Applications of Hydrogels for Next-Generation Batteries
Abstract
1. Introduction
2. Hydrogel Fundamentals for Battery Applications
2.1. Matrix Structures and Crosslinking Mechanisms
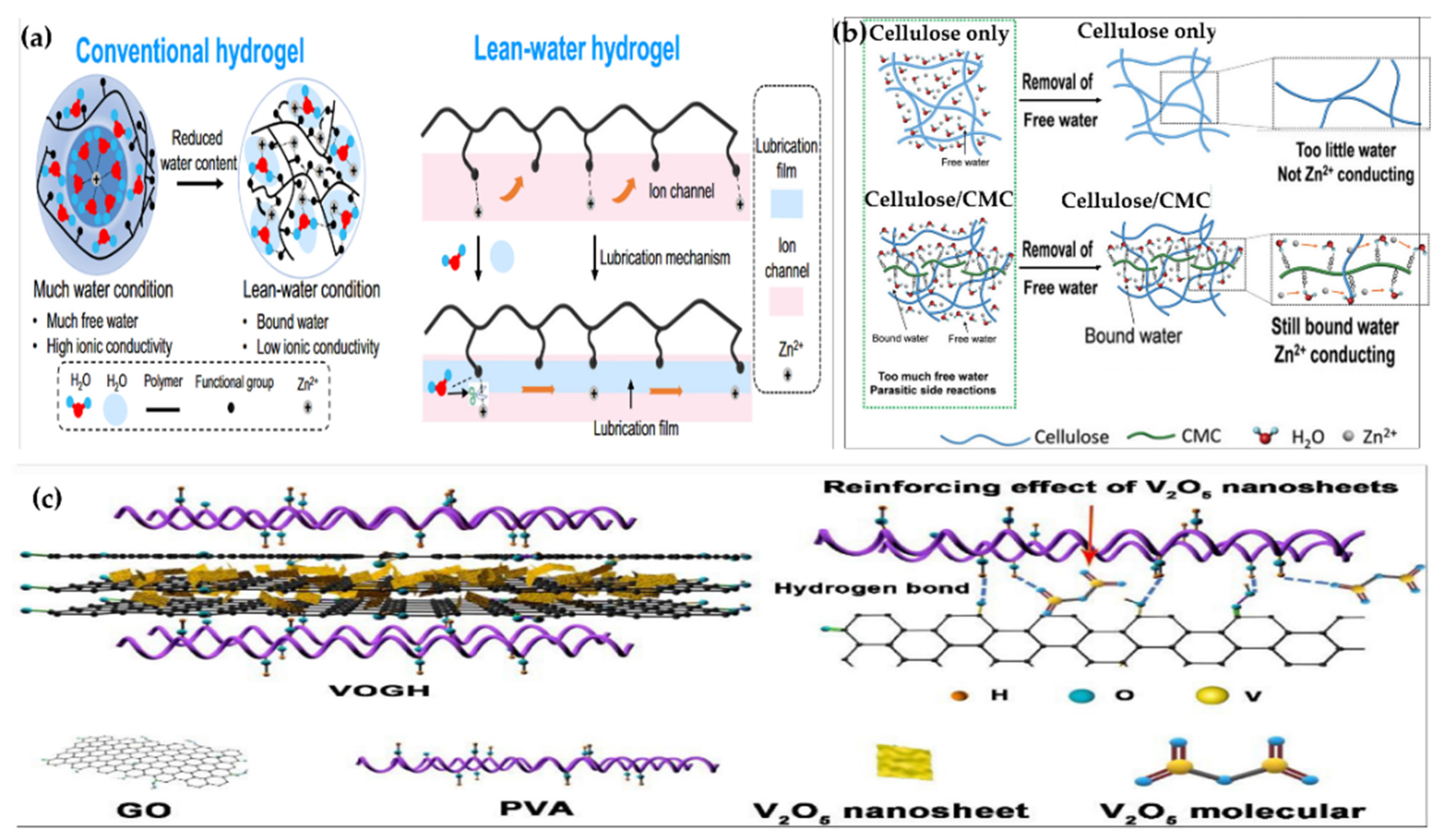
2.1.1. Physical CrossLinking
- Thermal cycling (heating or cooling of polymer solutions);
- Ionic interactions between charged polymer segments;
- Complex coacervation, involving phase separation of oppositely charged polymers;
- Hydrogen bonding between functional groups;
- Maturation, or heat-induced aggregation;
- Freeze–thaw cycles, which promote crystallite formation and physical entanglement.
2.1.2. Chemical CrossLinking
2.1.3. Radiation Crosslinking
| Crosslinking Method | Hydrogel Composition | Key Synthesis Parameters | Characterization Techniques | Ref. |
|---|---|---|---|---|
| Physical Crosslinking | Chitosan–Silver Nanocomposite | Polymer ratio, pH, ionic strength | Sol–gel transition, swelling behavior, antimicrobial activity, mechanical strength, SEM analysis | [86] |
| Catechol–Chitosan–Thiolated Pluronic F-127 | Polymer concentration, temperature | Tissue adhesion, mechanical stability, rheology | [87] | |
| Chitosan-based Hydrogel | pH, concentration, temperature | FTIR, rheological properties, mechanical testing, degradation profile | [88] | |
| Chemical Crosslinking | Polyvinyl Alcohol/Carbomer/Glabridin | Crosslinker concentration, mixing time | Swelling ratio, mechanical strength, shape retention | [89] |
| TiO2–Chitosan–Poly(acrylic acid) | Nanoparticle loading, pH | Dye adsorption, FTIR, SEM, thermal analysis | [90] | |
| Polyethylene Glycol Hydrogel | Mixing duration, temperature | Rheology, mechanical testing, swelling kinetics | [91] | |
| Acylated Xylan–Graphene Oxide | pH, polymer ratio | FTIR, SEM, particle size distribution | [91] | |
| Chitosan/Dopamine/Inulin Aldehyde | Composition ratio, reaction time | FTIR, DSC, mechanical testing, cytotoxicity assays | [92] | |
| Polyvinyl Alcohol Hydrogel | Polymer concentration, crosslinking agent | Tensile strength, rheology, SEM, FTIR, structural analysis | [93] | |
| Irradiation Crosslinking | Chitosan–Silver Nanoparticles | Radiation dose, composition | SEM, UV–Vis absorbance, particle size, antimicrobial testing | [94] |
| Sterculia Gum–Graphene Oxide | Radiation intensity, polymer ratio | SEM, swelling behavior, drug release profile, and biomedical evaluation | [95] |
2.2. Organic Functional Groups Relevant to Battery Chemistry
2.3. Interfacial Traits and Ion Transport Mechanisms
2.4. Mechanical Properties and Durability Factors
3. Classification of Hydrogels Used in Battery Systems
3.1. Natural Hydrogels
3.2. Synthetic Hydrogels
3.3. Composite Hydrogels
3.4. Carbon-Based Hydrogels
3.5. Conductive Polymer Hydrogels
3.6. MOF Hydrogels
| Hydrogel Type | Source/Composition | Properties | Applications | Advantages | Challenges | Ref. |
|---|---|---|---|---|---|---|
| Natural Hydrogels | Alginate, chitosan, cellulose | Biocompatibility, biodegradability, high gelation ability | Electrolytes, separators in batteries and supercapacitors | Renewable, environmentally friendly | Mechanical strength, scalability | [114] |
| Synthetic Hydrogels | Polyacrylamide (PAM), polyethylene oxide (PEO), polyvinyl alcohol (PVA) | High water content, tunable mechanical properties, high ionic conductivity | Electrolytes, separators in batteries and supercapacitors | Customizable properties, high ionic conductivity | Environmental impact, mechanical robustness | [112] |
| Composite Hydrogels | Graphene oxide + polymer matrix, nanoparticles + polymer matrix | Enhanced mechanical properties, high electrical conductivity | Electrodes in batteries, supercapacitors | Enhanced properties, multifunctionality | Complexity in synthesis, cost | [112] |
| Carbon-based Hydrogels | Graphene, carbon nanotubes (CNTs) | High electrical conductivity, mechanical strength | Electrodes in batteries, supercapacitors | High electrical conductivity, mechanical strength | Scalability, cost | [188] |
| Conductive Polymer Hydrogels | Polyaniline, polypyrrole | High electrical conductivity, flexibility | Electrodes, sensors, supercapacitors | High electrical conductivity, flexibility | Stability, environmental impact | [189] |
| Metal–Organic Framework (MOF) Hydrogels | MOFs + polymer matrix | High porosity, tunable properties | Electrolytes, gas storage, sensors | High porosity, tunable properties | Synthesis complexity, cost | [190] |
4. Structural and Morphological Characterization
4.1. Microstructural Performance
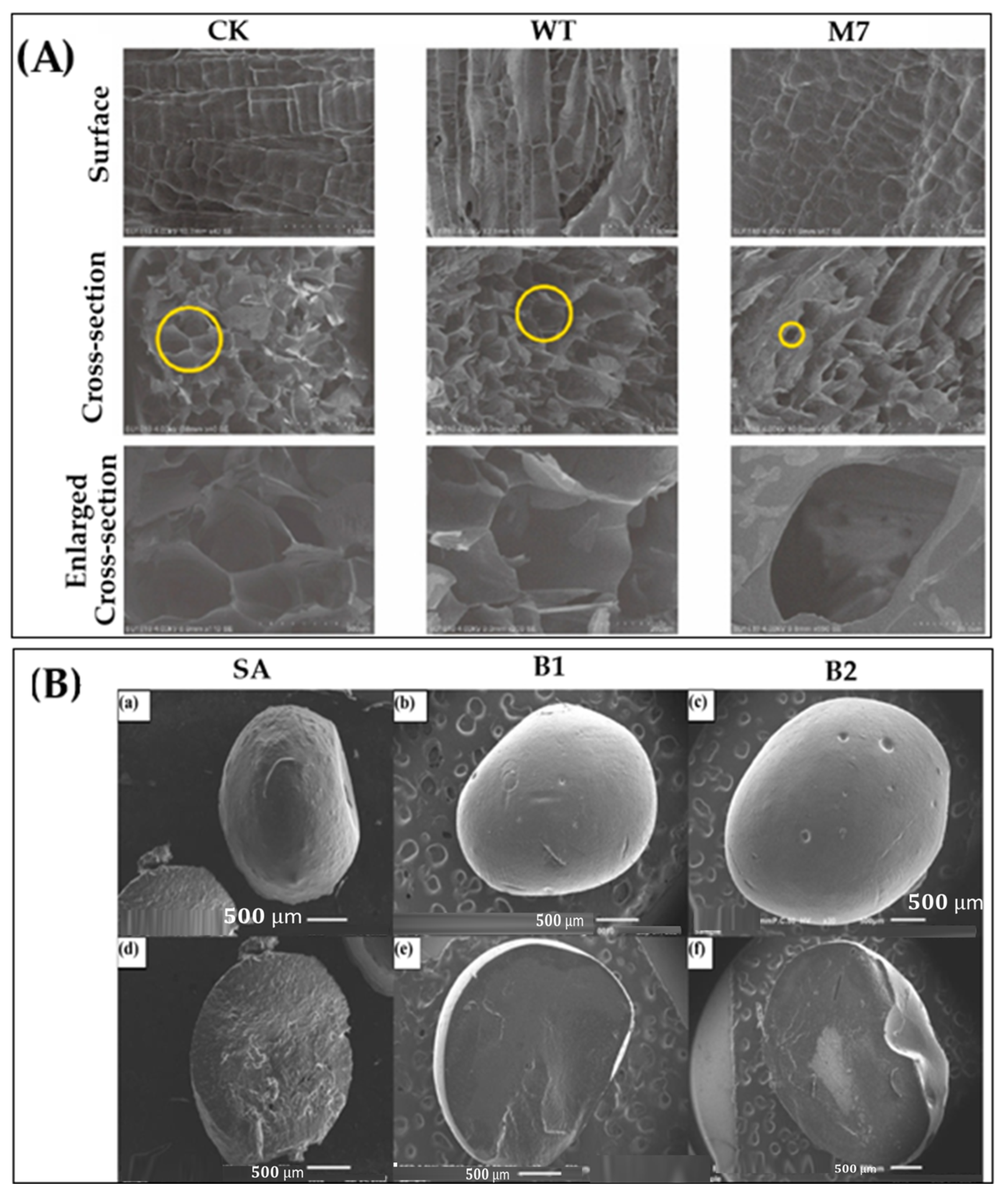
4.2. Morphology Study
4.3. Mechanical Properties and Performance of Hydrogels
4.4. Fourier Transform Infrared Spectroscopy (FTIR)
4.5. Viscoelastic Properties
5. Hydrogel-Based Materials in Battery Applications
5.1. Synthesis and Fabrication of Hydrogel Battery Components
5.1.1. Fabrication of Hydrogel Electrolytes
5.1.2. Integration with Electrodes
5.1.3. Assembly of Full Cells
5.2. Functional Properties and Mechanisms
5.2.1. Hydrolytic Behavior and Water Absorption
5.2.2. Porosity and Ion Transport
5.2.3. Safety and Non-Toxicity
5.2.4. Self-Healing Capability
5.2.5. Tunable Mechanical Properties and Design Flexibility
5.3. Advantages and Challenges
- Improved safety due to non-flammable, water-based electrolytes [230].
6. Hydrogels in Specific Battery Chemistries
- Molecular Backbone: Synthetic polymers such as polyvinyl alcohol (PVA), polyethylene oxide (PEO), and acrylamide are commonly used due to their hydrophilicity and ability to coordinate with metal ions, facilitating ion transport. Natural polymers like alginate, chitosan, and gelatin offer biocompatibility and biodegradability, making them ideal for wearable and implantable devices [33,127,147,242,243,244,245].
- Extent of Crosslinking: Hydrogels can be physically crosslinked (via hydrogen bonding or ionic interactions), chemically crosslinked (via covalent bonding using agents like glutaraldehyde or borax), or radiation crosslinked (using gamma or electron beams). The degree and type of crosslinking determine the hydrogel’s mechanical strength, swelling behavior, and electrochemical stability [248,249].
6.1. Hydrogels in Li-Ion Batteries
6.1.1. Hydrogel-Derived Electrodes
6.1.2. Hydrogel-Derived Binders
6.1.3. Hydrogel Electrolytes for Aqueous Lithium-Ion Batteries
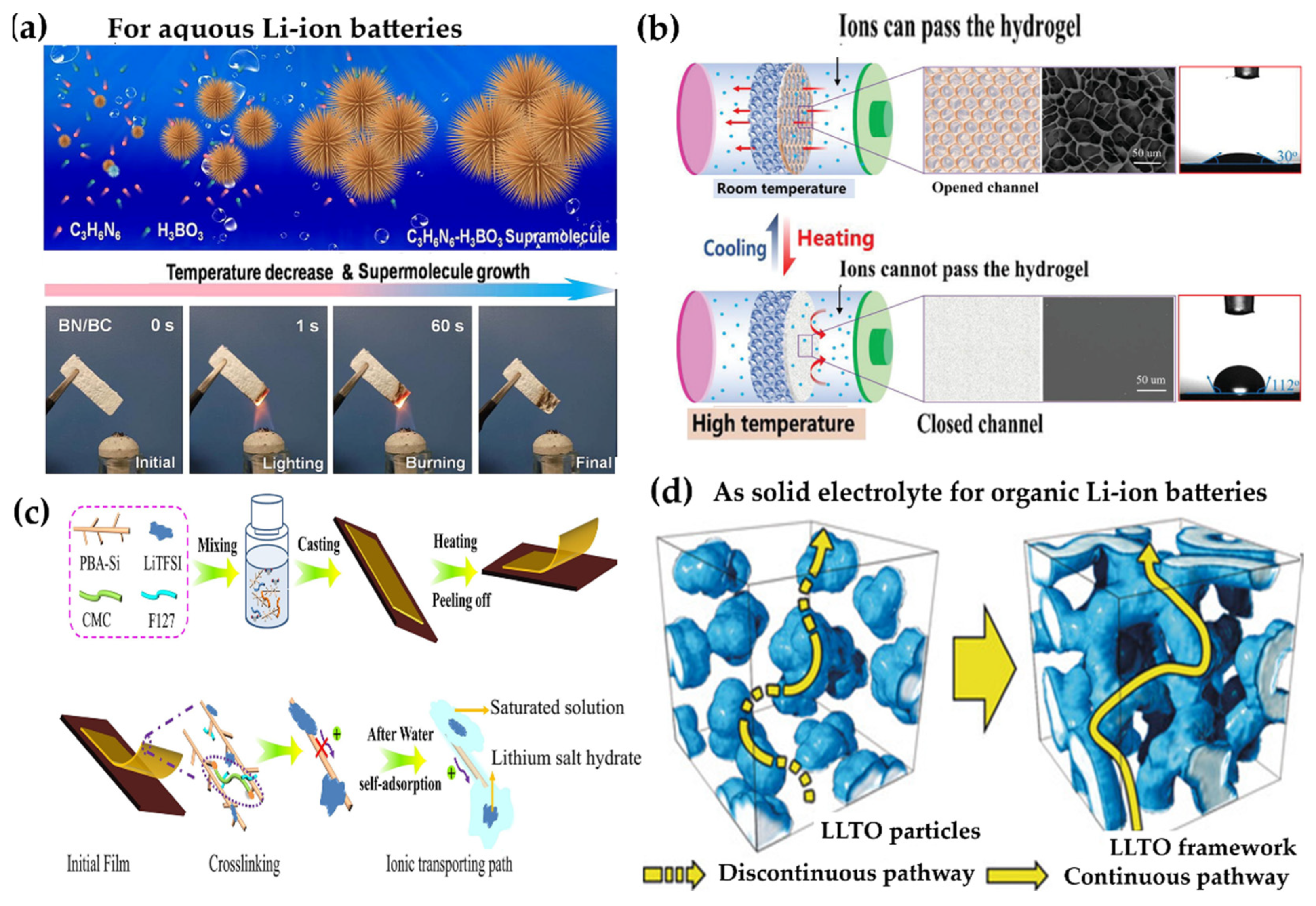
6.1.4. Hydrogels Under Extreme Conditions
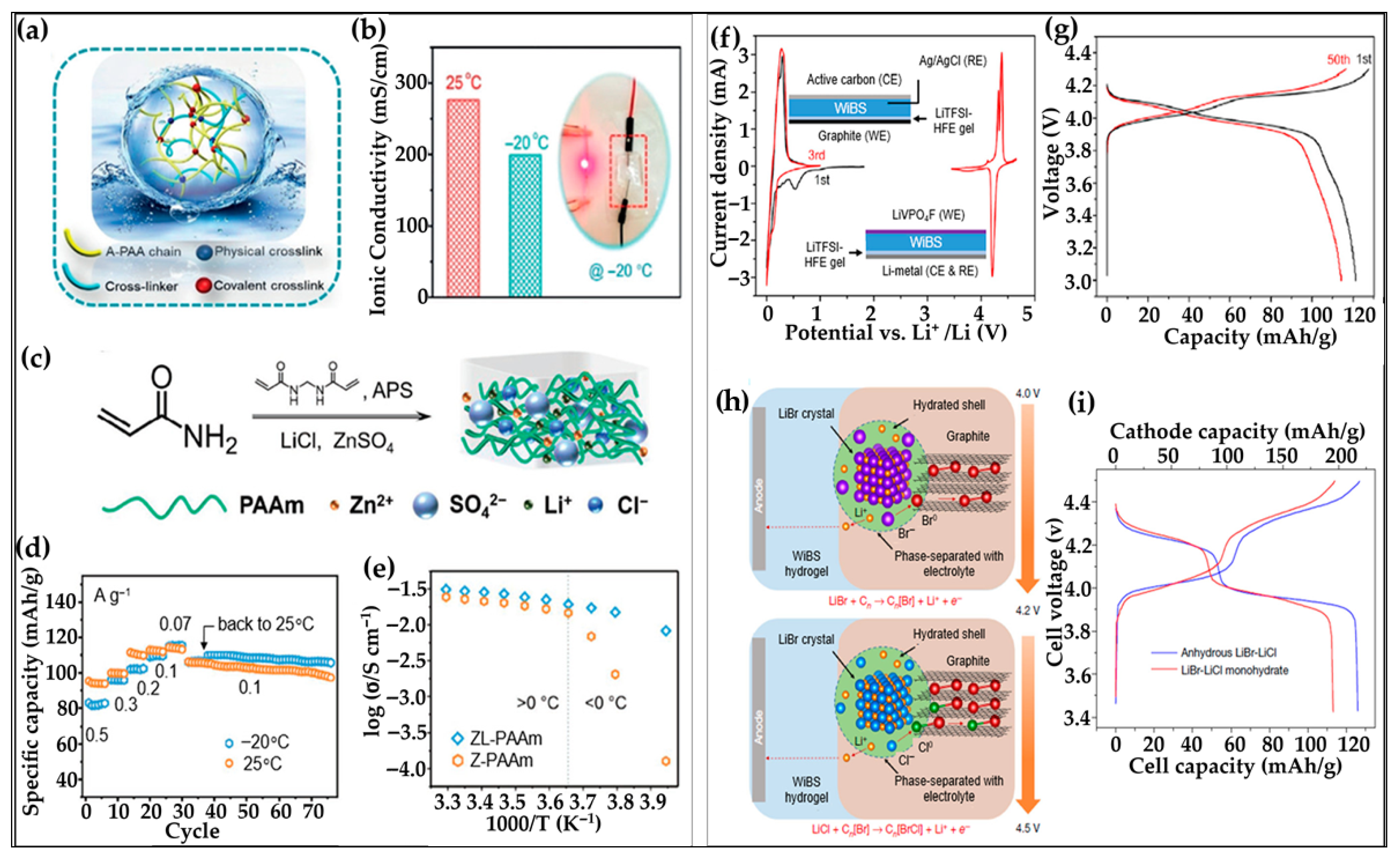
6.2. Hydrogels for Sodium-Ion Batteries
6.2.1. Hydrogel Electrolytes for Sodium-Ion Batteries
6.2.2. Hydrogel Anodes for Sodium-Ion Batteries
6.2.3. Hydrogel Cathodes for Sodium-Ion Batteries
6.3. Hydrogel Electrolytes for Zinc-Ion Battery
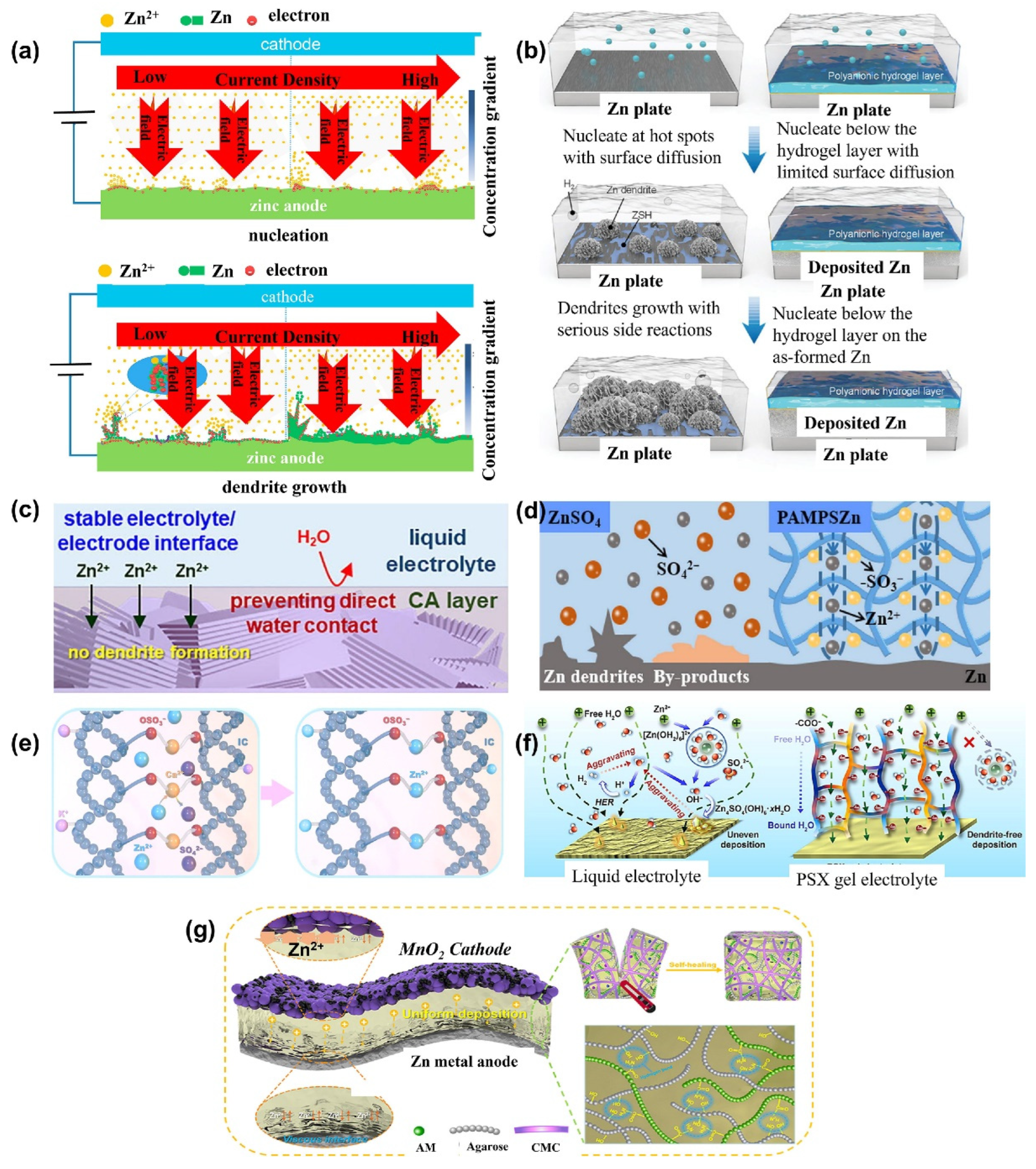
6.3.1. High-Voltage Hydrogel-Based Zinc-Ion Batteries
6.3.2. Self-Healing Hydrogel-Based Zn-Ion Batteries
6.4. Hydrogels for Magnesium-Ion Batteries
6.4.1. Hydrogels as Electrolyte for Mg-Ion Battery (MIBs)
6.4.2. Hydrogel-Derived Anodes for MIBs
6.5. Hydrogels for Aluminum-Ion Batteries
Hydrogels as Electrolytes for AIBs
| Battery Type | Electrodes | Electrolyte | Ionic Conductivity | Specific Capacity | Cyclability | Tensile Strength | Elastic Modulus | Maximum Strain | Capacity Retention Under Deformation | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Li-ion | Anode: prelithiated V2O5, cathode: LiMn2O4 | PAM-WiS gel | 103 S cm−1 | 60 mAh/g at 1C/40 mAh/g at 5C | 95% after 100 cycles | — | — | 100% | 28 mAh/g under 50% stretch | [367] |
| Li-ion | Anode: activated carbon, cathode: LiMn2O4 | PAAm–chitosan WiS gel | 51.3 mS cm−1 | 110.7 mAh/g at 0.1 A/g | 90.1% after 2000 cycles | — | — | 2540% | ~95% under 90° bending and twisting | [368] |
| Li-ion | Anode: SiO microparticles, cathode: lithium foil | TA–PAA cobweb-like hydrogel binder | — | 901.2 mAh/g after 1200 cycles at 2 A/g | 619.2 mAh/g at 5 A/g (rate performance) | High (via nanoindentation) | High (via rheology) | Up to 1000% | Maintains structure; self-healing; minimal volume expansion | [281] |
| Li-ion | Anode: SiP2/C composite, cathode: lithium foil | Li-PAA@PEDOT:PSS hydrogel binder | — | 1520 mAh/g at 2000 mA/g | >450 cycles, ICE >93.8% | — | — | — | Self-healing and structural recombination | [277] |
| Li–S | Cathode: 3D carbon-HKUST-1/S, Anode: lithium metal | Conducting polymer hydrogel with MOF domains | — | >16 mAh/cm2; 1230.8 mAh/cm3 | 82% after 300 cycles at 0.2C | — | — | — | Maintains structural integrity under high sulfur loading | [284] |
| Li-ion | Anode: Si nanoparticles, cathode: lithium foil | 3D meshlike PAM hydrogel binder | — | ≈1526 mAh/g | Stable over 500 cycles | — | Tunable via crosslinker | — | Maintains structure under deformation | [369] |
| Li-ion | Anode: Si particles, cathode: lithium foil | ESVCA hydrogel binder | — | ~1743 mAh/g after 200 cycles | 74.1% retention | — | — | — | Self-healing and stretchable up to 400% | [370] |
| Li-ion | Cathode: standard LIB cathode, Anode: lithium metal | BN/BC composite aerogel separator | — | 146.5 mAh/g | 500 cycles, 0.012% degradation/cycle | High (BN aerogel) | — | — | Flame-retardant, flexible, wettable | [259] |
| Li-ion | Cathode: high-loading LIB cathode, Anode: lithium foil | Low-water-content hydrogel film (6.63 wt%) | 2.6 mS/cm | 119 mAh/g (2.5 mAh/cm2 areal) | 81.8% after 500 cycles at RT; 66.2% at −30 °C | — | — | High (foldable) | Anti-freezing, low swelling, flexible | [371] |
| Li-ion | Cathode: stretchable nanowire network | Crosslinked hydrogel | — | 119 mAh/g | 91.6% after 250 tensile strains | — | — | 100% stretchable | Stable under repeated deformation | [291] |
| Li-ion | Full cell (flexible LIB) | Moisture self-absorbing hydrogel film (4.62% water) | 1.15 mS/cm | — | 73.2% after 1000 cycles at 1 A/g | — | — | >300% | Maintains OCV under 50–125% stretch and after cutting | [230] |
| Na-ion (aqueous) | Anode: NaTi2(PO4)3@C, Cathode: Activated Carbon | Na2SO4–SiO2 hydrogel with methanol additive | 0.070 mS/cm at −30 °C | 61.8 mAh/g at −30 °C | High stability at 0.13 A/g | — | — | — | Stable operation at −30 °C | [313] |
| Na-ion | Anode: metallic Na, Cathode: Na2Fe2(SO4)3 | Hierarchical nanocellulose-based GPE | 2.32 mS/cm | 69.7 mAh/g after 50 cycles at 1C | Stable Na plating/stripping up to ±500 µA/cm2 | — | — | — | Mesoporous structure ensures uniform ion flux and dendrite-free deposition | [314] |
| Na-ion (aqueous) | Anode: NaTi2(PO4)3, Cathode: Na3V2(PO4)3 | Salt-concentrated methylated hydrogel | — | — | 82.8% after 580 cycles | — | — | — | Suppressed water activity; stable cycling | [372] |
| Na-ion | Urchin-like MoS2/MoO2 microspheres coated with GO hydrogel | Na-ion compatible electrolyte (e.g., NaPF6 in EC/DEC) | Moderate to high (depending on GO dispersion) | ~400–600 mAh/g (initial) | Excellent (>500 cycles with high retention) | Moderate (due to GO hydrogel) | Moderate | High (flexible hydrogel matrix) | High (GO hydrogel maintains integrity under strain) | [315] |
| Zn-ion | Na3V2(PO4)3@C@CNT porous network via hydrogel templating | Na-ion compatible (e.g., NaClO4 in EC/DEC) | High (due to CNT network) | ~110–120 mAh/g (theoretical ~117 mAh/g) | Excellent (>1000 cycles with >90% retention) | High (CNT reinforcement) | High | Moderate to High | Very High (flexible and conductive network) | [312] |
| Zn-ion | Ultrathin Zn-based fiber electrodes | Solid-state gel or polymer electrolyte (e.g., PVA/ZnSO4) | Moderate to High (solid-state) | ~100–150 mAh/g (depending on design) | Excellent (stable over hundreds of cycles) | High (fiber structure) | Moderate to High | High (textile-compatible) | Very High (maintains performance under bending/stretching) | [373] |
| Zn-ion | Zn-based electrodes with flexible current collectors | Hierarchical structured polymer electrolyte (e.g., PVA-based with Zn salts) | Moderate to High (solid-state polymer) | ~100–140 mAh/g | Excellent (stable over 500+ cycles) | High (polymer matrix) | Moderate | High (wearable and flexible) | Very High (maintains capacity under bending/stretching) | [374] |
| Zn-ion | Zn-based electrodes with biopolymeric hydrogel coating | Aqueous ZnSO4 with hydrogel interphase | High (enhanced by hydrogel interface) | ~120–160 mAh/g | Excellent (dendrite suppression enables long-term cycling) | Moderate to High (biopolymer matrix) | Moderate | High | Very High (stable under mechanical stress and deformation) | [324] |
| Al-ion | Mg-based electrodes (e.g., Mg foil or Mg alloy) | Modified aqueous electrolyte with antifreeze additives (e.g., Cl−/NO3− coordination) | High (even at −50 °C) | ~100–120 mAh/g | Excellent (stable at sub-zero temperatures) | Moderate | Moderate | Moderate | High (electrolyte remains functional under cold and stress) | [360] |
| Zn-ion | Hybrid Al-compatible electrodes (e.g., MnO2 cathode, Al anode) | Quasi-solid-state aqueous hybrid electrolyte (Al3⁺/H⁺ based) | High (optimized for dual-ion transport) | ~250–300 mAh/g | Exceptional (10,000+ cycles with minimal degradation) | Moderate to High (gel matrix) | Moderate | High | Very High (smart switching maintains performance under stress) | [363] |
| Zn-ion | Fiber-shaped Al-compatible electrodes (e.g., MnO2 or conductive polymers) | Aqueous Al-ion electrolyte (e.g., AlCl3 or Al(NO3)3) | High (optimized for fiber geometry) | ~200–300 mAh/g | Excellent (stable over many cycles under strain) | High (fiber structure) | Moderate to High | Very High (stretchable design) | Very High (maintains capacity under stretching and bending) | [364] |
| Zn-ion | Flexible Al-compatible electrodes (e.g., MnO2 cathode, Al anode) | Aqueous Al-ion electrolyte (e.g., Al(NO3)3 or AlCl3) | High (optimized for flexibility and ion transport) | ~250–300 mAh/g | Excellent (long cycle life, >1000 cycles) | High (flexible substrate materials) | Moderate to High | High | Very High (maintains capacity under bending/stretching) | [365] |
| Zn-ion | Copper and zinc foil | Polyanion hydrogels: APTQ+/AAm | — | ~160 mAh/g at 2 mA/cm2 | — | 50 kPa | — | ~200% | — | [375] |
| Zn-ion | Anode: zinc, cathode: CoFe(CN)6 | Zn(OTf)2 | — | 173.4 mAh/g at 0.3 A/g | No capacity decline after 2200 cycles | — | — | — | ~100% under 180° bending | [376] |
| Zn-ion | Anode: zinc@graphite, cathode: α-MnO2 | PAAm/ZnSO4/MnSO4 | — | 277.5 mAh/g at 1C | 69.02% after 1000 cycles at 4C | — | — | — | >85% under 25% compression for 30 cycles | [377] |
7. Challenges and Future Perspectives
7.1. Current Limitations in Hydrogel Applications
- 1.
- Mechanical Strength and Durability: Despite their impressive flexibility, many hydrogels face limitations in mechanical strength and durability, particularly when subjected to harsh operating conditions such as temperature extremes, high humidity, or mechanical deformation. This results in performance degradation, which limits their use in long-term applications like wearable electronics or energy storage systems [378].
- 2.
- Ionic Conductivity: The ionic conductivity of hydrogels, while suitable for some applications like supercapacitors, is often lower than that of traditional solid-state electrolytes or metal-based conductors. The challenge lies in optimizing the hydrogel matrix to improve ion transport without compromising other desirable properties such as biocompatibility and environmental.
- 3.
- Scalability and Manufacturing: The scalability of hydrogel-based devices, especially for large-scale energy storage applications, remains a significant hurdle. Many hydrogel-based systems are difficult to manufacture uniformly at a large scale while maintaining consistent performance.
- 4.
- Environmental and Biodegradability Concerns: While hydrogels are often considered eco-friendly, the degradation products of some synthetic hydrogels may raise concerns regarding their long-term environmental impact. Research is ongoing to develop fully biodegradable hydrogels that can break down harmlessly in natural environments.
7.2. Potential Solutions and Advancements
- 1.
- Composite Hydrogels: The incorporation of conductive materials such as carbon nanotubes, graphene, and metallic nanoparticles into hydrogel matrices has shown promise in enhancing mechanical strength and conductivity. These composite hydrogels can offer the dual benefits of improved performance and flexibility, addressing the mechanical and conductivity issues simultaneously.
- 2.
- 3D Printing and Smart Fabrication Techniques: Advances in 3D printing and other smart fabrication methods are enabling the precise control of hydrogel structures, allowing for the creation of hydrogels with tailored properties for specific energy applications. This includes optimizing pore structures for ion transport or adjusting the polymer networks for improved mechanical integrity.
- 3.
- Self-Healing Hydrogels: Self-healing hydrogels, which can repair damage autonomously, offer an exciting avenue to overcome the durability challenges faced by hydrogels. By integrating dynamic covalent bonds or reversible crosslinking strategies, hydrogels can recover their function after being subjected to mechanical or environmental stress, which is crucial for ensuring long-term device reliability.
- 4.
- Biodegradable and Sustainable Hydrogels: Research into biodegradable and bio-based hydrogels, such as those derived from polysaccharides, is advancing rapidly. These hydrogels not only mitigate environmental concerns but also possess excellent biocompatibility, which is essential for energy devices that interact with the human body, such as wearable sensors and bioelectronics.
7.3. Future Research Directions
- 1.
- Interdisciplinary Collaboration: There is a growing need for interdisciplinary research that combines materials science, chemistry, and engineering to create hydrogels with optimized properties for specific applications. Collaborations between researchers in fields such as nanotechnology, organic electronics, and biomaterials are key to developing next-generation hydrogels for energy storage and conversion systems.
- 2.
- High-Performance Batteries: Further research into the optimization of hydrogels for batteries, particularly for hybrid systems that combine both, is an exciting prospect. Focus should be on improving the energy density, stability, and cycling life of these devices through innovative hydrogel formulations.
- 3.
- Mechanistic Insights via Simulation: Although experimental studies have revealed the role of functional groups in facilitating ion transport within hydrogel electrolytes, deeper mechanistic insights are needed. Theoretical approaches such as density functional theory (DFT) and molecular dynamics (MD) simulations have been increasingly utilized to validate and explore ion–polymer interactions. DFT-based tight-binding methods can model electrochemical interfaces and predict ion–functional group interactions, while MD simulations reveal how polymer composition, crosslinking density, and hydration levels influence ion mobility and transport pathways [379,380]. Incorporating these computational tools in future studies will enhance the predictive design of hydrogel electrolytes and support the development of high-performance energy storage systems.
8. Conclusions
8.1. Summary of Key Points
8.2. Impact of Hydrogels on the Future of Energy Materials and Devices
8.3. Final Thoughts
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Elbinger, L.; Enke, M.; Ziegenbalg, N.; Brendel, J.C.; Schubert, U.S. Beyond Lithium-Ion Batteries: Recent Developments in Polymer-Based Electrolytes for Alternative Metal-Ion-Batteries. Energy Storage Mater. 2024, 65, 103063. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, P.; Xiang, H.; Liang, X.; Yu, Y. High-Safety Nonaqueous Electrolytes and Interphases for Sodium-Ion Batteries. Small 2019, 15, e1805479. [Google Scholar] [CrossRef]
- Gao, H.; Xue, L.; Xin, S.; Goodenough, J.B. A High-Energy-Density Potassium Battery with a Polymer-Gel Electrolyte and a Polyaniline Cathode. Angew. Chem. 2018, 130, 5547–5551. [Google Scholar] [CrossRef]
- Oh, J.S.; Ko, J.M.; Kim, D.W. Preparation and Characterization of Gel Polymer Electrolytes for Solid State Magnesium Batteries. Electrochim. Acta 2004, 50, 903–906. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, H.; Alfred, M.; Ke, H.; Cai, Y.; Wang, Q.; Huang, F.; Liu, B.; Lv, P.; Wei, Q. Reaction Modifier System Enable Double-Network Hydrogel Electrolyte for Flexible Zinc-Air Batteries with Tolerance to Extreme Cold Conditions. Energy Storage Mater. 2021, 42, 88–96. [Google Scholar] [CrossRef]
- Kanbua, C.; Sirichaibhinyo, T.; Rattanawongwiboon, T.; Lertsarawut, P.; Chanklinhorm, P.; Ummartyotin, S. Gamma Radiation-Induced Crosslinking of Ca2+ Loaded Poly(Acrylic Acid) and Poly(Ethylene Glycol) Diacrylate Networks for Polymer Gel Electrolytes. S. Afr. J. Chem. Eng. 2022, 39, 90–96. [Google Scholar] [CrossRef]
- Mohammad, A.; Köhler, T.; Biswas, S.; Stöcker, H.; Meyer, D.C. A Flexible Solid-State Ionic Polymer Electrolyte for Application in Aluminum Batteries. ACS Appl. Energy Mater. 2023, 6, 2914–2923. [Google Scholar] [CrossRef]
- Ju, Y.X.; Song, P.; Wang, P.Y.; Chen, X.X.; Chen, T.; Yao, X.H.; Zhao, W.G.; Zhang, D.Y. Cartilage Structure-Inspired Elastic Silk Nanofiber Network Hydrogel for Stretchable and High-Performance Supercapacitors. Int. J. Biol. Macromol. 2023, 242, 124912. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, Q.; Mugo, S.M.; An, L.; Zhang, Q.; Lu, Y. Self-Healing and Shape-Editable Wearable Supercapacitors Based on Highly Stretchable Hydrogel Electrolytes. Adv. Sci. 2022, 9, e2201039. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, M.G.; Lübben, J.F. Review on Hydrogel-Based Flexible Supercapacitors for Wearable Applications. Gels 2023, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, C.; Răileanu, S.; Zgârian, R.; Tihan, G.; Burnei, C. State-of-the-Art Advances and Current Applications of Gel-Based Membranes. Gels 2024, 10, 39. [Google Scholar] [CrossRef]
- Huang, J.; Wu, C.H.; Li, F.; Wang, X.; Chen, S.C. Enhancing the Proton Exchange Membrane in Tubular Air-Cathode Microbial Fuel Cells through a Hydrophobic Polymer Coating on a Hydrogel. Materials 2024, 17, 1286. [Google Scholar] [CrossRef]
- Bae, S.; Kim, M.; Jo, N.; Kim, K.M.; Lee, C.; Kwon, T.H.; Nam, Y.S.; Ryu, J. Amine-Rich Hydrogels for Molecular Nanoarchitectonics of Photosystem II and Inverse Opal TiO2 toward Solar Water Oxidation. ACS Appl. Mater. Interfaces 2024, 16, 16086–16095. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.M. Building Better Batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.M. Nanomaterials for Rechargeable Lithium Batteries. Angew. Chem. Int. Ed. 2008, 47, 2930–2946. [Google Scholar] [CrossRef]
- Russo, R.; Becuwe, M.; Frayret, C.; Stevens, P.; Toussaint, G. Optimization of Disodium Naphthalene Dicarboxylates Negative Electrode for Organic-Inorganic Hybrid Sodium Batteries. ECS Meet. Abstr. 2022, MA2022-01, 94. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Tang, Z.; Liu, Z.; Ruan, Z.; Ma, L.; Yang, Q.; Wang, D.; Zhi, C. Hydrogel Electrolytes for Flexible Aqueous Energy Storage Devices. Adv. Funct. Mater. 2018, 28, 1804560. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, N.; Cui, Y. Promises and Challenges of Nanomaterials for Lithium-Based Rechargeable Batteries. Nat. Energy 2016, 1, 16071. [Google Scholar] [CrossRef]
- Lee, C.; Huang, H.S.; Wang, Y.Y.; Zhang, Y.S.; Chakravarthy, R.D.; Yeh, M.Y.; Lin, H.C.; Wei, J. Stretchable, Adhesive, and Biocompatible Hydrogel Based on Iron–Dopamine Complexes. Polymers 2023, 15, 4378. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, Y.; Xing, G.; Chou, S.L.; Tang, Y. Electrochemical Energy Storage Devices Working in Extreme Conditions. Energy Environ. Sci. 2021, 14, 3323–3351. [Google Scholar] [CrossRef]
- Segura Zarate, A.Y.; Gontrani, L.; Galliano, S.; Bauer, E.M.; Donia, D.T.; Barolo, C.; Bonomo, M.; Carbone, M. Green Zinc/Galactomannan-Based Hydrogels Push up the Photovoltage of Quasi Solid Aqueous Dye Sensitized Solar Cells. Sol. Energy 2024, 272, 112460. [Google Scholar] [CrossRef]
- Ünlü, B.; Türk, S.; Özacar, M. Novel Anti-Freeze and Self-Adhesive Gellan Gum/P3HT/LiCl Based Gel Electrolyte for Quasi Solid Dye Sensitized Solar Cells. Colloids Surf. A Physicochem. Eng. Asp. 2023, 674, 131869. [Google Scholar] [CrossRef]
- Kanti, P.K.; Deepthi, J.K.; Swapnalin, J.; Vicki Wanatasanappan, V. Advancements and Prospects of MXenes in Emerging Solar Cell Technologies. Sol. Energy Mater. Sol. Cells 2025, 285, 113540. [Google Scholar] [CrossRef]
- Cheng, T.; Liu, Z.; Qu, J.; Meng, C.; He, L.; Li, L.; Yang, X.; Cao, Y.; Han, K.; Zhang, Y. High-Performance Organic–Inorganic Hybrid Conductive Hydrogels for Stretchable Elastic All-Hydrogel Supercapacitors and Flexible Self-Powered Integrated Systems. Adv. Sci. 2024, 11, 2403358. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, Y.; Kawamura, A.; Takashima, Y.; Miyata, T. Design of Molecularly Imprinted Hydrogels with Thermoresponsive Drug Binding Sites. J. Mater. Chem. B 2022, 10, 6644–6654. [Google Scholar] [CrossRef]
- Costa, D.C.S.; Costa, P.D.C.; Gomes, M.C.; Chandrakar, A.; Wieringa, P.A.; Moroni, L.; Mano, J.F. Universal Strategy for Designing Shape Memory Hydrogels. ACS Mater. Lett. 2022, 4, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Dai, Z.; Sheng, X.; Xia, D.; Shao, P.; Yang, L.; Luo, X. Conducting Polymer Hydrogels as a Sustainable Platform for Advanced Energy, Biomedical and Environmental Applications. Sci. Total Environ. 2021, 786, 147430. [Google Scholar] [CrossRef]
- Song, H.-S.; Rumon, M.M.H.; Rahman Khan, M.M.; Jeong, J.-H. Toward Intelligent Materials with the Promise of Self-Healing Hydrogels in Flexible Devices. Polymers 2025, 17, 542. [Google Scholar] [CrossRef]
- Liao, H.; Zhong, W.; Li, T.; Han, J.; Sun, X.; Tong, X.; Zhang, Y. A Review of Self-Healing Electrolyte and Their Applications in Flexible/Stretchable Energy Storage Devices. Electrochim. Acta 2022, 404, 139730. [Google Scholar] [CrossRef]
- Narayan, R.; Laberty-Robert, C.; Pelta, J.; Tarascon, J.M.; Dominko, R. Self-Healing: An Emerging Technology for Next-Generation Smart Batteries. Adv. Energy Mater. 2022, 12, 2102652. [Google Scholar] [CrossRef]
- Li, H.; Zhao, H.; Song, K.; Han, F.; Liu, Z.; Tian, Q. Flexible and Stretchable Implantable Devices for Peripheral Neuromuscular Electrophysiology. Nanoscale 2024, 16, 6402–6428. [Google Scholar] [CrossRef]
- Guo, Y.; Bae, J.; Zhao, F.; Yu, G. Functional Hydrogels for Next-Generation Batteries and Supercapacitors. Trends Chem. 2019, 1, 335–348. [Google Scholar] [CrossRef]
- Shen, Z.; Zhai, Z.; Liu, Y.; Bao, X.; Zhu, Y.; Zhang, T.; Li, L.; Hong, G.; Zhang, N. Hydrogel Electrolytes-Based Rechargeable Zinc-Ion Batteries under Harsh Conditions. Nano-micro Lett. 2025, 17, 227. [Google Scholar] [CrossRef]
- Vandeginste, V.; Wang, J. A Review of the Synthesis of Biopolymer Hydrogel Electrolytes for Improved Electrode–Electrolyte Interfaces in Zinc-Ion Batteries. Energies 2024, 17, 310. [Google Scholar] [CrossRef]
- Chen, Y.; He, S.; Rong, Q. Recent Progress in Environment-Adaptable Hydrogel Electrolytes for Flexible Energy Storage Devices. J. Energy Storage 2023, 73, 109023. [Google Scholar] [CrossRef]
- Elbinger, L.; Schröter, E.; Zimmer, P.; Friebe, C.; Osenberg, M.; Manke, I.; Schubert, U.S. Flexible Hydrogel Electrolytes for Organic Batteries with High Cyclability. J. Phys. Chem. C 2024, 128, 11465–11476. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Deng, L.; Li, J.-T.; Huang, Q.-S.; Lu, Y.; Liu, J.; Zhang, T.; Huang, L.; Sun, S.-G. Multiple Hydrogel Alginate Binders for Si Anodes of Lithium-Ion Battery. Electrochim. Acta 2017, 245, 371–378. [Google Scholar] [CrossRef]
- Rakhman, D.; Batyrbekuly, D.; Myrzakhmetov, B.; Zhumagali, K.; Issabek, K.; Sultan-Akhmetov, O.; Umirov, N.; Konarov, A.; Bakenov, Z. Polyacrylamide-Based Hydrogel Electrolyte for Modulating Water Activity in Aqueous Hybrid Batteries. RSC Adv. 2024, 14, 40222–40233. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Islam, M.; Raut, B.; Yun, S.; Kim, H.Y.; Nam, K.-W. A Comprehensive Review of Functional Gel Polymer Electrolytes and Applications in Lithium-Ion Battery. Gels 2024, 10, 563. [Google Scholar] [CrossRef]
- Xue, W.; Ahangaran, F.; Wang, H.; Theato, P.; Cheng, Y.-J. Gel Polymer Electrolytes for Lithium Batteries: Advantages, Challenges, and Perspectives. Macromol. Rapid Commun. 2025, 2500207. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, J.; Pathak, T.S.; Santos, D.M.F. Applications of Polymer Electrolytes in Lithium-Ion Batteries: A Review. Polymers 2023, 15, 3907. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Lv, L.; Xue, S.; Yan, D.; Ma, G.; Wang, H.; Wan, H.; Wang, H. Emerging Advanced Design Strategies and Engineering toward Polymer-Hydrogel Electrolytes for Flexible Zinc-Based Rechargeable Batteries: A Review From an All-Around Insight. Small 2025, 2505804. [Google Scholar] [CrossRef]
- Cui, K.; Gong, J.P. Aggregated Structures and Their Functionalities in Hydrogels. Aggregate 2021, 2, e33. [Google Scholar] [CrossRef]
- Kim, B.; Park, J.; Lee, J.Y. Conductive Double-Network Hydrogel Composed of Sodium Alginate, Polyacrylamide, and Reduced Graphene Oxide. Korean J. Chem. Eng. 2023, 40, 352–360. [Google Scholar] [CrossRef]
- Zhou, Y.; Fei, X.; Tian, J.; Xu, L.; Li, Y. A Ionic Liquid Enhanced Conductive Hydrogel for Strain Sensing Applications. J. Colloid Interface Sci. 2022, 606, 192–203. [Google Scholar] [CrossRef]
- Cholewinski, A.; Si, P.; Uceda, M.; Pope, M.; Zhao, B. Polymer Binders: Characterization and Development toward Aqueous Electrode Fabrication for Sustainability. Polymers 2021, 13, 631. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gui, C.; Zhang, Y.; Nan, J.; Li, Y.; Wang, Z.; Sun, L.; Tan, X.; Wang, C.; Yang, F.; et al. An Adhesive Interface between Hydrogel Electrolyte and Electrode for Low-Temperature Solid-State Capacitive Devices. J. Energy Storage 2024, 102, 114061. [Google Scholar] [CrossRef]
- Yoon, J.; Lee, J.; Kim, H.; Kim, J.; Jin, H.-J. Polymeric Binder Design for Sustainable Lithium-Ion Battery Chemistry. Polymers 2024, 16, 254. [Google Scholar] [CrossRef]
- Srivastava, M.; Anil Kumar, M.R.; Zaghib, K. Binders for Li-Ion Battery Technologies and Beyond: A Comprehensive Review. Batteries 2024, 10, 268. [Google Scholar] [CrossRef]
- Wang, F.; Ma, X.; Li, Y.; Liu, H.; Wu, Q.; Guan, X.; Liu, H.; Wang, X.X.; Xu, J. Room-Temperature Rapid Self-Healing Polymer Binders for Si Anodes in Highly Cycling-Stable and Capacity-Maintained Lithium-Ion Batteries. ACS Appl. Energy Mater. 2023, 6, 3538–3548. [Google Scholar] [CrossRef]
- Bao, D.; Guan, F.; Ji, X.; Zhang, X.; Xu, Y.; Yang, Q.; Yao, Q.; Zhang, S.; Guo, J. Unidirectionally Arranged Layered Structured Hydrogels with High Strength, Multifunctional Integration, and Somatosensory Actuators. Chem. Eng. J. 2025, 505, 159294. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, B.; Li, J.; Shen, M.; Liu, H.; Xu, X.; Shang, S. Self-Healing, Self-Adhesive, and Stretchable Conductive Hydrogel for Multifunctional Sensor Prepared by Catechol Modified Nanocellulose Stabilized Poly(α-Thioctic Acid). Carbohydr. Polym. 2023, 313, 120813. [Google Scholar] [CrossRef]
- Zhao, R.; Yan, X.; Lin, H.; Zhao, Z.; Song, S. Mechanical Tough, Stretchable, and Adhesive PEDOT:PSS-Based Hydrogel Flexible Electronics towards Multi-Modal Wearable Application. Chem. Eng. J. 2025, 510, 161645. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, W.; Wang, D.; Jiang, Z.; Wang, X.; Dong, M.; Chen, K. Highly Stretchable, Self-Healing, Anti-Freezing, and Moisturizing Hydrogel with Efficient Conductive Pathways for Self-Powered Sensing Skin Electronics. Chem. Eng. J. 2025, 520, 165986. [Google Scholar] [CrossRef]
- Wu, J.; Xue, W.; Yun, Z.; Liu, Q.; Sun, X. Biomedical Applications of Stimuli-Responsive “Smart” Interpenetrating Polymer Network Hydrogels. Mater. Today Bio 2024, 25, 100998. [Google Scholar] [CrossRef]
- Li, M.; Hicks, R.P.; Chen, Z.; Luo, C.; Guo, J.; Wang, C.; Xu, Y. Electrolytes in Organic Batteries. Chem. Rev. 2023, 123, 1712–1773. [Google Scholar] [CrossRef] [PubMed]
- Surendran, V.; Thangadurai, V. Solid-State Lithium Metal Batteries for Electric Vehicles: Critical Single Cell Level Assessment of Capacity and Lithium Necessity. ACS Energy Lett. 2025, 10, 991–1001. [Google Scholar] [CrossRef]
- Wang, S.; Wen, X.; Huang, Z.; Xu, H.; Fan, F.; Wang, X.; Tian, G.; Liu, S.; Liu, P.; Wang, C.; et al. High-Entropy Strategy Flattening Lithium Ion Migration Energy Landscape to Enhance the Conductivity of Garnet-Type Solid-State Electrolytes. Adv. Funct. Mater. 2025, 35, 2416389. [Google Scholar] [CrossRef]
- Ullah, C.; Kim, A.; Lim, D.Y.; Ullah, A.; Kim, D.Y.; Lim, S.I.; Lim, H.-R. Hydrogel-Based Biointerfaces: Recent Advances, Challenges, and Future Directions in Human-Machine Integration. Gels 2025, 11, 232. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Gund, G.S.; Qi, K.; Nakhanivej, P.; Liu, H.; Li, F.; Xia, B.Y.; Park, H.S. Hybridization Design of Materials and Devices for Flexible Electrochemical Energy Storage. Energy Storage Mater. 2019, 19, 212–241. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Y.; Xu, X. Evolution and Application of All-in-One Electrochemical Energy Storage System. Energy Storage Mater. 2021, 41, 677–696. [Google Scholar] [CrossRef]
- Shi, Y.; Feng, A.; Mao, S.; Onggowarsito, C.; Stella Zhang, X.; Guo, W.; Fu, Q. Hydrogels in Solar-Driven Water and Energy Production: Recent Advances and Future Perspectives. Chem. Eng. J. 2024, 492, 152303. [Google Scholar] [CrossRef]
- Duan, H.; Zhang, Y.; Zhang, Y.; Zhu, P.; Mao, Y. Recent Advances of Stretchable Nanomaterial-Based Hydrogels for Wearable Sensors and Electrophysiological Signals Monitoring. Nanomaterials 2024, 14, 1398. [Google Scholar] [CrossRef]
- Liu, D.; Huyan, C.; Wang, Z.; Guo, Z.; Zhang, X.; Torun, H.; Mulvihill, D.; Xu, B.B.; Chen, F. Conductive Polymer Based Hydrogels and Their Application in Wearable Sensors: A Review. Mater. Horiz. 2023, 10, 2800–2823. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Lee, H.S.; Jeong, H.; Kim, D.-H. Recent Advances in Conductive Hydrogels for Soft Biointegrated Electronics: Materials, Properties, and Device Applications. Wearable Electron. 2024, 1, 255–280. [Google Scholar] [CrossRef]
- Gashti, M.P.; María, J.; Moreno, C.; Chelu, M.; Popa, M. Massimo Mariello Eco-Friendly Conductive Hydrogels: Towards Green Wearable Electronics. Gels 2025, 11, 220. [Google Scholar] [CrossRef]
- Akhtar, M.F.; Hanif, M.; Ranjha, N.M. Methods of Synthesis of Hydrogels … A Review. Saudi Pharm. J. 2016, 24, 554–559. [Google Scholar] [CrossRef]
- Voorhaar, L.; Hoogenboom, R. Supramolecular Polymer Networks: Hydrogels and Bulk Materials. Chem. Soc. Rev. 2016, 45, 4013–4031. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Chen, X.; Mohy Eldin, M.S.; Kenawy, E.-R.S. Crosslinked Poly(Vinyl Alcohol) Hydrogels for Wound Dressing Applications: A Review of Remarkably Blended Polymers. Arab. J. Chem. 2015, 8, 1–14. [Google Scholar] [CrossRef]
- MohanKumar, B.S.; Priyanka, G.; Rajalakshmi, S.; Sankar, R.; Sabreen, T.; Ravindran, J. Hydrogels: Potential Aid in Tissue Engineering—A Review. Polym. Bull. 2022, 79, 7009–7039. [Google Scholar] [CrossRef]
- Cui, W.; Pi, M.; Li, Y.; Shi, L.-Y.; Ran, R. Multimechanism Physical Cross-Linking Results in Tough and Self-Healing Hydrogels for Various Applications. ACS Appl. Polym. Mater. 2020, 2, 3378–3389. [Google Scholar] [CrossRef]
- Palantöken, S.; Bethke, K.; Zivanovic, V.; Kalinka, G.; Kneipp, J.; Rademann, K. Cellulose Hydrogels Physically Crosslinked by Glycine: Synthesis, Characterization, Thermal and Mechanical Properties. J. Appl. Polym. Sci. 2020, 137, 48380. [Google Scholar] [CrossRef]
- Sunarti, T.C.; Febrian, M.I.; Ruriani, E.; Yuliasih, I. Some Properties of Chemical Cross-Linking Biohydrogel from Starch and Chitosan. Int. J. Biomater. 2019, 2019, 1542128. [Google Scholar] [CrossRef]
- Singh, A.; Narvi, S.S.; Dutta, P.K.; Pandey, N.D. External Stimuli Response on a Novel Chitosan Hydrogel Crosslinked with Formaldehyde. Bull. Mater. Sci. 2006, 29, 233–238. [Google Scholar] [CrossRef]
- Kawase, M.; Michibayashi, N.; Nakashima, Y.; Kurikawa, N.; Yagi, K.; Mizoguchi, T. Application of Glutaraldehyde-Crosslinked Chitosan as a Scaffold for Hepatocyte Attachment. Biol. Pharm. Bull. 1997, 20, 708–710. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Méndez, J.A.; Fernández-Gutiérrez, M.; Vázquez, B.; San Román, J. Oxidized Dextrins as Alternative Crosslinking Agents for Polysaccharides: Application to Hydrogels of Agarose–Chitosan. Acta Biomater. 2014, 10, 798–811. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Weng, Y.; Xu, L.; Chen, H. Sustained Release of Avastin® from Polysaccharides Cross-Linked Hydrogels for Ocular Drug Delivery. Int. J. Biol. Macromol. 2013, 60, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Coviello, T.; Grassi, M.; Lapasin, R.; Marino, A.; Alhaique, F. Scleroglucan/Borax: Characterization of a Novel Hydrogel System Suitable for Drug Delivery. Biomaterials 2003, 24, 2789–2798. [Google Scholar] [CrossRef]
- Abdel-Raouf, M.E.; El-Saeed, S.M.; Zaki, E.G.; Al-Sabagh, A.M. Green Chemistry Approach for Preparation of Hydrogels for Agriculture Applications through Modification of Natural Polymers and Investigating Their Swelling Properties. Egypt. J. Pet. 2018, 27, 1345–1355. [Google Scholar] [CrossRef]
- Hazer, B.; Demirel, S.I.; Borcakli, M.; Eroglu, M.S.; Cakmak, M.; Erman, B. Free Radical Crosslinking of Unsaturated Bacterial Polyesters Obtained from Soybean Oily Acids. Polym. Bull. 2001, 46, 389–394. [Google Scholar] [CrossRef]
- Ovais, M.; Nadhman, A.; Khalil, A.T.; Raza, A.; Khuda, F.; Sohail, M.F.; Islam, N.U.; Sarwar, H.S.; Shahnaz, G.; Ahmad, I.; et al. Biosynthesized Colloidal Silver and Gold Nanoparticles As Emerging Leishmanicidal Agents: An Insight. Nanomedicine 2017, 12, 2807–2819. [Google Scholar] [CrossRef]
- Singh, B.; Pal, L. Radiation Crosslinking Polymerization of Sterculia Polysaccharide–PVA–PVP for Making Hydrogel Wound Dressings. Int. J. Biol. Macromol. 2011, 48, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Gulrez, S.K.H.; Al-Assaf, S.; Phillips, G.O. Hydrogels: Methods of Preparation, Characterisation and Applications. In Progress in Molecular and Environmental Bioengineering—From Analysis and Modeling to Technology Applications; IntechOpen: London, UK, 2011; p. 117150. [Google Scholar]
- Wach, R.A.; Mitomo, H.; Yoshii, F.; Kume, T. Hydrogel of Radiation-Induced Cross-Linked Hydroxypropylcellulose. Macromol. Mater. Eng. 2002, 287, 285–295. [Google Scholar] [CrossRef]
- Wasikiewicz, J.M.; Mitomo, H.; Nagasawa, N.; Yagi, T.; Tamada, M.; Yoshii, F. Radiation Crosslinking of Biodegradable Carboxymethylchitin and Carboxymethylchitosan. J. Appl. Polym. Sci. 2006, 102, 758–767. [Google Scholar] [CrossRef]
- Kozicki, M.; Kołodziejczyk, M.; Szynkowska, M.; Pawlaczyk, A.; Leśniewska, E.; Matusiak, A.; Adamus, A.; Karolczak, A. Hydrogels Made from Chitosan and Silver Nitrate. Carbohydr. Polym. 2016, 140, 74–87. [Google Scholar] [CrossRef]
- Ryu, J.H.; Lee, Y.; Kong, W.H.; Kim, T.G.; Park, T.G.; Lee, H. Catechol-Functionalized Chitosan/Pluronic Hydrogels for Tissue Adhesives and Hemostatic Materials. Biomacromolecules 2011, 12, 2653–2659. [Google Scholar] [CrossRef]
- Wang, T.; Chen, L.; Shen, T.; Wu, D. Preparation and Properties of a Novel Thermo-Sensitive Hydrogel Based on Chitosan/Hydroxypropyl Methylcellulose/Glycerol. Int. J. Biol. Macromol. 2016, 93, 775–782. [Google Scholar] [CrossRef]
- Pan, X.; Li, Y.; Pang, W.; Xue, Y.; Wang, Z.; Jiang, C.; Shen, C.; Liu, Q.; Liu, L. Preparation, Characterisation and Comparison of Glabridin-Loaded Hydrogel-Forming Microneedles by Chemical and Physical Cross-Linking. Int. J. Pharm. 2022, 617, 121612. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Bai, H.; Zhao, Y.; Kang, S.; Yi, H.; Zhang, T.; Song, S. Synthesis of Chitosan Cross-Linked 3D Network-Structured Hydrogel for Methylene Blue Removal. Int. J. Biol. Macromol. 2019, 141, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-F.; Xie, Y.; Shan, S.; Li, W.; Sun, L. Chemically-Crosslinked Xylan/Graphene Oxide Composite Hydrogel for Copper Ions Removal. J. Polym. Environ. 2022, 30, 3999–4013. [Google Scholar] [CrossRef]
- Rahnama, H.; Nouri Khorasani, S.; Aminoroaya, A.; Molavian, M.R.; Allafchian, A.; Khalili, S. Facile Preparation of Chitosan-Dopamine-Inulin Aldehyde Hydrogel for Drug Delivery Application. Int. J. Biol. Macromol. 2021, 185, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, T.; Shi, Y.; Wang, H. Dramatically Enhancing Mechanical Properties of Hydrogels by Drying Reactive Polymers at Elevated Temperatures to Introduce Strong Physical and Chemical Crosslinks. Polymer 2022, 249, 124842. [Google Scholar] [CrossRef]
- Nguyen, N.T.-P.; Nguyen, L.V.-H.; Thanh, N.T.; Toi, V.V.; Ngoc Quyen, T.; Tran, P.A.; David Wang, H.-M.; Nguyen, T.-H. Stabilization of Silver Nanoparticles in Chitosan and Gelatin Hydrogel and Its Applications. Mater. Lett. 2019, 248, 241–245. [Google Scholar] [CrossRef]
- Singh, B.; Singh, B. Modification of Sterculia Gum Polysaccharide via Network Formation by Radiation Induced Crosslinking Polymerization for Biomedical Applications. Int. J. Biol. Macromol. 2018, 116, 91–99. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y.; Fu, J.; Shi, L.; Li, C.; Ma, R. Advances in Functional Cellulose Hydrogels as Electrolytes for Flexible Zinc-Ion Batteries. Nanomaterials 2024, 14, 1645. [Google Scholar] [CrossRef] [PubMed]
- Day, G.J.; Zhang, Q.; Remillat, C.D.L.; Comandini, G.; Perriman, A.W.; Scarpa, F. Tunable Network Architecture in a Hydrogel with Extreme Vibration Damping Properties. Commun. Mater. 2025, 6, 148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yuan, D.; Zhao, J.; Ji, X.; Zhang, Y. Gel Electrolytes: Chemistry and Applications. Chem. Asian J. 2023, 18, e202300360. [Google Scholar] [CrossRef]
- Oyen, M.L. Mechanical Characterisation of Hydrogel Materials. Int. Mater. Rev. 2014, 59, 44–59. [Google Scholar] [CrossRef]
- Lu, K.; Jiang, T.; Hu, H.; Wu, M. Hydrogel Electrolytes for Quasi-Solid Zinc-Based Batteries. Front. Chem. 2020, 8, 546728. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, X.; Liu, J.; Zhang, H.; Fu, D. Advances in the Application of Natural/Synthetic Hybrid Hydrogels in Tissue Engineering and Delivery Systems: A Comprehensive Review. Int. J. Pharm. 2025, 672, 125323. [Google Scholar] [CrossRef]
- Xu, M.-W.; Jia, W.; Bao, S.-J.; Su, Z.; Dong, B. Novel Mesoporous MnO2 for High-Rate Electrochemical Capacitive Energy Storage. Electrochim. Acta 2010, 55, 5117–5122. [Google Scholar] [CrossRef]
- Rymsha, K.V.; Yevchuk, I.Y.; Zhyhailo, M.M.; Demchyna, O.I.; Maksymych, V.M.; Ivashchyshyn, F.O. Hydrogels and Their Composites Based on Sulfo-Containing Acrylates: Preparation, Properties, and Proton Conductivity. J. Solid State Electrochem. 2024, 28, 555–563. [Google Scholar] [CrossRef]
- Kang, S.H.; Lee, G.Y.; Lim, J.; Kim, S.O. CNT–RGO Hydrogel-Integrated Fabric Composite Synthesized via an Interfacial Gelation Process for Wearable Supercapacitor Electrodes. ACS Omega 2021, 6, 19578–19585. [Google Scholar] [CrossRef]
- Saeed, A.; Alwafi, R.; Alenizi, M.A.; Al-Marhaby, F.A.; Al-Rasheedi, A.; Asnag, G.M.; Al-Hakimi, A.N.; Ghalab, S.; Al-Ghamdi, S.A. Influence of Zinc Acetate on HPMC/CMC Polymer Blend: Investigation of Their Composites’ Structural, Optical, and Dielectric Properties for Dielectric Capacitor Applications. Inorg. Chem. Commun. 2025, 171, 113536. [Google Scholar] [CrossRef]
- Xu, Z.-H.; Zhao, Z.-Y.; Wang, H.; Wang, S.-M.; Chen, H.-Y.; Xu, J.-J. CRISPR-Cas12a-Based Efficient Electrochemiluminescence Biosensor for ATP Detection. Anal. Chim. Acta 2021, 1188, 339180. [Google Scholar] [CrossRef]
- Chen, J.-X.; Ou, K.-J.; Wu, Y.-C.; Li, J.-W.; Wang, J.-H.; Kuo, C.-F.J.; Cheng, C.-C.; Tseng, Y.-H.; Chiu, C.-W. Highly Compressible and Efficient CNT/RGO/PDMS Thermoelectric Generator Based on a 3D Sponge-Structured Network for Harvesting Energy from a Shoe Sole. ACS Appl. Electron. Mater. 2025, 7, 1871–1882. [Google Scholar] [CrossRef]
- Wanniarachchi, P.C.; Paranagama, I.T.; Idangodage, P.A.; Nallaperuma, B.; Samarasinghe, T.T.; Jayathilake, C. Natural Polymer-Based Hydrogels: Types, Functionality, Food Applications, Environmental Significance and Future Perspectives: An Updated Review. Food Biomacromol. 2025, 2, 84–105. [Google Scholar] [CrossRef]
- Timaeva, O.; Pashkin, I.; Mulakov, S.; Kuzmicheva, G.; Konarev, P.; Terekhova, R.; Sadovskaya, N.; Czakkel, O.; Prevost, S. Synthesis and Physico-Chemical Properties of Poly(N-Vinyl Pyrrolidone)-Based Hydrogels with Titania Nanoparticles. J. Mater. Sci. 2020, 55, 3005–3021. [Google Scholar] [CrossRef] [PubMed]
- Timaeva, O.I.; Kuz’micheva, G.M.; Pashkin, I.I.; Czakkel, O.; Prevost, S. Structure and Dynamics of Titania—Poly(N-Vinyl Caprolactam) Composite Hydrogels. Soft Matter 2020, 16, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Y.; Pang, Y.; Wang, Y.; Lai, C.; Zhang, D.; Liu, Y. Facile Design of Multiscale Cellulose-Enhanced Hydrogel Electrolytes for Flexible Zn-Ion Capacitors in Wearable Electronics. Macromol. Rapid Commun. 2025, 46, 2500295. [Google Scholar] [CrossRef]
- Priya, A.S.; Kannan, K.; Henry, J.; Aepuru, R.; Shanmugaraj, K.; Pabba, D.P.; Sathish, M. Advancements in Hydrogel Materials for Next-Generation Energy Devices: Properties, Applications, and Future Prospects. Cellulose 2025, 32, 6307–6335. [Google Scholar] [CrossRef]
- Nanda, D.; Behera, D.; Pattnaik, S.S.; Behera, A.K. Advances in Natural Polymer-Based Hydrogels: Synthesis, Applications, and Future Directions in Biomedical and Environmental Fields. Discov. Polym. 2025, 2, 6. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sharma, R.; Pani, B.; Sarkar, A.; Tripathi, M. Engineering the Future with Hydrogels: Advancements in Energy Storage Devices and Biomedical Technologies. New J. Chem. 2024, 48, 10347–10369. [Google Scholar] [CrossRef]
- Jain, A.; Mahata, K.; Onkarnath; Banerjee, S. Biopolymer Derived Gel Polymer Electrolytes: Current Status and Future Perspectives. Macromol. Rapid Commun. 2025, e00472. [Google Scholar] [CrossRef] [PubMed]
- Otgonbayar, Z.; Yang, S.; Kim, I.-J.; Oh, W.-C. Recent Advances in 2D MXene and Solid State Electrolyte for Energy Storage Applications: Comprehensive Review. Chem. Eng. J. 2023, 472, 144801. [Google Scholar] [CrossRef]
- Seleka, W.M.; Makhado, E.; Kganyakgo, L.K.; Mofokeng, L.E.; Makwakwa, D.; Botlhoko, O.J. Development of a Rapid Responsive Conductive Electrochemical Sensor for Sensitive Hydrogen Detection: Chitosan-Based GO/Fe3O4/PANi Hydrogel Nanocomposite. Int. J. Hydrogen Energy 2025, 142, 498–510. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, M.; Li, J.; Hu, C.; Li, J.; Xiong, R.; Huang, C. Nanocellulose Based Hydrogel for Flexible Sensors: Current Progress and Future Perspective. Nano Energy 2024, 129, 109974. [Google Scholar] [CrossRef]
- Tamo, A.K. Nanocellulose-Based Hydrogels as Versatile Materials with Interesting Functional Properties for Tissue Engineering Applications. J. Mater. Chem. B 2024, 12, 7692–7759. [Google Scholar] [CrossRef] [PubMed]
- Dutta, T.; Chaturvedi, P.; Llamas-Garro, I.; Velázquez-González, J.S.; Dubey, R.; Mishra, S.K. Smart Materials for Flexible Electronics and Devices: Hydrogel. RSC Adv. 2024, 14, 12984–13004. [Google Scholar] [CrossRef]
- Guo, L.; Ma, W.-B.; Wang, Y.; Song, X.-Z.; Ma, J.; Han, X.-D.; Tao, X.-Y.; Guo, L.-T.; Fan, H.-L.; Liu, Z.-S.; et al. A Chemically Crosslinked Hydrogel Electrolyte Based All-in-One Flexible Supercapacitor with Superior Performance. J. Alloys Compd. 2020, 843, 155895. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Yang, Y.; Qian, C.; Chen, C.; Han, L.; Han, Q. A Stretchable and Self-Healable Conductive Hydrogels Based on Gelation/Polyacrylamide/Polypyrrole for All-in-One Flexible Supercapacitors with High Capacitance. Colloids Surf. A Physicochem. Eng. Asp. 2022, 636, 128145. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, K.; Zheng, X.; Liu, Q.; Han, Y.; Liu, Z. Research Progress of Photo-Crosslink Hydrogels in Ophthalmology: A Comprehensive Review Focus on the Applications. Mater. Today Bio 2024, 26, 101082. [Google Scholar] [CrossRef]
- Zainulabdeen, K.W.Y.Z. Development of Flexible, Durable and Ionic Materials Based on Poly(Acrylamide) Hydrogels for Soft Conducting and Sensing Applications. Ph.D. Thesis, University of Wollongong, Wollongong, Australia, 2020. [Google Scholar]
- Gao, Y.; Peng, K.; Mitragotri, S. Covalently Crosslinked Hydrogels via Step-Growth Reactions: Crosslinking Chemistries, Polymers, and Clinical Impact. Adv. Mater. 2021, 33, 2006362. [Google Scholar] [CrossRef] [PubMed]
- Kharkar, P.; Kiick, K.; Kloxin, A. Designing Degradable Hydrogels for Orthogonal Control of Cell Microenvironments. Chem. Soc. Rev. 2013, 42, 7335–7372. [Google Scholar] [CrossRef] [PubMed]
- Romero García, A.; Bercea, M. Recent Advances in Poly(Vinyl Alcohol)-Based Hydrogels. Polymers 2024, 16, 2021. [Google Scholar] [CrossRef]
- Deutsch Lukatsky, A.T.; Dan, Y.; Mizrahi, L.; Amir, E. Hydrogels Based on Crosslinked Polyethylene Glycol Diacrylate and Fish Skin Gelatin. Eur. Polym. J. 2024, 210, 112990. [Google Scholar] [CrossRef]
- Kang, G.; Lu, N.; Li, L.; Wang, S.; Liu, S.; Yang, Y.; Luan, J.; Zhang, S.; Wang, G. Stretchable/Compressible Supercapacitors Based on High-Elasticity and Fatigue-Resistant Hydrogel Electrolyte Cross-Linked by Hydrophobic Nanospheres. Nano Lett. 2025, 25, 3858–3866. [Google Scholar] [CrossRef]
- Shin, S.-H.; Lee, W.; Kim, S.-M.; Lee, M.; Koo, J.M.; Hwang, S.Y.; Oh, D.X.; Park, J. Ion-Conductive Self-Healing Hydrogels Based on an Interpenetrating Polymer Network for a Multimodal Sensor. Chem. Eng. J. 2019, 371, 452–460. [Google Scholar] [CrossRef]
- Wang, J.; Yang, B.; Jiang, Z.; Liu, Y.; Zhou, L.; Liu, Z.; Tang, L. Recent Advances of Conductive Hydrogels for Flexible Electronics. Electron. Mater. 2024, 5, 101–131. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Wang, Y.; Huang, Y. Advances in Conductive Filler-Integrated Hydrogels and Derived Aerogels: Innovative Strategies for Electromagnetic Interference Shielding. Mater. Horizons 2025. [Google Scholar] [CrossRef]
- Shin, M.; Lim, J.; An, J.; Yoon, J.; Choi, J.-W. Nanomaterial-Based Biohybrid Hydrogel in Bioelectronics. Nano Converg. 2023, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Y.; Ma, Y.; Wang, M.; Pan, G. Nano-Crosslinked Dynamic Hydrogels for Biomedical Applications. Mater. Today Bio 2023, 20, 100640. [Google Scholar] [CrossRef]
- Hanyková, L.; Šťastná, J.; Krakovský, I. Responsive Acrylamide-Based Hydrogels: Advances in Interpenetrating Polymer Structures. Gels 2024, 10, 414. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, F.; Li, M.; Yang, M.; Zhang, D.; Han, L.; Li, X.; Zhang, Y.; Cao, A.; Shang, Y. Fast Gelling, High Performance MXene Hydrogels for Wearable Sensors. J. Colloid Interface Sci. 2024, 658, 137–147. [Google Scholar] [CrossRef]
- Song, Y.; Ao, Q.; Jiang, T.; Tong, X.; Ding, R.; Li, X.; Tang, J. Sustainable and High Performance MXene Hydrogel with Interlocked Structure for Machine Learning-Facilitated Human-Interactive Sensing. Chem. Eng. J. 2024, 499, 156432. [Google Scholar] [CrossRef]
- Munasir; Prapanca, A.; Aliansah, M.F.; Paramudhita, F.A.; Faaizatunnisa, N.; Ariesta, M.N.; Taufiq, A. Self-Healing Graphene-Based Composite Hydrogels for Motion Sensing: Source, Fabrication, and Applications in Assistive Technologies—A Review. Sensors Int. 2025, 6, 100338. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, B.; Zhang, D.; Yang, M.; Huang, X.; Han, L.; Chen, K.; Li, X.; Pang, R.; Shang, Y.; et al. Conductive Hydrogels Incorporating Carbon Nanoparticles: A Review of Synthesis, Performance and Applications. Particuology 2023, 83, 212–231. [Google Scholar] [CrossRef]
- Hajalilou, A. Liquid Metal–Polymer Hydrogel Composites for Sustainable Electronics: A Review. Molecules 2025, 30, 905. [Google Scholar] [CrossRef]
- Li, N.; Yuan, X.; Li, Y.; Zhang, G.; Yang, Q.; Zhou, Y.; Guo, M.; Liu, J. Bioinspired Liquid Metal Based Soft Humanoid Robots. Adv. Mater. 2024, 36, 2404330. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wu, A.; Zhang, Y.; Duan, H.; Zhu, P.; Mao, Y. Recent Progress of Nanomaterials-Based Composite Hydrogel Sensors for Human–Machine Interactions. Discov. Nano 2025, 20, 60. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Wang, Y.; Zhang, Q.; Wang, H.; Duan, X.; Yan, J.; Xia, Z.; Wang, R.; Shou, W.; Li, X. Ultra-Stretchable and Anti-Freezing Ionic Conductive Hydrogels as High Performance Strain Sensors and Flexible Triboelectric Nanogenerator in Extreme Environments. Nano Energy 2024, 126, 109633. [Google Scholar] [CrossRef]
- Lu, C.-H.; Yu, C.-H.; Yeh, Y.-C. Engineering Nanocomposite Hydrogels Using Dynamic Bonds. Acta Biomater. 2021, 130, 66–79. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Z.; Lu, B. Self-Healing and Tough Polyacrylic Acid-Based Hydrogels for Micro-Strain Sensors. Gels 2025, 11, 475. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, R.; Wang, R.; Chen, B.; Li, H.; Shen, A.; Mao, Y. Recent Advances in Stretchable Hydrogel-Based Triboelectric Nanogenerators for on-Skin Electronics. Mater. Chem. Front. 2024, 8, 4003–4028. [Google Scholar] [CrossRef]
- Li, Y.; Tan, S.; Zhang, X.; Li, Z.; Cai, J.; Liu, Y. Design Strategies and Emerging Applications of Conductive Hydrogels in Wearable Sensing. Gels 2025, 11, 258. [Google Scholar] [CrossRef]
- Chen, B.; Zhu, Y.; Yu, R.; Feng, Y.; Han, Z.; Liu, C.; Zhu, P.; Lu, L.; Mao, Y. Recent Progress of Biomaterial-Based Hydrogels for Wearable and Implantable Bioelectronics. Gels 2025, 11, 442. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, Q. Novel Structural Janus Hydrogels for Battery Applications: Structure Design, Properties, and Prospects. Colloids Interfaces 2025, 9, 48. [Google Scholar] [CrossRef]
- Liu, X.; Miller II, A.L.; Park, S.; Waletzki, B.E.; Terzic, A.; Yaszemski, M.J.; Lu, L. Covalent Crosslinking of Graphene Oxide and Carbon Nanotube into Hydrogels Enhances Nerve Cell Responses. J. Mater. Chem. B 2016, 4, 6930–6941. [Google Scholar] [CrossRef]
- Anjali, J.; Jose, V.K.; Lee, J.-M. Carbon-Based Hydrogels: Synthesis and Their Recent Energy Applications. J. Mater. Chem. A 2019, 7, 15491–15518. [Google Scholar] [CrossRef]
- Fattah, I.M.R.; Alom, J.; Zaman, J.U.; Ban, S.; Veza, I.; Kalam, M.A.; Hessel, V.; Ahmed, M.B. Hydrogel-Derived Materials for Microbial Fuel Cell. J. Power Sources 2025, 625, 235688. [Google Scholar] [CrossRef]
- Yang, G.; Zhou, Z.; Liu, X.; Zhang, Y.; Wang, S.; Yan, W.; Ding, S. Bowl-Shaped Hollow Carbon Wrapped in Graphene Grown in Situ by Chemical Vapor Deposition as an Advanced Anode Material for Sodium-Ion Batteries. J. Colloid Interface Sci. 2023, 637, 283–290. [Google Scholar] [CrossRef]
- Kougkolos, G.; Golzio, M.; Laudebat, L.; Valdez-Nava, Z.; Flahaut, E. Hydrogels with electrically conductive nanomaterials for biomedical applications. J. Mater. Chem. B 2023, 11, 2036–2062. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Qu, D.; Liu, W.; An, L.; Wang, X.; Sun, Z. Fabrication of a Bilayer Structural Carbon-Based Hydrogel Material with Excellent Energy Conversion Efficiency. Sci. China Mater. 2023, 66, 4834–4840. [Google Scholar] [CrossRef]
- Islam, M.S.; Kundu, S.; Samsunnahar, M.; Khandaker, T.; Ibrahim, A.B.M.; Anik, M.A.-A.M.; Hasan, M.K.; Hossain, M.S. Carbon Gel Materials: Synthesis, Structural Design, and Emerging Applications in Energy and Environmental Technologies. Mater. Adv. 2025. [Google Scholar] [CrossRef]
- Sun, X.; Qin, Z.; Ye, L.; Zhang, H.; Yu, Q.; Wu, X.; Li, J.; Yao, F. Carbon Nanotubes Reinforced Hydrogel as Flexible Strain Sensor with High Stretchability and Mechanically Toughness. Chem. Eng. J. 2020, 382, 122832. [Google Scholar] [CrossRef]
- Wang, L.; Luo, M.; Zhang, Z.; Ji, D.; Chang, X.; Zhu, Y. Ultra-Stretchable, Robust, Self-Healable Conductive Hydrogels Enabled by the Synergistic Effects of Hydrogen Bonds and Ionic Coordination Bonds toward High-Performance e-Skins. Chem. Eng. J. 2024, 500, 156800. [Google Scholar] [CrossRef]
- Kim, H.; Kim, D.; Kim, J.; Lee, Y.; Shin, M.; Kim, J.; Bossuyt, F.M.; Lee, G.-H.; Lee, B.; Taylor, W.R.; et al. Advances and Perspectives in Fiber-Based Electronic Devices for next-Generation Soft Systems. npj Flex. Electron. 2025, 9, 84. [Google Scholar] [CrossRef]
- Gao, L.; Liu, F.; Qi, J.; Gao, W.; Xu, G. Recent Advances and Challenges in Hybrid Supercapacitors Based on Metal Oxides and Carbons. Inorganics 2025, 13, 49. [Google Scholar] [CrossRef]
- Indriyati, I.; Primadona, I.; Permatasari, F.; Irham, M.; Nasir, M.; Iskandar, F. Recent Advances and Rational Design Strategies of Carbon Dots towards Highly Efficient Solar Evaporation. Nanoscale 2021, 13, 7523–7532. [Google Scholar] [CrossRef]
- Shahid, M.A.; Rahman, M.M.; Hossain, M.T.; Hossain, I.; Sheikh, M.S.; Rahman, M.S.; Uddin, N.; Donne, S.W.; Hoque, M.I. Advances in Conductive Polymer-Based Flexible Electronics for Multifunctional Applications. J. Compos. Sci. 2025, 9, 42. [Google Scholar] [CrossRef]
- Yang, T.; Xu, C.; Liu, C.; Ye, Y.; Sun, Z.; Wang, B.; Luo, Z. Conductive Polymer Hydrogels Crosslinked by Electrostatic Interaction with PEDOT:PSS Dopant for Bioelectronics Application. Chem. Eng. J. 2021, 429, 132430. [Google Scholar] [CrossRef]
- Tomczykowa, M.; Plonska-Brzezinska, M.E. Conducting Polymers, Hydrogels and Their Composites: Preparation, Properties and Bioapplications. Polymers 2019, 11, 350. [Google Scholar] [CrossRef] [PubMed]
- Rahman Khan, M.M.; Chakraborty, N. Conducting Polymer-Based Gel Materials: Synthesis, Morphology, Thermal Properties, and Applications in Supercapacitors. Gels 2024, 10, 553. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Ma, Z.; Pan, L.J.; Shi, Y. Properties of Conductive Polymer Hydrogels and Their Application in Sensors. J. Polym. Sci. Part B Polym. Phys. 2019, 57, 1606–1621. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Wang, F.; Zhang, J.-H.; Zhang, J.; Shi, Y.; Pan, L. Application of Conductive Polymer Hydrogels in Flexible Electronics. J. Polym. Sci. 2022, 60, 2635–2662. [Google Scholar] [CrossRef]
- Li, L.; Meng, J.; Zhang, M.; Liu, T.; Zhang, C. Recent Advances in Conductive Polymer Hydrogel Composites and Nanocomposites for Flexible Electrochemical Supercapacitors. Chem. Commun. 2022, 58, 185–207. [Google Scholar] [CrossRef]
- Oh, S.-G.; Im, S.-S. Electroconductive Polymer Nanoparticles Preparation and Characterization of PANI and PEDOT Nanoparticles. Curr. Appl. Phys. 2002, 2, 273–277. [Google Scholar] [CrossRef]
- He, H.; Chen, Y.; Pu, A.; Wang, L.; Li, W.; Zhou, X.; Tang, C.Y.; Ban, K.; Yang, M.; Xu, L. Strong and High-Conductivity Hydrogels with All-Polymer Nanofibrous Networks for Applications as High-Capacitance Flexible Electrodes. npj Flex. Electron. 2024, 8, 56. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, L.; Guo, M.; Sun, Z.; Chen, Y.; Wu, Y.; Li, Y.; Xiang, D.; Li, H.; Li, Z. Double-Network Hydrogel-Based Stretchable, Adhesive, and Conductive E-Skin Sensor Coupled with Human Skin-like Biocompatible and Protective Properties. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129803. [Google Scholar] [CrossRef]
- Zhao, M.; Wu, T.; Wang, X.; Liang, L.; Lu, H.; Xie, Z.; Yuan, T.; Fang, G. Intrinsically Conductive Polymer Reinforced Hydrogel with Synergistic Strength, Toughness, and Sensitivity for Flexible Motion-Monitoring Sensors. Cell Rep. Phys. Sci. 2024, 5, 102178. [Google Scholar] [CrossRef]
- Wang, T.; Liu, J.; Zhao, Y.; Lu, Y. Synergistic Mastery: Advancing Mechanical and Electrical Harmony in Conducting Polymer Hydrogel Bioelectronics. Bioact. Mater. 2025, 52, 300–317. [Google Scholar] [CrossRef]
- Jain, N.; Waidi, Y.O. The Multifaceted Role of 3D Printed Conducting Polymers in Next-Generation Energy Devices: A Critical Perspective. JACS Au 2025, 5, 411–425. [Google Scholar] [CrossRef]
- Peng, Y.; Yuan, W.; Liu, X.; Xie, P.; Yang, F.; Zhao, H.; Lu, D.; Yin, Y.; Wu, Z. All-in-One Integration of Polyaniline-Polyvinyl Alcohol Electrode/Electrolyte Interface for Tailorable Solid-State Supercapacitors. J. Energy Storage 2023, 61, 106701. [Google Scholar] [CrossRef]
- Virumbrales, C.; Hernández-Ruiz, R.; Trigo-López, M.; Vallejos, S.; García, J.M. Sensory Polymers: Trends, Challenges, and Prospects Ahead. Sensors 2024, 24, 3852. [Google Scholar] [CrossRef]
- Lim, J.Y.C.; Goh, L.; Otake, K.; Goh, S.S.; Loh, X.J.; Kitagawa, S. Biomedically-Relevant Metal Organic Framework-Hydrogel Composites. Biomater. Sci. 2023, 11, 2661–2677. [Google Scholar] [CrossRef]
- Durán-Egido, V.; García-Giménez, D.; Martínez-López, J.C.; Pérez-Vidal, L.; Carretero-González, J. Metal–Organic Frameworks as Fillers in Porous Organic Polymer-Based Hybrid Materials: Innovations in Composition, Processing, and Applications. Polymers 2025, 17, 1941. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhao, X.; Webb, E.; Xu, G.; Zhang, W.; Wang, Y. Advances in Metal–Organic Framework-Based Hydrogel Materials: Preparation, Properties and Applications. J. Mater. Chem. A 2023, 11, 2092–2127. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Abbas, Q.; Mouselly, M.; Alawadhi, H.; Olabi, A.G. High-Performance Effective Metal–Organic Frameworks for Electrochemical Applications. J. Sci. Adv. Mater. Devices 2022, 7, 100465. [Google Scholar] [CrossRef]
- Song, Y.; Ma, S. Pore Engineering in Metal-Organic Frameworks and Covalent Organic Frameworks: Strategies and Applications. Chem. Sci. 2025, 16, 11740–11767. [Google Scholar] [CrossRef]
- Zhou, W.; Tian, M.; Wang, H.; Qi, Z.; Yuan, H.; Zhong, L.; Sun, X. Integration of Metal-Organic Frameworks into Hydrogels: Optimizing Their Properties and Applications. Z. für Anorg. Und Allg. Chem. 2024, 650, e202400157. [Google Scholar] [CrossRef]
- He, R.; He, J.; Shen, J.; Fu, H.; Zhang, Y.; Wang, B. Recent Advances in Multifaceted Applications of MOF-Based Hydrogels. Soft Sci. 2024, 4, 37. [Google Scholar] [CrossRef]
- Nazir, M.A.; Ullah, S.; Jamil, A.; Shaaban, I.A.; Gurbanova, L.; Khan, K.; Shah, S.S.A.; Bao, S.-J. Advances in Metal–Organic Framework-Based Materials for Sustainable Energy Solutions. J. Mater. Chem. A 2025, 13, 25258–25303. [Google Scholar] [CrossRef]
- Zou, M.; Dong, M.; Zhao, T. Advances in Metal-Organic Frameworks MIL-101(Cr). Int. J. Mol. Sci. 2022, 23, 9396. [Google Scholar] [CrossRef]
- Utpalla, P.; Mor, J.; Pujari, P.K.; Sharma, S.K. High Ionic Conductivity and Ion Conduction Mechanism in ZIF-8 Based Quasi-Solid-State Electrolytes: A Positron Annihilation and Broadband Dielectric Spectroscopy Study. Phys. Chem. Chem. Phys. 2022, 24, 24999–25009. [Google Scholar] [CrossRef]
- Saeidi, M.; Chenani, H.; Orouji, M.; Adel Rastkhiz, M.; Bolghanabadi, N.; Vakili, S.; Mohamadnia, Z.; Hatamie, A.; Simchi, A. Electrochemical Wearable Biosensors and Bioelectronic Devices Based on Hydrogels: Mechanical Properties and Electrochemical Behavior. Biosensors 2023, 13, 823. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, R. Hydrogels: Fundamentals to Advanced Energy Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar] [CrossRef]
- Chen, J.; Liu, F.; Abdiryim, T.; Liu, X. An Overview of Conductive Composite Hydrogels for Flexible Electronic Devices. Adv. Compos. Hybrid Mater. 2024, 7, 35. [Google Scholar] [CrossRef]
- Sayed, M.M. El Production of Polymer Hydrogel Composites and Their Applications. J. Polym. Environ. 2023, 31, 2855–2879. [Google Scholar] [CrossRef]
- Aigoin, J.; Payré, B.; Moncla, J.M.; Escudero, M.; Goudouneche, D.; Ferri-Angulo, D.; Calmon, P.-F.O.; Vaysse, L.; Kemoun, P.; Malaquin, L.; et al. Comparative Analysis of Electron Microscopy Techniques for Hydrogel Microarchitecture Characterization: SEM, Cryo-SEM, ESEM, and TEM. ACS Omega 2025, 10, 14687–14698. [Google Scholar] [CrossRef]
- Zewail, T.M.M.; Saad, M.A.; AbdelRazik, S.M.; Eldakiky, B.M.; Sadik, E.R. Synthesis of sodium alginate/polyvinyl alcohol/polyethylene glycol semi-interpenetrating hydrogel as a draw agent for forward osmosis desalination. BMC Chem. 2024, 18, 134. [Google Scholar] [CrossRef]
- Lambrecht, S.; Biermann, M.; Kara, S.; Jopp, S.; Meyer, J. A Novel Characterization Technique for Hydrogels-in Situ Rheology-Raman Spectroscopy for Gelation and Polymerization Tracking. Mater. Adv. 2024, 5, 6957. [Google Scholar] [CrossRef]
- Peppas, N.A.; Keys, K.B.; Torres-Lugo, M.; Lowman, A.M. Poly(Ethylene Glycol)-Containing Hydrogels in Drug Delivery. J. Control. Release 1999, 62, 81–87. [Google Scholar] [CrossRef]
- Zhang, Z.; Long, M.; Zheng, N.; Deng, Y.; Wang, Q.; Osire, T.; Xia, X. Microstructural, Physicochemical Properties, and Interaction Mechanism of Hydrogel Nanoparticles Modified by High Catalytic Activity Transglutaminase Crosslinking. Food Hydrocoll. 2024, 147, 109384. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, W.; Yuan, C.; Morioka, K.; Chen, S.; Liu, D.; Ye, X. Enhancement of the Gelation Properties of Hairtail (Trichiurus Haumela) Muscle Protein with Curdlan and Transglutaminase. Food Chem. 2015, 176, 115–122. [Google Scholar] [CrossRef]
- Cortés-Camargo, S.; Román-Guerrero, A.; Alvarez-Ramirez, J.; Alpizar-Reyes, E.; Velázquez-Gutiérrez, S.K.; Pérez-Alonso, C. Microstructural Influence on Physical Properties and Release Profiles of Sesame Oil Encapsulated into Sodium Alginate-Tamarind Mucilage Hydrogel Beads. Carbohydr. Polym. Technol. Appl. 2023, 5, 100302. [Google Scholar] [CrossRef]
- Lozano-Vazquez, G.; Lobato-Calleros, C.; Escalona-Buendia, H.; Chavez, G.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. Effect of the Weight Ratio of Alginate-Modified Tapioca Starch on the Physicochemical Properties and Release Kinetics of Chlorogenic Acid Containing Beads. Food Hydrocoll. 2015, 48, 301–311. [Google Scholar] [CrossRef]
- Sharma, R.; Lalhall, A.; Puri, S.; Wangoo, N. Design of Fmoc-Phenylalanine Nanofibrillar Hydrogel and Mechanistic Studies of Its Antimicrobial Action against Both Gram-Positive and Gram-Negative Bacteria. ACS Appl. Bio Mater. 2023, 6, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, A.; Mashayekhan, S.; Baheiraei, N.; Pourjavadi, A. Biohybrid Oxidized Alginate/Myocardial Extracellular Matrix Injectable Hydrogels with Improved Electromechanical Properties for Cardiac Tissue Engineering. Int. J. Biol. Macromol. 2021, 180, 692–708. [Google Scholar] [CrossRef]
- Navaee, A.; Salimi, A. Efficient Amine Functionalization of Graphene Oxide through the Bucherer Reaction: An Extraordinary Metal-Free Electrocatalyst for the Oxygen Reduction Reaction. RSC Adv. 2015, 5, 59874–59880. [Google Scholar] [CrossRef]
- Hou, Y.; Chang, Y.; Zhao, Z.; Zhang, M.; Zhao, Q.; Guo, M.; Zhao, J.; Wu, S.; Ma, Y. Advanced In-Situ Functionalized Conductive Hydrogels with High Mechanical Strength for Hypersensitive Soft Strain Sensing Applications. Chem. Eng. J. 2024, 497, 154731. [Google Scholar] [CrossRef]
- Bouhadir, K.H.; Alsberg, E.; Mooney, D.J. Hydrogels for Combination Delivery of Antineoplastic Agents. Biomaterials 2001, 22, 2625–2633. [Google Scholar] [CrossRef]
- Medha; Sethi, S.; Mahajan, P.; Thakur, S.; Sharma, N.; Singh, N.; Kumar, A.; Kaur, A.; Kaith, B.S. Design and Evaluation of Fluorescent Chitosan-Starch Hydrogel for Drug Delivery and Sensing Applications. Int. J. Biol. Macromol. 2024, 274, 133486. [Google Scholar] [CrossRef]
- Enoch, K.; Rakavi, C.S.; Somasundaram, A.A. Tuning the Rheological Properties of Chitosan/Alginate Hydrogels for Tissue Engineering Application. Colloids Surf. A Physicochem. Eng. Asp. 2024, 697, 134434. [Google Scholar] [CrossRef]
- Nam, S.; Hu, K.H.; Butte, M.J.; Chaudhuri, O. Strain-Enhanced Stress Relaxation Impacts Nonlinear Elasticity in Collagen Gels. Proc. Natl. Acad. Sci. USA 2016, 113, 5492–5497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, S.; Wang, L.; Li, B.; Xie, M.; Deng, Y.; Zhang, J.; Zeng, H.; Qiu, L.; Huang, L.; et al. Triple-Crosslinked Double-Network Alginate/Dextran/Dendrimer Hydrogel with Tunable Mechanical and Adhesive Properties: A Potential Candidate for Sutureless Keratoplasty. Carbohydr. Polym. 2024, 344, 122538. [Google Scholar] [CrossRef]
- Cacopardo, L.; Guazzelli, N.; Nossa, R.; Mattei, G.; Ahluwalia, A. Engineering Hydrogel Viscoelasticity. J. Mech. Behav. Biomed. Mater. 2019, 89, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Lim, H.; Ko, J.; Cho, J. Unlocking High-Efficiency Energy Storage and Conversion with Biocompatible Electrodes: The Key Role of Interfacial Interaction Assembly and Structural Design. Energy Adv. 2024, 3, 2152–2174. [Google Scholar] [CrossRef]
- Tang, J.; Xu, X.; Fang, Z.; Shao, Z. Advances in Reaction Interface Engineering for 3D Hydrogel and Aerogel Derived Electrodes in Energy Conversion Devices. Energy Rev. 2025, 4, 100153. [Google Scholar] [CrossRef]
- Ning, L.; Zhou, J.; Xue, T.; Yan, X.-H.; Zou, Z.-L.; Wang, B.-P.; Lu, Y.-J.; Han, F.-L. Freeze-Thawed Polyacrylamide-Polyvinyl Alcohol Double Network with Enhanced Mechanical Properties as Hydrogel Electrolyte for Zinc-Ion Battery. J. Energy Storage 2023, 74, 109508. [Google Scholar] [CrossRef]
- Jagota, S.; Pandey, S.S.; Hakkarainen, M.; Chandra, P.; Yadav, M.; Warkar, S.G.; Sand, A. Superabsorbent Polymers: Synthesis, Applications, and Challenges. ChemistrySelect 2025, 10, e02854. [Google Scholar] [CrossRef]
- Mo, F.; Cui, M.; He, N.; Chen, L.; Fei, J.; Ma, Z.; Yu, S.; Wei, J.; Huang, Y. Recent Progress and Perspectives on Advanced Flexible Zn-Based Batteries with Hydrogel Electrolytes. Mater. Res. Lett. 2022, 10, 501–520. [Google Scholar] [CrossRef]
- Uyanga, K.A. Development of Sustainable Hydrogels with Enhanced Hydrolytic and Thermal Stability. Ph.D. Thesis, School of Energy and Environment, City University of Hong Kong, Hong Kong, China, 2021. [Google Scholar]
- Majcher, M.J.; Hoare, T. Hydrogel Properties and Characterization Techniques. In Functional Biopolymers; Jafar Mazumder, M.A., Sheardown, H., Al-Ahmed, A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–25. ISBN 978-3-319-92066-5. [Google Scholar]
- Durpekova, S.; Bergerova, E.D.; Hanusova, D.; Dusankova, M.; Sedlarik, V. Eco-Friendly Whey/Polysaccharide-Based Hydrogel with Poly (Lactic Acid) for Improvement of Agricultural Soil Quality and Plant Growth. Int. J. Biol. Macromol. 2022, 212, 85–96. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Savina, I.N.; Mikhalovsky, S. V Properties of Water Bound in Hydrogels. Gels 2017, 3, 37. [Google Scholar] [CrossRef]
- Harinarayanan, A.; Ummer, R.P.; Thomas, S.; Kandasubramanian, B. Recent Progress in Functional Hydrogel Electrolyte-Based Flexible Lithium-Ion Batteries: Preparation, Modifications, Fabrication, and Applications. J. Electron. Mater. 2025, 54, 2497–2517. [Google Scholar] [CrossRef]
- Zhang, H.; Gan, X.; Yan, Y.; Zhou, J. A Sustainable Dual Cross-Linked Cellulose Hydrogel Electrolyte for High-Performance Zinc-Metal Batteries. Nano-Micro Lett. 2024, 16, 106. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Xu, Z.; Wang, X. Polymer Hydrogel Electrolytes for Flexible and Multifunctional Zinc-Ion Batteries and Capacitors. Energy Environ. Mater. 2023, 6, e12464. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, L.; Peng, M.; Shen, D.; Zhu, C.; Qian, S.; Liu, J.; Cao, Y.; Yan, C.; Zhou, J. Developing Thermoregulatory Hydrogel Electrolyte to Overcome Thermal Runaway in Zinc-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2206653. [Google Scholar] [CrossRef]
- Devi, V.K.A.; Shyam, R.; Palaniappan, A.; Jaiswal, A.K.; Oh, T.H.; Nathanael, A.J. Self-Healing Hydrogels: Preparation, Mechanism and Advancement in Biomedical Applications. Polymers 2021, 13, 3782. [Google Scholar] [CrossRef]
- Wu, H.; Yu, G.; Pan, L.; Liu, N.; McDowell, M.T.; Bao, Z.; Cui, Y. Stable Li-Ion Battery Anodes by in-Situ Polymerization of Conducting Hydrogel to Conformally Coat Silicon Nanoparticles. Nat. Commun. 2013, 4, 1943. [Google Scholar] [CrossRef]
- Wan, X.; Mu, T.; Yin, G. Intrinsic Self-Healing Chemistry for Next-Generation Flexible Energy Storage Devices. Nano-Micro Lett. 2023, 15, 99. [Google Scholar] [CrossRef]
- Long, J.; Han, T.; Lin, X.; Zhu, Y.; Ding, Y.; Liu, J.; Zhang, H. An Integrated Flexible Self-Healing Zn-Ion Battery Using Dendrite-Suppressible Hydrogel Electrolyte and Free-Standing Electrodes for Wearable Electronics. Nano Res. 2023, 16, 11000–11011. [Google Scholar] [CrossRef]
- Cheng, S.; Zhu, R.; Xu, X. Hydrogels for next Generation Neural Interfaces. Commun. Mater. 2024, 5, 99. [Google Scholar] [CrossRef]
- Liu, J.; Lu, D.; Chen, B. Tuning Mechanical Behaviors of Highly Entangled Hydrogels with the Random Distribution of Mobile Entanglements. Appl. Math. Mech. 2024, 45, 277–294. [Google Scholar] [CrossRef]
- Uyanga, K.A.; Li, W.; Daoud, W.A. Exploiting Cellulose-Based Hydrogels for Sustainable, Intelligent Wearables in Pandemic Preparedness and Control. Eur. Polym. J. 2024, 212, 113041. [Google Scholar] [CrossRef]
- Chen, Z.; To, J.W.F.; Wang, C.; Lu, Z.; Liu, N.; Chortos, A.; Pan, L.; Wei, F.; Cui, Y.; Bao, Z. A Three-Dimensionally Interconnected Carbon Nanotube-Conducting Polymer Hydrogel Network for High-Performance Flexible Battery Electrodes. Adv. Energy Mater. 2014, 4, 1400207. [Google Scholar] [CrossRef]
- Li, X.; Zeng, S.; Li, W.; Lin, H.; Zhong, H.; Zhu, H.; Mai, Y. Stretchable Hydrogel Electrolyte Films Based on Moisture Self-Absorption for Flexible Quasi-Solid-State Batteries. Chem. Eng. J. 2022, 439, 135741. [Google Scholar] [CrossRef]
- Raza, B.; Shamraiz, U.; Hu, M.; Xu, X.; Yu, B.; Jing, S.; Tao, Y.; Wang, J. Advances in Organic Electrolytes for High-Performance Zinc Batteries: Enhancing Zinc Anode Robustness and Efficiency. Chem. Eng. J. 2025, 515, 163617. [Google Scholar] [CrossRef]
- Huang, F.; Guo, Y.; Zhao, W.; Wu, R.; Dong, Y.; Long, G.; Du, P. Advanced Hydrogel Electrolyte with Enhanced Interfacial Adhesion and Low-Temperature Resistant for Flexible Zinc-Ion Batteries. Chem. Eng. J. 2024, 498, 155248. [Google Scholar] [CrossRef]
- Gong, Y.; Zhao, C.; Wang, D.; Wang, X.; Wang, Z.; Wu, Y.; Xia, Y.; Jing, Q.; Ji, Y.; Jiang, Y.; et al. Advancing High-Voltage Halide-Based Solid-State Batteries: Interfacial Challenges, Material Innovations, and Applications. Energy Storage Mater. 2025, 74, 103980. [Google Scholar] [CrossRef]
- Janek, J.; Zeier, W.G. A Solid Future for Battery Development. Nat. Energy 2016, 1, 16141. [Google Scholar] [CrossRef]
- Muldoon, J.; Bucur, C.B.; Gregory, T. Quest for Nonaqueous Multivalent Secondary Batteries: Magnesium and Beyond. Chem. Rev. 2014, 114, 11683–11720. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, L. Cellulose-Based Hydrogels: Present Status and Application Prospects. Carbohydr. Polym. 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Hwang, S.; Kim, D.H.; Shin, J.H.; Jang, J.E.; Ahn, K.H.; Lee, C.; Lee, H. Ionic Conduction and Solution Structure in LiPF6 and LiBF4 Propylene Carbonate Electrolytes. J. Phys. Chem. C 2018, 122, 19438–19446. [Google Scholar] [CrossRef]
- Bae, J.; Qian, Y.; Li, Y.; Zhou, X.; Goodenough, J.B.; Yu, G. Polar Polymer-Solvent Interaction Derived Favorable Interphase for Stable Lithium Metal Batteries. Energy Environ. Sci. 2019, 12, 3319–3327. [Google Scholar] [CrossRef]
- Yang, C.; Chen, J.; Qing, T.; Fan, X.; Sun, W.; von Cresce, A.; Ding, M.S.; Borodin, O.; Vatamanu, J.; Schroeder, M.A.; et al. 4.0 V Aqueous Li-Ion Batteries. Joule 2017, 1, 122–132. [Google Scholar] [CrossRef]
- Feig, V.R.; Tran, H.; Lee, M.; Bao, Z. Mechanically Tunable Conductive Interpenetrating Network Hydrogels That Mimic the Elastic Moduli of Biological Tissue. Nat. Commun. 2018, 9, 2740. [Google Scholar] [CrossRef]
- Lopez, J.; Sun, Y.; Mackanic, D.G.; Lee, M.; Foudeh, A.M.; Song, M.S.; Cui, Y.; Bao, Z. A Dual-Crosslinking Design for Resilient Lithium-Ion Conductors. Adv. Mater. 2018, 30, e1804142. [Google Scholar] [CrossRef]
- Bari, G.A.K.M.R.; Jeong, J.-H.; Barai, H.R. Conductive Gels for Energy Storage, Conversion, and Generation: Materials Design Strategies, Properties, and Applications. Materials 2024, 17, 2268. [Google Scholar] [CrossRef]
- Vikraman, V.K.; Kumar, D.P.; Boopathi, G.; Komalabharathi, P.; Subramanian, P. Bio-Based Polymer Electrolytes for Supercapacitor Applications. In Encyclopedia of Green Materials; Baskar, C., Ramakrishna, S., La Rosa, A.D., Eds.; Springer Nature Singapore: Singapore, 2022; pp. 1–7. ISBN 978-981-16-4921-9. [Google Scholar]
- Assis, R.G.; Ramos, M.K.; Zarbin, A.J.G. Designing Self-Sustained Hydrogel Electrolytes for Aqueous, Transparent, and Flexible Batteries. J. Braz. Chem. Soc. 2024, 35, e20240086. [Google Scholar] [CrossRef]
- Guo, B.; Jia, J.; Zhao, Y.; Zhang, J.; Li, G.; Chen, K.; Wang, A.; Liu, C. Innovative Zwitterionic Polymers in Advanced Batteries. Energy Storage Mater. 2025, 78, 104253. [Google Scholar] [CrossRef]
- Guo, Y.; Bae, J.; Fang, Z.; Li, P.; Zhao, F.; Yu, G. Hydrogels and Hydrogel-Derived Materials for Energy and Water Sustainability. Chem. Rev. 2020, 120, 7642–7707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Z.; Sun, J.; Yao, Z.; Lu, H. The Formation and Performance Tuning Mechanism of Starch-Based Hydrogels. Carbohydr. Polym. 2025, 350, 123048. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Wang, S.; Chen, X.; Jiang, Y.; Su, J. Fabrication of Physical and Chemical Crosslinked Hydrogels for Bone Tissue Engineering. Bioact. Mater. 2022, 12, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Huebsch, N.; Mooney, D.J.; Suo, Z. Stress-Relaxation Behavior in Gels with Ionic and Covalent Crosslinks. J. Appl. Phys. 2010, 107, 63509. [Google Scholar] [CrossRef]
- Sandu, G.; Ernould, B.; Rolland, J.; Cheminet, N.; Brassinne, J.; Das, P.R.; Filinchuk, Y.; Cheng, L.; Komsiyska, L.; Dubois, P. Mechanochemical Synthesis of PEDOT: PSS Hydrogels for Aqueous Formulation of Li-Ion Battery Electrodes. ACS Appl. Mater. Interfaces 2017, 9, 34865–34874. [Google Scholar] [CrossRef]
- Tian, M.; Chen, X.; Sun, S.; Yang, D.; Wu, P. A Bioinspired High-Modulus Mineral Hydrogel Binder for Improving the Cycling Stability of Microsized Silicon Particle-Based Lithium-Ion Battery. Nano Res. 2019, 12, 1121–1127. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, M.; Jiang, C.; Zhu, P.; Sun, B.; Gao, Q.; Gao, C.; Liu, R. Highly Adhesive and Self-Healing γ-PGA/PEDOT: PSS Conductive Hydrogels Enabled by Multiple Hydrogen Bonding for Wearable Electronics. Nano Energy 2022, 95, 106991. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, X.; Ding, J.; Huang, B.; Wang, P.; Zhao, Y.; Mu, Q.; Zhang, S.; Ren, C.; Xu, W. Hydrogels for Underwater Adhesion: Adhesion Mechanism, Design Strategies and Applications. J. Mater. Chem. A 2022, 10, 11823–11853. [Google Scholar] [CrossRef]
- Li, X.; Wang, D.; Ran, F. Key Approaches and Challenges in Fabricating Advanced Flexible Zinc-Ion Batteries with Functional Hydrogel Electrolytes. Energy Storage Mater. 2023, 56, 351–393. [Google Scholar] [CrossRef]
- Hou, X.; Pollard, T.P.; He, X.; Du, L.; Ju, X.; Zhao, W.; Li, M.; Wang, J.; Paillard, E.; Lin, H.; et al. “Water-in-Eutectogel” Electrolytes for Quasi-Solid-State Aqueous Lithium-Ion Batteries. Adv. Energy Mater. 2022, 12, 2200401. [Google Scholar] [CrossRef]
- Lv, D.; Chai, J.; Wang, P.; Zhu, L.; Liu, C.; Nie, S.; Li, B.; Cui, G. Pure Cellulose Lithium-Ion Battery Separator with Tunable Pore Size and Improved Working Stability by Cellulose Nanofibrils. Carbohydr. Polym. 2021, 251, 116975. [Google Scholar] [CrossRef]
- Kalaj, M.; Cohen, S.M. Postsynthetic Modification: An Enabling Technology for the Advancement of Metal–Organic Frameworks. ACS Cent. Sci. 2020, 6, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Bai, Z.; Liu, S.; Zhu, Y.; Zheng, J.; Chen, G.; Huang, B. Self-Protecting Aqueous Lithium-Ion Batteries. Small 2022, 18, e2203035. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, Z.; Lv, T.; Dong, K.; Liu, Y.; Qi, Y.; Cao, S.; Chen, T. Ultrafast Self-Assembly of Supramolecular Hydrogels toward Novel Flame-Retardant Separator for Safe Lithium Ion Battery. J. Colloid Interface Sci. 2023, 649, 591–600. [Google Scholar] [CrossRef]
- Li, Y.; Ding, P.; Gu, Y.; Qian, S.; Pang, Y.; Wang, L.; Feng, J.; Liu, B.; Wan, Q.; Li, P.; et al. Self-Healable Gels in Electrochemical Energy Storage Devices. Nano Res. 2024, 17, 3302–3323. [Google Scholar] [CrossRef]
- Ohzuku, T.; Ueda, A. Solid-State Redox Reactions of LiCoO2 (R3m) for 4 Volt Secondary Lithium Cells. J. Electrochem. Soc. 1994, 141, 2972–2977. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Y.; Rui, X.; Qi, D.; Luo, Y.; Leow, W.R.; Chen, S.; Guo, J.; Wei, J.; Li, W.; et al. Conductive Inks Based on a Lithium Titanate Nanotube Gel for High-Rate Lithium-Ion Batteries with Customized Configuration. Adv. Mater. 2016, 28, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shi, Y.; Ding, Y.; Zhang, W.; Yu, G. In Situ Reactive Synthesis of Polypyrrole-MnO2 Coaxial Nanotubes as Sulfur Hosts for High-Performance Lithium-Sulfur Battery. Nano Lett. 2016, 16, 7276–7281. [Google Scholar] [CrossRef]
- Hasegawa, G.; Ishihara, Y.; Kanamori, K.; Miyazaki, K.; Yamada, Y.; Nakanishi, K.; Abe, T. Facile Preparation of Monolithic LiFePO4/Carbon Composites with Well-Defined Macropores for a Lithium-Ion Battery. Chem. Mater. 2011, 23, 5208–5216. [Google Scholar] [CrossRef]
- Wu, H.; Wang, X.; Jiang, L.; Wu, C.; Zhao, Q.; Liu, X.; Hu, B.; Yi, L. The Effects of Electrolyte on the Supercapacitive Performance of Activated Calcium Carbide-Derived Carbon. J. Power Sources 2013, 226, 202–209. [Google Scholar] [CrossRef]
- Xiao, W.; Zhou, W.; Yu, H.; Pu, Y.; Zhang, Y.; Hu, C. Template Synthesis of Hierarchical Mesoporous δ-MnO2 Hollow Microspheres as Electrode Material for High-Performance Symmetric Supercapacitor. Electrochim. Acta 2018, 264, 1–11. [Google Scholar] [CrossRef]
- Ojeda, M.; Chen, B.; Leung, D.Y.C.; Xuan, J.; Wang, H. A Hydrogel Template Synthesis of TiO2 Nanoparticles for Aluminium-Ion Batteries. Energy Procedia 2017, 105, 3997–4002. [Google Scholar] [CrossRef]
- Kwon, K.A.; Lim, H.S.; Sun, Y.K.; Suh, K. Do α-Fe2O3 Submicron Spheres with Hollow and Macroporous Structures as High-Performance Anode Materials for Lithium Ion Batteries. J. Phys. Chem. C 2014, 118, 2897–2907. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, H.; Bae, J.; Chung, S.H.; Zhang, W.; Manthiram, A.; Yu, G. Nanostructured Host Materials for Trapping Sulfur in Rechargeable Li–S Batteries: Structure Design and Interfacial Chemistry. Small Methods 2018, 2, 1700279. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, G.; Cha, J.J.; Wu, H.; Vosgueritchian, M.; Yao, Y.; Bao, Z.; Cui, Y. Improving the Performance of Lithium-Sulfur Batteries by Conductive Polymer Coating. ACS Nano 2011, 5, 9187–9193. [Google Scholar] [CrossRef]
- Kim, W.J.; Kang, J.G.; Kim, D.W. Fibrin Biopolymer Hydrogel-Templated 3D Interconnected Si@C Framework for Lithium Ion Battery Anodes. Appl. Surf. Sci. 2021, 551, 149439. [Google Scholar] [CrossRef]
- Ebnesajjad, S.; Landrock, A. Introduction and Adhesion Theories. In Adhesives Technology Handbook; William Andrew Publishing: Norwich, NY, USA, 2009. [Google Scholar]
- Li, G.; Li, C.; Li, G.; Yu, D.; Song, Z.; Wang, H.; Liu, X.; Liu, H.; Liu, W. Development of Conductive Hydrogels for Fabricating Flexible Strain Sensors. Small 2022, 18, 2101518. [Google Scholar] [CrossRef]
- Herrmann, A.; Haag, R.; Schedler, U. Hydrogels and Their Role in Biosensing Applications. Adv. Healthc. Mater. 2021, 10, 2100062. [Google Scholar]
- Zou, F.; Manthiram, A. A Review of the Design of Advanced Binders for High-Performance Batteries. Adv. Energy Mater. 2020, 10, 2002508. [Google Scholar] [CrossRef]
- Chen, C.; Lee, S.H.; Cho, M.; Kim, J.; Lee, Y. Cross-Linked Chitosan as an Efficient Binder for Si Anode of Li-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 2658–2665. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yao, R.; Wang, S.; Wei, Y.; Chen, B.; Liang, W.; Tian, C.; Nie, C.; Li, D.; Chen, Y. Rational Design of Stretchable and Conductive Hydrogel Binder for Highly Reversible SiP2 Anode. J. Energy Chem. 2023, 83, 564–573. [Google Scholar] [CrossRef]
- Yang, B.; Liu, F.; Liu, Y.; Dong, J.; Liu, M.; Wang, S.; Zhang, L. Self-Assembled Three-Dimensional Si/Carbon Frameworks as Promising Lithium-Ion Battery Anode. J. Power Sources 2023, 553, 232274. [Google Scholar] [CrossRef]
- Weng, Z.; Di, S.; Chen, L.; Wu, G.; Zhang, Y.; Jia, C.; Zhang, N.; Liu, X.; Chen, G. Random Copolymer Hydrogel as Elastic Binder for the SiOX Microparticle Anode in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 42494–42503. [Google Scholar] [CrossRef]
- Mu, P.; Sun, C.; Gao, C.; Li, L.; Zhang, H.; Li, J.; Li, C.; Dong, S.; Cui, G. Dual Network Electrode Binder toward Practical Lithium-Sulfur Battery Applications. ACS Energy Lett. 2023, 8, 3733–3741. [Google Scholar] [CrossRef]
- Wang, W.; Ding, J.; Liu, Z.; Wei, Y.; Cheng, W.; Liu, Q.; Zhang, W.; Wang, X.C.; Zhang, W.; Wang, B.; et al. Novel-Designed Cobweb-like Binder by “Four-in-One” Strategy for High Performance SiO Anode. Chem. Eng. J. 2023, 458, 141387. [Google Scholar] [CrossRef]
- Wang, J.; Ye, T.; Li, Y.; Wang, L.; Li, L.; Li, F.; He, E.; Zhang, Y. Ultrasoft All-Hydrogel Aqueous Lithium-Ion Battery with a Coaxial Fiber Structure. Polym. J. 2022, 54, 1383–1389. [Google Scholar] [CrossRef]
- Zhao, M.; Li, B.Q.; Zhang, X.Q.; Huang, J.Q.; Zhang, Q. A Perspective toward Practical Lithium-Sulfur Batteries. ACS Cent. Sci. 2020, 6, 1095–1104. [Google Scholar] [CrossRef]
- Liu, B.; Bo, R.; Taheri, M.; Di Bernardo, I.; Motta, N.; Chen, H.; Tsuzuki, T.; Yu, G.; Tricoli, A. Metal-Organic Frameworks/Conducting Polymer Hydrogel Integrated Three-Dimensional Free-Standing Monoliths as Ultrahigh Loading Li-S Battery Electrodes. Nano Lett. 2019, 19, 4391–4399. [Google Scholar] [CrossRef]
- Xu, G.L.; Gong, Y.D.; Miao, C.; Wang, Q.; Nie, S.Q.; Xin, Y.; Wen, M.Y.; Liu, J.; Xiao, W. Sn Nanoparticles Embedded into Porous Hydrogel-Derived Pyrolytic Carbon as Composite Anode Materials for Lithium-Ion Batteries. Rare Met. 2022, 41, 3421–3431. [Google Scholar] [CrossRef]
- Xu, T.; Liu, K.; Sheng, N.; Zhang, M.; Liu, W.; Liu, H.; Dai, L.; Zhang, X.; Si, C.; Du, H.; et al. Biopolymer-Based Hydrogel Electrolytes for Advanced Energy Storage/Conversion Devices: Properties, Applications, and Perspectives. Energy Storage Mater. 2022, 48, 244–262. [Google Scholar] [CrossRef]
- Li, H.; Lv, T.; Sun, H.; Qian, G.; Li, N.; Yao, Y.; Chen, T. Ultrastretchable and Superior Healable Supercapacitors Based on a Double Cross-Linked Hydrogel Electrolyte. Nat. Commun. 2019, 10, 536. [Google Scholar] [CrossRef]
- Lu, C.; Chen, X. All-Temperature Flexible Supercapacitors Enabled by Antifreezing and Thermally Stable Hydrogel Electrolyte. Nano Lett. 2020, 20, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, S.; Wang, D.; Yang, Q.; Mo, F.; Liang, G.; Li, N.; Zhang, H.; Zapien, J.A.; Zhi, C. Super-Stretchable Zinc–Air Batteries Based on an Alkaline-Tolerant Dual-Network Hydrogel Electrolyte. Adv. Energy Mater. 2019, 9, 1803046. [Google Scholar] [CrossRef]
- Liu, J.; Yang, C.; Chi, X.; Wen, B.; Wang, W.; Liu, Y. Water/Sulfolane Hybrid Electrolyte Achieves Ultralow-Temperature Operation for High-Voltage Aqueous Lithium-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2106811. [Google Scholar] [CrossRef]
- Cao, X.; Tan, D.; Guo, Q.; Zhang, T.; Hu, F.; Sun, N.; Huang, J.; Fang, C.; Ji, R.; Bi, S.; et al. High-Performance Fully-Stretchable Solid-State Lithium-Ion Battery with a Nanowire-Network Configuration and Crosslinked Hydrogel. J. Mater. Chem. A 2022, 10, 11562–11573. [Google Scholar] [CrossRef]
- Bae, J.; Li, Y.; Zhang, J.; Zhou, X.; Zhao, F.; Shi, Y.; Goodenough, J.B.; Yu, G. A 3D Nanostructured Hydrogel-Framework-Derived High-Performance Composite Polymer Lithium-Ion Electrolyte. Angew. Chem. Int. Ed. 2018, 57, 2096–2100. [Google Scholar] [CrossRef]
- Yang, S.-J.; Jiang, F.-N.; Hu, J.-K.; Yuan, H.; Cheng, X.-B.; Kaskel, S.; Zhang, Q.; Huang, J.-Q. Life Cycle Safety Issues of Lithium Metal Batteries: A Perspective. Electron 2023, 1, e8. [Google Scholar] [CrossRef]
- Yang, M.; Chen, L.; Li, H.; Wu, F. Air/Water Stability Problems and Solutions for Lithium Batteries. Energy Mater. Adv. 2025, 2022, 9842651. [Google Scholar] [CrossRef]
- Xiao, K.; Ren, P.; Wang, X.; Chen, H.; Zhou, Q. Polymer/Ceramic Interfacial Layer Enables Stable Cycling of All-Solid-State Li-Metal Batteries with Sulfide Electrolyte. Mater. Lett. 2024, 362, 136221. [Google Scholar] [CrossRef]
- Mishra, R.N.; Madikere Raghunatha Reddy, A.K.; Goulet, M.-A.; Zaghib, K. Water-in-Salt Electrolytes: Advances and Chemistry for Sustainable Aqueous Monovalent-Metal-Ion Batteries. Batteries 2025, 11, 120. [Google Scholar] [CrossRef]
- Shen, Z.; Huang, J.; Xie, Y.; Wei, D.; Chen, J.; Shi, Z. Solid Electrolyte Interphase on Lithium Metal Anodes. ChemSusChem 2024, 17, e202301777. [Google Scholar] [CrossRef]
- Kulathuvayal, A.S.; Su, Y. Ionic Transport through the Solid Electrolyte Interphase in Lithium-Ion Batteries: A Review from First-Principles Perspectives. ACS Appl. Energy Mater. 2023, 6, 5628–5645. [Google Scholar] [CrossRef]
- Pei, Z.; Yuan, Z.; Wang, C.; Zhao, S.; Fei, J.; Wei, L.; Chen, J.; Wang, C.; Qi, R.; Liu, Z.; et al. A Flexible Rechargeable Zinc–Air Battery with Excellent Low-Temperature Adaptability. Angew. Chem. Int. Ed. 2020, 59, 4793–4799. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, X.; Tang, H.; Wang, J.; Hao, Q.; Liu, L.; Li, Y.; Zhang, K.; Schmidt, O.G. Antifreezing Hydrogel with High Zinc Reversibility for Flexible and Durable Aqueous Batteries by Cooperative Hydrated Cations. Adv. Funct. Mater. 2020, 30, 1907218. [Google Scholar] [CrossRef]
- Strachan, N.; Fais, B.; Daly, H. Reinventing the Energy Modelling–Policy Interface. Nat. Energy 2016, 1, 16012. [Google Scholar] [CrossRef]
- Le Floch, P.; Yao, X.; Liu, Q.; Wang, Z.; Nian, G.; Sun, Y.; Jia, L.; Suo, Z. Wearable and Washable Conductors for Active Textiles. ACS Appl. Mater. Interfaces 2017, 9, 25542–25552. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.; Zhang, T.; Parada, G.A.; Liu, X.; Zhao, X. Skin-Inspired Hydrogel-Elastomer Hybrids with Robust Interfaces and Functional Microstructures. Nat. Commun. 2016, 7, 12028. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Y.; Keplinger, C.; Whitesides, G.M.; Suo, Z. Ionic Skin. Adv. Mater. 2014, 26, 7608–7614. [Google Scholar] [CrossRef]
- Suo, L.; Borodin, O.; Gao, T.; Olguin, M.; Ho, J.; Fan, X.; Luo, C.; Wang, C.; Xu, K. “Water-in-Salt” Electrolyte Enables High-Voltage Aqueous Lithium-Ion Chemistries. Science 2015, 350, 938–943. [Google Scholar] [CrossRef]
- Yang, C.; Chen, J.; Ji, X.; Pollard, T.P.; Lü, X.; Sun, C.J.; Hou, S.; Liu, Q.; Liu, C.; Qing, T.; et al. Aqueous Li-Ion Battery Enabled by Halogen Conversion–Intercalation Chemistry in Graphite. Nature 2019, 569, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zheng, Y.; Zartashia, M.; Shan, Y.; Noor, H.; Lou, H.; Hou, X. Aqueous Dual-Electrolyte Full-Cell System for Improving Energy Density of Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 34835–34843. [Google Scholar] [CrossRef]
- Yang, M.; Luo, J.; Guo, X.; Chen, J.; Cao, Y.; Chen, W. Aqueous Rechargeable Sodium-Ion Batteries: From Liquid to Hydrogel. Batteries 2022, 8, 180. [Google Scholar] [CrossRef]
- Casas, X.; Niederberger, M.; Lizundia, E. A Sodium-Ion Battery Separator with Reversible Voltage Response Based on Water-Soluble Cellulose Derivatives. ACS Appl. Mater. Interfaces 2020, 12, 29264–29274. [Google Scholar] [CrossRef]
- Cheng, C.; Zang, X.; Hou, W.; Li, C.; Huang, Q.; Hu, X.; Sun, C.; Zhang, Y.; Yang, J.; Ma, F. Construction of Three-Dimensional Electronic Interconnected Na3V2(PO4)3/C as Cathode for Sodium Ion Batteries. J. Alloys Compd. 2022, 899, 163363. [Google Scholar] [CrossRef]
- Zhong, L.; Lu, Y.; Li, H.; Tao, Z.; Chen, J. High-Performance Aqueous Sodium-Ion Batteries with Hydrogel Electrolyte and Alloxazine/CMK-3 Anode. ACS Sustain. Chem. Eng. 2018, 6, 7761–7768. [Google Scholar] [CrossRef]
- Yan, Y.; Ma, Z.; Lin, H.; Rui, K.; Zhang, Q.; Wang, Q.; Du, M.; Li, D.; Zhang, Y.; Zhu, J.; et al. Hydrogel Self-Templated Synthesis of Na3V2(PO4)3 @C@CNT Porous Network as Ultrastable Cathode for Sodium-Ion Batteries. Compos. Commun. 2019, 13, 97–102. [Google Scholar] [CrossRef]
- Cheng, Y.; Chi, X.; Yang, J.; Liu, Y. Cost Attractive Hydrogel Electrolyte for Low Temperature Aqueous Sodium Ion Batteries. J. Energy Storage 2021, 40, 102701. [Google Scholar] [CrossRef]
- Mittal, N.; Tien, S.; Lizundia, E.; Niederberger, M. Hierarchical Nanocellulose-Based Gel Polymer Electrolytes for Stable Na Electrodeposition in Sodium Ion Batteries. Small 2022, 18, e2107183. [Google Scholar] [CrossRef]
- Zhang, S.; Li, S.; Li, L.; Wen, D.; Peng, X.; Zhang, H. Urchin-like MoS2/MoO2 Microspheres Coated with GO Hydrogel: Achieve a Long-Term Cyclability as Anode for Sodium Ion Batteries. Mater. Today Sustain. 2023, 21, 100289. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, J.P.; Fu, N.; Zhou, W.B.; Liu, B.; Deng, Q.; Wu, X.W. Comprehensive Review on Zinc-Ion Battery Anode: Challenges and Strategies. InfoMat 2022, 4, e12306. [Google Scholar] [CrossRef]
- Yan, J.; Ang, E.H.; Yang, Y.; Zhang, Y.; Ye, M.; Du, W.; Li, C.C. High-Voltage Zinc-Ion Batteries: Design Strategies and Challenges. Adv. Funct. Mater. 2021, 31, 2010213. [Google Scholar] [CrossRef]
- Zuo, Y.; Wang, K.; Pei, P.; Wei, M.; Liu, X.; Xiao, Y.; Zhang, P. Zinc Dendrite Growth and Inhibition Strategies. Mater. Today Energy 2021, 20, 100692. [Google Scholar] [CrossRef]
- Lv, Y.; Xiao, Y.; Ma, L.; Zhi, C.; Chen, S. Recent Advances in Electrolytes for “Beyond Aqueous” Zinc-Ion Batteries. Adv. Mater. 2022, 34, 2106409. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Chen, Y.; Shi, L.; Wang, K.; Wang, B.; Li, L.; Ma, Y.; Li, Y.; Sun, Z.; Ali, W.; et al. An Overview and Future Perspectives of Rechargeable Zinc Batteries. Small 2020, 16, e2000730. [Google Scholar] [CrossRef]
- Selis, L.A.; Seminario, J.M. Dendrite Formation in Li-Metal Anodes: An Atomistic Molecular Dynamics Study. RSC Adv. 2019, 9, 27835–27848. [Google Scholar] [CrossRef]
- Li, B.; Chao, Y.; Li, M.; Xiao, Y.; Li, R.; Yang, K.; Cui, X.; Xu, G.; Li, L.; Yang, C.; et al. A Review of Solid Electrolyte Interphase (SEI) and Dendrite Formation in Lithium Batteries. Electrochem. Energy Rev. 2023, 6, 7. [Google Scholar] [CrossRef]
- Mittal, N.; Ojanguren, A.; Kundu, D.; Lizundia, E.; Niederberger, M. Bottom-Up Design of a Green and Transient Zinc-Ion Battery with Ultralong Lifespan. Small 2023, 19, e2206249. [Google Scholar] [CrossRef]
- Park, J.H.; Hyun Park, S.; Joung, D.; Kim, C. Sustainable Biopolymeric Hydrogel Interphase for Dendrite-Free Aqueous Zinc-Ion Batteries. Chem. Eng. J. 2022, 433, 133532. [Google Scholar] [CrossRef]
- Cong, J.; Shen, X.; Wen, Z.; Wang, X.; Peng, L.; Zeng, J.; Zhao, J. Ultra-Stable and Highly Reversible Aqueous Zinc Metal Anodes with High Preferred Orientation Deposition Achieved by a Polyanionic Hydrogel Electrolyte. Energy Storage Mater. 2021, 35, 586–594. [Google Scholar] [CrossRef]
- Liu, Y.; He, H.; Gao, A.; Ling, J.; Yi, F.; Hao, J.; Li, Q.; Shu, D. Fundamental Study on Zn Corrosion and Dendrite Growth in Gel Electrolyte towards Advanced Wearable Zn-Ion Battery. Chem. Eng. J. 2022, 446, 137021. [Google Scholar] [CrossRef]
- Ji, S.; Qin, J.; Yang, S.; Shen, P.; Hu, Y.; Yang, K.; Luo, H.; Xu, J. A Mechanically Durable Hybrid Hydrogel Electrolyte Developed by Controllable Accelerated Polymerization Mechanism towards Reliable Aqueous Zinc-Ion Battery. Energy Storage Mater. 2023, 55, 236–243. [Google Scholar] [CrossRef]
- Zhu, R.; Yang, H.; Cui, W.; Fadillah, L.; Huang, T.; Xiong, Z.; Tang, C.; Kowalski, D.; Kitano, S.; Zhu, C.; et al. High Strength Hydrogels Enable Dendrite-Free Zn Metal Anodes and High-Capacity Zn-MnO2batteries via a Modified Mechanical Suppression Effect. J. Mater. Chem. A 2022, 10, 3122–3133. [Google Scholar] [CrossRef]
- Yang, J.L.; Li, J.; Zhao, J.W.; Liu, K.; Yang, P.; Fan, H.J. Stable Zinc Anodes Enabled by a Zincophilic Polyanionic Hydrogel Layer. Adv. Mater. 2022, 34, e2202382. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, L.; Guo, W.; Chang, C.; Wang, J.; Cong, Z.; Pu, X. High-Performance Dual-Ion Zn Batteries Enabled by a Polyzwitterionic Hydrogel Electrolyte with Regulated Anion/Cation Transport and Suppressed Zn Dendrite Growth. J. Mater. Chem. A 2021, 9, 24325–24335. [Google Scholar] [CrossRef]
- Chan, C.Y.; Wang, Z.; Li, Y.; Yu, H.; Fei, B.; Xin, J.H. Single-Ion Conducting Double-Network Hydrogel Electrolytes for Long Cycling Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 30594–30602. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Wang, Y.; Lu, C.; Zhou, S.; He, Q.; Hu, Y.; Feng, M.; Wan, Y.; Lin, J.; Zhang, Y.; et al. Modulation of Hydrogel Electrolyte Enabling Stable Zinc Metal Anode. Energy Storage Mater. 2022, 51, 588–598. [Google Scholar] [CrossRef]
- Ling, W.; Mo, F.; Wang, J.; Liu, Q.; Liu, Y.; Yang, Q.; Qiu, Y.; Huang, Y. Self-Healable Hydrogel Electrolyte for Dendrite-Free and Self-Healable Zinc-Based Aqueous Batteries. Mater. Today Phys. 2021, 20, 100458. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Q.; Hong, H.; Yang, S.; Zhang, R.; Wang, X.; Jin, X.; Xiong, B.; Bai, S.; Zhi, C. Lean-Water Hydrogel Electrolyte for Zinc Ion Batteries. Nat. Commun. 2023, 14, 3890. [Google Scholar] [CrossRef]
- Xu, L.; Meng, T.; Zheng, X.; Li, T.; Brozena, A.H.; Mao, Y.; Zhang, Q.; Clifford, B.C.; Rao, J.; Hu, L. Nanocellulose-Carboxymethylcellulose Electrolyte for Stable, High-Rate Zinc-Ion Batteries. Adv. Funct. Mater. 2023, 33, 2302098. [Google Scholar] [CrossRef]
- Ma, D.; Yuan, D.; Ponce de León, C.; Jiang, Z.; Xia, X.; Pan, J. Current Progress and Future Perspectives of Electrolytes for Rechargeable Aluminum-Ion Batteries. Energy Environ. Mater. 2023, 6, e12301. [Google Scholar] [CrossRef]
- Wu, H.; Yan, C.; Xu, L.; Xu, N.; Wu, X.; Jiang, Z.; Zhu, S.; Diao, G.; Chen, M. Super Flexible Cathode Material with 3D Cross-Linking System Based on Polyvinyl Alcohol Hydrogel for Boosting Aqueous Zinc Ion Batteries. ChemElectroChem 2022, 9, e202200288. [Google Scholar] [CrossRef]
- Lv, R.; Wu, H.; Jiang, Z.; Zheng, A.; Yu, H.; Chen, M. Flexible Hydrogel Compound of V2O5/GO/PVA for Enhancing Mechanical and Zinc Storage Performances. Electrochim. Acta 2022, 429, 140998. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, G.; Ye, L.; He, J.; Xu, W.; Hong, S.; Chen, W.; Li, M.C.; Liu, C.; Mei, C. High-Energy and Dendrite-Free Solid-State Zinc Metal Battery Enabled by a Dual-Network and Water-Confinement Hydrogel Electrolyte. Chem. Eng. J. 2023, 472, 144992. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, R.; Hu, Y.; Wang, R.; Shen, J.; Mao, Y.; Wu, Q.; Wang, B. Achieving Highly Reversible Zn Anodes by Inhibiting the Activity of Free Water Molecules and Manipulating Zn Deposition in a Hydrogel Electrolyte. ACS Appl. Energy Mater. 2023, 6, 7468–7476. [Google Scholar] [CrossRef]
- Lu, H.; Hu, J.; Wei, X.; Zhang, K.; Xiao, X.; Zhao, J.; Hu, Q.; Yu, J.; Zhou, G.; Xu, B. A Recyclable Biomass Electrolyte towards Green Zinc-Ion Batteries. Nat. Commun. 2023, 14, 4435. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Li, H.; Qin, B.; Passerini, S. High-Voltage Operation of a V2O5 Cathode in a Concentrated Gel Polymer Electrolyte for High-Energy Aqueous Zinc Batteries. ACS Appl. Mater. Interfaces 2020, 12, 15305–15312. [Google Scholar] [CrossRef]
- Sun, L.; Yao, Y.; Dai, L.; Jiao, M.; Ding, B.; Yu, Q.; Tang, J.; Liu, B. Sustainable and High-Performance Zn Dual-Ion Batteries with a Hydrogel-Based Water-in-Salt Electrolyte. Energy Storage Mater. 2022, 47, 187–194. [Google Scholar] [CrossRef]
- Li, C.; Zhang, K.; Cheng, X.; Li, J.; Jiang, Y.; Li, P.; Wang, B.; Peng, H. Polymers for Flexible Energy Storage Devices. Prog. Polym. Sci. 2023, 143, 101714. [Google Scholar] [CrossRef]
- Huang, S.; Wan, F.; Bi, S.; Zhu, J.; Niu, Z.; Chen, J. A Self-Healing Integrated All-in-One Zinc-Ion Battery. Angew. Chem. Int. Ed. 2019, 58, 4313–4317. [Google Scholar] [CrossRef]
- Wang, X.; Wang, B.; Cheng, J. Multi-Healable, Mechanically Durable Double Cross-Linked Polyacrylamide Electrolyte Incorporating Hydrophobic Interactions for Dendrite-Free Flexible Zinc-Ion Batteries. Adv. Funct. Mater. 2023, 33, 2304470. [Google Scholar] [CrossRef]
- Liu, J.; Long, J.; Shen, Z.; Jin, X.; Han, T.; Si, T.; Zhang, H. A Self-Healing Flexible Quasi-Solid Zinc-Ion Battery Using All-In-One Electrodes. Adv. Sci. 2021, 8, 2004689. [Google Scholar] [CrossRef]
- Li, Q.; Cui, X.; Pan, Q. Self-Healable Hydrogel Electrolyte toward High-Performance and Reliable Quasi-Solid-State Zn-MnO2 Batteries. ACS Appl. Mater. Interfaces 2019, 11, 38762–38770. [Google Scholar] [CrossRef]
- Liu, D.; Tang, Z.; Luo, L.; Yang, W.; Liu, Y.; Shen, Z.; Fan, X.H. Self-Healing Solid Polymer Electrolyte with High Ion Conductivity and Super Stretchability for All-Solid Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 36320–36329. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Wu, X.; Liu, Y.S.; Yu, C.; Liu, Y.; Sun, K.; Shen, C.; Huang, W.; Zhou, Y.; Chen, J.S.; et al. Self-Adapting and Self-Healing Hydrogel Interface with Fast Zn2+ Transport Kinetics for Highly Reversible Zn Anodes. Adv. Funct. Mater. 2023, 33, 2300952. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, A.; Hao, J.; Li, X.; Ling, J.; Yi, F.; Li, Q.; Shu, D. Soaking-Free and Self-Healing Hydrogel for Wearable Zinc-Ion Batteries. Chem. Eng. J. 2023, 452, 139605. [Google Scholar] [CrossRef]
- Shen, P.; Hu, Y.; Ji, S.; Luo, H.; Zhai, C.; Yang, K. A Self-Healing Nanocomposite Hydrogel Electrolyte for Rechargeable Aqueous Zn-MnO2 Battery. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 647, 129195. [Google Scholar] [CrossRef]
- He, X.; Cheng, R.; Sun, X.; Xu, H.; Li, Z.; Sun, F.; Zhan, Y.; Zou, J.; Laine, R.M. Organic Cathode Materials for Rechargeable Magnesium-Ion Batteries: Fundamentals, Recent Advances, and Approaches to Optimization. J. Magnes. Alloy. 2023, 11, 4359–4389. [Google Scholar] [CrossRef]
- Asif, M.; Kilian, S.; Rashad, M. Uncovering Electrochemistries of Rechargeable Magnesium-Ion Batteries at Low and High Temperatures. Energy Storage Mater. 2021, 42, 129–144. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, H.; Jiang, C.; Tang, Y. Multi-Ion Strategies towards Emerging Rechargeable Batteries with High Performance. Energy Storage Mater. 2019, 23, 566–586. [Google Scholar] [CrossRef]
- Wu, X.; Dou, Y.; Lian, R.; Wang, Y.; Wei, Y. Understanding Rechargeable Magnesium Ion Batteries via First-Principles Computations: A Comprehensive Review. Energy Storage Mater. 2022, 48, 344–355. [Google Scholar] [CrossRef]
- Ahn, H.; Kim, D.; Lee, M.; Nam, K.W. Challenges and Possibilities for Aqueous Battery Systems. Commun. Mater. 2023, 4, 37. [Google Scholar] [CrossRef]
- Deivanayagam, R.; Ingram, B.J.; Shahbazian-Yassar, R. Progress in Development of Electrolytes for Magnesium Batteries. Energy Storage Mater. 2019, 21, 136–153. [Google Scholar] [CrossRef]
- Li, M.; Ding, Y.; Sun, Y.; Ren, Y.; Yang, J.; Yin, B.; Li, H.; Zhang, S.; Ma, T. Emerging Rechargeable Aqueous Magnesium Ion Battery. Mater. Rep. Energy 2022, 2, 100161. [Google Scholar] [CrossRef]
- Yang, G.; Xu, X.; Qu, G.; Deng, J.; Zhu, Y.; Fang, C.; Fontaine, O.; Hiralal, P.; Zheng, J.; Zhou, H. An Aqueous Magnesium-Ion Battery Working at −50 °C Enabled by Modulating Electrolyte Structure. Chem. Eng. J. 2023, 455, 140806. [Google Scholar] [CrossRef]
- Cheng, M.; Liu, J.; Wang, X.; Li, Y.; Xia, W.; Liu, Q.; Hu, J.; Wei, T.; Ling, Y.; Liu, B.; et al. In-Situ Synthesis of Bi Nanospheres Anchored in 3D Interconnected Cellulose Nanocrystal Derived Carbon Aerogel as Anode for High-Performance Mg-Ion Batteries. Chem. Eng. J. 2023, 451, 138824. [Google Scholar] [CrossRef]
- Kwakernaak, M.C.; Kiriinya, L.K.; Legerstee, W.J.; Berghmans, W.M.J.; Hofman, C.G.T.; Kelder, E.M. Magnesium Alginate as an Electrolyte for Magnesium Batteries. Batteries 2025, 11, 16. [Google Scholar] [CrossRef]
- Wang, H.; Wang, P.; Ji, Z.; Chen, Z.; Wang, J.; Ling, W.; Liu, J.; Hu, M.; Zhi, C.; Huang, Y. Rechargeable Quasi-Solid-State Aqueous Hybrid Al3+/H+ Battery with 10,000 Ultralong Cycle Stability and Smart Switching Capability. Nano Res. 2021, 14, 4154–4162. [Google Scholar] [CrossRef]
- Wang, P.; Chen, Z.; Wang, H.; Ji, Z.; Feng, Y.; Wang, J.; Liu, J.; Hu, M.; Fei, J.; Gan, W.; et al. A High-Performance Flexible Aqueous Al Ion Rechargeable Battery with Long Cycle Life. Energy Storage Mater. 2020, 25, 426–435. [Google Scholar] [CrossRef]
- Xiong, T.; He, B.; Zhou, T.; Wang, Z.; Wang, Z.; Xin, J.; Zhang, H.; Zhou, X.; Liu, Y.; Wei, L. Stretchable Fiber-Shaped Aqueous Aluminum Ion Batteries. EcoMat 2022, 4, e12218. [Google Scholar] [CrossRef]
- Tay, I.R.; Xue, J.; Lee, W.S.V. Methods for Characterizing Intercalation in Aqueous Zinc Ion Battery Cathodes: A Review. Adv. Sci. 2023, 10, 2303211. [Google Scholar] [CrossRef]
- Chen, X.; Huang, H.; Pan, L.; Liu, T.; Niederberger, M. Fully Integrated Design of a Stretchable Solid-state Lithium-ion Full Battery. Adv. Mater. 2019, 31, 1904648. [Google Scholar] [CrossRef]
- Dai, L.; Arcelus, O.; Sun, L.; Wang, H.; Carrasco, J.; Zhang, H.; Zhang, W.; Tang, J. Embedded 3D Li+ Channels in a Water-in-Salt Electrolyte to Develop Flexible Supercapacitors and Lithium-Ion Batteries. J. Mater. Chem. A 2019, 7, 24800–24806. [Google Scholar] [CrossRef]
- Woo, H.; Park, K.; Kim, J.; Yun, A.J.; Nam, S.; Park, B. 3D Meshlike Polyacrylamide Hydrogel as a Novel Binder System via in Situ Polymerization for High-Performance Si-Based Electrode. Adv. Mater. Interfaces 2020, 7, 1901475. [Google Scholar] [CrossRef]
- Hu, S.; Wang, L.; Huang, T.; Yu, A. A Conductive Self-Healing Hydrogel Binder for High-Performance Silicon Anodes in Lithium-Ion Batteries. J. Power Sources 2020, 449, 227472. [Google Scholar] [CrossRef]
- Liu, W.; Huang, Y.; Cai, Z.; Tan, Y.; Huang, B.; Zhong, H.; Mai, Y. Flexible All-in-One Quasi-Solid-State Batteries Enabled by Low-Water-Content Hydrogel Films. Small 2023, 19, e2303480. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Du, X.; Wu, H.; Ren, Y.; Wang, J.; Wang, H.; Chen, Z.; Zhao, J.; Cui, G. A Bio-Inspired Methylation Approach to Salt-Concentrated Hydrogel Electrolytes for Long-Life Rechargeable Batteries. Angew. Chem. Int. Ed. 2023, 62, e202311589. [Google Scholar] [CrossRef]
- Xiao, X.; Xiao, X.; Zhou, Y.; Zhao, X.; Chen, G.; Liu, Z.; Wang, Z.; Lu, C.; Hu, M.; Nashalian, A. An Ultrathin Rechargeable Solid-State Zinc Ion Fiber Battery for Electronic Textiles. Sci. Adv. 2021, 7, eabl3742. [Google Scholar] [CrossRef]
- Li, H.; Han, C.; Huang, Y.; Huang, Y.; Zhu, M.; Pei, Z.; Xue, Q.; Wang, Z.; Liu, Z.; Tang, Z.; et al. An Extremely Safe and Wearable Solid-State Zinc Ion Battery Based on a Hierarchical Structured Polymer Electrolyte. Energy Environ. Sci. 2018, 11, 941–951. [Google Scholar] [CrossRef]
- Duan, J.; Xie, W.; Yang, P.; Li, J.; Xue, G.; Chen, Q.; Yu, B.; Liu, R.; Zhou, J. Tough Hydrogel Diodes with Tunable Interfacial Adhesion for Safe and Durable Wearable Batteries. Nano Energy 2018, 48, 569–574. [Google Scholar] [CrossRef]
- Ma, L.; Chen, S.; Long, C.; Li, X.; Zhao, Y.; Liu, Z.; Huang, Z.; Dong, B.; Zapien, J.A.; Zhi, C. Achieving High-Voltage and High-Capacity Aqueous Rechargeable Zinc Ion Battery by Incorporating Two-Species Redox Reaction. Adv. Energy Mater. 2019, 9, 1902446. [Google Scholar] [CrossRef]
- Wang, Z.; Mo, F.; Ma, L.; Yang, Q.; Liang, G.; Liu, Z.; Li, H.; Li, N.; Zhang, H.; Zhi, C. Highly Compressible Cross-Linked Polyacrylamide Hydrogel-Enabled Compressible Zn–MnO2 Battery and a Flexible Battery–Sensor System. ACS Appl. Mater. Interfaces 2018, 10, 44527–44534. [Google Scholar] [CrossRef]
- Wang, Y.T.; Zhao, Y.G.; Xu, X.F.; Pan, D.; Jiang, W.Q.; Yang, X.H.; Wang, Z. Superior Mechanical Properties Induced by the Interaction between Dislocations and Precipitates in the Electro-Pulsing Treated Al-Mg-Si Alloys. Mater. Sci. Eng. A 2018, 735, 154–161. [Google Scholar] [CrossRef]
- Quaino, P.; Nuñez, J.L.; Aradi, B.; van Der Heide, T.; Santos, E.; Schmickler, W. Why DFT-Based Tight Binding Gives a Better Representation of the Potential at Metal-Solution Interfaces than DFT Does. ChemElectroChem 2023, 10, e202300230. [Google Scholar] [CrossRef]
- Li, W.; Zhang, J.; Nith, R.; Yue, J.; Kamath, A.; Yang, C.; Wei, C.; Lee, B.; Li, P.; Tsai, H.-M. Hydrogen Evolution and Dynamics in Hydrogel Electrochemical Cells for Ischemia–Reperfusion Therapy. Nat. Chem. Eng. 2025, 2, 484–497. [Google Scholar] [CrossRef]

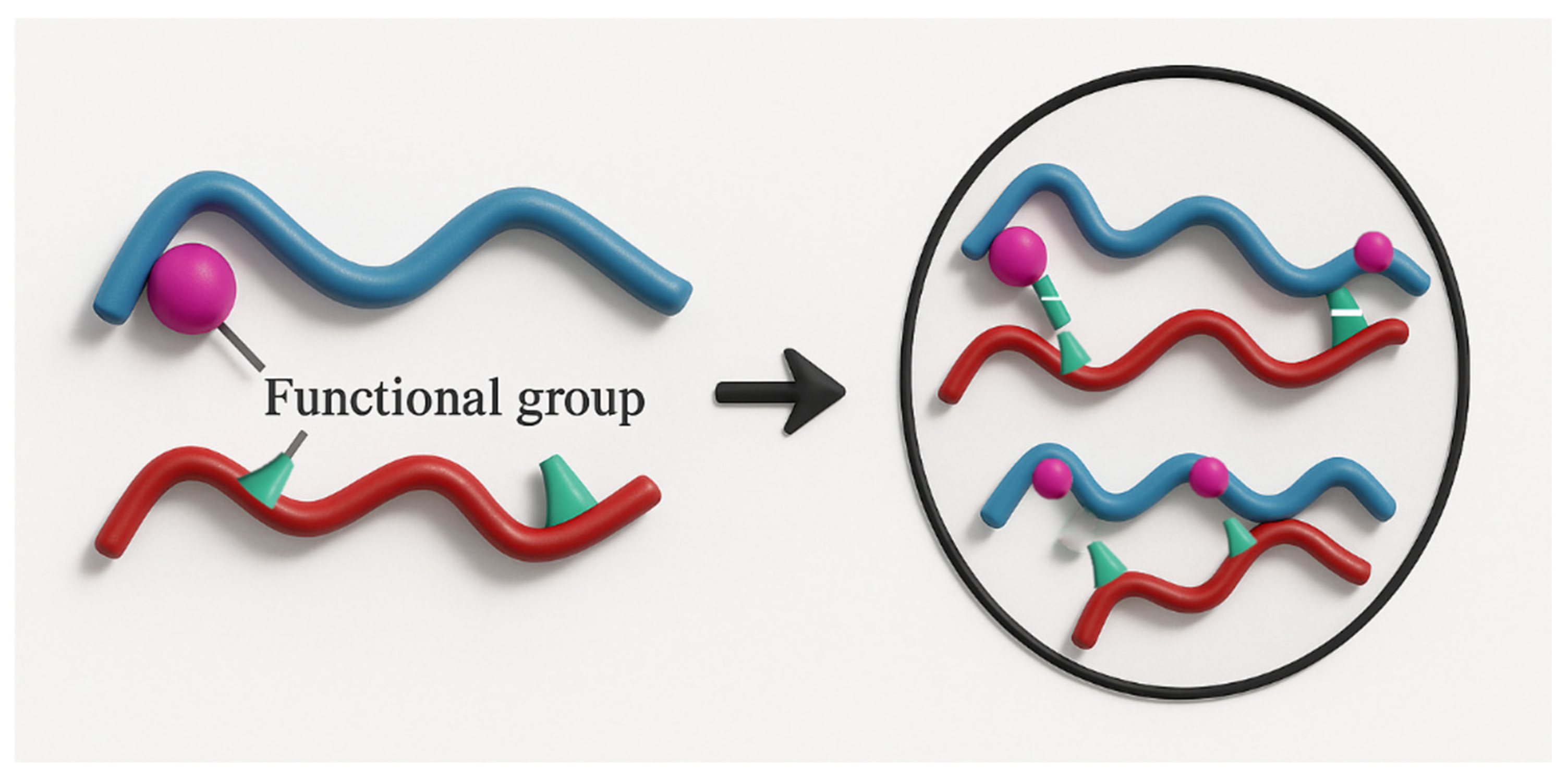
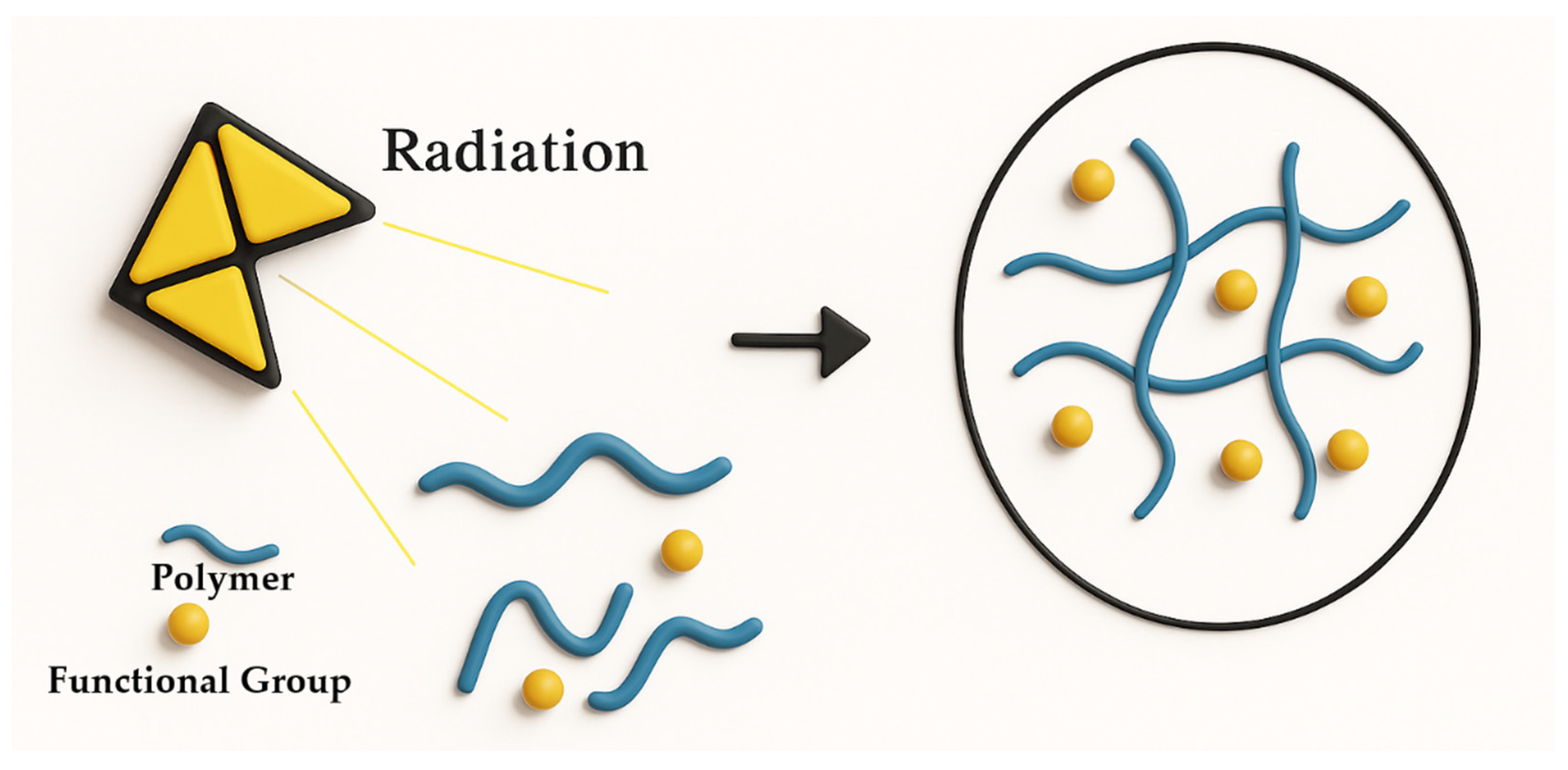
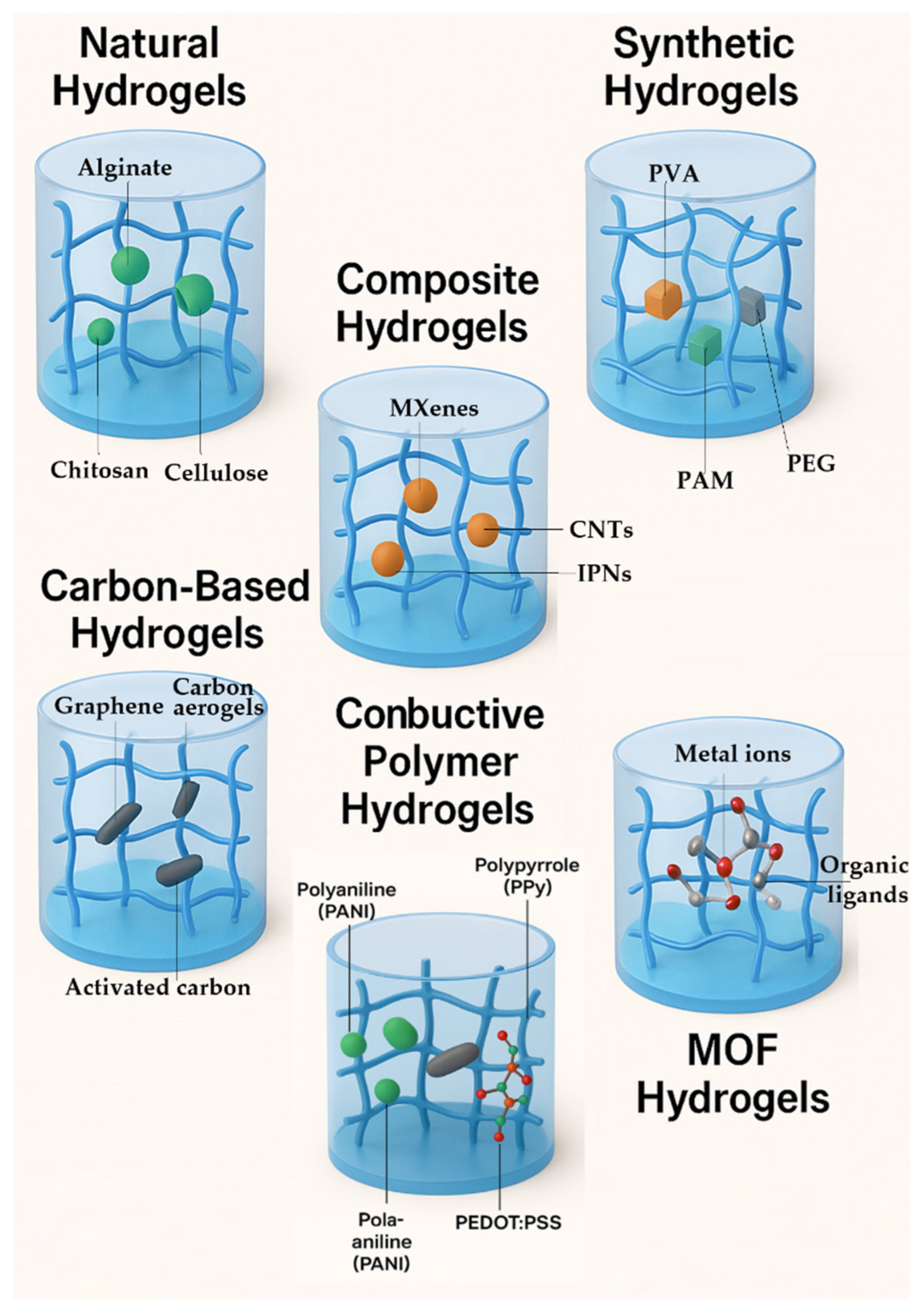
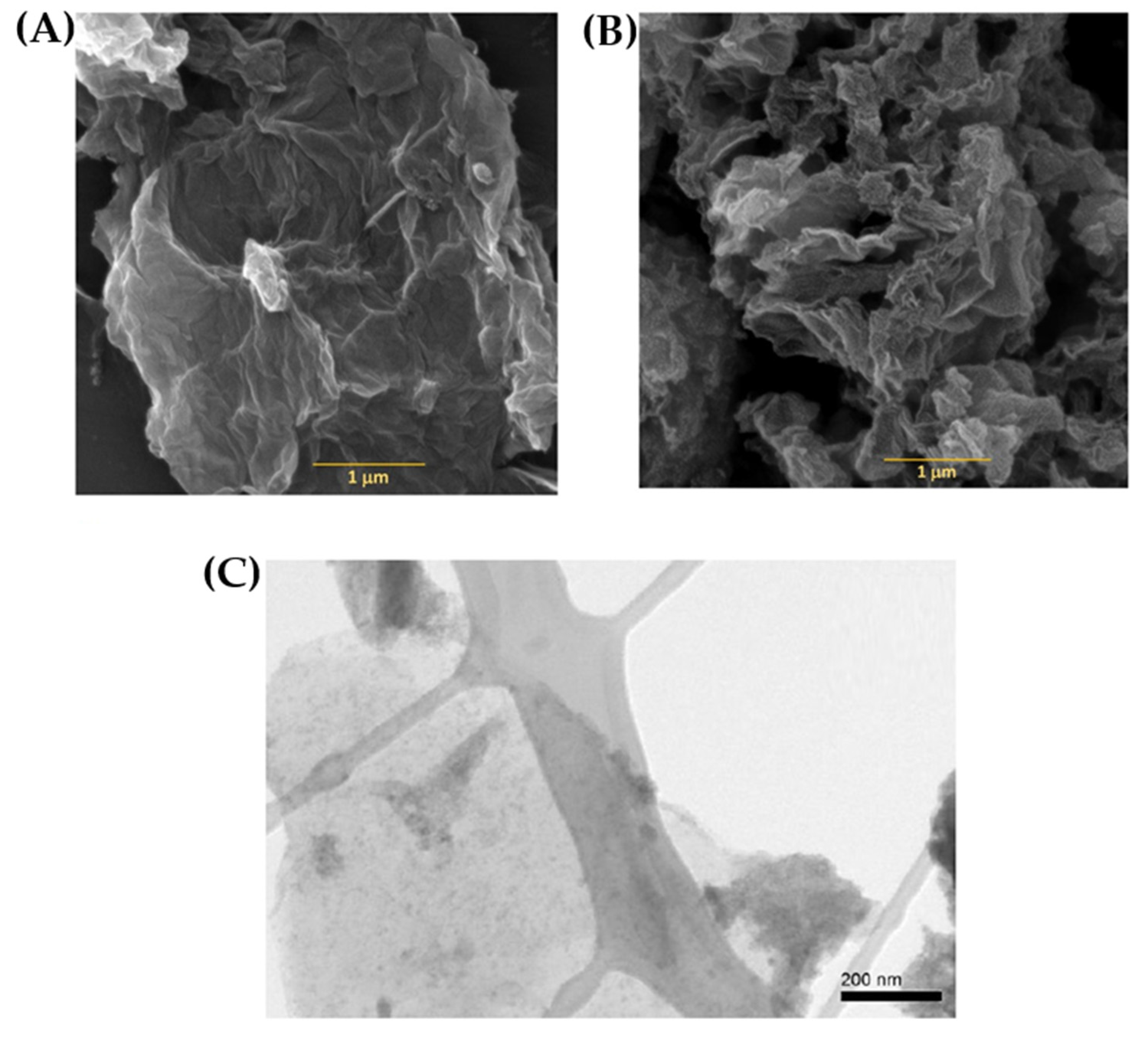
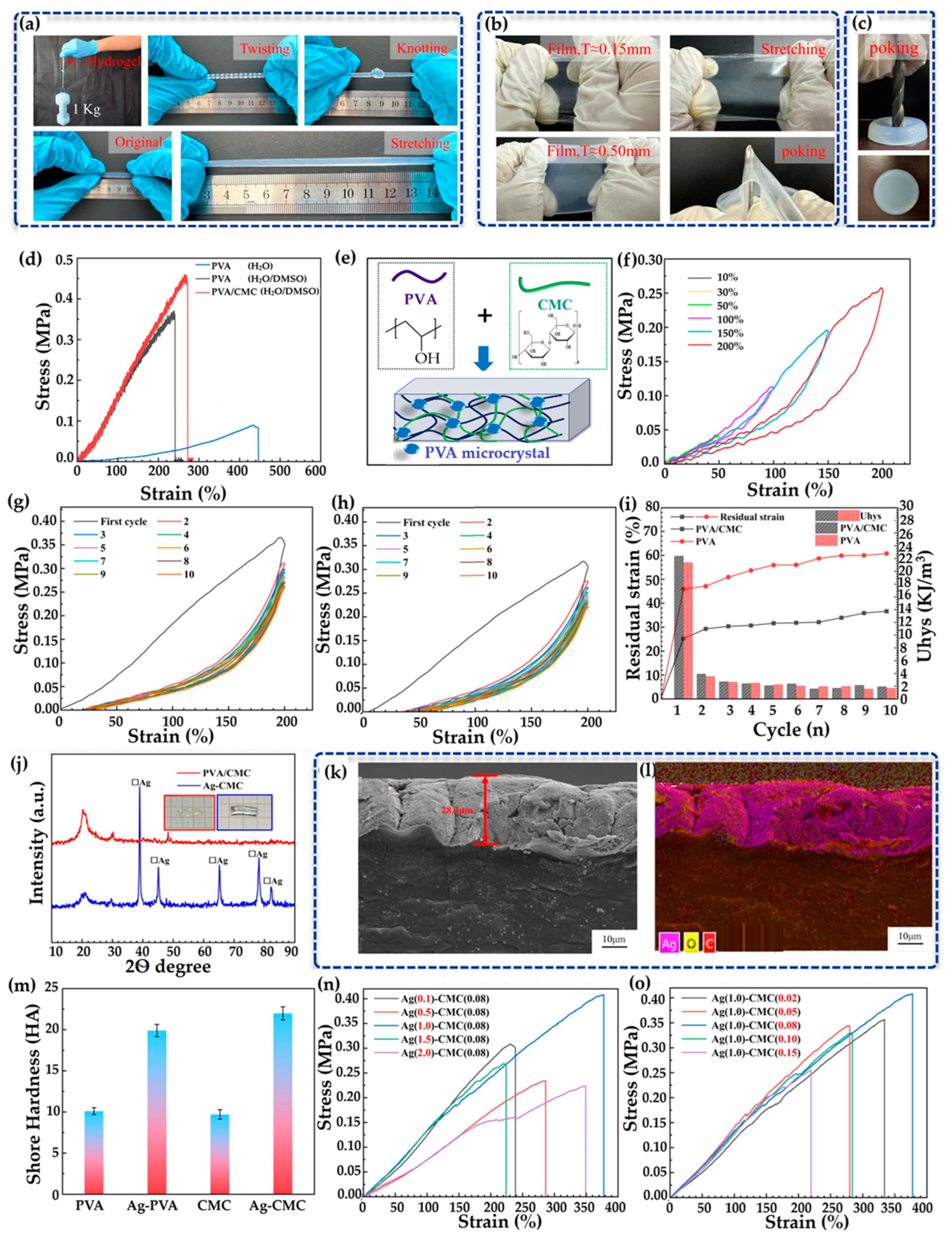
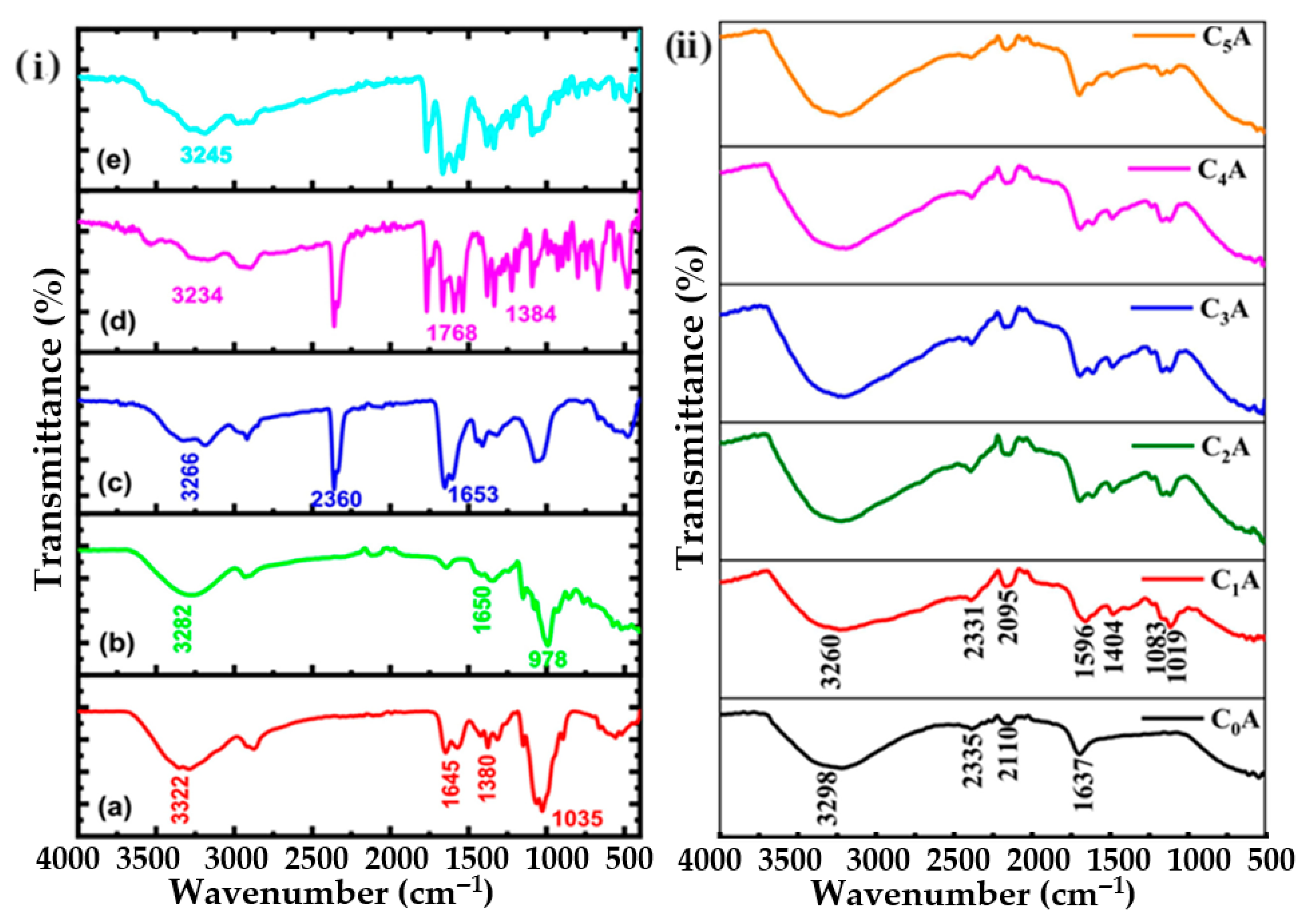
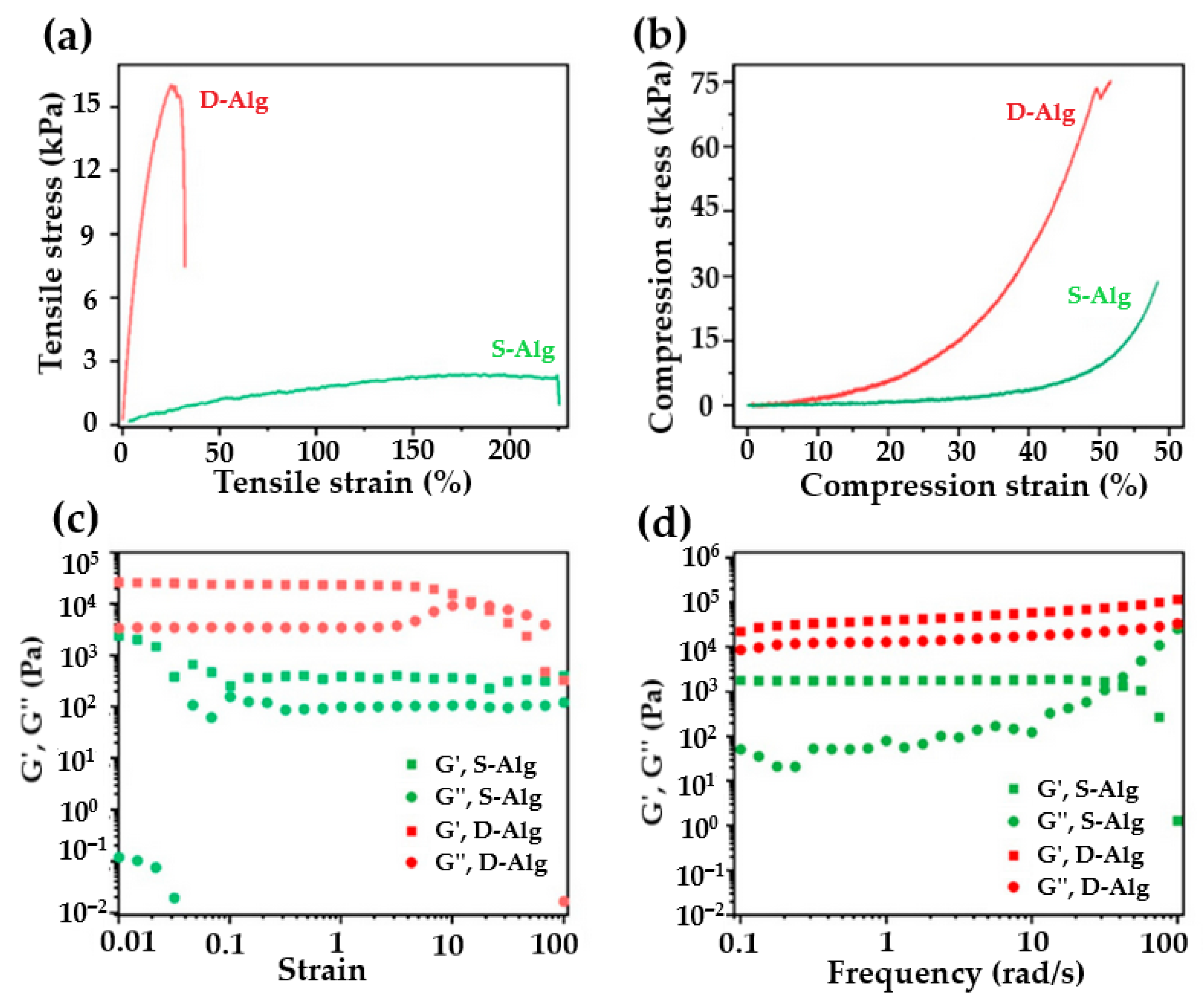

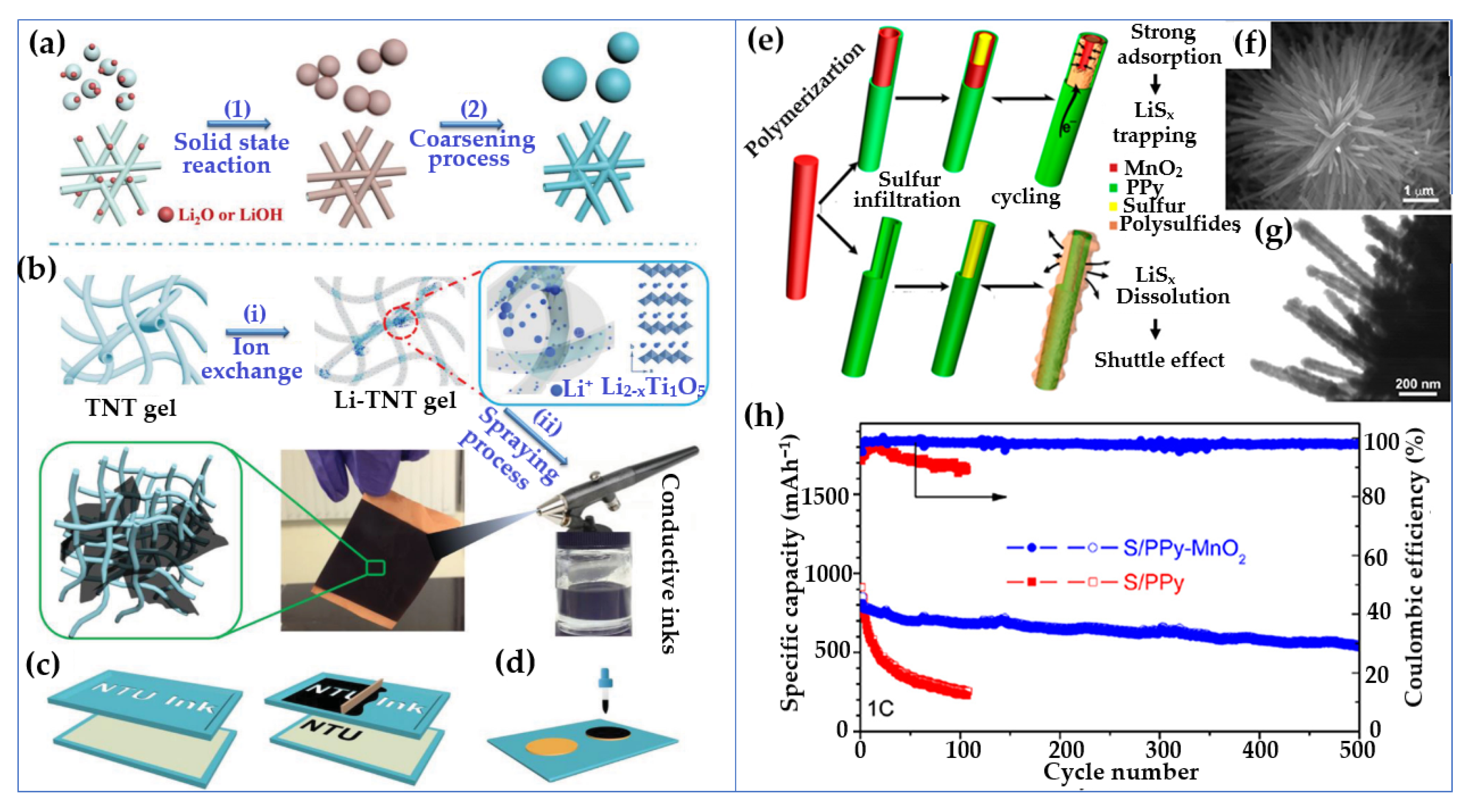
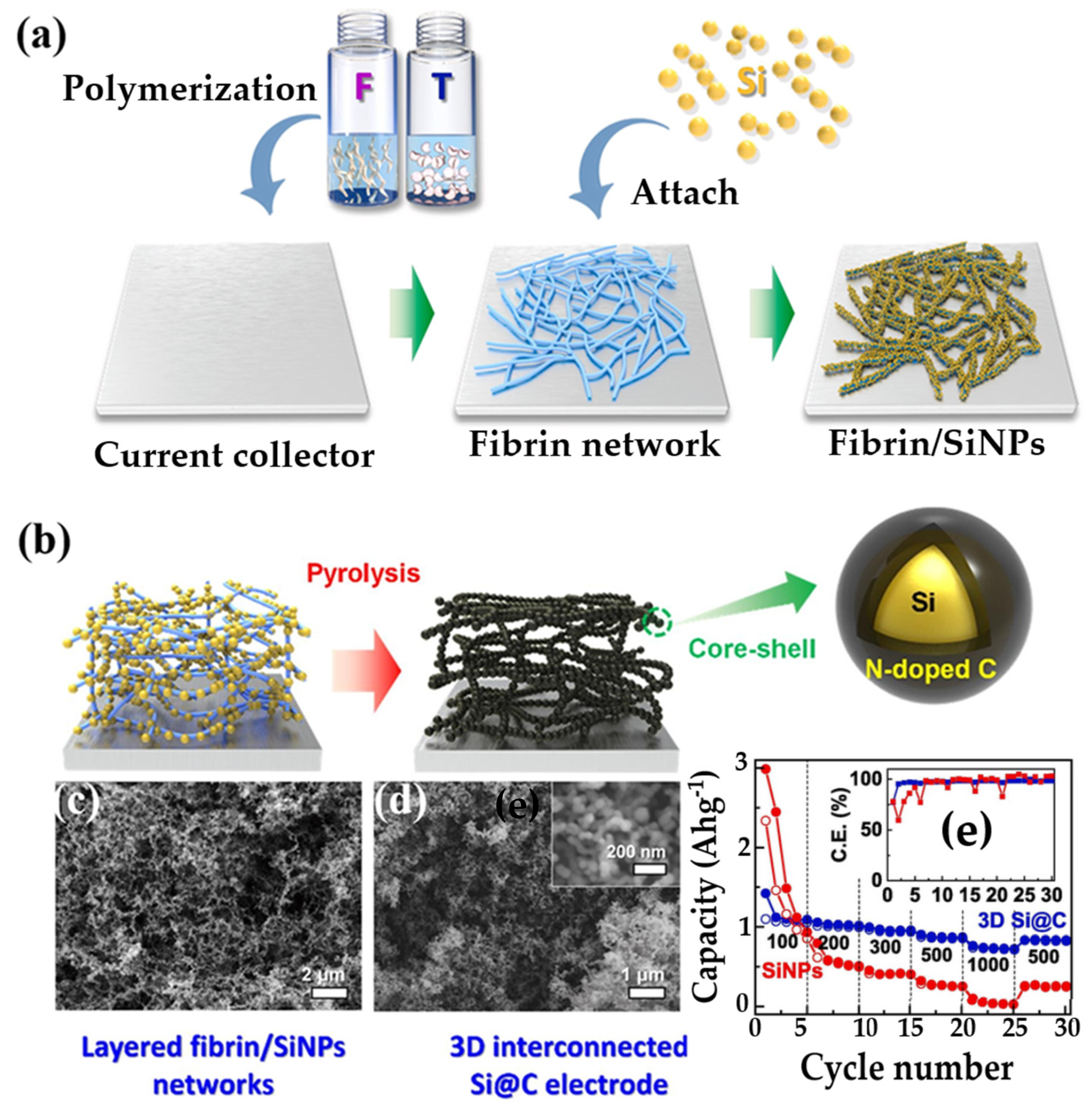
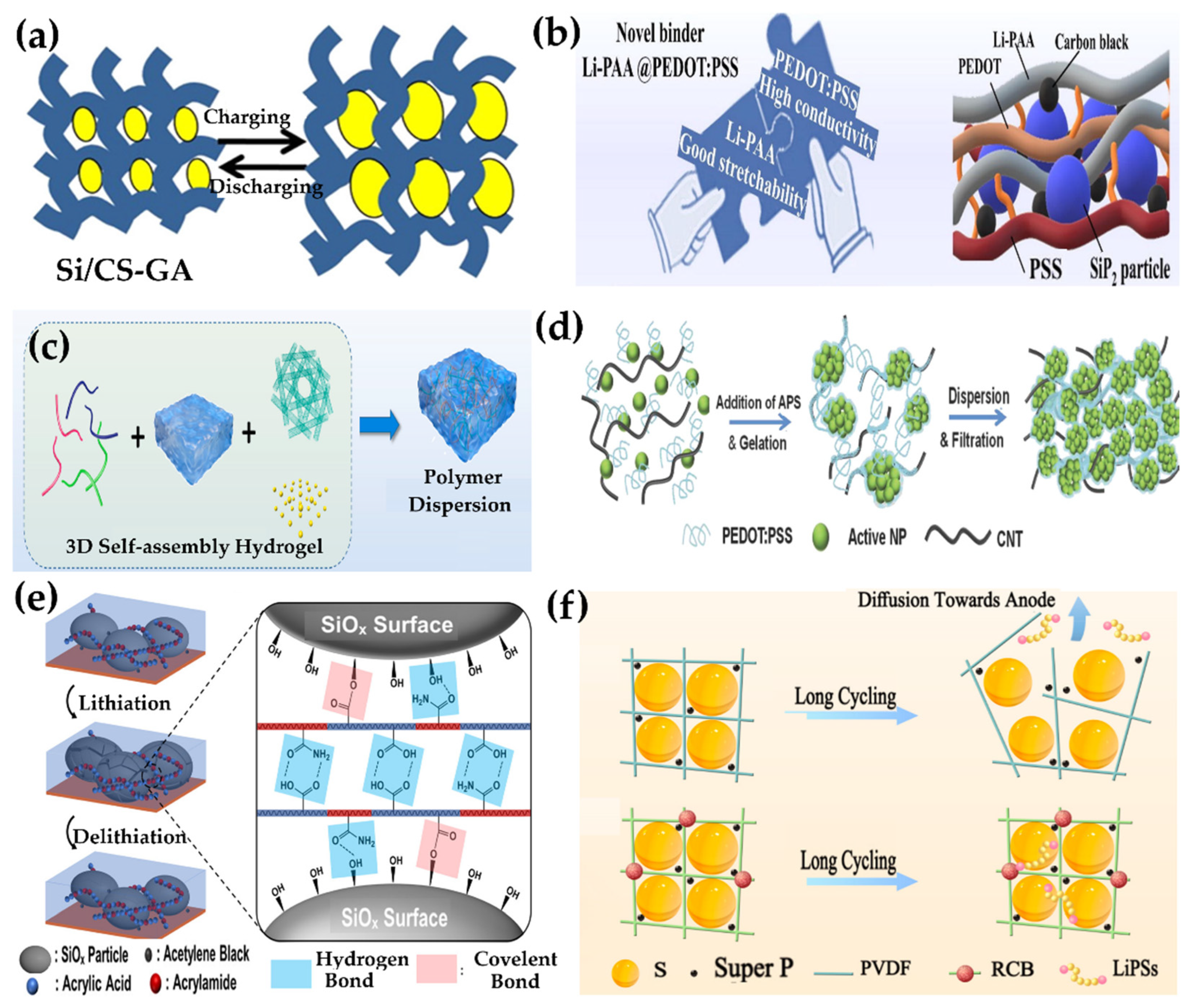
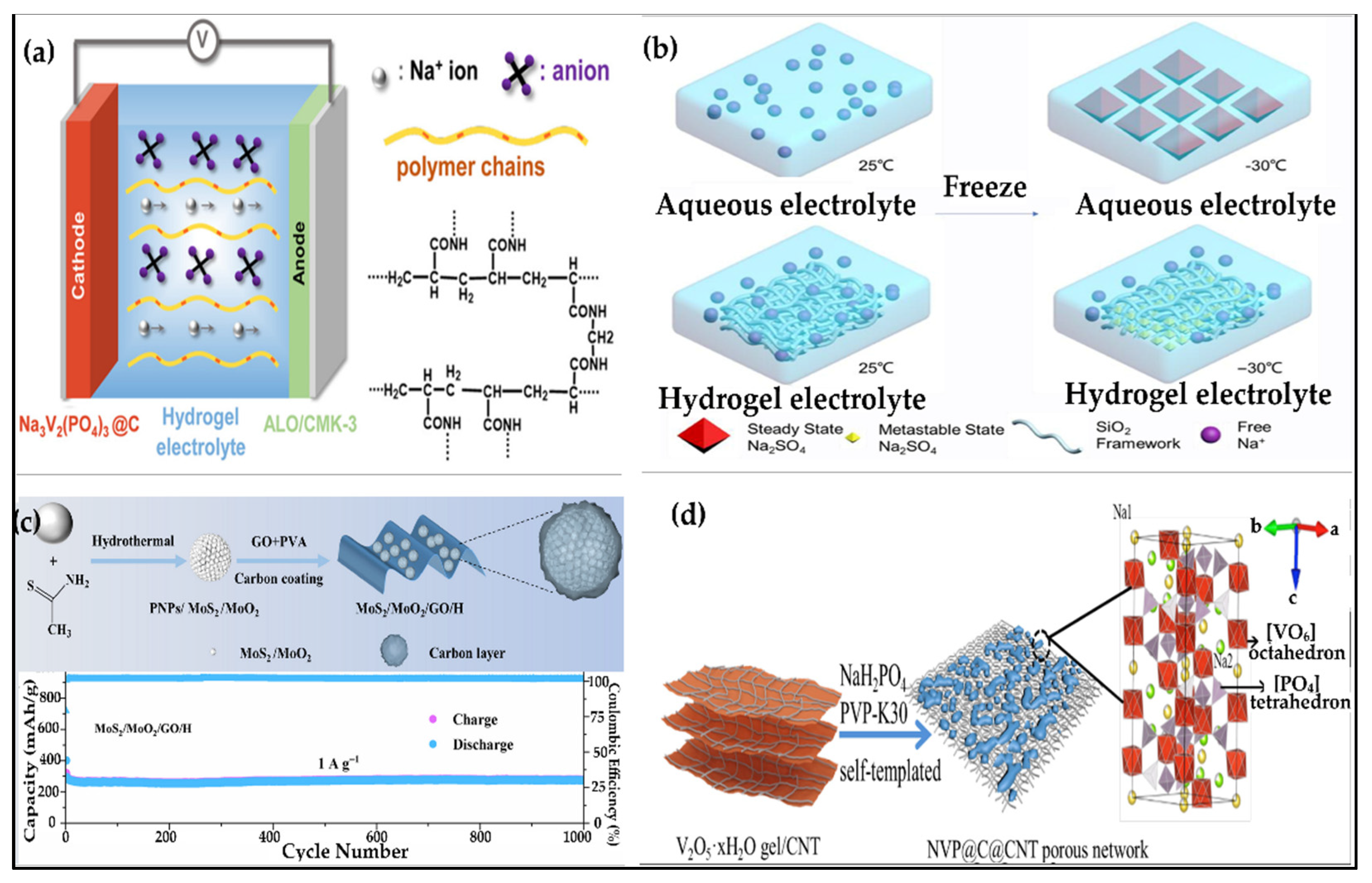
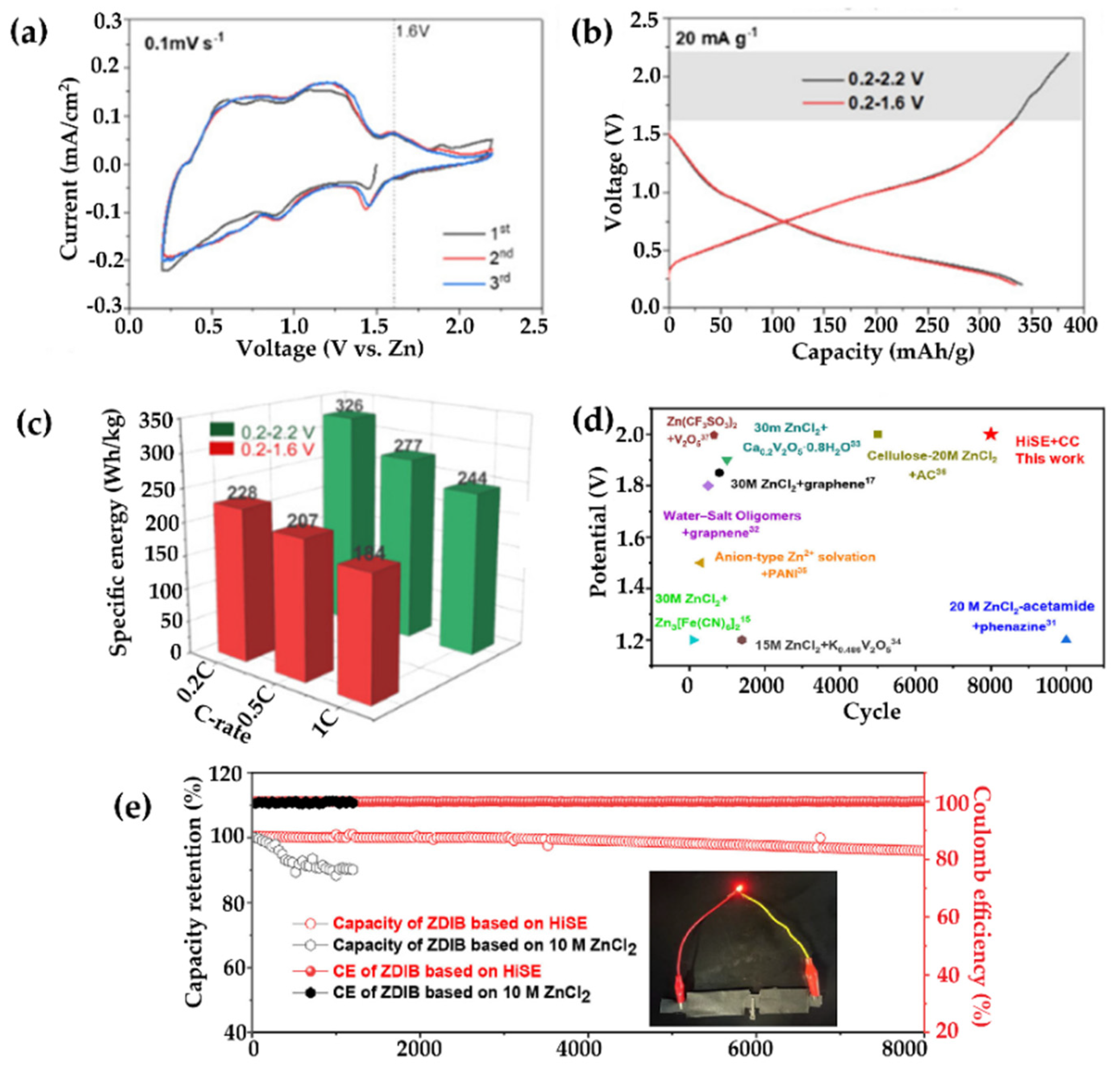
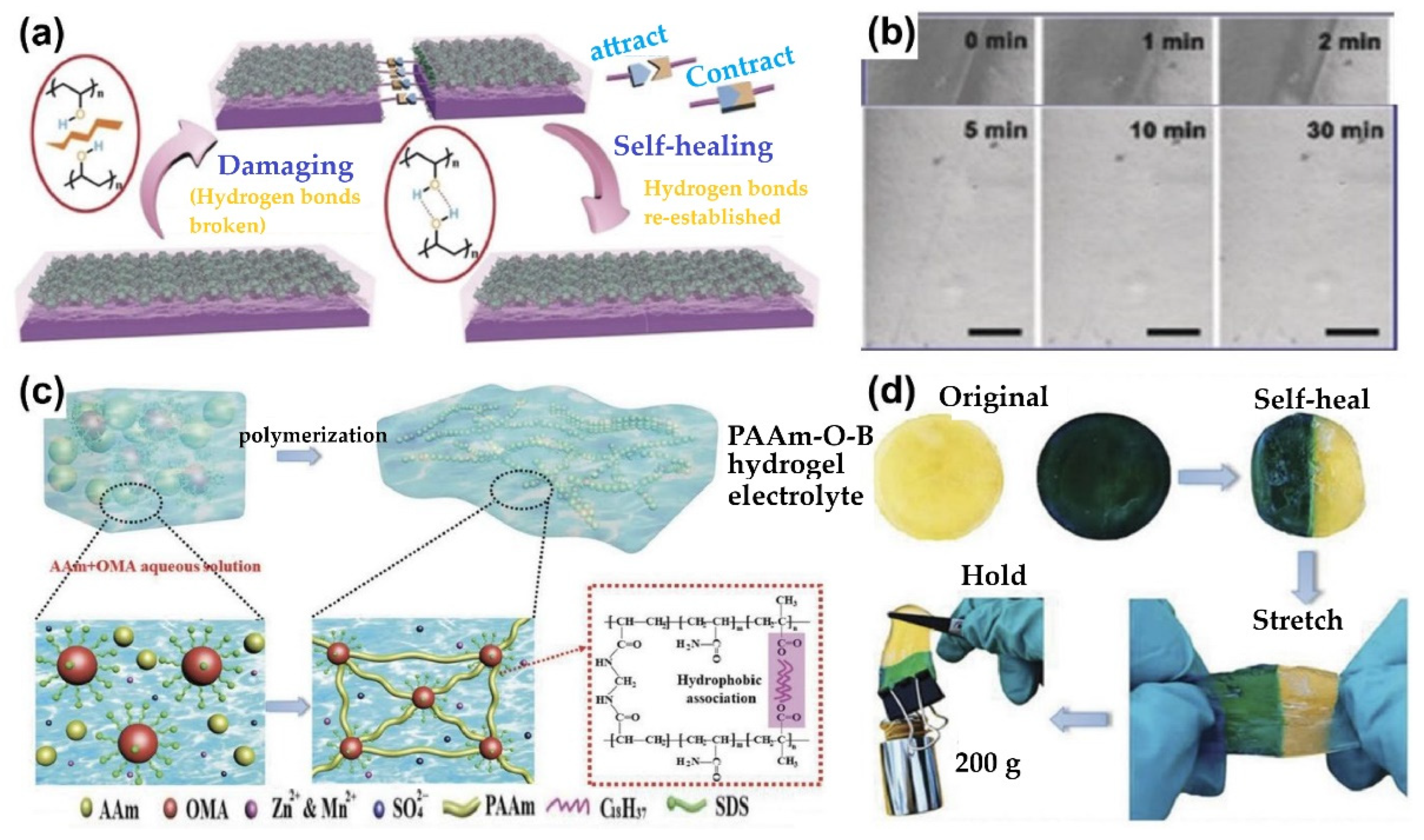
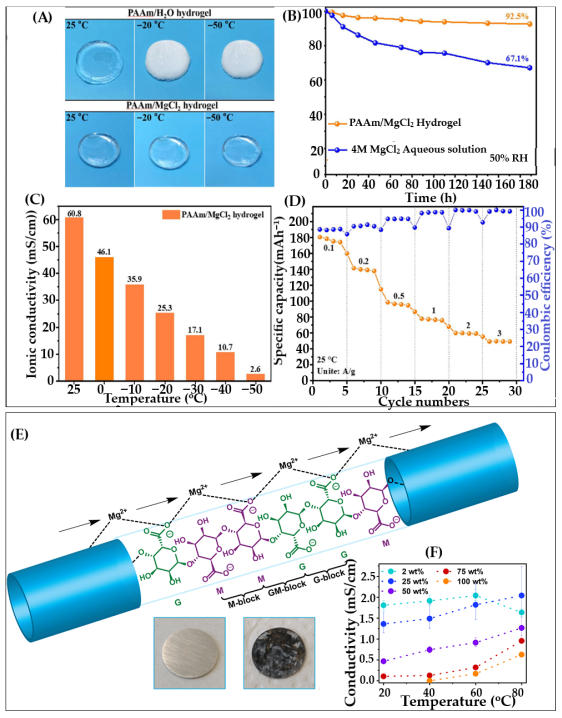
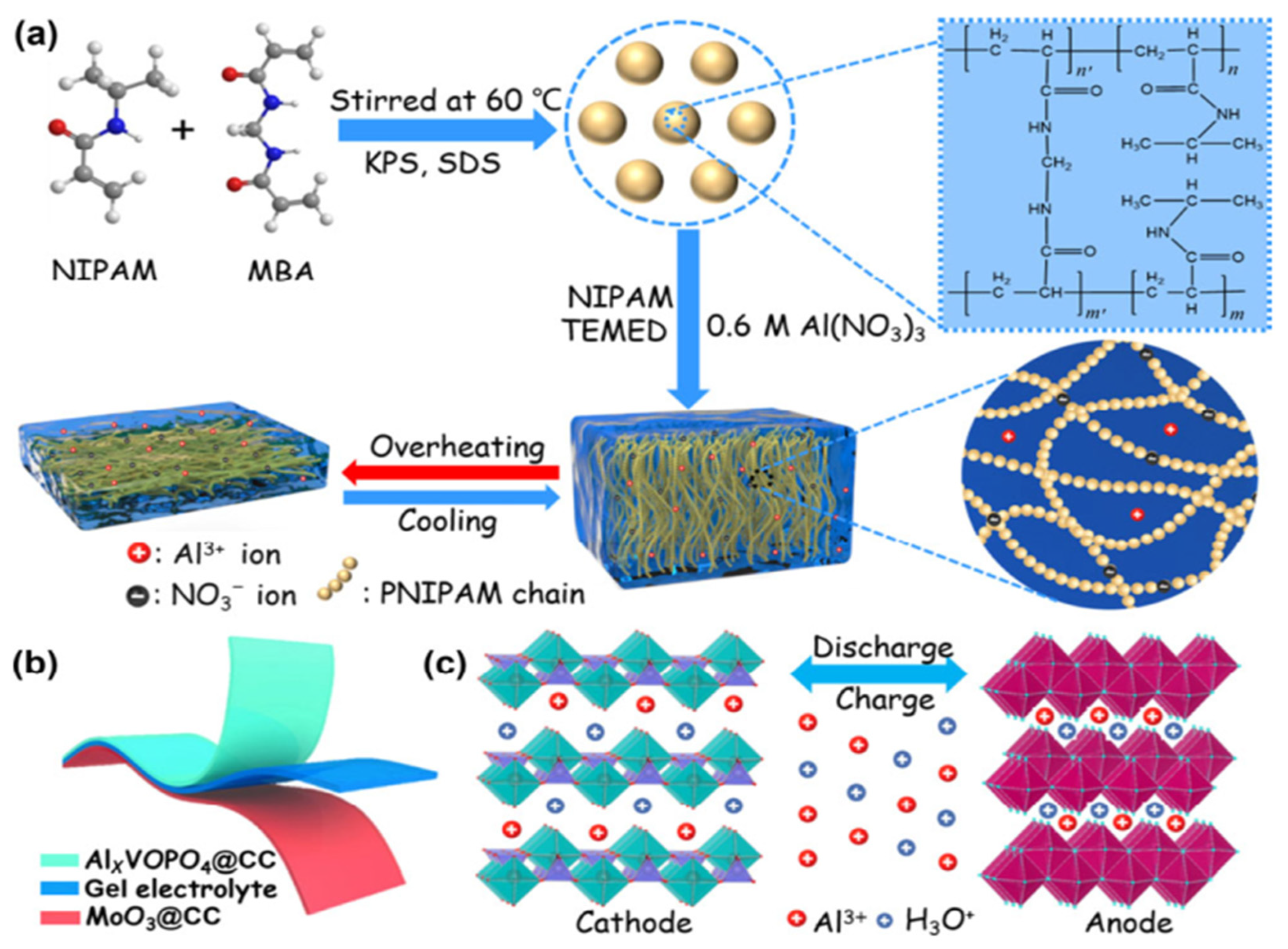
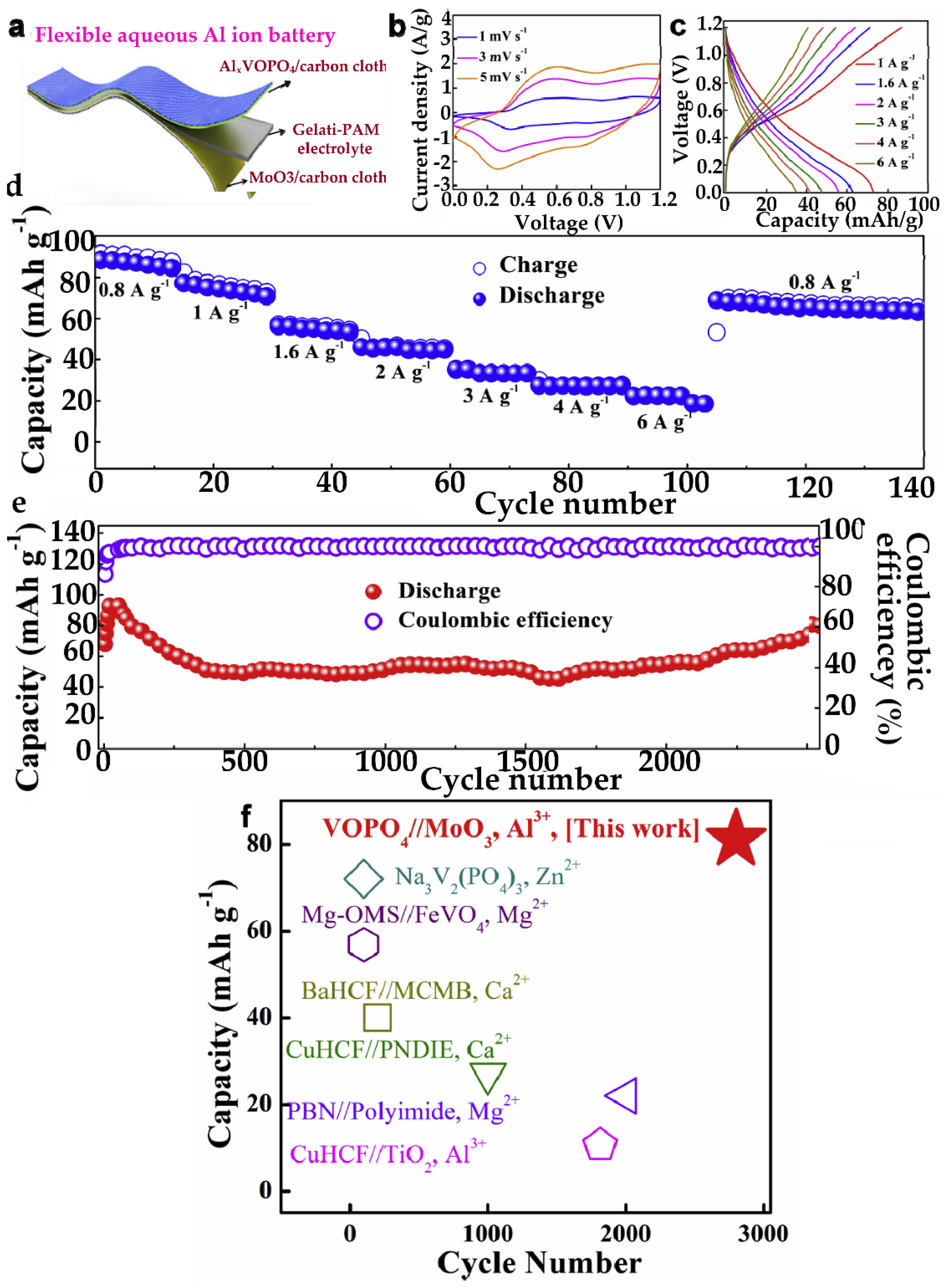
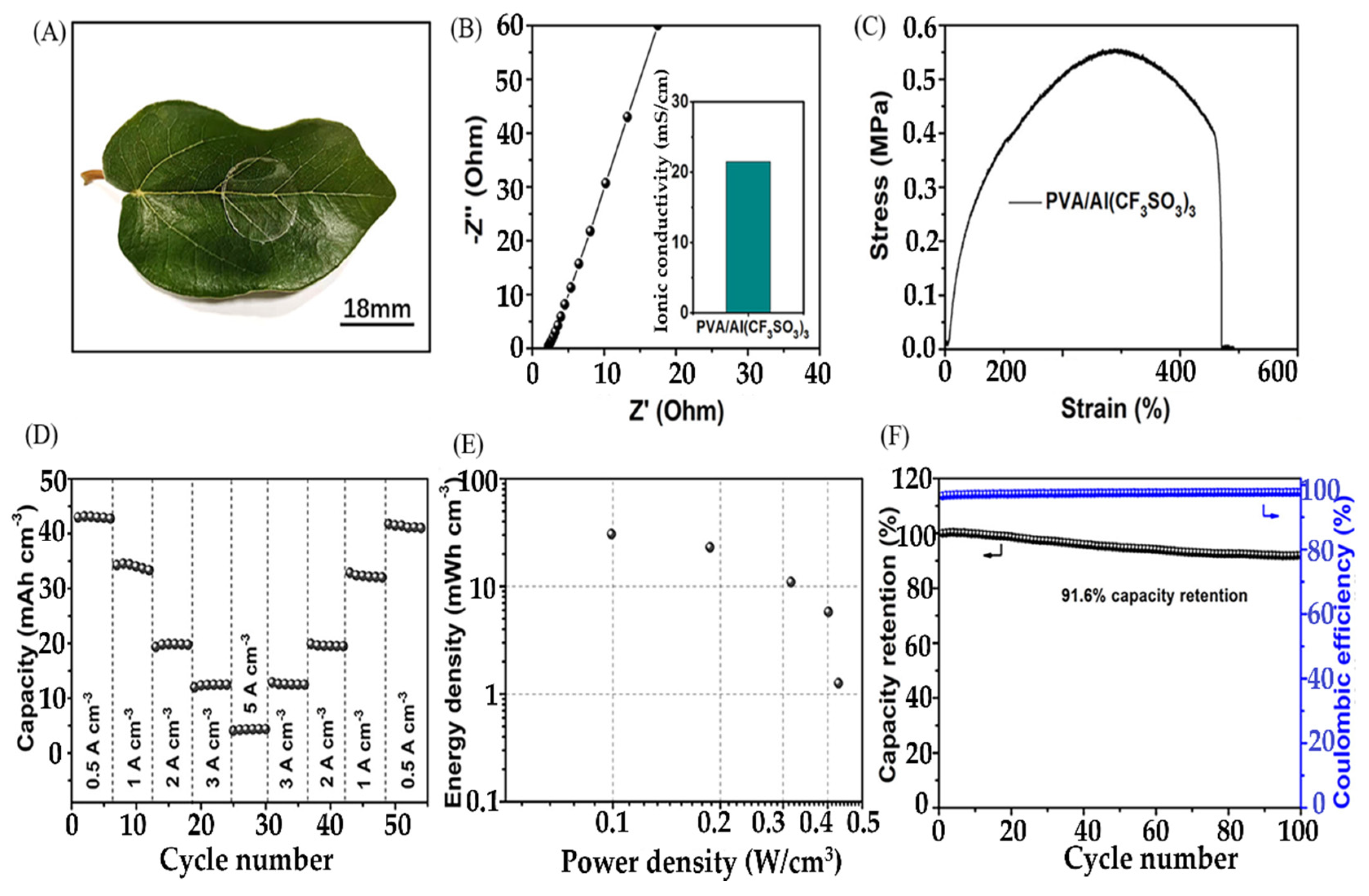
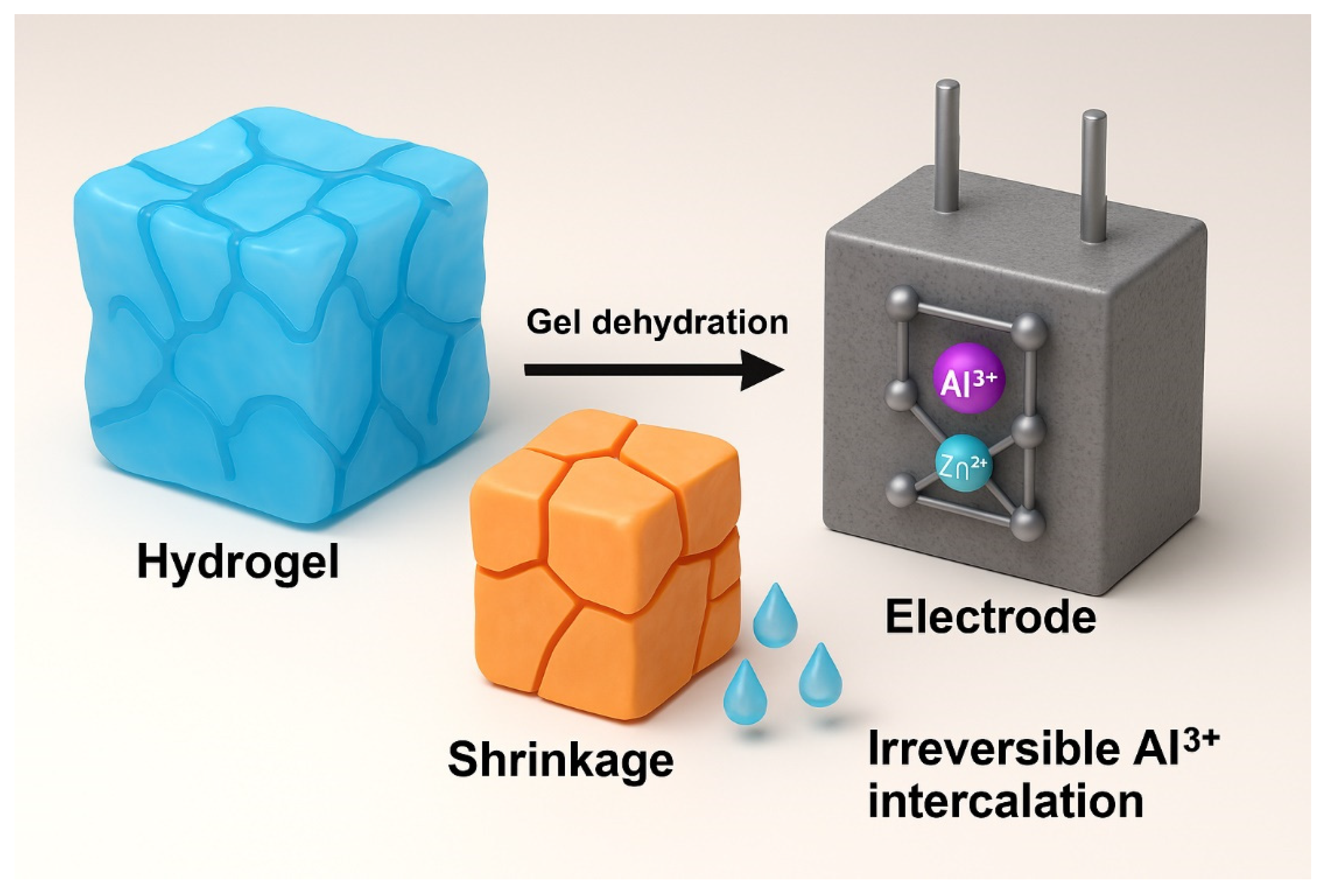
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutradhar, S.C.; Banik, N.; Ahmed, M.S.; Jang, H.; Nam, K.-W.; Islam, M. Applications of Hydrogels for Next-Generation Batteries. Gels 2025, 11, 757. https://doi.org/10.3390/gels11090757
Sutradhar SC, Banik N, Ahmed MS, Jang H, Nam K-W, Islam M. Applications of Hydrogels for Next-Generation Batteries. Gels. 2025; 11(9):757. https://doi.org/10.3390/gels11090757
Chicago/Turabian StyleSutradhar, Sabuj Chandra, Nipa Banik, Md. Shahriar Ahmed, Hohyoun Jang, Kyung-Wan Nam, and Mobinul Islam. 2025. "Applications of Hydrogels for Next-Generation Batteries" Gels 11, no. 9: 757. https://doi.org/10.3390/gels11090757
APA StyleSutradhar, S. C., Banik, N., Ahmed, M. S., Jang, H., Nam, K.-W., & Islam, M. (2025). Applications of Hydrogels for Next-Generation Batteries. Gels, 11(9), 757. https://doi.org/10.3390/gels11090757










