Abstract
Caffeine (a stimulant) and ethanol (a depressant) may have opposite effects in our body, but under in vitro conditions they can “gel” together. Caffeine, being one of the widely used stimulants, continued to surprise the scientific community with its unprecedented biological, medicinal and physicochemical properties. Here, we disclose the supramolecular self-assembly of anhydrous caffeine in a series of alcoholic and aromatic solvents, rendering a highly entangled microcrystalline network facilitating the encapsulation of the solvents as illustrated using direct imaging, microscopy analysis and NMR studies.
1. Introduction
Coffee, with its flavor, aroma and stimulant properties, is one of the extensively consumed beverages in the human history [1]. The stimulant properties of coffee and tea are attributed to the presence of caffeine, which represents one of the widely used alkaloids [2]. The presence of caffeine is not restricted to coffee beans and tea leaves, but found in a number of other plants and plant products [3,4,5,6,7]. In spite of having several centuries of history, numerous biological, medicinal, physicochemical, as well as materials properties of caffeine have remained undisclosed and continued to surprise the scientific community [8,9,10]. Recently, caffeine has gained importance in genetics as an elegant example for convergent evolution [11]. The biological importance of caffeine as an anticancer agent, in prevention of Alzheimer disease, controlling the amyloid fibril formation and as a potential neuroactive drug has attracted the modern research community [12,13,14,15]. In supramolecular chemistry, the studies dealing with solid-state structural properties of caffeine and its derivatives continued to emerge rapidly [16,17,18]. The polymorphic forms, solvates, co-crystals and inclusion complexes of caffeine have been well documented in the literature [19,20,21]. The first X-ray diffraction studies by June Sutor showed that caffeine readily crystallizes in its monohydrate form [22]. The monohydrate form readily undergoes a transformation under ambient conditions into an anhydrous β-form. However, at higher temperatures the β-form undergoes phase transition into anhydrous α-form [23]. During this transition process, the single crystals are often destroyed, making the structure determination a challenging task. Therefore, the X-ray crystal structure determination of pure anhydrous form of caffeine remained a mystery until recently. In 2007, the first successful crystal structure was reported for anhydrous caffeine by Lehman et al. [24]. Using X-ray powder diffraction and high resolution solid state NMR, Enright and co-workers studied the anhydrous forms of caffeine [25]. Eddleston et al. showed that caffeine undergoes crystallization into tubular microcrystals in a wide range of solvents [26]. A vast number of crystallization of caffeine were carried out using solvent evaporation method. However, the self-assembly of caffeine leading to immobilization of solvent has not been reported in the literature.
Self-assembled supramolecular gels derived from low molecular weight organic gelators (LMOG) are attractive building blocks for drug delivery, tissue engineering and nanoscience [27,28,29]. The tremendous interest in this field over the past two decades is attributed to the ability of small molecules to generate micro- and nanofibers, tapes or rod-like self-assembled superstructures, which trap and immobilize a wide variety of solvents [30,31,32,33]. This property of small molecules offers a unique opportunity towards the careful and systematic design of gelators with tunable material properties [34,35]. The gelation ability of peptides, steroidal derivatives, aromatic charge transfer complexes, supramolecular metallogelators, have been well documented in the literature [27,28,29,30,31,32,33,34,35,36,37,38,39]. The morphological details have been studied using TEM, Cryo-TEM, environmental SEM, AFM and optical microscope [40,41,42,43]. Several non-invasive methods such as fluorescence spectroscopy, UV-Vis, circular dichroism, solution NMR, as well as Solid state NMR, have been utilized to study gels in their native state [44,45,46,47,48]. One of the hypotheses that, the gelation is due to 3D fibrillar network established via supramolecular self-assembly of small molecules. The size of the network varies significantly from one gelator to another as well as from one solvent to another for a given gelator. When the gel networks are at nanometer regime, it poses a challenge to visualize and provide a direct evidence towards 3D network formation leading to gelation in its native state. We point out that, the microscopy studies require a different imaging environment, or surface to study the self-assembly compared to a native gel, as a result the growth of fibers at its native gelation environment is limited. Here, we show that an anhydrous form of caffeine undergo supramolecular self-assembly leading to microcrystalline network and immobilize the solvents at a concentration as low as 1.0 w/v%. The microcrystalline (d = >2 µm) network allows the direct visualization of the evolution and solvent encapsulation during gelation in its native state.
2. Results and Discussion
Caffeine (Figure 1a) being one of widely used alkaloids, can either be extracted from various sources or obtained commercially [34,35]. Caffeine is readily soluble in water (20 g/L at room temperature and 660 g/L at boiling temperature). The solubility in ethanol is relatively high (15 g/L). However, in our study when the commercial caffeine was recrystallized from ethanol, it resulted in an anhydrous form of caffeine. Surprisingly, when the recrystallized caffeine (see experimental Secion 4.2 for details) was dissolved in ethanol (2.0 w/v%) by heating and allowed to attain room temperature, the solution turned into a gel (Figure 1) and showed the resistance to flow upon inversion. More importantly, this property of caffeine has never been reported in the literature. Similar studies using other xanthines such as theobromine and theophylline showed that the gelation is unique to caffeine (Figure 1a).
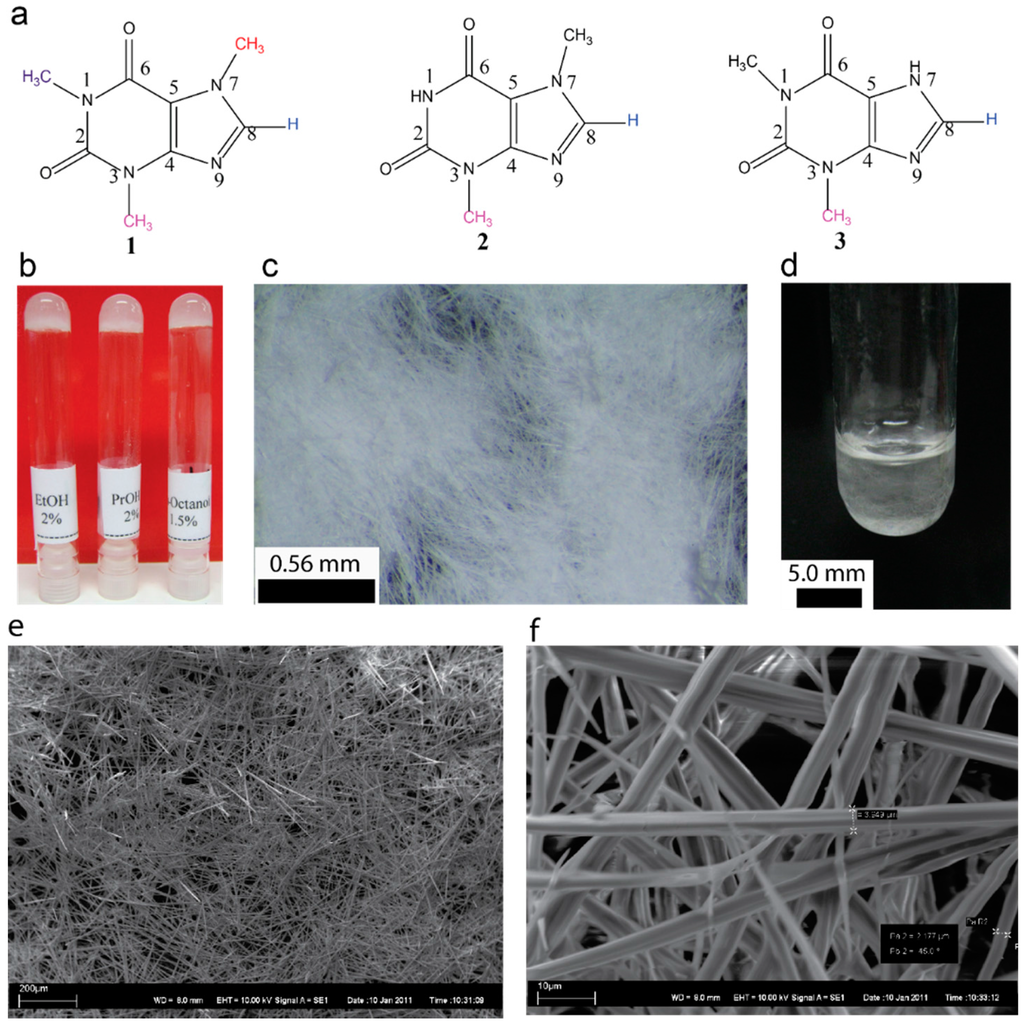
Figure 1.
(a) Chemical structures of molecules used in this study, caffeine 1, theobromine 2, and theophylline 3; (b) representative photographs of gels (2.0 w/v%) derived from caffeine; (c) 1-propanol gel upon placing on a glass slide shows entangled microfibrillar network; (d) photograph of 2.0 w/v% of caffeine in 1-propanol upon cooling a hot solution shows the fiber network before forming a stable gel; (e,f) SEM micrographs of 1-Octanol xerogel (2.0%).
Inspired by this observation, we undertook a systematic study on recrystallized form of caffeine. The gelation was observed in a number of alcohols with minimum gelation concentration of 1.0 w/v%. We point out that the aggregation of small molecules above 2.0% leading to gel like structure is common, therefore we do not include any solvent where the gels are formed above 2.0%. The gelation properties of caffeine was studied in 15 different alcoholic solvents and aromatic solvents (see Supplementary Materials, Table S1 for details).
We observed that the gels obtained from alcoholic solvents are opaque or translucent depending on the chain length (Figure 1b). The gelation occurred within 10–30 min upon allowing a hot solution containing a known amount of caffeine to attain the room temperature. Gelation at 1.0% required mild sonication of the hot solution for one minute. On the other hand, for 2.0% gels in alcoholic solvents, no sonication was required. Similarly, aromatic solvents such as chlorobenzene and toluene furnished a gelatinous fluid upon attaining the room temperature. However, sonicating the hot solutions for 1.0 min stable gels were obtained. Typical macroscopic and microscopic appearances of the selected organogels are shown in Figure 1b–f (see supplementary Figures S1 and S2). The gel melting temperature increased with increasing the chain length of alcohols (see Supplementary Figure S3). For a given solvent the gel melting temperature increased linearly with increasing the concentration (see Supplementary Figure S4).
The gels remained stable for several months and placing the gel over a glass surface shows the presence of high aspect ratio microcrystalline network (Figure 1c). Interestingly, the formation of crystalline network when a hot solution was allowed cool can be visualized directly in a test tube used for gelation studies as shown in Figure 1d. The scanning electron micrographs of xerogels displayed the micron sized self-assembled tubular structures, which are highly entangled (Figure 1e,f). The self-assembly of caffeine and other pharmaceutical compounds into a tubular structure have been well studied in the literature. This prompted us to further investigate the self-assembly in detail. The diameter of the microcrystalline structures varied from 2 to 4 µm in diameter. Higher order structures do allow more detailed observation thereby providing evidence for an existing hypothesis that the gelation is due to self-assembled 3D network formation. Studying the native gel is a challenge, especially when the nanoscale structures are formed. On the other hand, optical microscopy has been used to directly visualize SAFIN formation, as well as their collapse [44,45,46,47,48]. Since, the caffeine fibers are relatively large it provides an opportunity to visualize gelation in its native state, where the network formation, its growth and entanglements can be followed in its native state.
The formation of microcrystalline network following by solvent immobilization is shown in Figure 1d. Figure 2a–f shows the selected photographs illustrating the growth and entanglement of microcrystalline network upon placing a hot solution of caffeine (2.0%) in 1-propanol on a glass slide. Figure 2g–i shows selected photographs of a hot solution in a Petri dish forming small crystallites, which nucleate the growth of entangled network in the presence of solvent.
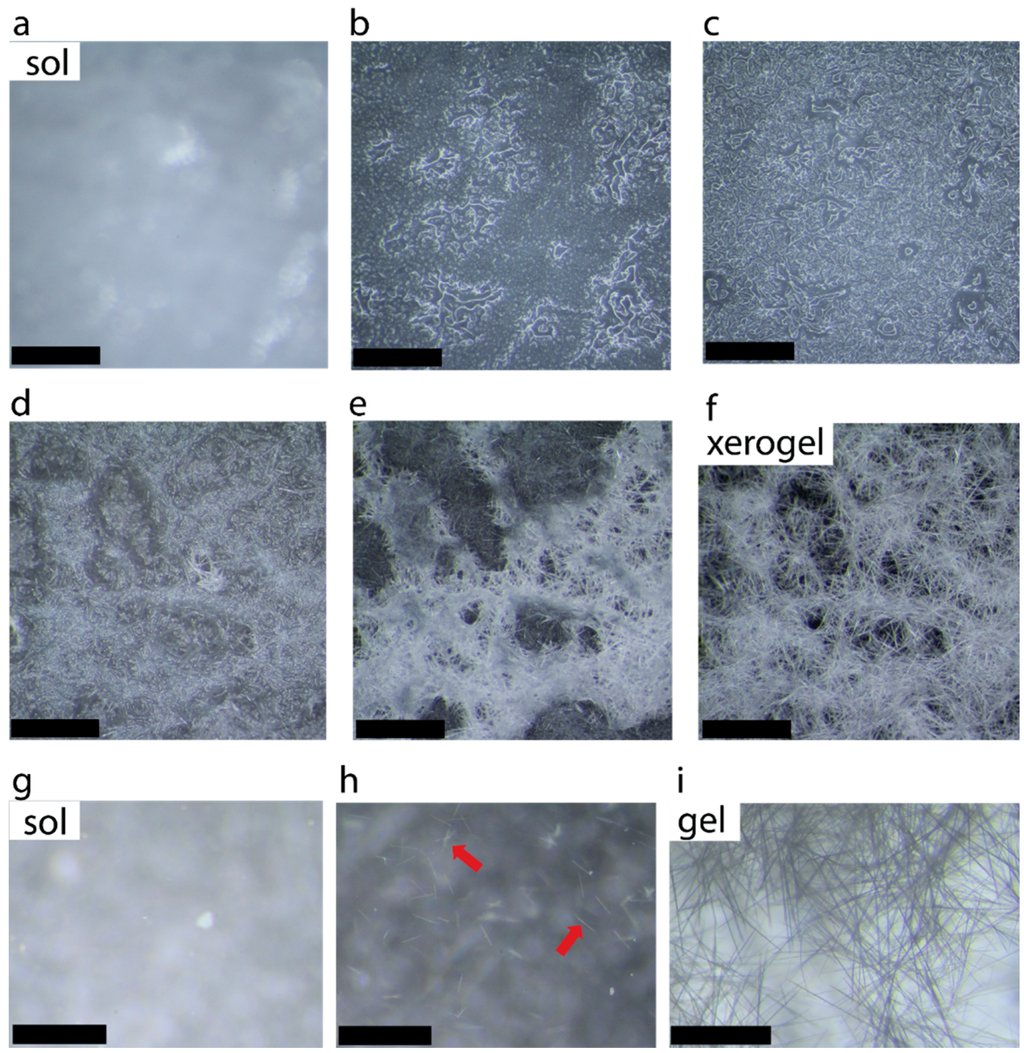
Figure 2.
Photographs of 2.0% caffeine in 1-propanol (a–f) when a hot solution was placed on a glass slide (scale bar 0.85 mm) and (g–i) selected images of 2-0% caffeine in 1-propanol hot solution placed in a petri dish showing fiber formation in the presence of solvent (scale bar: 0.92 mm). Red arrows indicate the formatin of microcrystals.
In order to compare the commercial caffeine, the recrystallized form and the xerogels, we used solid state 13C CPMAS NMR spectroscopy (Figure 3, see Supplementary Figure S5). The 13C CPMAS NMR results suggest that the recrystallized form of caffeine, and the xerogels display similar patterns in their 13C spectra and are comparable to the 13C CPMAS spectral patterns reported for the high temperature anhydrous form reported by Enright et al. [25]. The commercial caffeine, also shows a similar spectral pattern but the presence of overlapping peaks between 145–155 ppm and 25–30 ppm clearly suggests the presence of more than one form (Figure 3). We further confirm that both the commercial, and the recrystallized caffeine are anhydrous form of caffeine by elemental analysis. Finally, the gels were studied using solution 1H NMR (see Supplementary Figure S6) and variable temperature (VT) NMR spectroscopy (see Supplementary Figures S7–S10). The 1H NMR of the toluene-d8 gel showed broad signals, apart from that the signals from 10-CH3 and 12-CH3 now moved downfield (shielded) compared to that in CDCl3 solution (Figure 4). Upon heating both 10-CH3 and 12-CH3 signals showed a significant shift and while the 10-CH3 signal moved upfield upon heating, the 12-CH3 signal moved in the opposite direction. Meanwhile 8-H also shifted upfield in the NMR spectrum. Further, the variable temperature (VT) NMR of 1-octanol (non-deuterated) gel was performed (without field lock) to study the gel in its native state and similar observations were made (see Supplementary Figures S9–S11).
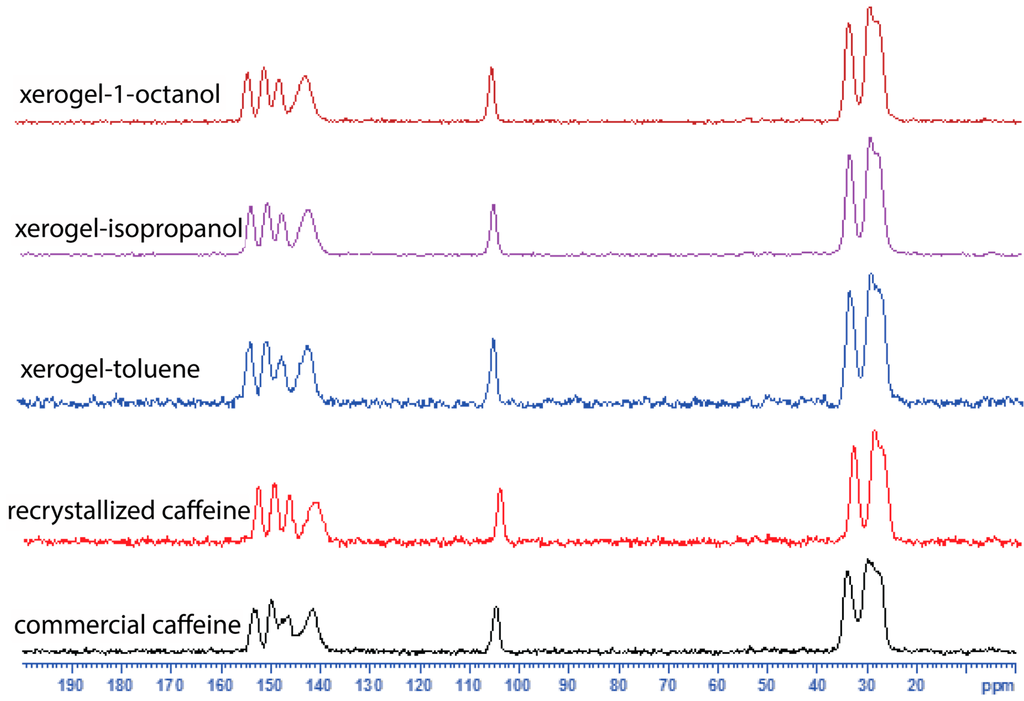
Figure 3.
Solid state 13C CPMAS NMR spectra of commercial caffeine, recrystallized form and xerogels.
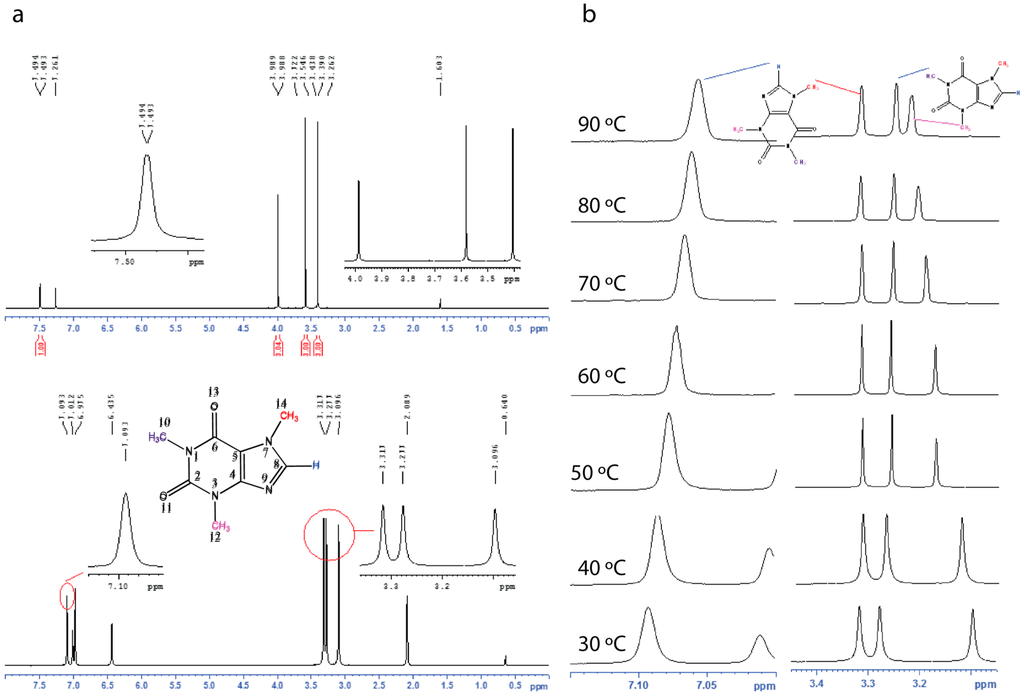
Figure 4.
Solution NMR Studies; (a) 1H NMR of caffeine in CDCl3 (top) and 2.0% toluene-d8 gel of caffeine and (b) variable temperature (VT) NMR of 2.0% toluene-d8 gel of caffeine. Also see supplementary Figures S6–S8.
3. Conclusions
In summary, we have shown that anhydrous caffeine has the ability to act as a low molecular weight gelator, a property which has not been reported earlier. We provide direct evidence for microcrystalline fiber formation leading to entangled 3D network leading to gelation. Caffeine being naturally abundant, commercially cheap and one of the highly consumed alkaloids with medicinal properties, we foresee that this property is a significant finding to generate both materials and pharmaceutically important gels in combination with suitable components. Further studies to use caffeine as a two-component gelator towards hydrogels are progress in our laboratory, and the results will be published elsewhere.
4. Experimental Section
4.1. Materials and Methods
Analytical grade reagents and solvents were used for the purification, crystallization and gelation studies. The solvents were dried and stored over 3 Å molecular sieves prior to use. Caffeine was obtained from Aldrich (99%). All the solvents, used for recrystallization and gelation tests were purchased from commercial suppliers (Sigma Aldrich, Helsinki, Finland), methanol, ethanol, 1-propanol, iso-propanol, 1-butanol, 1-pentanol, 1-hexanol, 1-heptanol, 1-octanol, 1-nonanol, 1-decanol, cyclohexanol, ethylene glycol, 1,2-pentanediol, toluene, benzene, xylenes, mesitylene, chlorobenzene and benzyl alcohol. 1H and 13C NMR experiments were measured in a Bruker Avance DRX 500 NMR spectrometer equipped with a 5 mm diameter broad-band inverse probe head working at 500.13 MHz for 1H and at 125.76 MHz for 13C. The 13C{1H} NMR spectra were measured using composite pulse, waltz16, decoupling. The solution state NMR spectra were measured in CDCl3 and toluene-d8. 1H NMR of 1-octanol gel was performed without lock. 1H and 13C chemical shifts were referenced to the solvent signals (δ = 7.26 for CDCl3, and δ = 2.09 for toluene-d8) for 1H and δ = 77.0 ppm for 13C from int. TMS (Helsinki, Finland). Elemental analyses experiments were carried out using Elementar Vario EL III-analyser (Hanau, Germany).
4.2. Recrystallization of Caffeine
In a 250 mL round-bottom flask, a mixture of commercial caffeine (200 mg) and ethanol (25 mL) was heated over an oil bath until the mixture turned clear solution. The hot solution was quickly filtered using Whatman No. 4 filter paper (VWR International Oy, Helsinki, Finland) and allowed to crystallize. The crystalline solid was dried under vacuum and used for further studies.
4.3. Gelation Tests
A typical gelation test was performed dissolving a known amount of sample in a test tube (l = 10 cm, d = 1.0 cm) in a solvent (0.5 mL) under investigation. The mixture was heated slowly until it turns into a clear solution (note: We avoid rapid heating due to sublimation properties of caffeine, which may lead to erroneous results). The solution was either allowed to attain room temperature (~22 °C) or subjected for sonication for 1 min. The formation of the gel was tested using the “resistance to flow upon inversion of the test tube”. Depending on the appearance the gels are denoted as, P = precipitate after attaining a hot solution to room temperature. G = gel, Gs = required sonication, S = solution.
4.4. Scanning Electron Microscopy (SEM) Studies
The sample preparation for SEM measurements was carried out by placing the hot sol (10 µL) on a carbon tape fixed over a sample stub. The sample was allowed to dry under ambient conditions for 24 h. The sample was sputter coated with gold in a JEOL Fine Coat Ion Sputter JFC-1100 (Tokyo, Japan) and the images were collected using Bruker Quantax400 EDS microscope (Berlin, Germany) equipped with a digital camera.
4.5. Solid-State NMR Studies
The 13C{1H}CP/MAS and 15N{1H}CP/MAS NMR spectra were recorded on a Bruker AV400 spectrometer (Bremen, Germany) equipped with a 4 mm standard bore CPMAS probe head whose X channel was tuned to 100.62 MHz for 13C and 40.55 MHz for 15N, respectively. The other channel was tuned to 400.13 MHz for broad band 1H decoupling. Approximately 100 mg of dried and finely powdered samples were packed in the ZrO2 rotor closed with Kel-F cap and spun at 10 KHz rate. The 13C{1H}CPMAS NMR was carried out for all samples under Hartmann-Hahn conditions with TPPM (tppm15) decoupling. The π/2 pulse for proton and carbons were found to be 4.0 μs and 5 μs at power levels of −5.0 dB and −4.0 dB, respectively. The experiments were conducted at contact time of 2 ms. A total of 20,000 scans were recorded with 5 s recycle delay for each sample. All free inducation decays (FIDs) were processed by exponential apodization function with line broadening of 20 Hz prior to FT. The 15N{1H}CP/MAS NMR experiments were carried out for all samples at a 10 kHz spinning rate under Hartmann-Hahn condition. The π/2pulses for proton and nitrogen were found to be 4.2 µs and 5 µs at power levels of −4.6 dB and −0.8 dB, respectively. The optimized contact time of 2 ms was used for efficient polarization transfer with a 5 s recycle delay to acquire the CP/MAS spectra. A total of 50,000 scans were acquired to obtain the CP/MAS spectra.
4.6. Variable Temperature NMR of Toulene-d8 Gel
Twelve milligrams of recrystallized caffeine was taken in NMR tube (d = 5 mm) and 600 µL of tolune-d8 was added. The sample was slowly heated until it turned into a clear solution. The solution was subjected for sonication and stabilized for 2 h. VTNMR was recorded from 30 °C to 90 °C with 10 °C increment at a time with 5 min of stabilizing time at each temperature.
4.7. Variable Temperature NMR of 1-Octanol Gel
Twelve milligrams of recrystallized caffeine was taken in NMR tube (d = 5 mm) and 600 µL of 1-octanol was added. The sample was slowly heated until it turned into a clear solution. The solution was subjected for sonication and stabilized for 2 h. VTNMR was recorded without lock from 30 °C to 90 °C with 10 °C increment at a time with 5 min of stabilizing time at each temperature.
4.8. Elemental Analysis
The elemental analyses were performed for the commercial and recrystallized form of caffeine using Elementar Vario EL III-analyser.
- Elemental analysis of commercial caffeine: C, 49.31; H, 5.20; N, 28.62.
- Elemental analysis of recrystallized form of caffeine: C, 49.66; H, 5.26; N, 28.78.
- Theoretical composition of caffeine (C8H10N4O2): C, 49.48; H, 5.19; N, 28.85.
Supplementary Materials
The following are available online at www.mdpi.com/2310-2861/2/1/9/s1, details of general experimental methods, recrystallization procedure, gelation tests, solid state NMR, electron microscopy and variable temperature NMR. Figures S1–S11; Table S1.
Acknowledgments
We thank the rector of the University of Jyväskylä and the Academy of Finland (Decision n:o 127006, 12 December 2008) for the financial support towards the post-doctoral fellowship and Centre for International Mobility (CIMO) Finland for a visiting researcher fellowship and Spectroscopy Laboratory Technician Reijo Kauppinen for his help with the solution NMR studies.
Author Contributions
Nonappa and Erkki Kolehmainen designed the project, interpreted the results and transformed it into practice.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Smith, A.R.F. A History of Coffee in Coffee: Botany, Biochemistry and Production of Beans and Beverage; Clifford, M.N., Willson, K.C., Eds.; Springer-Verlag: New York, NY, US, 1985; pp. 1–12. [Google Scholar]
- Weinberg, B.A.; Bealer, B.K. The World of Caffeine: The Science and Culture of the World’s Most Popular Drug; Routledge: New York, NY, USA, 2001. [Google Scholar]
- Suzuki, T.; Ashihara, H.; Waller, G.R. Purine and purine alkaloid metabolism in Camellia and Coffea plants. Phytochemistry 1992, 31, 2575–2584. [Google Scholar] [CrossRef]
- Alikaridis, F. Natural constituents of Ilex species. J. Ethnopharmacol. 1987, 20, 121–144. [Google Scholar] [CrossRef]
- Clifford, M.N.; Ramirez, J.R. Phenols and caffeine in wet-processed coffee beans and coffee pulp. Food Chem. 1991, 40, 35–42. [Google Scholar] [CrossRef]
- Mehr, C.B.; Biswal, R.N.; Collins, J.L.; Cochran, H.D. Supercritical carbon dioxide extraction of caffeine from guarana. J. Supercrit. Fluids 1996, 9, 185–191. [Google Scholar] [CrossRef]
- Li, S.; Hartland, S. A new industrial process for extracting cocoa butter and xanthines with supercritical carbon dioxide. J. Am. Oil Chem. Soc. 1996, 73, 423–429. [Google Scholar] [CrossRef]
- Nathanson, J. Caffeine and related methylxanthines: Possible naturally occurring pesticides. Science 1984, 226, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, R.G.; Armstrong, J.W.; Campbell, E. Pest control: Caffeine as a repellent for slugs and snails. Nature 2002, 417, 915–916. [Google Scholar] [CrossRef] [PubMed]
- Grobbee, D.E.; Rimm, E.B.; Giovannucci, E.; Colditz, G.; Stampfer, M.; Willett, W. Coffee, caffeine, and cardiovascular disease in men. N. Engl. J. Med. 1990, 323, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Denoeud, F.; Carretero-Paulet, L.; Dereeper, A.; Droc, G.; Guyot, R.; Pietrella, M.; Zheng, C.; Alberti, A.; Anthony, F.; Aprea, G.; et al. The coffee genome provides insight into the convergent evolution of caffeine biosynthesis. Science 2014, 345, 1181–1184. [Google Scholar] [CrossRef] [PubMed]
- Eskelinen, M.H.; Kivipelto, M. Caffeine as a protective factor in dementia and Alzheimer’s disease. J. Alzheimer’s Dis. 2010, 20, S167–S174. [Google Scholar]
- Song, F.; Qureshi, A.A.; Han, J. Increased caffeine intake is associated with reduced risk of basal cell carcinoma of the skin. Cancer Res. 2012, 72, 3282–3289. [Google Scholar] [CrossRef] [PubMed]
- Kerzendorfer, C.; O’Driscoll, M. UVB and Caffeine: Inhibiting the DNA Damage Response to Protect Against the Adverse Effects of UVB. J. Invest. Dermatol. 2009, 129, 1611–1613. [Google Scholar] [CrossRef] [PubMed]
- Sabisz, M.; Skladanowski, A. Modulation of cellular response to anticancer treatment by caffeine: Inhibition of cell-cycle checkpoints, DNA repair and more. Curr. Pharm. Biotechnol. 2008, 9, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Bučar, D.K.; Henry, R.F.; Lou, X.; Duers, R.W.; MacGillivray, L.R.; Zhang, G.G.Z. Cocrystals of caffeine and hydroxybenzoic acids composed of multiple supramolecular heterosynthons: Screening via solution-mediated phase transformation and structural characterization. Cryst. Growth Des. 2009, 9, 1932–1943. [Google Scholar] [CrossRef]
- Leyssens, T.; Tumanova, N.; Robeyns, K.; Candoni, N.; Veesler, S. Solution cocrystallization, an effective tool to explore the variety of cocrystal systems: Caffeine/dicarboxylic acid cocrystals. CrystEngComm 2014, 16, 9603–9611. [Google Scholar] [CrossRef]
- Carlucci, L.; Gavezzotti, A. Molecular recognition and crystal energy landscapes: An X-ray and computational study of caffeine and other methylxanthines. Chem. Eur. J. 2004, 11, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, A.; Simo, A.; Fenger, R.; Christen, W.; Rademann, K.; Panne, U.; Emmerling, F. Morphological diversity of caffeine on surfaces: needles and hexagons. Cryst. Growth Des. 2012, 12, 583–588. [Google Scholar] [CrossRef]
- Schultheiss, N.; Roe, M.; Boerrigter, S.X.M. Cocrystals of nutraceutical p-coumaric acid with caffeine and theophylline: Polymorphism and solid-state stability explored in detail using their crystal graphs. CrystEngComm 2011, 13, 611–619. [Google Scholar] [CrossRef]
- Hedoux, A.; Guinet, Y.; Paccou, L.; Danede, F.; Derollez, P. Polymorphic transformation of anhydrous caffeine upon grinding and hydrostatic pressurizing analyzed by low-frequency raman spectroscopy. J. Pharm. Sci. 2013, 102, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Sutor, J. The structures of the pyrimidines and purines. VII. The crystal structure of caffeine. Acta Cryst. 1958, 11, 453–458. [Google Scholar] [CrossRef]
- Edwards, H.G.M.; Lawson, E.; de Matas, M.; Shields, L.; York, P. Metamorphosis of caffeine hydrate and anhydrous caffeine. J. Chem. Soc., Perkin Trans. 1997, 2, 1985–1990. [Google Scholar] [CrossRef]
- Lehmann, C.W.; Stowasser, F. The crystal structure of anhydrous β-caffeine as determined from X-ray powder-diffraction data. Chem. Eur. J. 2007, 13, 2908–2911. [Google Scholar] [CrossRef] [PubMed]
- Enright, G.D.; Terskikh, V.V.; Brouwer, D.H.; Ripmeester, J.A. The Structure of Two Anhydrous Polymorphs of Caffeine from single-crystal diffraction and ultrahigh-field solid-state 13C NMR Spectroscopy. Cryst. Growth Des. 2007, 7, 1406–1410. [Google Scholar] [CrossRef]
- Eddleston, M.D.; Jones, W. Formation of tubular crystals of pharmaceutical compounds. Cryst. Growth Des. 2010, 10, 365–370. [Google Scholar] [CrossRef]
- Hirst, A.R.; Escuder, B.; Miravet, J.F.; Smith, D.K. High-tech applications of self-assembling supramolecular nanostructured gel-phase materials: From regenerative medicine to electronic devices. Angew. Chem. Int. Ed. 2008, 47, 8002–8018. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.G.; Terech, P. Molecular Gels: Materials With Self-Assembled Fibrillar Networks; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Nonappa; Maitra, U. Unlocking the potential of bile acids in synthesis, supramolecular/materials chemistry and nanoscience. Org. Biomol. Chem. 2008, 6, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Zinic, M.; Vögtle, F.; Fages, F. Cholesterol-based gelators. Top. Curr. Chem. 2005, 256, 39–76. [Google Scholar] [PubMed]
- Smith, D.K. Organic Nanostructures; Atwood, J.L., Steed, J.W., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; pp. 111–154. [Google Scholar]
- Weiss, R.G. Preface to the molecular and polymer gels; materials with self-assembled fibrillar networks special issue. Langmuir 2009, 25, 8369. [Google Scholar] [CrossRef] [PubMed]
- Estroff, L.A.; Hamilton, A.D. Water gelation by small organic molecules. Chem. Rev. 2004, 104, 1201–1218. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.G. The past, present, and future of molecular gels. What is the status of the field, and where is it going? J. Am. Chem. Soc. 2014, 136, 7519–7530. [Google Scholar] [CrossRef] [PubMed]
- Lana, Y.; Corradinia, M.G.; Weiss, R.G.; Raghavan, S.R.; Rogers, M.A. To gel or not to gel: Correlating molecular gelation with solvent parameters. Chem. Soc. Rev. 2015, 44, 6035–6058. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Llansola, F.; Miravet, J.F.; Escuder, B. Aldehyde responsive supramolecular hydrogels: Towards biomarker-specific delivery system. Chem. Commun. 2011, 47, 4706–4708. [Google Scholar] [CrossRef] [PubMed]
- Bunzen, H.; Nonappa; Kalenius, E.; Hietala, S.; Kolehmainen, E. Subcomponent self-assembly: A quick way to novel metallogels. Chem. Eur. J. 2013, 19, 12978–12981. [Google Scholar] [CrossRef] [PubMed]
- Svobodová, H.; Nonappa; Lahtinen, M.; Wimmer, Z.; Kolehmainen, E. A steroid-based gelator of A(LS)2 type: Tuning gel properties by metal coordination. Soft Matter 2012, 8, 7840–7847. [Google Scholar] [CrossRef]
- Svobodová, H.; Nonappa; Wimmer, Z.; Kolehmainen, E. Design, synthesis and stimuli responsive gelation of novel stigmasterol-amino acid conjugates. J. Colloid Interface Sci. 2011, 361, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Estroff, L.A.; Leiserowitz, L.; Addadi, L.; Weiner, S.; Hamilton, A.D. Characterization of an organic hydrogel: A cryo-TEM and X- ray diffraction study. Adv. Mater. 2003, 15, 38–42. [Google Scholar] [CrossRef]
- Wang, R.; Geiger, C.; Chen, L.; Swanson, B.; Whitten, D.G. Direct Observation of sol−gel conversion: The role of the solvent in organogel formation. J. Am. Chem. Soc. 2000, 122, 2399–2400. [Google Scholar] [CrossRef]
- Wang, R.; Liu, X.-Y.; Xiong, J.; Li, J. Real-time observation of fiber network formation in molecular organogel: Supersaturation-dependent microstructure and its related rheological property. J. Phys. Chem. B 2006, 110, 7275–7280. [Google Scholar] [CrossRef] [PubMed]
- Nonappa; Maitra, U. Simple esters of cholic acid as potent organogelators: Direct imaging of the collapse of SAFINs. Soft Matter 2007, 3, 1428–1433. [Google Scholar] [CrossRef]
- Iqbal, S.; Rodríguez-Lansola, F.; Escuder, B.; Miravet, J.F.; Verbruggen, I.; Willem, R. HRMAS 1H NMR as a tool for the study of supramolecular gels. Soft Matter 2010, 6, 1875–1878. [Google Scholar] [CrossRef]
- Nonappa; Lahtinen, M.; Behera, B.; Kolehmainen, E.; Maitra, U. Unraveling the packing pattern leading to gelation using SS NMR and X-ray diffraction: Direct observation of the evolution of self-assembled fibers. Soft Matter 2010, 6, 1748–1757. [Google Scholar] [CrossRef]
- Nonappa; Šaman, D.; Kolehmainen, E. Studies on supramolecular gel formation using DOSY NMR. Mag. Res. Chem. 2015, 53, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Noponen, V.; Nonappa; Lahtinen, M.; Valkonen, A.; Salo, H.; Kolehmainen, E.; Sievänen, E. Bile acid–amino acid ester conjugates: Gelation, structural properties, and thermoreversible solid to solid phase transition. Soft Matter 2010, 6, 3789–3796. [Google Scholar] [CrossRef]
- Ikonen, S.; Nonappa; Lahtinen, M.; Valkonen, A.; Salo, H.; Kolehmainen, E. Bile acid-derived mono- and diketals—Synthesis, structural characterization and self-assembling properties. Org. Biomol. Chem. 2010, 8, 2784–2794. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).