Tissue-like Fracture Toughness and Stress–Relaxation Ability in PVA-Agar-Based Hydrogels for Biomedical Applications
Abstract
1. Introduction
2. Results and Discussion
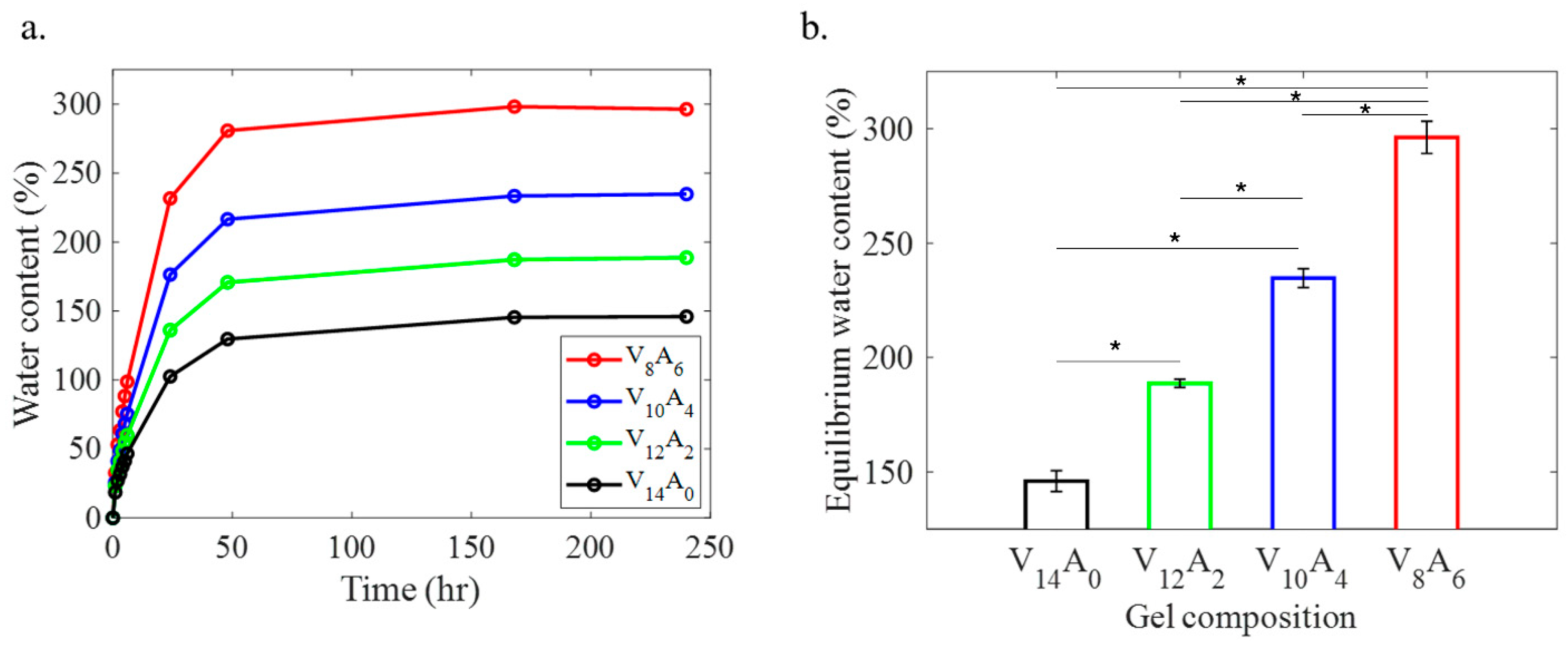
2.1. Swelling Behavior
2.2. Mechanical Properties
2.2.1. Tension and Compression Behavior
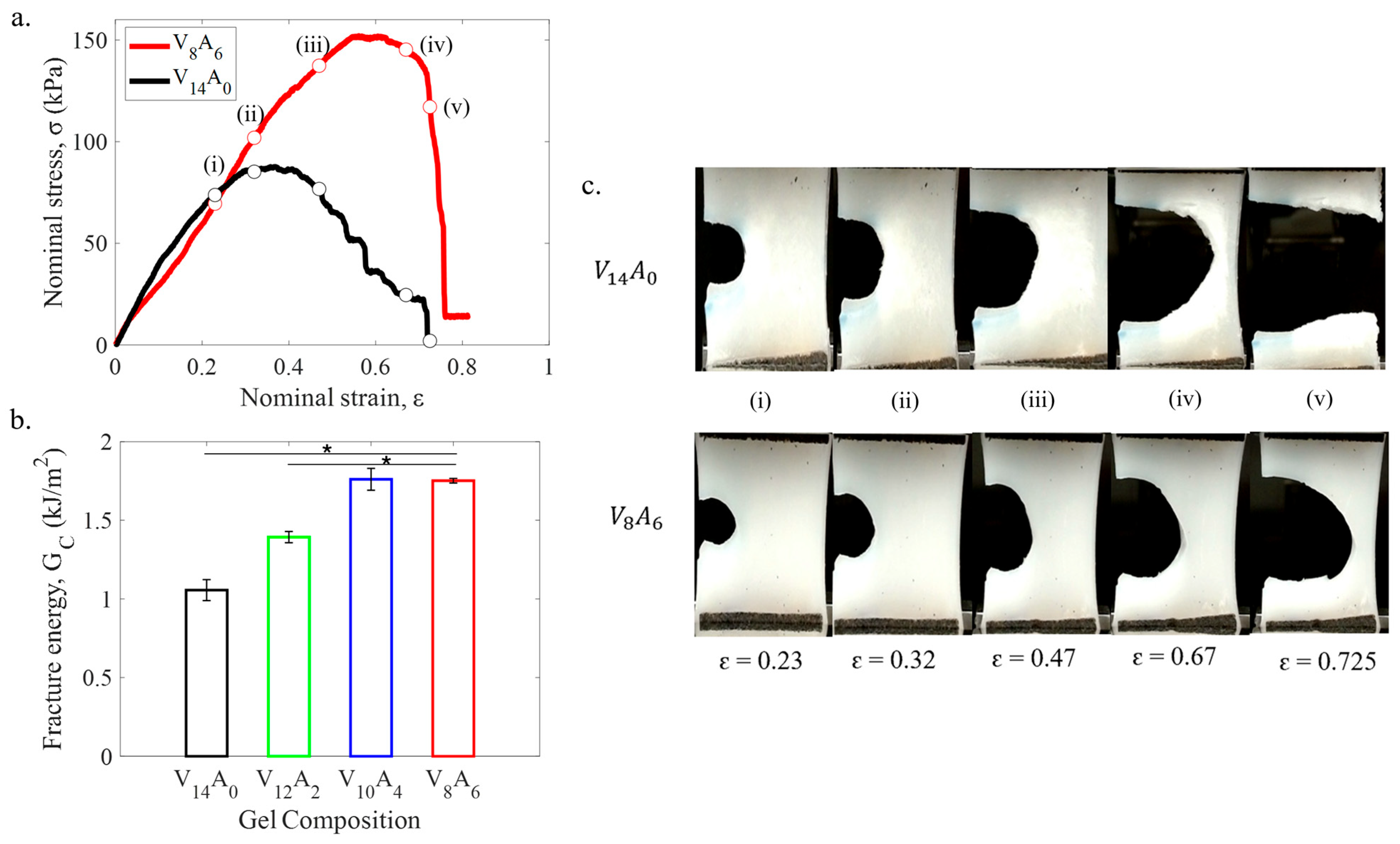
2.2.2. Fracture Behavior
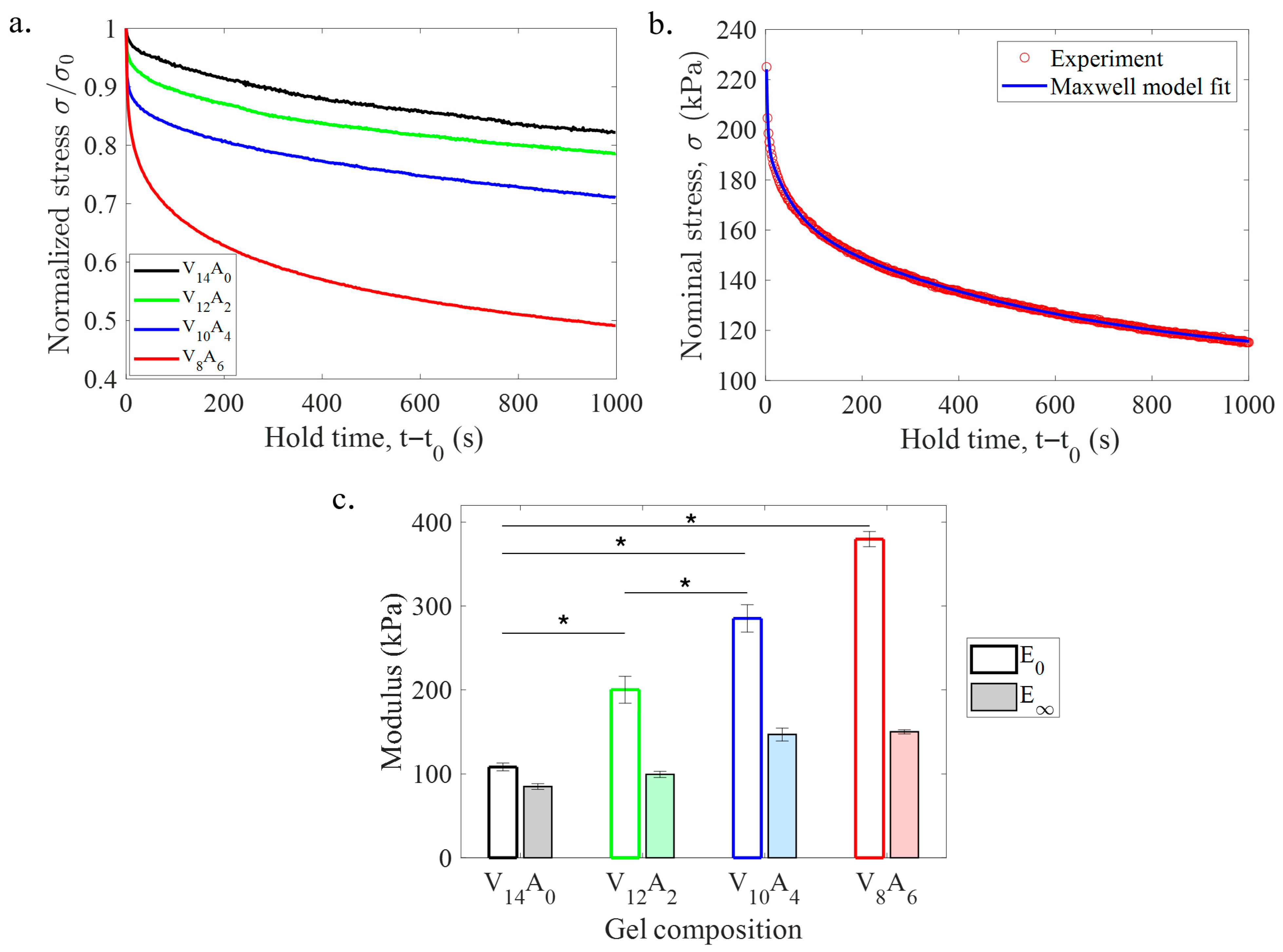
2.2.3. Stress–Relaxation Behavior
2.3. Cytotoxicity
2.4. Discussion
3. Conclusions
4. Materials and Methods
4.1. Hydrogel Fabrication
4.2. Swelling Study
4.3. Mechanical Testing
4.3.1. Compression and Tension Tests
4.3.2. Fracture Tests
4.3.3. Stress–Relaxation Tests
4.4. Cytotoxicity Test
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oyen, M.L. Mechanical characterisation of hydrogel materials. Int. Mater. Rev. 2014, 59, 44–59. [Google Scholar] [CrossRef]
- Simha, N.; Carlson, C.; Lewis, J. Evaluation of fracture toughness of cartilage by micropenetration. J. Mater. Sci. Mater. Med. 2004, 15, 631–639. [Google Scholar] [CrossRef]
- Taylor, D.; O’Mara, N.; Ryan, E.; Takaza, M.; Simms, C. The fracture toughness of soft tissues. J. Mech. Behav. Biomed. Mater. 2012, 6, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.; Gaillard, E.; Mongrain, R.; Reiter, S.; Tardif, J.-C. Characterization of fracture toughness exhaustion in pig aorta. J. Mech. Behav. Biomed. Mater. 2013, 17, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Virag, J.; Oyen, M.L. Micromechanical poroelastic and viscoelastic properties of ex-vivo soft tissues. J. Biomech. 2020, 113, 110090. [Google Scholar] [CrossRef]
- Wang, C.C.; Hung, C.T.; Mow, V.C. An analysis of the effects of depth-dependent aggregate modulus on articular cartilage stress-relaxation behavior in compression. J. Biomech. 2001, 34, 75–84. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Brandl, F.; Sommer, F.; Goepferich, A. Rational design of hydrogels for tissue engineering: Impact of physical factors on cell behavior. Biomaterials 2007, 28, 134–146. [Google Scholar] [CrossRef]
- De Leon-Oliva, D.; Boaru, D.L.; Perez-Exposito, R.E.; Fraile-Martinez, O.; García-Montero, C.; Diaz, R.; Bujan, J.; García-Honduvilla, N.; Lopez-Gonzalez, L.; Álvarez-Mon, M. Advanced hydrogel-based strategies for enhanced bone and cartilage regeneration: A comprehensive review. Gels 2023, 9, 885. [Google Scholar] [CrossRef]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-based hydrogels applied in drug delivery: An overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef]
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Versatility of hydrogels: From synthetic strategies, classification, and properties to biomedical applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between structure and rheology of hydrogels for various applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef] [PubMed]
- Surowiecka, A.; Strużyna, J.; Winiarska, A.; Korzeniowski, T. Hydrogels in burn wound management—A review. Gels 2022, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Liu, Z. Revealing novel insights into the toughening mechanism of double network hydrogels via uniaxial tensile tests. J. Mech. Phys. Solids 2024, 190, 105710. [Google Scholar] [CrossRef]
- Song, R.; Wang, X.; Johnson, M.; Milne, C.; Lesniak-Podsiadlo, A.; Li, Y.; Lyu, J.; Li, Z.; Zhao, C.; Yang, L. Enhanced strength for double network hydrogel adhesive through cohesion-adhesion balance. Adv. Funct. Mater. 2024, 34, 2313322. [Google Scholar] [CrossRef]
- Nonoyama, T.; Gong, J.P. Double-network hydrogel and its potential biomedical application: A review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2015, 229, 853–863. [Google Scholar] [CrossRef]
- Gong, J.P. Why are double network hydrogels so tough? Soft Matter 2010, 6, 2583–2590. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Haque, M.A.; Kurokawa, T.; Gong, J.P. Super tough double network hydrogels and their application as biomaterials. Polymer 2012, 53, 1805–1822. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Zhao, X.; Illeperuma, W.R.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Suo, Z.; Vlassak, J.J. Stiff, strong, and tough hydrogels with good chemical stability. J. Mater. Chem. B 2014, 2, 6708–6713. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Rahbar, N.; Calvert, P.D. Strong fiber-reinforced hydrogel. Acta Biomater. 2013, 9, 5313–5318. [Google Scholar] [CrossRef]
- Dubey, N.; Ferreira, J.A.; Daghrery, A.; Aytac, Z.; Malda, J.; Bhaduri, S.B.; Bottino, M.C. Highly tunable bioactive fiber-reinforced hydrogel for guided bone regeneration. Acta Biomater. 2020, 113, 164–176. [Google Scholar] [CrossRef]
- Yi, J.; Choe, G.; Park, J.; Lee, J.Y. Graphene oxide-incorporated hydrogels for biomedical applications. Polym. J. 2020, 52, 823–837. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Xu, C.; Li, Y.; Gao, J.; Wang, W.; Liu, Y. High strength graphene oxide/polyvinyl alcohol composite hydrogels. J. Mater. Chem. 2011, 21, 10399–10406. [Google Scholar] [CrossRef]
- Argun, A.; Can, V.; Altun, U.; Okay, O. Nonionic double and triple network hydrogels of high mechanical strength. Macromolecules 2014, 47, 6430–6440. [Google Scholar] [CrossRef]
- Li, X.; Tang, C.; Liu, D.; Yuan, Z.; Hung, H.C.; Luozhong, S.; Gu, W.; Wu, K.; Jiang, S. High-strength and nonfouling zwitterionic triple-network hydrogel in saline environments. Adv. Mater. 2021, 33, 2102479. [Google Scholar] [CrossRef]
- Islam, M.; Oyen, M. Load-relaxation characteristics of chemical and physical hydrogels as soft tissue mimics. Exp. Mech. 2021, 61, 939–949. [Google Scholar] [CrossRef]
- Zhao, X.; Huebsch, N.; Mooney, D.J.; Suo, Z. Stress-relaxation behavior in gels with ionic and covalent crosslinks. J. Appl. Phys. 2010, 107, 063509. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Gu, L.; Klumpers, D.; Darnell, M.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Lee, H.-p.; Lippens, E.; Duda, G.N. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2016, 15, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Richardson, B.M.; Anseth, K.S. Dynamic covalent hydrogels as biomaterials to mimic the viscoelasticity of soft tissues. Prog. Mater. Sci. 2021, 120, 100738. [Google Scholar] [CrossRef]
- Islam, M.R.; Oyen, M.L. Mechanical characterization of hydrogels. In The Mechanics of Hydrogels; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–24. [Google Scholar]
- Adelnia, H.; Ensandoost, R.; Moonshi, S.S.; Gavgani, J.N.; Vasafi, E.I.; Ta, H.T. Freeze/thawed polyvinyl alcohol hydrogels: Present, past and future. Eur. Polym. J. 2022, 164, 110974. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and morphology of freeze/thawed PVA hydrogels. Macromolecules 2000, 33, 2472–2479. [Google Scholar] [CrossRef]
- Subhash, G.; Liu, Q.; Moore, D.; Ifju, P.; Haile, M. Concentration dependence of tensile behavior in agarose gel using digital image correlation. Exp. Mech. 2011, 51, 255–262. [Google Scholar] [CrossRef]
- Sabzi, M.; Samadi, N.; Abbasi, F.; Mahdavinia, G.R.; Babaahmadi, M. Bioinspired fully physically cross-linked double network hydrogels with a robust, tough and self-healing structure. Mater. Sci. Eng. C 2017, 74, 374–381. [Google Scholar]
- Sun, X.; Luo, C.; Luo, F. Preparation and properties of self-healable and conductive PVA-agar hydrogel with ultra-high mechanical strength. Eur. Polym. J. 2020, 124, 109465. [Google Scholar]
- Li, H.; Wu, C.-w.; Wang, S.; Zhang, W. Mechanically strong poly (vinyl alcohol) hydrogel with macropores and high porosity. Mater. Lett. 2020, 266, 127504. [Google Scholar] [CrossRef]
- Weizel, A.; Distler, T.; Schneidereit, D.; Friedrich, O.; Bräuer, L.; Paulsen, F.; Detsch, R.; Boccaccini, A.; Budday, S.; Seitz, H. Complex mechanical behavior of human articular cartilage and hydrogels for cartilage repair. Acta Biomater. 2020, 118, 113–128. [Google Scholar] [CrossRef]
- Wheatley, B.B.; Fischenich, K.M.; Button, K.D.; Haut, R.C.; Donahue, T.L.H. An optimized transversely isotropic, hyper-poro-viscoelastic finite element model of the meniscus to evaluate mechanical degradation following traumatic loading. J. Biomech. 2015, 48, 1454–1460. [Google Scholar] [CrossRef] [PubMed]
- Mattice, J.M.; Lau, A.G.; Oyen, M.L.; Kent, R.W. Spherical indentation load-relaxation of soft biological tissues. J. Mater. Res. 2006, 21, 2003–2010. [Google Scholar] [CrossRef]
- Webber, R.E.; Creton, C.; Brown, H.R.; Gong, J.P. Large strain hysteresis and mullins effect of tough double-network hydrogels. Macromolecules 2007, 40, 2919–2927. [Google Scholar] [CrossRef]
- Armisen, R.; Gaiatas, F. Agar. In Handbook of Hydrocolloids; Elsevier: Amsterdam, The Netherlands, 2009; pp. 82–107. [Google Scholar]
- Rivlin, R.S.; Thomas, A.G. Rupture of rubber. I. Characteristic energy for tearing. J. Polym. Sci. 1953, 10, 291–318. [Google Scholar] [CrossRef]
- Tschoegl, N.W. The Phenomenological Theory of Linear Viscoelastic Behavior: An Introduction; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamas, I., Jr.; Chandrashekar, B.L.; Biguetti, C.C.; Islam, M.R. Tissue-like Fracture Toughness and Stress–Relaxation Ability in PVA-Agar-Based Hydrogels for Biomedical Applications. Gels 2025, 11, 747. https://doi.org/10.3390/gels11090747
Lamas I Jr., Chandrashekar BL, Biguetti CC, Islam MR. Tissue-like Fracture Toughness and Stress–Relaxation Ability in PVA-Agar-Based Hydrogels for Biomedical Applications. Gels. 2025; 11(9):747. https://doi.org/10.3390/gels11090747
Chicago/Turabian StyleLamas, Ismael, Jr., Bhuvana L. Chandrashekar, Claudia C. Biguetti, and Mohammad R. Islam. 2025. "Tissue-like Fracture Toughness and Stress–Relaxation Ability in PVA-Agar-Based Hydrogels for Biomedical Applications" Gels 11, no. 9: 747. https://doi.org/10.3390/gels11090747
APA StyleLamas, I., Jr., Chandrashekar, B. L., Biguetti, C. C., & Islam, M. R. (2025). Tissue-like Fracture Toughness and Stress–Relaxation Ability in PVA-Agar-Based Hydrogels for Biomedical Applications. Gels, 11(9), 747. https://doi.org/10.3390/gels11090747







