Comparison of Flavor Stability of Yuja (Citrus junos Tanaka) Oil-Based Nano-Carriers and Dried Gels
Abstract
1. Introduction
2. Results and Discussion
2.1. Particle Size and PDI of Y-NE and Y-NLC
2.2. Characterization of Yuja Dried Gels
2.2.1. Particle Size and Size Distribution
2.2.2. Moisture Content and Color
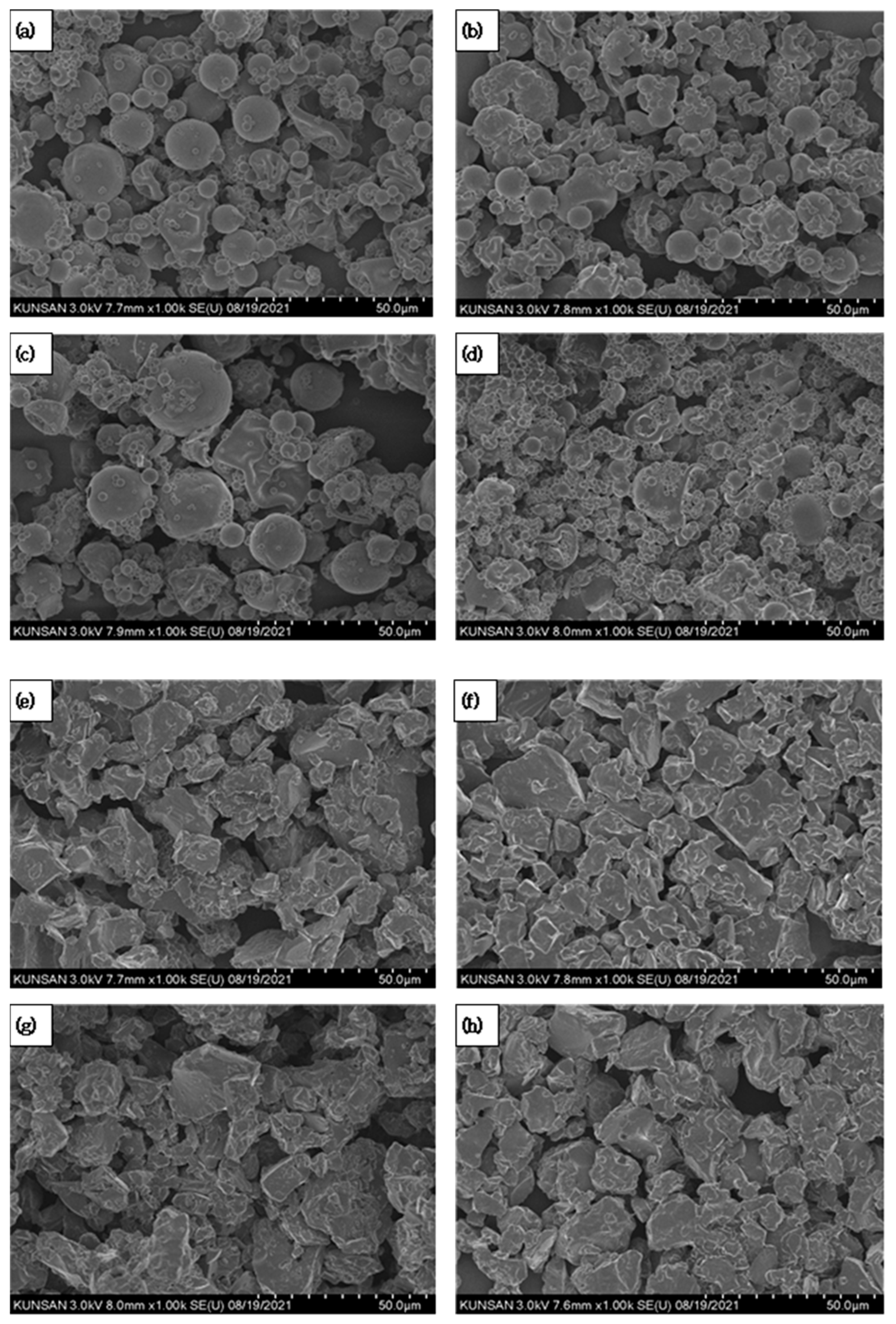
2.2.3. Field Emission Scanning Electron Microscopy (FE-SEM)
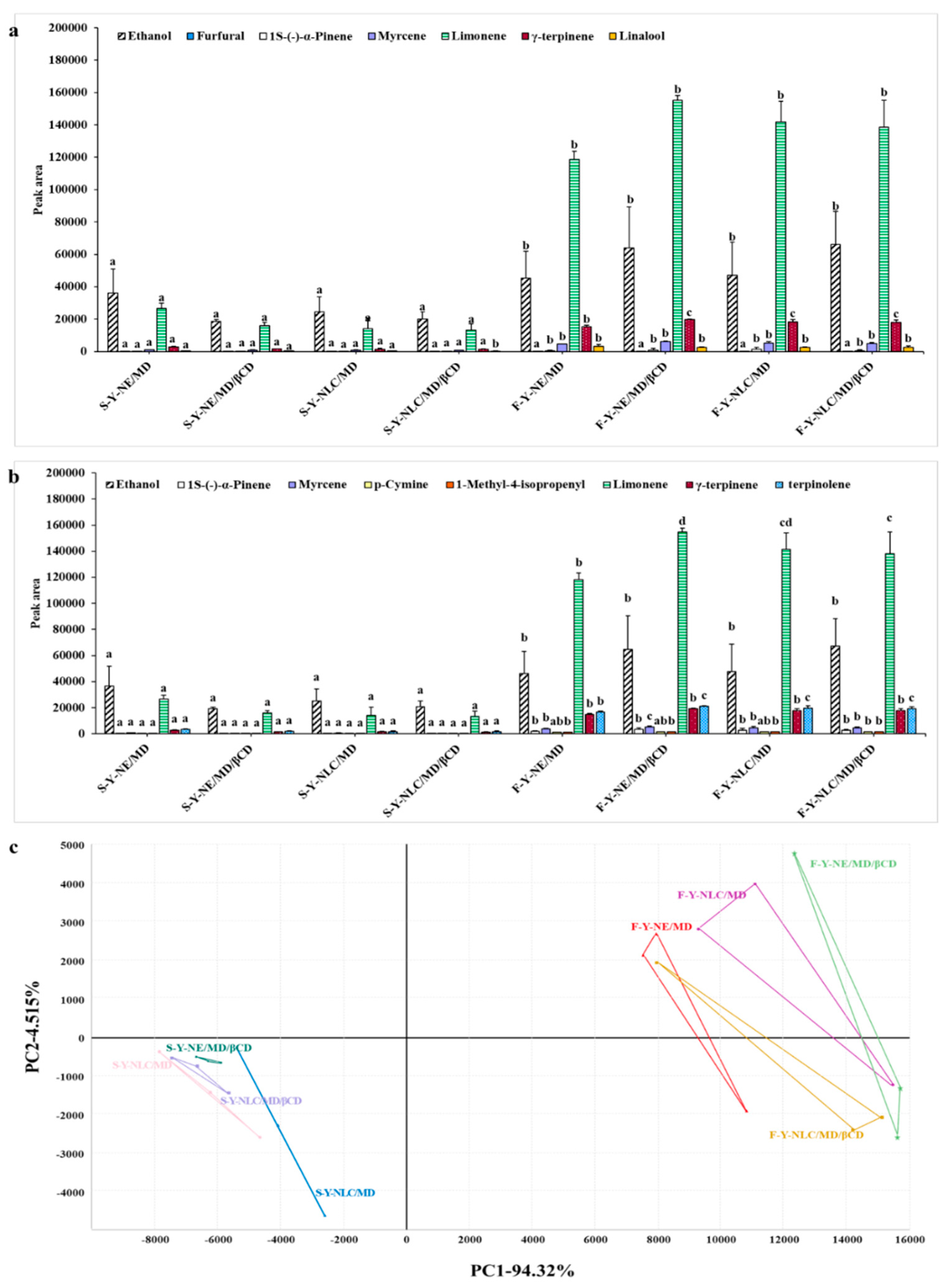
2.2.4. Volatile Profiles of Yuja Dried Gels Analyzed Using E-Nose-Based Fast GC
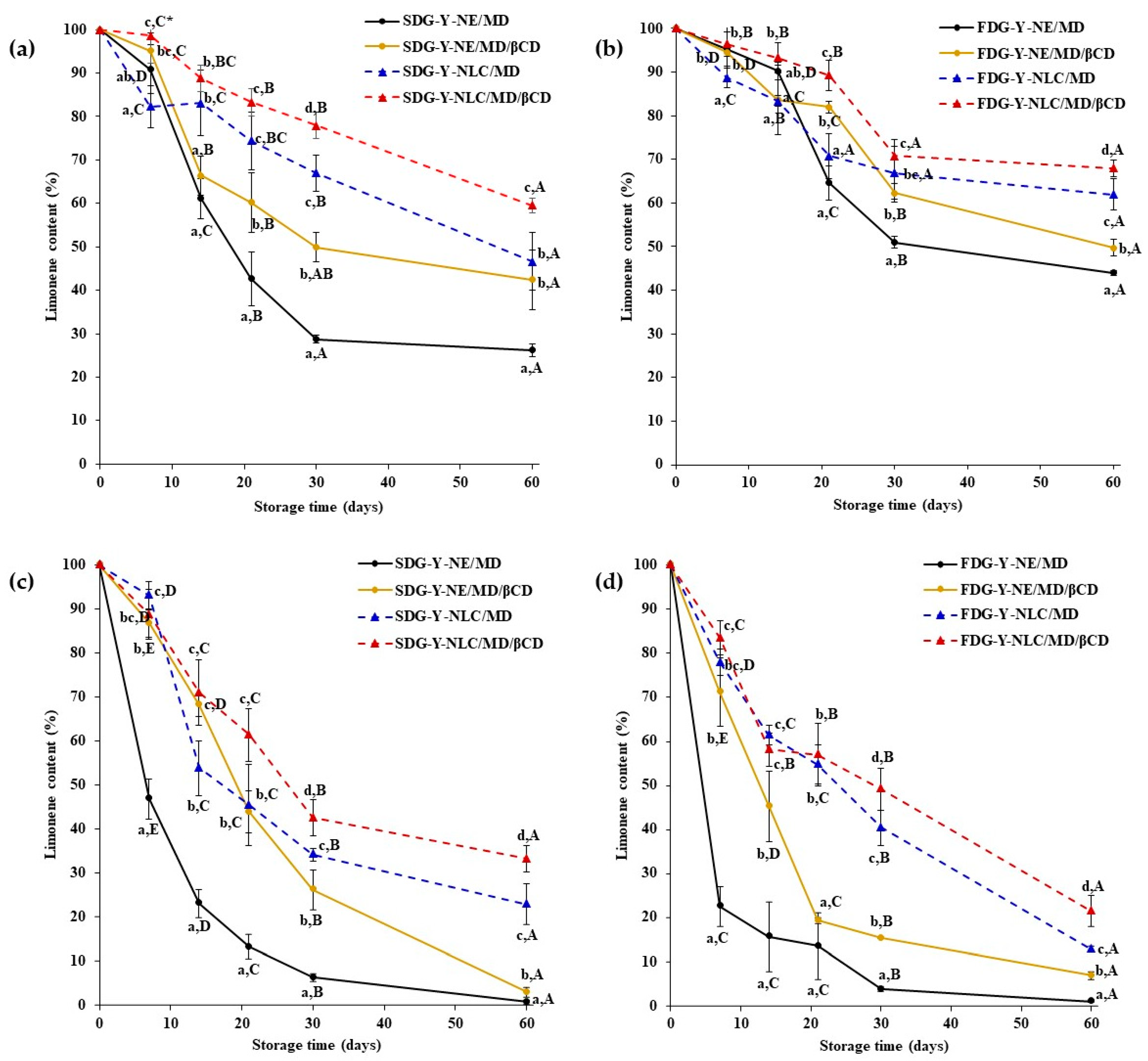
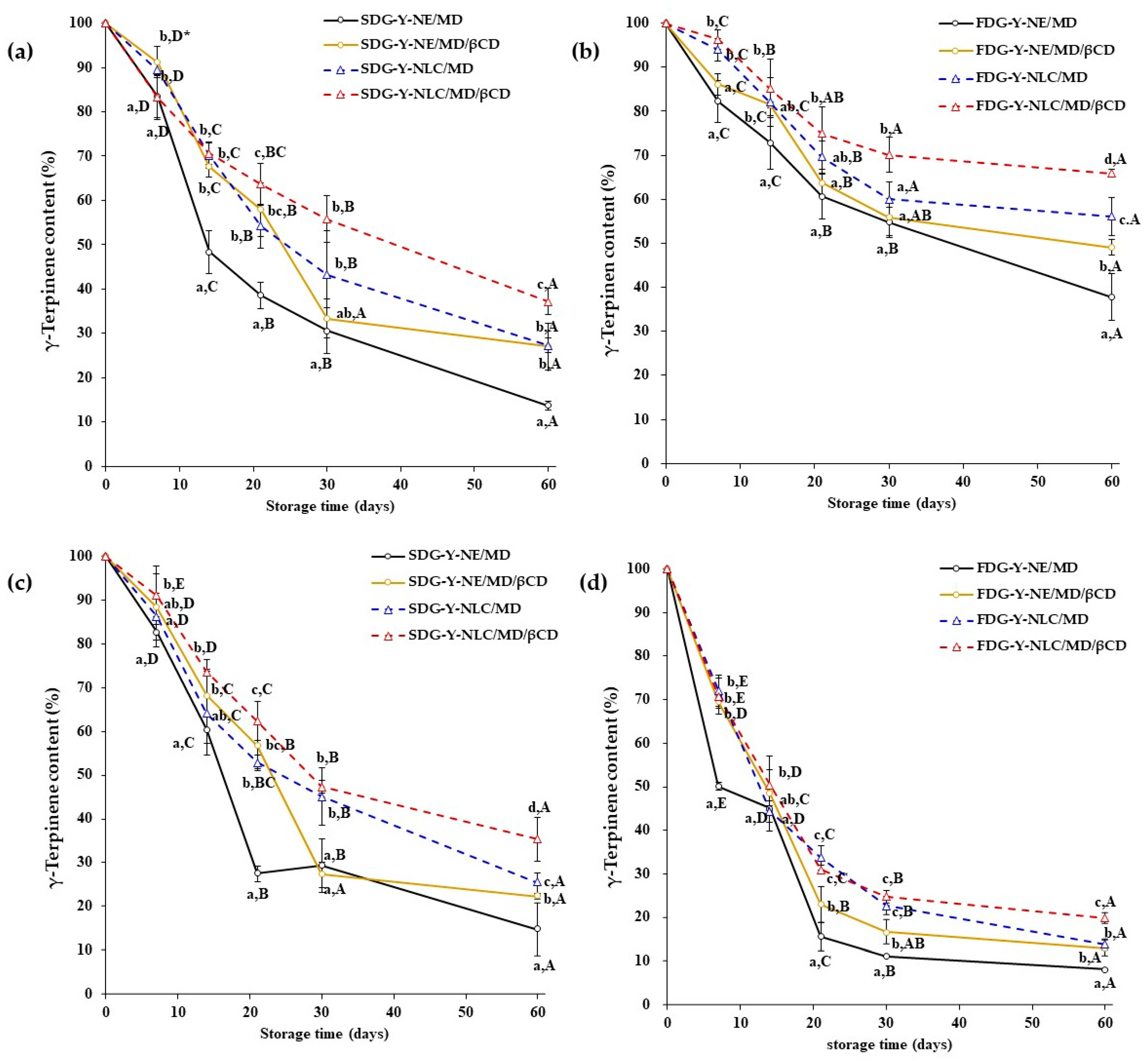
2.3. Storage Stability of Limonene and γ-Terpinene Contained in Yuja Dried Gels
2.3.1. Limonene Stability
2.3.2. γ-Terpinene Stability
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Analysis of Yuja Oil
4.3. Preparation and Characterization of O/W Yuja Oil-NE and Yuja Oil-NLC
4.4. Coating of Yuja Oil-Based NE and NLC
4.4.1. Preparation of Y-NE and Y-NLC
4.4.2. Spray-Dried Yuja Gels
4.4.3. Freeze-Dried Yuja Gels
4.5. Characterization of Physicochemical Properties of Yuja Dried Gels
4.5.1. Measurement of Particle Size and Distribution
4.5.2. Moisture Content
4.5.3. Color
4.5.4. Field Emission Scanning Electron Microscope (FE-SEM)
4.5.5. Electronic Nose-Based Fast GC Analysis
4.5.6. Stability of Volatile Compounds Contained in Yuja Dried Gels During Storage
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, J.A.; Oh, J.E.; Cho, M.S. Development of Yuja (Citrus junos) Beverage Based on Antioxidant Properties and Sensory Attributes Using Response Surface Methodology. J. Food Sci. Technol. 2019, 56, 1854–1863. [Google Scholar] [CrossRef]
- Ferhat, M.A.; Meklati, B.Y.; Smadja, J.; Chemat, F. An Improved Microwave Clevenger Apparatus for Distillation of Essential Oils from Orange Peel. J. Chromatogr. A 2006, 1112, 121–126. [Google Scholar] [CrossRef]
- Rezzadori, K.; Benedetti, S.; Amante, E.R. Proposals for The Residues Recovery: Orange Waste as Raw Material for New Products. Food Bioprod. Process 2012, 90, 606–614. [Google Scholar] [CrossRef]
- Rodríguez, A.; Peris, J.E.; Redondo, A.; Shimada, T.; Costell, E.; Carbonell, I.; Rojas, C.; Peňa, L. Impact of Limonene Synthase Up- or Down- Regulation on Sweet Orange Fruit and Juice Odor Perception. Food Chem. 2017, 217, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Bousbia, N.; Vian, M.A.; Ferhat, M.A.; Meklati, B.Y.; Chemat, F. A New Process for Extraction of Essential Oil from Citrus Peels: Microwave Hydrodiffusion and Gravity. J. Food Eng. 2009, 90, 409–413. [Google Scholar] [CrossRef]
- Wang, M.; Doi, T.; McClements, D.J. Encapsulation and controlled release of hydrophobic flavors using biopolymer-based microgel delivery systems: Sustained release of garlic flavor during simulated cooking. Food Res. Int. 2019, 119, 6–14. [Google Scholar] [CrossRef]
- Chuesiang, P.; Siripatrawan, U.; Sanguandeekul, R.; Yang, J.S.; McLandsborough, L.; McClements, D.J. Antimicrobial activity and chemical stability of cinnamon oil in oil-in-water nanoemulsions fabricated using the phase inversion temperature method. LWT 2019, 110, 190–196. [Google Scholar] [CrossRef]
- Katopodi, A.; Detsi, A. Solid lipid nanoparticles and nanostructured lipid carriers of natural products as promising systems for their bioactivity enhancement: The case of essential oils and flavonoids. Colloids Surfaces A 2021, 630, 127529. [Google Scholar] [CrossRef]
- Chuesiang, P.; Siripatrawan, U.; Sanguandeekul, R.; McLandsborough, L.; McClements, D.J. Optimization of cinnamon oil nanoemulsions using phase inversion temperature method: Impact of oil phase composition and surfactant concentration. J. Colloid Interface Sci. 2018, 514, 208–216. [Google Scholar] [CrossRef]
- Park, S.J.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Development of nanostructured lipid carriers for the encapsulation and controlled release of vitamin D3. Food Chem. 2017, 225, 213–219. [Google Scholar] [CrossRef]
- Shin, G.H.; Kim, J.T.; Park, H.J. Recent developments in nanoformulations of liphophilic functional foods. Trends Food Sci. Technol. 2015, 46, 144–157. [Google Scholar] [CrossRef]
- Ryu, V.; McClements, D.J.; Corradini, M.; McLandsborough, L. Effect of ripening inhibitor type on formation, stability, and antimicrobial activity of thyme oil nanoemulsion. Food Chem. 2018, 205, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Witayaudom, P.; Klinkesorn, U. Effect of surfactant concentration and solidification temperature on the characteristics and stability of nanostructured lipid carrier (NLC) prepared from rambutan (Nephelium lappaceum L.) kernel fat. J. Colloid Interface Sci. 2017, 505, 1082–1092. [Google Scholar] [CrossRef] [PubMed]
- Boruah, M.; Sarma, A. Dry gels: Concept, current trends, and new avenues in drug delivery and biomedical application. Adv. Healthc. Mater. 2025; in press. [Google Scholar] [CrossRef]
- Boss, E.A.; Filho, R.M.; de Toledo, E.C.V. Freeze drying process: Real time model and optimization. Chem. Eng. Process Process Intensif. 2004, 43, 1475–1485. [Google Scholar] [CrossRef]
- Yu, J.Y.; Chuesiang, P.; Shin, G.H.; Park, H.J. Post-processing techniques for the improvement of liposome stability. Pharmaceutics 2021, 13, 1023. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols-a review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Özkan, G.; Bilek, S.E. Microencapsulation of natural food colourants. Int. J. Nutr. Food Sci. 2014, 3, 145–156. [Google Scholar] [CrossRef]
- Pudziuvelyte, L.; Marksa, M.; Jakstas, V.; Ivanauskas, L.; Kopustinskiene, D.M.; Bernatoniene, J. Microencapsulation of Elsholtzia ciliata herb ethanolic extract by spray-drying: Impact of resistant-maltodextrin complemented with sodium caseinate, skim milk, and beta-cyclodextrin on the quality of spray-dried powders. Molecules 2019, 24, 1461. [Google Scholar] [CrossRef]
- Preis, M.; EcKert, C.; Häusler, O.; Breitkreutz, J. A comparative study on solubilizing and taste-masking capacities of hydroxypropyl-β-cyclodextrin and maltodextrins with high amylose content. Sens. Actuators B 2014, 193, 442–450. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, P.; Wang, J.; Wu, Y.; Han, Y.; Zhou, J. Combining various wall materials for encapsulation of blueberry anthocyanin extracts: Optimization by artificial neural network and genetic algorithm and a comprehensive analysis of anthocyanin powder properties. Powder Technol. 2017, 311, 77–87. [Google Scholar] [CrossRef]
- Kharat, M.; McClements, D.J. Fabrication and characterization of nanostructured lipid carriers (NLC) using a plant-based emulsifier: Quillaja saponin. Food Res. Int. 2019, 126, 108601. [Google Scholar] [CrossRef]
- Elversson, J.; Millqvist-Fureby, A.; Alderborn, G.; Elofsson, U. Droplet and particle size relationship and shell thickness of inhalable lactose particles during spray drying. J. Pharm. Sci. 2003, 92, 900–910. [Google Scholar] [CrossRef]
- Pasrija, D.; Ezhilarasi, P.N.; Indrani, D.; Anandharamakrishnan, C. Microencapsulation of green tea polyphenols and its effect on incorporated bread quality. LWT 2015, 64, 289–296. [Google Scholar] [CrossRef]
- Pellicer, J.A.; Fortea, M.I.; Trabal, J.; Rodríguez-López, M.I.; Gabaldón, J.A.; Núnez-Delicado, E. Stability of microencapsulated strawberry flavour by spray drying, freeze drying and fluid bed. Powder Technol. 2019, 347, 179–185. [Google Scholar] [CrossRef]
- Quispe-Condori, S.; Saldaña, M.D.A.; Temelli, F. Microencapsulation of flax oil with zein using spray and freeze drying. LWT 2011, 44, 1880–1887. [Google Scholar] [CrossRef]
- Klinkesorn, U.; Sophanodora, P.; Chinachoti, P.; Decker, E.A.; McClements, D.J. Characterization of spray-dried tuna oil emulsified in two-layered interfacial membranes prepared using electrostatic layer-by-layer deposition. Food Res. Int. 2006, 39, 449–457. [Google Scholar] [CrossRef]
- Haas, K.; Obernberger, J.; Zehetner, E.; Kiesslich, A.; Volkert, M.; Jaeger, H. Impact of powder particle structure on the oxidation stability and color of encapsulated crystalline and emulsified carotenoids in carrot concentrate powders. J. Food Eng. 2019, 263, 398–408. [Google Scholar] [CrossRef]
- Lund, E.D.; Dinsmore, H.L. Determination of citrus volatiles by headspace analysis. In Analysis of Foods and Beverages; Charalambous, G., Ed.; Academic Press: London, UK; New York, NY, USA, 1978; pp. 135–185. [Google Scholar]
- Mahanta, B.P.; Bora, P.K.; Kemprai, P.; Borah, G.; Lal, M.; Haldar, S. Thermolabile essential oils, aromas and flavours: Degradation pathways, effect of thermal processing and alteration of sensory quality. Food Res. Int. 2021, 145, 110404. [Google Scholar] [CrossRef]
- Zhang, L.; Hayes, D.G.; Chen, G.; Zhong, Q. Transparent dispersions of milk-fat-based nanostructured lipid carriers for delivery of β-carotene. J. Agric. Food Chem. 2013, 61, 9435–9443. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, Y.; Ye, Q.; Baker, B.; Selomulya, C. Enhancing volatile retention and storage stability for encapsulated raspberry flavour powder. Food Chem. 2025, 481, 144005. [Google Scholar] [CrossRef] [PubMed]
- Rudbäck, J.; Bergström, M.A.; Börje, A.; Nilsson, U.; Karlberg, A.T. α-Terpinene, an antioxidant in tea tree oil, autoxidizes rapidly to skin allergens on air exposure. Chem. Res. Toxicol. 2012, 25, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Purushothaman, D.N.; Ravi, S. GC-MS analysis of essential oil obtained from Heracleum candolleanum (Wight et Arn). J. Pharm. Res. 2013, 6, 155–157. [Google Scholar] [CrossRef]
- Hong, S.J.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Enhanced bioaccessibility and stability of iron through W/O/W double emulsion-based solid lipid nanoparticles and coating with water-soluble chitosan. Int. J. Biol. Macromol. 2022, 209, 895–903. [Google Scholar] [CrossRef]
- Tonon, R.V.; Grosso, C.R.F.; Hubinger, M.D. Influence of emulsion composition and inlet air temperature on the microencapsulation of flaxseed oil by spray drying. Food Res. Int. 2011, 44, 282–289. [Google Scholar] [CrossRef]
- Kim, D.; Lee, Y.Y.; Kim, H.J.; Choi, M.; Lee, S.; Kim, H.E.; Kim, E.; Jo, M.; Choi, Y.J. Enhanced storage and gastrointestinal stability of spray-dried whey protein emulsions with chitosan and gum Arabic. Int. J. Biol. Macromol. 2025, 299, 140260. [Google Scholar] [CrossRef]
- Jo, S.M.; Moon, H.S.; Hong, S.J.; Yoon, S.; Jeong, H.; Park, H.; Ban, Y.; Youn, M.Y.; Lee, Y.; Park, S.-S.; et al. Exploring flavor patterns in the peel of tangor: A new citrus variety based on electronic sensors and GC-MS/O. Food Chem. 2025, 485, 144415. [Google Scholar] [CrossRef]
- Li, Y.; Fei, C.; Mao, C.; Ji, D.; Gong, J.; Qin, Y.; Qu, L.; Zhang, W.; Bian, Z.; Su, L.; et al. Physicochemical parameters combined flash GC e-nose and artificial neural network for quality and volatile characterization of vinegar with different brewing techniques. Food Chem. 2022, 374, 131658. [Google Scholar] [CrossRef] [PubMed]



| Formulations | Average Particle Size (nm) | PDI |
|---|---|---|
| Y-NE | 37.36 ± 0.43 b | 0.24 ± 0.00 b |
| Y-NLC | 32.03 ± 0.46 a | 0.19 ± 0.00 a |
| Samples | D[4,3] (μm) | Span | Moisture Content (%) | Color Values | |||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | ∆E | ||||
| SDG-Y-NE/MD * | 11.76 ± 0.40 a | 1.69 ± 0.07 b | 2.80 ± 0.54 ab | 94.32 ± 0.07 de | −0.56 ± 0.06 e | 9.48 ± 0.28 b | 9.48 ± 0.28 b |
| SDG-Y-NE/MD/βCD | 11.84 ± 0.32 a | 1.49 ± 0.07 a | 2.72 ± 0.45 ab | 94.21 ± 0.41 d | −0.80 ± 0.03 d | 8.21 ± 0.04 a | 8.21 ± 0.04 a |
| SDG-Y-NLC/MD | 12.11 ± 0.15 a | 1.86 ± 0.03 c | 2.33 ± 0.46 a | 94.36 ± 0.17 de | −0.44 ± 0.06 f | 10.72 ± 0.10 d | 10.72 ± 0.10 d |
| SDG-Y-NLC/MD/βCD | 12.20 ± 0.18 a | 1.70 ± 0.08 b | 2.32 ± 0.23 a | 94.76 ± 0.34 e | −0.85 ± 0.02 d | 9.82 ± 0.12 c | 9.82 ± 0.12 c |
| FDG-Y-NE/MD | 18.59 ± 0.35 b | 2.49 ± 0.06 e | 3.31 ± 0.21 b | 92.15 ± 0.36 b | −1.66 ± 0.01 c | 14.29 ± 0.11 e | 14.29 ± 0.11 e |
| FDG-Y-NE/MD/βCD | 24.63 ± 0.11 c | 2.17 ± 0.02 d | 3.27 ± 0.11 b | 92.53 ± 0.25 b | −1.77 ± 0.03 b | 14.22 ± 0.21 e | 14.22 ± 0.21 e |
| FDG-Y-NLC/MD | 28.52 ± 0.22 d | 2.23 ± 0.02 d | 3.17 ± 0.33 b | 93.02 ± 0.01 c | −1.73 ± 0.02 b | 14.57 ± 0.13 f | 14.57 ± 0.13 f |
| FDG-Y-NLC/MD/βCD | 29.16 ± 0.40 e | 2.22 ± 0.00 d | 3.06 ± 0.10 b | 93.27 ± 0.27 c | −1.86 ± 0.03 a | 14.16 ± 0.11 e | 14.16 ± 0.11 e |
| Column | Drying Type | RT | Samples | Compound | Description | |||
|---|---|---|---|---|---|---|---|---|
| Y-NE/MD | Y-NE/MD/β-CD | Y-NLC/MD | Y-NLC/MD/β-CD | |||||
| MTX-5 | SD | 63.64 | 26,605 a ± 3901 | 15,987 a ± 2382 | 14,181 a ± 7895 | 13,273 a ± 4839 | Limonene | Citrus, Fruity, Lemon |
| FD | 118,644 b ± 6199 | 155,237 c ± 3572 | 141,951 c ± 15,350 | 138,551 c ± 20,194 | ||||
| SD | 66.37 | 2874 a ± 432 | 1573 a ± 245 | 1380 a ± 829 | 1259 a ± 439 | γ-Terpinene | Citrus, Fruity | |
| FD | 15,349 b ± 977 | 19,751 c ± 568 | 18,383 c ± 1872 | 18,032 c ± 1932 | ||||
| SD | 67.95 | 600 a ± 52 | 375 a ± 44 | 359 a ± 161 | 308 a ± 90 | Linalool | Floral, Fruity, Lemon | |
| FD | 3231 b ± 1304 | 2703 b ± 171 | 2567 b ± 319 | 2697 b ± 1046 | ||||
| MTX-1701 | SD | 59.49 | 859 a ± 123 | 527 a ± 98 | 475 a ± 264 | 471 a ± 161 | Myrcene | Ethereal, Fruity, Geranium |
| FD | 59.49 | 3993 b ± 129 | 5344 c ± 680 | 4722 b ± 1053 | 4729 b ± 582 | |||
| SD | 60.96 | 301 a ± 94 | 213 a ± 86 | 220 a ± 125 | 209 a ± 105 | p-Cymene | Citrus, Fruity | |
| FD | 60.96 | 1288 ab ± 19 | 1597 ab ± 125 | 1557 ab ± 181 | 1499 b ± 117 | |||
| SD | 63.34 | 17,362 a ± 12537 | 15,957 a ± 2440 | 14,238 a ± 7755 | 13,416 a ± 4970 | Limonene | Citrus, Fruity, Orange | |
| FD | 63.34 | 118,128 b ± 6259 | 154,519 d ± 3632 | 141,382 cd ± 15,306 | 137,940 c ± 20,286 | |||
| SD | 66.28 | 3283 a ± 469 | 1906 a ± 337 | 1737 a ± 854 | 1618 a ± 714 | Terpinolene | Fruity, Herbaceous, Pine | |
| FD | 66.28 | 16,558 b ± 1245 | 21,163 c ± 558 | 19,721 c ± 1999 | 19,244 c ± 2038 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.A.; Chuesiang, P.; Kim, J.T.; Shin, G.H. Comparison of Flavor Stability of Yuja (Citrus junos Tanaka) Oil-Based Nano-Carriers and Dried Gels. Gels 2025, 11, 751. https://doi.org/10.3390/gels11090751
Jung SA, Chuesiang P, Kim JT, Shin GH. Comparison of Flavor Stability of Yuja (Citrus junos Tanaka) Oil-Based Nano-Carriers and Dried Gels. Gels. 2025; 11(9):751. https://doi.org/10.3390/gels11090751
Chicago/Turabian StyleJung, Seo A., Piyanan Chuesiang, Jun Tae Kim, and Gye Hwa Shin. 2025. "Comparison of Flavor Stability of Yuja (Citrus junos Tanaka) Oil-Based Nano-Carriers and Dried Gels" Gels 11, no. 9: 751. https://doi.org/10.3390/gels11090751
APA StyleJung, S. A., Chuesiang, P., Kim, J. T., & Shin, G. H. (2025). Comparison of Flavor Stability of Yuja (Citrus junos Tanaka) Oil-Based Nano-Carriers and Dried Gels. Gels, 11(9), 751. https://doi.org/10.3390/gels11090751






