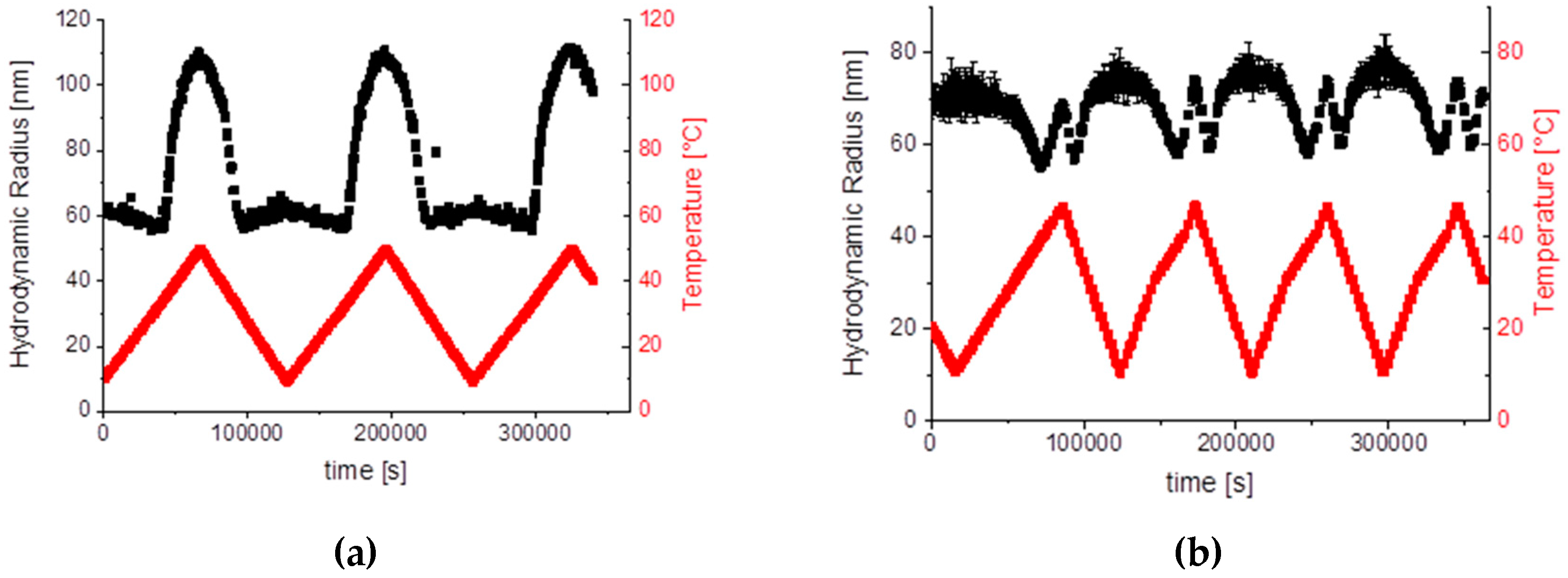
2.2.1. Sample SiPN-1
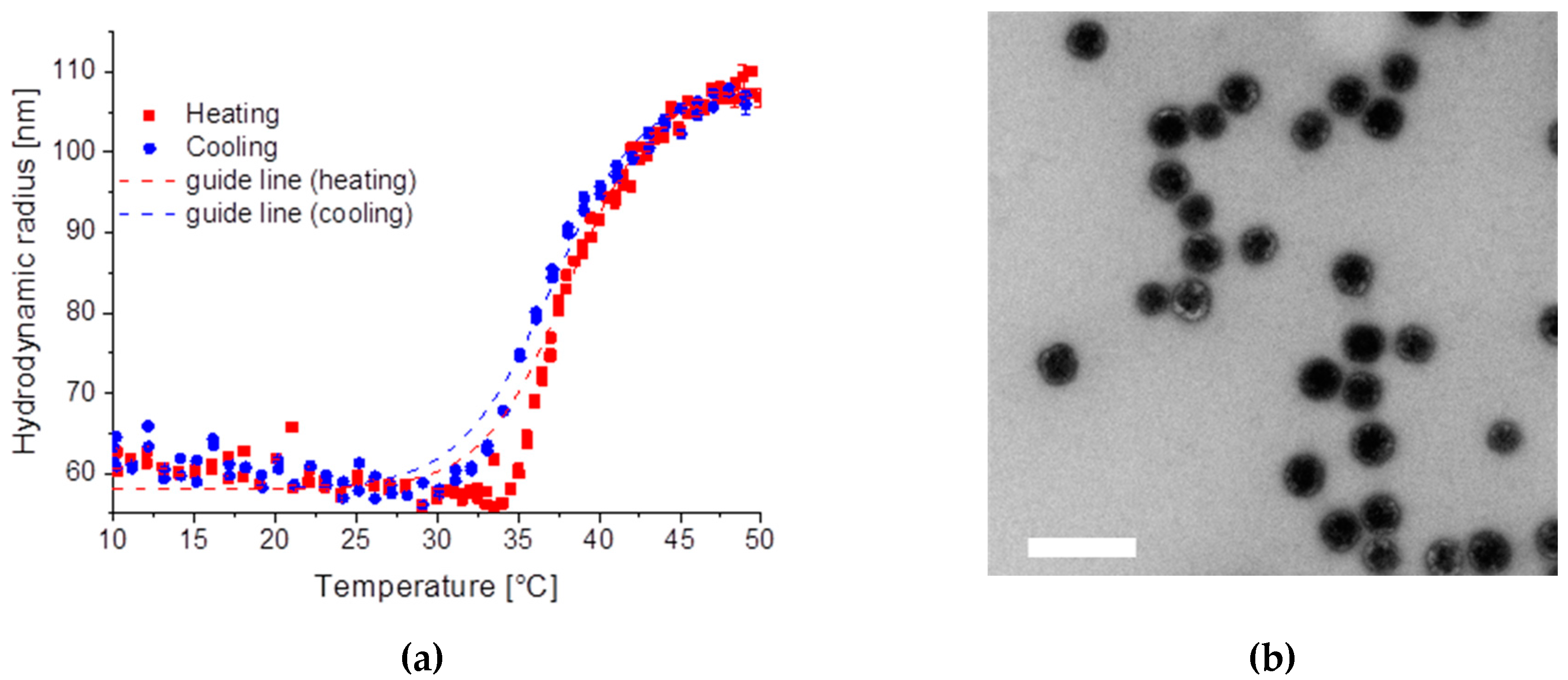
2.2.2. Sample SiPN-2
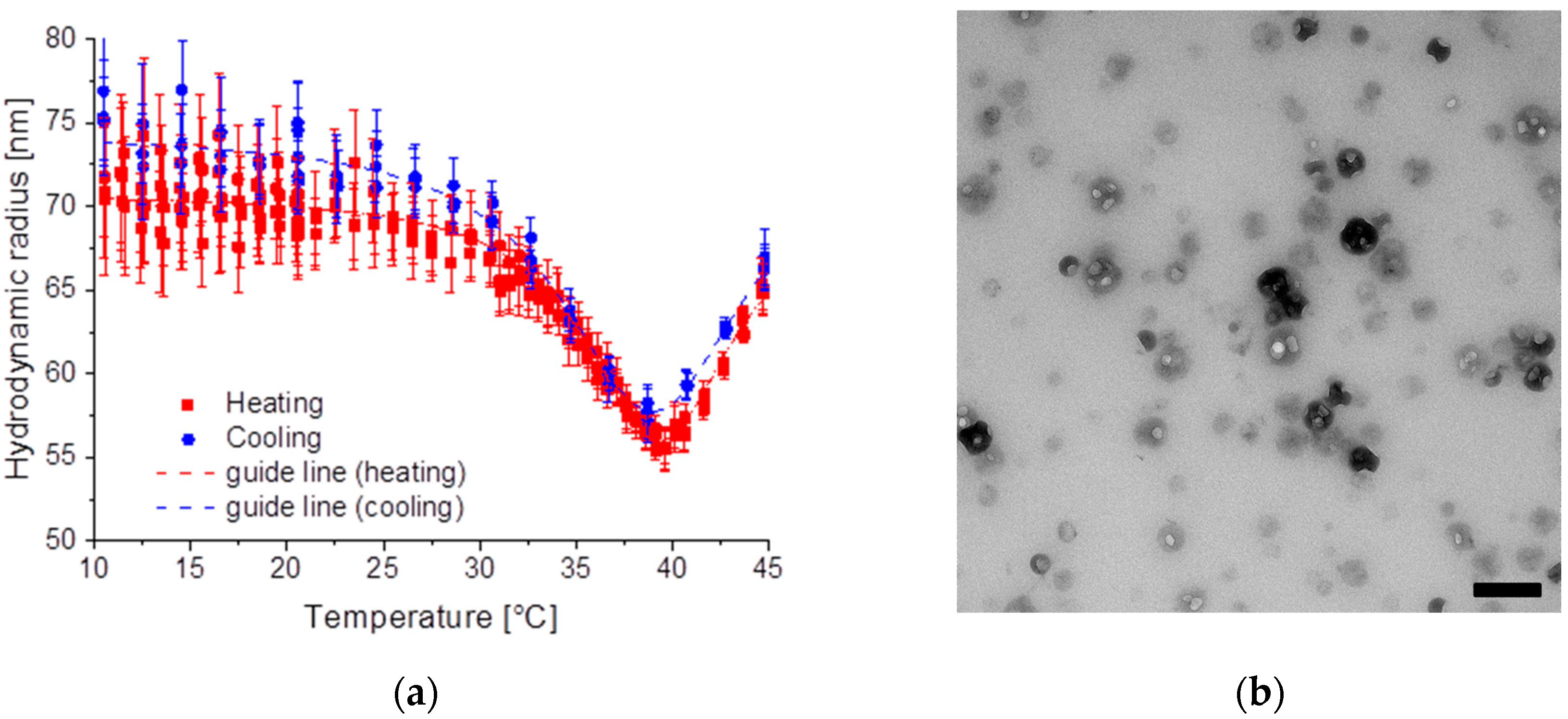
2.2.3. Sample SiPN-3
2.3. Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis
4.2.1. TPM-Grafted Silica Particles
4.2.2. pNipam-Grafted Silica Microgels
SiPN-1
SiPN-2
SiPN-3
4.3. Characterization Methods
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haq, M.A.; Su, Y.; Wang, D. Mechanical properties of pnipam based hydrogels: A review. Mater. Sci. Eng. C 2017, 70, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Doring, A.; Birnbaum, W.; Kuckling, D. Responsive hydrogels—Structurally and dimensionally optimized smart frameworks for applications in catalysis, micro-system technology and material science. Chem. Soc. Rev. 2013, 42, 7391–7420. [Google Scholar] [CrossRef] [PubMed]
- Lyon, L.A.; Meng, Z.; Singh, N.; Sorrell, C.D.; St John, A. Thermoresponsive microgel-based materials. Chem. Soc. Rev. 2009, 38, 865–874. [Google Scholar] [CrossRef] [PubMed]
- De Las Heras Alarcon, C.; Pennadam, S.; Alexander, C. Stimuli responsive polymers for biomedical applications. Chem. Soc. Rev. 2005, 34, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Varga, I.; Gilányi, T.; Mészáros, R.; Filipcsei, G.; Zrínyi, M. Effect of cross-link density on the internal structure of poly(N-isopropylacrylamide) microgels. J. Phys. Chem. B 2001, 105, 9071–9076. [Google Scholar] [CrossRef]
- Pelton, R. Temperature-sensitive aqueous microgels. Adv. Colloid Int. Sci. 2000, 85, 1–33. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, Y. Pnipam microgels for biomedical applications: From dispersed particles to 3D assemblies. Soft Matter 2011, 7, 6375. [Google Scholar] [CrossRef]
- Tiktopulo, E.I.; Uversky, V.N.; Lushchik, V.B.; Klenin, S.I.; Bychkova, V.E.; Ptitsyn, O.B. “Domain” coil-globule transition in homopolymers. Macromolecules 1995, 28, 7519–7524. [Google Scholar] [CrossRef]
- Schroer, M.A.; Michalowsky, J.; Fischer, B.; Smiatek, J.; Grubel, G. Stabilizing effect of tmao on globular pnipam states: Preferential attraction induces preferential hydration. Phys. Chem. Chem. Phys. 2016, 18, 31459–31470. [Google Scholar] [CrossRef] [PubMed]
- Micciulla, S.; Michalowsky, J.; Schroer, M.A.; Holm, C.; von Klitzing, R.; Smiatek, J. Concentration dependent effects of urea binding to poly(N-isopropylacrylamide) brushes: A combined experimental and numerical study. Phys. Chem. Chem. Phys. 2016, 18, 5324–5335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hoogenboom, R. Polymers with upper critical solution temperature behavior in alcohol/water solvent mixtures. Prog. Polym. Sci. 2015, 48, 122–142. [Google Scholar] [CrossRef]
- Liu, L.; Wang, T.; Liu, C.; Lin, K.; Liu, G.; Zhang, G. Specific anion effect in water-nonaqueous solvent mixtures: Interplay of the interactions between anion, solvent, and polymer. J. Phys. Chem. B 2013, 117, 10936–10943. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.O.R.; Freitas, R.F.S. Phase behavior of poly(N-isopropylacrylamide) in binary aqueous solutions. Polymer 2002, 43, 5879–5885. [Google Scholar] [CrossRef]
- Kratz, K.; Hellweg, T.; Eimer, W. Structural changes in pnipam microgel particles as seen by sans, dls, and em techniques. Polymer 2001, 42, 6631–6639. [Google Scholar] [CrossRef]
- Fernandez-Barbero, A.; Fernandez-Nieves, A.; Grillo, I.; Lopez-Cabarcos, E. Structural modifications in the swelling of inhomogeneous microgels by light and neutron scattering. Phys. Rev. E 2002, 66, 051803. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.J.; Dubbert, J.; Rudov, A.A.; Pedersen, J.S.; Lindner, P.; Karg, M.; Potemkin, I.I.; Richtering, W. Multi-shell hollow nanogels with responsive shell permeability. Sci. Rep. 2016, 6, 22736. [Google Scholar] [CrossRef] [PubMed]
- Hertle, Y.; Zeiser, M.; Hasenöhrl, C.; Busch, P.; Hellweg, T. Responsive P(NIPAM-co-NTBAM) microgels: Flory–Rehner description of the swelling behaviour. Colloid Polym. Sci. 2010, 288, 1047–1059. [Google Scholar] [CrossRef]
- Acciaro, R.; Gilanyi, T.; Varga, I. Preparation of monodisperse poly(N-isopropylacrylamide) microgel particles with homogenous cross-link density distribution. Langmuir 2011, 27, 7917–7925. [Google Scholar] [CrossRef] [PubMed]
- Graf, C.; Vossen, D.L.J.; Imhof, A.; van Blaaderen, A. A general method to coat colloidal particles with silica. Langmuir 2003, 19, 8. [Google Scholar] [CrossRef]
- Rittikulsittichai, S.; Kolhatkar, A.G.; Sarangi, S.; Vorontsova, M.A.; Vekilov, P.G.; Brazdeikis, A.; Randall Lee, T. Multi-responsive hybrid particles: Thermo-, ph-, photo-, and magneto-responsive magnetic hydrogel cores with gold nanorod optical triggers. Nanoscale 2016, 8, 11851–11861. [Google Scholar] [CrossRef] [PubMed]
- Messing, R.; Schmidt, A.M. Perspectives for the mechanical manipulation of hybrid hydrogels. Polym. Chem. 2011, 2, 18. [Google Scholar] [CrossRef]
- Dagallier, C.; Dietsch, H.; Schurtenberger, P.; Scheffold, F. Thermoresponsive hybrid microgel particles with intrinsic optical and magnetic anisotropy. Soft Matter 2010, 6, 2174. [Google Scholar] [CrossRef]
- Hinrichs, S.; Nun, N.; Fischer, B. Synthesis and characterization of anisotropic magnetic hydrogels. J. Magn. Magn. Mater. 2017, 431, 237–240. [Google Scholar] [CrossRef]
- Dulle, M.; Jaber, S.; Rosenfeldt, S.; Radulescu, A.; Forster, S.; Mulvaney, P.; Karg, M. Plasmonic gold-poly(N-isopropylacrylamide) core-shell colloids with homogeneous density profiles: A small angle scattering study. Phys. Chem. Chem. Phys. 2015, 17, 1354–1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karg, M.; Pastoriza-Santos, I.; Perez-Juste, J.; Hellweg, T.; Liz-Marzan, L.M. Nanorod-coated pnipam microgels: Thermoresponsive optical properties. Small 2007, 3, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Karg, M.; Pastoriza-Santos, I.; Liz-Marzan, L.M.; Hellweg, T. A versatile approach for the preparation of thermosensitive pnipam core-shell microgels with nanoparticle cores. Chem. Phys. Chem. 2006, 7, 2298–2301. [Google Scholar] [CrossRef] [PubMed]
- Karg, M.; Wellert, S.; Prevost, S.; Schweins, R.; Dewhurst, C.; Liz-Marzán, L.M.; Hellweg, T. Well defined hybrid pnipam core-shell microgels: Size variation of the silica nanoparticle core. Colloid Polym. Sci. 2010, 289, 699–709. [Google Scholar] [CrossRef]
- Contreras-Cáceres, R.; Pacifico, J.; Pastoriza-Santos, I.; Pérez-Juste, J.; Fernández-Barbero, A.; Liz-Marzán, L.M. Au@pnipam thermosensitive nanostructures: Control over shell cross-linking, overall dimensions, and core growth. Adv. Funct. Mater. 2009, 19, 3070–3076. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Roldan, P.S.; Alcântara, I.L.; Rocha, J.C.; Padilha, C.C.F.; Padilha, P.M. Determination of copper, iron, nickel and zinc in fuel kerosene by faas after adsorption and pre-concentration on 2-aminothiazole-modified silica gel. Eclet. Quím. São Paulo 2004, 29. [Google Scholar] [CrossRef]
- Buga, M.R.; Zaharia, C.; Balan, M.; Bressy, C.; Ziarelli, F.; Margaillan, A. Surface modification of silk fibroin fibers with poly(methyl methacrylate) and poly(tributylsilyl methacrylate) via raft polymerization for marine antifouling applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 51, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Popescu, M.-C.; Filip, D.; Vasile, C.; Cruz, C.; Rueff, J.M.; Marcos, M.; Serrano, J.L.; Singurel, G. Characterization by fourier transform infrared spectroscopy (FT-IR) and 2D IR correlation spectroscopy of PAMAM dendrimer. J. Phys. Chem. B 2006, 110, 14198–14211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Hou, G.; Dai, S.; Lu, R.; Wang, C. Preparation of thermosensitive pnipam microcontainers and a versatile method to fabricate pnipam shell on particles with silica surface. Colloid Polym. Sci. 2012, 290, 1341–1346. [Google Scholar] [CrossRef]
- Cejkova, J.; Hanus, J.; Stepanek, F. Investigation of internal microstructure and thermo-responsive properties of composite pnipam/silica microcapsules. J. Colloid Interface Sci. 2010, 346, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ma, J.; Zhang, B.; Zhao, S.; Li, Y.; Xu, Y.; Yu, W.; Wang, J. Synthesis of thermoresponsive silica nanoparticle/pnipam hybrids by aqueous surface-initiated atom transfer radical polymerization. Mater. Lett. 2007, 61, 949–952. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, Y.-H.; Oh, S.-G. Preparation of thermosensitive pnipam-grafted mesoporous silica particles. Macromol. Chem. Phys. 2007, 208, 2419–2427. [Google Scholar] [CrossRef]
- Guo, J.; Yang, W.; Deng, Y.; Wang, C.; Fu, S. Organic-dye-coupled magnetic nanoparticles encaged inside thermoresponsive pnipam microcapsules. Small 2005, 1, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Marcelo, G.; Areias, L.R.P.; Viciosa, M.T.; Martinho, J.M.G.; Farinha, J.P.S. Pnipam based microgels with a ucst response. Polymer 2017, 116, 261–267. [Google Scholar] [CrossRef]
- Wang, Q.; Biswas, C.S.; Galluzzi, M.; Wu, Y.; Du, B.; Stadler, F.J. Random copolymer gels of N-isopropylacrylamide and N-ethylacrylamide: Effect of synthesis solvent compositions on their properties. RSC Adv. 2017, 7, 9381–9392. [Google Scholar] [CrossRef]
- Bischofberger, I.; Calzolari, D.C.E.; Trappe, V. Co-nonsolvency of pnipam at the transition between solvation mechanisms. Soft Matter 2014, 10, 8288–8295. [Google Scholar] [CrossRef] [PubMed]
- Bischofberger, I.; Calzolari, D.C.; De Los Rios, P.; Jelezarov, I.; Trappe, V. Hydrophobic hydration of poly-N-isopropyl acrylamide: A matter of the mean energetic state of water. Sci. Rep. 2014, 4, 4377. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Name | Ethanol | NH3 (A) | TEOS | NH3 (B) | TPM |
|---|---|---|---|---|---|
| mL | mL | mL | mL | mL | |
| Si-1 | 200 | 15 | 5 | 10 | 1 |
| Si-2 | 450 * | 20 | 7.5 | 15 | 2 |
| Si-3 | 750 | 35 | 5 | 10 | 1 |
| Chemical | Nipam mmol | BIS mmol |
|---|---|---|
| First addition | 17.67 | 0.57 |
| Second addition | 17.67 | 0.57 |
| Third addition | 17.67 | 0.65 |
| sum | 53.01 | 1.79 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nun, N.; Hinrichs, S.; Schroer, M.A.; Sheyfer, D.; Grübel, G.; Fischer, B. Tuning the Size of Thermoresponsive Poly(N-Isopropyl Acrylamide) Grafted Silica Microgels. Gels 2017, 3, 34. https://doi.org/10.3390/gels3030034
Nun N, Hinrichs S, Schroer MA, Sheyfer D, Grübel G, Fischer B. Tuning the Size of Thermoresponsive Poly(N-Isopropyl Acrylamide) Grafted Silica Microgels. Gels. 2017; 3(3):34. https://doi.org/10.3390/gels3030034
Chicago/Turabian StyleNun, Nils, Stephan Hinrichs, Martin A. Schroer, Dina Sheyfer, Gerhard Grübel, and Birgit Fischer. 2017. "Tuning the Size of Thermoresponsive Poly(N-Isopropyl Acrylamide) Grafted Silica Microgels" Gels 3, no. 3: 34. https://doi.org/10.3390/gels3030034
APA StyleNun, N., Hinrichs, S., Schroer, M. A., Sheyfer, D., Grübel, G., & Fischer, B. (2017). Tuning the Size of Thermoresponsive Poly(N-Isopropyl Acrylamide) Grafted Silica Microgels. Gels, 3(3), 34. https://doi.org/10.3390/gels3030034