Novel Approaches for the 3D Printing of Collagen-Sourced Biomaterials Against Infectious and Cardiovascular Diseases
Abstract
1. Introduction
2. Preparation of Collagen and General Usage
2.1. Major Resources of Collagen
2.2. Extraction Techniques for Collagen
2.3. Artificial Intelligence (AI)-Based Tailoring and Optimization of Collagen
3. Application of Collagen Against Infectious Diseases
3.1. Major Role of Collagen in Infectious Diseases
3.2. Major Role of Collagen in Cardiovascular Diseases
4. Hydrogel in 3D Printing and Tissue Regeneration
4.1. 3D Printing and Tissue Regeneration
4.2. Natural Polymer Hydrogels as 3D-Printing Bioinks
4.3. Collagen-Based Hydrogels as 3D-Printing Bioinks
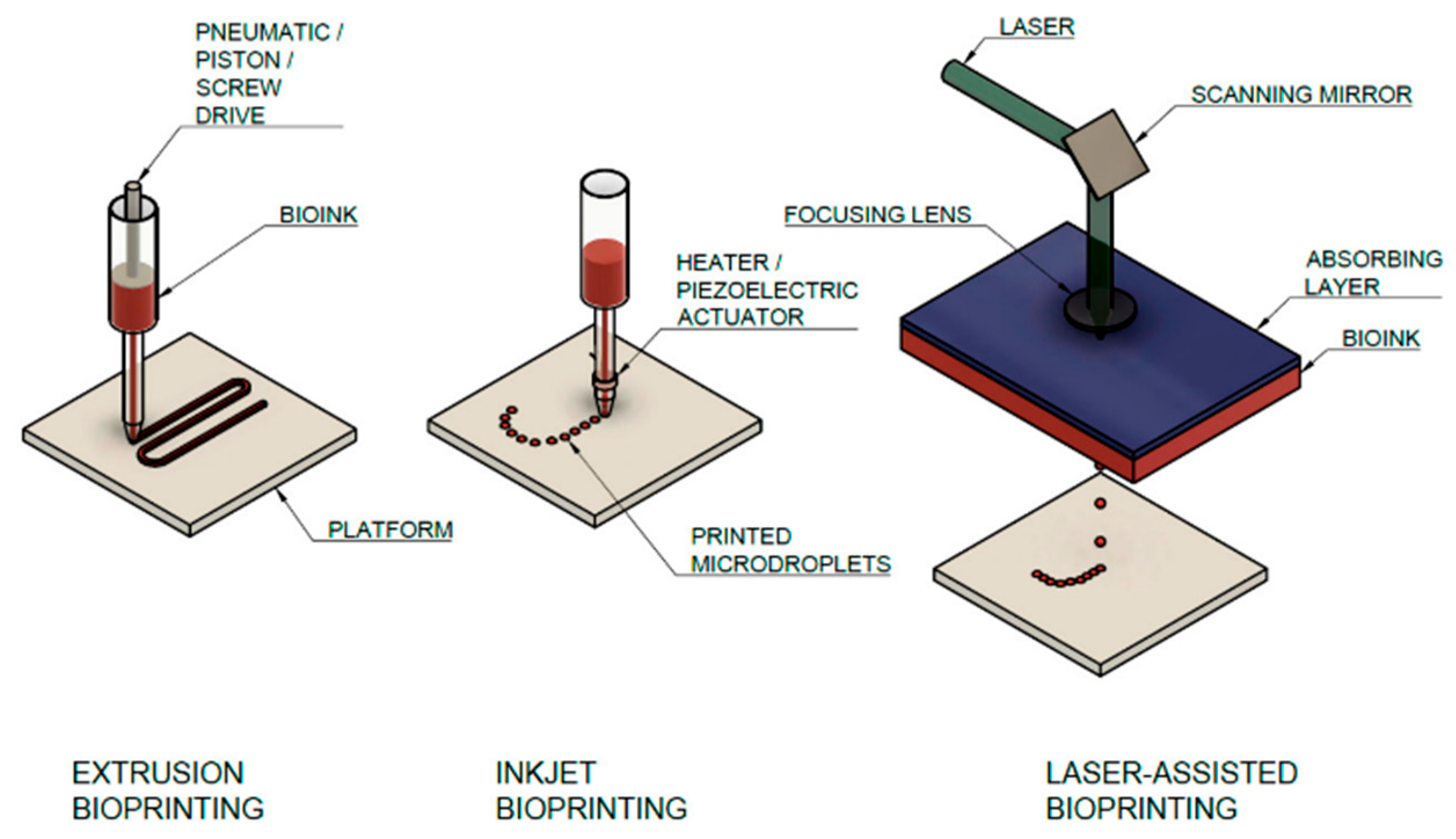
| Modality/Strategy | Strengths | Weakness | Applications | References |
|---|---|---|---|---|
| Extrusion bioprinting | Handles high-viscosity collagen blends | Lower resolution; shear stress | Lung epithelium models; vascular patches | [71] |
| Inkjet bioprinting | High throughput; multi-material | Requires low viscosity | Drug-screening microtissues | [58] |
| Laser-assisted (LAB) | High precision; gentle to cells | Cost, setup complexity | Patterned myocardium, Endothelial lattices | [14] |
| Light-based (SLA/DLP) | Fine features, rapid curing | Photo-initiator cytotoxicity | Microvasculature, cardiac valves | [14] |
| Collagen + chitosan | Printability, barrier mimicry | Batch variability | Lung tissue infection models | [71] |
| Collagen + hyaluronic acid | Angiogenesis, ECM-like | Needs crosslinking for strength | Cardiac/wound regeneration | [69] |
| Collagen + gelatin (GelMA) | Tunable gelation; cell adhesion | Thermosensitive | Myocardial patches, skin | [70] |
| Smart additives (e.g., black phosphorus) | 4D stimuli-responsive, adaptive | Potential safety issue | 4D bioinks for remodeling | [72] |
4.4. Collagen in 3D Printing Against Infectious Diseases
4.5. Collagen in 3D Printing Against Cardiovascular Diseases
4.6. Bioavailability of the 3D-Printed Collagen Products
5. Challenges and Future Directions
Funding
Data Availability Statement
Conflicts of Interest
References
- Duval, K.; Grover, H.; Han, L.H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling physiological events in 2D vs. 3D cell culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Veerubhotla, R.; Clark, J. 3D printing for regenerative medicine: Technologies and tools for clinical translation. Expert Opin. Biol. Ther. 2021, 21, 621–633. [Google Scholar]
- Veerubhotla, R.; Tandon, B.; Clark, J. Role of artificial intelligence and quantum computing in bioengineering: Advances and future applications. Biogen. Transl. Med. 2022, 7, e10280. [Google Scholar]
- Yi, C.; Sun, X.; Lin, Y.; Gu, C.; Ding, L.; Lu, X.; Fan, H. COVID-19: What has been learned and to be learned about the novel coronavirus disease. Int. J. Biol. Sci. 2021, 17, 1497–1506. [Google Scholar] [CrossRef]
- Lee, W.; Debasitis, J.C.; Lee, V.K.; Lee, J.H.; Fischer, K.; Edminster, K.; Yoo, S.S. Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication. Biomaterials 2016, 32, 1204–1211. [Google Scholar] [CrossRef]
- Zimmerling, A.; Chen, X. 3D bioprinting for skin tissue engineering: Current status and perspectives. J. Tissue Eng. 2020, 11, 2041731420985256. [Google Scholar]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Marques, C.F.; Diogo, G.S.; Pina, S.; Oliveira, J.M.; Reis, R.L. Collagen-based bioinks for hard tissue engineering applications: A comprehensive review. J. Mater. Sci. Mater. Med. 2019, 30, 32. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.; Bhowmick, S.; Das, A. Emerging trends in collagen-based biomaterials for soft and hard tissue engineering. Tissue Eng. Regen. Med. 2025, 17, 204. [Google Scholar]
- Wang, X.; Zhang, Y.; He, J.; Liu, M.; Li, J. Recent advances of collagen-based bioinks in 3D bioprinting. Biomater. Sci. 2023, 11, 1158–1176. [Google Scholar]
- Cavallo, C.; Desando, G.; Martini, L.; Roffi, A.; Zini, N.; Bartolotti, I.; Grigolo, B. Collagen-based bioinks for 3D bioprinting: Advances and applications in regenerative medicine. J. Clin. Med. 2023, 12, 777. [Google Scholar]
- Vidler, T.; Jayasuriya, S.; Atif, A. Light-assisted 3D printing technologies for biomedical applications: A review. Biofabrication 2024, 16, 023001. [Google Scholar]
- Advanced Biomatrix Report. Available online: https://advancedbiomatrix.com (accessed on 28 July 2025).
- Stepanovska, J.; Stachurova, T.; Raska, M. Bioink development for 3D bioprinting: A biomaterial perspective. Materials 2021, 14, 5283. [Google Scholar]
- Allan, J.T.; Watt, F.M. Influence of extracellular matrix composition and organization on cell shape, cytoskeleton, and adhesion. Nat. Cell Biol. 2001, 3, E256–E259. [Google Scholar]
- Long, C.; Li, X.; Zhang, Y.; Zhao, Y. Self-assembly of Type I collagen: Morphology regulation, molecular packing, and growth mechanism. Biomacromolecules 2015, 16, 2102–2111. [Google Scholar]
- Gauza-Wlodarczyk, M.; Kubisz, L.; Wlodarczyk, D. Amino acid composition in determining collagen type for the production of biomedical materials. Adv. Clin. Exp. Med. 2017, 26, 561–566. [Google Scholar]
- Drago, D.; Cossetti, C.; Iraci, N.; Gaude, E.; Musco, G.; Pluchino, S. The stem cell secretome and its role in brain repair. Biochimie 2014, 95, 2271–2285. [Google Scholar] [CrossRef]
- Brett, D. A review of collagen and collagen-based wound dressings. Wounds 2008, 20, 347–356. [Google Scholar]
- Liu, T.; Hao, Y.; Zhang, Z.; Zhou, H.; Peng, S.; Zhang, D.; Li, K.; Chen, Y.; Chen, M. Advanced Cardiac Patches for the Treatment of Myocardial Infarction. Circulation 2024, 149, 2002–2020. [Google Scholar] [CrossRef]
- Ponugoti, N.; Xu, F.; Nguyen, K.C.; Zhou, Q.; Zhang, X. Mechanical characterization of collagen-based scaffolds prepared from tendon and skin. Mater. Sci. Eng. C 2013, 33, 2623–2631. [Google Scholar]
- Coraca-Huber, D.C.; Hausdorfer, J.; Fille, M.; Steidl, M.; Nogler, M. Collagen from equine tendon for application in medicine and tissue engineering. Sci. World J. 2014, 2014, 817603. [Google Scholar]
- Moon, H.J.; Ko, D.Y.; Kim, J.; Jung, M.; Min, S.K.; Kim, J.H. Physical properties and biocompatibility of horse tendon collagen as a potential scaffold for tissue engineering. Biotechnol. Bioprocess Eng. 2015, 20, 1102–1110. [Google Scholar]
- Gaikwad, K.K.; Kim, M. Fish-derived collagen and its role in regenerative medicine: Recent insights and future directions. Mar. Drugs 2024, 22, 60. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, D.; Liu, J.; Xu, S.; Qiu, F.; Hu, L.; Liu, Y.; Ke, C.; Ruan, C. 4D printing polymeric biomaterials for adaptive tissue regeneration. Bioact. Mater. 2025, 22, 370–399. [Google Scholar] [CrossRef]
- Sakai, S.; Kawakami, K.; Hirose, M. Production of collagen scaffolds from tendon and their properties. J. Biomater. Appl. 2015, 30, 324–332. [Google Scholar]
- Zhou, Q.; Lu, Y. Structural differences in collagen from various mammalian tissues and their implications for biomedical use. Biomed. Mater. 2016, 11, 055007. [Google Scholar]
- Liu, J.; Xie, M.; Gao, Y.; Chen, X. Comparative analysis of marine and mammalian collagens: Mechanical properties and antibacterial performance. J. Biomed. Mater. Res. A 2025, 113, 987–998. [Google Scholar]
- Riley, G.P.; Herman, S.M. Extraction and solubilization of collagen from tendon tissue. Matrix Biol. 2005, 24, 131–139. [Google Scholar]
- Zhao, Y.; Zhang, K.; Wang, J. Enzymatic degradation of collagen: An insight into collagenase activity. J. Enzyme Inhib. Med. Chem. 2012, 27, 692–699. [Google Scholar]
- Franciosi, E.; Alessandri, S.; Mari, A.; Tursi, A. Enzyme-assisted extraction and characterization of collagen. Int. J. Biol. Macromol. 2007, 40, 255–261. [Google Scholar]
- Matsushita, O.; Koide, T.; Kobayashi, R.; Nagata, K.; Okabe, A.; Maeda, H. Substrate recognition and cleavage site of Clostridium histolyticum class I collagenase. J. Biol. Chem. 1994, 269, 5763–5768. [Google Scholar]
- Rawlings, N.D.; Barrett, A.J. Evolutionary families of metallopeptidases. Methods Enzymol. 1995, 248, 183–228. [Google Scholar] [PubMed]
- Srinivasan, R.; Kim, S.; Gupta, R. Artificial intelligence–driven optimization of collagen valorization from food and biomedical waste. Biomater. Adv. 2025, 142, 213876. [Google Scholar]
- Li, J.; Zhang, Y.; Zhang, W.; Liu, H. Angiogenesis in tissue engineering: From mechanistic understanding to advanced biofabrication. Adv. Healthc. Mater. 2021, 10, 2002300. [Google Scholar]
- Harder, D.R. AI in biomedical modeling: From mechanistic pathways to personalized medicine. Nat. Rev. Bioeng. 2023, 1, 15–27. [Google Scholar]
- Salvante, A.; Giacobazzi, R.; Martini, L.; Candiani, G. AI-assisted angiogenesis quantification on the CAM assay for scaffold evaluation. Mater. Sci. Eng. C 2024, 158, 114064. [Google Scholar]
- Masrouri, M.; Qin, Z. Towards data-efficient mechanical design of bicontinuous composites using generative AI. Theor. Appl. Mech. Lett. 2024, 141, 100492. [Google Scholar] [CrossRef]
- Singh, A.; Dwivedi, S.; Srivastava, R.; Verma, P. Collagen in infectious diseases: From barrier function to immune modulation. Front. Immunol. 2023, 14, 1210085. [Google Scholar]
- Myllyharju, J.; Kivirikko, K.I. Collagens, modifying enzymes, and their mutations in humans, flies, and worms. Trends Genet. 2001, 17, 33–41. [Google Scholar] [CrossRef]
- Arseni, L.; Lombardi, A.; Orioli, D. From collagen biosynthesis to collagenopathies: Challenges and opportunities. J. Biol. Chem. 2018, 293, 10418–10430. [Google Scholar]
- Zaarour, B.; Younes, I.; Rinaudo, M. Bioactive effects of hydrolyzed collagen on skin fibroblast proliferation. Mar. Drugs 2022, 20, 164. [Google Scholar]
- Ortiz-López, M.; Peredo-Escárcega, A.; de Jesús Delgado-López, D. The impact of marine-derived collagen on human keratinocyte function and wound healing. Tissue Eng. Part A 2022, 28, 25–35. [Google Scholar]
- Brandao-Rangel, E.; Nascimento, J.A.; Cruz, M.S. Anti-inflammatory properties of hydrolyzed collagen peptides in human cell cultures. Biomed. Pharmacother. 2022, 149, 112909. [Google Scholar]
- Wareham, L.K.; Baratta, R.O.; Del Buono, B.J.; Schlumpf, E.; Calkins, D.J. Collagen in the central nervous system: Contributions to neurodegeneration and promise as a therapeutic target. Mol. Neurodegener. 2024, 19, 11. [Google Scholar] [CrossRef]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen fragments and wound healing: From bench to bedside. Adv. Wound Care 2021, 10, 151–167. [Google Scholar]
- Tronci, G.; Russell, S.J.; Wood, D.J. Biomimetic approaches to designing collagen-based cardiovascular scaffolds. Front. Bioeng. Biotechnol. 2019, 7, 340. [Google Scholar]
- Harsha, V.K.; Brundha, M.P. Inflammatory mechanisms in cardiac tissue remodeling: A histopathological review. Res. J. Pharm. Technol. 2020, 13, 3840–3843. [Google Scholar]
- Konta, A.A.; García-Piña, M.; Serrano, D.R. Personalised 3D printed medicines: Which techniques and polymers are more successful? Bioengineering 2017, 4, 79. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2022, 7, 824–840. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Cui, F.Z.; Zhang, H.Y. Functionalized natural hydrogels for tissue engineering. Adv. Healthc. Mater. 2021, 10, 2001271. [Google Scholar]
- Ho, C.M.; Ng, S.H.; Li, K.H.H.; Yoon, Y.J. 3D printed hydrogels for tissue engineering applications. Adv. Drug Deliv. Rev. 2022, 174, 60–78. [Google Scholar]
- Moss, E.A.; Nanduri, M.; Collins, M.N. Hydrogel-based bioinks for 3D bioprinting in regenerative medicine. Adv. Mater. Interfaces 2025, 12, 2201645. [Google Scholar]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef]
- Lee, J.M.; Sing, S.L.; Zhou, M.; Yeong, W.Y. 3D bioprinting processes: A perspective on classification and terminology. Int. J. Bioprinting 2015, 1, 3–14. [Google Scholar] [CrossRef]
- Abelardo, E. Synthetic material bioinks. In 3D Bioprinting for Reconstructive Surgery; Thomas, D.J., Jessop, Z.M., Whitaker, I.S., Eds.; Woodhead Publishing: Cambridge, MA, USA, 2018; pp. 137–144. [Google Scholar]
- Veerubhotla, S.; Lee, Y. Smart collagen–alginate hydrogel: Fabrication and biomedical applications. Bioengineering 2022, 9, 615. [Google Scholar]
- Wong, K.H.K.; Chan, J.M.W.; Lo, A.C.Y. Fabrication of collagen-based hydrogel using salt-induced phase inversion for tissue engineering applications. Mater. Sci. Eng. C 2011, 31, 1003–1010. [Google Scholar]
- Norris, K.; Alvarez, Z.; Matson, J.B. Ultrasound-assisted formation of injectable collagen-based hydrogels for localized therapy. ACS Biomater. Sci. Eng. 2019, 5, 4070–4078. [Google Scholar]
- Jiang, T.; Xu, G.; Wang, Q.; Pan, H. Molecular self-assembly of collagen-mimetic hydrogels with tunable mechanical properties. Biomacromolecules 2019, 20, 1641–1650. [Google Scholar]
- Islam, M.M.; Fukunaga, Y.; Sato, M. Fabrication of biomimetic collagen scaffold and its application in tissue engineering. J. Biomed. Mater. Res. A 2015, 103, 2225–2233. [Google Scholar]
- Deng, C.; Li, X.; Zhang, L. Injectable hydrogel with enhanced angiogenesis and tissue remodeling for burn wound healing. Biomater. Sci. 2021, 9, 77–86. [Google Scholar]
- Jridi, M.; Abdelhedi, O.; Nasri, M. Composite hydrogel from fish skin collagen and polysaccharides: Application in wound healing. Colloids Surf. B 2015, 136, 133–142. [Google Scholar]
- Ying, Y.; Liu, Y.; Wu, Y. Collagen I-hydroxybenzoic acid hydrogel enhances vasculature and epithelial regeneration. ACS Omega 2019, 4, 17315–17325. [Google Scholar]
- Gao, Y.; Zhu, Y.; Zhang, Y. Injectable collagen–hyaluronic acid hydrogels for wound healing. Acta Biomater. 2018, 78, 63–73. [Google Scholar]
- Kim, B.S.; Kim, H.; Gao, G.; Cho, D.W. 3D bioprinting of functional tissue constructs using bioink based on gelatin and collagen. Biofabrication 2016, 8, 035027. [Google Scholar]
- Suo, H.; Wang, Y.; Chen, G. Collagen–chitosan hydrogel scaffolds for 3D printing of lung tissue constructs. Mater. Sci. Eng. C 2021, 127, 112223. [Google Scholar]
- Bai, Z.; Liu, W.; Wang, Y. Intelligent black phosphorus–collagen bioink for smart tissue regeneration. Adv. Funct. Mater. 2024, 34, 2308922. [Google Scholar]
- Yin, J.; Yan, M.; Wang, Y.; Fu, J.; Suo, H. 3D printing of low concentration alginate-based scaffolds for cell expansion and transplantation. Biofabrication 2018, 10, 045004. [Google Scholar]
- Shin, J.H.; Kang, H.W. The development of gelatin-based bio-ink for use in 3D hybrid bioprinting. Int. J. Precis. Eng. Manuf. 2018, 19, 767–771. [Google Scholar] [CrossRef]
- Delgado, L.M.; Bayon, Y.; Pandit, A.; Zeugolis, D.I. In vitro biological characterization of collagen scaffolds. J. Biomed. Mater. Res. B 2015, 103, 539–556. [Google Scholar]
- Adamiak, K.; Sionkowska, A. Current methods of collagen crosslinking: Review. Int. J. Biol. Macromol. 2020, 161, 550–560. [Google Scholar] [CrossRef]
- Fullana, S.G.; Torres-Giner, S.; Lagaron, J.M. Improved collagen nanofibers through blending with natural polysaccharides. Carbohydr. Polym. 2012, 89, 1220–1228. [Google Scholar]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2015, 356, eaaf3627. [Google Scholar] [CrossRef]
- Bhowmick, S.; Dinda, A.K.; Mandal, T.K. Lung tissue engineering: Recent developments and future prospects. Adv. Healthc. Mater. 2018, 7, 1700981. [Google Scholar]
- Hiller, M.; von der Helm, C.; Behr, J.M. Development of ready-to-use bioinks for diverse 3D bioprinting applications. Biofabrication 2018, 10, 044102. [Google Scholar]
- Hibino, N.; Duncan, D.R.; Nalbandian, A.; Shinoka, T. Challenges to myocardial tissue engineering: Cell delivery, biomaterial selection, and immunogenicity. Circ. J. 2017, 81, 1225–1233. [Google Scholar]
- Wei, Z.; Lei, M.; Wang, Y. Hydrogels with tunable mechanical plasticity regulate endothelial cell outgrowth in vasculogenesis and angiogenesis. Nat. Commun. 2023, 14, 8307. [Google Scholar] [CrossRef]
- Isaeva, E.V.; Beketov, E.E.; Demyashkin, G.A.; Yakovleva, N.D.; Arguchinskaya, N.V.; Kisel, A.A. Cartilage formation in vivo using high concentration collagen-based bioink with MSC and decellularized ECM granules. Int. J. Mol. Sci. 2022, 23, 2703. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ruan, C.; Niu, X. Collagen-based bioinks for regenerative medicine: Fabrication, application and prospective. Med. Nov. Technol. Devices 2023, 17, 100211. [Google Scholar] [CrossRef]
- Osidak, E.O.; Kozhukhov, V.I.; Osidak, M.S.; Domogatsky, S.P. Collagen as Bioink for Bioprinting: A Comprehensive Review. Int. J. Bioprint 2020, 6, 270. [Google Scholar] [CrossRef]
- Faruque, A.V.; Alam, S.; Biswas, K. Artificial intelligence-enhanced 3D bioprinting of collagen scaffolds for regenerative medicine. J. Biomed. Mater. Res. A 2023, 111, 987–1002. [Google Scholar]
- Shelke, N.B.; James, R.; Laurencin, C.T. AI-assisted control in collagen-based biofabrication: A pathway to real-time optimization. Adv. Healthc. Mater. 2024, 13, 2301567. [Google Scholar]
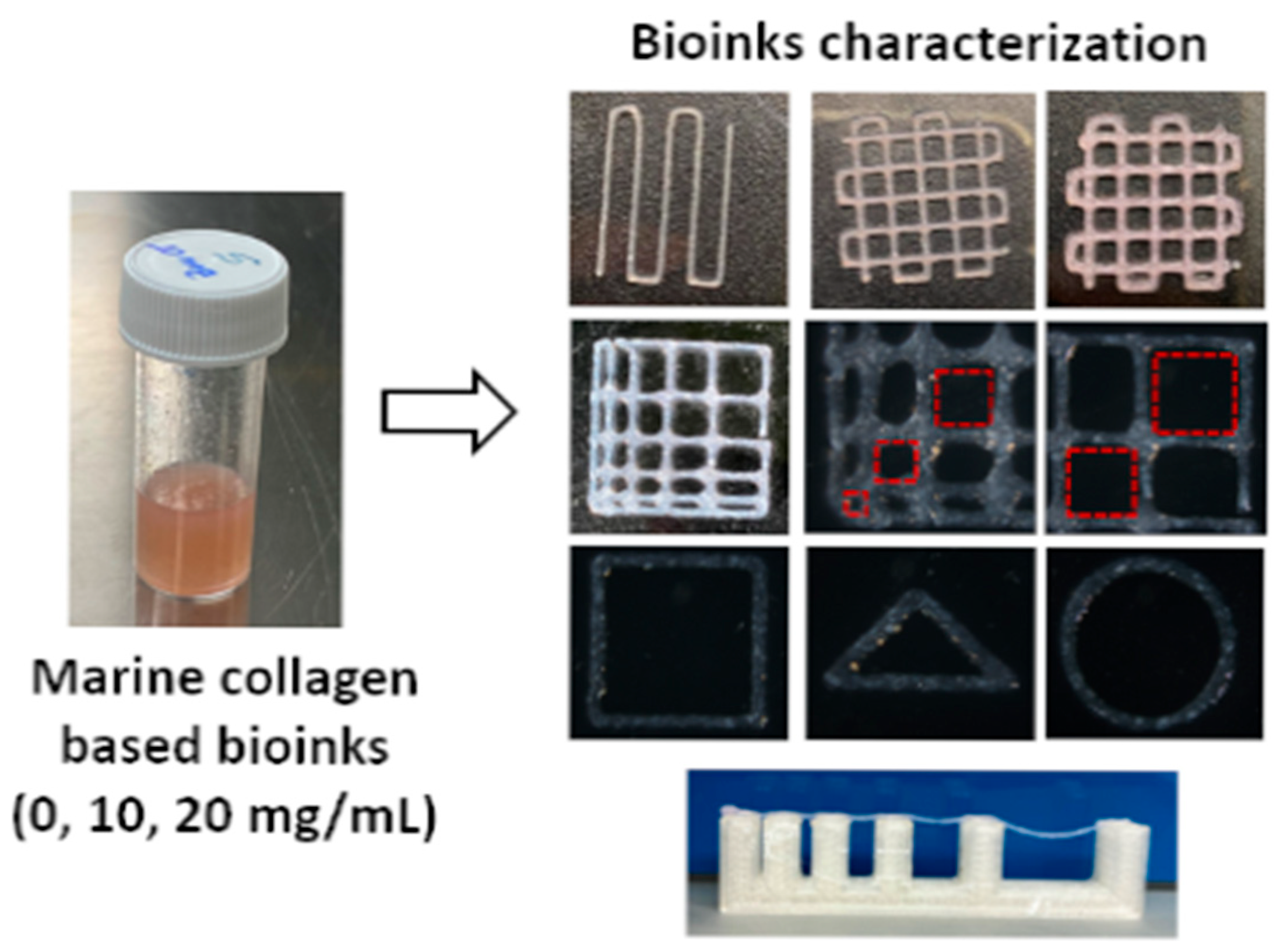
| Source | Dominant Type(s) | Key Attributes | Extraction Method | Pros/Cons | Major Usages | References |
|---|---|---|---|---|---|---|
| Bovine/Porcine Skin | I, III | Random fiber orientation; moderate strength | Acid-soluble or pepsin-assisted | Pros: readily available; cons: fast degradation, fibrillogenic | Wound dressings, Dermal scaffolds | [8,12,13,21,22] |
| Bovine/Porcine Tendon | I | Residual packing; high tensile strength | Pepsin-assisted | Pros: mechanical robustness; cons: sourcing issues | Load-bearing scaffolds, surgical meshes | [23] |
| Equine Tendon | I | High purity; slow degradation | Pepsin-assisted | Pros: biocompatibility; cons: limited supply | Regenerative implants, grafts | [24,25] |
| Fish Skin/Scales | I | Lower temp denaturation; bioactive peptides | Acid or mild enzymes | Pros: sustainable, by-product use; cons: lower strength, antibacterial gap | Wound care, nutraceuticals | [26] |
| Recombinant/Engineered | I variants | Tunable, batch consistency | Microbial/plant expression | Pros: customizable; cons: cost, yield | Advanced bioinks, disease models | [12,13,27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.; Lee, C.H. Novel Approaches for the 3D Printing of Collagen-Sourced Biomaterials Against Infectious and Cardiovascular Diseases. Gels 2025, 11, 745. https://doi.org/10.3390/gels11090745
Lee Y, Lee CH. Novel Approaches for the 3D Printing of Collagen-Sourced Biomaterials Against Infectious and Cardiovascular Diseases. Gels. 2025; 11(9):745. https://doi.org/10.3390/gels11090745
Chicago/Turabian StyleLee, Yugyung, and Chi H. Lee. 2025. "Novel Approaches for the 3D Printing of Collagen-Sourced Biomaterials Against Infectious and Cardiovascular Diseases" Gels 11, no. 9: 745. https://doi.org/10.3390/gels11090745
APA StyleLee, Y., & Lee, C. H. (2025). Novel Approaches for the 3D Printing of Collagen-Sourced Biomaterials Against Infectious and Cardiovascular Diseases. Gels, 11(9), 745. https://doi.org/10.3390/gels11090745







