Microstructure and Photocatalytic Performance of BaTi5O11 Nanocrystals Synthesized via Sol-Gel Method Mediated by Organic Solvents
Abstract
1. Introduction
2. Results and Discussions
3. Conclusions
4. Materials and Methods
4.1. BaTi5O11 Nanocrystal Preparation
4.2. Material Characterization
4.3. Photocatalytic MB Degradation
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Masekela, D.; Hintsho-Mbita, N.; Sam, S.; Yusuf, T.; Mabuba, N. Application of BaTiO3-based catalysts for piezocatalytic, photocatalytic and piezo-photocatalytic degradation of organic pollutants and bacterial disinfection in wastewater: A comprehensive review. Arab. J. Chem. 2023, 16, 104473. [Google Scholar] [CrossRef]
- Ray, S.; Cho, J.; Hur, J. A critical review on strategies for improving efficiency of BaTiO3-based photocatalysts for wastewater treatment. J. Environ. Manage. 2021, 290, 112679. [Google Scholar] [CrossRef]
- Taghreed, A.; Riham, S.; Muftah, H. Organic Contaminants in Industrial Wastewater: Prospects of Waste Management by Integrated Approaches. In Combined Application of Physico-Chemical & Microbiological Processes for Industrial Effluent Treatment Plant; Springer: Singapore, 2020; pp. 205–235. [Google Scholar]
- Yarbay, R.; Şimşek, V.; Bogdan, L.; Tomašić, V. Photocatalytic Degradation of Neonicotinoids—A Comparative Study of the Efficacy of Hybrid Photocatalysts. Processes 2024, 12, 489. [Google Scholar] [CrossRef]
- Bollinger, J.C.; Lima, E.C.; Mouni, L.; Salvestrini, S.; Tran, H.N. Molecular properties of methylene blue, a common probe in sorption and degradation studies: A review. Environ. Chem. Lett. 2025, 23, 1403–1424. [Google Scholar] [CrossRef]
- Liu, M.; You, X.; Li, Y.; Yang, Y. Critical review on development of methylene blue degradation by wet catalytic methods. Rev. Chem. Eng. 2025, 41, 179–195. [Google Scholar] [CrossRef]
- Yamashita, Y.; Tada, M.; Kakihana, M.; Osada, M.; Yoshida, K. Synthesis of RuO2-loaded BaTinO2n+1 (n = 1, 2 and 5) using a polymerizable complex method and its photocatalytic activity for the decomposition of water. J. Mater. Chem. 2002, 12, 1782–1786. [Google Scholar] [CrossRef]
- Kohno, M.; Kaneko, T.; Ogura, S.; Sato, K.; Inoue, Y. Dispersion of ruthenium oxide on barium titanates (Ba6Ti17O40, Ba4Ti13O30, BaTi4O9 and Ba2Ti9O20) and photocatalytic activity for water decomposition. J. Chem. Soc. Faraday Trans. 1998, 94, 89–94. [Google Scholar] [CrossRef]
- Manar, M.; Mohamed, F.; Mohamed, E.; Nabil, E.; Moritz, R.; Ghada, B. Studying the kinetic parameters of BaTi5O11 by using the thermoluminescence technique. Arab. J. Chem. 2023, 16, 105247. [Google Scholar]
- Álvarez, D.; Carmen, M.; Reinosa, J.; Canu, G.; Buscaglia, M.; Buscaglia, M.; Fernández, J. Revealing the Role of the Intermediates during the Synthesis of BaTi5O11. Inorg. Chem. 2019, 58, 8120–8129. [Google Scholar] [CrossRef]
- Chen, Y.; Li, E.; Duan, S.; Zhang, S. Low temperature sintering kinetics and microwave dielectric properties of BaTi5O11 ceramic. ACS Sustain. Chem. Eng. 2017, 5, 10606–10613. [Google Scholar] [CrossRef]
- Fukui, T.; Sakurai, C.; Okuyama, M. Effects of heating rate on sintering of alkoxide-derived BaTi5O11 powder. J. Mater. Res. 1992, 7, 192–196. [Google Scholar] [CrossRef]
- Tangjuank, S.; Tunkasiri, T. Effects of heat-treatment on structural evolution and morphology of BaTi5O11 powder synthesized by the sol-gel method. Mater. Sci. Eng. B 2004, 108, 223–226. [Google Scholar] [CrossRef]
- Sözen, A.P. Synthesis of BaTi5O11 by an aqueous co-precipitation method via a stable organic titanate precursor. J. Serb. Chem. Soc. 2021, 86, 415–427. [Google Scholar]
- Zhang, W.; Han, J.; Cheng, X.; Guo, D. Photocatalytic activity of BaTi5O11 nanocrystals synthesized by hydrothermal method. Funct. Mater. Lett. 2024, 249, 2450015. [Google Scholar] [CrossRef]
- Cheng, X.; Lu, G.; Huang, Y.; Guo, D. Photocatalytic mechanism of BaTi5O11 nanocrystals for methylene blue degradation. Mater. Lett. 2024, 376, 137302. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, X.; Che, J.; Huang, K.; Peng, S.; Wang, J.; Guo, D. Photocatalytic performance of BaTi5O11 nanocrystals synthesized by sol-gel method for methylene blue degradation. Funct. Mater. Lett. 2025, 18, 2550013. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, C.; Guo, D.; Wang, C.; Shen, Q.; Zhang, L. Effect of Zr content on microstructure of BaTi2O5 powders prepared by sol-gel method. Mater. Lett. 2012, 73, 72–74. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Manuspiya, H. Correlation between size and phase structure of crystalline BaTiO3 particles synthesized by sol-gel method. Mater. Res. Exp. 2019, 6, 065062. [Google Scholar] [CrossRef]
- Bokov, D.; Jalil, A.T.; Chupradit, S.; Suksatan, W.; Ansari, M.J.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by sol-gel method: Synthesis and application. Adv. Mater. Sci. Eng. 2021, 2021, 5102014. [Google Scholar] [CrossRef]
- Zarkov, A. Sol–Gel Technology Applied to Materials Science: Synthesis, Characterization and Applications. Materials 2024, 17, 462. [Google Scholar] [CrossRef]
- Niederberger, M. Nonaqueous sol-gel routes to metal oxide nanoparticles. Acc. Chem. Res. 2007, 9, 793–800. [Google Scholar] [CrossRef]
- Rezapour, M.; Talebian, N. Comparison of structural, optical properties and photocatalytic activity of ZnO with different morphologies: Effect of synthesis methods and reaction media. Mater. Chem. Phys. 2011, 129, 249–255. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, S.; Zeng, X.; Lin, M.; Lin, C.; Lin, T.; Gao, M.; Zhao, C.; Wu, X. Microstructural manipulation and enhanced piezo-photocatalytic performance of sol-gel-derived pure BaTiO3 particles. Ceram. Int. 2025, 51, 3960–3969. [Google Scholar] [CrossRef]
- Mageste, F.; Sampaio, D.; Mayrink, T.; Alcamand, H.; Palhares, H.; Nunes, E.; Houmard, M. Influence of the alcoholic solvent and gelation temperature on the structural and water vapor adsorption properties of silica adsorbents synthesized by sol-gel method without catalyst. Microporous Mesoporous Mater. 2022, 342, 112114. [Google Scholar] [CrossRef]
- Dutta, A.; Behera, R.K.; Pradhan, N. Solvent Polarity: How Does This Influence the Precursor Activation, Reaction Rate, Crystal Growth, and Doping in Perovskite Nanocrystals? ACS Energy Lett. 2019, 4, 926–932. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, W.; Li, Y.; Meng, S.; Liu, Y.; Cao, Y.; You, J.; Zhang, B.; Zang, J. The influence of solvent-temperature parameters of sodium titanium phosphate anode materials prepared by sol-gel method. Mater. Chem. Phys. 2024, 315, 128946. [Google Scholar] [CrossRef]
- Gholami, T.; Seifi, H.; Dawi, E.A.; Pirsaheb, M.; Seifi, S.; Aljeboree, A.M.; Hamoody, A.M.; Altimari, U.S.; Abass, M.A.; Salavati-Niasari, M. A review on investigating the effect of solvent on the synthesis, morphology, shape and size of nanostructures. Mater. Sci. Eng. B 2024, 304, 117370. [Google Scholar] [CrossRef]
- Donohue, M.D.; Aranovich, G.L. Adsorption hysteresis in porous solids. J. Colloid Interface Sci. 1998, 205, 121–130. [Google Scholar] [CrossRef]
- Wan, S.; Wang, W.; Cheng, B.; Luo, G.; Shen, Q.; Yu, J.; Zhang, J.; Cao, S.; Zhang, L. A superlattice interface and S-scheme heterojunction for ultrafast charge separation and transfer in photocatalytic H2 evolution. Nat. Commun. 2024, 15, 9612. [Google Scholar] [CrossRef]
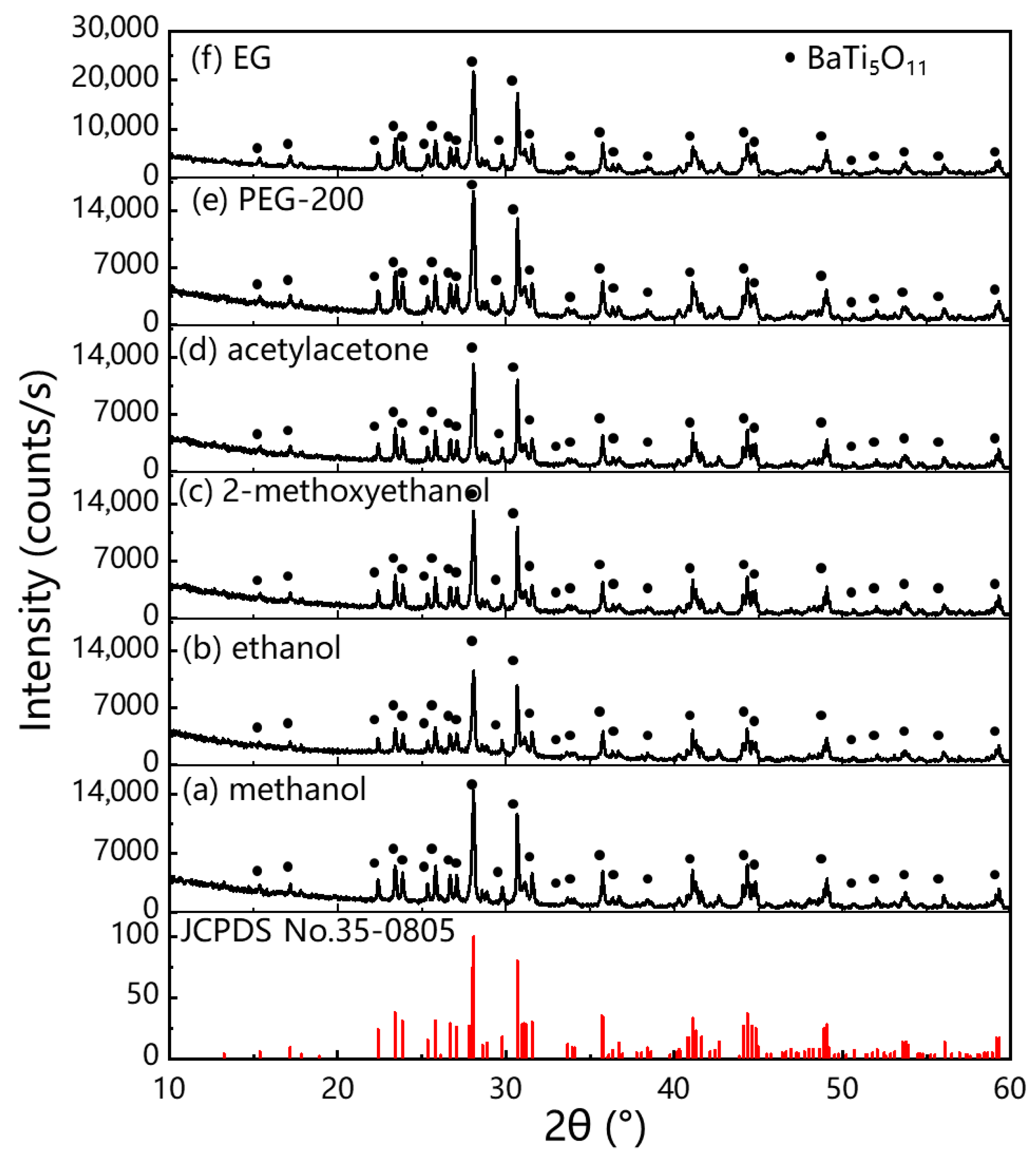



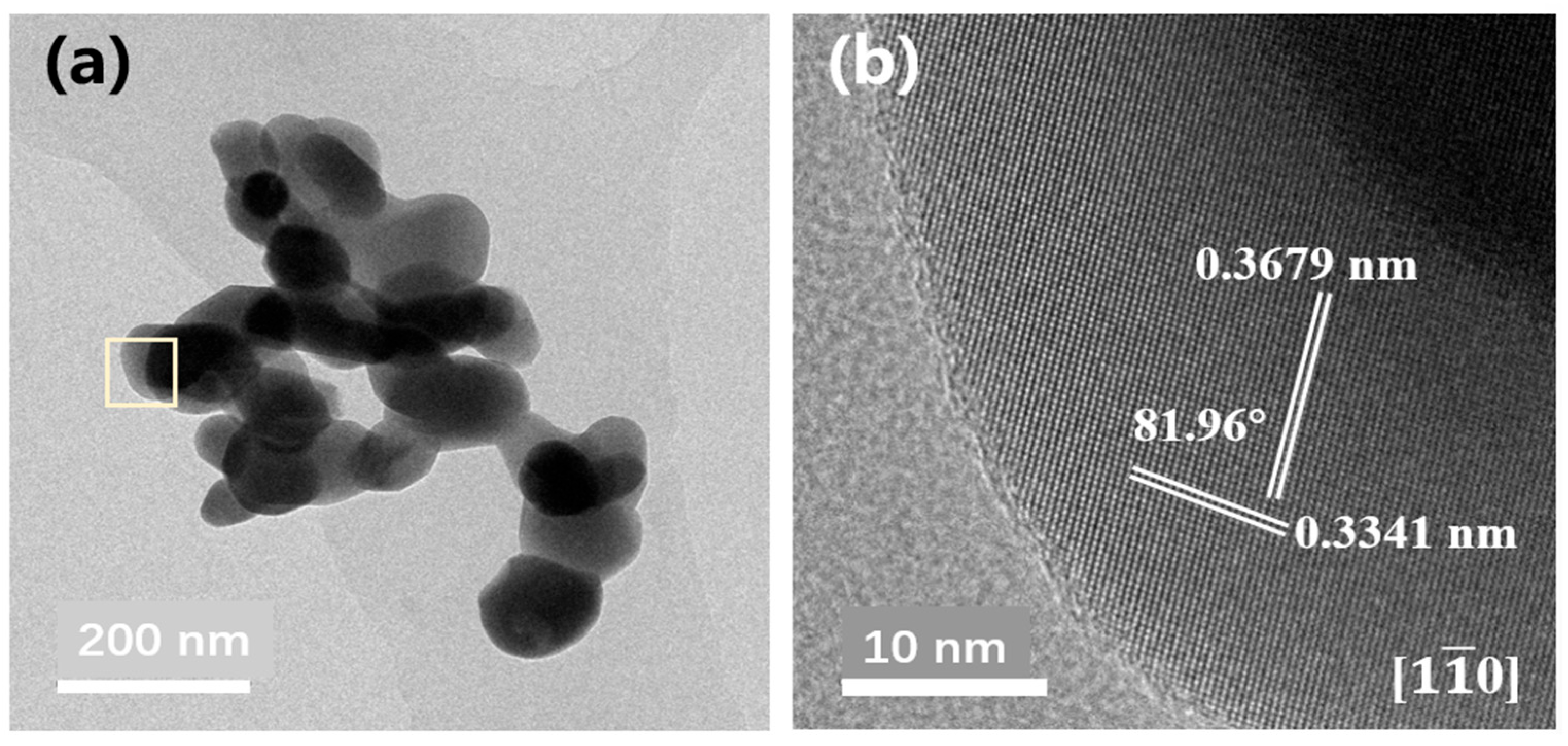


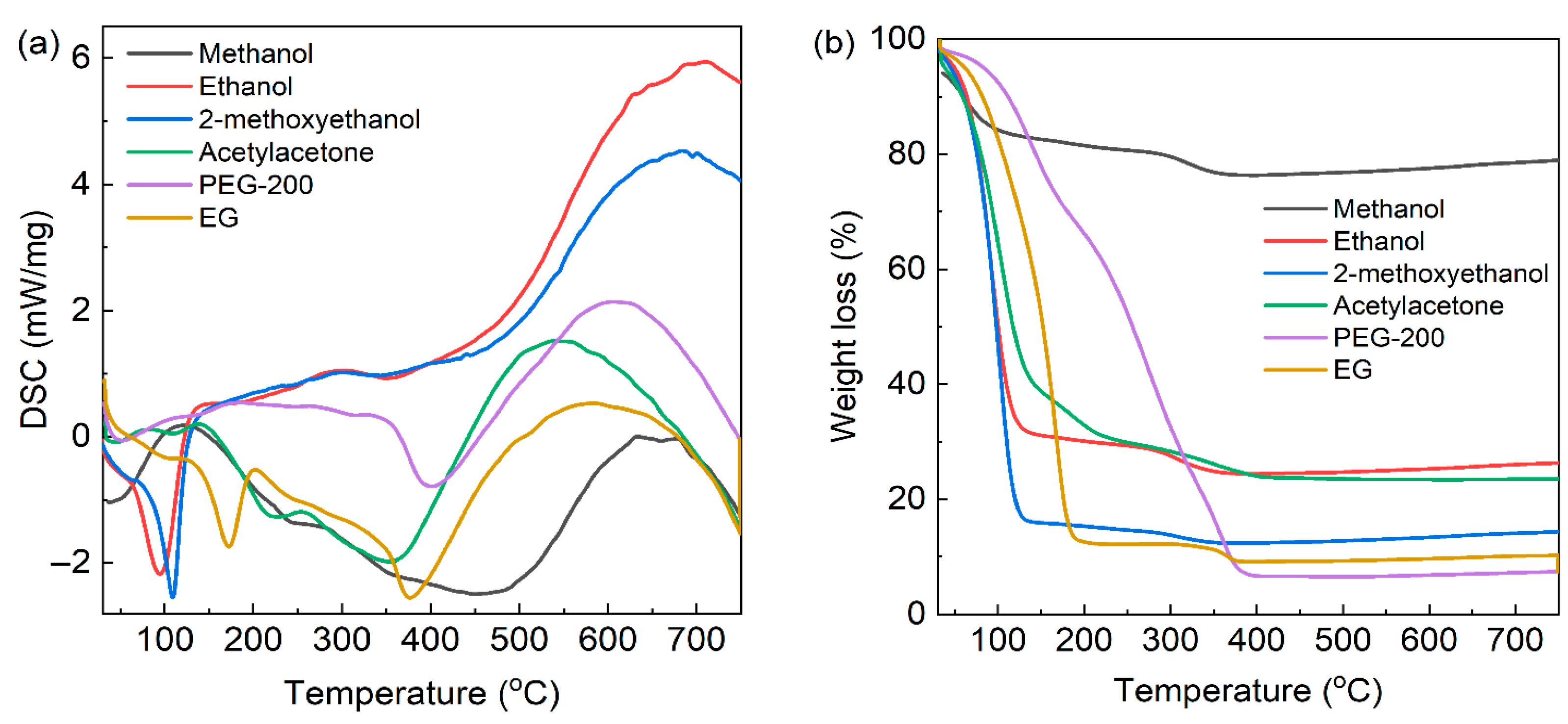
| Solvents | Methanol | Ethanol | 2-Methoxyethanol | Acetylacetone | EG | PEG-200 |
|---|---|---|---|---|---|---|
| Molecular formula | CH4O | C2H6O | C3H8O2 | C5H8O2 | (CH2OH)2 | (CH2OH)n |
| Baking temperature (℃) | 150 | 150 | 150 | 150 | 220 | 250 |
| Viscosity (mPa·s, 25 °C) | 0.550 | 1.07 | 1.80 | 1.48 | 17.3 | 22.0 |
| Specific surface area (m2/g) | 3.69 | 3.78 | 3.97 | 4.69 | 8.69 | 9.78 |
| Average pore size (nm) | 43.8 | 40.5 | 42.9 | 28.9 | 27.8 | 17.8 |
| Eg (eV) | 3.45 | 3.61 | 3.44 | 3.45 | 3.59 | 3.61 |
| K Value (min−1) | 4.98 × 10−2 | 9.12 × 10−2 | 5.13 × 10−2 | 0.13 | 3.98 × 10−2 | 0.28 |
| Solvents | Acetic Acid | Methanol | Ethanol | 2-Methoxyethanol | Acetylacetone | EG | PEG-200 |
|---|---|---|---|---|---|---|---|
| Molecular Formula | C2H4O2 | CH4O | C2H6O | C3H8O2 | C5H8O2 | (CH2OH)2 | (CH2OH)n |
| Molecular Weight | 60 | 32 | 46 | 76 | 100 | 62 | 200 |
| Boiling Point (℃) | 117.9 | 64.7 | 78.3 | 124 | 141 | 197 | 250 |
| Permittivity | 6.15 | 32.7 | 25.8 | 16.93 | 23.1 | 37 | 20.5 |
| Viscosity (mPa·s, 25 °C) | 1.22 | 0.550 | 1.07 | 1.80 | 1.48 | 17.3 | 22.0 |
| Density (g/cm3) | 1.05 | 0.791 | 0.790 | 0.965 | 0.975 | 1.11 | 1.27 |
| Surface Tension | 31.9 | 20.1 | 22.3 | 27.6 | 27.5 | 48.4 | 49.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Gao, T.; Li, X.; Huang, Y.; Wang, J.; Huang, Z.; Guo, D. Microstructure and Photocatalytic Performance of BaTi5O11 Nanocrystals Synthesized via Sol-Gel Method Mediated by Organic Solvents. Gels 2025, 11, 706. https://doi.org/10.3390/gels11090706
Wang H, Gao T, Li X, Huang Y, Wang J, Huang Z, Guo D. Microstructure and Photocatalytic Performance of BaTi5O11 Nanocrystals Synthesized via Sol-Gel Method Mediated by Organic Solvents. Gels. 2025; 11(9):706. https://doi.org/10.3390/gels11090706
Chicago/Turabian StyleWang, Honghua, Tianchen Gao, Xinyi Li, Yuci Huang, Junjie Wang, Zhixiong Huang, and Dongyun Guo. 2025. "Microstructure and Photocatalytic Performance of BaTi5O11 Nanocrystals Synthesized via Sol-Gel Method Mediated by Organic Solvents" Gels 11, no. 9: 706. https://doi.org/10.3390/gels11090706
APA StyleWang, H., Gao, T., Li, X., Huang, Y., Wang, J., Huang, Z., & Guo, D. (2025). Microstructure and Photocatalytic Performance of BaTi5O11 Nanocrystals Synthesized via Sol-Gel Method Mediated by Organic Solvents. Gels, 11(9), 706. https://doi.org/10.3390/gels11090706






