Bio-Aerogels as Materials for Active Food Packaging: Emerging Trends in Food Preservation
Abstract
1. Introduction
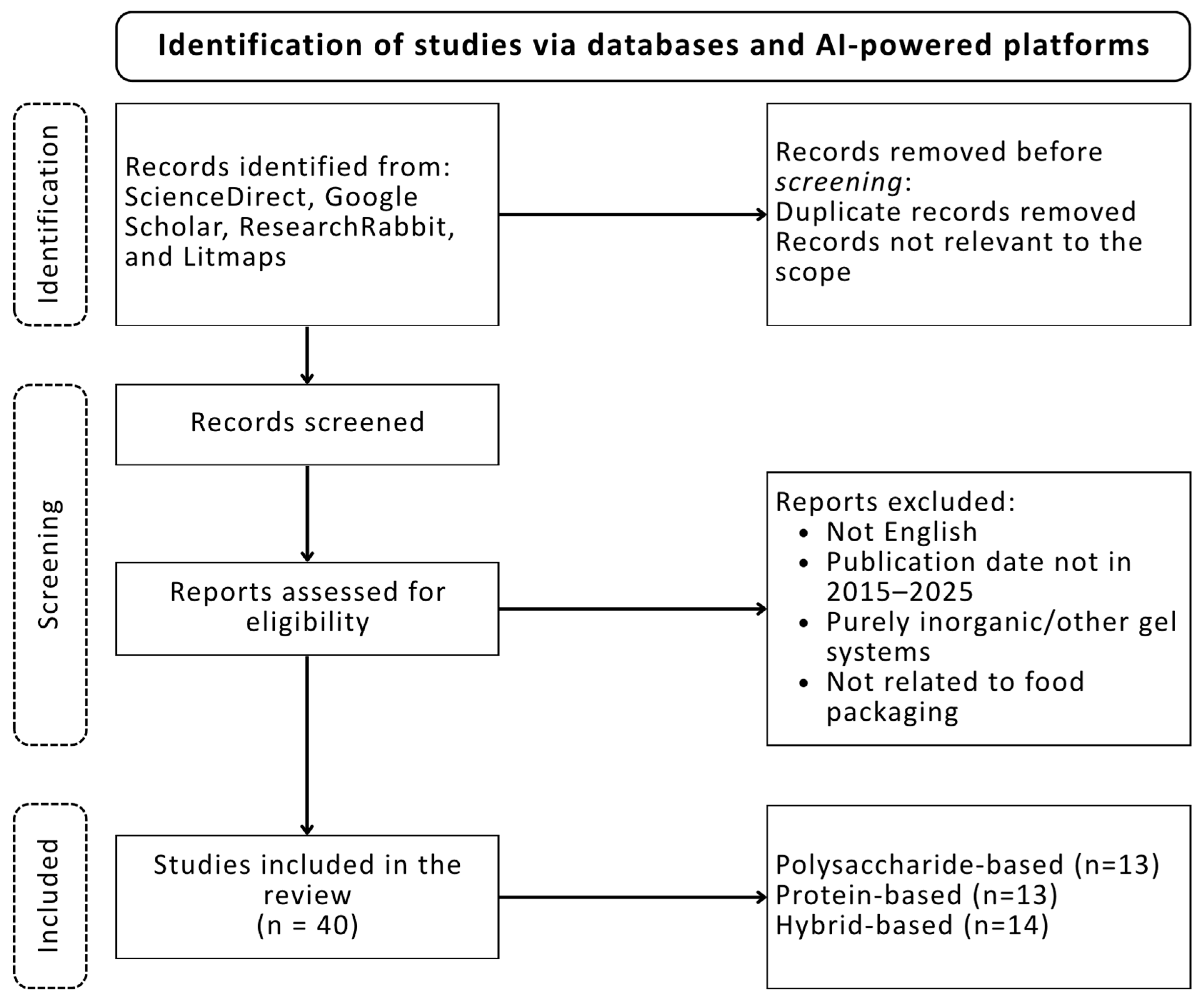
2. Review Methodology
3. Origin and Evolution of Aerogels
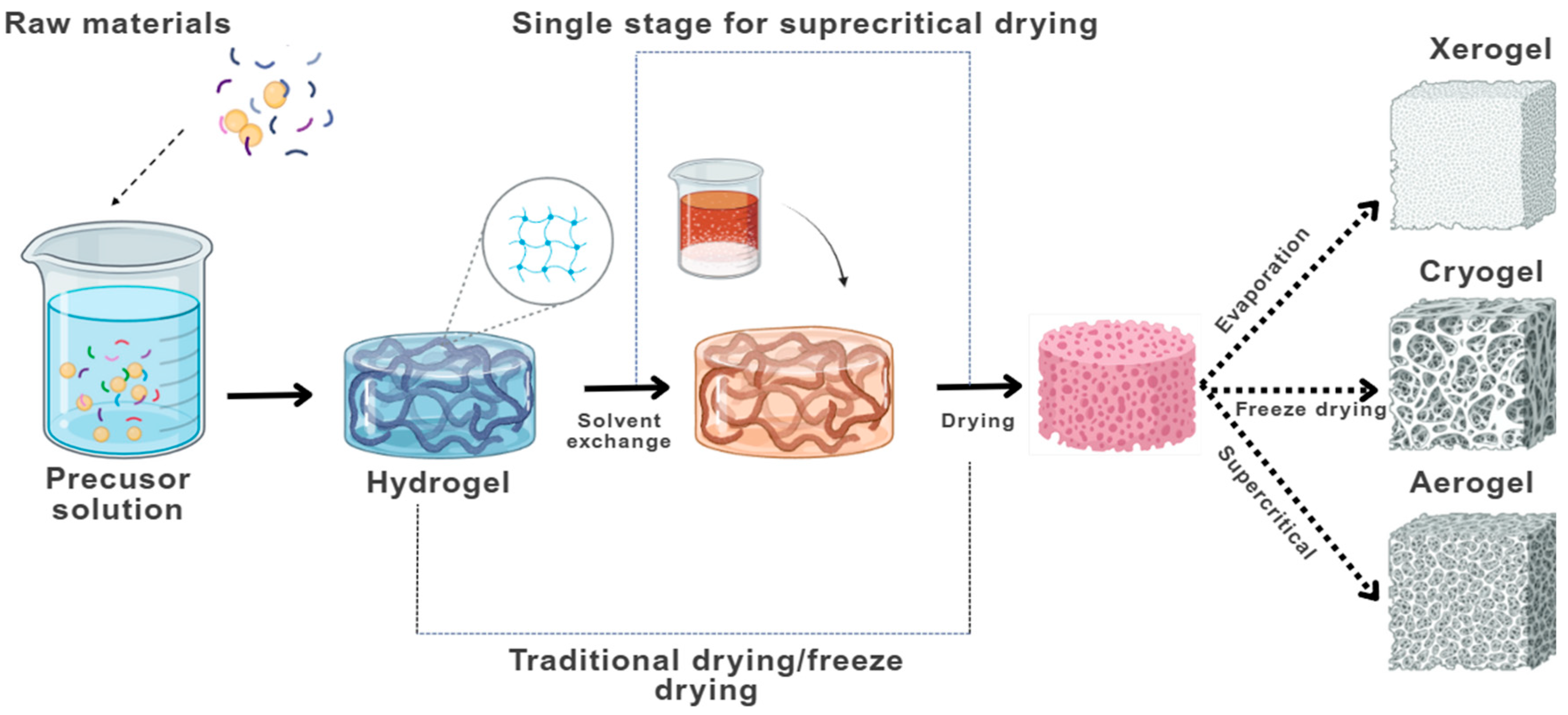
- Aerogels, obtained via supercritical drying, generally exhibit a mesoporous structure, with pore diameters between 2 and 50 nm.
- Xerogels, formed through ambient pressure drying, tend to be microporous, with pores smaller than 2 nm.
- Cryogels, produced by freeze-drying, are typically macroporous, with pore sizes exceeding 50 nm.
4. Properties of Bio-Aerogels and Their Applications
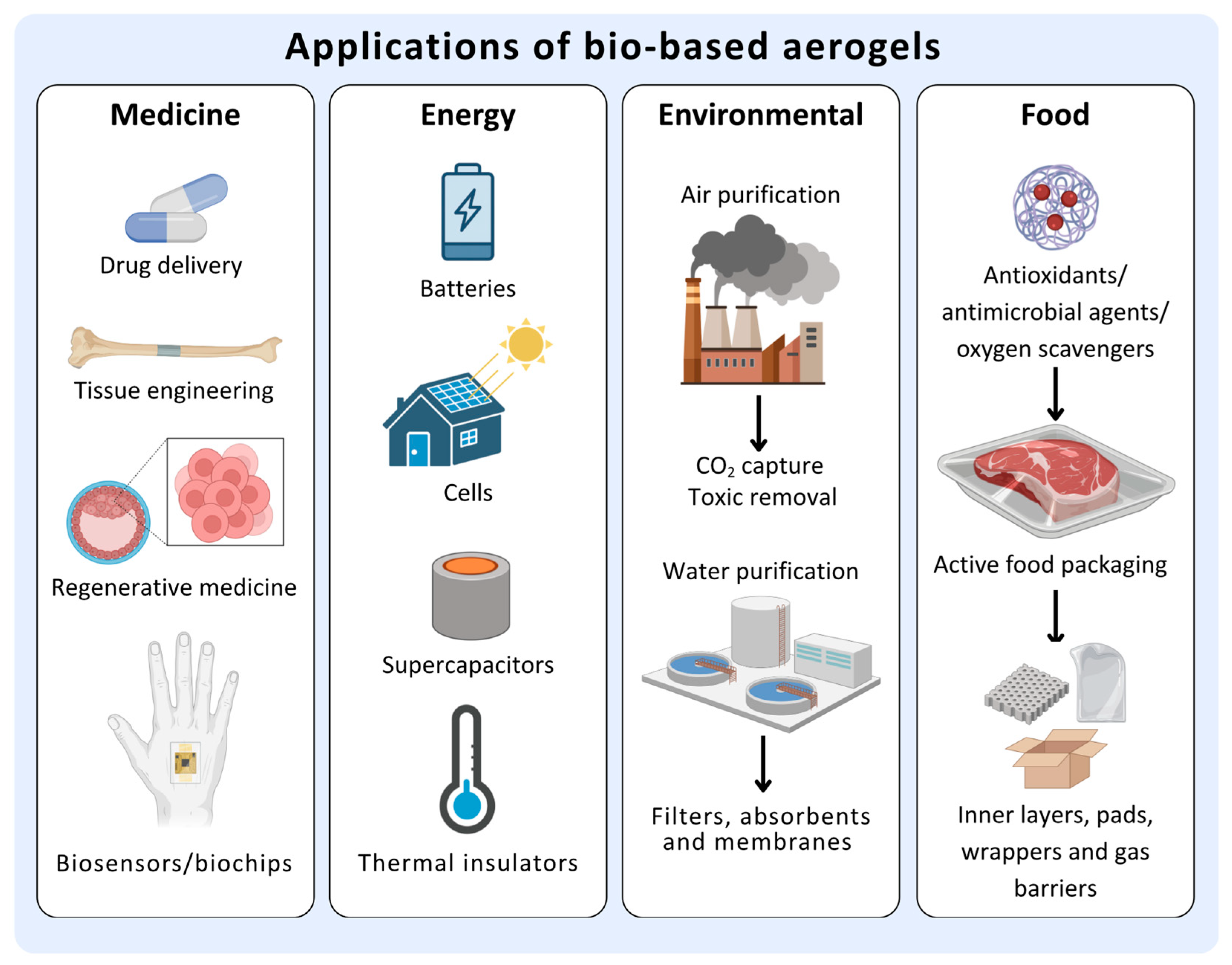
5. Bio-Aerogels Manufacturing Stages for Food Applications
5.1. Hydrogel Formation
5.2. Solvent Exchange
5.3. Drying
- a.
- Air drying
- b.
- Freeze-drying
- c.
- Supercritical drying
- d.
- Other drying techniques
| Drying Method | Advantages | Disadvantages |
|---|---|---|
| Air Drying |
|
|
| Freeze Drying |
|
|
| Supercritical Drying |
|
|
| Spray Drying |
|
|
| Microwave Drying |
|
|
6. Biodegradable Precursors for the Fabrication of Aerogels
6.1. Polysaccharide-Based Aerogels
| Polysaccharide | Properties | Dry Method | References |
|---|---|---|---|
| Corn starch (52.6% amylose) | Surface area: 1.3–1.7 m2/g; Pore volume: 0.0017–0.0054 cm3/g; Pore size: 14.9–15.1 nm; Porosity: 66.2–70.4% | Supercritical CO2 drying | [32] |
| Wheat starch | Surface area: 49.4–45.4 m2/g; Pore size: 0.09–0.27 cm3/g; Density: 0.03–0.05 g/cm3; Porosity: 91.3% | Supercritical CO2 drying | [33] |
| Alginate/pectin | Surface area: 16.76–21.27 m2/g; Pore size: 183–1081 nm; Density: 0.19–0.297 g/cm3; Porosity: 65.6–79% | Freeze-drying | [34,35] |
| Alginate/hyaluronic acid and Sodium alginate–grapefruit | Surface area: 446–611 m2/g; Density: 0.035–0.063 g/cm3; Porosity: 97–98% Thermal conductivity: 0.027–0.040 W · m−1 · K−1; Density: 0.030–0.042 g/cm3; Compressive strength: 317 kPa | Supercritical CO2 drying | [36,37] |
| Carrageenan (various types) | Surface area: 34–174 m2/g; Pore volume: 0.10–0.54 cm3/g; Pore size: 7.4–16.5 nm; Porosity: >94.3% | Supercritical CO2 drying | [38] |
| Konjac glucomannan/ soy protein | Density: 0.0201–0.0524 g/cm3; Porosity: 92.49–97.17% | Freeze-drying | [39] |
| Xanthan gum, gellan, and dextran | Lightweight, porous structures suitable for encapsulation and delivery | Supercritical CO2 drying | [26] |
| Chitosan | Surface area: 178 m2/g; Pore volume: 0.98 cm3/g; Porosity: ~96%; Density:0.034–0.063 g/cm3 | Supercritical CO2 drying | [17] |
| Microcrystalline cellulose-based carbon | Surface area: 38 m2/g; Pore volume: 0.3–2.4 cm3/g; Pore size: 10–100 nm | Supercritical CO2 drying | [40] |
| Nanofibrillated cellulose | Pore size: surface area: 80–100 m2/g; nm; Density: 0.012–0.033 g/cm3; Porosity: 98–99% | Freeze-drying | [41] |
| k-Carrageenan | Density: 0.129–0.237 g/cm3; Porosity: 98–99% | Supercritical CO2 drying | [42] |
| Starch/cellulose | Pore size: 24.73–100 nm; Density: 0.012–0.033 g/cm3; Porosity: 64–87% | Freeze-drying | [43] |
6.2. Protein-Based Aerogels
| Protein | Properties | Dry Method | References |
|---|---|---|---|
| Whey protein isolate | Surface area: 354 m2/g, Pore volume: 1.55 cm3/g, Density: 0.28 g/cm3, Pore size: 79.1 | Supercritical CO2 drying | [9,44] |
| Egg white protein | Surface area: 232 m2/g, Pore volume: 2.28 cm3/g, Density: 0.179 g/cm3, Pore size: 41.7 nm, Oil absorption: 0.74 g oil/g aerogel | Supercritical CO2 drying | [45] |
| Egg white protein isolate | Surface area: 154 m2/g, Pore volume: 0.33 cm3/g, Pore size: 7.1 nm | Supercritical CO2 drying | [46] |
| Egg white protein | Surface area: 390–422 m2/g, Pore volume: 1.27–1.69 cm3/g, Pore size: 9.2–14 nm | Supercritical CO2 drying | [19] |
| Soy protein | Surface area: 222–278 m2/g, Pore volume: 1.88–3.13 cm3/g, Density: 0.21 g/cm3, Pore size: 8–11 nm | Supercritical CO2 drying | [47] |
| Silk fibroin | Surface area: 424 m2/g, Pore size: 5–130 nm, Density: 0.19–0.25 g/cm3 | Supercritical CO2 drying | [48] |
| Silk fibroin | Surface area: 260–308 m2/g, Pore size: 17 nm, Pore volume: 1.8–1.7 cm3/g | Supercritical CO2 drying | [49] |
| Plant-based isolates (pea, soy, chia seed, wheat, zein, lentil) | Protein-based aerogels with biocompatibility and porosity | Supercritical CO2 drying | [26,50,51,52] |
| Soy protein | Surface area: 384–478 m2/g, P: 17 nm, Pore volume: 0.12–0.15 cm3/g (micropore), 1.72–2.29 cm3/g (mesopore), 1.41–2.72 cm3/g (macropore), Density: 0.19–0.25 g/cm3 | Supercritical CO2 drying | [24] |
6.3. Hybrid Aerogels
- i.
- Organic–Organic Bio-aerogels
- ii.
- Organic–Inorganic Bio-aerogels
| Matrix | Functional Material | Properties | Drying Method | Targeted Applications | References |
|---|---|---|---|---|---|
| Whey protein isolate (WPI)/Chitosan | Citric acid (CA), ε-polylysine hydrochloride (ε-PLH) | Superabsorbent (1486% water absorption); Antibacterial (≈80% against S. aureus, E. coli); Improves meat shelf-life (7 days) | Freeze-drying | Chicken meat preservation (absorbent pads) | [54] |
| Whey protein isolate (WPI) | Tannins | Reduced water absorption (219–559% vs. 4794% for pure WPI); Surface area: 216–353 m2/g | Supercritical CO2 drying | Food packaging (moisture-resistant) | [7] |
| WPI/Tannin | Bis(trimethylsilyl)amine (HMDS) | Hydrophobized (water absorption: 39–84%); Surface area: 87–242 m2/g | Supercritical CO2 drying | Food packaging (aqueous stability) | [7] |
| Dialdehyde nanocellulose (NCF)/Collagen | Sodium periodate (NaIO4) | High porosity (90–95%), Superabsorbent (>4000% water absorption); Low density: 0.025 g/cm3 | Freeze-drying | Biological compatibility applications | [55] |
| Gelatin, Dialdehyde Starch, Bacterial Cellulose | Curcumin | Super absorbent (water: 30.86 g/g, oil: 27.67 g/g); Antibacterial (survival rate <45% for E. coli, S. aureus, L. monocytogenes); Resilience under 70% compression strain | Freeze-drying | Fresh pork preservation: extends shelf life to 12 days (absorbent pads) | [56] |
| Pectin/Alginate | Zinc oxide nanoparticles (ZnO) | Pore size: 0.18–0.54 μm, Antimicrobial activity against S. aureus, E. coli; Thermal stability; Water absorption (472–791%) | Supercritical CO2 drying | Antimicrobial food packaging | [57] |
| Whey Proteins | Spirulin (SP) cells | Low density: 0.23–0.29 g/cm3; High porosity; Firmness: 10–47.5 N, Absorption capacity (oil: 5.6 g/g, water: 5 g/g) | Supercritical CO2 drying | Food applications | [58] |
| Whey Proteins | Hydrophilic (alginate, agar) or hydrophobic (ethylcellulose) coatings | Low density: 0.28–0.35 g/cm3; Porosity: 74–79%; Firmness: 10–90 N; Absorption capacity (oil: 2–6.2 g/g, water: 6.5–8.5 g/g) | Supercritical CO2 drying | Active coatings/layers for food packaging and smart food ingredients | [9] |
| Starch/Cellulose | Thymus daenensis essential oil (TDEO) | Low density: 18.42–54.77 mg/cm3; Pore size: 24.73–95.5 μm; Antimicrobial activity against E. coli O157:H7, psychrophiles, and yeast-mold | Freeze-drying | Antimicrobial packaging for cheese | [43] |
| Chitosan | Copper nanoparticles (CuNPs) encapsulated in liposomes | Antimicrobial against Gram-positive and Gram-negative bacteria; Absorption capacity (oil: 17–25 g/g, water: 3–25 g/g); Density: 25–30 mg/cm3 | Freeze-drying | Fresh pork preservation: extends shelf life to 14 days at 4 °C | [17] |
| Chitosan | Morillonite, clove essential oil, nanocellulose immobilized copper nanoparticles (CuNPs) fibers | Water absorption: ≈20%; Low density: 0.04–0.06 g/cm3; Porosity: 54.4–77.4%; Antimicrobial activity against E. coli, S. aureus and mold; Resilience under 30% compression strain | Freeze-drying | Active packaging and buffers for food (fruits and vegetables): protects blueberries from damage during transport and extends the storage by 3 days at 20 °C and 85% humidity | [28] |
| Poly(vinyl alcohol) (PVA) | Silica aerogel (SA) | Thermal conductivity: 0.068 W m−1·K−1); Tensile strength: 18.05–42.32 MPa; Water vapor transmission rate: 1.28–1.76 g m−2 d−1; Thermal stability | Not described | Multilayer packaging system for temperature-sensitive foodstuff packaging applications: chocolate | [10] |
| Galactoglucomannan (GGM), Cellulose Nanofibrils | Sunflower oil (SFO) | Density: ≈0.02 g/cm3; Surface area: 2–4 m2/g; Hexanal release for at least three weeks: 7–23 µmol/g | Freeze-drying | Food packaging materials with a system for in situ production and release hexanal: tests of blueberries and cherry tomatoes | [59] |
| Carboxymethyl Nanocellulose (CMC)/Chitosan/glycerol | Silver nanoparticles (AgNPs) | Cushioning coefficient: 5.04; Compression resilience (>90%); Antibacterial against E. coli, S. aureus; Biodegradation of >70% within 14 days; Swelling rate: 116.67% | Freeze-drying | Cushioning and antibacterial packaging for the storage and transportation of fruits and vegetables | [60] |
| Alginate | Oxidized nanocellulose | Porosity: 81–97.4%; Water absorption: 793–1468%; Water retention: 221.1–846.7%; Thermal stability | Freeze-drying | Food packaging for temperature-sensitive foods | [61] |
7. Applications of Bio-Aerogels in Food Packaging
7.1. Application in Fruit and Vegetable Packaging
7.2. Application in Fresh Meat Packaging
7.3. Other Applications of Bio-Aerogels in Food Packaging
8. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dis Huang, K.; Wang, Y. Advances in bio-based smart food packaging for enhanced food safety. Trends Food Sci. Technol. 2025, 159, 104960. [Google Scholar] [CrossRef]
- Taherimehr, M.; Yousefnia Pasha, H.; Tabatabaeekoloor, R.; Pesaranhajiabbas, E. Trends and challenges of biopolymer-based nanocomposites in food packaging. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5321–5348. [Google Scholar] [CrossRef]
- Grand View Research, Inc. Biopolymers Market Size, Share and Growth Report, 2030. Available online: https://www.grandviewresearch.com/industry-analysis/biopolymers-market-report (accessed on 20 July 2025).
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. Environmental impact of food packaging materials: A review of contemporary development from conventional plastics to polylactic acid based materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef] [PubMed]
- Siddaway, A.P.; Wood, A.M.; Hedges, L.V. How to do a systematic review: A best practice guide for conducting and reporting narrative reviews, meta-analyses, and meta-syntheses. Annu. Rev. Psychol. 2019, 70, 747–770. [Google Scholar] [CrossRef]
- Kistler, S.S. Coherent expanded aerogels and jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Effraimopoulou, E.; Kalmár, J.; Paul, G.; Marchese, L.; Ioannou, D.; Paraskevopoulou, P.; Gurikov, P. Whey protein isolate-based aerogels with improved hydration properties for food packaging applications. ACS Appl. Nano Mater. 2024, 7, 618–627. [Google Scholar] [CrossRef]
- Leite, A.C.; Pereira, R.N.; Rodrigues, R.M. Protein aerogels as food-grade delivery systems—A comprehensive review. Food Hydrocoll. 2025, 163, 111138. [Google Scholar] [CrossRef]
- De Berardinis, L.; Plazzotta, S.; Magnan, M.; Manzocco, L. Hydrophilic or hydrophobic coating of whey protein aerogels obtained by supercritical-CO2-drying: Effect on physical properties, moisture adsorption and interaction with water and oil in food systems. Innov. Food Sci. Emerg. Technol. 2024, 91, 103530. [Google Scholar] [CrossRef]
- Chen, C.; Ding, R.; Yang, S.; Wang, J.; Chen, W.; Zong, L.; Xie, J. Development of thermal insulation packaging film based on poly(vinyl alcohol) incorporated with silica aerogel for food packaging application. LWT—Food Sci. Technol. 2020, 129, 109568. [Google Scholar] [CrossRef]
- Dhua, S.; Gupta, A.K.; Mishra, P. Aerogel: Functional emerging material for potential application in food: A review. Food Bioprocess Technol. 2022, 15, 2396–2421. [Google Scholar] [CrossRef]
- Wei, G.; Zhang, J.; Usuelli, M.; Zhang, X.; Liu, B.; Mezzenga, R. Biomass vs inorganic and plastic-based aerogels: Structural design, functional tailoring, resource-efficient applications and sustainability analysis. Prog. Mater. Sci. 2022, 125, 100915. [Google Scholar] [CrossRef]
- Santos, P.D.; Viganó, J.; De Figueiredo Furtado, G.; Cunha, R.L.; Hubinger, M.D.; Rezende, C.A.; Martínez, J. Production of resveratrol-loaded alginate aerogel: Characterization, mathematical modeling, and study of impregnation. J. Supercrit. Fluids 2020, 163, 104882. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, X.; Zhang, Y.; Xu, E.; Yan, S.; Xu, H.; Li, M. Recent advances in the fabrication, characterization and application of starch-based materials for active food packaging: Hydrogels and aerogels. Sustain. Food Technol. 2024, 2, 615–634. [Google Scholar] [CrossRef]
- Kazemi-Taskooh, Z.; Varidi, M. Designation and characterization of cold-set whey protein-gellan gum hydrogel for iron entrapment. Food Hydrocoll. 2021, 111, 106205. [Google Scholar] [CrossRef]
- Vrabič-Brodnjak, U. Hybrid Materials of Bio-Based Aerogels for Sustainable Packaging Solutions. Gels 2024, 10, 27. [Google Scholar] [CrossRef]
- Chen, L.; Niu, X.; Fan, X.; Liu, Y.; Yang, J.; Xu, X.; Zhou, G.; Zhu, B.; Ullah, N.; Feng, X. Highly absorbent antibacterial chitosan-based aerogels for shelf-life extension of fresh pork. Food Control 2022, 136, 108644. [Google Scholar] [CrossRef]
- Falua, K.J.; Babaei-Ghazvini, A.; Acharya, B. Comparative study of the structure and mechanical properties of starch aerogels fabricated from air-classified and isolated pulse starches. Int. J. Biol. Macromol. 2024, 257, 128478. [Google Scholar] [CrossRef] [PubMed]
- Kleemann, C.; Schuster, R.; Rosenecker, E.; Selmer, I.; Smirnova, I.; Kulozik, U. In-vitro-digestion and swelling kinetics of whey protein, egg white protein and sodium caseinate aerogels. Food Hydrocoll. 2020, 101, 105534. [Google Scholar] [CrossRef]
- Alavi, F.; Ciftci, O.N. Effect of starch type and chitosan supplementation on physicochemical properties, morphology, and oil structuring capacity of composite starch bioaerogels. Food Hydrocoll. 2023, 141, 108637. [Google Scholar] [CrossRef]
- Franco, P.; Aliakbarian, B.; Perego, P.; Reverchon, E.; De Marco, I. Supercritical adsorption of quercetin on aerogels for active packaging applications. Ind. Eng. Chem. Res. 2018, 57, 15155–15161. [Google Scholar] [CrossRef]
- Klost, M.; Keil, C.; Gurikov, P. Dried Porous Biomaterials from Mealworm Protein Gels: Proof of Concept and Impact of Drying Method on Structural Properties and Zinc Retention. Gels 2024, 10, 275. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Singhal, R.S. The potential of supercritical drying as a “Green” method for the production of food-grade bioaerogels: A comprehensive critical review. Food Hydrocoll. 2023, 141, 108738. [Google Scholar] [CrossRef]
- Amaral-Labat, G.; Grishechko, L.; Szczurek, A.; Fierro, V.; Pizzi, A.; Kuznetsov, B.; Celzard, A. Highly mesoporous organic aerogels derived from soy and tannin. Green Chem. 2012, 14, 3099–3106. [Google Scholar] [CrossRef]
- Liu, H.; Xing, F.; Yu, P.; Zhe, M.; Shakya, S.; Liu, M.; Ritz, U. Multifunctional aerogel: A unique and advanced biomaterial for tissue regeneration and repair. Mater. Des. 2024, 243, 113091. [Google Scholar] [CrossRef]
- Selvasekaran, P.; Chidambaram, R. Food-grade aerogels obtained from polysaccharides, proteins, and seed mucilages: Role as a carrier matrix of functional food ingredients. Trends Food Sci. Technol. 2021, 112, 208–220. [Google Scholar] [CrossRef]
- Baudron, V.; Gurikov, P.; Smirnova, I.; Whitehouse, S. Porous starch materials via supercritical- and freeze-drying. Gels 2019, 5, 12. [Google Scholar] [CrossRef]
- Wang, X.; Guo, J.; Zhou, H.; Hou, Y.; Jin, P.; Zheng, Y.; Wu, Z. Chitosan-based aerogel food active packaging with integrated preservation and buffering functions. J. Adv. Res. 2025; in press. [Google Scholar] [CrossRef]
- Chel, A.; Hazarika, M. Application of aerogels in the packaging of fresh meat: A review. Packag. Technol. Sci. 2020, 33, 213–224. [Google Scholar] [CrossRef]
- Nita, L.E.; Ghilan, A.; Rusu, A.G.; Neamtu, I.; Chiriac, A.P. New trends in bio-based aerogels. Pharmaceutics 2020, 12, 449. [Google Scholar] [CrossRef]
- Wang, Y.; Su, Y.; Wang, W.; Fang, Y.; Riffat, S.B.; Jiang, F. The advances of polysaccharide-based aerogels: Preparation and potential application. Carbohydr. Polym. 2019, 226, 115242. [Google Scholar] [CrossRef]
- Goimil, L.; Braga, M.E.M.; Dias, A.M.A.; Gómez-Amoza, J.L.; Concheiro, A.; Alvarez-Lorenzo, C.; de Sousa, H.C.; García-González, C.A. Supercritical processing of starch aerogels and aerogel-loaded poly(ε-caprolactone) scaffolds for sustained release of ketoprofen for bone regeneration. J. CO2 Util. 2017, 18, 237–249. [Google Scholar] [CrossRef]
- Ubeyitogullari, A.; Ciftci, O.N. Formation of nanoporous aerogels from wheat starch. Carbohydr. Polym. 2016, 147, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Y.; Wang, J.; Zhang, H.; Zhang, W.; Liu, J.; Zhang, Y.; Liu, X. Advanced alginate-based nanofiber aerogels. Compos. Sci. Technol. 2024, 221, 109368. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, H. Alginate/pectin aerogel microspheres for controlled release of proanthocyanidins. Int. J. Biol. Macromol. 2019, 136, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Athamneh, T.; Amin, A.; Benke, E.; Ambrus, R.; Leopold, C.S.; Gurikov, P.; Smirnova, I. Alginate and hybrid alginate–hyaluronic acid aerogel microspheres as potential carrier for pulmonary drug delivery. J. Supercrit. Fluids 2019, 150, 49–55. [Google Scholar] [CrossRef]
- Jing, N.; Feng, Y.; Ge, H.; Tang, Q.; Xie, Y.; Li, S. Sodium alginate–grapefruit peel aerogels with outstanding thermal conductivity, excellent compressive strength, and low density. Mater. Today Commun. 2025, 45, 112427. [Google Scholar] [CrossRef]
- Alnaief, M.; Obaidat, R.; Mashaqbeh, H. Effect of processing parameters on preparation of carrageenan aerogel microparticles. Carbohydr. Polym. 2018, 180, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.; Yang, H. Promoted strain-hardening and crystallinity of a soy protein-konjac glucomannan complex gel by konjac glucomannan. Food Hydrocoll. 2022, 133, 107959. [Google Scholar] [CrossRef]
- Alatalo, S.; Pileidis, F.D.; Mäkilä, E.; Sevilla, M.; Salonen, J.J.; Sillanpää, M. Versatile cellulose based carbon aerogel for the removal of both cationic and anionic metal contaminants from water. ACS Appl. Mater. Interfaces 2015, 7, 25875–25883. [Google Scholar] [CrossRef]
- Jiménez-Saelices, C.; Seantier, B.; Cathala, B.; Grohens, Y. Spray freeze-dried nanofibrillated cellulose aerogels with thermal superinsulating properties. Carbohydr. Polym. 2017, 157, 105–113. [Google Scholar] [CrossRef]
- Manzocco, L.; Valoppi, F.; Calligaris, S.; Andreatta, F.; Spilimbergo, S.; Nicoli, M. Exploitation of κ-carrageenan aerogels as template for edible oleogel preparation. Food Hydrocoll. 2017, 71, 68–75. [Google Scholar] [CrossRef]
- Mirmoeini, S.S.; Hosseini, S.H.; Lotfi Javid, A.; Esmaeili Koutamehr, M.; Sharafi, H.; Molaei, R.; Moradi, M. Essential oil-loaded starch/cellulose aerogel: Preparation, characterization and application in cheese packaging. Int. J. Biol. Macromol. 2023, 244, 125356. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Madadlou, A.; Sabouri, A.A. Whey protein aerogel as blended with cellulose crystalline particles or loaded with fish oil. Food Chem. 2015, 174, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Selmer, I.; Karnetzke, J.; Kleemann, C.; Lehtonen, M.; Mikkonen, K.S.; Kulozik, U.; Smirnova, I. Encapsulation of fish oil in protein aerogel micro-particles. J. Food Eng. 2019, 260, 1–11. [Google Scholar] [CrossRef]
- Selmer, I.; Kleemann, C.; Kulozik, U.; Heinrich, S.; Smirnova, I. Development of egg white protein aerogels as new matrix material for microencapsulation in food. J. Supercrit. Fluids 2015, 106, 42–49. [Google Scholar] [CrossRef]
- Kaur, S.; Singh, S.; Saini, R.; Sharma, S.; Kaur, A.; Kaur, A.; Kaur, M.; Kaur, P.; Kaur, J.; Kaur, G. Transforming soy proteins into nanoporous aerogels using supercritical CO2 drying. J. Food Sci. 2025, 90, e70011. [Google Scholar] [CrossRef]
- Marín, M.; Mallepally, R.; McHugh, M.A. Silk fibroin aerogels for drug delivery applications. J. Supercrit. Fluids 2014, 91, 84–89. [Google Scholar] [CrossRef]
- Mallepally, R.R.; Marin, M.A.; Surampudi, V.; Subia, B.; Rao, R.R. Silk fibroin aerogels: Potential scaffolds for tissue engineering applications. Biomed. Mater. 2015, 10, 035002. [Google Scholar] [CrossRef]
- Chen, Q.; Guan, J.; Wang, Z.; Wang, Y.; Wang, X.; Chen, Z. Improving the Gelation Properties of Pea Protein Isolates Using Psyllium Husk Powder: Insight into the Underlying Mechanism. Foods 2024, 13, 3413. [Google Scholar] [CrossRef] [PubMed]
- Mekala, S.; Saldaña, M.D.A. Lentil protein concentrate–pectin gels dried with supercritical CO2: Influence of protein–polysaccharide interactions on the characteristics of aerogels. J. Supercrit. Fluids 2023, 201, 106006. [Google Scholar] [CrossRef]
- Yang, C.; Li, A.; Guo, T.; Cheng, J.; Liu, Z.; Hu, H.; Wang, J. Novel organic-inorganic composite pea protein silica food-grade aerogel materials: Fabrication, mechanisms, high oil-holding property and curcumin delivery capacity. Int. J. Biol. Macromol. 2024, 273, 132832. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, H.; Peng, C.; Ma, J.; Huang, S.; Wang, R.; Chen, J. Application of protein/polysaccharide aerogels in drug delivery system: A review. Int. J. Biol. Macromol. 2023, 247, 125727. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Guo, X.; Chai, Y.; Li, X.; Chen, L.; Feng, X. Superabsorbent whey protein isolates/chitosan-based antibacterial aerogels: Preparation, characterization and application in chicken meat preservation. Int. J. Biol. Macromol. 2024, 259, 128961. [Google Scholar] [CrossRef]
- Lu, T.; Li, Q.; Chen, W.; Yu, H. Composite aerogels based on dialdehyde nanocellulose and collagen for potential applications as wound dressing and tissue engineering scaffold. Compos. Sci. Technol. 2014, 94, 132–138. [Google Scholar] [CrossRef]
- Wang, F.; Xu, Z.; Chen, L.; Qiao, Z.; Hu, Y.; Fan, X.; Liu, Y.; Kang, Z.; Huang, F.; Han, M.; et al. Super absorbent resilience antibacterial aerogel with curcumin for fresh pork preservation. Food Control 2024, 159, 110289. [Google Scholar] [CrossRef]
- Mottola, S.; Viscusi, G.; Oliva, G.; Vigliotta, G.; Cardea, S.; Gorrasi, G.; De Marco, I. Pectin/alginate aerogel containing ZnO produced from beetroot extract mediated green synthesis for potential applications in food packaging. J. CO2 Util. 2025, 91, 103003. [Google Scholar] [CrossRef]
- De Berardinis, L.; Plazzotta, S.; Magnan, M.; Manzocco, L. Hybrid aerogels of spirulina and whey proteins as novel cellular solids. LWT 2024, 191, 117078. [Google Scholar] [CrossRef]
- Lehtonen, M.; Kekäläinen, S.; Nikkilä, I.; Kilpeläinen, P.; Tenkanen, M.; Mikkonen, K.S. Active food packaging through controlled in situ production and release of hexanal. Food Chem. X 2020, 5, 100074. [Google Scholar] [CrossRef]
- Zhou, W.; Fang, J.; Tang, S.; Wu, Z.; Wang, X. 3D-printed nanocellulose-based cushioning-antibacterial dual-function food packaging aerogel. Molecules 2021, 26, 3543. [Google Scholar] [CrossRef]
- Lin, N.; Bruzzese, C.; Dufresne, A. TEMPO-oxidized nanocellulose participating as crosslinking aid for alginate-based sponges. ACS Appl. Mater. Interfaces 2012, 4, 4948–4959. [Google Scholar] [CrossRef]
- Ezati, P.; Khan, A.; Priyadarshi, R.; Bhattacharya, T.; Tammina, S.K.; Rhim, J.W. Biopolymer-based UV protection functional films for food packaging. Food Hydrocoll. 2023, 142, 108771. [Google Scholar] [CrossRef]
- Fontes-Candia, C.; Erboz, E.; Martínez-Abad, A.; López-Rubio, A.; Martínez-Sanz, M. Superabsorbent food packaging bioactive cellulose-based aerogels from Arundo donax waste biomass. Food Hydrocoll. 2019, 96, 151–160. [Google Scholar] [CrossRef]
- Da Silva, F.T.; de Oliveira, J.P.; Fonseca, L.M.; Bruni, G.P.; Da Rosa Zavareze, E.; Dias, A.R.G. Physically cross-linked aerogels based on germinated and non-germinated wheat starch and PEO for application as water absorbers for food packaging. Int. J. Biol. Macromol. 2020, 155, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Dhua, S.; Mishra, P. Development of highly reusable, mechanically stable corn starch-based aerogel using glycerol for potential application in the storage of fresh spinach leaves. Int. J. Biol. Macromol. 2023, 242, 125102. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Herrejón, Y.G.; Vargas-Almaraz, J.; Castañeda-Salazar, A.; Mendoza, S. Bio-Aerogels as Materials for Active Food Packaging: Emerging Trends in Food Preservation. Gels 2025, 11, 756. https://doi.org/10.3390/gels11090756
Morales-Herrejón YG, Vargas-Almaraz J, Castañeda-Salazar A, Mendoza S. Bio-Aerogels as Materials for Active Food Packaging: Emerging Trends in Food Preservation. Gels. 2025; 11(9):756. https://doi.org/10.3390/gels11090756
Chicago/Turabian StyleMorales-Herrejón, Yuliza G., Jorge Vargas-Almaraz, Adolfo Castañeda-Salazar, and Sandra Mendoza. 2025. "Bio-Aerogels as Materials for Active Food Packaging: Emerging Trends in Food Preservation" Gels 11, no. 9: 756. https://doi.org/10.3390/gels11090756
APA StyleMorales-Herrejón, Y. G., Vargas-Almaraz, J., Castañeda-Salazar, A., & Mendoza, S. (2025). Bio-Aerogels as Materials for Active Food Packaging: Emerging Trends in Food Preservation. Gels, 11(9), 756. https://doi.org/10.3390/gels11090756







