Click Chemistry-Based Hydrogels for Tissue Engineering
Abstract
1. Introduction
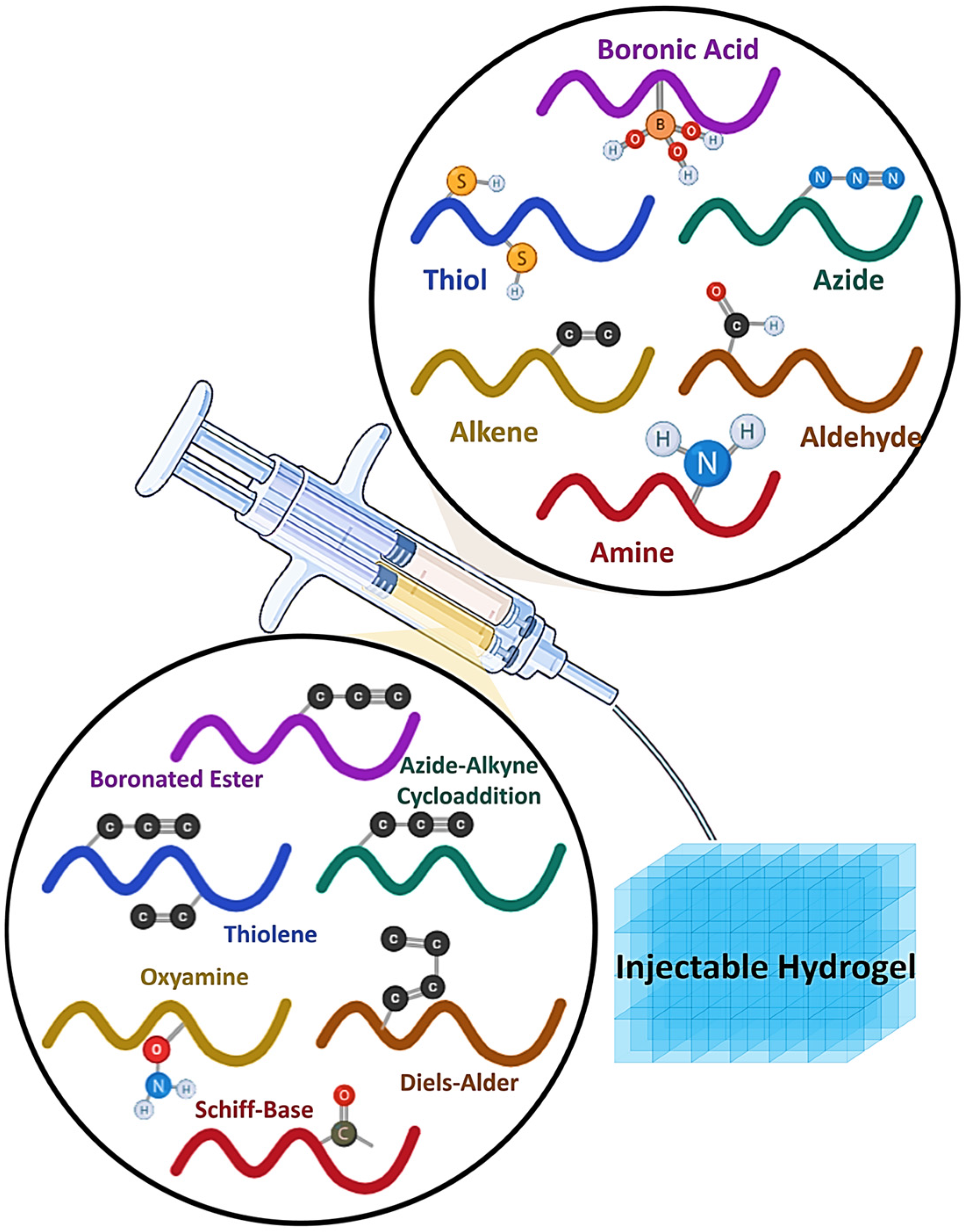
2. Fundamentals of Click Chemistry in Hydrogel Design
Definition and Key Principles of Click Chemistry
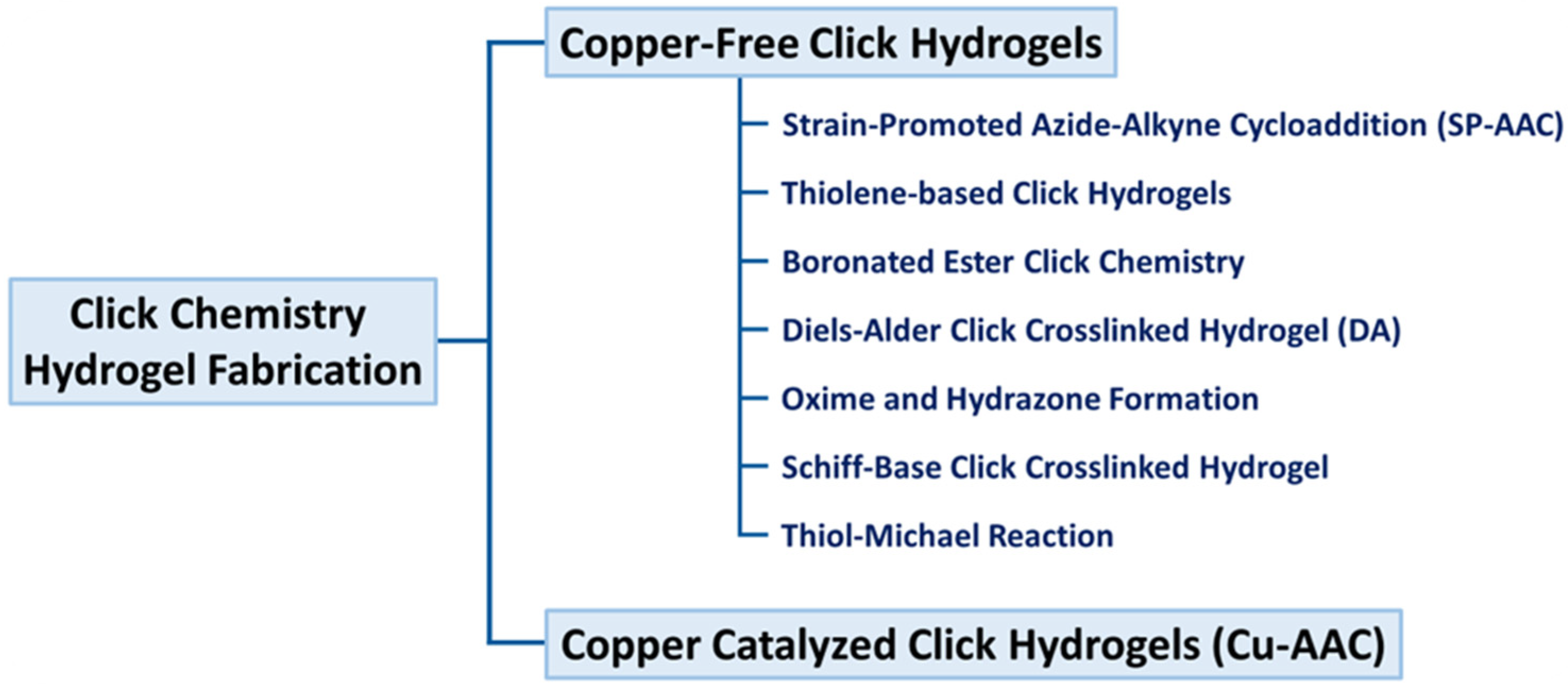
3. Major Click Reactions Used in Hydrogels
3.1. Azide–Alkyne Cycloaddition (Cu/SPAAC)
3.2. Thiol-Ene Based Click Hydrogels
3.3. Boronated Ester Click Chemistry
3.4. Diels–Alder Click Chemistry
3.5. Oxime and Hydrazone Formation
3.6. Pseudo-Click Hydrogels
3.7. Schiff Base Reaction
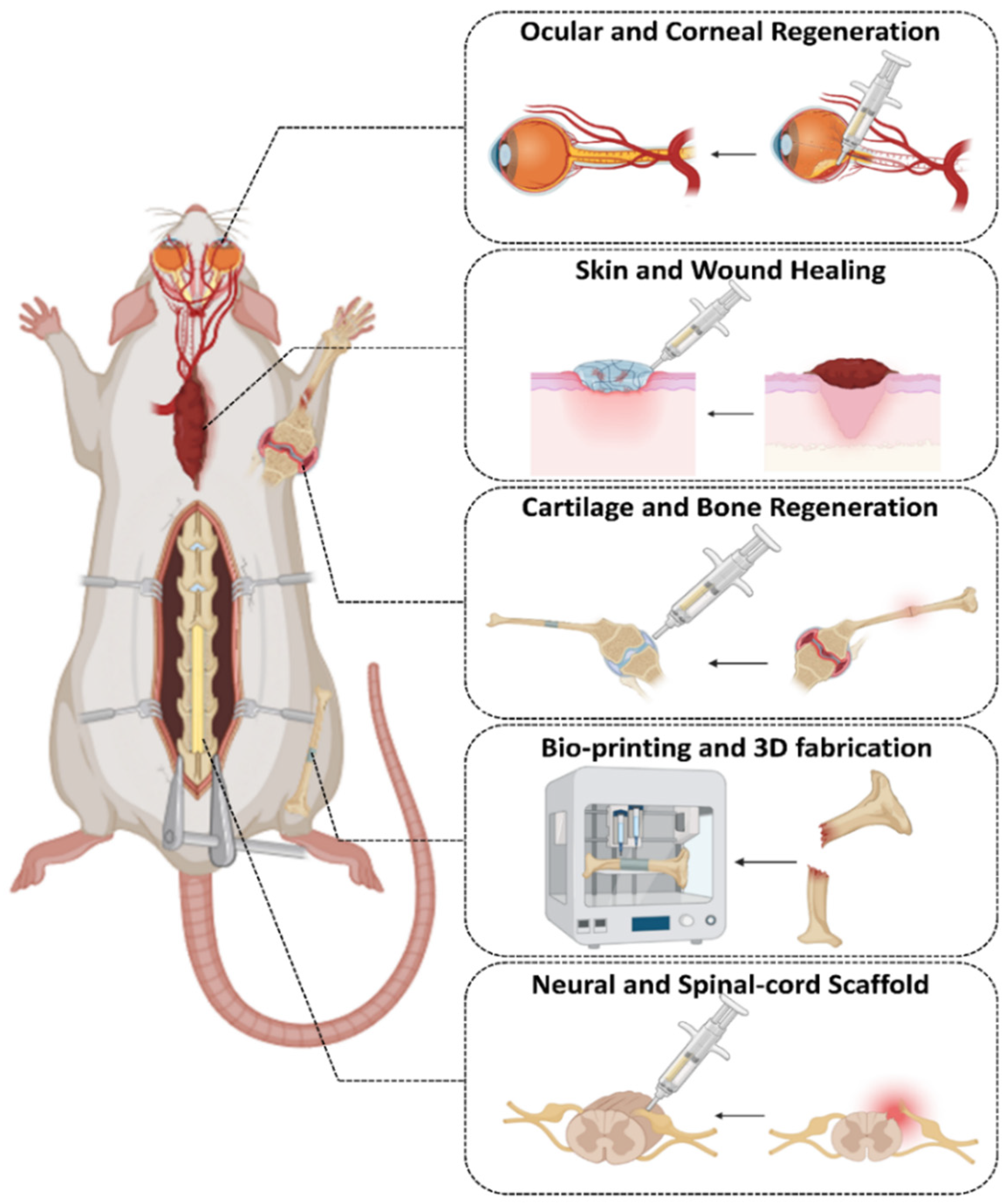
4. Applications in Tissue Engineering
4.1. Cartilage and Bone Tissue Engineering
4.2. Skin and Wound Healing
4.3. Neural and Spinal Cord Scaffolds
4.4. Ocular and Corneal Regeneration
4.5. Bioprinting and 3D Fabrication Using Click-Based Inks
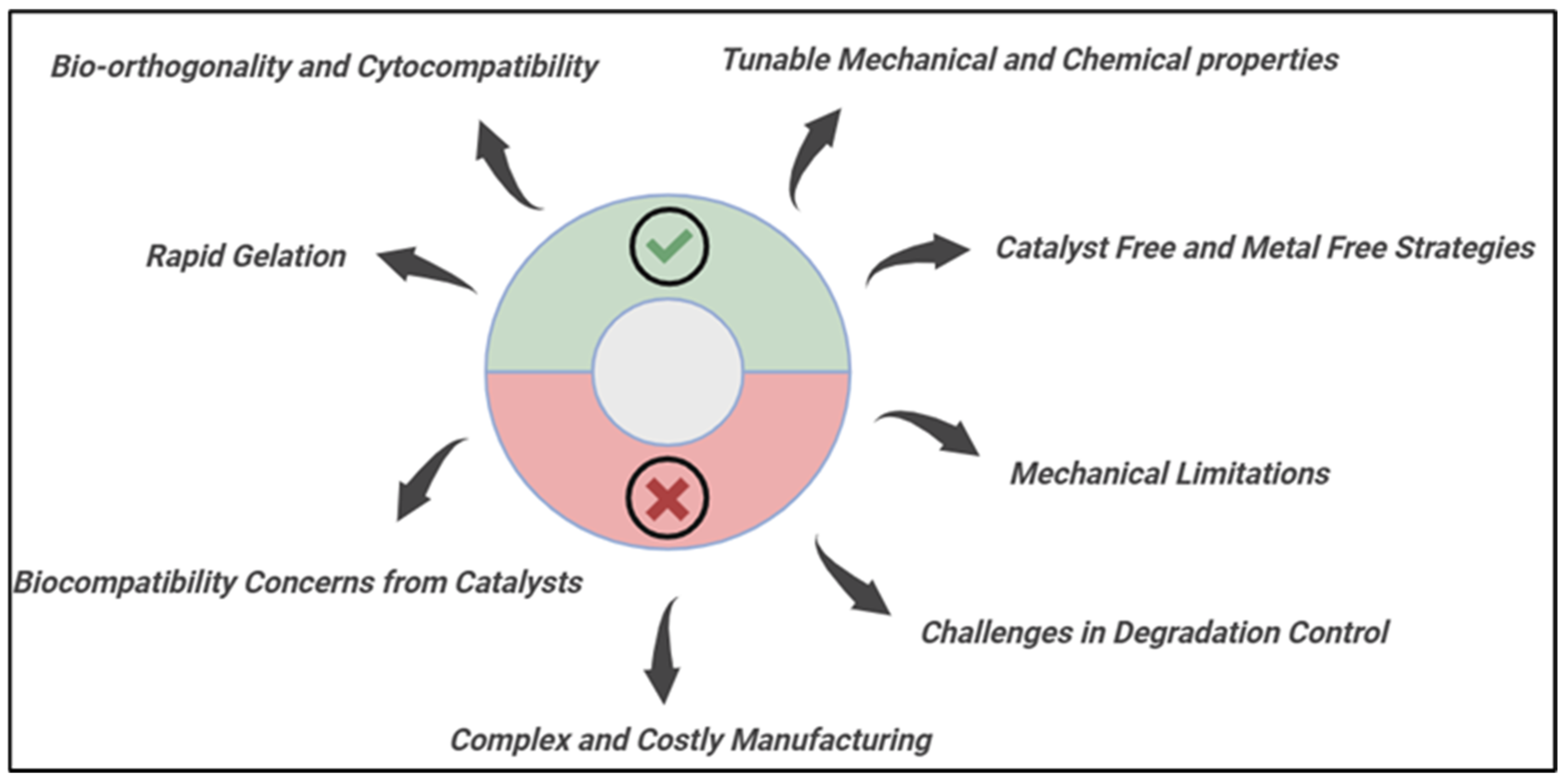
5. Advantages and Limitations of Click Chemistry in Hydrogels
5.1. Advantages
5.2. Disadvantages
6. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arabpour, Z.; Salehi, M.; An, S.; Moghtader, A.; Anwar, K.N.; Baharnoori, S.M.; Shah, R.J.; Abedi, F.; Djalilian, A.R. Exploring hydrogel nanoparticle systems for enhanced ocular drug delivery. Gels 2024, 10, 589. [Google Scholar] [CrossRef]
- Waqar, M.A.; Mubarak, N.; Khan, A.M.; Shaheen, F.; Mustafa, M.A.; Riaz, T. Recent advances in polymers, preparation techniques, applications and future perspectives of hydrogels. Int. J. Polym. Mater. Polym. Biomater. 2025, 74, 265–284. [Google Scholar] [CrossRef]
- Mahheidari, N.; Alizadeh, M.; Farahani, M.K.; Arabpour, Z.; Djalilian, A.R.; Salehi, M. Regeneration of the skin wound by two different crosslinkers: In vitro and in vivo studies. Iran. J. Basic Med. Sci. 2025, 28, 194. [Google Scholar]
- El-Sayed, N.S.; Fahmy, T.Y.; Kamel, S. Stimuli-responsive hydrogels derived from agricultural residues: An overview. Chem. Pap. 2025, 79, 3475–3491. [Google Scholar] [CrossRef]
- Sojdeh, S.; Panjipour, A.; Bejandi, Z.B.; Salehi, M.; Yaghmour, A.; Arabpour, Z.; Djalilian, A.R.; Chan, R.V.P. Hydrogel-Based Vitreous Substitutes. Int. J. Mol. Sci. 2025, 26, 8406. [Google Scholar] [CrossRef]
- Arabpour, Z.; Youseffi, M.; Soon, C.F.; Sultana, N.; Bazgeir, M.R.; Masoud, M.; Sefat, F. Designing biomaterials for regenerative medicine: State-of-the-art and future perspectives. In Tissue Engineering Strategies for Organ Regeneration; CRC Press: Boca Raton, FL, USA, 2020; pp. 1–9. [Google Scholar]
- Nie, L.; Sun, Y.; Okoro, O.V.; Deng, Y.; Jiang, G.; Shavandi, A. Click chemistry for 3D bioprinting. Mater. Horiz. 2023, 10, 2727–2763. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Han, Y.; Sun, H.-Y.; Hilborn, J.; Shi, L. Click chemistry-based biopolymeric hydrogels for regenerative medicine. Biomed. Mater. 2021, 16, 022003. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, L.; Peng, J.; Xing, M.; Han, Y.; Wang, X.; Xu, Y.; Chang, J. Bioglass activated albumin hydrogels for wound healing. Adv. Healthc. Mater. 2018, 7, 1800144. [Google Scholar] [CrossRef]
- Gao, G.; Jiang, Y.-W.; Jia, H.-R.; Wu, F.-G. Near-infrared light-controllable on-demand antibiotics release using thermo-sensitive hydrogel-based drug reservoir for combating bacterial infection. Biomaterials 2019, 188, 83–95. [Google Scholar] [CrossRef]
- Ullah, A.; Lim, S.I. Bioinspired tunable hydrogels: An update on methods of preparation, classification, and biomedical and therapeutic applications. Int. J. Pharm. 2022, 612, 121368. [Google Scholar] [CrossRef]
- Haider, A.; Kortz, U.; Afridi, S.; Sohail, M.; Joshi, S.A.; Iqbal, J. Novel pH responsive supramolecular hydrogels of chitosan hydrochloride and polyoxometalate: In-vitro, in-vivo and preliminary safety evaluation. Int. J. Pharm. 2017, 533, 125–137. [Google Scholar]
- Li, S.; Xia, Y.; Qiu, Y.; Chen, X.; Shi, S. Preparation and property of starch nanoparticles reinforced aldehyde–hydrazide covalently crosslinked PNIPAM hydrogels. J. Appl. Polym. Sci. 2018, 135, 45761. [Google Scholar] [CrossRef]
- Patel, P.; Thareja, P. Hydrogels differentiated by length scales: A review of biopolymer-based hydrogel preparation methods, characterization techniques, and targeted applications. Eur. Polym. J. 2022, 163, 110935. [Google Scholar] [CrossRef]
- Lowe, A.B. Thiol-ene “click” reactions and recent applications in polymer and materials synthesis. Polym. Chem. 2010, 1, 17–36. [Google Scholar] [CrossRef]
- DeForest, C.A.; Anseth, K.S. Cytocompatible click-based hydrogels with dynamically tunable properties through orthogonal photoconjugation and photocleavage reactions. Nat. Chem. 2011, 3, 925–931. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, J.; Deng, C.; Suuronen, E.J.; Zhong, Z. Click hydrogels, microgels and nanogels: Emerging platforms for drug delivery and tissue engineering. Biomaterials 2014, 35, 4969–4985. [Google Scholar] [CrossRef]
- Truong, V.X.; Tsang, K.M.; Forsythe, J.S. Nonswelling click-cross-linked gelatin and PEG hydrogels with tunable properties using pluronic linkers. Biomacromolecules 2017, 18, 757–766. [Google Scholar] [CrossRef]
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef]
- Li, X.; Xiong, Y. Application of “click” chemistry in biomedical hydrogels. ACS Omega 2022, 7, 36918–36928. [Google Scholar] [CrossRef]
- Moradi, M.; Parvizpour, F.; Arabpour, Z.; Zargarzadeh, N.; Nazari, M.; Rashnavadi, H.; Sefat, F.; Dehghani, S.; Latifi, M.; Jafarian, A. Articular cartilage injury; current status and future direction. Curr. Stem Cell Res. Ther. 2024, 19, 653–661. [Google Scholar] [CrossRef]
- Sawhney, A.S.; Pathak, C.P.; van Rensburg, J.J.; Dunn, R.C.; Hubbell, J.A. Optimization of photopolymerized bioerodible hydrogel properties for adhesion prevention. J. Biomed. Mater. Res. 1994, 28, 831–838. [Google Scholar] [CrossRef]
- Hubbell, J.A. Hydrogel systems for barriers and local drug delivery in the control of wound healing. J. Control. Release 1996, 39, 305–313. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Barner-Kowollik, C.; Du Prez, F.E.; Espeel, P.; Hawker, C.J.; Junkers, T.; Schlaad, H.; Van Camp, W. “Clicking” polymers or just efficient linking: What is the difference. Angew. Chem. Int. Ed. 2011, 50, 60–62. [Google Scholar] [CrossRef]
- Yang, W.; Chen, J.; Yan, J.; Liu, S.; Yan, Y.; Zhang, Q. Advance of click chemistry in anion exchange membranes for energy application. J. Polym. Sci. 2022, 60, 627–649. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, J.; Mi, Y.; Dong, F.; Li, Q.; Guo, Z. Enhanced antifungal activity of novel cationic chitosan derivative bearing triphenylphosphonium salt via azide-alkyne click reaction. Int. J. Biol. Macromol. 2020, 165, 1765–1772. [Google Scholar] [CrossRef]
- Fan, J.; Bao, B.; Wang, Z.; Xu, R.; Wang, W.; Yu, D. High tri-stimulus response photochromic cotton fabrics based on spiropyran dye by thiol-ene click chemistry. Cellulose 2020, 27, 493–510. [Google Scholar] [CrossRef]
- Franco, C.A.; da Silva, T.I.; Dias, M.G.; Ferreira, B.W.; de Sousa, B.L.; Bousada, G.M.; Barreto, R.W.; Vaz, B.G.; Lima, G.d.S.; Dos Santos, M.H. Synthesis of tyrosol 1, 2, 3-triazole derivatives and their phytotoxic activity against Euphorbia heterophylla. J. Agric. Food Chem. 2022, 70, 2806–2816. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, J.; Noh, I. Click chemistry-based injectable hydrogels and bioprinting inks for tissue engineering applications. Tissue Eng. Regen. Med. 2018, 15, 531–546. [Google Scholar] [CrossRef]
- Mueller, E.; Poulin, I.; Bodnaryk, W.J.; Hoare, T. Click chemistry hydrogels for extrusion bioprinting: Progress, challenges, and opportunities. Biomacromolecules 2022, 23, 619–640. [Google Scholar] [CrossRef]
- Sletten, E.M.; Bertozzi, C.R. Bioorthogonal chemistry: Fishing for selectivity in a sea of functionality. Angew. Chem. Int. Ed. 2009, 48, 6974–6998. [Google Scholar] [CrossRef] [PubMed]
- Debets, M.F.; Van Berkel, S.S.; Schoffelen, S.; Rutjes, F.P.; Van Hest, J.C.; Van Delft, F.L. Aza-dibenzocyclooctynes for fast and efficient enzyme PEGylation via copper-free (3 + 2) cycloaddition. Chem. Commun. 2010, 46, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Hoyle, C.E.; Bowman, C.N. Thiol–ene click chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef]
- Fairbanks, B.D.; Schwartz, M.P.; Bowman, C.N.; Anseth, K.S. Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2, 4, 6-trimethylbenzoylphosphinate: Polymerization rate and cytocompatibility. Biomaterials 2009, 30, 6702–6707. [Google Scholar] [CrossRef]
- Briou, B.; Améduri, B.; Boutevin, B. Trends in the Diels–Alder reaction in polymer chemistry. Chem. Soc. Rev. 2021, 50, 11055–11097. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Hubbell, J. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Phelps, E.A.; Landázuri, N.; Thulé, P.M.; Taylor, W.R.; García, A.J. Bioartificial matrices for therapeutic vascularization. Proc. Natl. Acad. Sci. USA 2010, 107, 3323–3328. [Google Scholar] [CrossRef]
- Dirksen, A.; Hackeng, T.M.; Dawson, P.E. Nucleophilic catalysis of oxime ligation. Angew. Chem. 2006, 118, 7743–7746. [Google Scholar] [CrossRef]
- Grover, G.N.; Lam, J.; Nguyen, T.H.; Segura, T.; Maynard, H.D. Biocompatible hydrogels by oxime click chemistry. Biomacromolecules 2012, 13, 3013–3017. [Google Scholar] [CrossRef]
- Oliveira, A.B.; Guo, Z.; Bernardes, G. Inverse electron demand Diels–Alder reactions in chemical biology. Chem. Soc. Rev. 2017, 46, 4895–4950. [Google Scholar] [CrossRef] [PubMed]
- Blackman, M.L.; Royzen, M.; Fox, J.M. Tetrazine ligation: Fast bioconjugation based on inverse-electron-demand Diels− Alder reactivity. J. Am. Chem. Soc. 2008, 130, 13518–13519. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Krasnova, L.; Finn, M.; Sharpless, K.B. Sulfur (VI) fluoride exchange (SuFEx): Another good reaction for click chemistry. Angew. Chem. Int. Ed. 2014, 53, 9430–9448. [Google Scholar] [CrossRef] [PubMed]
- Barrow, A.; Smedley, C.; Zheng, Q.; Li, S.; Dong, J.; Moses, J. The growing applications of SuFEx click chemistry. Chem. Soc. Rev. 2019, 48, 4731–4758. [Google Scholar] [CrossRef]
- Lee, S.M.; Jang, W.-D. Polyion complex micelle formed from tetraphenylethene containing block copolymer. Biomater. Res. 2017, 21, 17. [Google Scholar] [CrossRef]
- Yi, G.; Son, J.; Yoo, J.; Park, C.; Koo, H. Application of click chemistry in nanoparticle modification and its targeted delivery. Biomater. Res. 2018, 22, 13. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, Y.; Le Thi, P.; Oh, D.H.; Park, K.D. Sulfobetaine methacrylate hydrogel-coated anti-fouling surfaces for implantable biomedical devices. Biomater. Res. 2018, 22, 3. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Vermonden, T.; Hennink, W.E. Hydrogels for therapeutic delivery: Current developments and future directions. Biomacromolecules 2017, 18, 316–330. [Google Scholar] [CrossRef]
- Agard, N.J.; Prescher, J.A.; Bertozzi, C.R. A strain-promoted [3 + 2] azide− alkyne cycloaddition for covalent modification of biomolecules in living systems. J. Am. Chem. Soc. 2004, 126, 15046–15047. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, L.; Yang, L.; Zhu, F.; Ding, M.; Lin, F.; Wang, Z.; Li, Y. “Click” chemistry in polymeric scaffolds: Bioactive materials for tissue engineering. J. Control. Release 2018, 273, 160–179. [Google Scholar] [CrossRef]
- Macdougall, L.J.; Wiley, K.L.; Kloxin, A.M.; Dove, A.P. Design of synthetic extracellular matrices for probing breast cancer cell growth using robust cyctocompatible nucleophilic thiol-yne addition chemistry. Biomaterials 2018, 178, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, L.J.; Truong, V.X.; Dove, A.P. Efficient in situ nucleophilic thiol-yne click chemistry for the synthesis of strong hydrogel materials with tunable properties. ACS Macro Lett. 2017, 6, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Farahani, P.E.; Adelmund, S.M.; Shadish, J.A.; DeForest, C.A. Photomediated oxime ligation as a bioorthogonal tool for spatiotemporally-controlled hydrogel formation and modification. J. Mater. Chem. B 2017, 5, 4435–4442. [Google Scholar] [CrossRef]
- Aleahmad, F.; Ebrahimi, S.; Salmannezhad, M.; Azarnia, M.; Jaberipour, M.; Hoseini, M.; Talaei-Khozani, T. Heparin/collagen 3D scaffold accelerates hepatocyte differentiation of Wharton’s jelly-derived mesenchymal stem cells. Tissue Eng. Regen. Med. 2017, 14, 443–452. [Google Scholar] [CrossRef]
- Jung, C.S.; Kim, B.K.; Lee, J.; Min, B.-H.; Park, S.-H. Development of printable natural cartilage matrix bioink for 3D printing of irregular tissue shape. Tissue Eng. Regen. Med. 2018, 15, 155–162. [Google Scholar] [CrossRef]
- Gopinathan, J.; Noh, I. Recent trends in bioinks for 3D printing. Biomater. Res. 2018, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Yang, J.; Song, J.; Cao, X.; Zhou, B.; Yang, L.; Li, C.; Wang, Y. A motion-responsive injectable lubricative hydrogel for efficient Achilles tendon adhesion prevention. Mater. Today Bio 2025, 30, 101458. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, Y.; Ma, S.; Li, X.; Wang, W.; Chen, X.; Zheng, J.; Fan, Z.; Jiang, Y.; Liao, Y. Multifunctional DNA hydrogels with light-triggered gas-therapy and controlled G-Exos release for infected wound healing. Bioact. Mater. 2025, 52, 422–437. [Google Scholar] [CrossRef] [PubMed]
- Hein, J.E.; Fokin, V.V. Copper-catalyzed azide–alkyne cycloaddition (CuAAC) and beyond: New reactivity of copper (I) acetylides. Chem. Soc. Rev. 2010, 39, 1302–1315. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, S.; Li, K.; Tan, L.; Cen, L.; Fu, G. Well-defined and biocompatible hydrogels with toughening and reversible photoresponsive properties. Soft Matter 2016, 12, 2192–2199. [Google Scholar] [CrossRef]
- Pickens, C.J.; Johnson, S.N.; Pressnall, M.M.; Leon, M.A.; Berkland, C.J. Practical considerations, challenges, and limitations of bioconjugation via azide–alkyne cycloaddition. Bioconjugate Chem. 2017, 29, 686–701. [Google Scholar] [CrossRef]
- Xu, Z.; Bratlie, K.M. Click chemistry and material selection for in situ fabrication of hydrogels in tissue engineering applications. ACS Biomater. Sci. Eng. 2018, 4, 2276–2291. [Google Scholar] [CrossRef]
- Tseng, T.-C.; Hsieh, F.-Y.; Theato, P.; Wei, Y.; Hsu, S.-h. Glucose-sensitive self-healing hydrogel as sacrificial materials to fabricate vascularized constructs. Biomaterials 2017, 133, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Kim, G.B.; Shan, D.; Kim, J.P.; Hu, J.; Wang, W.; Hamad, F.G.; Qian, G.; Rizk, E.B.; Yang, J. Click chemistry improved wet adhesion strength of mussel-inspired citrate-based antimicrobial bioadhesives. Biomaterials 2017, 112, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Meng, F.; Jing, X.; Huang, Y. Combination of Anti-Biofouling and Ion-Interaction by Click Chemistry for Endotoxin Selective Removal from Protein Solution. Adv. Healthc. Mater. 2013, 2, 784–789. [Google Scholar] [CrossRef]
- Kennedy, D.C.; McKay, C.S.; Legault, M.C.; Danielson, D.C.; Blake, J.A.; Pegoraro, A.F.; Stolow, A.; Mester, Z.; Pezacki, J.P. Cellular consequences of copper complexes used to catalyze bioorthogonal click reactions. J. Am. Chem. Soc. 2011, 133, 17993–18001. [Google Scholar] [CrossRef]
- Baskin, J.M.; Prescher, J.A.; Laughlin, S.T.; Agard, N.J.; Chang, P.V.; Miller, I.A.; Lo, A.; Codelli, J.A.; Bertozzi, C.R. Copper-free click chemistry for dynamic in vivo imaging. Proc. Natl. Acad. Sci. USA 2007, 104, 16793–16797. [Google Scholar] [CrossRef]
- Han, S.-S.; Yoon, H.Y.; Yhee, J.Y.; Cho, M.O.; Shim, H.-E.; Jeong, J.-E.; Lee, D.-E.; Kim, K.; Guim, H.; Lee, J.H. In situ cross-linkable hyaluronic acid hydrogels using copper free click chemistry for cartilage tissue engineering. Polym. Chem. 2018, 9, 20–27. [Google Scholar] [CrossRef]
- Fan, M.; Ma, Y.; Mao, J.; Zhang, Z.; Tan, H. Cytocompatible in situ forming chitosan/hyaluronan hydrogels via a metal-free click chemistry for soft tissue engineering. Acta Biomater. 2015, 20, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Le, P.; Fernandes-Cunha, G.M.; Heilshorn, S.C.; Myung, D. Bio-orthogonally crosslinked hyaluronate-collagen hydrogel for suture-free corneal defect repair. Biomaterials 2020, 255, 120176. [Google Scholar] [CrossRef]
- Liu, X.; Camilleri, E.T.; Li, L.; Gaihre, B.; Rezaei, A.; Park, S.; Miller II, A.L.; Tilton, M.; Waletzki, B.E.; Terzic, A. Injectable catalyst-free “click” organic-inorganic nanohybrid (click-ON) cement for minimally invasive in vivo bone repair. Biomaterials 2021, 276, 121014. [Google Scholar] [CrossRef]
- Zhou, P.; Brown, L.; Madl, C.M. Bioorthogonal Engineering of Cellular Microenvironments Using Isonitrile Ligations. Adv. Funct. Mater. 2025, 2422047. [Google Scholar] [CrossRef]
- Chang, S.-Y.; McAnena, A.P.; Kim, J.; Song, J. Tuning Hyaluronic Acid Microstructures by Engineered Amphiphilicity: From Dynamically Cross-Linked Gels to Multilayered Nanoparticles. ACS Appl. Mater. Interfaces 2025, 17, 31909–31922. [Google Scholar] [CrossRef]
- Jain, E.; Neal, S.; Graf, H.; Tan, X.; Balasubramaniam, R.; Huebsch, N. Copper-free azide–alkyne cycloaddition for peptide modification of alginate hydrogels. ACS Appl. Bio Mater. 2021, 4, 1229–1237. [Google Scholar] [CrossRef]
- Neves, M.I.; Magalhães, M.V.; Bidarra, S.J.; Moroni, L.; Barrias, C.C. Versatile click alginate hydrogels with protease-sensitive domains as cell responsive/instructive 3D microenvironments. Carbohydr. Polym. 2023, 320, 121226. [Google Scholar] [CrossRef]
- Degirmenci, A.; Sanyal, R.; Sanyal, A. Metal-free click-chemistry: A powerful tool for fabricating hydrogels for biomedical applications. Bioconjugate Chem. 2024, 35, 433–452. [Google Scholar] [CrossRef]
- Luu, T.; Gristwood, K.; Knight, J.C.; Jorg, M. Click chemistry: Reaction rates and their suitability for biomedical applications. Bioconjugate Chem. 2024, 35, 715–731. [Google Scholar] [CrossRef]
- Neves, M.I.; Bidarra, S.J.; Magalhães, M.V.; Torres, A.L.; Moroni, L.; Barrias, C.C. Microstructured click hydrogels for cell contact guidance in 3D. Mater. Today Bio 2023, 19, 100604. [Google Scholar] [CrossRef] [PubMed]
- Testore, D.; Zoso, A.; Paoletti, C.; Groppo, S.; Marcello, E.; Chiono, V. Bio-orthogonally double cross-linked alginate-gelatin hydrogels with tunable viscoelasticity for cardiac tissue engineering. Mater. Today Bio 2025, 34, 102121. [Google Scholar] [CrossRef] [PubMed]
- Rydholm, A.E.; Bowman, C.N.; Anseth, K.S. Degradable thiol-acrylate photopolymers: Polymerization and degradation behavior of an in situ forming biomaterial. Biomaterials 2005, 26, 4495–4506. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.; Scott, T.F.; Kloxin, C.J.; Bowman, C.N. Click chemistry in materials science. Adv. Funct. Mater. 2014, 24, 2572–2590. [Google Scholar] [CrossRef]
- Pereira, R.F.; Barrias, C.C.; Bártolo, P.J.; Granja, P.L. Cell-instructive pectin hydrogels crosslinked via thiol-norbornene photo-click chemistry for skin tissue engineering. Acta Biomater. 2018, 66, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.E.; Carberry, B.J.; Worrell, B.T.; Dudaryeva, O.Y.; McBride, M.K.; Bowman, C.N.; Anseth, K.S. Photopolymerized dynamic hydrogels with tunable viscoelastic properties through thioester exchange. Biomaterials 2018, 178, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Raza, A.; Shih, H. PEG hydrogels formed by thiol-ene photo-click chemistry and their effect on the formation and recovery of insulin-secreting cell spheroids. Biomaterials 2011, 32, 9685–9695. [Google Scholar] [CrossRef] [PubMed]
- Colak, B.; Di Cio, S.; Gautrot, J.E. Biofunctionalized patterned polymer brushes via thiol–ene coupling for the control of cell adhesion and the formation of cell arrays. Biomacromolecules 2018, 19, 1445–1455. [Google Scholar] [CrossRef]
- Sharma, S.; Floren, M.; Ding, Y.; Stenmark, K.R.; Tan, W.; Bryant, S.J. A photoclickable peptide microarray platform for facile and rapid screening of 3-D tissue microenvironments. Biomaterials 2017, 143, 17–28. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, X.; Chen, Z.; Li, S.; Yan, C. Thiol–Ene click reaction initiated rapid gelation of PEGDA/silk fibroin hydrogels. Polymers 2019, 11, 2102. [Google Scholar] [CrossRef]
- Lueckgen, A.; Garske, D.S.; Ellinghaus, A.; Mooney, D.J.; Duda, G.N.; Cipitria, A. Enzymatically-degradable alginate hydrogels promote cell spreading and in vivo tissue infiltration. Biomaterials 2019, 217, 119294. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Li, T.; Zhang, L.; Azhar, U.; Ma, J.; Zhai, C.; Zong, C.; Zhang, S. Cytocompatible and non-fouling zwitterionic hyaluronic acid-based hydrogels using thiol-ene “click” chemistry for cell encapsulation. Carbohydr. Polym. 2020, 236, 116021. [Google Scholar] [CrossRef]
- Ding, H.; Li, B.; Liu, Z.; Liu, G.; Pu, S.; Feng, Y.; Jia, D.; Zhou, Y. Decoupled pH-and thermo-responsive injectable chitosan/PNIPAM hydrogel via thiol-ene click chemistry for potential applications in tissue engineering. Adv. Healthc. Mater. 2020, 9, 2000454. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, S.; Zhang, C.; Liang, K.; Li, J.; Yang, H.; Gu, S.; Bai, Z.; Ye, D.; Xu, W. Photopolymerized maleilated chitosan/thiol-terminated poly (vinyl alcohol) hydrogels as potential tissue engineering scaffolds. Carbohydr. Polym. 2018, 184, 383–389. [Google Scholar] [CrossRef]
- Nagahama, K.; Kimura, Y.; Takemoto, A. Living functional hydrogels generated by bioorthogonal cross-linking reactions of azide-modified cells with alkyne-modified polymers. Nat. Commun. 2018, 9, 2195. [Google Scholar] [CrossRef]
- Ding, Y.; Xu, X.; Sharma, S.; Floren, M.; Stenmark, K.; Bryant, S.J.; Neu, C.P.; Tan, W. Biomimetic soft fibrous hydrogels for contractile and pharmacologically responsive smooth muscle. Acta Biomater. 2018, 74, 121–130. [Google Scholar] [CrossRef]
- Troncoso-Afonso, L.; Henríquez-Banegas, Y.M.; Vinnacombe-Willson, G.A.; Gutierrez, J.; Gallastegui, G.; Liz-Marzán, L.M.; García-Astrain, C. Using thiol–ene click chemistry to engineer 3D printed plasmonic hydrogel scaffolds for SERS biosensing. Biomater. Sci. 2025, 13, 2936–2950. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.J.; Eckman, N.; Brunel, E.S.; Jons, C.K.; Sen, S.; Appel, E.A. A thiol–ene click-based strategy to customize injectable polymer–nanoparticle hydrogel properties for therapeutic delivery. Biomater. Sci. 2025, 13, 1323–1334. [Google Scholar] [CrossRef]
- Atmani, Z.; Steindorfer, T.; Kargl, R.; Kleinschek, K.S.; Heinze, T.; Gericke, M. Allyl-Functionalized Polysaccharides for 3D Printable Hydrogels Through Thiol–Ene Click Chemistry. Polysaccharides 2025, 6, 13. [Google Scholar] [CrossRef]
- Bakaic, E.; Smeets, N.M.; Hoare, T. Injectable hydrogels based on poly (ethylene glycol) and derivatives as functional biomaterials. RSC Adv. 2015, 5, 35469–35486. [Google Scholar] [CrossRef]
- Hu, B.-H.; Su, J.; Messersmith, P.B. Hydrogels cross-linked by native chemical ligation. Biomacromolecules 2009, 10, 2194–2200. [Google Scholar] [CrossRef]
- Gyarmati, B.; Némethy, Á.; Szilágyi, A. Reversible disulphide formation in polymer networks: A versatile functional group from synthesis to applications. Eur. Polym. J. 2013, 49, 1268–1286. [Google Scholar] [CrossRef]
- Zhang, B.Y.; He, W.D.; Li, L.Y.; Sun, X.L.; Li, W.T.; Zhang, K.R. Reducibly degradable hydrogels of PNIPAM and PDMAEMA: Synthesis, stimulus-response and drug release. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 3604–3612. [Google Scholar] [CrossRef]
- Meng, F.; Hennink, W.E.; Zhong, Z. Reduction-sensitive polymers and bioconjugates for biomedical applications. Biomaterials 2009, 30, 2180–2198. [Google Scholar] [CrossRef]
- Beaupre, D.M.; Weiss, R.G. Thiol-and disulfide-based stimulus-responsive soft materials and self-assembling systems. Molecules 2021, 26, 3332. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Yuan, J.; Jiang, Z.; Wang, S.; Wang, P.; Wang, Q.; Cui, L. Thiol-ene photoclick reaction: An eco-friendly and facile approach for preparation of MPEG-g-keratin biomaterial. Eng. Life Sci. 2020, 20, 17–25. [Google Scholar] [CrossRef]
- Wojda, S.J.; Marozas, I.A.; Anseth, K.S.; Yaszemski, M.J.; Donahue, S.W. Thiol-ene Hydrogels for Local Delivery of PTH for Bone Regeneration in Critical Size defects. J. Orthop. Res. 2020, 38, 536–544. [Google Scholar] [CrossRef]
- Kudaibergen, G.; Akhmetkarimova, Z.; Yildirim, E.; Baidarbekov, M. Thiol-ene clickable gelatin–hyaluronic acid cryogels. J. Mater. Sci. 2023, 58, 10821–10831. [Google Scholar] [CrossRef]
- Choh, S.-Y.; Cross, D.; Wang, C. Facile synthesis and characterization of disulfide-cross-linked hyaluronic acid hydrogels for protein delivery and cell encapsulation. Biomacromolecules 2011, 12, 1126–1136. [Google Scholar] [CrossRef]
- Huang, Z.; Delparastan, P.; Burch, P.; Cheng, J.; Cao, Y.; Messersmith, P.B. Injectable dynamic covalent hydrogels of boronic acid polymers cross-linked by bioactive plant-derived polyphenols. Biomater. Sci. 2018, 6, 2487–2495. [Google Scholar] [CrossRef]
- Marco-Dufort, B.; Tibbitt, M.W. Design of moldable hydrogels for biomedical applications using dynamic covalent boronic esters. Mater. Today Chem. 2019, 12, 16–33. [Google Scholar] [CrossRef]
- Amaral, A.J.; Gaspar, V.M.; Mano, J.F. Responsive laminarin-boronic acid self-healing hydrogels for biomedical applications. Polym. J. 2020, 52, 997–1006. [Google Scholar] [CrossRef]
- Cho, S.; Hwang, S.Y.; Oh, D.X.; Park, J. Recent progress in self-healing polymers and hydrogels based on reversible dynamic B–O bonds: Boronic/boronate esters, borax, and benzoxaborole. J. Mater. Chem. A 2021, 9, 14630–14655. [Google Scholar] [CrossRef]
- Wu, D.; Wang, W.; Diaz-Dussan, D.; Peng, Y.-Y.; Chen, Y.; Narain, R.; Hall, D.G. In situ forming, dual-crosslink network, self-healing hydrogel enabled by a bioorthogonal nopoldiol–benzoxaborolate click reaction with a wide pH range. Chem. Mater. 2019, 31, 4092–4102. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Wang, Q.; Han, Y.; Chen, H.; Tan, Y. Doubly dynamic hydrogel formed by combining boronate ester and acylhydrazone bonds. Polymers 2020, 12, 487. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.M.; Kim, S.H.; Phan, V.G.; Thambi, T.; Lee, D.S. Therapeutic effects of boronate ester cross-linked injectable hydrogels for the treatment of hepatocellular carcinoma. Biomater. Sci. 2021, 9, 7275–7286. [Google Scholar] [CrossRef]
- Wang, T.; Ma, L.; Zhang, H.; Cui, L.; Bai, X.; Kong, L.; Yang, S.; Wang, J. Preparation and Properties of Direct Ink Writing 3D Printing Hydrogel Ink Based on Dynamic Boronic Ester Bond. J. Appl. Polym. Sci. 2025, e57632. [Google Scholar] [CrossRef]
- Miki, R.; Yamaki, T.; Uchida, M.; Natsume, H. Phenylboronate-salicylate ester cross-linked self-healing hydrogel composed of modified hyaluronan at physiological pH. Soft Matter 2024, 20, 2926–2936. [Google Scholar] [CrossRef] [PubMed]
- Miki, R.; Yamaki, T.; Uchida, M.; Natsume, H. pH-responsive in situ gelation via phenylboronate-glucamine ester-crosslinking in modified hyaluronan at physiological pH. Colloid Polym. Sci. 2025, 1–11. [Google Scholar] [CrossRef]
- An, H.; Bo, Y.; Chen, D.; Wang, Y.; Wang, H.; He, Y.; Qin, J. Cellulose-based self-healing hydrogel through boronic ester bonds with excellent biocompatibility and conductivity. RSC Adv. 2020, 10, 11300–11310. [Google Scholar] [CrossRef]
- Banach, Ł.; Williams, G.T.; Fossey, J.S. Insulin delivery using dynamic covalent boronic acid/ester-controlled release. Adv. Ther. 2021, 4, 2100118. [Google Scholar] [CrossRef]
- Unger, K.; Coclite, A.M. Glucose-responsive boronic acid hydrogel thin films obtained via initiated chemical vapor deposition. Biomacromolecules 2022, 23, 4289–4295. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, Z.; Liu, S.; Zhong, X.; Wang, Y.; Xie, R.; Song, W.; Ren, L. Bioactive Phenylboronic Acid-Functionalized Hyaluronic Acid Hydrogels Induce Chondro-Aggregates and Promote Chondrocyte Phenotype. Macromol. Biosci. 2023, 23, 2300153. [Google Scholar] [CrossRef]
- Zhang, X.; Nan, K.; Zhang, Y.; Song, K.; Geng, Z.; Shang, D.; Guan, X.; Fan, L. A novel injectable hydrogel prepared from phenylboronic acid modified gelatin and oxidized-dextran for bone tissue engineering. Int. J. Biol. Macromol. 2024, 261, 129666. [Google Scholar] [CrossRef]
- Liu, F.; Li, G.; An, Z.; Wang, S.; Xu, S.; Liu, H. Dynamic Boronate Ester Based Hydrogel with Enhanced Mechanical Properties and Multi-Stimuli-Triggered Release for Tissue Repair and Antioxidant Therapy. Gels 2025, 11, 370. [Google Scholar] [CrossRef] [PubMed]
- Terriac, L.; Helesbeux, J.-J.; Maugars, Y.; Guicheux, J.; Tibbitt, M.W.; Delplace, V. Boronate ester hydrogels for biomedical applications: Challenges and opportunities. Chem. Mater. 2024, 36, 6674–6695. [Google Scholar] [CrossRef]
- Yigit, S.; Sanyal, R.; Sanyal, A. Fabrication and functionalization of hydrogels through “click” chemistry. Chem.–Asian J. 2011, 6, 2648–2659. [Google Scholar] [CrossRef]
- Yu, F.; Cao, X.; Du, J.; Wang, G.; Chen, X. Multifunctional hydrogel with good structure integrity, self-healing, and tissue-adhesive property formed by combining Diels–Alder click reaction and acylhydrazone bond. ACS Appl. Mater. Interfaces 2015, 7, 24023–24031. [Google Scholar] [CrossRef]
- Nimmo, C.M.; Owen, S.C.; Shoichet, M.S. Diels− Alder click cross-linked hyaluronic acid hydrogels for tissue engineering. Biomacromolecules 2011, 12, 824–830. [Google Scholar] [CrossRef]
- Morozova, S.M. Recent advances in hydrogels via Diels–Alder crosslinking: Design and applications. Gels 2023, 9, 102. [Google Scholar] [CrossRef]
- Koehler, K.C.; Alge, D.L.; Anseth, K.S.; Bowman, C.N. A Diels–Alder modulated approach to control and sustain the release of dexamethasone and induce osteogenic differentiation of human mesenchymal stem cells. Biomaterials 2013, 34, 4150–4158. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.J.; Taimoory, S.M.; Tam, R.Y.; Baker, A.E.; Binth Mohammad, N.; Trant, J.F.; Shoichet, M.S. Diels–Alder click-cross-linked hydrogels with increased reactivity enable 3D cell encapsulation. Biomacromolecules 2018, 19, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Owen, S.C.; Fisher, S.A.; Tam, R.Y.; Nimmo, C.M.; Shoichet, M.S. Hyaluronic acid click hydrogels emulate the extracellular matrix. Langmuir 2013, 29, 7393–7400. [Google Scholar] [CrossRef]
- Bai, X.; Lü, S.; Cao, Z.; Gao, C.; Duan, H.; Xu, X.; Sun, L.; Gao, N.; Feng, C.; Liu, M. Self-reinforcing injectable hydrogel with both high water content and mechanical strength for bone repair. Chem. Eng. J. 2016, 288, 546–556. [Google Scholar] [CrossRef]
- Fan, M.; Ma, Y.; Zhang, Z.; Mao, J.; Tan, H.; Hu, X. Biodegradable hyaluronic acid hydrogels to control release of dexamethasone through aqueous Diels–Alder chemistry for adipose tissue engineering. Mater. Sci. Eng. C 2015, 56, 311–317. [Google Scholar] [CrossRef]
- Madl, C.M.; Heilshorn, S.C. Rapid diels–alder cross-linking of cell encapsulating hydrogels. Chem. Mater. 2019, 31, 8035–8043. [Google Scholar] [CrossRef]
- Lü, S.; Bai, X.; Liu, H.; Ning, P.; Wang, Z.; Gao, C.; Ni, B.; Liu, M. An injectable and self-healing hydrogel with covalent cross-linking in vivo for cranial bone repair. J. Mater. Chem. B 2017, 5, 3739–3748. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, L.; Yu, X.; Wang, C.; Wang, Z. Synthesis and characterization of a novel double cross-linked hydrogel based on Diels-Alder click reaction and coordination bonding. Mater. Sci. Eng. C 2018, 82, 299–309. [Google Scholar] [CrossRef]
- Abandansari, H.S.; Ghanian, M.H.; Varzideh, F.; Mahmoudi, E.; Rajabi, S.; Taheri, P.; Nabid, M.R.; Baharvand, H. In situ formation of interpenetrating polymer network using sequential thermal and click crosslinking for enhanced retention of transplanted cells. Biomaterials 2018, 170, 12–25. [Google Scholar] [CrossRef]
- Bai, X.; Lü, S.; Liu, H.; Cao, Z.; Ning, P.; Wang, Z.; Gao, C.; Ni, B.; Ma, D.; Liu, M. Polysaccharides based injectable hydrogel compositing bio-glass for cranial bone repair. Carbohydr. Polym. 2017, 175, 557–564. [Google Scholar] [CrossRef]
- Madl, C.M.; Heilshorn, S.C. Bioorthogonal strategies for engineering extracellular matrices. Adv. Funct. Mater. 2018, 28, 1706046. [Google Scholar] [CrossRef] [PubMed]
- Haiber, L.M.; Kufleitner, M.; Wittmann, V. Application of the inverse-electron-demand Diels-Alder reaction for metabolic glycoengineering. Front. Chem. 2021, 9, 654932. [Google Scholar] [CrossRef] [PubMed]
- Pagel, M. Inverse electron demand Diels–Alder (IEDDA) reactions in peptide chemistry. J. Pept. Sci. 2019, 25, e3141. [Google Scholar] [CrossRef]
- Handula, M.; Chen, K.-T.; Seimbille, Y. IEDDA: An attractive bioorthogonal reaction for biomedical applications. Molecules 2021, 26, 4640. [Google Scholar] [CrossRef]
- Kalia, J.; Raines, R.T. Hydrolytic stability of hydrazones and oximes. Angew. Chem. Int. Ed. 2008, 47, 7523–7526. [Google Scholar] [CrossRef]
- Christman, K.L.; Broyer, R.M.; Schopf, E.; Kolodziej, C.M.; Chen, Y.; Maynard, H.D. Protein nanopatterns by oxime bond formation. Langmuir 2011, 27, 1415–1418. [Google Scholar] [CrossRef] [PubMed][Green Version]
- He, W.; Wang, Y.; Li, X.; Ji, Y.; Yuan, J.; Yang, W.; Yan, S.; Yan, J. Sealing the Pandora’s vase of pancreatic fistula through entrapping the digestive enzymes within a dextrorotary (D)-peptide hydrogel. Nat. Commun. 2024, 15, 7235. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Jing, X.; Hu, Y.; Wu, P.; Zhang, X.; Li, Y.; Zhao, H.; Ma, Q.; Dong, X.; Mahadevan, C. Electrospun green fluorescent-highly anisotropic conductive Janus-type nanoribbon hydrogel array film for multiple stimulus response sensors. Compos. Part B Eng. 2025, 288, 111933. [Google Scholar] [CrossRef]
- Baskin, J.M.; Dehnert, K.W.; Laughlin, S.T.; Amacher, S.L.; Bertozzi, C.R. Visualizing enveloping layer glycans during zebrafish early embryogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 10360–10365. [Google Scholar] [CrossRef]
- Baker, A.E.; Cui, H.; Ballios, B.G.; Ing, S.; Yan, P.; Wolfer, J.; Wright, T.; Dang, M.; Gan, N.Y.; Cooke, M.J. Stable oxime-crosslinked hyaluronan-based hydrogel as a biomimetic vitreous substitute. Biomaterials 2021, 271, 120750. [Google Scholar] [CrossRef]
- Sánchez-Morán, H.; Ahmadi, A.; Vogler, B.; Roh, K.-H. Oxime cross-linked alginate hydrogels with tunable stress relaxation. Biomacromolecules 2019, 20, 4419–4429. [Google Scholar] [CrossRef]
- Boehnke, N.; Cam, C.; Bat, E.; Segura, T.; Maynard, H.D. Imine hydrogels with tunable degradability for tissue engineering. Biomacromolecules 2015, 16, 2101–2108. [Google Scholar] [CrossRef]
- Hardy, J.G.; Lin, P.; Schmidt, C.E. Biodegradable hydrogels composed of oxime crosslinked poly (ethylene glycol), hyaluronic acid and collagen: A tunable platform for soft tissue engineering. J. Biomater. Sci. Polym. Ed. 2015, 26, 143–161. [Google Scholar] [CrossRef]
- Hentzen, N.B.; Smeenk, L.E.; Witek, J.; Riniker, S.; Wennemers, H. Cross-linked collagen triple helices by oxime ligation. J. Am. Chem. Soc. 2017, 139, 12815–12820. [Google Scholar] [CrossRef]
- Tamura, T.; Song, Z.; Amaike, K.; Lee, S.; Yin, S.; Kiyonaka, S.; Hamachi, I. Affinity-guided oxime chemistry for selective protein acylation in live tissue systems. J. Am. Chem. Soc. 2017, 139, 14181–14191. [Google Scholar] [CrossRef]
- Fujita, M.; Policastro, G.M.; Burdick, A.; Lam, H.T.; Ungerleider, J.L.; Braden, R.L.; Huang, D.; Osborn, K.G.; Omens, J.H.; Madani, M.M. Preventing post-surgical cardiac adhesions with a catechol-functionalized oxime hydrogel. Nat. Commun. 2021, 12, 3764. [Google Scholar] [CrossRef]
- Borelli, A.N.; Young, M.W.; Kirkpatrick, B.E.; Jaeschke, M.W.; Mellett, S.; Porter, S.; Blatchley, M.R.; Rao, V.V.; Sridhar, B.V.; Anseth, K.S. Stress relaxation and composition of hydrazone-crosslinked hybrid biopolymer-synthetic hydrogels determine spreading and secretory properties of MSCs. Adv. Healthc. Mater. 2022, 11, 2200393. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, X.; Yang, K.; Dong, Q.; Yang, H.; Gu, S.; Xu, W.; Zhou, Y. Dual-crosslinked hyaluronic acid hydrogel with self-healing capacity and enhanced mechanical properties. Carbohydr. Polym. 2023, 301, 120372. [Google Scholar] [CrossRef]
- Wang, S.; Nawale, G.N.; Oommen, O.P.; Hilborn, J.; Varghese, O.P. Influence of ions to modulate hydrazone and oxime reaction kinetics to obtain dynamically cross-linked hyaluronic acid hydrogels. Polym. Chem. 2019, 10, 4322–4327. [Google Scholar] [CrossRef]
- Mather, B.D.; Viswanathan, K.; Miller, K.M.; Long, T.E. Michael addition reactions in macromolecular design for emerging technologies. Prog. Polym. Sci. 2006, 31, 487–531. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Lowe, A.B.; Bowman, C.N. Thiol-click chemistry: A multifaceted toolbox for small molecule and polymer synthesis. Chem. Soc. Rev. 2010, 39, 1355–1387. [Google Scholar] [CrossRef] [PubMed]
- Elbert, D.L.; Pratt, A.B.; Lutolf, M.P.; Halstenberg, S.; Hubbell, J.A. Protein delivery from materials formed by self-selective conjugate addition reactions. J. Control. Release 2001, 76, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Choi, Y.J.; Noh, I.; Tae, G. Synthesis and characterization of in situ chitosan-based hydrogel via grafting of carboxyethyl acrylate. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2007, 83, 674–682. [Google Scholar]
- Young, J.L.; Engler, A.J. Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials 2011, 32, 1002–1009. [Google Scholar] [CrossRef]
- Pupkaite, J.; Rosenquist, J.; Hilborn, J.; Samanta, A. Injectable shape-holding collagen hydrogel for cell encapsulation and delivery cross-linked using thiol-michael addition click reaction. Biomacromolecules 2019, 20, 3475–3484. [Google Scholar] [CrossRef]
- Tortora, M.; Cavalieri, F.; Chiessi, E.; Paradossi, G. Michael-type addition reactions for the in situ formation of poly (vinyl alcohol)-based hydrogels. Biomacromolecules 2007, 8, 209–214. [Google Scholar] [CrossRef]
- Chatani, S.; Nair, D.P.; Bowman, C.N. Relative reactivity and selectivity of vinyl sulfones and acrylates towards the thiol–Michael addition reaction and polymerization. Polym. Chem. 2013, 4, 1048–1055. [Google Scholar] [CrossRef]
- Jin, R.; Teixeira, L.M.; Krouwels, A.; Dijkstra, P.J.; Van Blitterswijk, C.; Karperien, M.; Feijen, J. Synthesis and characterization of hyaluronic acid–poly (ethylene glycol) hydrogels via Michael addition: An injectable biomaterial for cartilage repair. Acta Biomater. 2010, 6, 1968–1977. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.D.; Kiick, K.L. Reversible maleimide–thiol adducts yield glutathione-sensitive poly (ethylene glycol)–heparin hydrogels. Polym. Chem. 2013, 4, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.; Jung, U.-W.; Noh, I. Synthesis and biocompatibility characterizations of in situ chondroitin sulfate–gelatin hydrogel for tissue engineering. Tissue Eng. Regen. Med. 2018, 15, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Wei, Z.; Zhu, X.L.; Huang, G.Y.; Xu, F.; Yang, J.H.; Osada, Y.; Zrínyi, M.; Li, J.H.; Chen, Y.M. Dextran-based hydrogel formed by thiol-Michael addition reaction for 3D cell encapsulation. Colloids Surf. B Biointerfaces 2015, 128, 140–148. [Google Scholar] [CrossRef]
- Bulpitt, P.; Aeschlimann, D. New strategy for chemical modification of hyaluronic acid: Preparation of functionalized derivatives and their use in the formation of novel biocompatible hydrogels. J. Biomed. Mater. Res. 1999, 47, 152–169. [Google Scholar] [CrossRef]
- Tian, W.; Zhang, C.; Hou, S.; Yu, X.; Cui, F.; Xu, Q.; Sheng, S.; Cui, H.; Li, H. Hyaluronic acid hydrogel as Nogo-66 receptor antibody delivery system for the repairing of injured rat brain: In vitro. J. Control. Release 2005, 102, 13–22. [Google Scholar] [CrossRef]
- Dahlmann, J.; Krause, A.; Möller, L.; Kensah, G.; Möwes, M.; Diekmann, A.; Martin, U.; Kirschning, A.; Gruh, I.; Dräger, G. Fully defined in situ cross-linkable alginate and hyaluronic acid hydrogels for myocardial tissue engineering. Biomaterials 2013, 34, 940–951. [Google Scholar] [CrossRef]
- Chang, J.; Tao, Y.; Wang, B.; Guo, B.-h.; Xu, H.; Jiang, Y.-r.; Huang, Y. An in situ-forming zwitterionic hydrogel as vitreous substitute. J. Mater. Chem. B 2015, 3, 1097–1105. [Google Scholar] [CrossRef]
- Martínez-Sanz, E.; Ossipov, D.A.; Hilborn, J.; Larsson, S.; Jonsson, K.B.; Varghese, O.P. Bone reservoir: Injectable hyaluronic acid hydrogel for minimal invasive bone augmentation. J. Control. Release 2011, 152, 232–240. [Google Scholar] [CrossRef]
- Karvinen, J.; Joki, T.; Ylä-Outinen, L.; Koivisto, J.T.; Narkilahti, S.; Kellomäki, M. Soft hydrazone crosslinked hyaluronan-and alginate-based hydrogels as 3D supportive matrices for human pluripotent stem cell-derived neuronal cells. React. Funct. Polym. 2018, 124, 29–39. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, H.; Trinh, P.; Heilshorn, S.C.; Yang, F. Elastin-like protein-hyaluronic acid (ELP-HA) hydrogels with decoupled mechanical and biochemical cues for cartilage regeneration. Biomaterials 2017, 127, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Highley, C.B.; Yeh, Y.C.; Galarraga, J.H.; Uman, S.; Burdick, J.A. Three-dimensional extrusion bioprinting of single-and double-network hydrogels containing dynamic covalent crosslinks. J. Biomed. Mater. Res. Part A 2018, 106, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Jiang, X. Injectable and degradable pH-responsive hydrogels via spontaneous amino–yne click reaction. ACS Appl. Mater. Interfaces 2018, 10, 361–370. [Google Scholar] [CrossRef]
- Schwarz, S.; Kuth, S.; Distler, T.; Gögele, C.; Stölzel, K.; Detsch, R.; Boccaccini, A.R.; Schulze-Tanzil, G. 3D printing and characterization of human nasoseptal chondrocytes laden dual crosslinked oxidized alginate-gelatin hydrogels for cartilage repair approaches. Mater. Sci. Eng. C 2020, 116, 111189. [Google Scholar] [CrossRef] [PubMed]
- Distler, T.; Polley, C.; Shi, F.; Schneidereit, D.; Ashton, M.D.; Friedrich, O.; Kolb, J.F.; Hardy, J.G.; Detsch, R.; Seitz, H. Electrically conductive and 3D-printable oxidized alginate-gelatin polypyrrole: PSS hydrogels for tissue engineering. Adv. Healthc. Mater. 2021, 10, 2001876. [Google Scholar] [CrossRef]
- Chen, H.; Liao, R.; Du, Q.; Li, C.; Xiao, X.; Shan, Y. Injectable hyaluronic acid/oxidized chitosan hydrogels with hypochlorous acid released for instant disinfection and antibacterial effects. Front. Mater. 2022, 9, 935096. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, J.H.; Liu, Z.Q.; Xu, F.; Zhou, J.X.; Zrínyi, M.; Osada, Y.; Chen, Y.M. Novel biocompatible polysaccharide-based self-healing hydrogel. Adv. Funct. Mater. 2015, 25, 1352–1359. [Google Scholar] [CrossRef]
- Yan, S.; Wang, W.; Li, X.; Ren, J.; Yun, W.; Zhang, K.; Li, G.; Yin, J. Preparation of mussel-inspired injectable hydrogels based on dual-functionalized alginate with improved adhesive, self-healing, and mechanical properties. J. Mater. Chem. B 2018, 6, 6377–6390. [Google Scholar] [CrossRef] [PubMed]
- Kisiel, M.; Martino, M.M.; Ventura, M.; Hubbell, J.A.; Hilborn, J.; Ossipov, D.A. Improving the osteogenic potential of BMP-2 with hyaluronic acid hydrogel modified with integrin-specific fibronectin fragment. Biomaterials 2013, 34, 704–712. [Google Scholar] [CrossRef]
- Chand, R.; Janarthanan, G.; Elkhoury, K.; Vijayavenkataraman, S. Digital light processing 3D bioprinting of biomimetic corneal stroma equivalent using gelatin methacryloyl and oxidized carboxymethylcellulose interpenetrating network hydrogel. Biofabrication 2025, 17, 025011. [Google Scholar] [CrossRef]
- Ma, W.; Yang, M.; Wu, C.; Wang, S.; Du, M. Bioinspired self-healing injectable nanocomposite hydrogels based on oxidized dextran and gelatin for growth-factor-free bone regeneration. Int. J. Biol. Macromol. 2023, 251, 126145. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, M.; Jamali, R.; Kiyani, T.; Mohamadnia, Z.; Moradi, A.-R. Characterization of Schiff base self-healing hydrogels by dynamic speckle pattern analysis. Sci. Rep. 2024, 14, 27950. [Google Scholar] [CrossRef]
- He, Q.; Ding, X.; Deng, J.; Zhang, Y.; Wang, X.; Zhan, D.; Okoro, O.V.; Yan, L.; Shavandi, A.; Nie, L. Fabrication of injectable, adhesive, self-healing, superabsorbent hydrogels based on quaternary ammonium chitosan and oxidized pullulan. Heliyon 2024, 10, e38577. [Google Scholar] [CrossRef]
- Nascimento, S.d.P.D.; de Souza, R.R.M.; Sobral, M.V.; Xavier-Junior, F.H.; da Silva, M.V.S.; Viana, M.M.; da Silva, F.F.; Serpe, M.J.; de Souza, A.L. Gelatin-Oxidized Alginate and Chitosan-Coated Zein Nanoparticle Hydrogel Composite to Enhance Breast Cancer Cytotoxicity in Dual-Drug Delivery. ACS Omega 2024, 9, 45190–45202. [Google Scholar] [CrossRef]
- Afewerki, S.; Bassous, N.; Harb, S.V.; Corat, M.A.F.; Maharjan, S.; Ruiz-Esparza, G.U.; de Paula, M.M.; Webster, T.J.; Tim, C.R.; Viana, B.C. Engineering multifunctional bactericidal nanofibers for abdominal hernia repair. Commun. Biol. 2021, 4, 233. [Google Scholar] [CrossRef]
- de Sousa Araújo, E.; Stocco, T.D.; de Sousa, G.F.; Afewerki, S.; Marciano, F.R.; Corat, M.A.F.; de Paula, M.M.M.; Verde, T.F.C.L.; Silva, M.C.M.; Lobo, A.O. Oxygen-generating microparticles in chondrocytes-laden hydrogels by facile and versatile click chemistry strategy. Colloids Surf. B Biointerfaces 2021, 205, 111850. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.H.; Cheon, S.Y.; Park, S.H.; Lee, H.; Yoo, S.-Y.; Ahn, M.Y.; Lee, D.; Kim, M.S.; Park, J.H.; Hollister, S.J. Lacuna-mimic human-derived nasal septal chondrocyte clusters encapsulated in click chemistry-based hydrogel for in vivo cartilage regeneration. Chem. Eng. J. 2025, 165965. [Google Scholar] [CrossRef]
- Liu, X.; Gaihre, B.; Li, L.; Rezaei, A.; Tilton, M.; Elder, B.D.; Lu, L. Bioorthogonal “click chemistry” bone cement with bioinspired natural mimicking microstructures for bone repair. ACS Biomater. Sci. Eng. 2023, 9, 1585–1597. [Google Scholar] [CrossRef]
- Arickx, U. Fabrication of Highly Robust Thiol-Ene Click Chemistry-Based Hydrogel Scaffold for 3D DLP Printing. Master’s Thesis, Maastricht University, Maastricht, The Netherlands, 2023. [Google Scholar]
- Qu, J.; Zhao, X.; Liang, Y.; Xu, Y.; Ma, P.X.; Guo, B. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem. Eng. J. 2019, 362, 548–560. [Google Scholar] [CrossRef]
- Liu, C.; Wu, J.; Gan, D.; Li, Z.; Shen, J.; Tang, P.; Luo, S.; Li, P.; Lu, X.; Zheng, W. The characteristics of mussel-inspired nHA/OSA injectable hydrogel and repaired bone defect in rabbit. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1814–1825. [Google Scholar] [CrossRef]
- Ocando, C.; Dinescu, S.; Samoila, I.; Ghitulica, C.D.; Cucuruz, A.; Costache, M.; Averous, L. Fabrication and properties of alginate-hydroxyapatite biocomposites as efficient biomaterials for bone regeneration. Eur. Polym. J. 2021, 151, 110444. [Google Scholar] [CrossRef]
- Fan, Z.; Liu, B.; Wang, J.; Zhang, S.; Lin, Q.; Gong, P.; Ma, L.; Yang, S. A novel wound dressing based on Ag/graphene polymer hydrogel: Effectively kill bacteria and accelerate wound healing. Adv. Funct. Mater. 2014, 24, 3933–3943. [Google Scholar] [CrossRef]
- Li, R.; Liu, K.; Huang, X.; Li, D.; Ding, J.; Liu, B.; Chen, X. Bioactive materials promote wound healing through modulation of cell behaviors. Adv. Sci. 2022, 9, 2105152. [Google Scholar] [CrossRef] [PubMed]
- Leclère, F.M.; Casoli, V. Use of bioartificial dermal regeneration template for skin restoration in combat casualty injuries. Regen. Med. 2016, 11, 359–360. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, R.; Jiao, J.; Li, Z.; Wang, P.; Liu, Y.; Li, S.; Chen, C.; Li, Z.; Qu, G. A click chemistry-mediated all-peptide cell printing hydrogel platform for diabetic wound healing. Nat. Commun. 2023, 14, 7856. [Google Scholar] [CrossRef]
- Hu, J.; Quan, Y.; Lai, Y.; Zheng, Z.; Hu, Z.; Wang, X.; Dai, T.; Zhang, Q.; Cheng, Y. A smart aminoglycoside hydrogel with tunable gel degradation, on-demand drug release, and high antibacterial activity. J. Control. Release 2017, 247, 145–152. [Google Scholar] [CrossRef]
- Xu, W.; He, M.; Lu, Q. Fibronectin connecting cell sheet based on click chemistry for wound repair. Adv. Sci. 2024, 11, 2306746. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; Xu, H.; Lei, B.; Mao, C. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics 2019, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Tian, J.; Liu, Y.; Cao, H.; Li, R.; Wang, J.; Wu, J.; Zhang, Q. Dynamic covalent constructed self-healing hydrogel for sequential delivery of antibacterial agent and growth factor in wound healing. Chem. Eng. J. 2019, 373, 413–424. [Google Scholar] [CrossRef]
- Selvarajah, S.; Hammond, E.R.; Haider, A.H.; Abularrage, C.J.; Becker, D.; Dhiman, N.; Hyder, O.; Gupta, D.; Black III, J.H.; Schneider, E.B. The burden of acute traumatic spinal cord injury among adults in the united states: An update. J. Neurotrauma 2014, 31, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Gandhamal, A.; Talbar, S.; Hani, A.F.M. Knee articular cartilage segmentation from MR images: A review. ACM Comput. Surv. (CSUR) 2018, 51, 97. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, J.; Huang, Y.; Wang, L.; Gao, H.; Yang, Y. Clickable immune-microenvironment modulated hydrogels for spinal cord injury repair. J. Colloid Interface Sci. 2025, 679, 1079–1092. [Google Scholar] [CrossRef]
- Li, L.; Xiao, B.; Mu, J.; Zhang, Y.; Zhang, C.; Cao, H.; Chen, R.; Patra, H.K.; Yang, B.; Feng, S. A MnO2 nanoparticle-dotted hydrogel promotes spinal cord repair via regulating reactive oxygen species microenvironment and synergizing with mesenchymal stem cells. ACS Nano 2019, 13, 14283–14293. [Google Scholar] [CrossRef]
- Tigner, T.J.; Dampf, G.; Tucker, A.; Huang, Y.C.; Jagrit, V.; Clevenger, A.J.; Mohapatra, A.; Raghavan, S.A.; Dulin, J.N.; Alge, D.L. Clickable granular hydrogel scaffolds for delivery of neural progenitor cells to sites of spinal cord injury. Adv. Healthc. Mater. 2024, 13, 2303912. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Mu, J.; Chen, J.; Feng, S.; Gao, J. Implantation of a functional TEMPO-hydrogel induces recovery from rat spinal cord transection through promoting nerve regeneration and protecting bladder tissue. Biomater. Sci. 2020, 8, 1695–1701. [Google Scholar] [CrossRef]
- Liu, W.; Xu, B.; Zhao, S.; Han, S.; Quan, R.; Liu, W.; Ji, C.; Chen, B.; Xiao, Z.; Yin, M. Spinal cord tissue engineering via covalent interaction between biomaterials and cells. Sci. Adv. 2023, 9, eade8829. [Google Scholar] [CrossRef]
- Tsai, I.-L.; Hsu, C.-C.; Hung, K.-H.; Chang, C.-W.; Cheng, Y.-H. Applications of biomaterials in corneal wound healing. J. Chin. Med. Assoc. 2015, 78, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Meller, D.; Peters, K.; Meller, K. Human cornea and sclera studied by atomic force microscopy. Cell Tissue Res. 1997, 288, 111–118. [Google Scholar] [CrossRef]
- Lei, L.; Hu, Y.; Shi, H.; Bao, Z.; Wu, Y.; Jiang, J.; Li, X. Biofunctional peptide-click PEG-based hydrogels as 3D cell scaffolds for corneal epithelial regeneration. J. Mater. Chem. B 2022, 10, 5938–5945. [Google Scholar] [CrossRef]
- Li, L.; Lu, C.; Wang, L.; Chen, M.; White, J.; Hao, X.; McLean, K.M.; Chen, H.; Hughes, T.C. Gelatin-based photocurable hydrogels for corneal wound repair. ACS Appl. Mater. Interfaces 2018, 10, 13283–13292. [Google Scholar] [CrossRef]
- Rosenquist, J.; Folkesson, M.; Hoglund, L.; Pupkaite, J.; Hilborn, J.n.; Samanta, A. An injectable, shape-retaining collagen hydrogel cross-linked using thiol-maleimide click chemistry for sealing corneal perforations. ACS Appl. Mater. Interfaces 2023, 15, 34407–34418. [Google Scholar] [CrossRef]
- Koivusalo, L.; Kauppila, M.; Samanta, S.; Parihar, V.S.; Ilmarinen, T.; Miettinen, S.; Oommen, O.P.; Skottman, H. Tissue adhesive hyaluronic acid hydrogels for sutureless stem cell delivery and regeneration of corneal epithelium and stroma. Biomaterials 2019, 225, 119516. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Fu, Z.; Li, H.; Wei, R.; Guo, J.; Wang, H.; Qi, J. Smart hydrogel: A new platform for cancer therapy. Adv. Colloid Interface Sci. 2025, 340, 103470. [Google Scholar] [CrossRef]
- Du, H.; Zhang, A.; Zhang, Q.; Sun, Y.; Zhu, H.; Wang, H.; Tan, Z.; Zhang, X.; Chen, G. Fabrication of recoverable Bi2O2S/Bi5O7I/ZA hydrogel beads for enhanced photocatalytic Hg0 removal in the presence of H2O2. Sep. Purif. Technol. 2025, 359, 130597. [Google Scholar] [CrossRef]
- Fu, S.; Dong, H.; Deng, X.; Zhuo, R.; Zhong, Z. Injectable hyaluronic acid/poly (ethylene glycol) hydrogels crosslinked via strain-promoted azide-alkyne cycloaddition click reaction. Carbohydr. Polym. 2017, 169, 332–340. [Google Scholar] [CrossRef]
- Truong, V.X.; Tsang, K.M.; Simon, G.P.; Boyd, R.L.; Evans, R.A.; Thissen, H.; Forsythe, J.S. Photodegradable gelatin-based hydrogels prepared by bioorthogonal click chemistry for cell encapsulation and release. Biomacromolecules 2015, 16, 2246–2253. [Google Scholar] [CrossRef]
- Bock, N.; Forouz, F.; Hipwood, L.; Clegg, J.; Jeffery, P.; Gough, M.; van Wyngaard, T.; Pyke, C.; Adams, M.N.; Bray, L.J. GelMA, click-chemistry gelatin and bioprinted polyethylene glycol-based hydrogels as 3D ex vivo drug testing platforms for patient-derived breast cancer organoids. Pharmaceutics 2023, 15, 261. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, Y.; Yu, J.; Becker, M.L. Enhanced osteogenic activity of poly (ester urea) scaffolds using facile post-3D printing peptide functionalization strategies. Biomaterials 2017, 141, 176–187. [Google Scholar] [CrossRef]
- Bebiano, L.B.; Presa, R.; Vieira, F.; Lourenço, B.N.; Pereira, R.F. Bioinspired and photo-clickable thiol-ene bioinks for the extrusion bioprinting of mechanically tunable 3D skin models. Biomimetics 2024, 9, 228. [Google Scholar] [CrossRef]
- Stichler, S.; Jungst, T.; Schamel, M.; Zilkowski, I.; Kuhlmann, M.; Böck, T.; Blunk, T.; Teßmar, J.; Groll, J. Thiol-ene clickable poly (glycidol) hydrogels for biofabrication. Ann. Biomed. Eng. 2017, 45, 273–285. [Google Scholar] [CrossRef]
- Yin, R.; Zhang, N.; Wang, K.; Long, H.; Xing, T.; Nie, J.; Zhang, H.; Zhang, W. Material design and photo-regulated hydrolytic degradation behavior of tissue engineering scaffolds fabricated via 3D fiber deposition. J. Mater. Chem. B 2017, 5, 329–340. [Google Scholar] [CrossRef]
- Jiang, P.; Lin, P.; Yang, C.; Qin, H.; Wang, X.; Zhou, F. 3D printing of dual-physical cross-linking hydrogel with ultrahigh strength and toughness. Chem. Mater. 2020, 32, 9983–9995. [Google Scholar] [CrossRef]
- Becer, C.R.; Hoogenboom, R.; Schubert, U.S. Click chemistry beyond metal-catalyzed cycloaddition. Angew. Chem. Int. Ed. 2009, 48, 4900–4908. [Google Scholar] [CrossRef]
- Fares, M.M.; Jabani, Z.H.; Abu-Haniyi, L.A. Synthesis of novel bioadhesive hydrogels via facile Thiol-Ene click chemistry for wound healing applications. Int. J. Biol. Macromol. 2024, 270, 132501. [Google Scholar] [CrossRef] [PubMed]
- Sousa, G.F.; Afewerki, S.; Dittz, D.; Santos, F.E.P.; Gontijo, D.O.; Scalzo, S.R.A.; Santos, A.L.C.; Guimaraes, L.C.; Pereira, E.M.; Barcelos, L.S.; et al. Catalyst-Free Click Chemistry for Engineering Chondroitin Sulfate-Multiarmed PEG Hydrogels for Skin Tissue Engineering. J. Funct. Biomater. 2022, 13, 45. [Google Scholar] [CrossRef]
- Collins, J.; Nadgorny, M.; Xiao, Z.; Connal, L.A. Doubly dynamic self-healing materials based on oxime click chemistry and boronic acids. Macromol. Rapid Commun. 2017, 38, 1600760. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Wang, M.; Chang, H.; Xu, F.; Yang, J. A self-healing cellulose nanocrystal-poly (ethylene glycol) nanocomposite hydrogel via Diels–Alder click reaction. ACS Sustain. Chem. Eng. 2017, 5, 6167–6174. [Google Scholar] [CrossRef]
- Tan, Y.; Huang, H.; Ayers, D.C.; Song, J. Modulating viscoelasticity, stiffness, and degradation of synthetic cellular niches via stoichiometric tuning of covalent versus dynamic noncovalent cross-linking. ACS Cent. Sci. 2018, 4, 971–981. [Google Scholar] [CrossRef]
- Tang, S.; Ma, H.; Tu, H.C.; Wang, H.R.; Lin, P.C.; Anseth, K.S. Adaptable fast relaxing boronate-based hydrogels for probing cell—Matrix interactions. Adv. Sci. 2018, 5, 1800638. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Yang, J.H.; Du, X.J.; Xu, F.; Zrinyi, M.; Osada, Y.; Li, F.; Chen, Y.M. Dextran-based self-healing hydrogels formed by reversible Diels–Alder reaction under physiological conditions. Macromol. Rapid Commun. 2013, 34, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Paton, R.S.; Kim, S.; Liang, Y.; Houk, K. Zee Fall Women’s 2018 Winter 19 Denim 34 Wi57 Yes P335 Jeans 0q4a0fdn--melodic.giftedhandscateringdc.com. J. Am. Chem. Soc 2013, 135, 15642–15649. [Google Scholar] [CrossRef] [PubMed]
- Soriano del Amo, D.; Wang, W.; Jiang, H.; Besanceney, C.; Yan, A.C.; Levy, M.; Liu, Y.; Marlow, F.L.; Wu, P. Biocompatible copper (I) catalysts for in vivo imaging of glycans. J. Am. Chem. Soc. 2010, 132, 16893–16899. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Z. Development and applications of the copper-catalyzed azide-alkyne cycloaddition (CuAAC) as a bioorthogonal reaction. Molecules 2016, 21, 1393. [Google Scholar] [CrossRef]
- Zhang, Z.; He, C.; Chen, X. Hydrogels based on pH-responsive reversible carbon–nitrogen double-bond linkages for biomedical applications. Mater. Chem. Front. 2018, 2, 1765–1778. [Google Scholar] [CrossRef]
- Han, G.S.; Domaille, D.W. Tuning the exchange dynamics of boronic acid hydrazones and oximes with pH and redox control. Org. Biomol. Chem. 2021, 19, 4986–4991. [Google Scholar] [CrossRef]

| Reaction Type | Mechanism | Advantages | Limitations | Applications | [Ref.] |
|---|---|---|---|---|---|
| Azide–Alkyne Cycloaddition | Copper(I)-catalyzed or strain-promoted reaction between azides and alkynes | High specificity, bioorthogonal, efficient under mild conditions | Copper catalyst may be cytotoxic (CuAAC); strain-promoted versions more expensive | Injectable hydrogels, cell encapsulation, drug delivery | [32,33] |
| Thiol–Ene Reaction | Radical-mediated addition of thiols to alkenes | Fast kinetics, no metal catalysts, tunable crosslinking | Requires photoinitiator or thermal initiator | In situ gelation, biofunctionalization, 3D cell culture | [34,35] |
| Diels–Alder Reaction | [4+2] Cycloaddition between a diene and a dienophile | Reversible under thermal control, catalyst-free | Temperature sensitivity may affect biological components | Injectable hydrogels, drug release systems | [16,36] |
| Michael Addition | Nucleophilic addition of thiols to electron-deficient vinyl groups | Mild conditions, high yield, no need for catalyst | Sensitivity to pH and competing nucleophiles | Cell-laden hydrogels, tissue engineering scaffolds | [37,38] |
| Oxime/Hydrazone Formation | Reaction between aldehydes/ketones and hydrazides or aminooxy groups | Chemoselective, compatible with aqueous environments | Hydrolysis-sensitive; slow gelation in some cases | Injectable matrices, bioadhesives | [39,40] |
| Tetrazine–Norbornene Reaction | Inverse electron-demand Diels–Alder reaction | Ultra-fast kinetics, catalyst-free, excellent bioorthogonality | Tetrazine compounds can be costly | Real-time cell encapsulation, dynamic biomaterials | [41,42] |
| SuFEx (Sulfur Fluoride Exchange) | Click reaction involving sulfur-fluoride bonds | High yield, stable products, emerging tool in bio-click chemistry | Limited current use in hydrogels; less biocompatibility data | Future directions in robust hydrogel crosslinking | [43,44] |
| Hydrogel Composition | Click Type | Application | Ref. |
|---|---|---|---|
| Alginate grafted with BCN/cyclooctyne; azide-peptides (e.g., Az-cRGD) | SPAAC | Peptide functionalisation of alginate preserving peptide bioactivity for 2D/3D cell assays; cell-friendly, in-presence-of-cells coupling. | [74] |
| Cyclooctyne-modified alginate (ALG-K) conjugated to bis-azide MMP-sensitive peptides (PVGLIG) | SPAAC | Tunable protease-sensitive alginate hydrogels that support cell spreading and matrix remodeling; modular and bioorthogonal. | [75] |
| Review covering many metal-free click reactions (SPAAC emphasis) applied to PEG, HA, alginate, gelatin hydrogels. | SPAAC & other metal-free clicks | Comprehensive summary of metal-free click chemistries for cell-laden hydrogels and translational potential; practical reagent notes. | [76] |
| Review/analysis of kinetics across click classes (CuAAC, SPAAC, IEDDA, etc.) relevant to hydrogel design. | CuAAC vs. SPAAC (comparison) | Practical guidance: CuAAC = fast but requires Cu(I) management; SPAAC = biocompatible (no copper) but reagent cost/kinetics tradeoffs—crucial when choosing for cell-laden gels. | [77] |
| Alginate hydrogels microstructured and functionalised via SPAAC for injectable cell delivery and morphogenesis guidance. | SPAAC | Demonstrated injectable, microstructured, cell-instructive hydrogels produced using bioorthogonal SPAAC; supports 3D cell patterning. | [78] |
| Alginate–gelatin hybrid hydrogels dual-crosslinked using SPAAC (bioorthogonal) plus ionic or secondary covalent crosslinks. | SPAAC (primary) + secondary crosslinks | Improved mechanics and cell-instructive environment; on viscoelastic tuning for cardiac TE (long-term in vivo results still pending) | [79] |
| Hydrogel Composition | Application/Highlight | Ref. |
|---|---|---|
| Methoxy-PEG grafted to keratin via thiol-ene photoclick | Eco-friendly fabrication of keratin-based hydrogel, potential for biomaterial scaffolding. | [104] |
| Thiol-ene hydrogel combined with poly (propylene fumarate) (PPF) scaffold | Local PTH delivery in critical-size bone defects, sustained bioactivity through 21 days, promoting bone healing. | [105] |
| Gelatin–hyaluronic acid modified for thiol-ene crosslinking | 3D-printable cryogel scaffolds, enhanced mechanical stability, biocompatibility, potentially useful in cartilage or soft tissue. | [106] |
| Hydroxypropyl methylcellulose (HPMC) derivatives via thiol-ene, forming PNP hydrogels with PEG–PLA nanoparticles | Injectable polymer–nanoparticle hydrogels featuring tunable mechanics, retention time, and bioactive peptide loading, ideal for cell/drug delivery. | [95] |
| Alginate/gelatin/CMC functionalized with thiol/norbornene for thiol-ene photo-crosslinking, incorporating SERS nanorods | 3D-printed plasmonic scaffolds enabling real-time metabolic sensing in 3D cell cultures, biosensing + structural scaffold in one. | [94] |
| Hydrogel Composition | Crosslink Type (Boronate-Based) | Application/Highlight | Ref. |
|---|---|---|---|
| Cellulose derivatives functionalized with boronic-acid/diol motifs to form reversible boronate esters. | Boronate-ester dynamic crosslinking (self-healing) | Self-healing, conductive hydrogel with good cytocompatibility, candidate for soft tissue repair and bioelectronic interfaces. | [117] |
| Various polymer backbones (PVA, PEG, polysaccharides) crosslinked by bis-boronic acids to give glucose-responsive networks. | Boronate ester crosslinks responsive to glucose/pH | Closed-loop insulin delivery platforms illustrating how BE hydrogels can be tuned for biomedical, implantable drug-release applications. | [118] |
| Thin-film hydrogels of polymers bearing phenylboronic acid (PBA) for glucose binding and reversible crosslinking. | PBA–diol boronate esters (glucose competitive) | Glucose-sensitive swelling/optical response; useful as biosensing layers and for smart biomaterials in regenerative medicine workflows. | [119] |
| Hyaluronic acid (HA) chemically modified with phenylboronic acid groups to form reversible networks with diol-bearing partners. | PBA–diol boronate ester network (dynamic) | Supports 2D/3D culture of articular chondrocytes, promising for cartilage tissue engineering and cell-friendly, dynamic ECM mimicry. | [120] |
| Gelatin modified with phenylboronic acid crosslinked to oxidized dextran (diol/aldehyde components present) to form an injectable gel. | Boronate ester (PBA–diol) dynamic crosslinks (plus secondary interactions) | Injectable, biodegradable hydrogel for bone TE; good cell viability and in vitro osteogenic markers reported. | [121] |
| Multi-component hydrogel combining PBA-functionalized polymers with catechol/diol units and ROS-scavenging moieties. | Boronate ester dynamic bonds (pH/glucose/ROS responsive) | Injectable, multi-stimuli-responsive system demonstrating controllable drug release, antioxidant effect and promising in vitro biocompatibility for wound/tissue repair. | [122] |
| Review summarizing PEG, PVA, HA, dextran, gelatin systems that exploit BE dynamics at physiological pH. | Survey of boronate ester chemistries, pH tuning, and strategies to operate near physiological conditions | State-of-the-art review covering materials design, pH/glucose responsiveness, self-healing, and translational challenges (stability, in vivo data, scale-up). Good starting point for application-directed design. | [123] |
| Hydrogel Composition | Crosslink Type | Application/Highlight | Ref. |
|---|---|---|---|
| Aldehyde-PEG + aminooxy-PEG (oxime), Rapid in situ gelation on wet tissue; physiological pH | Oxime | Strong wet-tissue adhesion/retention; anti-fouling surface | [153] |
| HA-aldehyde + aminooxy crosslinker, Physiological pH/ionic strength | Oxime | Transparent, vitreous-like modulus; enzymatic stability | [147] |
| HA-aldehyde/HA-hydrazide + benzaldehyde/hydrazide, Mild, aqueous; tunable stress relaxation | Hydrazone (acylhydrazone) | Viscoelastic (stress-relaxing) networks modulate MSC spreading & secretome | [154] |
| HA-aldehyde + dihydrazide crosslinker, Fast gelation; pH-responsive | Hydrazone (acylhydrazone) | Self-healing, enhanced mechanics; cytocompatible | [155] |
| Hydrogel Composition | Crosslink Type | Application/Highlight | Ref. |
|---|---|---|---|
| Alginate dialdehyde (ADA) + gelatin (GEL) | Schiff base (imine) between ADA aldehydes and GEL amines; printable grids | 3D-printed ADA–GEL scaffolds for cartilage tissue engineering; supports chondrocyte function. | [178] |
| Oxidized alginate + chitosan (plus conductive phase) | Schiff base (aldehyde–amine) gives self-healing; printable under mild conditions | Conductive, self-healing, 3D-printable hydrogels aimed at TE scaffolds (soft tissues). | [179] |
| Hyaluronic acid + oxidized chitosan (OCS) | Schiff base; physiological, tissue-adhesive, self-healing | Antibacterial, adhesive injectable hydrogel; promising wound-healing/soft-tissue TE dressing. | [180] |
| Oxidized dextran (ODex) + gelatin (GEL) + nano-fillers | Schiff base; injectable, self-healing | Growth-factor-free bone regeneration in vivo; enhanced osteogenesis from dynamic imine network. | [185] |
| Oxidized alginate + gelatin (with microspheres) | Schiff base; in situ gelation | Biocompatible composite scaffolds with improved mechanics for TE applications. | [185] |
| Gelatin + hyaluronic acid (one oxidized) | Dynamic mine; self-healing | Biodegradable, conductive, self-healing hydrogel; broadly relevant to TE and wound repair. | [186] |
| Quaternary-ammonium chitosan + oxidized partner | Schiff base; mild, injectable | Adhesive, antibacterial hydrogels suitable for tissue repair dressings and cell delivery. | [187] |
| Gelatin + oxidized alginate (Schiff base) + NPs | Imine crosslinking; injectable | Localized therapy platform: ADA–GEL imine network is a canonical TE matrix (drug/cell co-delivery). | [188] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sojdeh, S.; Panjipour, A.; Yaghmour, A.; Arabpour, Z.; Djalilian, A.R. Click Chemistry-Based Hydrogels for Tissue Engineering. Gels 2025, 11, 724. https://doi.org/10.3390/gels11090724
Sojdeh S, Panjipour A, Yaghmour A, Arabpour Z, Djalilian AR. Click Chemistry-Based Hydrogels for Tissue Engineering. Gels. 2025; 11(9):724. https://doi.org/10.3390/gels11090724
Chicago/Turabian StyleSojdeh, Soheil, Amirhosein Panjipour, Amal Yaghmour, Zohreh Arabpour, and Ali R. Djalilian. 2025. "Click Chemistry-Based Hydrogels for Tissue Engineering" Gels 11, no. 9: 724. https://doi.org/10.3390/gels11090724
APA StyleSojdeh, S., Panjipour, A., Yaghmour, A., Arabpour, Z., & Djalilian, A. R. (2025). Click Chemistry-Based Hydrogels for Tissue Engineering. Gels, 11(9), 724. https://doi.org/10.3390/gels11090724







