Printable Conductive Hydrogels and Elastomers for Biomedical Application
Abstract
1. Introduction
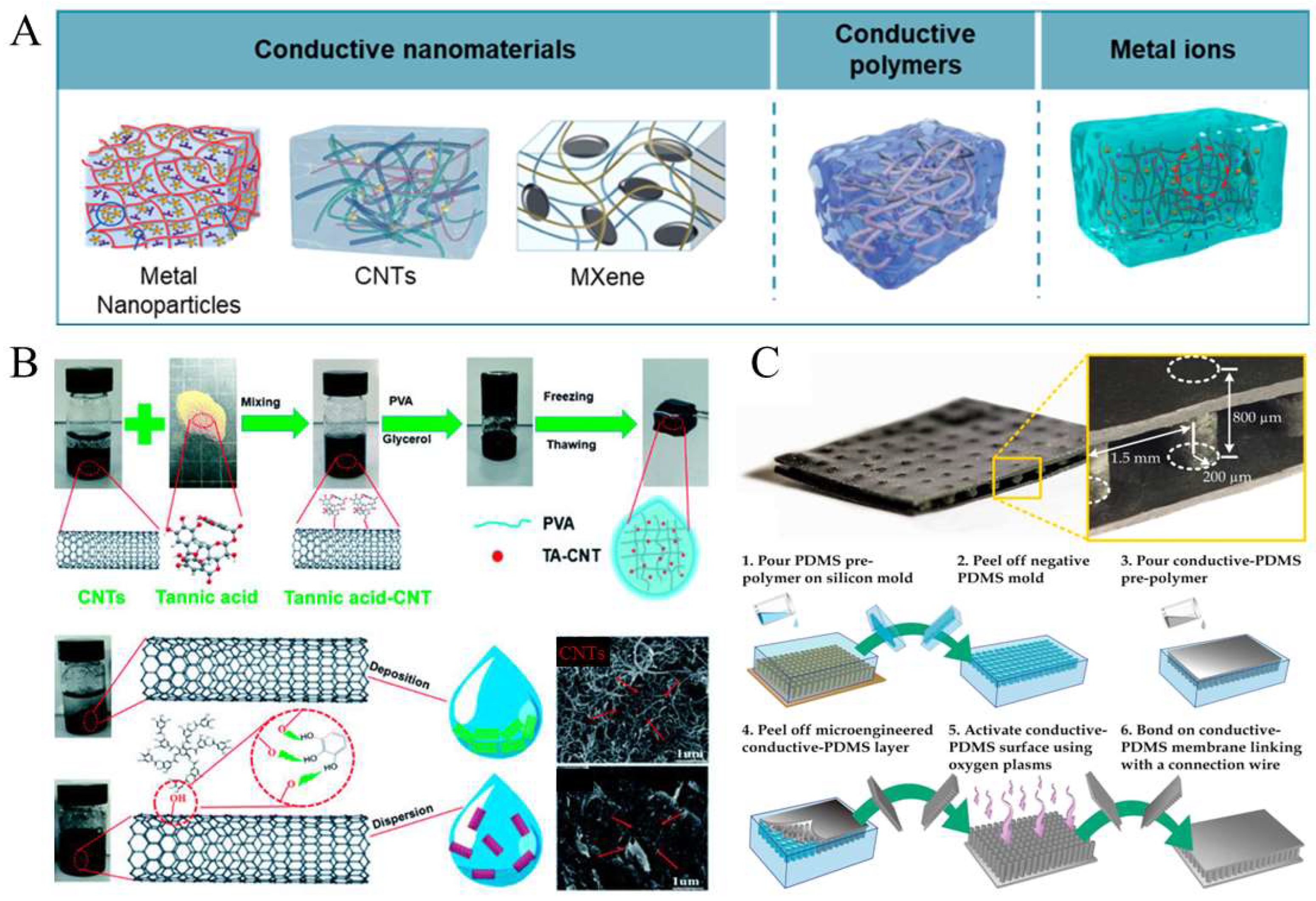
2. Fabrication of Conductive Soft Materials
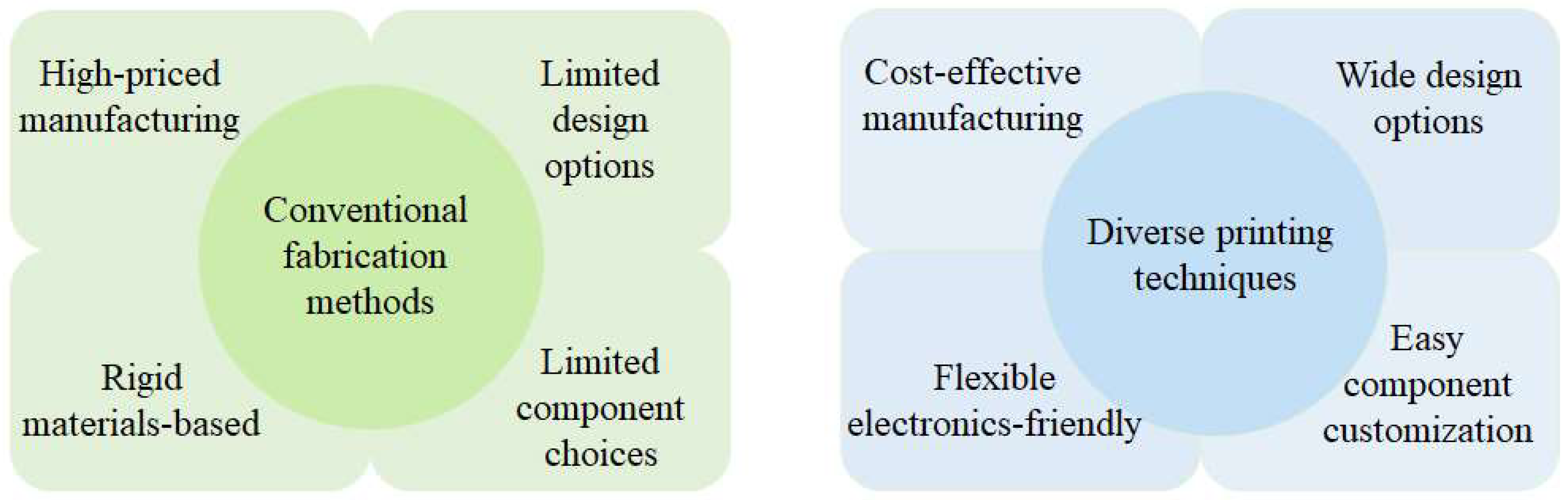
3. Significance of Printing Techniques
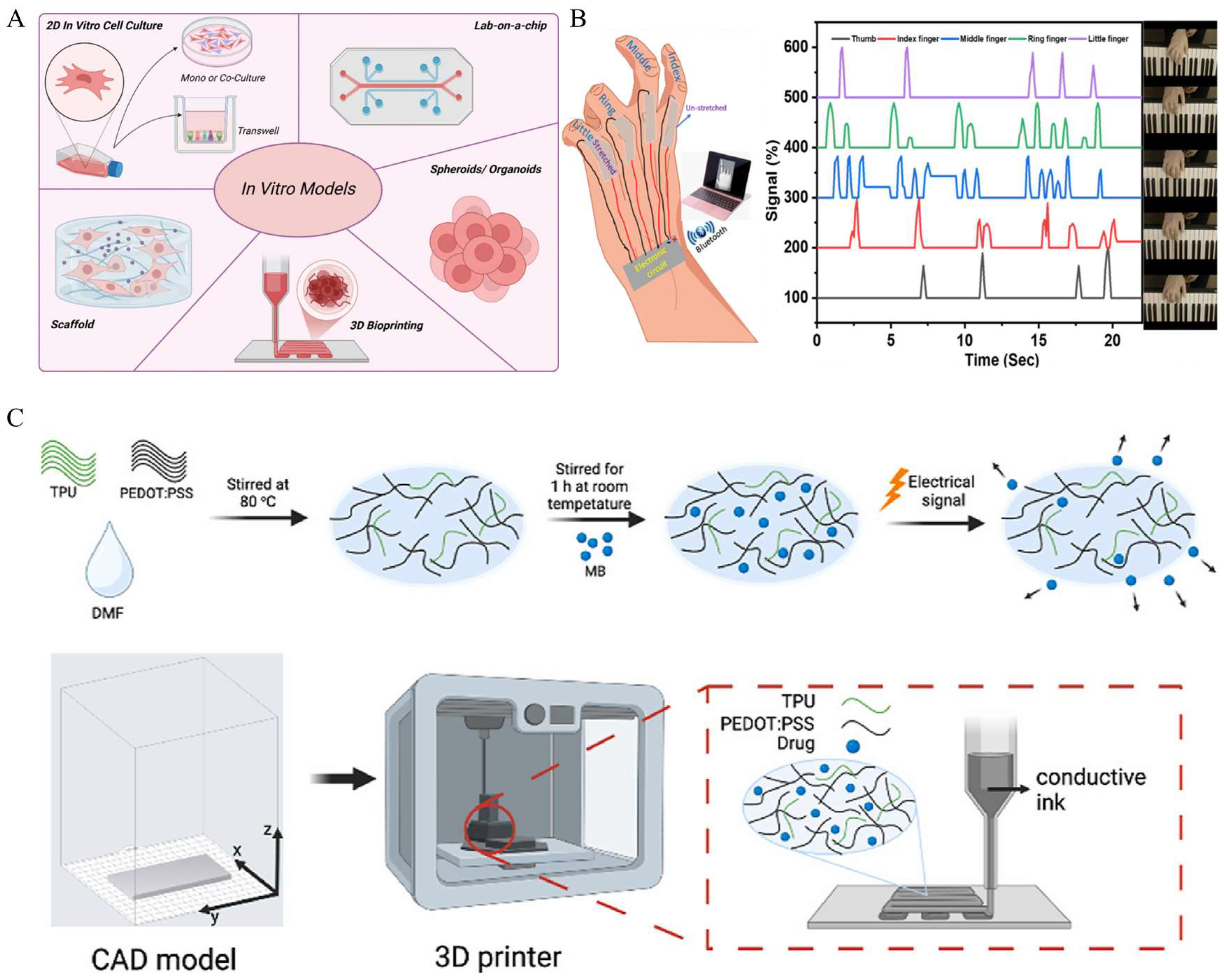
4. Application of Printed Conductive Elastomers In Vitro
5. Application of Printed Conductive Elastomers In Vivo
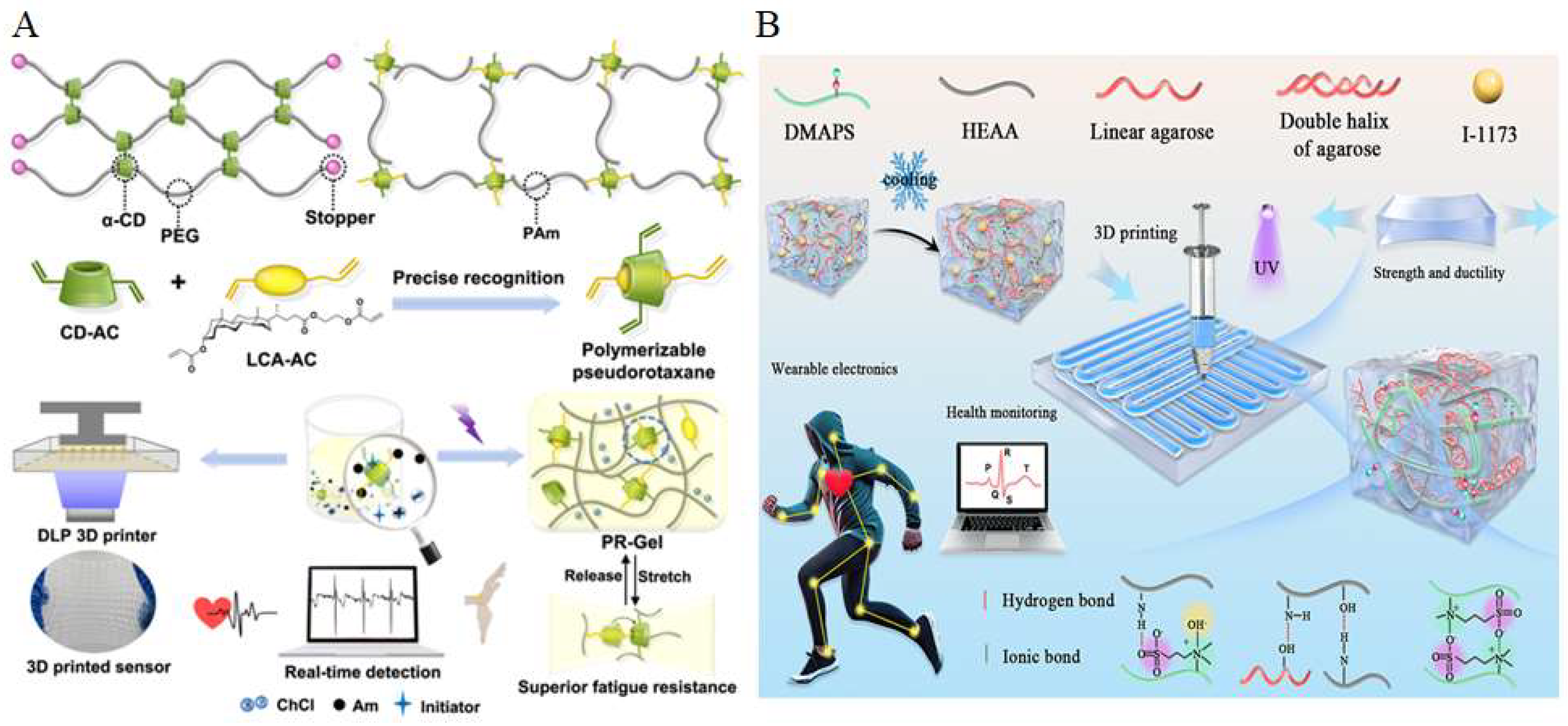
6. Application of Printed Conductive Hydrogels In Vitro
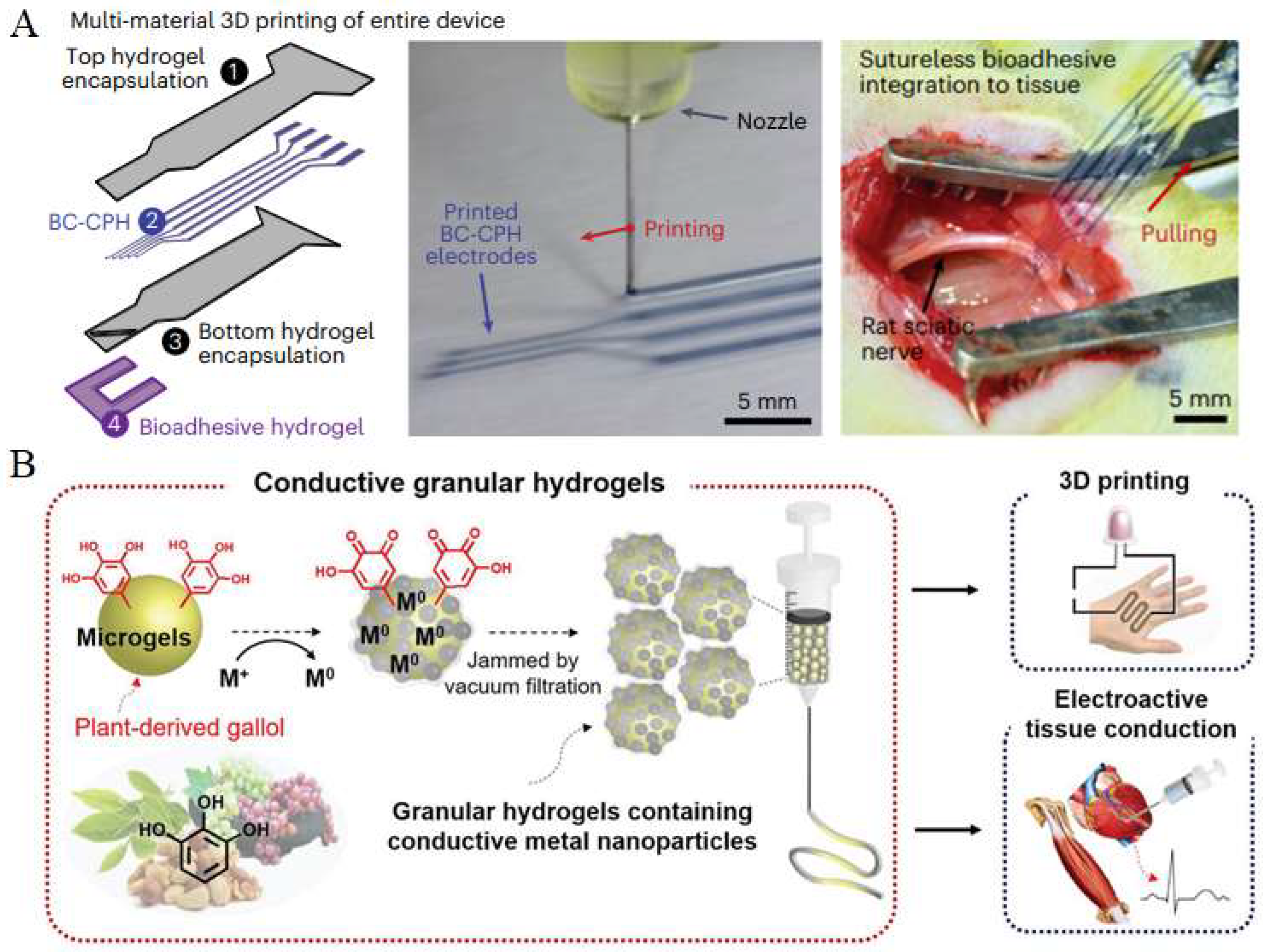
7. Application of Printed Conductive Hydrogels In Vivo
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, T.; Ni, Y.; Biesold, G.M.; Cheng, Y.; Ge, M.; Li, H.; Huang, J.; Lin, Z.; Lai, Y. Recent Advances in Conductive Hydrogels: Classifications, Properties, and Applications. Chem. Soc. Rev. 2022, 52, 473–509. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhou, X.; Dong, Y.; Li, J. Flexible Self-Repairing Materials for Wearable Sensing Applications: Elastomers and Hydrogels. Macromol. Rapid Commun. 2020, 41, 2000444. [Google Scholar] [CrossRef]
- Peng, Q.; Chen, J.; Wang, T.; Peng, X.; Liu, J.; Wang, X.; Wang, J.; Zeng, H. Recent Advances in Designing Conductive Hydrogels for Flexible Electronics. InfoMat 2020, 2, 843–865. [Google Scholar] [CrossRef]
- Rong, Q.; Lei, W.; Liu, M. Conductive Hydrogels as Smart Materials for Flexible Electronic Devices. Chem. Eur. J. 2018, 24, 16930–16943. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, D.; Liu, B.; Nian, G.; Li, X.; Yin, J.; Qu, S.; Yang, W. 3D printing of multifunctional hydrogels. Adv. Funct. Mater. 2019, 29, 1900971. [Google Scholar] [CrossRef]
- Deng, J.; Yuk, H.; Wu, J.; Varela, C.E.; Chen, X.; Roche, E.T.; Guo, C.F.; Zhao, X. Electrical bioadhesive interface for bioelectronics. Nat. Mater. 2021, 20, 229–236. [Google Scholar] [CrossRef]
- Wang, X.; Sun, X.; Gan, D.; Soubrier, M.; Chiang, H.-Y.; Yan, L.; Li, Y.; Li, J.; Yu, S.; Xia, Y.; et al. Bioadhesive and conductive hydrogel-integrated brain-machine interfaces for conformal and immune-evasive contact with brain tissue. Matter 2022, 5, 1204–1223. [Google Scholar] [CrossRef]
- Yuk, H.; Zhang, T.; Lin, S.; Parada, G.A.; Zhao, X. Touch bonding of hydrogels to diverse non-porous surfaces. Nat. Mater. 2016, 15, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D Printing of Hydrogels: Rational Design Strategies and Emerging Biomedical Applications. Mater. Sci. Eng. R Rep. 2020, 140, 100543. [Google Scholar] [CrossRef]
- Zhou, T.; Yuk, H.; Hu, F.; Wu, J.; Tian, F.; Roh, H.; Shen, Z.; Gu, G.; Xu, J.; Lu, B.; et al. 3D Printable High-Performance Conducting Polymer Hydrogel for All-Hydrogel Bioelectronic Interfaces. Nat. Mater. 2023, 22, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wu, B.; Yuan, P.; Liu, Y.; Hu, C. Progress of Research on Conductive Hydrogels in Flexible Wearable Sensors. Gels 2024, 10, 144. [Google Scholar] [CrossRef]
- Zhao, L.; Li, X.; Li, Y.; Wang, X.; Yang, W.; Ren, J. Polypyrrole-doped conductive self-healing composite hydrogels with high toughness and stretchability. Biomacromolecules 2021, 22, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, K.; Li, Y.; Lan, J.; Yan, B.; Shi, L.; Ran, R. High-strength, self-healable, temperature-sensitive, MXene-containing composite hydrogel as a smart compression sensor. ACS Appl. Mater. Interfaces 2019, 11, 47350–47357. [Google Scholar] [CrossRef] [PubMed]
- Borbolla-Jiménez, F.V.; Peña-Corona, S.I.; Farah, S.J.; Jiménez-Valdés, M.T.; Pineda-Pérez, E.; Romero-Montero, A.; Del Prado-Audelo, M.L.; Bernal-Chávez, S.A.; Magaña, J.J.; Leyva-Gómez, G. Films for wound healing fabricated using a solvent casting technique. Pharmaceutics 2023, 15, 1914. [Google Scholar] [CrossRef] [PubMed]
- Albdiry, M. Effect of melt blending processing on mechanical properties of polymer nanocomposites: A review. Polym. Bull. 2024, 81, 5793–5821. [Google Scholar] [CrossRef]
- Teng, Y.; Chi, J.; Huang, J.; Li, Z.; Li, S.; Wu, X.; Zhu, L.; Ren, J. Hydrogel toughening resets biomedical application boundaries. Prog. Polym. Sci. 2025, 161, 101929. [Google Scholar] [CrossRef]
- Fuchs, S.; Shariati, K.; Ma, M. Specialty tough hydrogels and their biomedical applications. Adv. Healthc. Mater. 2020, 9, 1901396. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, X.; Zhao, J.; Ling, G.; Zhang, P. Biomedical applications of supramolecular hydrogels with enhanced mechanical properties. Adv. Colloid Interface Sci. 2023, 321, 103000. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Han, W.; Lin, H.; Li, R.; Zhu, J.; Huang, W. 3D Printed Flexible Strain Sensors: From Printing to Devices and Signals. Adv. Mater. 2021, 33, e2004782. [Google Scholar] [CrossRef]
- Kumi, M.; Wang, T.; Ejeromedoghene, O.; Wang, J.; Li, P.; Huang, W. Exploring the Potentials of Chitin and Chitosan-Based Bioinks for 3D-Printing of Flexible Electronics: The Future of Sustainable Bioelectronics. Small Methods 2024, 8, e2301341. [Google Scholar] [CrossRef]
- Rim, Y.S.; Bae, S.H.; Chen, H.; De Marco, N.; Yang, Y. Recent Progress in Materials and Devices toward Printable and Flexible Sensors. Adv. Mater. 2016, 28, 4415–4440. [Google Scholar] [CrossRef]
- Placone, J.K.; Engler, A.J. Recent advances in extrusion-based 3D printing for biomedical applications. Adv. Healthc. Mater. 2018, 7, 1701161. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Yang, Z.; Zhang, H.; Wang, Y. Inkjet printing quality improvement research progress: A review. Heliyon 2024, 10, e30163. [Google Scholar] [CrossRef]
- Jeong, Y.G.; Yoo, J.J.; Lee, S.J.; Kim, M.S. 3D digital light process bioprinting: Cutting-edge platforms for resolution of organ fabrication. Mater. Today Bio 2024, 29, 101284. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, E.; Rego, G.P.; Ghosh, R.N.; Sahithi, V.B.; Poojary, D.P.; Namboothiri, P.K.; Peter, M. Advances in In Situ Bioprinting: A Focus on Extrusion and Inkjet-Based Bioprinting Techniques. Regen. Eng. Transl. Med. 2025, 1–19. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, Z.; Lu, X.; Dai, J.; Zhou, C.; Yan, J.; Wang, L.; Wang, Z.; Zang, J. Minimally invasive bioprinting for in situ liver regeneration. Bioact. Mater. 2023, 26, 465–477. [Google Scholar] [CrossRef]
- Hu, D.; Cheng, T.K.; Xie, K.; Lam, R.H.W. Microengineered Conductive Elastomeric Electrodes for Long-Term Electrophysiological Measurements with Consistent Impedance under Stretch. Sensors 2015, 15, 26906–26920. [Google Scholar] [CrossRef]
- Xu, L.; Du, X.; Zhou, Y.; Cao, X.; Shen, Y.; Zhu, H.; Huang, H. Polyaspartic Acid-Stabilized CaCO3-Containing In Situ Hydrogel for Protection and Treatment of Gastric Ulcer. Mol. Pharm. 2023, 20, 2105–2118. [Google Scholar] [CrossRef]
- Skardal, A.; Atala, A. Biomaterials for Integration with 3-D Bioprinting. Ann. Biomed. Eng. 2015, 43, 730–746. [Google Scholar] [CrossRef]
- Khan, B.; Abdullah, S.; Khan, S. Current Progress in Conductive Hydrogels and Their Applications in Wearable Bioelectronics and Therapeutics. Micromachines 2023, 14, 1005. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Chen, Y.; Wang, Z.; Liu, H.; Le, M.; Lin, C.; Wu, G.; Wang, L.; Shi, X.; Jia, Y.G.; et al. Polymerizable Rotaxane Hydrogels for Three-Dimensional Printing Fabrication of Wearable Sensors. Nat. Commun. 2023, 14, 1331. [Google Scholar] [CrossRef]
- Chong, J.; Sung, C.; Nam, K.S.; Kang, T.; Kim, H.; Lee, H.; Park, H.; Park, S.; Kang, J. Highly Conductive Tissue-like Hydrogel Interface through Template-Directed Assembly. Nat. Commun. 2023, 14, 2206. [Google Scholar] [CrossRef]
- Bai, J.; Wang, R.; Wang, X.; Liu, S.; Wang, X.; Ma, J.; Qin, Z.; Jiao, T. Biomineral calcium-ion-mediated conductive hydrogels with high stretchability and self-adhesiveness for sensitive iontronic sensors. Cell Rep. Phys. Sci. 2021, 2, 100623. [Google Scholar] [CrossRef]
- Liao, H.; Guo, X.; Wan, P.; Yu, G. Conductive MXene nanocomposite organohydrogel for flexible, healable, low-temperature tolerant strain sensors. Adv. Funct. Mater. 2019, 29, 1904507. [Google Scholar] [CrossRef]
- Alkahtani, M.E.; Sun, S.; Chapman, C.A.R.; Gaisford, S.; Orlu, M.; Elbadawi, M.; Basit, A.W. 3D Printed Electro-Responsive System with Programmable Drug Release. Mater. Today Adv. 2024, 23, 100509. [Google Scholar] [CrossRef]
- Duran, M.M.; Moro, G.; Zhang, Y.; Islam, A. 3D Printing of Silicone and Polyurethane Elastomers for Medical Device Application: A Review. Adv. Ind. Manuf. Eng. 2023, 7, 100125. [Google Scholar] [CrossRef]
- Park, J.; Lee, Y.; Kim, T.Y.; Hwang, S.; Seo, J. Functional bioelectronic materials for long-term biocompatibility and functionality. ACS Appl. Electron. Mater. 2022, 4, 1449–1468. [Google Scholar] [CrossRef]
- Cuttaz, E.; Goding, J.; Vallejo-Giraldo, C.; Aregueta-Robles, U.; Lovell, N.; Ghezzi, D.; Green, R.A. Conductive elastomer composites for fully polymeric, flexible bioelectronics. Biomater. Sci. 2019, 7, 1372–1385. [Google Scholar] [CrossRef]
- Kim, S.; Kang, J.; Lee, I.; Jang, J.; Park, C.B.; Lee, W.; Bae, B.S. An intrinsically stretchable multi-biochemical sensor for sweat analysis using photo-patternable ecoflex. npj Flex. Electron. 2023, 7, 33. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhong, W. Recent progress in advanced polyester elastomers for tissue engineering and bioelectronics. Molecules 2023, 28, 8025. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z. Printing Practice for the Fabrication of Flexible and Stretchable Electronics. Sci. China Technol. Sci. 2019, 62, 224–232. [Google Scholar] [CrossRef]
- Tazwar, H.T.; Antora, M.F.; Nowroj, I.; Rashid, A.B. Conductive polymer composites in soft robotics, flexible sensors and energy storage: Fabrication, applications and challenges. Biosens. Bioelectron. X 2025, 24, 100597. [Google Scholar] [CrossRef]
- Shin, Y.; Lee, H.S.; Jeong, H.; Kim, D.-H. Recent advances in conductive hydrogels for soft biointegrated electronics: Materials, properties, and device applications. Wearable Electron. 2024, 1, 255–280. [Google Scholar] [CrossRef]
- Iqbal, J.D.; Biller-Andorno, N. The regulatory gap in digital health and alternative pathways to bridge it. Health Policy Technol. 2022, 11, 100663. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Lu, T.; Luo, J.; Xue, H. Prostate-Specific Membrane Antigen Targeted, Glutathione-Sensitive Nanoparticles Loaded with Docetaxel and Enzalutamide for the Delivery to Prostate Cancer. Drug Deliv. 2022, 29, 2705–2712. [Google Scholar] [CrossRef]
- Xia, D.; Zhang, X.; Hao, H.; Jiang, W.; Chen, C.; Li, H.; Feng, L.; Li, J.; Wu, Y.; Zhang, L.; et al. Strategies to Prolong Drug Retention in Solid Tumors by Aggregating Endo-CMC Nanoparticles. J. Control. Release 2023, 360, 705–717. [Google Scholar] [CrossRef]
- Ke, Y.; Huang, L.; Song, Y.; Liu, Z.; Liang, L. Preparation and Pharmacological Effects of Minor Ginsenoside Nanoparticles: A Review. Front. Pharmacol. 2022, 13, 974274. [Google Scholar] [CrossRef]
- Tang, B.; Qian, Y.; Fang, G. Development of Lipid–Polymer Hybrid Nanoparticles for Improving Oral Absorption of Enoxaparin. Pharmaceutics 2020, 12, 607. [Google Scholar] [CrossRef]
- Xu, Y.; Dong, X.; Xu, H.; Jiao, P.; Zhao, L.X.; Su, G. Nanomaterial-Based Drug Delivery Systems for Pain Treatment and Relief: From the Delivery of a Single Drug to Co-Delivery of Multiple Therapeutics. Pharmaceutics 2023, 15, 2309. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fu, X.; Lin, M.; Jiang, X. Azeotropic Distillation-Induced Self-Assembly of Mesostructured Spherical Nanoparticles as Drug Cargos for Controlled Release of Curcumin. Pharmaceuticals 2022, 15, 275. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Han, G.; Chen, Q.; Yu, L.; Wang, P.; Zhang, Q.; Dong, J.; Zhang, W.; Huang, J. Au-Pt Nanoparticle Formulation as a Radiosensitizer for Radiotherapy with Dual Effects. Int. J. Nanomed. 2021, 16, 239–248. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, Y.; Xu, Y.; Zhu, C.; Liu, T.; Wang, K. Chitosan Nanoparticles for Oral Photothermally Enhanced Photodynamic Therapy of Colon Cancer. Int. J. Pharm. 2020, 589, 119763. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.W.; Fan, L.Y.; Shen, Z.Y. Application Research Progress of Nanomaterial Graphene and Its Derivative Complexes in Tumor Diagnosis and Therapy. Curr. Med. Chem. 2024, 31, 6436–6459. [Google Scholar] [CrossRef]
- Hu, Y. Superparamagnetic Iron Oxide Nanoparticles: Cytotoxicity, Metabolism, and Cellular Behavior in Biomedicine Applications. Int. J. Nanomed. 2021, 16, 6097–6113. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Deng, P.; Yin, R.; Zhu, Z.; Ling, C.; Ma, M.; Wang, J.; Li, S.; Liu, R. Effect and Mechanism of Paclitaxel Loaded on Magnetic Fe3O4@mSiO2-NH2-FA Nanocomposites to MCF-7 Cells. Drug Deliv. 2023, 30, 64–82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Meng, C.; Hou, K.; Wang, Z.; Huang, Y. The Cytological and Electrophysiological Effects of Silver Nanoparticles on Neuron-Like. PLoS ONE 2022, 17, e0277942. [Google Scholar] [CrossRef]
- Sui, A.; Yao, C.; Chen, Y.; Li, Y.; Yu, S.; Qu, J.; Wei, H.; Tang, J.; Chen, G. Polystyrene Nanoplastics Inhibit StAR Expression by Activating HIF-1α via ERK1/2 MAPK and AKT Pathways in TM3 Leydig Cells and Testicular Tissues of Mice. Food Chem. Toxicol. 2023, 173, 113634. [Google Scholar] [CrossRef]
- Huang, C.; Wu, D.; Ahmed, F.; Wang, Y.; Xu, J.; Luo, C.; Zhang, K.; Sun, F.; Huo, L. Zinc Oxide Nanoparticle Causes Toxicity to the Development of Mouse Oocyte and Early Embryo. Toxicol. Lett. 2022, 358, 48–58. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, X.; Lu, X.; Yang, Y.; Zhao, L.; Zhou, L.; Wang, K.; Fu, H. Mannose-Decorated Ginsenoside Rb1 Albumin Nanoparticles for Targeted Anti-Inflammatory Therapy. Front. Bioeng. Biotechnol. 2022, 10, 962380. [Google Scholar] [CrossRef]
- Xiong, Y.; Shen, S.; Yi, Z. Synthesis of Uniform Zinc Peroxide Nanoparticles for Antibacterial Application. Indian J. Pharm. Sci. 2024, 86, 923. [Google Scholar] [CrossRef]
- Hu, Y.; Li, D.; Wei, H.; Zhou, S.; Chen, W.; Yan, X.; Cai, J.; Chen, X.; Chen, B.; Liao, M.; et al. Neurite Extension and Orientation of Spiral Ganglion Neurons Can Be Directed by Superparamagnetic Iron Oxide Nanoparticles in a Magnetic Field. Int. J. Nanomed. 2021, 16, 4515–4526. [Google Scholar] [CrossRef]
- Dou, X.; Sun, K.; Chen, H.; Jiang, Y.; Wu, L.; Mei, J.; Ding, Z.; Xie, J. Nanoscale Metal-Organic Frameworks as Fluorescence Sensors for Food Safety. Antibiotics 2021, 10, 358. [Google Scholar] [CrossRef] [PubMed]
- Saquib, M.; Shetty, S.; Rathod, A.; Naik, K.; Nayak, R.; Selvakumar, M. Challenges in Carbon Ink Formulation and Strategies for Fabrication of Flexible Supercapacitors. Carbon. Trends 2025, 19, 100458. [Google Scholar] [CrossRef]
- Li, Z.; LeBlanc, J.; Kumar, H.; Zhang, H.; Yang, W.; He, X.; Lu, Q.; Van Humbeck, J.; Kim, K.; Hu, J. Super-Anti-Freezing, Tough and Adhesive Titanium Carbide and L-Ornithine-Enhanced Hydrogels. J. Bioresour. Bioprod. 2023, 8, 136–145. [Google Scholar] [CrossRef]
- Hart, J.L.; Hantanasirisakul, K.; Lang, A.C.; Anasori, B.; Pinto, D.; Pivak, Y.; van Omme, J.T.; May, S.J.; Gogotsi, Y.; Taheri, M.L. Control of MXenes’ Electronic Properties through Termination and Intercalation. Nat. Commun. 2019, 10, 522. [Google Scholar] [CrossRef]
- Ram, R.; Rahaman, M.; Aldalbahi, A.; Khastgir, D. Determination of percolation threshold and electrical conductivity of polyvinylidene fluoride (PVDF)/short carbon fiber (SCF) composites: Effect of SCF aspect ratio. Polym. Int. 2017, 66, 573–582. [Google Scholar] [CrossRef]
- Shahid, M.A.; Rahman, M.M.; Hossain, M.T.; Hossain, I.; Sheikh, M.S.; Rahman, M.S.; Uddin, N.; Donne, S.W.; Hoque, M.I.U. Advances in Conductive Polymer-Based Flexible Electronics for Multifunctional Applications. J. Compos. Sci. 2025, 9, 42. [Google Scholar] [CrossRef]
- Sui, X.; Guo, H.; Chen, P.; Zhu, Y.; Wen, C.; Gao, Y.; Yang, J.; Zhang, X.; Zhang, L. Zwitterionic Osmolyte-Based Hydrogels with Antifreezing Property, High Conductivity, and Stable Flexibility at Subzero Temperature. Adv. Funct. Mater. 2020, 30, 201907986. [Google Scholar] [CrossRef]
- He, P.; Wu, J.; Pan, X.; Chen, L.; Liu, K.; Gao, H.; Wu, H.; Cao, S.; Huang, L.; Ni, Y. Anti-Freezing and Moisturizing Conductive Hydrogels for Strain Sensing and Moist-Electric Generation Applications. J. Mater. Chem. A 2020, 8, 3109–3118. [Google Scholar] [CrossRef]
- Jose, J.; Peter, A.; Thajudeen, K.Y.; Gomes Pereira, M.D.L.; Bhat, S.G.; Michel, H. Recent Advances in the Design and Development of Bioink Formulations for Various Biomedical Applications. Results Eng. 2024, 22, 102060. [Google Scholar] [CrossRef]
- Athukorala, S.S.; Tran, T.S.; Balu, R.; Truong, V.K.; Chapman, J.; Dutta, N.K.; Roy Choudhury, N. 3D printable electrically conductive hydrogel scaffolds for biomedical applications: A review. Polymers 2021, 13, 474. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, M.; Chong, C.M.; Lin, D.; Chen, S.; Zhen, Y.; Ding, H.; Zhong, H.J. Recent Advances in the 3D Printing of Conductive Hydrogels for Sensor Applications: A Review. Polymers 2024, 16, 2131. [Google Scholar] [CrossRef]
- Cai, L.; Wang, C. Carbon Nanotube Flexible and Stretchable Electronics. Nanoscale Res. Lett. 2015, 10, 320. [Google Scholar] [CrossRef]
- Van Hazendonk, L.S.; Pinto, A.M.; Arapov, K.; Pillai, N.; Beurskens, M.R.C.; Teunissen, J.P.; Sneck, A.; Smolander, M.; Rentrop, C.H.A.; Bouten, P.C.P.; et al. Printed Stretchable Graphene Conductors for Wearable Technology. Chem. Mater. 2022, 34, 8031–8042. [Google Scholar] [CrossRef] [PubMed]
- Koch, B.; Rubino, I.; Quan, F.S.; Yoo, B.; Choi, H.J. Microfabrication for Drug Delivery. Materials 2016, 9, 646. [Google Scholar] [CrossRef] [PubMed]
- Faustino, V.; Catarino, S.O.; Lima, R.; Minas, G. Biomedical Microfluidic Devices by Using Low-Cost Fabrication Techniques: A Review. J. Biomech. 2016, 49, 2280–2292. [Google Scholar] [CrossRef] [PubMed]
- Morbioli, G.G.; Speller, N.C.; Stockton, A.M. A Practical Guide to Rapid-Prototyping of PDMS-Based Microfluidic Devices: A Tutorial. Anal. Chim. Acta 2020, 1135, 150–174. [Google Scholar] [CrossRef]
- Sun, H.; Zettl, J.; Willenbacher, N. Highly Conductive and Stretchable Filament for Flexible Electronics. Addit. Manuf. 2023, 78, 103872. [Google Scholar] [CrossRef]
- Ke, S.; Liu, Y.; Chen, F.; Ni, X.; Ma, Y. Recent Advances in Conductive Materials for Printed Electronics and Printed Technology. Polym. Adv. Technol. 2024, 35, 6581. [Google Scholar] [CrossRef]
- Tran, T.S.; Dutta, N.K.; Choudhury, N.R. Graphene Inks for Printed Flexible Electronics: Graphene Dispersions, Ink Formulations, Printing Techniques and Applications. Adv. Colloid Interface Sci. 2018, 261, 41–61. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Huang, X.; Chen, J.; Wu, K.; Wang, J.; Zhang, X. A Review of Conductive Carbon Materials for 3d Printing: Materials, Technologies, Properties, and Applications. Materials 2021, 14, 3911. [Google Scholar] [CrossRef]
- Lage-Rivera, S.; Ares-Pernas, A.; Becerra Permuy, J.C.; Gosset, A.; Abad, M.J. Enhancement of 3D Printability by FDM and Electrical Conductivity of PLA/MWCNT Filaments Using Lignin as Bio-Dispersant. Polymers 2023, 15, 999. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.R.G.; Lopes, C.E.C.; Tanaka, A.A.; Dantas, L.M.F.; Silva, I.S.; Stefano, J.S. Electrochemical Biosensors 3D Printed by Fused Deposition Modeling: Actualities, Trends, and Challenges. Biosensors 2025, 15, 57. [Google Scholar] [CrossRef]
- Aloqalaa, Z. Electrically conductive fused deposition modeling filaments: Current status and medical applications. Crystals 2022, 12, 1055. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, L.; Wang, J. Recent Developments in Conductive Polymer Composites for Fused Deposition Modeling. Compos. Part A Appl. Sci. Manuf. 2023, 174, 107739. [Google Scholar] [CrossRef]
- Bozkurt, Y.; Karayel, E. 3D printing technology; methods, biomedical applications, future opportunities and trends. J. Mater. Res. Technol. 2021, 14, 1430–1450. [Google Scholar] [CrossRef]
- Devi, M.G.; Amutheesan, M.; Govindhan, R.; Karthikeyan, B. A review of three-dimensional printing for biomedical and tissue engineering applications. Open Biotechnol. J. 2018, 12, 241–255. [Google Scholar] [CrossRef]
- Heo, D.N.; Lee, S.J.; Timsina, R.; Qiu, X.; Castro, N.J.; Zhang, L.G. Development of 3D Printable Conductive Hydrogel with Crystallized PEDOT:PSS for Neural Tissue Engineering. Mater. Sci. Eng. C 2019, 99, 582–590. [Google Scholar] [CrossRef]
- Ryan, K.R.; Down, M.P.; Hurst, N.J.; Keefe, E.M.; Banks, C.E. Additive Manufacturing (3D Printing) of Electrically Conductive Polymers and Polymer Nanocomposites and Their Applications. eScience 2022, 2, 365–381. [Google Scholar] [CrossRef]
- Zhou, J.; Vijayavenkataraman, S. 3D-Printable Conductive Materials for Tissue Engineering and Biomedical Applications. Bioprinting 2021, 24, e00166. [Google Scholar] [CrossRef]
- Kim, J.; Jang, J. 3D Printable Conductive Composite Inks for the Fabrication of Biocompatible Electrodes in Tissue Engineering Application. Int. J. Bioprint. 2022, 9, 287–298. [Google Scholar] [CrossRef]
- Hashemi, A.; Ezati, M.; Zumberg, I.; Chmelíková, L.; Fohlerová, Z.; Provazník, V. In Vitro Evaluation of 3D-Printed Conductive Chitosan-Polyaniline Scaffolds with Exosome Release for Enhanced Angiogenesis and Cardiomyocyte Protection. RSC Adv. 2025, 15, 16826–16844. [Google Scholar] [CrossRef]
- Serafin, A.; Casanova, C.R.; Chandel, A.K.S.; Reis, R.L.; Oliveira, J.M.; Collins, M.N. Conductive Biological Materials for in Vitro Models: Properties and Sustainability Implications. Vitr. Model. 2025, 4, 89–110. [Google Scholar] [CrossRef]
- Borayek, R.; Foroughi, F.; Xin, X.; Mohamed, A.M.; Abdelrahman, M.M.; Zedan, M.; Zhang, D.; Ding, J. Near-Zero Hysteresis Ionic Conductive Elastomers with Long-Term Stability for Sensing Applications. ACS Appl. Mater. Interfaces 2022, 14, 11727–11738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wen, H.; Zhu, J.; Xia, Z.; Zhang, Z. 3D Printing Dielectric Elastomers for Advanced Functional Structures: A Mini-Review. J. Appl. Polym. Sci. 2024, 141, 55015. [Google Scholar] [CrossRef]
- e Silva, E.P.; Huang, B.; Helaehil, J.V.; Nalesso, P.R.L.; Bagne, L.; de Oliveira, M.A.; Albiazetti, G.C.C.; Aldalbahi, A.; El-Newehy, M.; Santamaria, M., Jr.; et al. In Vivo Study of Conductive 3D Printed PCL/MWCNTs Scaffolds with Electrical Stimulation for Bone Tissue Engineering. Bio-Des. Manuf. 2021, 4, 190–202. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, Y.; Wang, J.; Wu, Q.; Gu, Z.; Zhou, Y.; Liu, X.; Yang, Y.; Tang, H.; Ling, Q.; et al. Ferromagnetic Soft Catheter Robots for Minimally Invasive Bioprinting. Nat. Commun. 2021, 12, 5072. [Google Scholar] [CrossRef]
- Park, Y.G.; Kwon, Y.W.; Koh, C.S.; Kim, E.; Lee, D.H.; Kim, S.; Mun, J.; Hong, Y.M.; Lee, S.; Kim, J.Y.; et al. In-Vivo Integration of Soft Neural Probes through High-Resolution Printing of Liquid Electronics on the Cranium. Nat. Commun. 2024, 15, 1772. [Google Scholar] [CrossRef]
- Dong, R.; Wang, L.; Hang, C.; Chen, Z.; Liu, X.; Zhong, L.; Qi, J.; Huang, Y.; Liu, S.; Wang, L.; et al. Printed Stretchable Liquid Metal Electrode Arrays for In Vivo Neural Recording. Small 2021, 17, 2006612. [Google Scholar] [CrossRef]
- Chen, G.; Ullah, A.; Xu, G.; Xu, Z.; Wang, F.; Liu, T.; Su, Y.; Zhang, T.; Wang, K. Topically Applied Liposome-in-Hydrogels for Systematically Targeted Tumor Photothermal Therapy. Drug Deliv. 2021, 28, 1923–1931. [Google Scholar] [CrossRef]
- Xu, L.; Bai, E.; Zhu, Y.; Qin, J.; Du, X.; Huang, H. PH-Responsive Hydrogel as a Potential Oral Delivery System of Baicalin for Prolonging Gastroprotective Activity. Pharmaceutics 2023, 15, 257. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, P.; Jiang, Y.; Li, L.; Ju, F.; Cheng, Q.; Zhou, Y.L.; Zhou, Y. Sustained-Release Esketamine Based Nanoparticle-Hydrogel Delivery System for Neuropathic Pain Management. Int. J. Nanomed. 2023, 18, 1131–1143. [Google Scholar] [CrossRef]
- Jie Li, Z.; Bing Luo, C.; Liang Wang, H.; Sun, J.; Qian Yang, Q.; Lang Zhou, Y. Metformin Suppressed Tendon Injury-Induced Adhesion via Hydrogel-Nanoparticle Sustained-Release System. Int. J. Pharm. 2023, 642, 123190. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tang, Q.; Lu, Y.; Chen, C.; Zhao, Y.L.; Xu, T.; Yang, C.W.; Chen, X.Q. Berberine-Loaded MSC-Derived SEVs Encapsulated in Injectable GelMA Hydrogel for Spinal Cord Injury Repair. Int. J. Pharm. 2023, 643, 123283. [Google Scholar] [CrossRef]
- Ji, J.; Cheng, J.; Chen, C.; Lu, Y.; Chen, X.; Zhang, F. Pirfenidone—Loaded Hyaluronic Acid Methacryloyl Hydrogel for Preventing Epidural Adhesions after Laminectomy. Drug Deliv. Transl. Res. 2023, 13, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Z.; Ge, Y.; Zhu, Y.; Tian, T.; Wei, J.; Jin, Y.; Zhao, Y.; Jia, Q.; Wu, J.; et al. Microenvironment Responsive Hydrogel Exerting Inhibition of Cascade Immune Activation and Elimination of Synovial Fibroblasts for Rheumatoid Arthritis Therapy. J. Control. Release 2024, 370, 747–762. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, J.; Gu, Y.; Liu, T.; Chen, L. Biomineralized MnO2 Nanoparticle-Constituted Hydrogels Promote Spinal Cord Injury Repair by Modulating Redox Microenvironment and Inhibiting Ferroptosis. Pharmaceutics 2024, 16, 1057. [Google Scholar] [CrossRef]
- Li, Z.; Kumar, H.; Guo, C.; Shin, J.; He, X.; Lu, Q.; Bai, H.; Kim, K.; Hu, J. Development of Antifreezing, Printable, Adhesive, Tough, Biocompatible, High-Water Content Hydrogel for Versatile Applications. ACS Appl. Mater. Interfaces 2023, 15, 16034–16045. [Google Scholar] [CrossRef]
- Li, Z.; He, X.; Kumar, H.; Shin, J.; Guo, C.; Abraham, B.; Shayesteh, A.; Kibria, M.; Lu, Q.; Bai, H.; et al. Visible Light Laser Direct-Writing of High-Resolution, Biocompatible, Super-Multifunctional and Tough Hydrogels without Photoinitiators in 30 S. Biomater. Adv. 2023, 147, 213318. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, Y.; Lu, J.; Cai, R.; Zhao, T.; Chen, Y.; Zhang, M.; Lu, X.; Chen, Y. Injectable, Tough and Adhesive Zwitterionic Hydrogels for 3D-Printed Wearable Strain Sensors. Chem. Eng. J. 2023, 475, 146340. [Google Scholar] [CrossRef]
- Shin, M.; Song, K.H.; Burrell, J.C.; Cullen, D.K.; Burdick, J.A. Injectable and Conductive Granular Hydrogels for 3D Printing and Electroactive Tissue Support. Adv. Sci. 2019, 6, 1901229. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Shen, C.; Chen, H.; Shin, J.; Dixit, K.; Lee, H.J. Printable Conductive Hydrogels and Elastomers for Biomedical Application. Gels 2025, 11, 707. https://doi.org/10.3390/gels11090707
Li Z, Shen C, Chen H, Shin J, Dixit K, Lee HJ. Printable Conductive Hydrogels and Elastomers for Biomedical Application. Gels. 2025; 11(9):707. https://doi.org/10.3390/gels11090707
Chicago/Turabian StyleLi, Zhangkang, Chenyu Shen, Hangyu Chen, Jaemyung Shin, Kartikeya Dixit, and Hyun Jae Lee. 2025. "Printable Conductive Hydrogels and Elastomers for Biomedical Application" Gels 11, no. 9: 707. https://doi.org/10.3390/gels11090707
APA StyleLi, Z., Shen, C., Chen, H., Shin, J., Dixit, K., & Lee, H. J. (2025). Printable Conductive Hydrogels and Elastomers for Biomedical Application. Gels, 11(9), 707. https://doi.org/10.3390/gels11090707






