Chitosan-Based Nanogels in Modern Drug Delivery: Focus on Protein and Gene Applications
Abstract
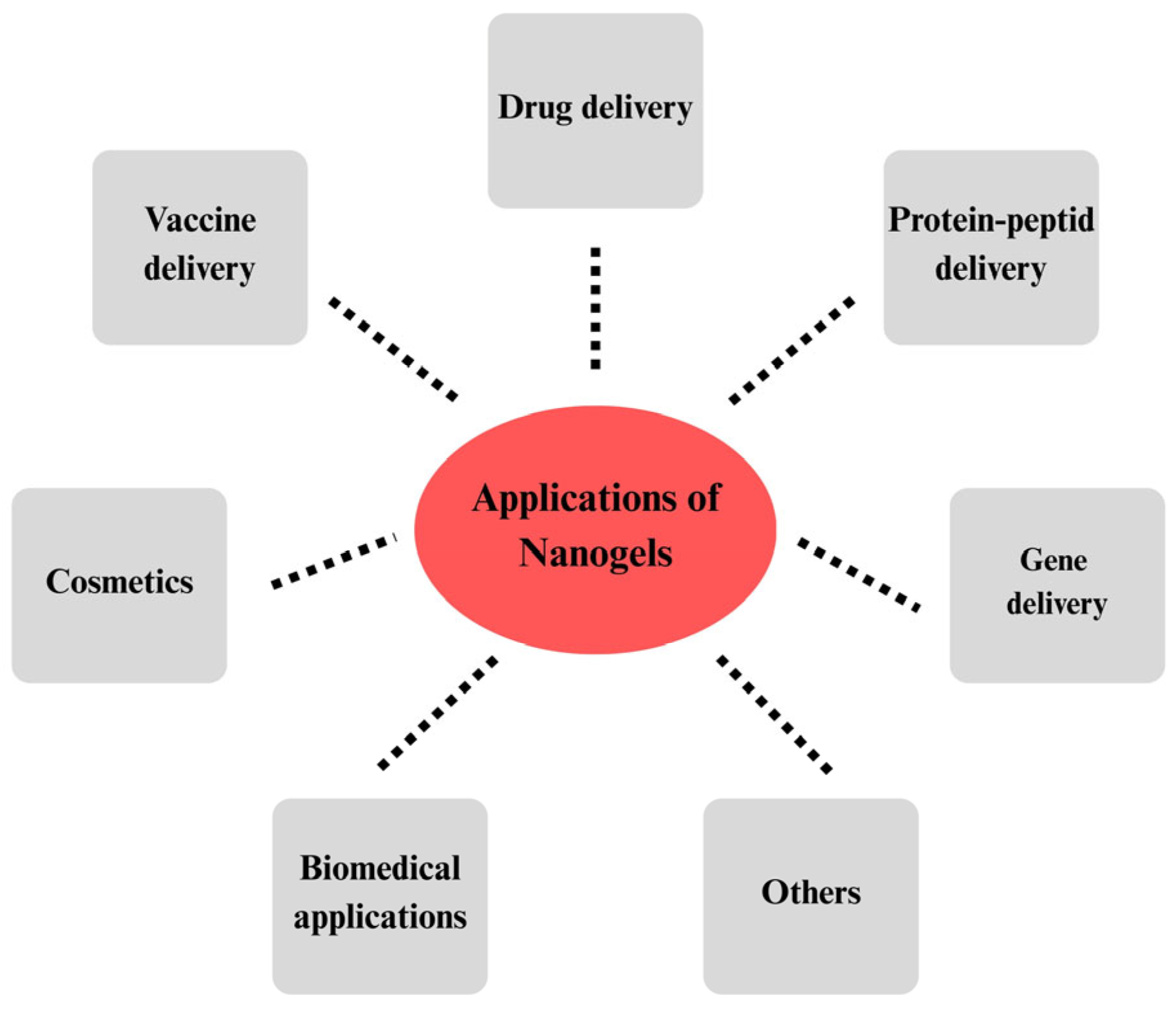
1. Introduction
2. Chitosan Nanogels
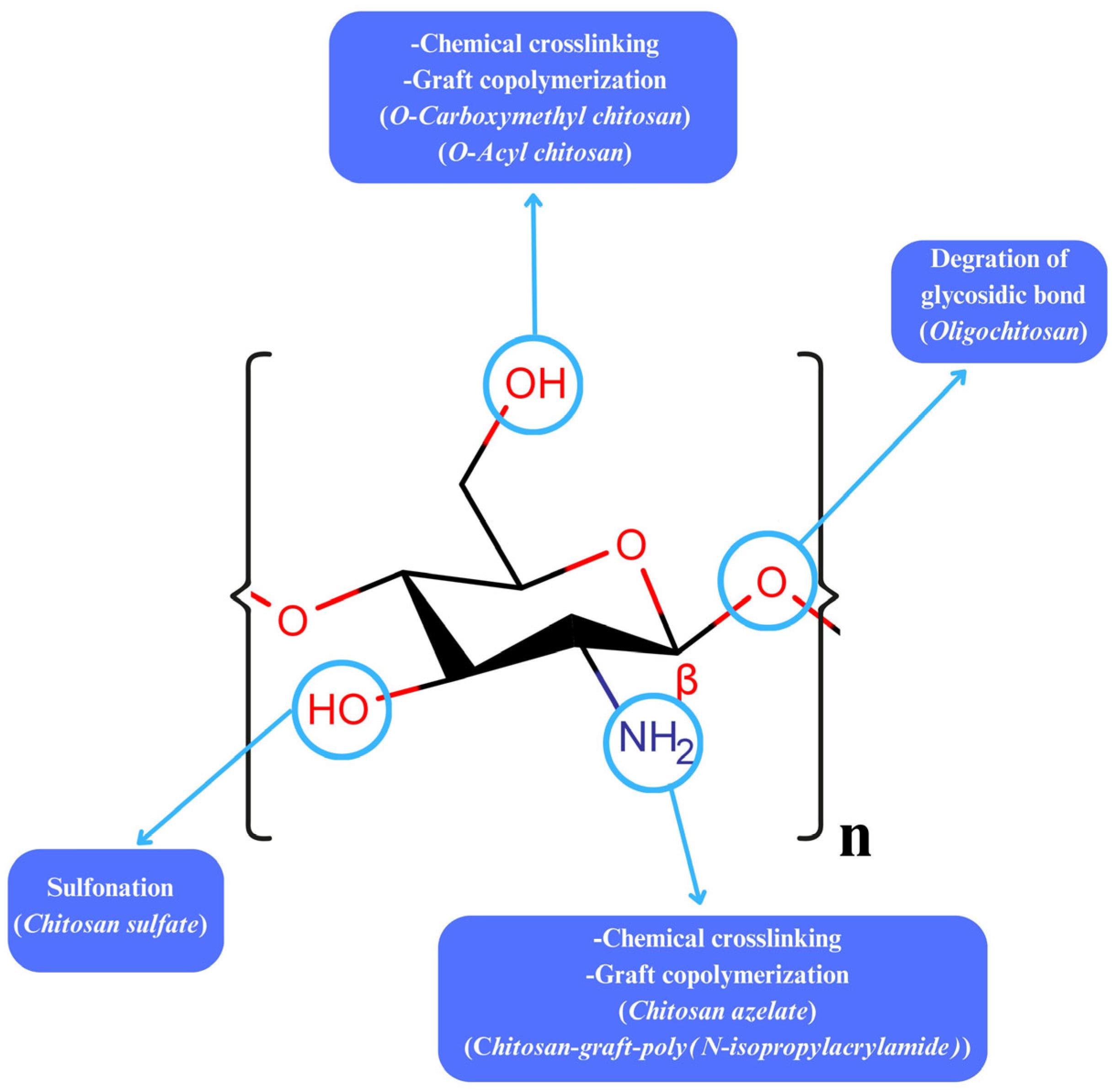
3. Modifications on Chitosan
4. Chitosan Nanogel Production
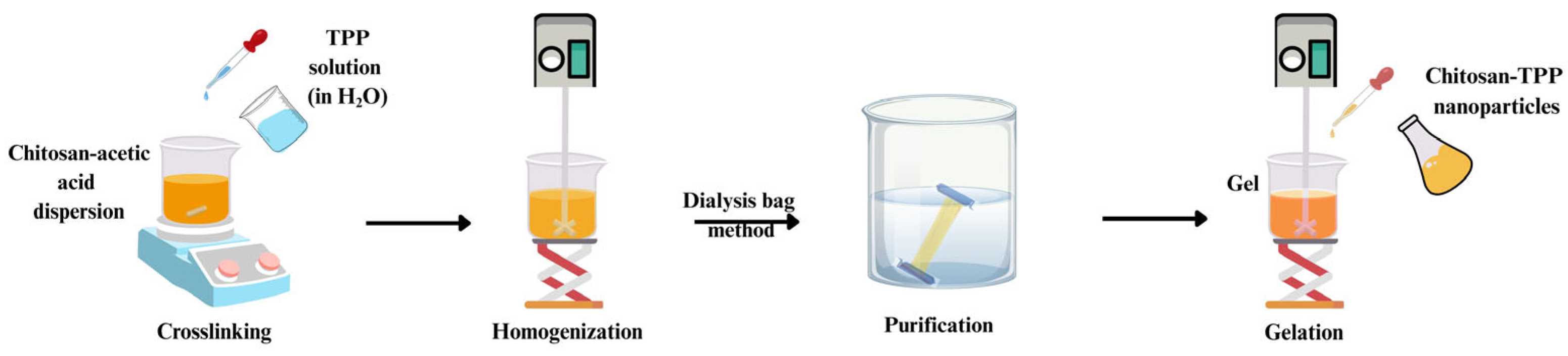
4.1. Ionic Gelation
4.2. Physical Gelation
4.3. Self-Assembly
4.4. Radical Polymerization
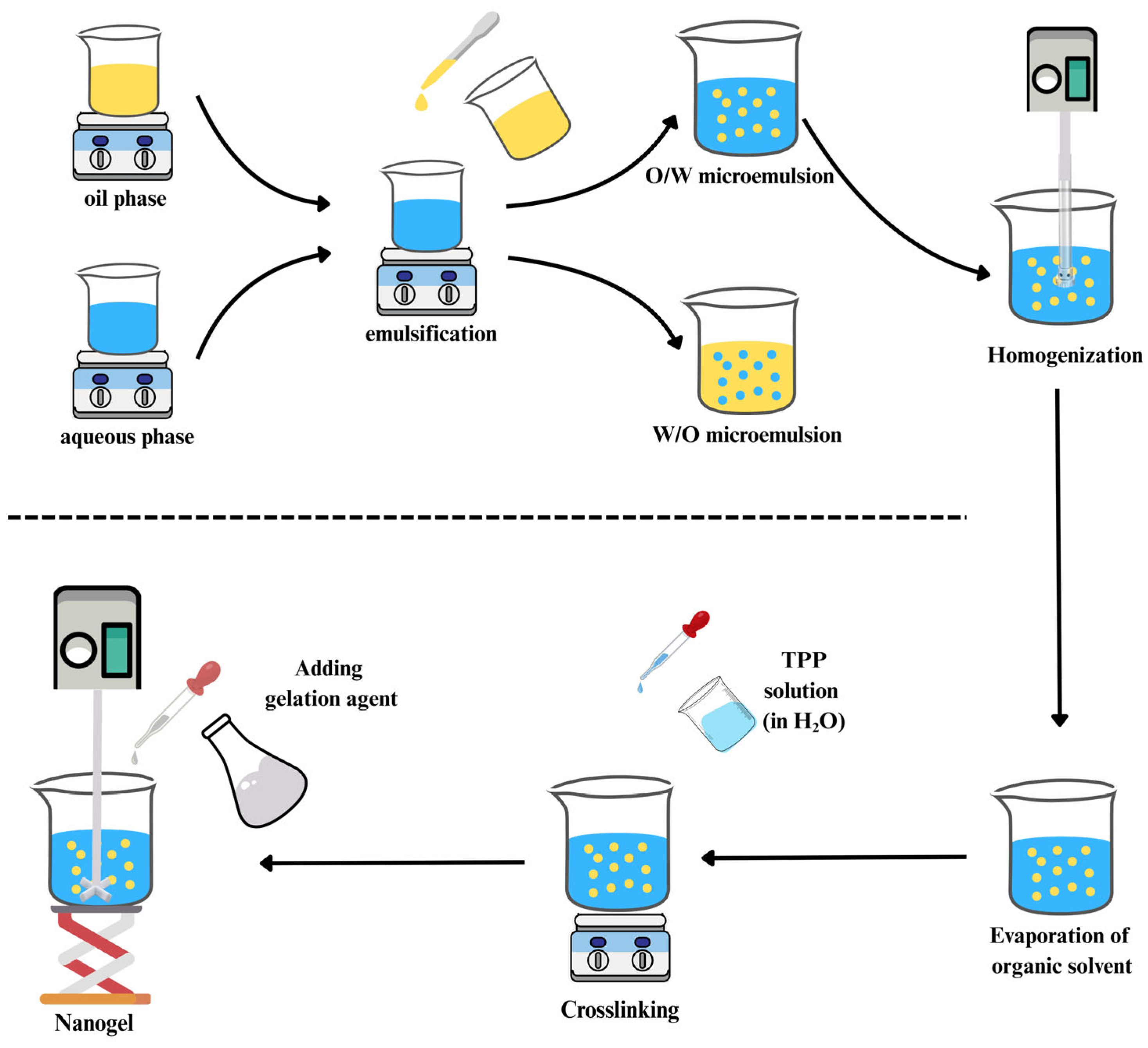
4.5. Microemulsion Technique
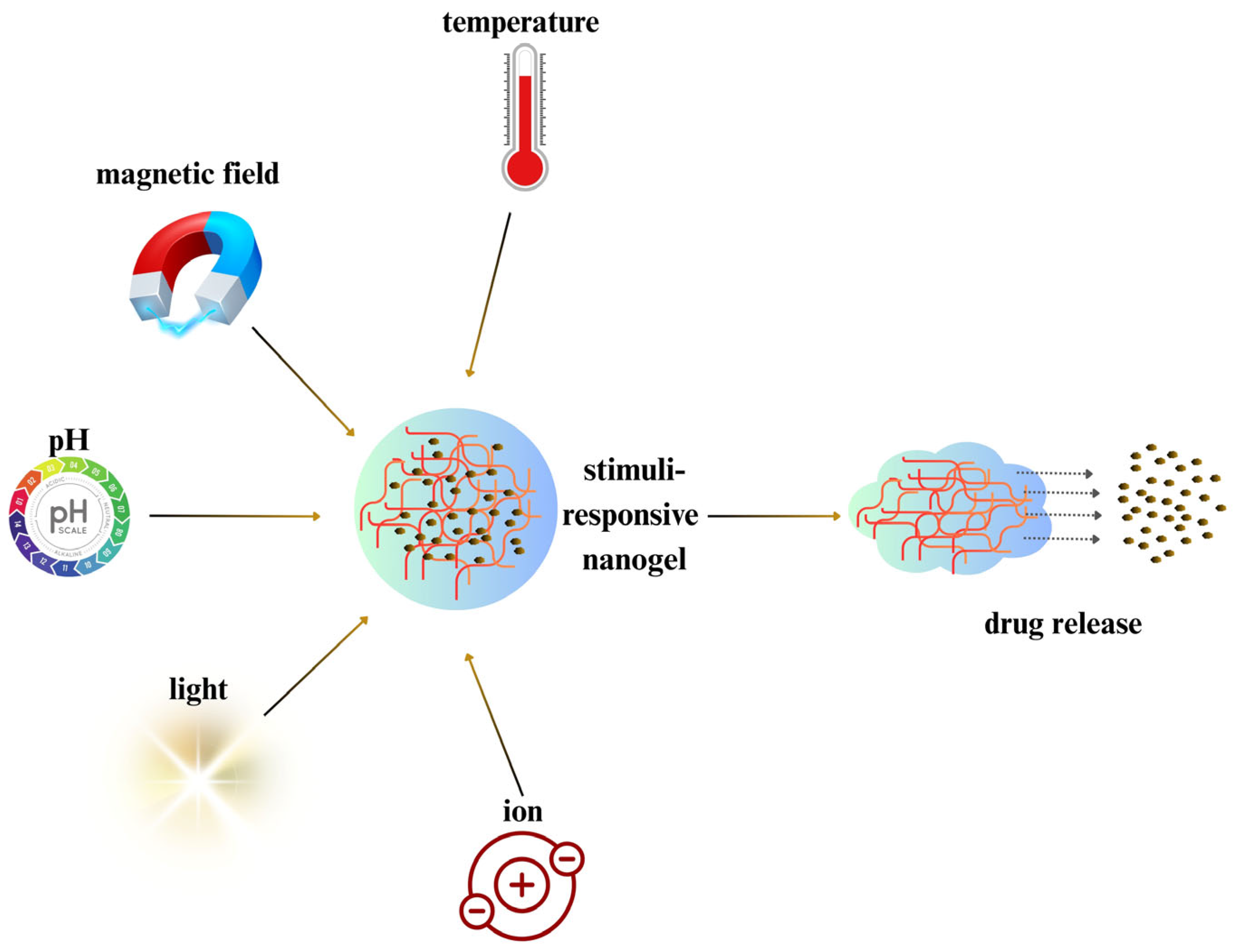
5. Smart Stimuli-Responsive Chitosan Nanogels
6. Chitosan Protein Hybrids
7. Protein–Peptide Delivery
8. Antigen Delivery
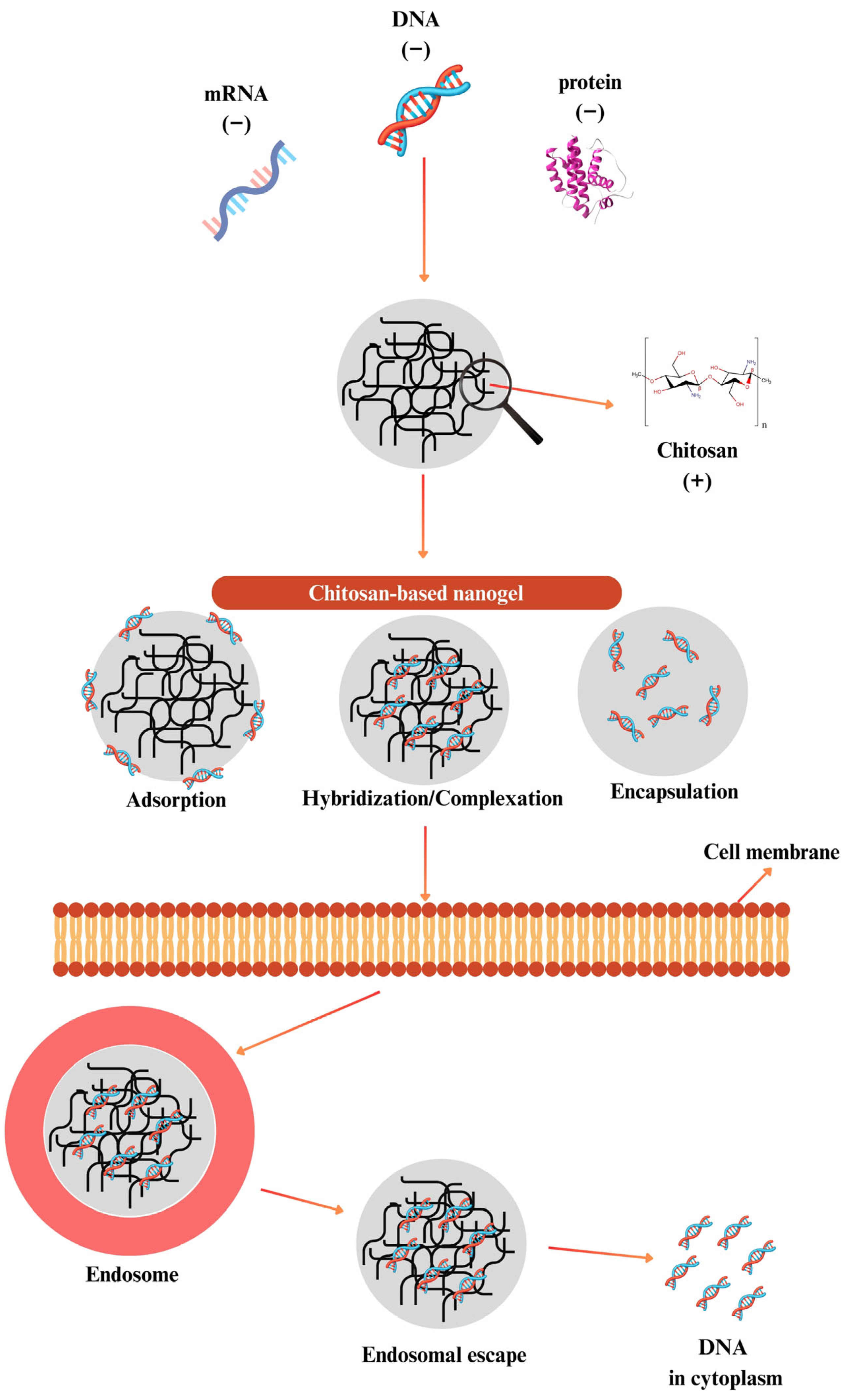
9. Gene Delivery
10. Genome Editing
11. Future Perspectives and Challenges
12. Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMP-2 | Human bone morphogenetic protein 2 |
| CH–GA | Chitosan-glycolic acid |
| CLM | Chitosan-based luminescent/magnetic |
| CMAs | Myristic acid and chitosan-based nanogels |
| CMC | Carboxymethyl chitosan |
| CMCS | Carboxymethyl chitosan |
| CS-g | Chitosan grafted |
| DD | Degree of deacetylation |
| DMA | 2,3-dimethylmaleic anhydride |
| DNA | Deoxyribonucleic acid |
| EMA | European Medicine Agency |
| FDA | Food and Drug Administration |
| GMP | Good Manufacturing Practices |
| LCST | Lower critical solution temperature |
| MA-CMCs | Methacrylic anhydride-modified carboxymethyl chitosan |
| mRNAs | Messenger RNAs |
| MW | Molecular weight |
| NAR/β-CD | Naringenin–β-cyclodextrin |
| NG | Nanogel |
| NLG | Nanolipogel |
| OPs | Oyster peptide |
| PCL | Polycaprolactone |
| PCS | Phosphorylated chitosan |
| PEG | Polyethylene glycol |
| PNIPAAm | Poly(N-isopropylacrylamide) |
| PVA | Polyvinyl alcohol |
| QbD | Quality-by-design |
| recNcPDI | Nanogel-encapsulated recombinant NcPDI |
| RNAi | RNA interference |
| RT-PCR | Reverse transcription polymerase chain reaction |
| siRNA | Small interfering RNA |
| TALENs | Transcription activator-like effector nucleases |
| TC | Trimethyl chitosan-cysteine |
| TPP | Tripolyphosphate |
| Tyr | Tyrosine |
| uPA | Urokinase-type plasminogen activator |
| ZFNs | Zinc finger nucleases |
References
- Zhang, H.F.; Ma, L.; Su, F.; Ma, X.F.; Li, T.; Wu, J.-Z.-X.; Zhao, G.H.; Wu, Z.M.; Hou, C.-L.; Yan, H.-J. PH and Reduction Dual-Responsive Feather Keratin—Sodium Alginate Nanogels with High Drug Loading Capacity for Tumor-Targeting DOX Delivery. Polym. Test. 2021, 103, 107375. [Google Scholar] [CrossRef]
- Preman, N.K.; Barki, R.R.; Vijayan, A.; Sanjeeva, S.G.; Johnson, R.P. Recent Developments in Stimuli-Responsive Polymer Nanogels for Drug Delivery and Diagnostics: A Review. Eur. J. Pharm. Biopharm. 2020, 157, 121–153. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.V.; Biswanath, S. Electrically Responsive Smart Hydrogels in Drug Delivery: A Review. J. Appl. Biomater. Biomech. 2007, 5, 125–139. [Google Scholar] [PubMed]
- Oh, Y.; Kim, S.-H. Hydrogel-Shelled Biodegradable Microspheres for Sustained Release of Encapsulants. J. Polym. Sci. 2022, 60, 1700–1709. [Google Scholar] [CrossRef]
- Duan, Q.Y.; Zhu, Y.X.; Jia, H.R.; Wang, S.H.; Wu, F.G. Nanogels: Synthesis, Properties, and Recent Biomedical Applications. Prog. Mater. Sci. 2023, 139, 101167. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Wang, Y.; Chen, H.; Zhang, X.; Luo, C.; Zhou, W.; Li, L.; Teng, L.; Yu, H.; et al. Smart Drug Delivery Systems for Precise Cancer Therapy. Acta Pharm. Sin. B 2022, 12, 4098–4121. [Google Scholar] [CrossRef]
- Wu, H.Q.; Wang, C.C. Biodegradable Smart Nanogels: A New Platform for Targeting Drug Delivery and Biomedical Diagnostics. Langmuir 2016, 32, 6211–6225. [Google Scholar] [CrossRef]
- Dalir Abdolahinia, E.; Barati, G.; Ranjbar-Navazi, Z.; Kadkhoda, J.; Islami, M.; Hashemzadeh, N.; Maleki Dizaj, S.; Sharifi, S. Application of Nanogels as Drug Delivery Systems in Multicellular Spheroid Tumor Model. J. Drug Deliv. Sci. Technol. 2022, 68, 103109. [Google Scholar] [CrossRef]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An Overview of Properties, Biomedical Applications and Obstacles to Clinical Translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef]
- Vinogradov, S.; Batrakova, E.; Kabanov, A. Poly(Ethylene Glycol)-Polyethyleneimine NanoGel(TM) Particles: Novel Drug Delivery Systems for Antisense Oligonucleotides. Colloids Surf. B Biointerfaces 1999, 16, 291–304. [Google Scholar] [CrossRef]
- Mastella, P.; Todaro, B.; Luin, S. Nanogels: Recent Advances in Synthesis and Biomedical Applications. Nanomaterials 2024, 14, 1300. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Özcan Bülbül, E.; Okur, M.E.; Karantas, I.D.; Üstündağ Okur, N. The Application of Nanogels as Efficient Drug Delivery Platforms for Dermal/Transdermal Delivery. Gels 2023, 9, 753. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Deng, H.; Gao, M.; Zhang, W. Self-Assembled Nanogels Based on Ionic Gelation of Natural Polysaccharides for Drug Delivery. Front. Bioeng. Biotechnol. 2021, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, S.; Xiong, W.; Pei, Y.; Li, B.; Chen, Y. Nanogels Fabricated from Bovine Serum Albumin and Chitosan via Self-Assembly for Delivery of Anticancer Drug. Colloids Surf. B Biointerfaces 2016, 146, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Papagiannopoulos, A.; Sotiropoulos, K. Current Advances of Polysaccharide-Based Nanogels and Microgels in Food and Biomedical Sciences. Polymers 2022, 14, 813. [Google Scholar] [CrossRef]
- Arpa, M.D.; Okur, N.Ü.; Gök, M.K.; Cevher, E. Chitosan-Based Buccal Mucoadhesive Bilayer Tablets Enhance the Bioavailability of Tizanidine Hydrochloride by Bypassing the First-Pass Metabolism. J. Drug Deliv. Sci. Technol. 2024, 97, 105739. [Google Scholar] [CrossRef]
- Noh, K.H.; Park, Y.M.; Kim, H.S.; Kang, T.H.; Song, K.H.; Lee, Y.H.; Byeon, Y.; Jeon, H.N.; Jung, I.D.; Shin, B.C.; et al. GM-CSF-Loaded Chitosan Hydrogel as an Immunoadjuvant Enhances Antigen-Specific Immune Responses with Reduced Toxicity. BMC Immunol. 2014, 15, 48. [Google Scholar] [CrossRef]
- Kim, S. Competitive Biological Activities of Chitosan and Its Derivatives: Antimicrobial, Antioxidant, Anticancer, and Anti-Inflammatory Activities. Int. J. Polym. Sci. 2018, 1708172. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, Y.; Pan, X.; Chen, S.; Zhuang, H.; Wang, S. A Composite Hydrogel of Chitosan/Heparin/Poly (γ-Glutamic Acid) Loaded with Superoxide Dismutase for Wound Healing. Carbohydr. Polym. 2018, 180, 168–174. [Google Scholar] [CrossRef]
- Takeya, H.; Itai, S.; Kimura, H.; Kurashina, Y.; Amemiya, T.; Nagoshi, N.; Iwamoto, T.; Sato, K.; Shibata, S.; Matsumoto, M.; et al. Schwann Cell-Encapsulated Chitosan-Collagen Hydrogel Nerve Conduit Promotes Peripheral Nerve Regeneration in Rodent Sciatic Nerve Defect Models. Sci. Rep. 2023, 13, 11932. [Google Scholar] [CrossRef]
- de Oliveira, W.F.; Sales Albuquerque, P.B.; Ribeiro Rodrigues, N.E.; dos Santos Silva, P.M.; Kennedy, J.F.; dos Santos Correia, M.T.; Breitenbach Barroso Coelho, L.C. Pharmaceutical Applications of Chitosan on Medical Implants: A Viable Alternative for Construction of New Biomaterials? Carbohydr. Polym. Technol. Appl. 2024, 7, 100407. [Google Scholar] [CrossRef]
- Dong, L.; Li, Y.; Cong, H.; Yu, B.; Shen, Y. A Review of Chitosan in Gene Therapy: Developments and Challenges. Carbohydr. Polym. 2024, 324, 121562. [Google Scholar] [CrossRef]
- Kim, Y.; Zharkinbekov, Z.; Raziyeva, K.; Tabyldiyeva, L.; Berikova, K.; Zhumagul, D.; Temirkhanova, K.; Saparov, A. Chitosan-Based Biomaterials for Tissue Regeneration. Pharmaceutics 2023, 15, 807. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Zangabad, P.S.; Majidi, S.; Barar, J.; Erfan-Niya, H.; Omidi, Y. Stimuli-Responsive Chitosan-Based Nanocarriers for Cancer Therapy. BioImpacts 2017, 7, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Marciello, M.; Rossi, S.; Caramella, C.; Remuñán-López, C. Freeze-Dried Cylinders Carrying Chitosan Nanoparticles for Vaginal Peptide Delivery. Carbohydr. Polym. 2017, 170, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Gao, Y.; Shu, J.; Zhang, C.; Zhao, K. Chitosan-Based Nanomaterial as Immune Adjuvant and Delivery Carrier for Vaccines. Vaccines 2022, 10, 1906. [Google Scholar] [CrossRef]
- Parmaksız, S.; Gül, A.; Erkunt Alak, S.; Karakavuk, M.; Can, H.; Gül, C.; Karakavuk, T.; López-Macías, C.; Puralı, N.; Döşkaya, M.; et al. Development of Multistage Recombinant Protein Vaccine Formulations against Toxoplasmosis Using a New Chitosan and Porin Based Adjuvant System. Int. J. Pharm. 2022, 626, 122199. [Google Scholar] [CrossRef]
- Yadav, H.; Malviya, R.; Kaushik, N. Chitosan in Biomedicine: A Comprehensive Review of Recent Developments. Carbohydr. Polym. Technol. Appl. 2024, 8, 100551. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Belogurova, N.G.; Poddubnaya, I.V.; Kudryashova, E.V. Mucosal Adhesive Chitosan Nanogel Formulations of Antibiotics and Adjuvants (Terpenoids, Flavonoids, Etc.) and Their Potential for the Treatment of Infectious Diseases of the Gastrointestinal Tract. Pharmaceutics 2023, 15, 2353. [Google Scholar] [CrossRef]
- Desu, P.K.; Karmakar, B.; Kondi, V.; Tiwari, O.N.; Halder, G. Optimizing Formulation of Green Tea Extract-Loaded Chitosan Nanogel. Biomass Convers. Biorefinery 2024, 14, 3209–3222. [Google Scholar] [CrossRef]
- Della Sala, F.; Barretta, M.; di Gennaro, M.; Paradiso, R.; Borriello, G.; Borzacchiello, A. Bio-Composite Nanogels Based on Chitosan and Hyaluronic Acid for the Treatment of Lung Infections. Gels 2024, 10, 709. [Google Scholar] [CrossRef]
- Suhail, M.; Chiu, I.H.; Ullah, A.; Khan, A.; Ullah, H.; Al-Sowayan, N.S.; Wu, P.C. Formulation and In Vitro Assessment of Polymeric PH-Responsive Nanogels of Chitosan for Sustained Delivery of Madecassoside. ACS Omega 2024, 9, 19345–19352. [Google Scholar] [CrossRef]
- Ramos, J.; Forcada, J.; Hidalgo-Alvarez, R. Cationic Polymer Nanoparticles and Nanogels: From Synthesis to Biotechnological Applications. Chem. Rev. 2014, 114, 367–428. [Google Scholar] [CrossRef]
- Pérez-Álvarez, L.; Laza, J.M.; Álvarez-Bautista, A. Covalently and Ionically Crosslinked Chitosan Nanogels for Drug Delivery. Curr. Pharm. Des. 2016, 22, 3380–3398. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Fan, Y.T.; Shen, L.J.; Yang, C.X.; Liu, X.Y.; Ma, Y.N.; Qi, L.Y.; Cho, K.H.; Cho, C.S.; Jiang, H.L. PH-Sensitive and Specific Ligand-Conjugated Chitosan Nanogels for Efficient Drug Delivery. Int. J. Biol. Macromol. 2019, 141, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Iacob, A.T.; Lupascu, F.G.; Apotrosoaei, M.; Vasincu, I.M.; Tauser, R.G.; Lupascu, D.; Giusca, S.E.; Caruntu, I.D.; Profire, L. Recent Biomedical Approaches for Chitosan Based Materials as Drug Delivery Nanocarriers. Pharmaceutics 2021, 13, 587. [Google Scholar] [CrossRef] [PubMed]
- Kayra, N.; Aytekin, A.Ö. Chapter 14—Chitosan Nanogel for Drug Delivery and Regenerative Medicine. In Polysaccharide Hydrogels for Drug Delivery and Regenerative Medicine; Giri, T.K., Ghosh, B., Badwaik, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 215–232. ISBN 978-0-323-95351-1. [Google Scholar]
- Ubaid, M.; Murtaza, G. Fabrication and Characterization of Genipin Cross-Linked Chitosan/Gelatin Hydrogel for PH-Sensitive, Oral Delivery of Metformin with an Application of Response Surface Methodology. Int. J. Biol. Macromol. 2018, 114, 1174–1185. [Google Scholar] [CrossRef]
- Manivong, S.; Garcia Ac, A.; Patten, S.A.; Fernandes, J.C.; Benderdour, M.; Banquy, X.; Moldovan, F.; Roullin, V.G. Chitosan-Based Nanogels: Synthesis and Toxicity Profile for Drug Delivery to Articular Joints. Nanomaterials 2022, 12, 1337. [Google Scholar] [CrossRef]
- Singh, T.P.; Chatli, M.K.; Sahoo, J. Development of Chitosan Based Edible Films: Process Optimization Using Response Surface Methodology. J. Food Sci. Technol. 2015, 52, 2530–2543. [Google Scholar] [CrossRef]
- Arpa, M.D.; Okur, N.Ü.; Gök, M.K.; Özgümüş, S.; Cevher, E. Chitosan-Based Buccal Mucoadhesive Patches to Enhance the Systemic Bioavailability of Tizanidine. Int. J. Pharm. 2023, 642, 123168. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Recent Advances in Engineered Chitosan-Based Nanogels for Biomedical Applications. J. Mater. Chem. B 2017, 5, 6986–7007. [Google Scholar] [CrossRef] [PubMed]
- Ullah, K.; Sohail, M.; Murtaza, G.; Khan, S.A. Natural and Synthetic Materials Based CMCh/PVA Hydrogels for Oxaliplatin Delivery: Fabrication, Characterization, In-Vitro and In-Vivo Safety Profiling. Int. J. Biol. Macromol. 2019, 122, 538–548. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Hornof, M.; Guggi, D. Thiolated Chitosans. Eur. J. Pharm. Biopharm. 2004, 57, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Chen, R.; Zhang, H.; Bryers, J.D. Injectable Biodegradable Chitosan-Alginate 3D Porous Gel Scaffold for MRNA Vaccine Delivery. Macromol. Biosci. 2019, 19, 1800242. [Google Scholar] [CrossRef] [PubMed]
- Karayianni, M.; Sentoukas, T.; Skandalis, A.; Pippa, N.; Pispas, S. Chitosan-Based Nanoparticles for Nucleic Acid Delivery: Technological Aspects, Applications, and Future Perspectives. Pharmaceutics 2023, 15, 1849. [Google Scholar] [CrossRef]
- Hanna, D.H.; Lotfy, V.F.; Basta, A.H.; Saad, G.R. Comparative Evaluation for Controlling Release of Niacin from Protein- and Cellulose-Chitosan Based Hydrogels. Int. J. Biol. Macromol. 2020, 150, 228–237. [Google Scholar] [CrossRef]
- Wang, X.; Niu, D.; Li, P.; Wu, Q.; Bo, X.; Liu, B.; Bao, S.; Su, T.; Xu, H.; Wang, Q. Dual-Enzyme-Loaded Multifunctional Hybrid Nanogel System for Pathological Responsive Ultrasound Imaging and T 2-Weighted Magnetic Resonance Imaging. ACS Nano 2015, 9, 5646–5656. [Google Scholar] [CrossRef]
- Sabir, F.; Asad, M.I.; Qindeel, M.; Afzal, I.; Dar, M.J.; Shah, K.U.; Zeb, A.; Khan, G.M.; Ahmed, N.; Din, F.U. Polymeric Nanogels as Versatile Nanoplatforms for Biomedical Applications. J. Nanomater. 2019, 2019, 1526186. [Google Scholar] [CrossRef]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef]
- Shoukat, H.; Pervaiz, F.; Rehman, S.; Noreen, S. Development of β-Cyclodextrin/Chitosan-Co-Poly (2-Acrylamide-2-Methylpropane Sulphonic Acid) Cross-Linked Hybrid IPN-Nanogels to Enhance the Solubility of Rosuvastatin: An in Vitro and in Vivo Attributes. J. Drug Deliv. Sci. Technol. 2022, 75, 103696. [Google Scholar] [CrossRef]
- Brianna; Anwar, A.; Teow, S.Y.; Wu, Y.S. Nanogel-Based Drug Delivery System as a Treatment Modality for Diverse Diseases: Are We There Yet? J. Drug Deliv. Sci. Technol. 2024, 91, 105224. [Google Scholar] [CrossRef]
- Kovacevic, B.; Ionescu, C.M.; Jones, M.; Wagle, S.R.; Lewkowicz, M.; Ðanić, M.; Mikov, M.; Mooranian, A.; Al-Salami, H. The Effect of Deoxycholic Acid on Chitosan-Enabled Matrices for Tissue Scaffolding and Injectable Nanogels. Gels 2022, 8, 358. [Google Scholar] [CrossRef]
- Quiñones, J.P.; Peniche, H.; Peniche, C. Chitosan Based Self-Assembled Nanoparticles in Drug Delivery. Polymers 2018, 10, 235. [Google Scholar] [CrossRef] [PubMed]
- Mateescu, M.A.; Ispas-Szabo, P.; Assaad, E. (Eds.) 3—Chitosan and Its Derivatives as Self-Assembled Systems for Drug Delivery. In Controlled Drug Delivery; Woodhead Publishing Series in Biomedicine; Woodhead Publishing: Sawston, UK, 2015; pp. 85–125. ISBN 978-1-907568-45-9. [Google Scholar]
- Lee, J.; Lee, C.; Kim, T.H.; Lee, E.S.; Shin, B.S.; Chi, S.C.; Park, E.S.; Lee, K.C.; Youn, Y.S. Self-Assembled Glycol Chitosan Nanogels Containing Palmityl-Acylated Exendin-4 Peptide as a Long-Acting Anti-Diabetic Inhalation System. J. Control. Release 2012, 161, 728–734. [Google Scholar] [CrossRef]
- Rajaei, A.; Salarbashi, D.; Tafaghodi, M.; Sabeti, Z.; Sabbagh, F.; Rakhshani, S.; Kamali, H.; Fahmideh-Rad, E. Evaluation of Antimicrobial and Structural Properties of Thyme Essential Oil-Loaded Chitosan-Capric Acid and Chitosan-Stearic Acid Nanogels. J. Food Qual. Hazards Control 2023, 10, 153–162. [Google Scholar] [CrossRef]
- Hajebi, S.; Rabiee, N.; Bagherzadeh, M.; Ahmadi, S.; Rabiee, M.; Roghani-Mamaqani, H.; Tahriri, M.; Tayebi, L.; Hamblin, M.R. Stimulus-Responsive Polymeric Nanogels as Smart Drug Delivery Systems. Acta Biomater. 2019, 92, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zha, L.; Banik, B.; Alexis, F. Stimulus Responsive Nanogels for Drug Delivery. Soft Matter 2011, 7, 5908–5916. [Google Scholar] [CrossRef]
- Ali, A.A.; Al-Othman, A.; Al-Sayah, M.H. Multifunctional Stimuli-Responsive Hybrid Nanogels for Cancer Therapy: Current Status and Challenges. J. Control. Release 2022, 351, 476–503. [Google Scholar] [CrossRef]
- Coşkunmeriç, N.; Üstündağ Okur, N.; Okur, M.E.; Ayla, Ş.; Yoltaş, A.; Karavana, S.Y. Promising Nanogels Loaded with Usnic Acid for Oral Ulcer Treatment: Development, Characterization, and in Vivo Evaluation in Rabbits. Pharm. Dev. Technol. 2021, 26, 431–443. [Google Scholar] [CrossRef]
- Aminu, N.; Chan, S.Y.; Yam, M.F.; Toh, S.M. A Dual-Action Chitosan-Based Nanogel System of Triclosan and Flurbiprofen for Localised Treatment of Periodontitis. Int. J. Pharm. 2019, 570, 118659. [Google Scholar] [CrossRef] [PubMed]
- Pathan, I.B.; Dwivedi, R.; Ambekar, W. Formulation and Evaluation of Ketoprofen Loaded Chitosan Nanogel for Pain Management: Ex-Vivo and In-Vivo Study. Ars Pharm. 2019, 60, 101–108. [Google Scholar]
- Abou-Taleb, H.A.; Fathalla, Z.; Naguib, D.M.; Fatease, A.A.; Abdelkader, H. Chitosan/Solid-Lipid Nanoparticles Hybrid Gels for Vaginal Delivery of Estradiol for Management of Vaginal Menopausal Symptoms. Pharmaceuticals 2023, 16, 1284. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rashid, R.S.; Helal, D.A.; Omar, M.M.; El Sisi, A.M. Nanogel Loaded with Surfactant Based Nanovesicles for Enhanced Ocular Delivery of Acetazolamide. Int. J. Nanomed. 2019, 14, 2973–2983. [Google Scholar] [CrossRef]
- Buosi, F.S.; Alaimo, A.; Di Santo, M.C.; Elías, F.; García Liñares, G.; Acebedo, S.L.; Castañeda Cataña, M.A.; Spagnuolo, C.C.; Lizarraga, L.; Martínez, K.D.; et al. Resveratrol Encapsulation in High Molecular Weight Chitosan-Based Nanogels for Applications in Ocular Treatments: Impact on Human ARPE-19 Culture Cells. Int. J. Biol. Macromol. 2020, 165, 804–821. [Google Scholar] [CrossRef]
- Arnfast, L.; Madsen, C.G.; Jorgensen, L.; Baldursdottir, S. Design and Processing of Nanogels as Delivery Systems for Peptides and Proteins. Ther. Deliv. 2014, 5, 691–708. [Google Scholar] [CrossRef]
- Gadziński, P.; Froelich, A.; Jadach, B.; Wojtyłko, M.; Tatarek, A.; Białek, A.; Krysztofiak, J.; Gackowski, M.; Otto, F.; Osmałek, T. Ionotropic Gelation and Chemical Crosslinking as Methods for Fabrication of Modified-Release Gellan Gum-Based Drug Delivery Systems. Pharmaceutics 2023, 15, 108. [Google Scholar] [CrossRef]
- Mutlu Agardan, B.M. Studies on the Formulation Optimization and Controlled Ionic Gelation of Chitosan Nanoparticles Using TPP-HP-Beta-CD Inclusion Complex. Istanb. J. Pharm. 2020, 50, 54–59. [Google Scholar]
- Sang, Z.; Qian, J.; Han, J.; Deng, X.; Shen, J.; Li, G.; Xie, Y. Comparison of Three Water-Soluble Polyphosphate Tripolyphosphate, Phytic Acid, and Sodium Hexametaphosphate as Crosslinking Agents in Chitosan Nanoparticle Formulation. Carbohydr. Polym. 2020, 230, 115577. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, Y.; Yu, Z.; Du, L. Synthesis, Characterization of Chitosan/Tripolyphosphate Nanoparticles Loaded with 4-Chloro-2-Methylphenoxyacetate Sodium Salt and Its Herbicidal Activity against Bidens pilosa L. Sci. Rep. 2024, 14, 18754. [Google Scholar] [CrossRef]
- Huang, Y.; Lapitsky, Y. On the Kinetics of Chitosan/Tripolyphosphate Micro- and Nanogel Aggregation and Their Effects on Particle Polydispersity. J. Colloid Interface Sci. 2017, 486, 27–37. [Google Scholar] [CrossRef]
- Ravi, H.; Baskaran, V. Biodegradable Chitosan-Glycolipid Hybrid Nanogels: A Novel Approach to Encapsulate Fucoxanthin for Improved Stability and Bioavailability. Food Hydrocoll. 2015, 43, 717–725. [Google Scholar] [CrossRef]
- Bruno, S.G.; Martínez, S.M.; Costa Gobbato, C.; Quinteros, D.A.; Alaimo, A.; Pérez, O.E. Chitosan-TPP Nanogels for Ocular Delivery of Folic Acid: Release Profile, Corneal Permeation, and Mucoadhesion Assessment. Pharmaceutics 2025, 17, 424. [Google Scholar] [CrossRef] [PubMed]
- Abreu, F.O.M.S.; Oliveira, E.F.; Paula, H.C.B.; De Paula, R.C.M. Chitosan/Cashew Gum Nanogels for Essential Oil Encapsulation. Carbohydr. Polym. 2012, 89, 1277–1282. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, M.A.; Hamidi, M. The Impact of Preparation Parameters on Typical Attributes of Chitosan-Heparin Nanohydrogels: Particle Size, Loading Efficiency, and Drug Release. Drug Dev. Ind. Pharm. 2013, 39, 1774–1782. [Google Scholar] [CrossRef]
- Abioye, A.O.; Issah, S.; Kola-Mustapha, A.T. Ex Vivo Skin Permeation and Retention Studies on Chitosan-Ibuprofen-Gellan Ternary Nanogel Prepared by in Situ Ionic Gelation Technique—A Tool for Controlled Transdermal Delivery of Ibuprofen. Int. J. Pharm. 2015, 490, 112–130. [Google Scholar] [CrossRef]
- Mi, F.L.; Kuan, C.Y.; Shyu, S.S.; Lee, S.T.; Chang, S.F. Study of Gelation Kinetics and Chain-Relaxation Properties of Glutaraldehyde-Cross-Linked Chitosan Gel and Their Effects on Microspheres Preparation and Drug Release. Carbohydr. Polym. 2000, 41, 389–396. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Rubini, K.; Roveri, N. Mechanical and Thermal Properties of Gelatin Films at Different Degrees of Glutaraldehyde Crosslinking. Biomaterials 2001, 22, 763–768. [Google Scholar] [CrossRef]
- Chen, T.; Embree, H.D.; Brown, E.M.; Taylor, M.M.; Payne, G.F. Enzyme-Catalyzed Gel Formation of Gelatin and Chitosan: Potential for in Situ Applications. Biomaterials 2003, 24, 2831–2841. [Google Scholar] [CrossRef]
- Jin, R.; Lin, C.; Cao, A. Enzyme-Mediated Fast Injectable Hydrogels Based on Chitosan-Glycolic Acid/Tyrosine: Preparation, Characterization, and Chondrocyte Culture. Polym. Chem. 2014, 5, 391–398. [Google Scholar] [CrossRef]
- Moreira Teixeira, L.S.; Feijen, J.; van Blitterswijk, C.A.; Dijkstra, P.J.; Karperien, M. Enzyme-Catalyzed Crosslinkable Hydrogels: Emerging Strategies for Tissue Engineering. Biomaterials 2012, 33, 1281–1290. [Google Scholar] [CrossRef]
- Lim, S.; Jeong, D.; Ki, M.R.; Pack, S.P.; Choi, Y.S. Tyrosinase-Mediated Rapid and Permanent Chitosan/Gelatin and Chitosan/Gelatin/Nanohydroxyapatite Hydrogel. Korean J. Chem. Eng. 2021, 38, 98–103. [Google Scholar] [CrossRef]
- Parhi, R. Cross-Linked Hydrogel for Pharmaceutical Applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Brunel, F.; Véron, L.; David, L.; Domard, A.; Delair, T. A Novel Synthesis of Chitosan Nanoparticles in Reverse Emulsion. Langmuir 2008, 24, 11370–11377. [Google Scholar] [CrossRef] [PubMed]
- Brunel, F.; Véron, L.; David, L.; Domard, A.; Verrier, B.; Delair, T. Self-Assemblies on Chitosan Nanohydrogels. Macromol. Biosci. 2010, 10, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Sacco, P.; Furlani, F.; De Marzo, G.; Marsich, E.; Paoletti, S.; Donati, I. Concepts for Developing Physical Gels of Chitosan and of Chitosan Derivatives. Gels 2018, 4, 67. [Google Scholar] [CrossRef]
- Oh, N.M.; Oh, K.T.; Baik, H.J.; Lee, B.R.; Lee, A.H.; Youn, Y.S.; Lee, E.S. A Self-Organized 3-Diethylaminopropyl-Bearing Glycol Chitosan Nanogel for Tumor Acidic PH Targeting: In Vitro Evaluation. Colloids Surf. B Biointerfaces 2010, 78, 120–126. [Google Scholar] [CrossRef]
- Chou, H.S.; Larsson, M.; Hsiao, M.H.; Chen, Y.C.; Röding, M.; Nydén, M.; Liu, D.M. Injectable Insulin-Lysozyme-Loaded Nanogels with Enzymatically-Controlled Degradation and Release for Basal Insulin Treatment: In Vitro Characterization and in Vivo Observation. J. Control. Release 2016, 224, 33–42. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, L.; Jiang, X.; Hu, Y.; Guo, J. Reversible Surface Switching of Nanogel Triggered by External Stimuli. Angew. Chemie Int. Ed. 2007, 46, 7104–7107. [Google Scholar] [CrossRef]
- Khalili, S.T.; Mohsenifar, A.; Beyki, M.; Zhaveh, S.; Rahmani-Cherati, T.; Abdollahi, A.; Bayat, M.; Tabatabaei, M. Encapsulation of Thyme Essential Oils in Chitosan-Benzoic Acid Nanogel with Enhanced Antimicrobial Activity against Aspergillus Flavus. LWT 2015, 60, 502–508. [Google Scholar] [CrossRef]
- Zhaveh, S.; Mohsenifar, A.; Beiki, M.; Khalili, S.T.; Abdollahi, A.; Rahmani-Cherati, T.; Tabatabaei, M. Encapsulation of Cuminum Cyminum Essential Oils in Chitosan-Caffeic Acid Nanogel with Enhanced Antimicrobial Activity against Aspergillus Flavus. Ind. Crops Prod. 2015, 69, 251–256. [Google Scholar] [CrossRef]
- Beyki, M.; Zhaveh, S.; Khalili, S.T.; Rahmani-Cherati, T.; Abollahi, A.; Bayat, M.; Tabatabaei, M.; Mohsenifar, A. Encapsulation of Mentha Piperita Essential Oils in Chitosan–Cinnamic Acid Nanogel with Enhanced Antimicrobial Activity against Aspergillus Flavus. Ind. Crops Prod. 2014, 54, 310–319. [Google Scholar] [CrossRef]
- Radnia, F.; Mohajeri, N.; Hashemi, F.; Imani, M.; Zarghami, N. Design and Development of Folate-Chitosan/CD Nanogel: An Efficient Fluorescent Platform for Cancer-Specific Delivery of AntimiR-21. React. Funct. Polym. 2021, 160, 104814. [Google Scholar] [CrossRef]
- Alsohaimi, I.H.; Alhumaimess, M.S.; Alqadami, A.A.; Hassan, H.M.A.; Chen, Q.; Alamri, M.S.; Alanzi, M.M.J.; Alraddadi, T.S. Chitosan-Carboxylic Acid Grafted Multifunctional Magnetic Nanocomposite as a Novel Adsorbent for Effective Removal of Methylene Blue Dye from Aqueous Environment. Chem. Eng. Sci. 2023, 280, 119017. [Google Scholar] [CrossRef]
- Goswami, N.; Lin, F.; Liu, Y.; Leong, D.T.; Xie, J. Highly Luminescent Thiolated Gold Nanoclusters Impregnated in Nanogel. Chem. Mater. 2016, 28, 4009–4016. [Google Scholar] [CrossRef]
- Yu, S.; Hu, J.; Pan, X.; Yao, P.; Jiang, M. Stable and PH-Sensitive Nanogels Prepared by Self-Assembly of Chitosan and Ovalbumin. Langmuir 2006, 22, 2754–2759. [Google Scholar] [CrossRef]
- Huang, Y.; Lapitsky, Y. Monovalent Salt Enhances Colloidal Stability during the Formation of Chitosan/Tripolyphosphate Microgels. Langmuir 2011, 27, 10392–10399. [Google Scholar] [CrossRef]
- Konstantinov, I.A.; Broadbelt, L.J. Chapter 2—A Quantum Mechanical Approach for Accurate Rate Parameters of Free-Radical Polymerization Reactions. In Computational Quantum Chemistry; Soroush, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 17–46. ISBN 978-0-12-815983-5. [Google Scholar]
- El-Sherbiny, I.M.; Elmahdy, M.M. Preparation, Characterization, Structure, and Dynamics of Carboxymethyl Chitosan Grafted with Acrylic Acid Sodium Salt. J. Appl. Polym. Sci. 2010, 118, 2134–2145. [Google Scholar] [CrossRef]
- Bulut, E. Ibuprofen Microencapsulation within Acrylamide-Grafted Chitosan and Methylcellulose Interpenetrating Polymer Network Microspheres: Synthesis, Characterization, and Release Studies. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1098–1108. [Google Scholar] [CrossRef]
- Povea, M.B.; Monal, W.A.; Cauich-Rodríguez, J.V.; Pat, A.M.; Rivero, N.B.; Covas, C.P. Interpenetrated Chitosan-Poly(Acrylic Acid-Co-Acrylamide) Hydrogels. Synthesis, Characterization and Sustained Protein Release Studies. Mater. Sci. Appl. 2011, 2, 509–520. [Google Scholar] [CrossRef]
- O’Reilly, R.K.; Hawker, C.J.; Wooley, K.L. Cross-Linked Block Copolymer Micelles: Functional Nanostructures of Great Potential and Versatility. Chem. Soc. Rev. 2006, 35, 1068–1083. [Google Scholar] [CrossRef] [PubMed]
- Keskin, D.; Zu, G.; Forson, A.M.; Tromp, L.; Sjollema, J.; van Rijn, P. Nanogels: A Novel Approach in Antimicrobial Delivery Systems and Antimicrobial Coatings. Bioact. Mater. 2021, 6, 3634–3657. [Google Scholar] [CrossRef] [PubMed]
- Maggi, F.; Ciccarelli, S.; Diociaiuti, M.; Casciardi, S.; Masci, G. Chitosan Nanogels by Template Chemical Cross-Linking in Polyion Complex Micelle Nanoreactors. Biomacromolecules 2011, 12, 3499–3507. [Google Scholar] [CrossRef] [PubMed]
- Babelyte, M.; Peciulyte, L.; Navikaite-Snipaitiene, V.; Bendoraitiene, J.; Samaryk, V.; Rutkaite, R. Synthesis and Characterization of Thermoresponsive Chitosan-Graft-Poly(N-Isopropylacrylamide) Copolymers. Polymers 2023, 15, 3154. [Google Scholar] [CrossRef]
- Vegesna, N.S.K.V.; Shivakumar, H.G.; Fathima, S.J.; Radha, V.; Khanum, F. PH and Thermosensitive 5-Fluorouracil Loaded Poly(NIPAM-: Co -AAc) Nanogels for Cancer Therapy. RSC Adv. 2016, 6, 105495–105507. [Google Scholar] [CrossRef]
- Arpa, M.D.; Çağlar, E.Ş.; Güreşçi, D.; Sipahi, H.; Üstündağ Okur, N. Novel Microemulsion Containing Benzocaine and Fusidic Acid Simultaneously: Formulation, Characterization, and In Vitro Evaluation for Wound Healing. AAPS PharmSciTech 2024, 25, 53. [Google Scholar] [CrossRef]
- Cao, S.; Deng, Y.; Zhang, L.; Aleahmad, M. Chitosan Nanoparticles, as Biological Macromolecule-Based Drug Delivery Systems to Improve the Healing Potential of Artificial Neural Guidance Channels: A Review. Int. J. Biol. Macromol. 2022, 201, 569–579. [Google Scholar] [CrossRef]
- Bagheri, F.; Darakhshan, S.; Mazloomi, S.; Shiri Varnamkhasti, B.; Tahvilian, R. Dual Loading of Nigella Sativa Oil-Atorvastatin in Chitosan–Carboxymethyl Cellulose Nanogel as a Transdermal Delivery System. Drug Dev. Ind. Pharm. 2021, 47, 569–578. [Google Scholar] [CrossRef]
- Nasr, F.H.; Khoee, S. Design, Characterization and in Vitro Evaluation of Novel Shell Crosslinked Poly(Butylene Adipate)-Co-N-Succinyl Chitosan Nanogels Containing Loteprednol Etabonate: A New System for Therapeutic Effect Enhancement via Controlled Drug Delivery. Eur. J. Med. Chem. 2015, 102, 132–142. [Google Scholar] [CrossRef]
- Cruz, A.; García-Uriostegui, L.; Ortega, A.; Isoshima, T.; Burillo, G. Radiation Grafting of N-Vinylcaprolactam onto Nano and Macrogels of Chitosan: Synthesis and Characterization. Carbohydr. Polym. 2017, 155, 303–312. [Google Scholar] [CrossRef]
- Ismail, R.; Baaity, Z.; Csóka, I. Regulatory Status Quo and Prospects for Biosurfactants in Pharmaceutical Applications. Drug Discov. Today 2021, 26, 1929–1935. [Google Scholar] [CrossRef]
- Sabourian, P.; Tavakolian, M.; Yazdani, H.; Frounchi, M.; van de Ven, T.G.M.; Maysinger, D.; Kakkar, A. Stimuli-Responsive Chitosan as an Advantageous Platform for Efficient Delivery of Bioactive Agents. J. Control. Release 2020, 317, 216–231. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Liu, J. Smart Stimuli-Responsive Chitosan Hydrogel for Drug Delivery: A Review. Int. J. Biol. Macromol. 2023, 235, 123902. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Jiang, J.; Kuang, L.; Jiang, J.; Zheng, W.; Liang, H. Smart Chitosan-Based Stimuli-Responsive Nanocarriers for the Controlled Delivery of Hydrophobic Pharmaceuticals. Macromolecules 2011, 44, 1298–1302. [Google Scholar] [CrossRef]
- Wang, C.Y.; Sun, M.; Fan, Z.; Du, J.Z. Intestine Enzyme-Responsive Polysaccharide-Based Hydrogel to Open Epithelial Tight Junctions for Oral Delivery of Imatinib against Colon Cancer. Chin. J. Polym. Sci. (Engl. Ed.) 2022, 40, 1154–1164. [Google Scholar] [CrossRef]
- Wang, F.; Li, J.; Chen, C.; Qi, H.; Huang, K.; Hu, S. Preparation and Synergistic Chemo-Photothermal Therapy of Redox-Responsive Carboxymethyl Cellulose/Chitosan Complex Nanoparticles. Carbohydr. Polym. 2022, 275, 118714. [Google Scholar] [CrossRef]
- Kostadinova, A.; Benkova, D.; Staneva, G.; Hazarosova, R.; Vitkova, V.; Yordanova, V.; Momchilova, A.; Angelova, M.I.; ElZorkany, H.E.S.; El-Sayed, K.; et al. Chitosan Hybrid Nanomaterials: A Study on Interaction with Biomimetic Membranes. Int. J. Biol. Macromol. 2024, 276, 133983. [Google Scholar] [CrossRef]
- Behrooznia, Z.; Nourmohammadi, J. Polysaccharide-Based Materials as an Eco-Friendly Alternative in Biomedical, Environmental, and Food Packaging. Giant 2024, 19, 100301. [Google Scholar] [CrossRef]
- Bealer, E.J.; Onissema-karimu, S.; Rivera-galletti, A.; Francis, M.; Wilkowski, J.; Hu, X. Protein—Polysaccharide Composite Materials: Fabrication and Applications. Polymers 2020, 12, 464. [Google Scholar] [CrossRef]
- Sun, Q.; Yang, Z.; Qi, X. Design and Application of Hybrid Polymer-Protein Systems in Cancer Therapy. Polymers 2023, 15, 2219. [Google Scholar] [CrossRef]
- Zhou, Y.; Petrova, S.P.; Edgar, K.J. Chemical Synthesis of Polysaccharide—Protein and Polysaccharide—Peptide Conjugates: A Review. Carbohydr. Polym. 2021, 274, 118662. [Google Scholar] [CrossRef]
- Nooshkam, M.; Varidi, M.; Zareie, Z.; Alkobeisi, F. Behavior of Protein-Polysaccharide Conjugate-Stabilized Food Emulsions under Various Destabilization Conditions. Food Chem. X 2023, 18, 100725. [Google Scholar] [CrossRef]
- He, N.; Wang, R.; He, Y.; Dang, X. Fabrication, Structure and Surface Charges of Albumin-Chitosan Hybrids. Sci. China Chem. 2012, 55, 1788–1795. [Google Scholar] [CrossRef]
- Yi, H.; Wu, L.Q.; Bentley, W.E.; Ghodssi, R.; Rubloff, G.W.; Culver, J.N.; Payne, G.F. Biofabrication with Chitosan. Biomacromolecules 2005, 6, 2881–2894. [Google Scholar] [CrossRef] [PubMed]
- Jiankang, H.; Dichen, L.; Yaxiong, L.; Bo, Y.; Hanxiang, Z.; Qin, L.; Bingheng, L.; Yi, L. Preparation of Chitosan-Gelatin Hybrid Scaffolds with Well-Organized Microstructures for Hepatic Tissue Engineering. Acta Biomater. 2009, 5, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Jafari, N.; Najavand, S.; Pazhang, M.; Matin, A.a. Entrapment of Papain in Chitosan–Polyethylene Glycol Hybrid Nanohydrogels: Presenting a Model for Protein Delivery Systems. Mol. Biotechnol. 2024, 67, 1433–1445. [Google Scholar] [CrossRef]
- Mao, Y.; Zhao, B.; Xu, L.; Wang, Y.; Qiu, X.; Sun, Y.; Yang, C. Protein-Polysaccharide Based Nanogel/Hydrogel Composite with Controlled Continuous Delivery of Drug for Enhanced Wound Healing. Carbohydr. Polym. 2025, 356, 123407. [Google Scholar] [CrossRef]
- Zucca, G.; Vigani, B.; Valentino, C.; Ruggeri, M.; Marchesi, N.; Pascale, A.; Giovilli, G.; Malavasi, L.; Sandri, G.; Rossi, S. Chondroitin Sulphate-Chitosan Based Nanogels Loaded with Naringenin-β-Cyclodextrin Complex as Potential Tool for the Treatment of Diabetic Retinopathy: A Formulation Study. Int. J. Nanomed. 2025, 20, 907–932. [Google Scholar] [CrossRef]
- Aranda-Barradas, M.E.; Coronado-Contreras, H.E.; Aguilar-Castañeda, Y.L.; Olivo-Escalante, K.D.; González-Díaz, F.R.; García-Tovar, C.G.; Álvarez-Almazán, S.; Miranda-Castro, S.P.; Del Real-López, A.; Méndez-Albores, A. Effect of Different Karyophilic Peptides on Physical Characteristics and In Vitro Transfection Efficiency of Chitosan-Plasmid Nanoparticles as Nonviral Gene Delivery Systems. Mol. Biotechnol. 2024, 67, 723–733. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as Pharmaceutical Carriers: Finite Networks of Infinite Capabilities. Angew. Chemie Int. Ed. 2009, 48, 5418–5429. [Google Scholar] [CrossRef]
- Delgado-Pujol, E.J.; Martínez, G.; Casado-Jurado, D.; Vázquez, J.; León-Barberena, J.; Rodríguez-Lucena, D.; Torres, Y.; Alcudia, A.; Begines, B. Hydrogels and Nanogels: Pioneering the Future of Advanced Drug Delivery Systems. Pharmaceutics 2025, 17, 215. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Alhareth, K.; Mignet, N. Advancement in Nanogel Formulations Provides Controlled Drug Release. Int. J. Pharm. 2020, 584, 119435. [Google Scholar] [CrossRef] [PubMed]
- Steinhilber, D.; Witting, M.; Zhang, X.; Staegemann, M.; Paulus, F.; Friess, W.; Küchler, S.; Haag, R. Surfactant Free Preparation of Biodegradable Dendritic Polyglycerol Nanogels by Inverse Nanoprecipitation for Encapsulation and Release of Pharmaceutical Biomacromolecules. J. Control. Release 2013, 169, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Kousalová, J.; Etrych, T. Polymeric Nanogels as Drug Delivery Systems. Physiol. Res. 2018, 67, s305–s317. [Google Scholar] [CrossRef]
- Lee, B.R.; Oh, K.T.; Baik, H.J.; Youn, Y.S.; Lee, E.S. A Charge-Switched Nano-Sized Polymeric Carrier for Protein Delivery. Int. J. Pharm. 2010, 392, 78–82. [Google Scholar] [CrossRef]
- Jin, H.; Tan, H.; Zhao, L.; Sun, W.; Zhu, L.; Sun, Y.; Hao, H.; Xing, H.; Liu, L.; Qu, X.; et al. Ultrasound-Triggered Thrombolysis Using Urokinase-Loaded Nanogels. Int. J. Pharm. 2012, 434, 384–390. [Google Scholar] [CrossRef]
- Cullier, A.; Cassé, F.; Manivong, S.; Contentin, R.; Legendre, F.; Garcia Ac, A.; Sirois, P.; Roullin, G.; Banquy, X.; Moldovan, F.; et al. Functionalized Nanogels with Endothelin-1 and Bradykinin Receptor Antagonist Peptides Decrease Inflammatory and Cartilage Degradation Markers of Osteoarthritis in a Horse Organoid Model of Cartilage. Int. J. Mol. Sci. 2022, 23, 8949. [Google Scholar] [CrossRef]
- Costa, B.; Alves, P.M.; Fonseca, D.R.; Campos, F.; Monteiro, A.C.; Shahrour, H.; Gomes, A.; Costa, F.; Gomes, P.; Martínez-de-Tejada, G.; et al. Dhvar5-Chitosan Nanogels and Their Potential to Improve Antibiotics Activity. Int. J. Biol. Macromol. 2024, 277, 134059. [Google Scholar] [CrossRef]
- Sundermann, J.; Oehmichen, S.; Sydow, S.; Burmeister, L.; Quaas, B.; Hänsch, R.; Rinas, U.; Hoffmann, A.; Menzel, H.; Bunjes, H. Varying the Sustained Release of BMP-2 from Chitosan Nanogel-Functionalized Polycaprolactone Fiber Mats by Different Polycaprolactone Surface Modifications. J. Biomed. Mater. Res. Part A 2021, 109, 600–614. [Google Scholar] [CrossRef]
- Nazem, H.; Mohsenifar, A.; Majdi, S. Chitosan-Myristate Nanogel as an Artificial Chaperone Protects Neuroserpin from Misfolding. Adv. Biomed. Res. 2016, 5, 170. [Google Scholar] [CrossRef]
- Wu, X.; He, C.; Wu, Y.; Chen, X.; Cheng, J. Nanogel-Incorporated Physical and Chemical Hybrid Gels for Highly Effective Chemo-Protein Combination Therapy. Adv. Funct. Mater. 2015, 25, 6744–6755. [Google Scholar] [CrossRef]
- Wu, C.; Zhi, Z.; Duan, M.; Sun, J.; Jiang, H.; Pang, J. Insights into the Formation of Carboxymethyl Chitosan-Nisin Nanogels for Sustainable Antibacterial Activity. Food Chem. 2023, 402, 134260. [Google Scholar] [CrossRef] [PubMed]
- Guaresti, O.; Maiz–Fernández, S.; Palomares, T.; Alonso-Varona, A.; Eceiza, A.; Pérez-Álvarez, L.; Gabilondo, N. Dual Charged Folate Labelled Chitosan Nanogels with Enhanced Mucoadhesion Capacity for Targeted Drug Delivery. Eur. Polym. J. 2020, 134, 109847. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, Z.; Zhou, L.; Gao, J.; Zheng, H.; Lin, H.; Zhu, G.; Qin, X.; Cao, W. Carboxymethyl Cellulose and Carboxymethyl Chitosan-Based Composite Nanogel as a Stable Delivery Vehicle for Oyster Peptides: Characterization, Absorption and Transport Mechanism. Food Chem. 2024, 442, 138464. [Google Scholar] [CrossRef]
- Wang, J.; Xu, M.; Cheng, X.; Kong, M.; Liu, Y.; Feng, C.; Chen, X. Positive/Negative Surface Charge of Chitosan Based Nanogels and Its Potential Influence on Oral Insulin Delivery. Carbohydr. Polym. 2016, 136, 867–874. [Google Scholar] [CrossRef]
- Shen, J.M.; Xu, L.; Lu, Y.; Cao, H.M.; Xu, Z.G.; Chen, T.; Zhang, H.X. Chitosan-Based Luminescent/Magnetic Hybrid Nanogels for Insulin Delivery, Cell Imaging, and Antidiabetic Research of Dietary Supplements. Int. J. Pharm. 2012, 427, 400–409. [Google Scholar] [CrossRef]
- Balde, A.; Benjakul, S.; Kim, S.K.; Nazeer, R.A. Development and in Vitro Effects of Thiolated Chitosan Nanoparticles for the Sustained Delivery of Inflammation Suppressing Bioactive Peptide. J. Drug Deliv. Sci. Technol. 2023, 88, 104971. [Google Scholar] [CrossRef]
- Wei, J.; Xue, W.; Yu, X.; Qiu, X.; Liu, Z. PH Sensitive Phosphorylated Chitosan Hydrogel as Vaccine Delivery System for Intramuscular Immunization. J. Biomater. Appl. 2017, 31, 1358–1369. [Google Scholar] [CrossRef]
- Debache, K.; Kropf, C.; Schütz, C.A.; Harwood, L.J.; Käuper, P.; Monney, T.; Rossi, N.; Laue, C.; Mccullough, K.C.; Hemphill, A. Vaccination of Mice with Chitosan Nanogel-Associated Recombinant NcPDI against Challenge Infection with Neospora Caninum Tachyzoites. Parasite Immunol. 2011, 33, 81–94. [Google Scholar] [CrossRef]
- Veilleux, D.; Nelea, M.; Biniecki, K.; Lavertu, M.; Buschmann, M.D. Preparation of Concentrated Chitosan/DNA Nanoparticle Formulations by Lyophilization for Gene Delivery at Clinically Relevant Dosages. J. Pharm. Sci. 2016, 105, 88–96. [Google Scholar] [CrossRef]
- Yuan, Y.; Tan, J.; Wang, Y.; Qian, C.; Zhang, M. Chitosan Nanoparticles as Non-Viral Gene Delivery Vehicles Based on Atomic Force Microscopy Study. Acta Biochim. Biophys. Sin. 2009, 41, 515–526. [Google Scholar] [CrossRef]
- Guliyeva, Ü.; Öner, F.; Özsoy, Ş.; Haziroǧlu, R. Chitosan Microparticles Containing Plasmid DNA as Potential Oral Gene Delivery System. Eur. J. Pharm. Biopharm. 2006, 62, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Akbuǧa, J.; Özbaş-Turan, S.; Erdoǧan, N. Plasmid-DNA Loaded Chitosan Microspheres for in Vitro IL-2 Expression. Eur. J. Pharm. Biopharm. 2004, 58, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Tan, Y.F.; Wong, Y.S.; Liew, M.W.J.; Venkatraman, S. Recent Advances in Chitosan-Based Carriers for Gene Delivery. Mar. Drugs 2019, 17, 381. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, M.; Tomaro-Duchesneau, C.; Prakash, S. Synthesis of TAT Peptide-Tagged PEGylated Chitosan Nanoparticles for SiRNA Delivery Targeting Neurodegenerative Diseases. Biomaterials 2013, 34, 1270–1280. [Google Scholar] [CrossRef]
- Altay Benetti, A.; Tan, E.Y.Z.; Chang, Z.W.; Bae, K.H.; Thwin, M.T.; Muthuramalingam, R.P.K.; Liao, K.C.; Wan, Y.; Ng, L.F.P.; Renia, L.; et al. Design and Characterization of a New Formulation for the Delivery of COVID-19-MRNA Vaccine to the Nasal Mucosa. Vaccines 2024, 12, 409. [Google Scholar] [CrossRef]
- Csaba, N.; Köping-Höggård, M.; Alonso, M.J. Ionically Crosslinked Chitosan/Tripolyphosphate Nanoparticles for Oligonucleotide and Plasmid DNA Delivery. Int. J. Pharm. 2009, 382, 205–214. [Google Scholar] [CrossRef]
- Şalva, E.; Kabasakal, L.; Eren, F.; Özkan, N.; Çakalaǧaoǧlu, F.; Akbuǧa, J. Local Delivery of Chitosan/VEGF SiRNA Nanoplexes Reduces Angiogenesis and Growth of Breast Cancer in Vivo. Nucleic Acid Ther. 2012, 22, 40–48. [Google Scholar] [CrossRef]
- Kaban, K.; Salva, E.; Akbuga, J. Modulation of the Dual-Faced Effects of MiR-141 with Chitosan/MiR-141 Nanoplexes in Breast Cancer Cells. J. Gene Med. 2019, 21, e3116. [Google Scholar] [CrossRef]
- Fathi-Karkan, S.; Mirinejad, S.; Ulucan-Karnak, F.; Mukhtar, M.; Ghahramani Almanghadim, H.; Sargazi, S.; Rahdar, A.; Díez-Pascual, A.M. Biomedical Applications of Aptamer-Modified Chitosan Nanomaterials: An Updated Review. Int. J. Biol. Macromol. 2023, 238, 124103. [Google Scholar] [CrossRef]
- Erbacher, P.; Zou, S.; Bettinger, T.; Steffan, A.M.; Remy, J.S. Chitosan-Based Vector/DNA Complexes for Gene Delivery: Biophysical Characteristics and Transfection Ability. Pharm. Res. 1998, 15, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Aral, C.; Özbas-Turan, S.; Kabasakal, L.; Keyer-Uysal, M.; Akbuga, J. Studies of Effective Factors of Plasmid DNA-Loaded Chitosan Microspheres I. Plasmid Size, Chitosan Concentration and Plasmid Addition Techniques. STP Pharma Sci. 2000, 10, 83–88. [Google Scholar]
- Genedy, H.H.; Delair, T.; Alcouffe, P.; Crépet, A.; Chatre, E.; Alhareth, K.; Montembault, A. Nanoassemblies of Chitosan-Based Polyelectrolyte Complexes as Nucleic Acid Delivery Systems. Biomacromolecules 2024, 25, 4780–4796. [Google Scholar] [CrossRef] [PubMed]
- Radeva, L.; Yoncheva, K. Nanogels—Innovative Drug Carriers for Overcoming Biological Membranes. Gels 2025, 11, 124. [Google Scholar] [CrossRef]
- Akbuğa, J.; Özbaş-Turan, S.; Ekentok, C. Chitosan Nanoparticles in Gene Delivery. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; Springer: Berlin/Heidelberg, Germany, 2016; pp. 337–351. [Google Scholar] [CrossRef]
- Pereira, P.; Morgado, D.; Crepet, A.; David, L.; Gama, F.M. Glycol Chitosan-Based Nanogel as a Potential Targetable Carrier for SiRNA. Macromol. Biosci. 2013, 13, 1369–1378. [Google Scholar] [CrossRef][Green Version]
- Cao, Y.; Tan, Y.F.; Wong, Y.S.; Aminuddin, M.; Ramya, B.; Liew, M.W.J.; Liu, J.; Venkatraman, S.S. Designing SiRNA/Chitosan-Methacrylate Complex Nanolipogel for Prolonged Gene Silencing Effects. Sci. Rep. 2022, 12, 3527. [Google Scholar] [CrossRef]
- Yu, Q.; Gao, Y.; Dai, W.; Li, D.; Zhang, L.; Hameed, M.M.A.; Guo, R.; Liu, M.; Shi, X.; Cao, X. Cell Membrane-Camouflaged Chitosan-Polypyrrole Nanogels Co-Deliver Drug and Gene for Targeted Chemotherapy and Bone Metastasis Inhibition of Prostate Cancer. Adv. Healthc. Mater. 2024, 13, e2400114. [Google Scholar] [CrossRef]
- Lee, J.I.; Kim, H.S.; Yoo, H.S. DNA Nanogels Composed of Chitosan and Pluronic with Thermo-Sensitive and Photo-Crosslinking Properties. Int. J. Pharm. 2009, 373, 93–99. [Google Scholar] [CrossRef]
- Tian, R.; Xian, L.; Li, Y.; Zheng, X. Silica Modified Chitosan/Polyethylenimine Nanogel for Improved Stability and Gene Carrier Ability. J. Nanosci. Nanotechnol. 2016, 16, 5426–5431. [Google Scholar] [CrossRef]
- Pereira, P.; Pedrosa, S.S.; Wymant, J.M.; Sayers, E.; Correia, A.; Vilanova, M.; Jones, A.T.; Gama, F.M. SiRNA Inhibition of Endocytic Pathways to Characterize the Cellular Uptake Mechanisms of Folate-Functionalized Glycol Chitosan Nanogels. Mol. Pharm. 2015, 12, 1970–1979. [Google Scholar] [CrossRef]
- Lin, Z.; Li, S.; Wu, Q.; Qu, H.; Shi, X.; Wang, K.; Tang, C.; Yin, C. In Situ Customized Apolipoprotein B48-Enriched Protein Corona Enhances Oral Gene Delivery of Chitosan-Based Nanoparticles. Biomaterials 2024, 311, 122704. [Google Scholar] [CrossRef]
- Naghib, S.M.; Ahmadi, B.; Mozafari, M.R. Smart Physicochemical-Triggered Chitosan-Based Nanogels for SiRNA Delivery and Gene Therapy: A Focus on Emerging Strategies and Paradigms for Cancer Therapy. Curr. Med. Chem. 2024, 32, 4913–4946. [Google Scholar] [CrossRef]
- Gaj, T.; Gersbach, C.A.; Barbas, C.F. ZFN, TALEN, and CRISPR/Cas-Based Methods for Genome Engineering. Trends Biotechnol. 2013, 31, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Doudna, J.A.; Charpentier, E. Genome Editing. The New Frontier of Genome Engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [Google Scholar] [CrossRef] [PubMed]
- Morshedzadeh, F.; Ghanei, M.; Lotfi, M.; Ghasemi, M.; Ahmadi, M.; Najari-Hanjani, P.; Sharif, S.; Mozaffari-Jovin, S.; Peymani, M.; Abbaszadegan, M.R. An Update on the Application of CRISPR Technology in Clinical Practice. Mol. Biotechnol. 2024, 66, 179–197. [Google Scholar] [CrossRef] [PubMed]
- ISBN 1262300109565. Available online: https://Crisprmedicinenews.Com/Clinical-Trials/ (accessed on 28 April 2025).
- Hirakawa, M.P.; Krishnakumar, R.; Timlin, J.A.; Carney, J.P.; Butler, K.S. Gene Editing and CRISPR in the Clinic: Current and Future Perspectives. Biosci. Rep. 2020, 40, BSR20200127. [Google Scholar] [CrossRef]
- Available online: www.Clinicaltrials.Gov (accessed on 21 April 2025).
- Nugrahaningsih, D.A.A.; Purnomo, E.; Wasityastuti, W.; Martien, R.; Arfian, N.; Hartatik, T. BMPR2 Editing in Fibroblast NIH3T3 Using CRISPR/Cas9 Affecting BMPR2 MRNA Expression and Proliferation. Indones. Biomed. J. 2022, 14, 45–51. [Google Scholar] [CrossRef]
- Zhang, B.C.; Wu, P.Y.; Zou, J.J.; Jiang, J.L.; Zhao, R.R.; Luo, B.Y.; Liao, Y.Q.; Shao, J.W. Efficient CRISPR/Cas9 Gene-Chemo Synergistic Cancer Therapy via a Stimuli-Responsive Chitosan-Based Nanocomplex Elicits Anti-Tumorigenic Pathway Effect. Chem. Eng. J. 2020, 393, 124688. [Google Scholar] [CrossRef]
- Li, Q.; Lv, X.; Tang, C.; Yin, C. Co-Delivery of Doxorubicin and CRISPR/Cas9 or RNAi-Expressing Plasmid by Chitosan-Based Nanoparticle for Cancer Therapy. Carbohydr. Polym. 2022, 287, 119315. [Google Scholar] [CrossRef]
- Khademi, Z.; Ramezani, M.; Alibolandi, M.; Zirak, M.R.; Salmasi, Z.; Abnous, K.; Taghdisi, S.M. A Novel Dual-Targeting Delivery System for Specific Delivery of CRISPR/Cas9 Using Hyaluronic Acid, Chitosan and AS1411. Carbohydr. Polym. 2022, 292, 119691. [Google Scholar] [CrossRef]
- Yoshinaga, N.; Zhou, J.K.; Xu, C.; Quek, C.H.; Zhu, Y.; Tang, D.; Hung, L.Y.; Najjar, S.A.; Shiu, C.Y.A.; Margolis, K.G.; et al. Phenylboronic Acid-Functionalized Polyplexes Tailored to Oral CRISPR Delivery. Nano Lett. 2023, 23, 757–764. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arpa, M.D.; Akbuğa, F.J. Chitosan-Based Nanogels in Modern Drug Delivery: Focus on Protein and Gene Applications. Gels 2025, 11, 735. https://doi.org/10.3390/gels11090735
Arpa MD, Akbuğa FJ. Chitosan-Based Nanogels in Modern Drug Delivery: Focus on Protein and Gene Applications. Gels. 2025; 11(9):735. https://doi.org/10.3390/gels11090735
Chicago/Turabian StyleArpa, Muhammet Davut, and Fatma Julide Akbuğa. 2025. "Chitosan-Based Nanogels in Modern Drug Delivery: Focus on Protein and Gene Applications" Gels 11, no. 9: 735. https://doi.org/10.3390/gels11090735
APA StyleArpa, M. D., & Akbuğa, F. J. (2025). Chitosan-Based Nanogels in Modern Drug Delivery: Focus on Protein and Gene Applications. Gels, 11(9), 735. https://doi.org/10.3390/gels11090735






