Rheology and Printability of Hydroxyapatite/Sodium Alginate Bioinks Added with Bovine or Fish Collagen Peptides
Abstract
1. Introduction
2. Results and Discussion
2.1. FTIR Analysis of Collagen Peptides
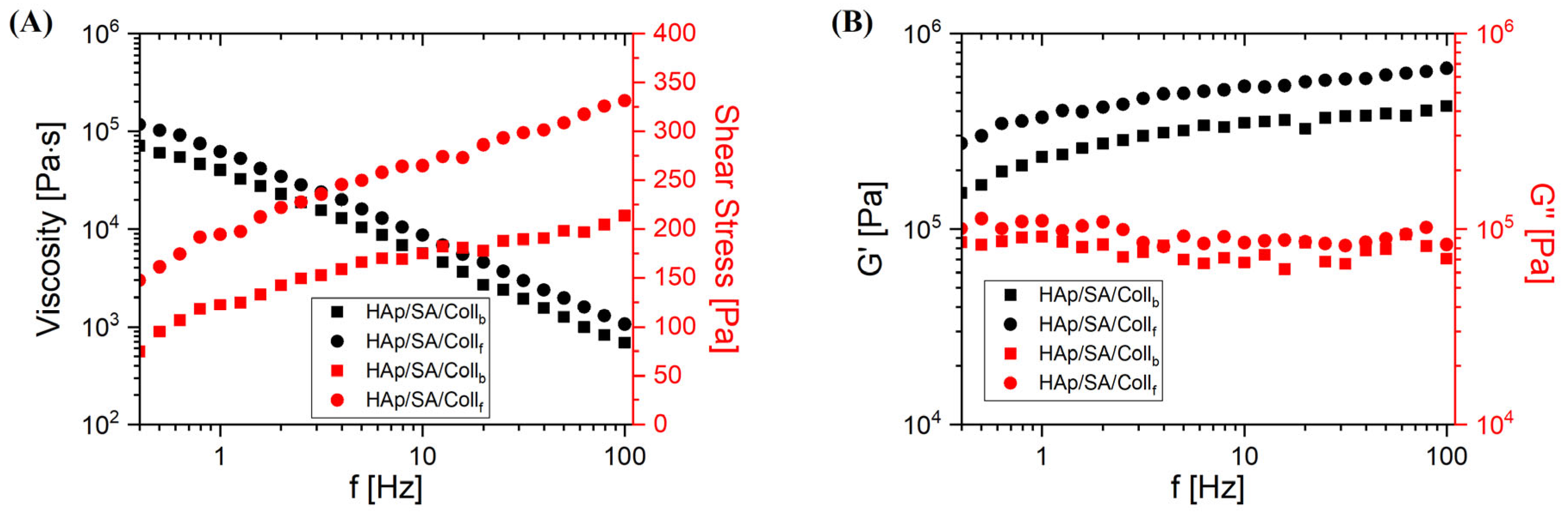
2.2. Rheological Characterization
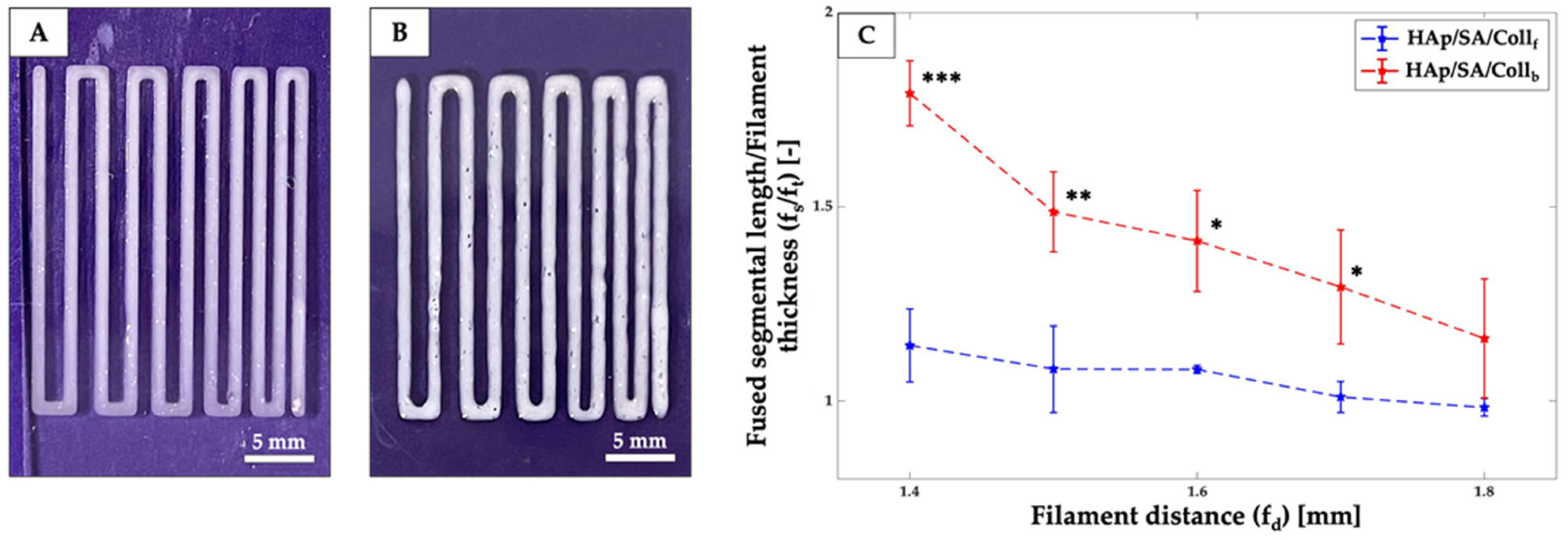
2.3. Printability Assessment
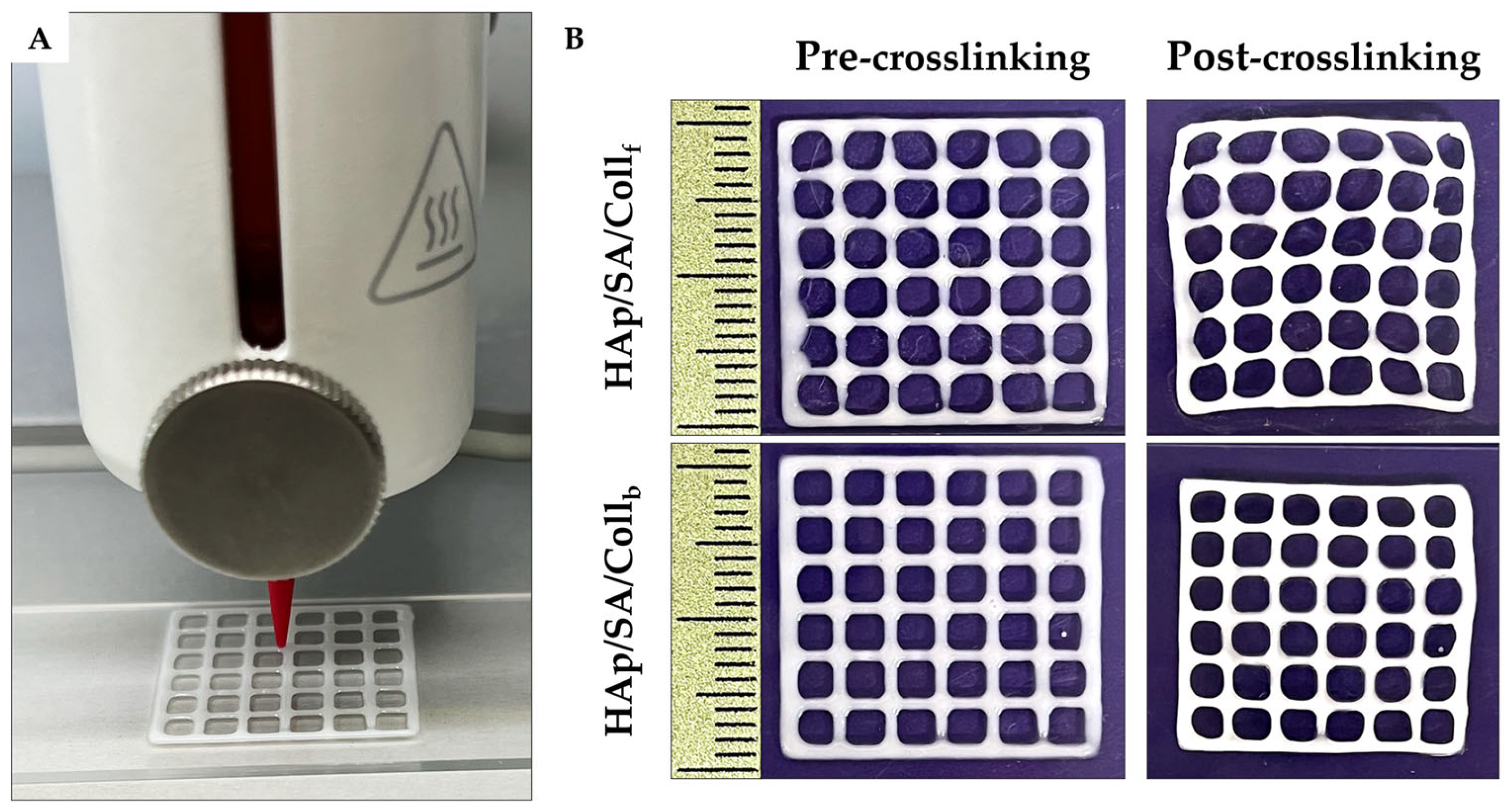
2.4. Gel Fraction Analysis
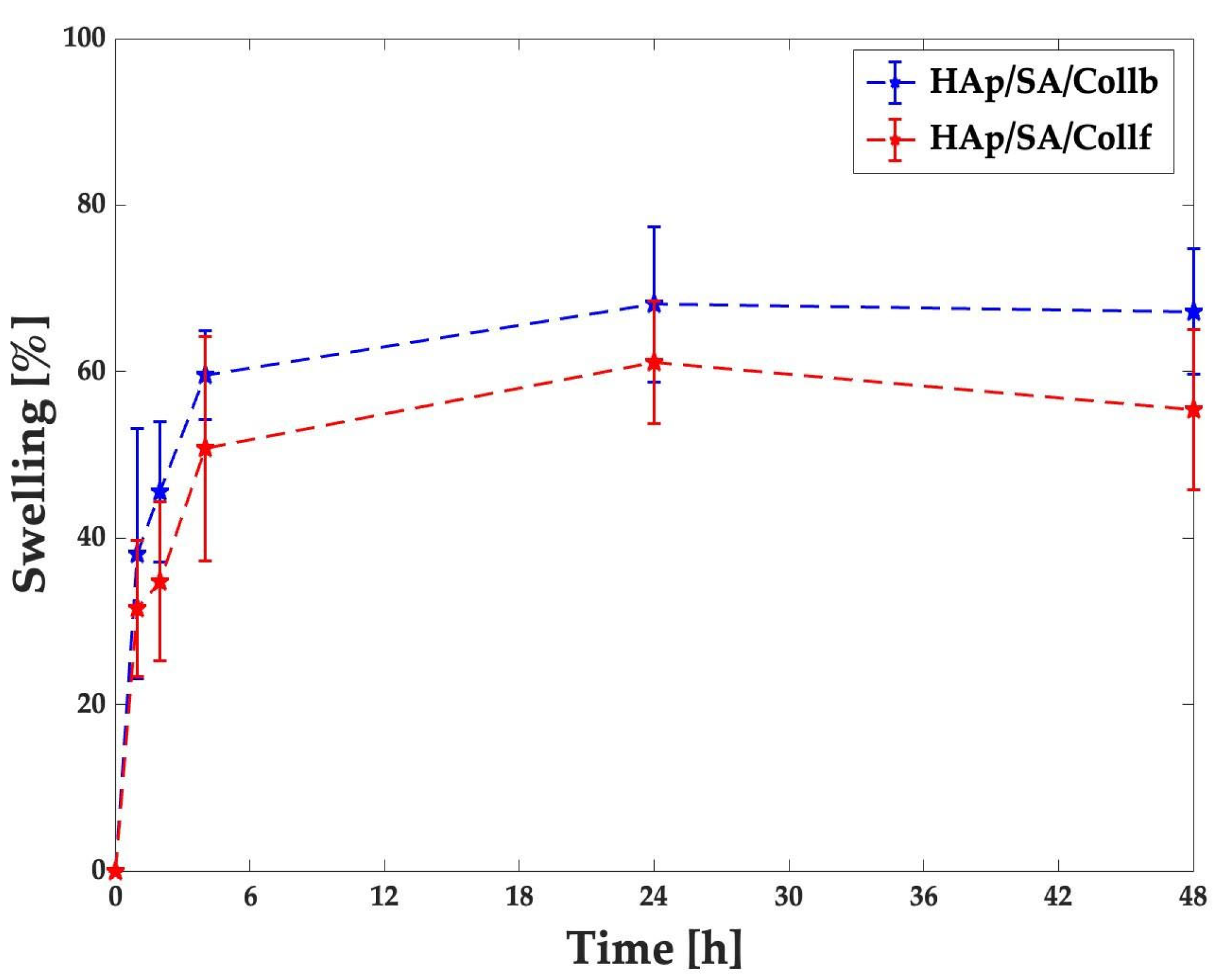
2.5. Swelling Test
2.6. Cytocompatibility Evaluation
2.7. Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Fourier Transform Infrared Spectroscopy
4.3. Preparation of HAp/SA/Coll Inks
4.4. Rheological Characterization of the Inks
4.5. The 3D Printing of Inks
4.6. Filament Fusion Test
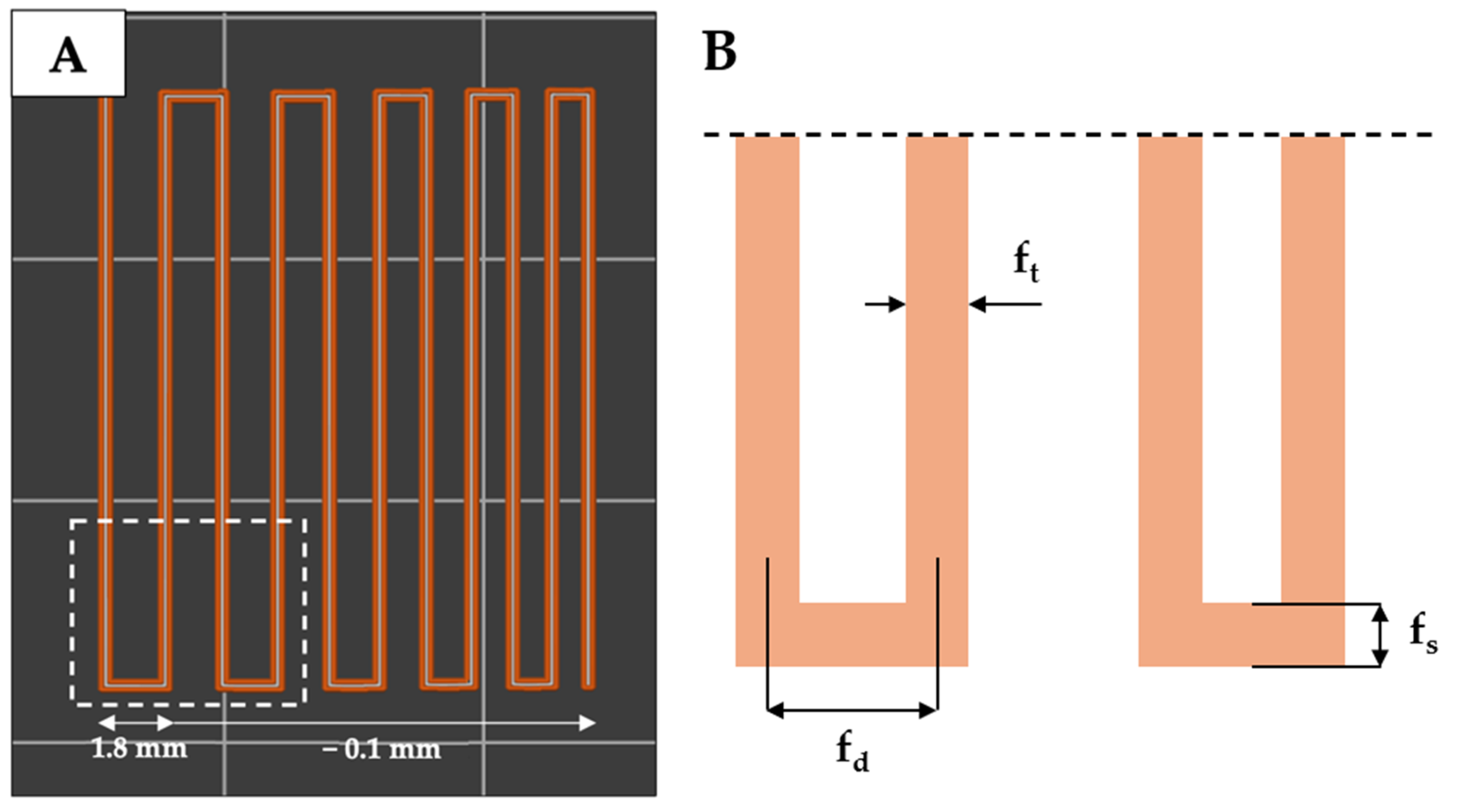
4.7. Semi-Quantification of Printability
4.8. Gel Fraction Analysis
4.9. Swelling Test
4.10. Cytocompatibility Assessment
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saunders, W.B.; Dejardin, L.M.; Soltys-Niemann, E.V.; Kaulfus, C.N.; Eichelberger, B.M.; Dobson, L.K.; Weeks, B.R.; Kerwin, S.C.; Gregory, C.A. Angle-Stable Interlocking Nailing in a Canine Critical-Sized Femoral Defect Model for Bone Regeneration Studies: In Pursuit of the Principle of the 3R’s. Front. Bioeng. Biotechnol. 2022, 10, 921486. [Google Scholar] [CrossRef] [PubMed]
- Leet, A.I.; Boyce, A.M.; Ibrahim, K.A.; Wientroub, S.; Kushner, H.; Collins, M.T. Bone-Grafting in Polyostotic Fibrous Dysplasia. JBJS 2016, 98, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Alonzo, M.; Primo, F.A.; Kumar, S.A.; Mudloff, J.A.; Dominguez, E.; Fregoso, G.; Ortiz, N.; Weiss, W.M.; Joddar, B. Bone Tissue Engineering Techniques, Advances, and Scaffolds for Treatment of Bone Defects. Curr. Opin. Biomed. Eng. 2021, 17, 100248. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Li, R.; Bai, H.; Zhu, Z.; Zhu, L.; Zhu, C.; Che, Z.; Liu, H.; Wang, J.; et al. Collagen-Based Biomaterials for Bone Tissue Engineering. Mater. Des. 2021, 210, 110049. [Google Scholar] [CrossRef]
- Guo, L.; Liang, Z.; Yang, L.; Du, W.; Yu, T.; Tang, H.; Li, C.; Qiu, H. The Role of Natural Polymers in Bone Tissue Engineering. J. Control. Release 2021, 338, 571–582. [Google Scholar] [CrossRef]
- Milazzo, M.; David, A.; Jung, G.S.; Danti, S.; Buehler, M.J. Molecular Origin of Viscoelasticity in Mineralized Collagen Fibrils. Biomater. Sci. 2021, 9, 3390–3400. [Google Scholar] [CrossRef]
- Milazzo, M.; Jung, G.S.; Danti, S.; Buehler, M.J. Mechanics of Mineralized Collagen Fibrils upon Transient Loads. ACS Nano 2020, 14, 8307–8316. [Google Scholar] [CrossRef]
- Fan, L.; Ren, Y.; Emmert, S.; Vučković, I.; Stojanovic, S.; Najman, S.; Schnettler, R.; Barbeck, M.; Schenke-Layland, K.; Xiong, X. The Use of Collagen-Based Materials in Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 3744. [Google Scholar] [CrossRef]
- Lee, J.M.; Suen, S.K.Q.; Ng, W.L.; Ma, W.C.; Yeong, W.Y. Bioprinting of Collagen: Considerations, Potentials, and Applications. Macromol. Biosci. 2021, 21, 2000280. [Google Scholar] [CrossRef]
- Cavallo, A.; Al Kayal, T.; Mero, A.; Mezzetta, A.; Pisani, A.; Foffa, I.; Vecoli, C.; Buscemi, M.; Guazzelli, L.; Soldani, G.; et al. Marine Collagen-Based Bioink for 3D Bioprinting of a Bilayered Skin Model. Pharmaceutics 2023, 15, 1331. [Google Scholar] [CrossRef]
- Silvipriya, K.S.; Kumar, K.K.; Bhat, A.R.; Kumar, B.D.; John, A.; Lakshmanan, P. Collagen: Animal Sources and Biomedical Application. J. Appl. Pharm. Sci. 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Liu, S.; Lau, C.-S.; Liang, K.; Wen, F.; Teoh, S.H. Marine Collagen Scaffolds in Tissue Engineering. Curr. Opin. Biotechnol. 2022, 74, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.-S.; Ok, Y.-J.; Hwang, S.-Y.; Kwak, J.-Y.; Yoon, S. Marine Collagen as a Promising Biomaterial for Biomedical Applications. Mar. Drugs 2019, 17, 467. [Google Scholar] [CrossRef] [PubMed]
- Pal, G.K.; Suresh, P. V Sustainable Valorisation of Seafood By-Products: Recovery of Collagen and Development of Collagen-Based Novel Functional Food Ingredients. Innov. food Sci. Emerg. Technol. 2016, 37, 201–215. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, X.; Li, T.; Yang, H.; Zhang, H.; Regenstein, J.M.; Zhou, P. Extraction and Characterization of Acid-and Pepsin-Soluble Collagens from the Scales, Skins and Swim-Bladders of Grass Carp (Ctenopharyngodon idella). Food Biosci. 2015, 9, 68–74. [Google Scholar] [CrossRef]
- Diogo, G.S.; Permuy, M.; Marques, C.F.; Sotelo, C.G.; Pérez-Martín, R.I.; Serra, J.; González, P.; Munõz, F.; Pirraco, R.P.; Reis, R.L.; et al. In Vivo Assessment of Marine vs Bovine Origin Collagen-Based Composite Scaffolds Promoting Bone Regeneration in a New Zealand Rabbit Model. Biomater. Adv. 2024, 159, 213813. [Google Scholar] [CrossRef]
- Pallela, R.; Venkatesan, J.; Janapala, V.R.; Kim, S.-K. Biophysicochemical Evaluation of Chitosan-Hydroxyapatite-Marine Sponge Collagen Composite for Bone Tissue Engineering. J. Biomed. Mater. Res. Part A 2012, 100, 486–495. [Google Scholar] [CrossRef]
- Elango, J.; Zhang, J.; Bao, B.; Palaniyandi, K.; Wang, S.; Wenhui, W.; Robinson, J.S. Rheological, Biocompatibility and Osteogenesis Assessment of Fish Collagen Scaffold for Bone Tissue Engineering. Int. J. Biol. Macromol. 2016, 91, 51–59. [Google Scholar] [CrossRef]
- Varma, M.V.; Kandasubramanian, B.; Ibrahim, S.M. 3D Printed Scaffolds for Biomedical Applications. Mater. Chem. Phys. 2020, 255, 123642. [Google Scholar] [CrossRef]
- Luo, Y.; Lin, X.; Huang, P. 3D Bioprinting of Artificial Tissues: Construction of Biomimetic Microstructures. Macromol. Biosci. 2018, 18, 1800034. [Google Scholar] [CrossRef]
- Chua, C.K.; Yeong, W.Y.; An, J. 3D Printing for Biomedical Engineering. Materials 2017, 10, 243. [Google Scholar] [CrossRef] [PubMed]
- Milazzo, M.; Contessi Negrini, N.; Scialla, S.; Marelli, B.; Farè, S.; Danti, S.; Buehler, M.J. Additive Manufacturing Approaches for Hydroxyapatite-Reinforced Composites. Adv. Funct. Mater. 2019, 29, 1903055. [Google Scholar] [CrossRef]
- Fatimi, A.; Okoro, O.V.; Podstawczyk, D.; Siminska-Stanny, J.; Shavandi, A. Natural Hydrogel-Based Bio-Inks for 3D Bioprinting in Tissue Engineering: A Review. Gels 2022, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.A.; Liu, W.; Jimenez, A.; Yang, J.; Akpek, A.; Liu, X.; Pi, Q.; Mu, X.; Hu, N.; Schiffelers, R.M.; et al. 3D Bioprinting: From Benches to Translational Applications. Small 2019, 15, 1805510. [Google Scholar] [CrossRef]
- Boonyagul, S.; Pukasamsombut, D.; Pengpanich, S.; Toobunterng, T.; Pasanaphong, K.; Sathirapongsasuti, N.; Tawonsawatruk, T.; Wangtueai, S.; Tanadchangsaeng, N. Bioink Hydrogel from Fish Scale Gelatin Blended with Alginate for 3D-Bioprinting Application. J. Food Process. Preserv. 2022, 46, e15864. [Google Scholar] [CrossRef]
- Govindharaj, M.; Roopavath, U.K.; Rath, S.N. Valorization of Discarded Marine Eel Fish Skin for Collagen Extraction as a 3D Printable Blue Biomaterial for Tissue Engineering. J. Clean. Prod. 2019, 230, 412–419. [Google Scholar] [CrossRef]
- Park, T.Y.; Yang, Y.J.; Ha, D.-H.; Cho, D.-W.; Cha, H.J. Marine-Derived Natural Polymer-Based Bioprinting Ink for Biocompatible, Durable, and Controllable 3D Constructs. Biofabrication 2019, 11, 35001. [Google Scholar] [CrossRef]
- Zhang, X.; Kim, G.J.; Kang, M.G.; Lee, J.K.; Seo, J.W.; Do, J.T.; Hong, K.; Cha, J.M.; Shin, S.R.; Bae, H. Marine Biomaterial-Based Bioinks for Generating 3D Printed Tissue Constructs. Mar. Drugs 2018, 16, 484. [Google Scholar] [CrossRef]
- Hu, T.; Lo, A.C.Y. Collagen--Alginate Composite Hydrogel: Application in Tissue Engineering and Biomedical Sciences. Polymers 2021, 13, 1852. [Google Scholar] [CrossRef]
- Tharakan, S.; Khondkar, S.; Lee, S.; Ahn, S.; Mathew, C.; Gresita, A.; Hadjiargyrou, M.; Ilyas, A. 3D Printed Osteoblast--Alginate/Collagen Hydrogels Promote Survival, Proliferation and Mineralization at Low Doses of Strontium Calcium Polyphosphate. Pharmaceutics 2022, 15, 11. [Google Scholar] [CrossRef]
- Diogo, G.S.; Marques, C.F.; Sotelo, C.G.; Pérez-Martín, R.I.; Pirraco, R.P.; Reis, R.L.; Silva, T.H. Cell-Laden Biomimetically Mineralized Shark-Skin-Collagen-Based 3D Printed Hydrogels for the Engineering of Hard Tissues. ACS Biomater. Sci. Eng. 2020, 6, 3664–3672. [Google Scholar] [CrossRef] [PubMed]
- Martínez Cortizas, A.; López-Costas, O. Linking Structural and Compositional Changes in Archaeological Human Bone Collagen: An FTIR-ATR Approach. Sci. Rep. 2020, 10, 17888. [Google Scholar] [CrossRef] [PubMed]
- Belbachir, K.; Noreen, R.; Gouspillou, G.; Petibois, C. Collagen Types Analysis and Differentiation by FTIR Spectroscopy. Anal. Bioanal. Chem. 2009, 395, 829–837. [Google Scholar] [CrossRef]
- Sun Pan, B.; En Chen, H.; Sung, W.E.N.C. Molecular and Thermal Characteristics of Acid-Soluble Collagen from Orbicular Batfish: Effects of Deep-Sea Water Culturing. Int. J. food Prop. 2018, 21, 1080–1090. [Google Scholar] [CrossRef]
- Maher, M.; Glattauer, V.; Onofrillo, C.; Duchi, S.; Yue, Z.; Hughes, T.C.; Ramshaw, J.A.M.; Wallace, G.G. Suitability of Marine-and Porcine-Derived Collagen Type I Hydrogels for Bioprinting and Tissue Engineering Scaffolds. Mar. Drugs 2022, 20, 366. [Google Scholar] [CrossRef]
- Iglesias-Mejuto, A.; García-González, C.A. 3D-Printed Alginate-Hydroxyapatite Aerogel Scaffolds for Bone Tissue Engineering. Mater. Sci. Eng. C 2021, 131, 112525. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, T.; Tenorio, A.J.; Campbell, K.T.; Silva, E.A.; Leach, J.K. Alginate-Based Bioinks for 3D Bioprinting and Fabrication of Anatomically Accurate Bone Grafts. Tissue Eng. Part A 2021, 27, 1168–1181. [Google Scholar] [CrossRef]
- Gorroñogoitia, I.; Urtaza, U.; Zubiarrain-Laserna, A.; Alonso-Varona, A.; Zaldua, A.M. A Study of the Printability of Alginate-Based Bioinks by 3D Bioprinting for Articular Cartilage Tissue Engineering. Polymers 2022, 14, 354. [Google Scholar] [CrossRef]
- Benedini, L.; Laiuppa, J.; Santillán, G.; Baldini, M.; Messina, P. Antibacterial Alginate/Nano-Hydroxyapatite Composites for Bone Tissue Engineering: Assessment of Their Bioactivity, Biocompatibility, and Antibacterial Activity. Mater. Sci. Eng. C 2020, 115, 111101. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Anaya-Sampayo, L.M.; Roa, N.S.; Martínez-Cardozo, C.; García-Robayo, D.A.; Rodríguez-Lorenzo, L.M. Influence of Hydroxyapatite and Gelatin Content on Crosslinking Dynamics and HDFn Cell Viability in Alginate Bioinks for 3D Bioprinting. Polymers 2024, 16, 3224. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.C.; Mcdermott, G.; Shields, F.; Alvites, R.; Lopes, B.; Sousa, P.; Moreira, A.; Coelho, A.; Santos, J.D.; Atayde, L.; et al. Innovative Ink-Based 3D Hydrogel Bioprinted Formulations for Tissue Engineering Applications. Gels 2024, 10, 831. [Google Scholar] [CrossRef] [PubMed]
- Călina, I.; Demeter, M.; Crăciun, G.; Scărișoreanu, A.; Mănăilă, E. The Influence of the Structural Architecture on the Swelling Kinetics and the Network Behavior of Sodium-Alginate-Based Hydrogels Cross-Linked with Ionizing Radiation. Gels 2024, 10, 588. [Google Scholar] [CrossRef] [PubMed]
- Azimi, B.; Ricci, C.; Macchi, T.; Günday, C.; Munafò, S.; Maleki, H.; Pratesi, F.; Tempesti, V.; Cristallini, C.; Bruschini, L.; et al. A Straightforward Method to Produce Multi-Nanodrug Delivery Systems for Transdermal/Tympanic Patches Using Electrospinning and Electrospray. Polymers 2023, 15, 3494. [Google Scholar] [CrossRef]
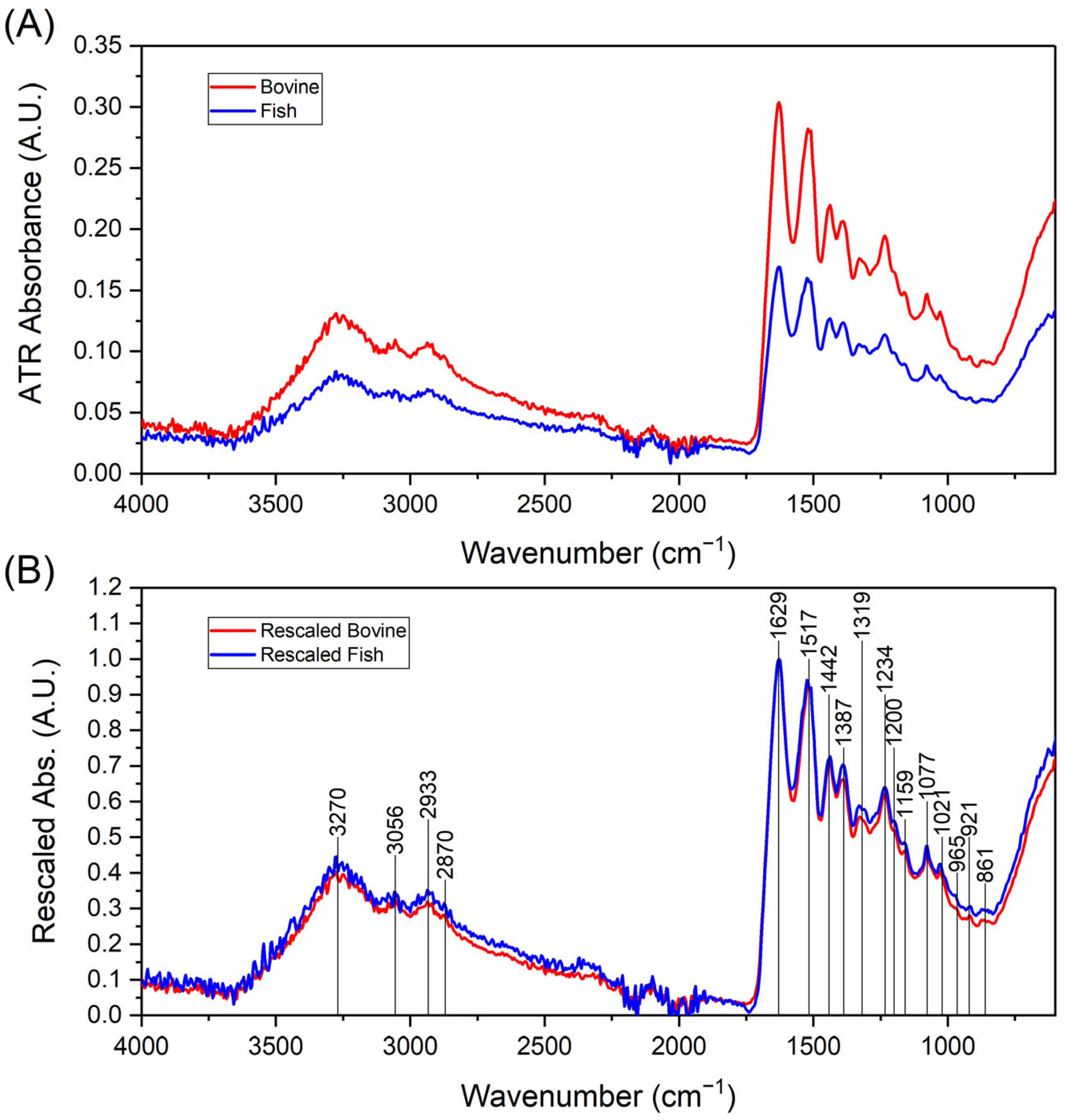
| Wavenumber [cm−1] | Bond Vibration |
|---|---|
| 861 | ν(C-O) ν(C-H) δ(C-O-H) δ(C-O-C) |
| 921 | ν(C-O) ν(C-H) δ(C-O-H) δ(C-O-C) |
| 965 | ν(C-O) ν(C-H) δ(C-O-H) δ(C-O-C) |
| 1021 | ν(C-O) |
| 1077 | ν(C-O) |
| 1200 | δ(N-H) ν(C-N) |
| 1234 | δ(N-H) ν(C-N) |
| 1319 | δ(CH2) δ(N-H) ν(C-N) |
| 1387 | δ(CH2) δ(CH3) |
| 1442 | δ(CH2) δ(CH3) |
| 1517 | δ(N-H) ν(C-N) |
| 1629 | ν(C=O) |
| 2870 | ν(C-H) ν(CH3) |
| 2933 | ν(C-H) ν(CH2) ν(O-H) |
| 3056 | ν(C-H) ν(N-H) ν(O-H) |
| 3270 | ν(O-H) |
| 3322 | ν(N-H) |
| Ink | Pr | Dr | S [%] |
|---|---|---|---|
| HAp/SA/Collf | 0.82 ± 0.01 | 39.51 ± 3.41 | 13.62 ± 1.41 |
| HAp/SA/Collb | 0.85 ± 0.01 | 43.72 ± 3.39 | 12.88 ± 0.85 |
| Ink | Gel Fraction [%] | Collagen Peptides Relased after 24 h [w%] | Crosslinked Alginate [%] |
|---|---|---|---|
| HAp/SA/Collf | 88.2 ± 10.1 | 17.1± 5.2 | 67.9 ± 29.2 |
| HAp/SA/Collb | 90.7 ± 6.7 | 23.8 ± 6.4 | 91.4 ± 11.0 |
| Ink | Nozzle Gauge | Extrusion Pressure [kPa] | Deposition Speed [mm/s] | z-Offset [mm] | Th [°C] | Tb [°C] |
|---|---|---|---|---|---|---|
| HAp/SA/Collf | 27G | 12–15 | 5 | 0.1 | RT | 20 |
| HAp/SA/Collb | 27G | 13–16 | 5 | 0.1 | RT | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milazzo, M.; Rovelli, R.; Ricci, C.; Macchi, T.; Gallone, G.; Danti, S. Rheology and Printability of Hydroxyapatite/Sodium Alginate Bioinks Added with Bovine or Fish Collagen Peptides. Gels 2025, 11, 209. https://doi.org/10.3390/gels11030209
Milazzo M, Rovelli R, Ricci C, Macchi T, Gallone G, Danti S. Rheology and Printability of Hydroxyapatite/Sodium Alginate Bioinks Added with Bovine or Fish Collagen Peptides. Gels. 2025; 11(3):209. https://doi.org/10.3390/gels11030209
Chicago/Turabian StyleMilazzo, Mario, Roberta Rovelli, Claudio Ricci, Teresa Macchi, Giuseppe Gallone, and Serena Danti. 2025. "Rheology and Printability of Hydroxyapatite/Sodium Alginate Bioinks Added with Bovine or Fish Collagen Peptides" Gels 11, no. 3: 209. https://doi.org/10.3390/gels11030209
APA StyleMilazzo, M., Rovelli, R., Ricci, C., Macchi, T., Gallone, G., & Danti, S. (2025). Rheology and Printability of Hydroxyapatite/Sodium Alginate Bioinks Added with Bovine or Fish Collagen Peptides. Gels, 11(3), 209. https://doi.org/10.3390/gels11030209









