Evaluation of the Effect of Oregano Essential Oil and Emulsifier Ratio on the Physicochemical, Mechanical, and Antioxidant Properties of Corn Starch Films Based on Gel Matrices
Abstract
1. Introduction
2. Results and Discussion
2.1. Yield and Physicochemical Characterization of OEO
2.1.1. Yield
2.1.2. Density
2.2. Quantification of Phenolic Compounds and Flavonoids
2.3. Antioxidant Activity Evaluated by DPPH, ABTS, and FRAP
2.4. Appearance of Starch Films Formed from Gel Matrices
2.5. Rheological Characterization of the Starch Films Formed from Gel Matrices Solutions (GFFS)
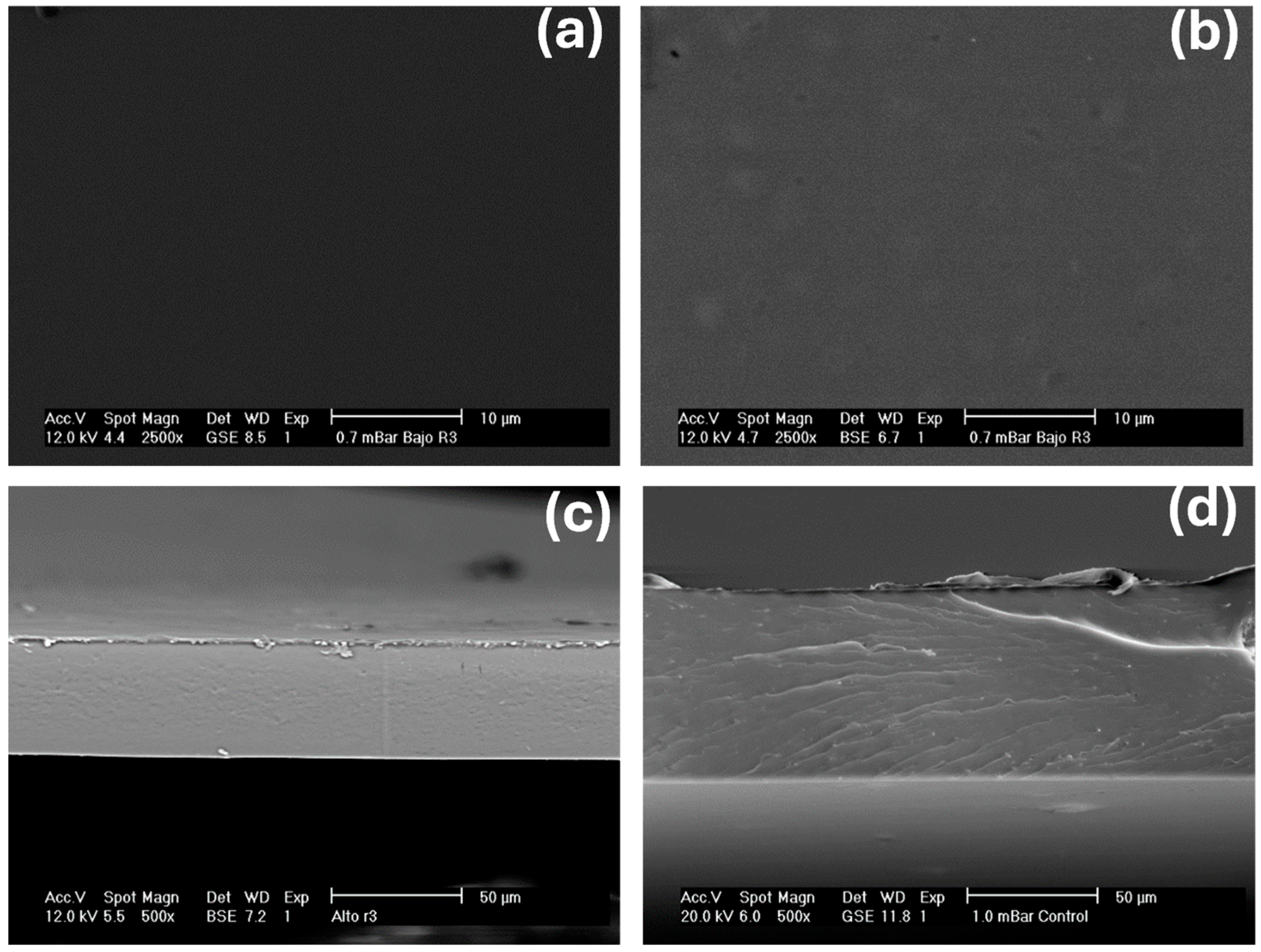
2.6. Scanning Electron Microscopy (SEM) Analysis
2.7. Mechanical Properties Evaluation of the Films
2.8. Water Vapor Permeability (WVP) Evaluation of the Films
2.9. Determination of Phenolic Compounds and Flavonoids in OEO-Containing Films
2.10. Antioxidant Activity Evaluation (DPPH, ABTS, and FRAP) in OEO-Containing Films
3. Conclusions
4. Materials and Methods
4.1. Plant Material
4.2. Extraction of OEO
4.3. Physicochemical Characterization of OEO
4.3.1. Essential Oil Yield Determination
4.3.2. Determination of Essential Oil Density
4.4. Determination of Total Phenolic Content in OEO
4.5. Determination of Total Flavonoid Content in OEO
4.6. Antioxidant Activity by the DPPH Method
4.7. Antioxidant Activity by the ABTS Method
4.8. Assessment of Antioxidant Capacity Using the FRAP Method
4.9. Development of OEO Starch Films Formed from Gel Matrices
4.10. Rheology of Starch Films Formed from Gel Matrices Solutions (GFFS)
4.11. Color Determination on Films
4.12. Evaluation of Mechanical Properties of the Films
4.13. Evaluation of Water Vapor Permeability (WVP) of Films
4.14. Extraction of Phenolic Compounds and Antioxidants from the Films
4.15. Quantification of Phenolic Compounds in Films
4.16. Scanning Electron Microscopy (SEM) Evaluation of Films
4.17. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghizdareanu, A.; Pasarin, D.; Banu, A.; Ionit, A.; Enascuta, C.E.; Vlaicu, A. Accelerated shelf-life and stability testing of hydrolyzed corn starch films. Polymers 2023, 15, 889. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Tye, Y.Y.; Saurabh, C.K.; Leh, C.P.; Lai, T.K.; Chong, E.W.N.; Nurul Fazita, M.R.; Mohd Hafidz, J.; Banerjee, A.; Syair, M.I. Biodegradable polymer films from seaweed polysaccharides: A review on cellulose as a reinforcement material. Express Polym. Lett. 2017, 11, 244–265. [Google Scholar] [CrossRef]
- Flores-Córdova, M.A.; Uribe-Cruz, G.; Salas-Salazar, N.A.; Sáenz-Mendoza, A.I.; Calderón-Loera, R.; Zamudio-Flores, P.B. Efecto de la temperatura de secado en las propiedades fisicoquímicas, mecánicas y de permeabilidad al vapor de agua de películas de almidón de maíz. Rev. Iberoam. Polímeros 2024, 25, 1–14. [Google Scholar]
- Gao, S.; Liu, R.; Song, H.; Huang, D.; Dai, Y.; Hou, H.; Wang, W. Regulation of fine structure of different types of natural waxes by octenyl succinate starch in starch bioplastics. Food Biosci. 2024, 59, 103920. [Google Scholar] [CrossRef]
- Hernández, M.S.; Ludueña, L.N.; Flores, S.K. Combined effect of oregano essential oil and glycerol on physicochemical properties of antimicrobial films based on chitosan and acetylated starch. Food Hydrocoll. 2024, 156, 110259. [Google Scholar] [CrossRef]
- Criollo-Feijoo, J.; Salas-Gómez, V.; Cornejo, F.; Auras, R.; Salazar, R. Cassava bagasse starch and oregano essential oil as a potential active food packaging material: A physicochemical, thermal, mechanical, antioxidant, and antimicrobial study. Heliyon 2024, 10, e36150. [Google Scholar] [CrossRef]
- Hernández, M.S.; Ludueña, L.N.; Flores, S.K. Citrid acid, chitosan and oregano essential oil impact on physical and antimicrobial properties of cassava starch films. Carbohydr. Polym. Technol. Appl. 2023, 5, 100307. [Google Scholar] [CrossRef]
- do Evangelho, J.A.; da Silva Dannenberg, G.; Biduski, B.; Halal, S.L.M.; Kringel, D.H.; Gularte, M.A.; Fiorentini, A.M.; da Rosa Zavareze, E. Antibacterial activity, optical, mechanical, and barrier properties of corn starch films containing orange essential oil. Carbohydr. Polym. 2019, 222, 114981. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, X.; Zhang, Y.; Xu, E.; Yan, S.; Huaide, X.; Mei, L. Recent advances in the fabrication, character-561 ization and application of starch-based materials for active food packaging: Hydrogels and aerogels. Sustain. Food Technol. 2024, 2, 615–634. [Google Scholar] [CrossRef]
- Monzón, L.T.; Cama, D.N. Estudio de la composición química del aceite esencial de orégano (Origanum vulgare spp.) de Tacna. Ing. Ind. 2017, 35, 195–205. [Google Scholar] [CrossRef][Green Version]
- Cáceres, M.B.; Rozo, V.F.; Del Valle, G.E. Estudio de la calidad de aceites esenciales de orégano, tomillo y romero cultivados en severino (el carmen, jujuy) recolectados en invierno y primavera. Rev. Cient. Fac. Cienc. Agrar. UNJu 2021, 14, 7–18. [Google Scholar][Green Version]
- Mera, M.C. Caracterización química del aceite esencial de orégano como agente bioconservador en alimentos. Univ. Cienc. Tecnol. 2020, 24, 54–62. [Google Scholar] [CrossRef]
- Costa, D.C.; Costa, H.S.; Alburqueque, T.G.; Ramos, F.; Castilho, M.C.; Sanches-Silva, A. Advances in phenolic compounds analysis of aromatic plants and their potential applications. Trends Food Sci. Technol. 2015, 45, 336–354. [Google Scholar] [CrossRef]
- Mohideen, M.; Hazmie, M.; Izzati, N.; Kamaruzaman, N. Review on extraction methods of essential oil from kaffir lime (Citrus hystrix) leaves. J. Acad. 2021, 9, 173–184. [Google Scholar] [CrossRef]
- Ifeanyichukwu, E.; Onoriode, O.S. Determining the Physicochemical Properties of Lemongrass oil Extract for Suitability in Various Applications. J. Nutr. Food Process. 2023, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Arcilla-Lozano, C.C.; Loarca-Piña, G.; Lecona-Uribe, S.; González de Mejía, E. El orégano: Propiedades, composición y actividad biológica de sus componentes. Arch. Latinoam. Nutr. 2004, 54, 100–111. [Google Scholar]
- Cardona, L.F.V.; Díaz, J.C.Q. Extracción y caracterización de aceite esencial de orégano, especie Plectranthus amboinicus, a partir de cultivos orgánicos del Magdalena Medio en Colombia/Extração e caracterização do óleo essencial de orégano, espécie Plectranthus amboinicus, de cultivos orgânicos de Magdalena Medio na Colômbia. Braz. J. Anim. Environ. Res. 2022, 5, 550–563. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants-Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef] [PubMed]
- Sarfaraz, D.; Rahimmalek, M.; Sabzalian, M.R.; Gharibi, S.; Matkowski, A.; Szumny, A. Essential Oil Composition and Antioxidant Activity of Oregano and Marjoram as Affected by Different Light-Emitting Diodes. Molecules 2023, 28, 3714. [Google Scholar] [CrossRef]
- Rios, S.J.C.; Rosales, S.R.; Velazquez-Quiñonez, S.E.; López, J.A.; Herrera, M.D. Phenolic Compounds and Antioxidant Capacity of Essential Oregano Oil from two Federal Entities in Mexico. Biotecnol. Sustentabilidad 2024, 9, 12–20. [Google Scholar] [CrossRef]
- Jung, W.S.; Chang, I.M.; Hwang, M.H.; Kim, S.H.; Yu, C.Y.; Chimire, B.K. Application of light-emiting diodes for improving the nutritional quality and bioactive compound levels of some crops and medicinal plantas. Molecules 2021, 26, 1477. [Google Scholar] [CrossRef]
- Malisch, C.S.; Salminen, J.P.; Kölliker, R.; Engström, M.T.; Suter, D.; Studer, B.; Lüscher, A. Drought effects on proanthocyanidins in sainfoin (Onobrychis viciifolia Scop.) are dependent on the plant’s ontogenetic stage. J. Agric. Food Chem. 2016, 64, 9307–9316. [Google Scholar] [CrossRef]
- Kotha, R.R.; Tareq, F.S.; Yildiz, E.; Luthria, D.L. Oxidative stress and antioxidants—A critical review on in vitro antioxidant assays. Antioxidants 2022, 11, 2388. [Google Scholar] [CrossRef]
- Kosakowska, O.; Węglarz, Z.; Pióro-Jabrucka, E.; Przybył, J.L.; Kraśniewska, K.; Gniewosz, M.; Bączek, K. Antioxidant and Antibacterial Activity of Essential Oils and Hydroethanolic Extracts of Greek Oregano (O. vulgare L. subsp. hirtum (Link) Ietswaart) and Common Oregano (O. vulgare L. subsp. vulgare). Molecules 2021, 26, 988. [Google Scholar] [CrossRef]
- Baranauskaite, J.; Kubiliene, A.; Marksa, M.; Petrikaite, V.; Vitkevičius, K.; Baranauskas, A.; Bernatoniene, J. HPLC postcolumn DPPH method and anticancer activity of carvacrol and rosmarinic acid. BioMed Res. Int. 2017, 26, 988. [Google Scholar] [CrossRef]
- Yañez, M.S.; Molina, R.R.; Paz, J.W.; Márquez, D.M. Estudio del potencial antioxidante de aceites esenciales de laurel, orégano y damiana pretratados con ultrasonido. Rev. Divulg. Cient. Tecnol. 2020, 7, 69–74. [Google Scholar]
- Arango, B.O.; Pantoja, D.D.; Santacruz, C.H.L.; Hurtado, B.A. Antioxidant activity of essential oils of oregano (Lippia origanoides h.b.k) grown in alto patia. Biotecnol. Sect. Agropecu. Agroind. 2012, 10, 79–86. [Google Scholar]
- Anaya-Esparza, L.M.; Vargas-Torres, A.; Palma-Rodríguez, H.M.; Castro-Mendoza, M.P.; Yahia, E.M.; Pérez-Larios, A.; Montalvo-González, E. Effect of mixed oxide-based TiO2 on the physicochemical properties of chitosan films. Period. Polytech. Chem. Eng. 2022, 66, 422–436. [Google Scholar] [CrossRef]
- Shah, A.; Masoodi, F.A.; Gani, A.; Ashwar, B.A. Physicochemical, rheological and structural characterization of acetylated starches. LWT-Food Sci. Technol. 2017, 80, 19–26. [Google Scholar] [CrossRef]
- Zamudio-Flores, P.B.; García-Amezquita, L.E.; Ramos-Martínez, A.; Gallegos-Tirado, J.M.; Bello-Pérez, L.A.; Salgado-Delgado, R. Soluciones formadoras de película a base de almidón oxidado de avena mezcladas con quitosano: Caracterización reológica y propiedades mecánicas de sus películas. Rev. Iberoam. Polímeros 2013, 14, 293–304. [Google Scholar]
- Edo, G.I.; Ndudi, W.; Ali, A.B.M.; Yousif, E.; Zainulabdeen, K.; Akpoghelie, P.O.; Isoje, E.F.; Igboku, U.A.; Opiti, R.A.; Athan Essaghah, A.E.; et al. Chitosan: An overview of its properties, solubility, functional technologies, food and health applications. Carbohydr. Res. 2025, 550, 109409. [Google Scholar] [CrossRef]
- Tirado-Gallegos, J.M.; Zamudio-Flores, P.B.; Ornelas-Paz, J.d.J.; Rios-Velasco, C.; Olivas Orozco, G.I.; Espino-Díaz, M.; Baeza-Jiménez, R.; Buenrostro-Figueroa, J.J.; Aguilar-González, M.A.; Lardizábal-Gutiérrez, D. Elaboration and Characterization of Active Apple Starch Films Incorporated with Ellagic Acid. Coatings 2018, 8, 384. [Google Scholar] [CrossRef]
- Hernández-Centeno, F.; Hernández-González, M.; Tirado-Gallegos, J.M.; Rodríguez-González, C.A.; Rios-Velasco, C.; Rodríguez-Hernández, A.M.; López-De la Peña, H.Y.; Zamudio-Flores, P.B. Biobased films from unconventionally sourced starch (Cucurbita foetidissima Kunth) and oregano essential oil (Lippia berlandieri Schauer): A look at their physicochemical properties. MRS Adv. 2024, 9, 523–530. [Google Scholar] [CrossRef]
- Ukwatta, R.H.; Yuan, R.; Ma, Y.; Xiong, X.; Hu, Y.; Li, C.; Xue, F. Effect of lipid addition on the physicochemical, structural, and photoactive antibacterial properties of cornstarch-chlorophyllin composite film. Food Res. Int. 2025, 202, 115699. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, R.; Ahmed, J.; Thakur, R.; Sarkar, P. Starch-based edible packaging: Rheological, thermal, mechanical, microstructural, and barrier properties—A review. Sustain. Food Technol. 2024, 2, 307. [Google Scholar] [CrossRef]
- Awang Wahab, D.N.; Siddique, M.B.M.; Khairuddin, N.; Chew, J.J.; Su, H.T. Mechanical, structural and barrier properties of starch-based film reinforced with cellulose microfibres extracted from midribs of Musa Saba’. Food Res. 2024, 8, 117–123. [Google Scholar] [CrossRef]
- Li, J.; Ye, F.; Lei, L.; Zhao, G. Combined effects of octenylsuccination and oregano essential oil on sweet potato starch films with emphasis on water resistance. Int. J. Biol. Macromol. 2018, 115, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Salgado, P.R.; Ortiz, C.M.; Musso, Y.S.; Di Giorgio, L.; Mauri, A.N. Edible films and coatings containing bioactives. Curr. Opin. Food Sci. 2015, 5, 86–92. [Google Scholar] [CrossRef]
- Singh, G.P.; Bangar, S.P.; Yang, T.; Trif, M.; Kumar, V.; Kumar, D. Effect on the Properties of Edible Starch-Based Films by the Incorporation of Additives: A Review. Polymers 2022, 14, 1987. [Google Scholar] [CrossRef]
- Lucinéia, C.; Monica, S.; Grasiele, M.; Fabio, Y.; Polyana, B.; Venicio, C.; Edineia, B. Production, antioxidant characterization and application of active starch-based films containing essential oils for beef packaging. Res. Soc. Dev. 2021, 10, e4310816903. [Google Scholar] [CrossRef]
- Majid, A.; Elham, A.; Zahra, A.; Hassan, H.; Mohammad, H. Evaluation of in vitro Antioxidant Characteristics of Corn Starch Bioactive Films Impregnated with Bunium persicum and Zataria multiflora Essential Oils. Annu. Res. Rev. Biol. 2017, 15, 1–9. [Google Scholar] [CrossRef]
- Martins, P.C.; Bagatini, D.C.; Martins, V.G. Oregano essential oil addition in rice starch films and its effects on the chilled fish storage. J. Food Sci. Technol. 2020, 58, 1562–1573. [Google Scholar] [CrossRef]
- Wei, A.; Shibamoto, T. Antioxidant/lipoxygenase inhibitory activities and chemical compositions of selected essential oils. J. Agric. Food Chem. 2010, 58, 7218–7225. [Google Scholar] [CrossRef] [PubMed]
- Sanches-Silva, A.; Costa, D.; Albuquerque, T.G.; Buonocore, G.G.; Ramos, F.; Castilho, M.C.; Machado, A.V.; Costa, H.S. Trends in the use of natural antioxidants in active food packaging: A review. Food Addit. Contam. 2014, 31, 374–395. [Google Scholar] [CrossRef] [PubMed]
- Nakas, A.; Giannarelli, G.; Fotopoulos, I.; Chainoglou, E.; Peperidou, A.; Kontogiannopoulos, K.N.; Tsiaprazi-Stamou, A.; Varsamis, V.; Gika, H.; Hadjipavlou-Litina, D.; et al. Optimizing the Distillation of Greek Oregano-Do Process Parameters Affect Bioactive Aroma Constituents and In Vitro Antioxidant Activity? Molecules 2023, 28, 971. [Google Scholar] [CrossRef]
- Ovares, J. Determinación de los Rendimientos y Caracterización de Aceites Esenciales Obtenidos por Hidrodestilación a Partir de Lippia alba y Rosmarinus officinalis. Ph.D. Thesis, Universidad de Costa Rica, San José, Costa Rica, 2016. [Google Scholar]
- Singleton, F.B.; Ross, C.W. Colorimetric of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Cardador-Martínez, A.; Albores, A.; Bah, M.; Calderón-Salinas, V.; Castaño-Tostado, E.; Guevara-González, R.; Shimada-Miyasaka, A.; Loarca-Piña, G. Relationship among antimutagenic, antioxidant and enzymatic activities of methanolic extract from common beans (Phaseolus vulgaris L.). Plant Foods Hum. Nutr. 2006, 61, 161–168. [Google Scholar] [CrossRef]
- Kuskoski, E.M.; Asuero, A.G.; Troncoso, A.M.; Mancini-Filho, J.; Fett, R. Aplicación de diversos métodos químicos para determinar actividad antioxidante en pulpa de frutos. Ciência Tecnol. Aliment. Camp. 2005, 25, 726–732. [Google Scholar] [CrossRef]
- Rubio, C.P.; Hernández-Ruiz, J.; Martínez-Sbuela, S.; Tvarijonaviciute, A.; Ceron, J.J. Spectrophotometric assays for total antioxidant capacity (TAC) in dog serum: An update. BMC Vet. Res. 2016, 12, 166. [Google Scholar] [CrossRef]
- Corsico, F.A.; Larrosa, V.J. Aceite esencial de orégano: Influencia en las propiedades de barrera en películas comestibles. In Proceedings of the XX Jornadas de Jovens Pesquisadores, Universidad Federal de Paraná: Curitiba, Brasil, 3 October 2012. [Google Scholar]
- Abedinia, A.; Motamedzadegan, A.; Ghavami, M.; Shahidi, S.A.; Rashidinejad, A. Development of alginate-based edible films using natural antimicrobial compounds: The influence on physicochemical properties and antibacterial activity. J. Food Process. Preserv. 2018, 42, e13301. [Google Scholar] [CrossRef]
- Zamudio-Flores, P.B.; Vargas-Torres, A.; Pérez-González, J.; Bosquez-Molina, E.; Bello-Pérez, L.A. Films prepared with oxidized banana starch: Mechanical and barrier properties. Starch/Stärke 2006, 58, 274–282. [Google Scholar] [CrossRef]
- ASTM E-96-80; Standard Test Methods for Water Vapor Transmission of Materials in Sheet Form. ASTM International: West Conshonhocken, PA, USA, 1989.
- Walpole, E.R.; Myers, H.R.; Myers, L.S. Probabilidad y Estadística Para Ingenieros, 6th ed.; Prentice-Hall Hispanoamericana, S.A.: Naucalpan de Juárez, Mexico, 1999; pp. 481–482. [Google Scholar]

| Analysis | Result |
|---|---|
| |
| Yield (% w/w) | 2.190 ± 0.360 |
| Density (g/cm3) | 0.899 ± 0.008 |
| |
| Phenols (mg g−1 GAE) | 12.010 ± 0.253 |
| Flavonoids (mg g−1 QE) | 0.875 ± 0.013 |
| DPPH (μmol TE g−1) | 6.070 ± 0.012 |
| ABTS (μmol TE g−1) | 6.640 ± 0.016 |
| FRAP (μmol TE g−1) | 225.410 ± 0.010 |
| Starch Films Formed from Gel Matrices Solution Sample | Rheological Variable | ||
|---|---|---|---|
| n | k | R2 | |
| Control | 0.528 ± 0.008 a | 0.841 ± 0.159 a | 0.997 ± 0.001 a |
| F1 | 0.625 ± 0.010 a | 1.018 ± 0.857 a | 0.975 ± 0.020 a |
| F2 | 0.705 ± 0.025 a | 1.257 ± 0.936 a | 0.984 ± 0.025 a |
| F3 | 0.683 ± 0.033 a | 1.198 ± 0.877 a | 0.989 ± 0.010 a |
| F4 | 0.711 ± 0.060 a | 1.308 ± 0.921 a | 0.995 ± 0.011 a |
| F5 | 0.608 ± 0.047 a | 1.382 ± 0.598 a | 0.991 ± 0.015 a |
| F6 | 0.721 ± 0.055 a | 1.405 ± 0.661 a | 0.988 ± 0.028 a |
| F7 | 0.587 ± 0.039 a | 1.411 ± 0.705 a | 0.993 ± 0.033 a |
| F8 | 0.477 ± 0.018 a | 1.466 ± 0.140 a | 0.995 ± 0.004 a |
| F9 | 0.491 ± 0.022 a | 1.328 ± 0.845 a | 0.990 ± 0.035 a |
| F10 | 0.501 ± 0.041 a | 1.675 ± 0.250 a | 0.986 ± 0.019 a |
| Film (Formulation and Nomenclature) | Formulation (To Prepare 100 g of Starch Films Formed from Gel Matrices-Forming Solution) |
|---|---|
| Formulation 1 (F1) | Starch = 4.00 g, Glycerol = 2.00 g, OEO = 0.129 g, Tween 80 = 0.0287 g, Distilled water = 93.80 g. |
| Formulation 2 (F2) | Starch = 4.00 g, Glycerol = 2.00 g, OEO = 0.129 g, Tween 80 = 0.0140 g, Distilled water = 93.90 g. |
| Formulation 3 (F3) | Starch = 4.00 g, Glycerol = 2.00 g, OEO = 0.0645 g, Tween 80 = 0.0140 g, Distilled water = 93.90 g. |
| Formulation 4 (F4) | Starch = 4.00 g, Glycerol = 2.00 g, OEO = 0.0323 g, Tween 80 = 0.0140 g, Distilled water = 93.90 g. |
| Formulation 5 (F5) | Starch = 4.00 g, Glycerol = 2.00 g, OEO = 0.1290 g, Tween 80 = 0.0140 g, Distilled water = 93.85 g. |
| Formulation 6 (F6) | Starch = 4.00 g, Glycerol = 2.00 g, OEO = 0.129 g, Tween 80 = 0.0140 g, Distilled water = 93.85 g. |
| Formulation 7 (F7) | Starch = 5.00 g, Glycerol = 2.50 g, OEO = 0.0046 g, Tween 80 = 0.0002 g, Distilled water = 92.49 g. |
| Formulation 8 (F8) | Starch = 4.00 g, Glycerol = 2.00 g, OEO = 0.0046 g, Tween 80 = 0.0010 g, and Distilled water = 93.99 g. |
| Formulation 9 (F9) | Starch = 8.00 g, Glycerol = 4.00 g, OEO = 0.800 g, Tween 80 = 0.0020 g, and Distilled water = 187.19 g. |
| Formulation 10 (F10) | Starch = 8.00 g, Glycerol = 4.00 g, OEO = 0.800 g, Tween 80 = 0.1738 g, and Distilled water = 187.03 g. |
| Analysis | Film | |
|---|---|---|
| F8 | Control | |
| ||
| L | 95.810 ± 0.040 a | 67.980 ± 9.590 b |
| a | −0.360 ± 0.010 a | −0.350 ± 0.060 a |
| b | 3.620 ± 0.040 a | 3.420 ± 0.440 a |
| C | 3.630 ± 0.040 a | 3.440 ± 0.440 a |
| °h | 95.590 ± 0.120 a | 69.590 ± 9.750 b |
| 0.155 ± 0.006 b | 0.208 ± 0.004 a |
| 1.000 ± 0.002 a | 1.001 ± 0.010 a |
| ||
| TS (MPa) | 7.440 ± 0.580 a | 5.250 ± 0.323 b |
| %E (%) | 45.470 ± 9.820 a | 51.380 ± 2.220 a |
| EM (MPa) | 181.600 ± 37.230 a | 220.330 ± 22.320 a |
| 1.230 ± 0.001 b | 2.910 ± 1.340 a |
| ||
| Total Phenolic Content (mg GAE g−1) | 2.337 ± 0.020 a | 0.250 ± 0.036 b |
| Total Flavonoid Content (mg CE g−1) | 0.025 ± 0.003 a | 0.027 ± 0.001 a |
| DPPH (μmol TE g−1) | 1.031 ± 0.067 a | 0.260 ± 0.012 b |
| ABTS (μmol TE g−1) | 1.188 ± 0.033 a | 0.570 ± 0.025 b |
| FRAP (μmol TE g−1) | 93.610 ± 3.776 a | 9.444 ± 0.694 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uribe-Cruz, G.; Flores-Córdova, M.A.; Soto-Caballero, M.C.; Salas-Salazar, N.A.; Rodríguez-Roque, M.J.; Acosta-Muñiz, C.H.; Romero-Bastida, C.A.; Zamudio-Flores, P.B. Evaluation of the Effect of Oregano Essential Oil and Emulsifier Ratio on the Physicochemical, Mechanical, and Antioxidant Properties of Corn Starch Films Based on Gel Matrices. Gels 2025, 11, 760. https://doi.org/10.3390/gels11090760
Uribe-Cruz G, Flores-Córdova MA, Soto-Caballero MC, Salas-Salazar NA, Rodríguez-Roque MJ, Acosta-Muñiz CH, Romero-Bastida CA, Zamudio-Flores PB. Evaluation of the Effect of Oregano Essential Oil and Emulsifier Ratio on the Physicochemical, Mechanical, and Antioxidant Properties of Corn Starch Films Based on Gel Matrices. Gels. 2025; 11(9):760. https://doi.org/10.3390/gels11090760
Chicago/Turabian StyleUribe-Cruz, Gabriela, María Antonia Flores-Córdova, Mayra Cristina Soto-Caballero, Nora Aideé Salas-Salazar, María Janeth Rodríguez-Roque, Carlos Horacio Acosta-Muñiz, Claudia Andrea Romero-Bastida, and Paul Baruk Zamudio-Flores. 2025. "Evaluation of the Effect of Oregano Essential Oil and Emulsifier Ratio on the Physicochemical, Mechanical, and Antioxidant Properties of Corn Starch Films Based on Gel Matrices" Gels 11, no. 9: 760. https://doi.org/10.3390/gels11090760
APA StyleUribe-Cruz, G., Flores-Córdova, M. A., Soto-Caballero, M. C., Salas-Salazar, N. A., Rodríguez-Roque, M. J., Acosta-Muñiz, C. H., Romero-Bastida, C. A., & Zamudio-Flores, P. B. (2025). Evaluation of the Effect of Oregano Essential Oil and Emulsifier Ratio on the Physicochemical, Mechanical, and Antioxidant Properties of Corn Starch Films Based on Gel Matrices. Gels, 11(9), 760. https://doi.org/10.3390/gels11090760







