Biological Models for Evaluating Hydrogel-Based Formulations in Wound Healing
Abstract
1. Introduction
2. Relevant Sections and Discussion
2.1. Hydrogels
2.2. Hydrogel Classification in the Context of Topical Wound Therapy
2.2.1. Origin of Polymeric Scaffold
2.2.2. Cross-Linking Mechanism
2.2.3. Stimuli-Responsive Hydrogels
2.2.4. Type of Therapeutic Agent Delivered
2.3. Biological Models Employed in the Evaluation of the Wound Healing Properties of Hydrogels
2.3.1. In Vitro Models
Monolayer Cultures
Scratch Wound Assays
Reconstructed Human Epidermis (RHE), 3D Models
2.3.2. Ex Vivo Models—Human or Porcine Skin Explants
2.3.3. In Vivo Wound Healing Models
Surgical Wounds
Burn Wounds
| No. | Localization/Species | Treatment | Main Results |
|---|---|---|---|
| 1 | Dorsal area—rats | Zinc alginate hydrogel | Faster wound healing, increased angiogenesis, and fibroblast migration [204] |
| 2 | Dorsal area—rats | Gel containing verapamil HCl-loaded nanofibers composed with alginate | Controlled release of verapamil leading to smooth, scar-free wound closure [205] |
| 3 | Dorsal area—mice | Zein/pectin/vitamin C scaffolds crosslinked to mimic hydrogel | Antioxidant effects, faster re-epithelialization, anti-inflammatory activity [206] |
| 4 | Second-degree burn on dorsal area—rats | Chitosan gel with epidermal growth factor | Increased cell proliferation and epithelialization, improved scar tissue formation [203] |
| 5 | Dorsal area—rats | Cream containing Clostridium Perfringens-derived wound-healing substance | Enhanced skin collagen formation and increased capillary formation [207] |
| 6 | Dorsal area—BALB/c mice | Ointment containing Aloe emodin and resveratrol | Faster wound healing by increasing blood vessel growth, upregulating IL-1β and VEGF, and stimulating immune cell response [208] |
| 7 | Second-degree burn on dorsal area—rats | Spray formulation of Olea europaea and Aloe vera leaves, Cocus nucifera fruit, and Chamomilla recutita flower plant extracts | Antioxidant and anti-inflammatory effects, increased angiogenesis, faster wound healing [209] |
Other Wounds
| Model/Species | Treatment | Main Results | |
|---|---|---|---|
| 1 | Ischemic wound in rabbits | TA Platelet-derived exosome product incorporated into a surgical fibrin sealant biogel | Boosted deep tissue repair by enhancing new vessel growth, structural rebuilding, and skin appendage renewal [210] |
| 2 | Fasciocutaneous flap on rats exposed to ischemia/revascularization of the vascular pedicle | LT photodynamic therapy mediated by Photofrin and 630 nm light | Inhibited revascularization in axial flaps, showing reduced blood flow [212] |
| 3 | Ischemic wound by using the Matrigel plug model on nu/nu mice | TA apoptotic extracellular vesicles from tooth pulp stem cells | Enhanced angiogenesis by collagen 1 delivery form vesicles and stimulation of the PI3K/AKT/VEGF pathway leading to faster wound healing [205] |
| 4 | X-ray irradiation on mice | LT-pulsed therapeutic ultrasound | No beneficial effects on wound closure rate were reported [211] |
2.4. Hydrogels in Clinical Practice
2.4.1. Hydrogels in Preclinical Studies
2.4.2. Hydrogels in Clinical Trials
2.4.3. Approved and Commercialized Gels
Synthetic Wound Dressings
Natural Wound Dressings
3. Conclusions
4. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yousef, H.; Alhajj, M.; Fakoya, A.O.; Sharma, S. Anatomy, Skin (Integument), Epidermis; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Wallace, H.A.; Basehore, B.M.; Zito, P.M. Wound Healing Phases; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Yazarlu, O.; Iranshahi, M.; Kashani, H.R.K.; Reshadat, S.; Habtemariam, S.; Iranshahy, M.; Hasanpour, M. Perspective on the Application of Medicinal Plants and Natural Products in Wound Healing: A Mechanistic Review. Pharmacol. Res. 2021, 174, 105841. [Google Scholar] [CrossRef]
- Brown, J.A. The Role of Hyaluronic Acid in Wound Healing’s Proliferative Phase. J. Wound Care 2004, 13, 48–51. [Google Scholar] [CrossRef] [PubMed]
- van Koppen, C.J.; Hartmann, R.W. Advances in the Treatment of Chronic Wounds: A Patent Review. Expert. Opin. Ther. Pat. 2015, 25, 931–937. [Google Scholar] [CrossRef]
- Kus, K.J.B.; Ruiz, E.S. Wound Dressings—A Practical Review. Curr. Dermatol. Rep. 2020, 9, 298–308. [Google Scholar] [CrossRef]
- Borda, L.J.; Macquhae, F.E.; Kirsner, R.S. Wound Dressings: A Comprehensive Review. Curr. Dermatol. Rep. 2016, 5, 287–297. [Google Scholar] [CrossRef]
- Ferraz, M.P. Wound Dressing Materials: Bridging Material Science and Clinical Practice. Appl. Sci. 2025, 15, 1725. [Google Scholar] [CrossRef]
- Brumberg, V.; Astrelina, T.; Malivanova, T.; Samoilov, A. Modern Wound Dressings: Hydrogel Dressings. Biomedicines 2021, 9, 1235. [Google Scholar] [CrossRef] [PubMed]
- Moradifar, F.; Sepahdoost, N.; Tavakoli, P.; Mirzapoor, A. Multi-Functional Dressings for Recovery and Screenable Treatment of Wounds: A Review. Heliyon 2025, 11, e41465. [Google Scholar] [CrossRef] [PubMed]
- Vowden, K.; Vowden, P. Wound Dressings: Principles and Practice. Surgery 2017, 35, 489–494. [Google Scholar] [CrossRef]
- Alberts, A.; Tudorache, D.-I.; Niculescu, A.-G.; Grumezescu, A.M. Advancements in Wound Dressing Materials: Highlighting Recent Progress in Hydrogels, Foams, and Antimicrobial Dressings. Gels 2025, 11, 123. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Thambi, T.; Jung, J.M.; Lee, D.S. Recent Strategies to Develop PH-Sensitive Injectable Hydrogels. Biomater. Sci. 2023, 11, 1948–1961. [Google Scholar] [CrossRef]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.; Selvanathan, V.; Sonsudin, F.; Abouloula, C. PH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Boon-in, S.; Theerasilp, M.; Crespy, D. Temperature-Responsive Double-Network Cooling Hydrogels. ACS Appl. Polym. Mater. 2023, 5, 2562–2574. [Google Scholar] [CrossRef]
- Guleria, S.; Chopra, L. Manikanika Temperature Responsive Hydrogels for Biomedical Applications. Mater. Today Proc. 2023, 92, 356–363. [Google Scholar] [CrossRef]
- Xing, Y.; Zeng, B.; Yang, W. Light Responsive Hydrogels for Controlled Drug Delivery. Front. Bioeng. Biotechnol. 2022, 10, 1075670. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, H.; Li, Z.; Xu, X.; Xing, H.; Wang, M.; Jia, H.; Liang, L.; Li, C.; Sun, L.; et al. Responsive Hydrogels Based on Triggered Click Reactions for Liver Cancer. Adv. Mater. 2022, 34, e2201651. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tan, Y.; Zeng, C.; Pan, W. Ultrafast Enzyme-Responsive Hydrogel for Real-Time Assessment and Treatment Optimization in Infected Wounds. J. Nanobiotechnol. 2025, 23, 9. [Google Scholar] [CrossRef]
- Sobczak, M. Enzyme-Responsive Hydrogels as Potential Drug Delivery Systems—State of Knowledge and Future Prospects. Int. J. Mol. Sci. 2022, 23, 4421. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Y.; Li, T.; Zhang, J.; Tian, H. Stimuli-responsive Hydrogels: Fabrication and Biomedical Applications. VIEW 2022, 3, 20200112. [Google Scholar] [CrossRef]
- Protsak, I.S.; Morozov, Y.M. Fundamentals and Advances in Stimuli-Responsive Hydrogels and Their Applications: A Review. Gels 2025, 11, 30. [Google Scholar] [CrossRef]
- Neumann, M.; di Marco, G.; Iudin, D.; Viola, M.; van Nostrum, C.F.; van Ravensteijn, B.G.P.; Vermonden, T. Stimuli-Responsive Hydrogels: The Dynamic Smart Biomaterials of Tomorrow. Macromolecules 2023, 56, 8377–8392. [Google Scholar] [CrossRef]
- Qiao, L.; Liang, Y.; Chen, J.; Huang, Y.; Alsareii, S.A.; Alamri, A.M.; Harraz, F.A.; Guo, B. Antibacterial Conductive Self-Healing Hydrogel Wound Dressing with Dual Dynamic Bonds Promotes Infected Wound Healing. Bioact. Mater. 2023, 30, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.I.; Park, K.M. Advances in Gelatin-Based Hydrogels for Wound Management. J. Mater. Chem. B 2021, 9, 1503–1520. [Google Scholar] [CrossRef]
- Yang, X.; Wang, B.; Peng, D.; Nie, X.; Wang, J.; Yu, C.-Y.; Wei, H. Hyaluronic Acid-Based Injectable Hydrogels for Wound Dressing and Localized Tumor Therapy: A Review. Adv. Nanobiomed Res. 2022, 2, 2200124. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, Y.; Zhang, Y.; Hu, J.; Ikegami, Y.; Aishima, S.; Ijima, H. Fast Wound Healing with a New Functional Hyaluronic Acid Dual Network Hydrogel. Gels 2025, 11, 266. [Google Scholar] [CrossRef]
- Gu, H.; Li, H.; Wei, L.; Lu, J.; Wei, Q. Collagen-Based Injectable and Self-Healing Hydrogel with Multifunction for Regenerative Repairment of Infected Wounds. Regen. Biomater. 2023, 10, rbad018. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Li, Y.; Yang, Y.; Jin, M.; Lin, X.; Zhuang, Z.; Guo, K.; Zhang, T.; Tan, W. Application of Collagen-Based Hydrogel in Skin Wound Healing. Gels 2023, 9, 185. [Google Scholar] [CrossRef]
- El-Sayed, N.S.; Helmy, N.M.; Kamel, S. Dual-Adhesive and Self-Healing Alginate-Based Hydrogel for Wound Healing. Chem. Pap. 2024, 78, 1021–1031. [Google Scholar] [CrossRef]
- Ishfaq, B.; Khan, I.U.; Khalid, S.H.; Asghar, S. Design and Evaluation of Sodium Alginate-Based Hydrogel Dressings Containing Betula Utilis Extract for Cutaneous Wound Healing. Front. Bioeng. Biotechnol. 2023, 11, 1042077. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, M.; Shamloo, A.; Nokoorani, Y.D. Development of a Polyvinyl Alcohol/Sodium Alginate Hydrogel-Based Scaffold Incorporating BFGF-Encapsulated Microspheres for Accelerated Wound Healing. Sci. Rep. 2020, 10, 7342. [Google Scholar] [CrossRef]
- Miguel, S.P.; Ribeiro, M.P.; Brancal, H.; Coutinho, P.; Correia, I.J. Thermoresponsive Chitosan–Agarose Hydrogel for Skin Regeneration. Carbohydr. Polym. 2014, 111, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, Processing and Application of Hydrogels: A Review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, A.M.; Ashour, S.A.; Hussien, R.M. A review on recent advances in hydrogels as drug delivery system. Int. J. Appl. Pharm. 2025, 17, 39–47. [Google Scholar] [CrossRef]
- Kundu, R.; Mahada, P.; Chhirang, B.; Das, B. Cellulose Hydrogels: Green and Sustainable Soft Biomaterials. Curr. Res. Green Sustain. Chem. 2022, 5, 100252. [Google Scholar] [CrossRef]
- Kushwaha, J.; Singh, R. Cellulose Hydrogel and Its Derivatives: A Review of Application in Heavy Metal Adsorption. Inorg. Chem. Commun. 2023, 152, 110721. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, Q.; Xu, W.; Yang, M.; Guo, W.; He, S.; Liu, W. Alginate-Based Hydrogels Mediated Biomedical Applications: A Review. Int. J. Biol. Macromol. 2024, 279, 135019. [Google Scholar] [CrossRef]
- Savić Gajić, I.M.; Savić, I.M.; Svirčev, Z. Preparation and Characterization of Alginate Hydrogels with High Water-Retaining Capacity. Polymers 2023, 15, 2592. [Google Scholar] [CrossRef]
- Tan, J.; Luo, Y.; Guo, Y.; Zhou, Y.; Liao, X.; Li, D.; Lai, X.; Liu, Y. Development of Alginate-Based Hydrogels: Crosslinking Strategies and Biomedical Applications. Int. J. Biol. Macromol. 2023, 239, 124275. [Google Scholar] [CrossRef]
- Mishra, A.; Omoyeni, T.; Singh, P.K.; Anandakumar, S.; Tiwari, A. Trends in Sustainable Chitosan-Based Hydrogel Technology for Circular Biomedical Engineering: A Review. Int. J. Biol. Macromol. 2024, 276, 133823. [Google Scholar] [CrossRef]
- Farasati Far, B.; Omrani, M.; Naimi Jamal, M.R.; Javanshir, S. Multi-Responsive Chitosan-Based Hydrogels for Controlled Release of Vincristine. Commun. Chem. 2023, 6, 28. [Google Scholar] [CrossRef]
- Hong, F.; Qiu, P.; Wang, Y.; Ren, P.; Liu, J.; Zhao, J.; Gou, D. Chitosan-Based Hydrogels: From Preparation to Applications, a Review. Food Chem. X 2024, 21, 101095. [Google Scholar] [CrossRef]
- An, S.; Choi, S.; Min, S.; Cho, S.-W. Hyaluronic Acid-Based Biomimetic Hydrogels for Tissue Engineering and Medical Applications. Biotechnol. Bioprocess Eng. 2021, 26, 503–516. [Google Scholar] [CrossRef]
- Gholamali, I.; Vu, T.T.; Jo, S.-H.; Park, S.-H.; Lim, K.T. Exploring the Progress of Hyaluronic Acid Hydrogels: Synthesis, Characteristics, and Wide-Ranging Applications. Materials 2024, 17, 2439. [Google Scholar] [CrossRef] [PubMed]
- Grieco, M.; Ursini, O.; Palamà, I.E.; Gigli, G.; Moroni, L.; Cortese, B. HYDRHA: Hydrogels of Hyaluronic Acid. New Biomedical Approaches in Cancer, Neurodegenerative Diseases, and Tissue Engineering. Mater. Today Bio 2022, 17, 100453. [Google Scholar] [CrossRef]
- Guan, D.; Li, W.; Zhao, S.; Tao, Z.; Liu, K.; Liu, Q.; Kong, W.; Zhu, S.; Sun, Y. Carboxymethyl Cellulose-Enhanced Recombinant Human Collagen Hydrogel Promotes Hemostasis and Wound Healing. Int. J. Biol. Macromol. 2025, 315, 144458. [Google Scholar] [CrossRef] [PubMed]
- Alberts, A.; Bratu, A.G.; Niculescu, A.-G.; Grumezescu, A.M. Collagen-Based Wound Dressings: Innovations, Mechanisms, and Clinical Applications. Gels 2025, 11, 271. [Google Scholar] [CrossRef]
- She, J.; Liu, J.; Mu, Y.; Lv, S.; Tong, J.; Liu, L.; He, T.; Wang, J.; Wei, D. Recent Advances in Collagen-Based Hydrogels: Materials, Preparation and Applications. React. Funct. Polym. 2025, 207, 106136. [Google Scholar] [CrossRef]
- Xiang, L.; Cui, W. Biomedical Application of Photo-Crosslinked Gelatin Hydrogels. J. Leather Sci. Eng. 2021, 3, 3. [Google Scholar] [CrossRef]
- Mohanto, S.; Narayana, S.; Merai, K.P.; Kumar, J.A.; Bhunia, A.; Hani, U.; Al Fatease, A.; Gowda, B.H.J.; Nag, S.; Ahmed, M.G.; et al. Advancements in Gelatin-Based Hydrogel Systems for Biomedical Applications: A State-of-the-Art Review. Int. J. Biol. Macromol. 2023, 253, 127143. [Google Scholar] [CrossRef]
- Andreazza, R.; Morales, A.; Pieniz, S.; Labidi, J. Gelatin-Based Hydrogels: Potential Biomaterials for Remediation. Polymers 2023, 15, 1026. [Google Scholar] [CrossRef]
- Li, S.; Dan, X.; Chen, H.; Li, T.; Liu, B.; Ju, Y.; Li, Y.; Lei, L.; Fan, X. Developing Fibrin-Based Biomaterials/Scaffolds in Tissue Engineering. Bioact. Mater. 2024, 40, 597–623. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of Natural Hydrogels for Regenerative Medicine Applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhao, X.; Xu, C.; Wang, L.; Xia, Y. Progress in the Mechanical Enhancement of Hydrogels: Fabrication Strategies and Underlying Mechanisms. J. Polym. Sci. 2022, 60, 2525–2542. [Google Scholar] [CrossRef]
- Shukla, A.; Syaifie, P.H.; Rochman, N.T.; Jaya Syaifullah, S.; Jauhar, M.M.; Mardliyati, E. A Recent Study of Natural Hydrogels: Improving Mechanical Properties for Biomedical Applications. Biomed. Mater. 2025, 20, 022010. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.-H.; Chen, X.-Y.; Fu, L.-Q.; Du, W.-L.; Yang, X.; Mou, X.-Z.; Hu, P.-Y. Design and Development of Hybrid Hydrogels for Biomedical Applications: Recent Trends in Anticancer Drug Delivery and Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 630943. [Google Scholar] [CrossRef]
- Sun, S.; Cui, Y.; Yuan, B.; Dou, M.; Wang, G.; Xu, H.; Wang, J.; Yin, W.; Wu, D.; Peng, C. Drug Delivery Systems Based on Polyethylene Glycol Hydrogels for Enhanced Bone Regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1117647. [Google Scholar] [CrossRef] [PubMed]
- Bakaic, E.; Smeets, N.M.B.; Hoare, T. Injectable Hydrogels Based on Poly(Ethylene Glycol) and Derivatives as Functional Biomaterials. RSC Adv. 2015, 5, 35469–35486. [Google Scholar] [CrossRef]
- Wu, S.; Hua, M.; Alsaid, Y.; Du, Y.; Ma, Y.; Zhao, Y.; Lo, C.; Wang, C.; Wu, D.; Yao, B.; et al. Poly(Vinyl Alcohol) Hydrogels with Broad-Range Tunable Mechanical Properties via the Hofmeister Effect. Adv. Mater. 2021, 33, 2007829. [Google Scholar] [CrossRef]
- Bercea, M. Recent Advances in Poly(Vinyl Alcohol)-Based Hydrogels. Polymers 2024, 16, 2021. [Google Scholar] [CrossRef]
- Penkavova, V.; Spalova, A.; Tomas, J.; Tihon, J. Polyacrylamide Hydrogels Prepared by Varying Water Content during Polymerization: Material Characterization, Reswelling Ability, and Aging Resistance. Polym. Eng. Sci. 2022, 62, 901–916. [Google Scholar] [CrossRef]
- Cresens, C.; Aytekin, S.; Shaghaghi, B.; Gerrits, L.; Montero-Calle, A.; Barderas, R.; Kouwer, P.; Rocha, S. Synthesis and Mechanical Characterization of Polyacrylamide (PAAm) Hydrogels with Different Stiffnesses for Large-Batch Cell Culture Applications. bioRxiv 2024. [Google Scholar] [CrossRef]
- Zare, M.; Bigham, A.; Zare, M.; Luo, H.; Rezvani Ghomi, E.; Ramakrishna, S. PHEMA: An Overview for Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 6376. [Google Scholar] [CrossRef] [PubMed]
- Halpenny, G.M.; Steinhardt, R.C.; Okialda, K.A.; Mascharak, P.K. Characterization of PHEMA-Based Hydrogels That Exhibit Light-Induced Bactericidal Effect via Release of NO. J. Mater. Sci. Mater. Med. 2009, 20, 2353–2360. [Google Scholar] [CrossRef] [PubMed]
- Visan, A.I.; Negut, I. Development and Applications of PLGA Hydrogels for Sustained Delivery of Therapeutic Agents. Gels 2024, 10, 497. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, F.; Atabaki, R.; Behrouzi, S.; Mohamadpour, F.; Kamali, H. The Recent Advancement in the PLGA-Based Thermo-Sensitive Hydrogel for Smart Drug Delivery. Int. J. Pharm. 2023, 631, 122484. [Google Scholar] [CrossRef]
- Hipwood, L.; Clegg, J.; Weekes, A.; Davern, J.W.; Dargaville, T.R.; Meinert, C.; Bock, N. Semi-Synthetic Click-Gelatin Hydrogels as Tunable Platforms for 3D Cancer Cell Culture. Gels 2022, 8, 821. [Google Scholar] [CrossRef]
- Lambrecht, S.; Gazizova, A.; Kara, S.; Meyer, J.; Jopp, S. Antimicrobial Properties and Biocompatibility of Semi-Synthetic Carbohydrate-Based Ionic Hydrogels. RSC Adv. 2024, 14, 30719–30731. [Google Scholar] [CrossRef]
- Alavarse, A.C.; Frachini, E.C.G.; da Silva, R.L.C.G.; Lima, V.H.; Shavandi, A.; Petri, D.F.S. Crosslinkers for Polysaccharides and Proteins: Synthesis Conditions, Mechanisms, and Crosslinking Efficiency, a Review. Int. J. Biol. Macromol. 2022, 202, 558–596. [Google Scholar] [CrossRef]
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking Method of Hyaluronic-Based Hydrogel for Biomedical Applications. J. Tissue Eng. 2017, 8, 2041731417726464. [Google Scholar] [CrossRef]
- Saeedi, M.; Moghbeli, M.R.; Vahidi, O. Chitosan/Glycyrrhizic Acid Hydrogel: Preparation, Characterization, and Its Potential for Controlled Release of Gallic Acid. Int. J. Biol. Macromol. 2023, 231, 123197. [Google Scholar] [CrossRef]
- de Siqueira, E.C.; de França, J.A.A.; de Souza, R.F.M.; Leoterio, D.M.d.S.; Cordeiro, J.N.; Doboszewski, B. Mecanisms of the Chemical Crosslinking to Obtain the Hydrogels: Synthesis, Conditions of Crosslinking and Biopharmaceutical Applications. Res. Soc. Dev. 2023, 12, e18312943072. [Google Scholar] [CrossRef]
- Cansu Tarakci, E.; Nihal Gevrek, T. Isocyanate Group Containing Reactive Hydrogels: Facile Synthesis and Efficient Biofunctionalization. Eur. Polym. J. 2022, 175, 111338. [Google Scholar] [CrossRef]
- You, Y.; Xie, Y.; Jiang, Z. Injectable and Biocompatible Chitosan-Alginic Acid Hydrogels. Biomed. Mater. 2019, 14, 025010. [Google Scholar] [CrossRef]
- Pappalardo, R.; Boffito, M.; Cassino, C.; Caccamo, V.; Chiono, V.; Ciardelli, G. Schiff-Base Cross-Linked Hydrogels Based on Properly Synthesized Poly(Ether Urethane)s as Potential Drug Delivery Vehicles in the Biomedical Field: Design and Characterization. ACS Omega 2024, 9, 45774–45788. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, H.A.; Moon, H.H.; Lee, D.-H.; Bhang, S.H.; Kim, Y.-C.; Song, C.; Kim, J.-H. Preparation of Polyaspartamide-Based Adhesive Hydrogels via Schiff Base Reaction with Aldehyde-Functionalized Dextran. Mater. Adv. 2023, 4, 1989–1997. [Google Scholar] [CrossRef]
- Buhus, G.; Popa, M.; Desbrieres, J. Hydrogels Based on Carboxymethylcellulose and Gelatin for Inclusion and Release of Chloramphenicol. J. Bioact. Compat. Polym. 2009, 24, 525–545. [Google Scholar] [CrossRef]
- Akakuru, O.U.; Isiuku, B.O. Chitosan Hydrogels and Their Glutaraldehyde-Crosslinked Counterparts as Potential Drug Release and Tissue Engineering Systems—Synthesis, Characterization, Swelling Kinetics and Mechanism. J. Phys. Chem. Biophys. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Demirbilek, C.; Dinç, C.Ö. Diethylaminoethyl Dextran/Epichlorohydrin (DEAE-D/ECH) Hydrogel as Adsorbent for Murexide. Desalination Water Treat. 2016, 57, 6884–6893. [Google Scholar] [CrossRef]
- Sarmah, D.; Borah, M.; Mandal, M.; Karak, N. Swelling Induced Mechanically Tough Starch–Agar Based Hydrogel as a Control Release Drug Vehicle for Wound Dressing Applications. J. Mater. Chem. B 2023, 11, 2927–2936. [Google Scholar] [CrossRef]
- Andrade del Olmo, J.; Alonso, J.M.; Sáez Martínez, V.; Ruiz-Rubio, L.; Pérez González, R.; Vilas-Vilela, J.L.; Pérez-Álvarez, L. Biocompatible Hyaluronic Acid-Divinyl Sulfone Injectable Hydrogels for Sustained Drug Release with Enhanced Antibacterial Properties against Staphylococcus Aureus. Mater. Sci. Eng. C 2021, 125, 112102. [Google Scholar] [CrossRef]
- Gomez, I.; Alesanco, Y.; Blázquez, J.A.; Viñuales, A.; Colmenares, L.C. Room-Temperature Self-Standing Cellulose-Based Hydrogel Electrolytes for Electrochemical Devices. Polymers 2020, 12, 2686. [Google Scholar] [CrossRef]
- Irvine, G.; Dawson, F.; George, A.; Kopeć, M. Are Polymer Gels Synthesized by Free Radical Polymerization with Cleavable Crosslinkers Really Degradable? Eur. Polym. J. 2024, 213, 113089. [Google Scholar] [CrossRef]
- Tran, H.D.N.; Park, K.D.; Ching, Y.C.; Huynh, C.; Nguyen, D.H. A Comprehensive Review on Polymeric Hydrogel and Its Composite: Matrices of Choice for Bone and Cartilage Tissue Engineering. J. Ind. Eng. Chem. 2020, 89, 58–82. [Google Scholar] [CrossRef]
- Shantha, K.L.; Harding, D.R.K. Synthesis and Evaluation of Sucrose-containing Polymeric Hydrogels for Oral Drug Delivery. J. Appl. Polym. Sci. 2002, 84, 2597–2604. [Google Scholar] [CrossRef]
- de Nooy, A.E.J.; Capitani, D.; Masci, G.; Crescenzi, V. Ionic Polysaccharide Hydrogels via the Passerini and Ugi Multicomponent Condensations: Synthesis, Behavior and Solid-State NMR Characterization. Biomacromolecules 2000, 1, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Tang, M.; Bowyer, A.; Eisenthal, R.; Hubble, J. A Novel PH- and Ionic-Strength-Sensitive Carboxy Methyl Dextran Hydrogel. Biomaterials 2005, 26, 4677–4683. [Google Scholar] [CrossRef]
- Cai, X.; Hu, S.; Yu, B.; Cai, Y.; Yang, J.; Li, F.; Zheng, Y.; Shi, X. Transglutaminase-Catalyzed Preparation of Crosslinked Carboxymethyl Chitosan/Carboxymethyl Cellulose/Collagen Composite Membrane for Postsurgical Peritoneal Adhesion Prevention. Carbohydr. Polym. 2018, 201, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.; Bao, B.; Zhu, Y.; Lin, H. Transglutaminase-Catalyzed Bottom-Up Synthesis of Polymer Hydrogel. Front. Bioeng. Biotechnol. 2022, 10, 824747. [Google Scholar] [CrossRef]
- Priya, A.S.; Premanand, R.; Ragupathi, I.; Bhaviripudi, V.R.; Aepuru, R.; Kannan, K.; Shanmugaraj, K. Comprehensive Review of Hydrogel Synthesis, Characterization, and Emerging Applications. J. Compos. Sci. 2024, 8, 457. [Google Scholar] [CrossRef]
- Zhang, P.; Qi, J.; Zhang, R.; Zhao, Y.; Yan, J.; Gong, Y.; Liu, X.; Zhang, B.; Wu, X.; Wu, X.; et al. Recent Advances in Composite Hydrogels: Synthesis, Classification, and Application in the Treatment of Bone Defects. Biomater. Sci. 2024, 12, 308–329. [Google Scholar] [CrossRef]
- Pita-López, M.L.; Fletes-Vargas, G.; Espinosa-Andrews, H.; Rodríguez-Rodríguez, R. Physically Cross-Linked Chitosan-Based Hydrogels for Tissue Engineering Applications: A State-of-the-Art Review. Eur. Polym. J. 2021, 145, 110176. [Google Scholar] [CrossRef]
- Taaca, K.L.M.; Prieto, E.I.; Vasquez, M.R. Current Trends in Biomedical Hydrogels: From Traditional Crosslinking to Plasma-Assisted Synthesis. Polymers 2022, 14, 2560. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Lu, J.; Ding, M.; Chen, Y. Synthesis and Properties of Poly(Vinyl Alcohol) Hydrogels with High Strength and Toughness. Polym. Test. 2022, 108, 107516. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in Crosslinking Strategies of Biomedical Hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef]
- Li, D.; Liang, R.; Wang, Y.; Zhou, Y.; Cai, W. Preparation of Silk Fibroin-derived Hydrogels and Applications in Skin Regeneration. Health Sci. Rep. 2024, 7, e2295. [Google Scholar] [CrossRef]
- Madappura, A.P.; Madduri, S. A Comprehensive Review of Silk-Fibroin Hydrogels for Cell and Drug Delivery Applications in Tissue Engineering and Regenerative Medicine. Comput. Struct. Biotechnol. J. 2023, 21, 4868–4886. [Google Scholar] [CrossRef]
- Liaqat, H.; Badshah, S.F.; Minhas, M.U.; Barkat, K.; Khan, S.A.; Hussain, M.D.; Kazi, M. PH-Sensitive Hydrogels Fabricated with Hyaluronic Acid as a Polymer for Site-Specific Delivery of Mesalamine. ACS Omega 2024, 9, 28827–28840. [Google Scholar] [CrossRef]
- Wang, Y.; He, C.; Chen, C.; Dong, W.; Yang, X.; Wu, Y.; Kong, Q.; Yan, B. Thermoresponsive Self-Healing Zwitterionic Hydrogel as an In Situ Gelling Wound Dressing for Rapid Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 55342–55353. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, W.; Shen, J.; Zhou, N.; Li, Y.; Tang, B.Z.; Zhang, M. A Thermosensitive Hydrogel with Efficient NIR Photothermal Conversion as Injectable Wound Dressing for Accelerating Skin Wound Healing. Adv. Funct. Mater. 2024, 34, 2312374. [Google Scholar] [CrossRef]
- Chen, H.; Xu, J.; Sun, J.; Jiang, Y.; Zheng, W.; Hu, W.; Qian, H. Recent Advances on Thermosensitive Hydrogels-Mediated Precision Therapy. Asian J. Pharm. Sci. 2024, 19, 100911. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, W.; Dong, Z.; Chao, Y.; Xu, L.; Chen, M.; Liu, Z. 1D Coordination Polymer Nanofibers for Low-Temperature Photothermal Therapy. Adv. Mater. 2017, 29, 1703588. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, W.; Liu, X.; Chen, Y.; Tian, S.; Fu, P. A Sol–Gel Transition and Self-Healing Hydrogel Triggered via Photodimerization of Coumarin. Gels 2023, 10, 21. [Google Scholar] [CrossRef]
- Qiu, W.; Gehre, C.; Nepomuceno, J.P.; Bao, Y.; Li, Z.; Müller, R.; Qin, X. Coumarin-Based Photodegradable Hydrogels Enable Two-Photon Subtractive Biofabrication at 300 Mm s−1. Angew. Chem. Int. Ed. 2024, 63, e202404599. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bian, Y.; Bai, J.; Gao, L. Visible-Light Responsive Hydrogel Based on Methoxy Azobenzene Amphiphilic Small Molecule. J. Photochem. Photobiol. A Chem. 2024, 457, 115893. [Google Scholar] [CrossRef]
- Homma, K.; Chang, A.C.; Yamamoto, S.; Tamate, R.; Ueki, T.; Nakanishi, J. Design of Azobenzene-Bearing Hydrogel with Photoswitchable Mechanics Driven by Photo-Induced Phase Transition for in Vitro Disease Modeling. Acta Biomater. 2021, 132, 103–113. [Google Scholar] [CrossRef]
- Tomatsu, I.; Peng, K.; Kros, A. Photoresponsive Hydrogels for Biomedical Applications. Adv. Drug Deliv. Rev. 2011, 63, 1257–1266. [Google Scholar] [CrossRef]
- Ge, Z.; Wu, H.; Wu, J.; He, Y.; Tan, R.; Wang, Y.; Xiao, T.; Dong, G.; Zhou, P.; Xing, Z. Photoresponsive Hydrogel Dressing Containing Nanoparticles with Excellent Synergetic Photodynamic, Photothermal, and Chemodynamic Therapies for Effective Infected Wound Healing. ACS Appl. Bio Mater. 2024, 7, 6970–6984. [Google Scholar] [CrossRef]
- Castrejón-Comas, V.; Mataró, N.; Resina, L.; Zanuy, D.; Nuñez-Aulina, Q.; Sánchez-Morán, J.; Enshaei, H.; Arnau, M.; Muñoz-Galán, H.; Worch, J.C.; et al. Electro-Responsive Hyaluronic Acid-Based Click-Hydrogels for Wound Healing. Carbohydr. Polym. 2025, 348, 122941. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Hao, L.; Mao, H. Magneto-Responsive Biocomposites in Wound Healing: From Characteristics to Functions. J. Mater. Chem. B 2024, 12, 7463–7479. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wang, M.; Chai, L.; Chen, H.; Chen, D.; Li, Y.; Liu, H.; Wu, Y.; Yang, X.; He, L.; et al. Glucose-Responsive, Self-Healing, Wet Adhesive and Multi-Biofunctional Hydrogels for Diabetic Wound Healing. Mater. Today Bio 2024, 27, 101159. [Google Scholar] [CrossRef]
- Cheng, Q.; Zhu, J.; Tu, S.; Fan, X. Enzyme-Crosslinked Hyaluronic Acid-Based Multifunctional Hydrogel Promotes Diabetic Wound Healing. Macromol. Res. 2025, 33, 779–788. [Google Scholar] [CrossRef]
- Maaz Arif, M.; Khan, S.M.; Gull, N.; Tabish, T.A.; Zia, S.; Ullah Khan, R.; Awais, S.M.; Arif Butt, M. Polymer-Based Biomaterials for Chronic Wound Management: Promises and Challenges. Int. J. Pharm. 2021, 598, 120270. [Google Scholar] [CrossRef]
- Hussain, Z.; Thu, H.E.; Shuid, A.N.; Katas, H.; Hussain, F. Recent Advances in Polymer-Based Wound Dressings for the Treatment of Diabetic Foot Ulcer: An Overview of State-of-the-Art. Curr. Drug Targets 2018, 19, 527–550. [Google Scholar] [CrossRef]
- Heremans, J.; Ballet, S.; Martin, C. The Versatility of Peptide Hydrogels: From Self-assembly to Drug Delivery Applications. J. Pept. Sci. 2025, 31, e3662. [Google Scholar] [CrossRef]
- Bakhtiary, N.; Ghalandari, B.; Ghorbani, F.; Varma, S.N.; Liu, C. Advances in Peptide-Based Hydrogel for Tissue Engineering. Polymers 2023, 15, 1068. [Google Scholar] [CrossRef]
- Cauwenbergh, T.; Ballet, S.; Martin, C. Peptide Hydrogel-Drug Conjugates for Tailored Disease Treatment. Mater. Today Bio 2025, 31, 101423. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xi, Z.; Fan, C.; Mei, Y.; Zhao, J.; Jiang, Y.; Zhao, M.; Xu, L. Hydrogels for Nucleic Acid Drugs Delivery. Adv. Healthc. Mater. 2024, 13, e2401895. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Li, W.; Yang, P.; Shen, N.; Yang, A.; Liu, X.; Ju, Y.; Lei, L.; Fang, B. DNA Hydrogels and Their Derivatives in Biomedical Engineering Applications. J. Nanobiotechnology 2024, 22, 518. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, W.; Wu, L.; Yu, Z.; Quan, Y.; Xie, X. Different Exosomes Are Loaded in Hydrogels for the Application in the Field of Tissue Repair. Front. Bioeng. Biotechnol. 2025, 13, 1545636. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Yao, J.; Sun, Z.; Mao, Y.; Wei, J.; Xie, Y.; Hu, X.K.; Li, X. Hydrogels as Carriers Deliver Stem Cells/Exosomes for Liver Injury. Mater. Adv. 2024, 5, 3587–3601. [Google Scholar] [CrossRef]
- Sille, I.E.; Pissinis, D.E.; Fagali, N.S.; Ghilini, F.; Urrutia, M.N.; Schilardi, P.L. Antimicrobial-Loaded Polyacrylamide Hydrogels Supported on Titanium as Reservoir for Local Drug Delivery. Pathogens 2023, 12, 202. [Google Scholar] [CrossRef]
- Ahmad, N.; Bukhari, S.N.A.; Hussain, M.A.; Ejaz, H.; Munir, M.U.; Amjad, M.W. Nanoparticles Incorporated Hydrogels for Delivery of Antimicrobial Agents: Developments and Trends. RSC Adv. 2024, 14, 13535–13564. [Google Scholar] [CrossRef]
- Moorcroft, S.C.T.; Roach, L.; Jayne, D.G.; Ong, Z.Y.; Evans, S.D. Nanoparticle-Loaded Hydrogel for the Light-Activated Release and Photothermal Enhancement of Antimicrobial Peptides. ACS Appl. Mater. Interfaces 2020, 12, 24544–24554. [Google Scholar] [CrossRef]
- Bâldea, I.; Soran, M.-L.; Stegarescu, A.; Opriș, O.; Kacso, I.; Tripon, S.; Adascalitei, A.; Fericel, I.G.; Decea, R.; Lung, I. Lilium Candidum Extract Loaded in Alginate Hydrogel Beads for Chronic Wound Healing. Gels 2025, 11, 22. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Boccaccini, A.R. Antibacterial Biohybrid Nanofibers for Wound Dressings. Acta Biomater. 2020, 107, 25–49. [Google Scholar] [CrossRef]
- Aderibigbe, B.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, X. Alginate Hydrogel Dressings for Advanced Wound Management. Int. J. Biol. Macromol. 2020, 162, 1414–1428. [Google Scholar] [CrossRef] [PubMed]
- Bâldea, I.; Lung, I.; Opriş, O.; Stegarescu, A.; Kacso, I.; Soran, M.-L. Antioxidant, Anti-Inflammatory Effects and Ability to Stimulate Wound Healing of a Common-Plantain Extract in Alginate Gel Formulations. Gels 2023, 9, 901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cheng, J.; Ao, Q. Preparation of Alginate-Based Biomaterials and Their Applications in Biomedicine. Mar. Drugs 2021, 19, 264. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Carvalho, A.; Vaz, D.C.; Gil, M.H.; Mendes, A.; Bártolo, P. Development of Novel Alginate Based Hydrogel Films for Wound Healing Applications. Int. J. Biol. Macromol. 2013, 52, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.; Correia, C.; Malpique, R.; Brito, C.; Jensen, J.; Bjorquist, P.; Carrondo, M.J.T.; Alves, P.M. Microencapsulation Technology: A Powerful Tool for Integrating Expansion and Cryopreservation of Human Embryonic Stem Cells. PLoS ONE 2011, 6, e23212. [Google Scholar] [CrossRef]
- Supramaniam, J.; Adnan, R.; Mohd Kaus, N.H.; Bushra, R. Magnetic Nanocellulose Alginate Hydrogel Beads as Potential Drug Delivery System. Int. J. Biol. Macromol. 2018, 118, 640–648. [Google Scholar] [CrossRef]
- Buket Basmanav, F.; Kose, G.T.; Hasirci, V. Sequential Growth Factor Delivery from Complexed Microspheres for Bone Tissue Engineering. Biomaterials 2008, 29, 4195–4204. [Google Scholar] [CrossRef]
- Ho, T.T.; Tran, H.A.; Doan, V.K.; Maitz, J.; Li, Z.; Wise, S.G.; Lim, K.S.; Rnjak-Kovacina, J. Natural Polymer-Based Materials for Wound Healing Applications. Adv. Nanobiomed Res. 2024, 4, 2300131. [Google Scholar] [CrossRef]
- Alberts, A.; Bratu, A.G.; Niculescu, A.-G.; Grumezescu, A.M. New Perspectives of Hydrogels in Chronic Wound Management. Molecules 2025, 30, 686. [Google Scholar] [CrossRef]
- Hodge, J.G.; Zamierowski, D.S.; Robinson, J.L.; Mellott, A.J. Evaluating Polymeric Biomaterials to Improve next Generation Wound Dressing Design. Biomater. Res. 2022, 26, 50. [Google Scholar] [CrossRef]
- Aderibigbe, B.A. Hybrid-Based Wound Dressings: Combination of Synthetic and Biopolymers. Polymers 2022, 14, 3806. [Google Scholar] [CrossRef]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New Developments in Medical Applications of Hybrid Hydrogels Containing Natural Polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Wang, Y.; Qi, H.; Shi, C.; Wei, G.; Xiao, L.; Huang, Z.; Liu, S.; Yu, H.; Teng, C.; et al. Nanocomposite Sponges of Sodium Alginate/Graphene Oxide/Polyvinyl Alcohol as Potential Wound Dressing: In Vitro and in Vivo Evaluation. Compos. B Eng. 2019, 167, 396–405. [Google Scholar] [CrossRef]
- Rana, M.M.; De la Hoz Siegler, H. Evolution of Hybrid Hydrogels: Next-Generation Biomaterials for Drug Delivery and Tissue Engineering. Gels 2024, 10, 216. [Google Scholar] [CrossRef]
- Liang, W.; Ni, N.; Huang, Y.; Lin, C. An Advanced Review: Polyurethane-Related Dressings for Skin Wound Repair. Polymers 2023, 15, 4301. [Google Scholar] [CrossRef]
- Eskandarinia, A.; Kefayat, A.; Agheb, M.; Rafienia, M.; Amini Baghbadorani, M.; Navid, S.; Ebrahimpour, K.; Khodabakhshi, D.; Ghahremani, F. A Novel Bilayer Wound Dressing Composed of a Dense Polyurethane/Propolis Membrane and a Biodegradable Polycaprolactone/Gelatin Nanofibrous Scaffold. Sci. Rep. 2020, 10, 3063. [Google Scholar] [CrossRef]
- Chen, H.; Xing, X.; Tan, H.; Jia, Y.; Zhou, T.; Chen, Y.; Ling, Z.; Hu, X. Covalently Antibacterial Alginate-Chitosan Hydrogel Dressing Integrated Gelatin Microspheres Containing Tetracycline Hydrochloride for Wound Healing. Mater. Sci. Eng. C 2017, 70, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, L.; Guo, C.; Qin, H.; Yu, X. A Promising Wound Dressing Material with Excellent Cytocompatibility and Proangiogenesis Action for Wound Healing: Strontium Loaded Silk Fibroin/Sodium Alginate (SF/SA) Blend Films. Int. J. Biol. Macromol. 2017, 104, 969–978. [Google Scholar] [CrossRef]
- Mokhena, T.C.; Luyt, A.S. Electrospun Alginate Nanofibres Impregnated with Silver Nanoparticles: Preparation, Morphology and Antibacterial Properties. Carbohydr. Polym. 2017, 165, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Jessy Mercy, D.; Thirumalai, A.; Udayakumar, S.; Deepika, B.; Janani, G.; Girigoswami, A.; Girigoswami, K. Enhancing Wound Healing with Nanohydrogel-Entrapped Plant Extracts and Nanosilver: An In Vitro Investigation. Molecules 2024, 29, 5004. [Google Scholar] [CrossRef] [PubMed]
- Ningrum, D.R.; Hanif, W.; Mardhian, D.F.; Asri, L.A.T.W. In Vitro Biocompatibility of Hydrogel Polyvinyl Alcohol/Moringa Oleifera Leaf Extract/Graphene Oxide for Wound Dressing. Polymers 2023, 15, 468. [Google Scholar] [CrossRef]
- Guo, C.; Zhou, Z.; Zhang, S.; Peng, P.; Liu, J.; Yan, Y.; Dong, J. Sulfonated, Quaternized, and Chlorogenic Acid Composited Sodium Alginate Hydrogels/Eucommia Ulmoides Rubber Films as in Vitro Antibacterial Wound Dressings for Accelerating Wound Healing. Ind. Crops Prod. 2022, 190, 115885. [Google Scholar] [CrossRef]
- Hofmann, E.; Fink, J.; Pignet, A.-L.; Schwarz, A.; Schellnegger, M.; Nischwitz, S.P.; Holzer-Geissler, J.C.J.; Kamolz, L.-P.; Kotzbeck, P. Human In Vitro Skin Models for Wound Healing and Wound Healing Disorders. Biomedicines 2023, 11, 1056. [Google Scholar] [CrossRef]
- Yadav, P.D.; Londhe, P.V.; Chavan, S.S.; Mohite, D.D.; Firame, G.B.; Kadam, S.S.; Patil, M.J.; Ansari, M.I. Electrospun Composite Nanofibers for Wound Healing: Synthesis, Characterization, and Clinical Potential of Biopolymer-Based Materials. Discov. Mater. 2024, 4, 99. [Google Scholar] [CrossRef]
- Joshi, A.S.; Madhusudanan, M.; Mijakovic, I. 3D Printed Inserts for Reproducible High Throughput Screening of Cell Migration. Front. Cell Dev. Biol. 2023, 11, 1256250. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.S. Advancing in Vitro Cell Migration Studies: A Review of Open-Source Analytical Platforms for Cancer and Wound Healing Research. Cell Adhes. Migr. 2025, 19, 2488116. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Brown, A.C. Characterizing Cell Migration Within Three-Dimensional In Vitro Wound Environments. J. Vis. Exp. 2017, 126, e56099. [Google Scholar] [CrossRef]
- Azzam, S.; Tomasova, L.; Danner, C.; Skiba, M.; Klein, M.; Guttenberg, Z.; Michaelis, S.; Wegener, J. A High-Precision Wound Healing Assay Based on Photosensitized Culture Substrates. Sci. Rep. 2024, 14, 9103. [Google Scholar] [CrossRef]
- Biglari, S.; Le, T.Y.L.; Tan, R.P.; Wise, S.G.; Zambon, A.; Codolo, G.; De Bernard, M.; Warkiani, M.; Schindeler, A.; Naficy, S.; et al. Simulating Inflammation in a Wound Microenvironment Using a Dermal Wound-on-a-Chip Model. Adv. Healthc. Mater. 2019, 8, e1801307. [Google Scholar] [CrossRef]
- Flynn, K.; Mahmoud, N.N.; Sharifi, S.; Gould, L.J.; Mahmoudi, M. Chronic Wound Healing Models. ACS Pharmacol. Transl. Sci. 2023, 6, 783–801. [Google Scholar] [CrossRef]
- Seiser, S.; Janker, L.; Zila, N.; Mildner, M.; Rakita, A.; Matiasek, J.; Bileck, A.; Gerner, C.; Paulitschke, V.; Elbe-Bürger, A. Octenidine-Based Hydrogel Shows Anti-Inflammatory and Protease-Inhibitory Capacities in Wounded Human Skin. Sci. Rep. 2021, 11, 32. [Google Scholar] [CrossRef]
- Liu, C.; Rinderknecht, H.; Histing, T.; Kolbenschlag, J.; Nussler, A.K.; Ehnert, S. Establishment of an In Vitro Scab Model for Investigating Different Phases of Wound Healing. Bioengineering 2022, 9, 191. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D Cell Cultures—A Comparison of Different Types of Cancer Cell Cultures. Arch. Med. Sci. 2016, 14, 910. [Google Scholar] [CrossRef]
- Haisma, E.M.; Rietveld, M.H.; de Breij, A.; van Dissel, J.T.; El Ghalbzouri, A.; Nibbering, P.H. Inflammatory and Antimicrobial Responses to Methicillin-Resistant Staphylococcus Aureus in an In Vitro Wound Infection Model. PLoS ONE 2013, 8, e82800. [Google Scholar] [CrossRef]
- Mieremet, A.; Rietveld, M.; Absalah, S.; van Smeden, J.; Bouwstra, J.A.; El Ghalbzouri, A. Improved Epidermal Barrier Formation in Human Skin Models by Chitosan Modulated Dermal Matrices. PLoS ONE 2017, 12, e0174478. [Google Scholar] [CrossRef]
- Folle, C.; Díaz-Garrido, N.; Mallandrich, M.; Suñer-Carbó, J.; Sánchez-López, E.; Halbaut, L.; Marqués, A.M.; Espina, M.; Badia, J.; Baldoma, L.; et al. Hydrogel of Thyme-Oil-PLGA Nanoparticles Designed for Skin Inflammation Treatment. Gels 2024, 10, 149. [Google Scholar] [CrossRef]
- Gross-Amat, O.; Guillen, M.; Salmon, D.; Nataf, S.; Auxenfans, C. Characterization of a Topically Testable Model of Burn Injury on Human Skin Explants. Int. J. Mol. Sci. 2020, 21, 6956. [Google Scholar] [CrossRef]
- O’Connor, N.A.; Syed, A.; Kastrat, E.; Cheng, H.-P. Antibacterial Silver Nanoparticle Containing Polydopamine Hydrogels That Enhance Re-Epithelization. Gels 2024, 10, 363. [Google Scholar] [CrossRef]
- Singh, V.; Marimuthu, T.; Lesotho, N.F.; Makatini, M.M.; Ntombela, T.; Van Eyk, A.; Choonara, Y.E. Synthesis of a Retro -GFOGER Adamantane-Based Collagen Mimetic Peptide Imbibed in a Hyaluronic Acid Hydrogel for Enhanced Wound Healing. ACS Appl. Bio Mater. 2025, 8, 4657–4672. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhao, C.; Wan, Y.; Majid, M.; Abbas, S.Q.; Wang, Y. In Vitro and in Vivo Evaluation of Alginate Hydrogel-Based Wound Dressing Loaded with Green Chemistry Cerium Oxide Nanoparticles. Front. Chem. 2023, 11, 1298808. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Grada, A.; Mervis, J.; Falanga, V. Research Techniques Made Simple: Animal Models of Wound Healing. J. Investig. Dermatol. 2018, 138, 2095–2105.e1. [Google Scholar] [CrossRef]
- Kurt, A.A.; Ibrahim, B.; Çınar, H.; Atsü, A.N.; Bursalıoğlu, E.O.; Bayır, İ.; Özmen, Ö.; Aslan, İ. Nanoemulsion Hydrogel Delivery System of Hypericum Perforatum L.: In Silico Design, In Vitro Antimicrobial–Toxicological Profiling, and In Vivo Wound-Healing Evaluation. Gels 2025, 11, 431. [Google Scholar] [CrossRef]
- Gagliardi, A.; Giuliano, E.; Voci, S.; Costa, N.; Bulotta, S.; Salvatici, M.C.; Ambrosio, N.; Paolino, D.; Siddique, F.; Majid, M.; et al. Rutin-Loaded Zein Gel as a Green Biocompatible Formulation for Wound Healing Application. Int. J. Biol. Macromol. 2024, 269, 132071. [Google Scholar] [CrossRef] [PubMed]
- Grover, P.; Khanna, K.; Bhatnagar, A.; Purkayastha, J. In Vivo-Wound Healing Studies of Sodium Thiosulfate Gel in Rats. Biomed. Pharmacother. 2021, 140, 111797. [Google Scholar] [CrossRef] [PubMed]
- Okur, M.E.; Karadağ, A.E.; Üstündağ Okur, N.; Özhan, Y.; Sipahi, H.; Ayla, Ş.; Daylan, B.; Demirci, B.; Demirci, F. In Vivo Wound Healing and In Vitro Anti-Inflammatory Activity Evaluation of Phlomis Russeliana Extract Gel Formulations. Molecules 2020, 25, 2695. [Google Scholar] [CrossRef]
- Akbari, F.; Azadbakht, M.; Bagheri, A.; Vahedi, L. In Vitro and In Vivo Wound Healing Activity of Astragalus Floccosus Boiss. (Fabaceae). Adv. Pharmacol. Pharm. Sci. 2022, 2022, 1–15. [Google Scholar] [CrossRef]
- Jabbar, A.A.; Abdul-Aziz Ahmed, K.; Abdulla, M.A.; Abdullah, F.O.; Salehen, N.A.; Mothana, R.A.; Houssaini, J.; Hassan, R.R.; Hawwal, M.F.; Fantoukh, O.I.; et al. Sinomenine Accelerate Wound Healing in Rats by Augmentation of Antioxidant, Anti-Inflammatory, Immunuhistochemical Pathways. Heliyon 2024, 10, e23581. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ziemer, M.; Stojanovic, I.; Saksida, T.; Maksimovic-Ivanic, D.; Mijatovic, S.; Djmura, G.; Gajic, D.; Koprivica, I.; Krajnovic, T.; et al. Mesenchymal Stem Cells From Mouse Hair Follicles Reduce Hypertrophic Scarring in a Murine Wound Healing Model. Stem Cell Rev. Rep. 2022, 18, 2028–2044. [Google Scholar] [CrossRef]
- Tunç, A.S.; Ercan, N. Effect of Topical Sildenafil on Wound Healing and Oxidative Stress in Rats. Injury 2024, 55, 111525. [Google Scholar] [CrossRef]
- Rodgers, K.; Xiong, S.; Felix, J.; Roda, N.; Espinoza, T.; Maldonado, S.; Dizerega, G. Development of Angiotensin (1-7) as an Agent to Accelerate Dermal Repair. Wound Repair Regen. 2001, 9, 238–247. [Google Scholar] [CrossRef]
- Jia, Y.; Zhao, G.; Jia, J. Preliminary Evaluation: The Effects of Aloe Ferox Miller and Aloe Arborescens Miller on Wound Healing. J. Ethnopharmacol. 2008, 120, 181–189. [Google Scholar] [CrossRef]
- Choi, J.; Park, Y.-G.; Yun, M.-S.; Seol, J.-W. Effect of Herbal Mixture Composed of Alchemilla Vulgaris and Mimosa on Wound Healing Process. Biomed. Pharmacother. 2018, 106, 326–332. [Google Scholar] [CrossRef]
- Oladejo, O.W.; Imosemi, I.O.; Osuagwu, F.C.; Oyedele, O.O.; Oluwadara, O.O.; Ekpo, O.E.; Aiku, A.; Adewoyin, O.; Akang, E.E.U. A Comparative Study of the Wound Healing Properties of Honey and Ageratum Conyzoides. Afr. J. Med. Med. Sci. 2003, 32, 193–196. [Google Scholar]
- Khosravian, P.; Motamedi, N.; Javdani, M.; Khorasgani, E.M. Histopathologic Evaluation of Skin Wound Healing Due to Local Application of Transdermal Chitosan Patch in Combination with Doxycycline, Zinc Nanoparticles, and Selenium Nanoparticles in Mice. BMC Vet. Res. 2025, 21, 221. [Google Scholar] [CrossRef]
- Nordback, P.H.; Miettinen, S.; Kääriäinen, M.; Haaparanta, A.-M.; Kellomäki, M.; Kuokkanen, H.; Seppänen, R. Chitosan Membranes in a Rat Model of Full-Thickness Cutaneous Wounds: Healing and IL-4 Levels. J. Wound Care 2015, 24, 245–251. [Google Scholar] [CrossRef]
- Ozkaya, H.; Omma, T.; Bag, Y.M.; Uzunoglu, K.; Isildak, M.; Duymus, M.E.; Kismet, K.; Senes, M.; Fidanci, V.; Celepli, P.; et al. Topical and Systemic Effects of N-Acetyl Cysteine on Wound Healing in a Diabetic Rat Model. Wounds 2019, 31, 91–96. [Google Scholar]
- Yang, H.; Song, L.; Sun, B.; Chu, D.; Yang, L.; Li, M.; Li, H.; Dai, Y.; Yu, Z.; Guo, J. Modulation of Macrophages by a Paeoniflorin-Loaded Hyaluronic Acid-Based Hydrogel Promotes Diabetic Wound Healing. Mater. Today Bio 2021, 12, 100139. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Zhang, Q.; Zhu, S.; Cai, J.; He, Y.; Lu, F. Delivery of Adipose-Derived Growth Factors from Heparinized Adipose Acellular Matrix Accelerates Wound Healing. Front. Bioeng. Biotechnol. 2023, 11, 1270618. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, H.; Wang, X.; Yang, Y.; Zhong, K.; Zhang, X.; Li, H. MSC-EVs and UCB-EVs Promote Skin Wound Healing and Spatial Transcriptome Analysis. Sci. Rep. 2025, 15, 4006. [Google Scholar] [CrossRef]
- Hong, S.E.; Hong, M.K.; Kang, S.R.; Young Park, B. Effects of Neodymium–Yttrium–Aluminum Garnet (Nd:YAG) Pulsed High-Intensity Laser Therapy on Full Thickness Wound Healing in an Experimental Animal Model. J. Cosmet. Laser Ther. 2016, 18, 432–437. [Google Scholar] [CrossRef]
- Kieran, I.; Knock, A.; Bush, J.; So, K.; Metcalfe, A.; Hobson, R.; Mason, T.; O’Kane, S.; Ferguson, M. Interleukin-10 Reduces Scar Formation in Both Animal and Human Cutaneous Wounds: Results of Two Preclinical and Phase II Randomized Control Studies. Wound Repair Regen. 2013, 21, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; He, Y.; Feng, J.; Dong, Z.; Yao, Y.; Mok, H.; Lin, M.; Feng, L. Extracellular Matrix/Stromal Vascular Fraction Gel Conditioned Medium Accelerates Wound Healing in a Murine Model. Wound Repair Regen. 2017, 25, 923–932. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Reza Farahpour, M.; Amjadi, S.; Mohammadi, M.; Hamishehkar, H. Nanoliposomal Peptides Derived from Spirulina Platensis Protein Accelerate Full-Thickness Wound Healing. Int. J. Pharm. 2023, 630, 122457. [Google Scholar] [CrossRef]
- Gil, E.S.; Panilaitis, B.; Bellas, E.; Kaplan, D.L. Functionalized Silk Biomaterials for Wound Healing. Adv. Healthc. Mater. 2013, 2, 206–217. [Google Scholar] [CrossRef]
- Huang, Y.; Qin, P.; Zhou, P.; Long, B.; Zhang, S.; Gao, R.; Zhu, B.; Li, Y.; Li, Q. Non-Pharmacological Interventions of Intermittent Fasting and Pulsed Radiofrequency Energy (PRFE) Combination Therapy Promote Diabetic Wound Healing. Nutr. Diabetes 2024, 14, 83. [Google Scholar] [CrossRef]
- Mehmandoust, F.G.; Torkaman, G.; Firoozabadi, M.; Talebi, G. Anodal and Cathodal Pulsed Electrical Stimulation on Skin Wound Healing in Guinea Pigs. J. Rehabil. Res. Dev. 2007, 44, 611. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.L.; Wyant, W.A.; Abdo Abujamra, B.; Kirsner, R.S.; Jozic, I. Diabetic Wound-Healing Science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, L.; Cheng, H.; Zhu, J.; Li, X.; Ye, S.; Li, X. Hydrogel-Based Dressings Designed to Facilitate Wound Healing. Mater. Adv. 2024, 5, 1364–1394. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Wang, R.; Yuan, P.; Fan, Z.; Yang, S. Preparation and Properties of ZnO/Sodium Alginate Bi-Layered Hydrogel Films as Novel Wound Dressings. New J. Chem. 2019, 43, 8684–8693. [Google Scholar] [CrossRef]
- Nešović, K.; Mišković-Stanković, V. A Comprehensive Review of the Polymer-based Hydrogels with Electrochemically Synthesized Silver Nanoparticles for Wound Dressing Applications. Polym. Eng. Sci. 2020, 60, 1393–1419. [Google Scholar] [CrossRef]
- Hajiali, H.; Summa, M.; Russo, D.; Armirotti, A.; Brunetti, V.; Bertorelli, R.; Athanassiou, A.; Mele, E. Alginate–Lavender Nanofibers with Antibacterial and Anti-Inflammatory Activity to Effectively Promote Burn Healing. J. Mater. Chem. B 2016, 4, 1686–1695. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Chung, H.; Lee, G.; Kim, D.; Jiang, Z.; Kim, S.-H.; Lee, K. Remendable Cross-Linked Alginate/Gelatin Hydrogels Incorporating Nanofibers for Wound Repair and Regeneration. Biomacromolecules 2024, 25, 4344–4357. [Google Scholar] [CrossRef]
- Alemdaroğlu, C.; Değim, Z.; Çelebi, N.; Zor, F.; Öztürk, S.; Erdoğan, D. An Investigation on Burn Wound Healing in Rats with Chitosan Gel Formulation Containing Epidermal Growth Factor. Burns 2006, 32, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.-G.; Bîrcă, A.C.; Mogoşanu, G.D.; Rădulescu, M.; Holban, A.M.; Manuc, D.; Alberts, A.; Grumezescu, A.M.; Mogoantă, L. Zinc Alginate Hydrogel-Coated Wound Dressings: Fabrication, Characterization, and Evaluation of Anti-Infective and In Vivo Performance. Gels 2025, 11, 427. [Google Scholar] [CrossRef]
- Barakat, H.S.; Freag, M.S.; Gaber, S.M.; Al Oufy, A.; Abdallah, O.Y. Development of Verapamil Hydrochloride-Loaded Biopolymer-Based Composite Electrospun Nanofibrous Mats: In Vivo Evaluation of Enhanced Burn Wound Healing without Scar Formation. Drug Des. Devel Ther. 2023, 17, 1211–1231. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, F.; Suarato, G.; Summa, M.; Miele, D.; Sandri, G.; Bertorelli, R.; Athanassiou, A. Plant-Based, Hydrogel-like Microfibers as an Antioxidant Platform for Skin Burn Healing. ACS Appl. Bio Mater. 2023, 6, 3103–3116. [Google Scholar] [CrossRef]
- Takahashi, M.; Morimoto, Y.; Kon, M.; Saga, K. A Histological Study of Cutaneous Thermal Wounds Using a Clostridium Perfringens-Derived Wound Healing Substance with Wound Healing Stimulation Activity. J. Dermatol. 1995, 22, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-X.; Wang, P.; Wang, Y.-T.; Huang, Y.; Jiang, L.; Wang, X.-M. Aloe Vera and Vitis Vinifera Improve Wound Healing in an in Vivo Rat Burn Wound Model. Mol. Med. Rep. 2016, 13, 1070–1076. [Google Scholar] [CrossRef]
- Askin, S.; Kaynarpinar, M. Efficacy of Formulation With Potential as Herbal Medicine on Second Degree Burn Wound: Biochemical and Molecular Evaluation. J. Cosmet. Dermatol. 2025, 24, e70122. [Google Scholar] [CrossRef]
- Fei, Y.; Ling, Z.; Tong, Q.; Wang, J. Apoptotic Extracellular Vesicles from Supernumerary Tooth-Derived Pulp Stem Cells Transfer COL1A1 to Promote Angiogenesis via PI3K/Akt/VEGF Pathway. Int. J. Nanomed. 2024, 19, 6811–6828. [Google Scholar] [CrossRef]
- Lowe, A.S.; Walker, M.D.; Cowan, R.; Baxter, G.D. Therapeutic Ultrasound and Wound Closure: Lack of Healing Effect on x-Ray Irradiated Wounds in Murine Skin. Arch. Phys. Med. Rehabil. 2001, 82, 1507–1511. [Google Scholar] [CrossRef]
- Belmont, M.J.; Marabelle, N.; Mang, T.S.; Hall, R.; Wax, M.K. Effect of Photodynamic Therapy on Revascularization of Fasciocutaneous Flaps. Laryngoscope 2000, 110, 942–945. [Google Scholar] [CrossRef]
- Sami, D.G.; Heiba, H.H.; Abdellatif, A. Wound Healing Models: A Systematic Review of Animal and Non-Animal Models. Wound Med. 2019, 24, 8–17. [Google Scholar] [CrossRef]
- Zomer, H.D.; Trentin, A.G. Skin Wound Healing in Humans and Mice: Challenges in Translational Research. J. Dermatol. Sci. 2018, 90, 3–12. [Google Scholar] [CrossRef]
- Parnell, L.K.S.; Volk, S.W. The Evolution of Animal Models in Wound Healing Research: 1993–2017. Adv. Wound Care 2019, 8, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, M.; Molahally, S.; Salwaji, S. Ethical Guidelines, Animal Profile, Various Animal Models Used in Periodontal Research with Alternatives and Future Perspectives. J. Indian. Soc. Periodontol. 2016, 20, 360. [Google Scholar] [CrossRef] [PubMed]
- Gounden, V.; Singh, M. Hydrogels and Wound Healing: Current and Future Prospects. Gels 2024, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef]
- Shu, W.; Wang, Y.; Zhang, X.; Li, C.; Le, H.; Chang, F. Functional Hydrogel Dressings for Treatment of Burn Wounds. Front. Bioeng. Biotechnol. 2021, 9, 788461. [Google Scholar] [CrossRef]
- Fan, F.; Saha, S.; Hanjaya-Putra, D. Biomimetic Hydrogels to Promote Wound Healing. Front. Bioeng. Biotechnol. 2021, 9, 718377. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Cen, Y.; Tian, M. Immunomodulatory Hydrogels for Skin Wound Healing: Cellular Targets and Design Strategy. J. Mater. Chem. B 2024, 12, 2435–2458. [Google Scholar] [CrossRef]
- Surowiecka, A.; Strużyna, J.; Winiarska, A.; Korzeniowski, T. Hydrogels in Burn Wound Management—A Review. Gels 2022, 8, 122. [Google Scholar] [CrossRef]
- Murphy, S.V.; Skardal, A.; Atala, A. Evaluation of Hydrogels for Bio-printing Applications. J. Biomed. Mater. Res. A 2013, 101A, 272–284. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Cai, K.; Zhang, B.; Tang, S.; Zhang, W.; Liu, W. Antibacterial Polysaccharide-Based Hydrogel Dressing Containing Plant Essential Oil for Burn Wound Healing. Burns Trauma. 2021, 9, tkab041. [Google Scholar] [CrossRef]
- Li, S.; Dong, S.; Xu, W.; Tu, S.; Yan, L.; Zhao, C.; Ding, J.; Chen, X. Antibacterial Hydrogels. Adv. Sci. 2018, 5, 1700527. [Google Scholar] [CrossRef]
- Manju, S.; Antony, M.; Sreenivasan, K. Synthesis and Evaluation of a Hydrogel That Binds Glucose and Releases Ciprofloxacin. J. Mater. Sci. 2010, 45, 4006–4012. [Google Scholar] [CrossRef]
- Sa, Y.; Wang, M.; Deng, H.; Wang, Y.; Jiang, T. Beneficial Effects of Biomimetic Nano-Sized Hydroxyapatite/Antibiotic Gentamicin Enriched Chitosan–Glycerophosphate Hydrogel on the Performance of Injectable Polymethylmethacrylate. RSC Adv. 2015, 5, 91082–91092. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.-R.S.; Tamer, T.M.; El-Meligy, M.A.; Mohy Eldin, M.S. Poly (Vinyl Alcohol)-Alginate Physically Crosslinked Hydrogel Membranes for Wound Dressing Applications: Characterization and Bio-Evaluation. Arab. J. Chem. 2015, 8, 38–47. [Google Scholar] [CrossRef]
- Lai, P.-L.; Hong, D.-W.; Ku, K.-L.; Lai, Z.-T.; Chu, I.-M. Novel Thermosensitive Hydrogels Based on Methoxy Polyethylene Glycol-Co-Poly(Lactic Acid-Co-Aromatic Anhydride) for Cefazolin Delivery. Nanomedicine 2014, 10, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, C.T.; Boakye-Agyeman, F.; Brinkman, C.L.; Reid, J.M.; Patel, R.; Bajzer, Z.; Dadsetan, M.; Yaszemski, M.J. Controlled Delivery of Vancomycin via Charged Hydrogels. PLoS ONE 2016, 11, e0146401. [Google Scholar] [CrossRef] [PubMed]
- Sawadkar, P.; Lali, F.; Garcia-Gareta, E.; Garrido, B.G.; Chaudhry, A.; Matharu, P.; Kyriakidis, C.; Greco, K. Innovative Hydrogels in Cutaneous Wound Healing: Current Status and Future Perspectives. Front. Bioeng. Biotechnol. 2025, 13, 1454903. [Google Scholar] [CrossRef]
- Li, Z.; Qu, T.; Ding, C.; Ma, C.; Sun, H.; Li, S.; Liu, X. Injectable Gelatin Derivative Hydrogels with Sustained Vascular Endothelial Growth Factor Release for Induced Angiogenesis. Acta Biomater. 2015, 13, 88–100. [Google Scholar] [CrossRef]
- Xiao, Z.; Zheng, X.; An, Y.; Wang, K.; Zhang, J.; He, H.; Wu, J. Zwitterionic Hydrogel for Sustained Release of Growth Factors to Enhance Wound Healing. Biomater. Sci. 2021, 9, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Bitterman-Deutsch, O.; Kogan, L.; Nasser, F. Delayed Immune Mediated Adverse Effects to Hyaluronic Acid Fillers: Report of Five Cases and Review of the Literature. Dermatol. Rep. 2015, 7, 5851. [Google Scholar] [CrossRef]
- Dam, P.; Celik, M.; Ustun, M.; Saha, S.; Saha, C.; Kacar, E.A.; Kugu, S.; Karagulle, E.N.; Tasoglu, S.; Buyukserin, F.; et al. Wound Healing Strategies Based on Nanoparticles Incorporated in Hydrogel Wound Patches. RSC Adv. 2023, 13, 21345–21364. [Google Scholar] [CrossRef] [PubMed]
- Kar, A.K.; Singh, A.; Dhiman, N.; Purohit, M.P.; Jagdale, P.; Kamthan, M.; Singh, D.; Kumar, M.; Ghosh, D.; Patnaik, S. Polymer-Assisted In Situ Synthesis of Silver Nanoparticles with Epigallocatechin Gallate (EGCG) Impregnated Wound Patch Potentiate Controlled Inflammatory Responses for Brisk Wound Healing. Int. J. Nanomed. 2019, 14, 9837–9854. [Google Scholar] [CrossRef]
- Zhang, B.; Lv, Y.; Yu, C.; Zhang, W.; Song, S.; Li, Y.; Chong, Y.; Huang, J.; Zhang, Z. Au–Pt Nanozyme-Based Multifunctional Hydrogel Dressing for Diabetic Wound Healing. Biomater. Adv. 2022, 137, 212869. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, N.; Godbey, W.T.; McPherson, G.L.; John, V.T. Microgel, Nanogel and Hydrogel–Hydrogel Semi-IPN Composites for Biomedical Applications: Synthesis and Characterization. Colloid. Polym. Sci. 2006, 284, 1121–1129. [Google Scholar] [CrossRef]
- Liang, C.; Dudko, V.; Khoruzhenko, O.; Hong, X.; Lv, Z.-P.; Tunn, I.; Umer, M.; Timonen, J.V.I.; Linder, M.B.; Breu, J.; et al. Stiff and Self-Healing Hydrogels by Polymer Entanglements in Co-Planar Nanoconfinement. Nat. Mater. 2025, 24, 599–606. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Yan, Z.; Ji, S.; Xiao, S.; Gao, J. Metal Nanoparticle Hybrid Hydrogels: The State-of-the-Art of Combining Hard and Soft Materials to Promote Wound Healing. Theranostics 2024, 14, 1534–1560. [Google Scholar] [CrossRef]
- Xue, H.; Hu, L.; Xiong, Y.; Zhu, X.; Wei, C.; Cao, F.; Zhou, W.; Sun, Y.; Endo, Y.; Liu, M.; et al. Quaternized Chitosan-Matrigel-Polyacrylamide Hydrogels as Wound Dressing for Wound Repair and Regeneration. Carbohydr. Polym. 2019, 226, 115302. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Hong, Y.-L.; Wu, T.-L. Novel Silver and Nanoparticle-Encapsulated Growth Factor Co-Loaded Chitosan Composite Hydrogel with Sustained Antimicrobility and Promoted Biological Properties for Diabetic Wound Healing. Mater. Sci. Eng. C 2021, 118, 111385. [Google Scholar] [CrossRef]
- Ying, H.; Zhou, J.; Wang, M.; Su, D.; Ma, Q.; Lv, G.; Chen, J. In Situ Formed Collagen-Hyaluronic Acid Hydrogel as Biomimetic Dressing for Promoting Spontaneous Wound Healing. Mater. Sci. Eng. C 2019, 101, 487–498. [Google Scholar] [CrossRef]
- Basha, S.I.; Ghosh, S.; Vinothkumar, K.; Ramesh, B.; Kumari, P.H.P.; Mohan, K.V.M.; Sukumar, E. Fumaric Acid Incorporated Ag/Agar-Agar Hybrid Hydrogel: A Multifunctional Avenue to Tackle Wound Healing. Mater. Sci. Eng. C 2020, 111, 110743. [Google Scholar] [CrossRef]
- Daristotle, J.L.; Lau, L.W.; Erdi, M.; Hunter, J.; Djoum, A.; Srinivasan, P.; Wu, X.; Basu, M.; Ayyub, O.B.; Sandler, A.D.; et al. Sprayable and Biodegradable, Intrinsically Adhesive Wound Dressing with Antimicrobial Properties. Bioeng. Transl. Med. 2020, 5, 10149. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Zhang, X.; Zhu, D.; Qi, X.; Cao, X.; Fang, Y.; Che, Y.; Han, Z.-C.; He, Z.-X.; et al. Prostaglandin E 2 Hydrogel Improves Cutaneous Wound Healing via M2 Macrophages Polarization. Theranostics 2018, 8, 5348–5361. [Google Scholar] [CrossRef]
- Liu, M.; Chen, Y.; Zhu, Q.; Tao, J.; Tang, C.; Ruan, H.; Wu, Y.; Loh, X.J. Antioxidant Thermogelling Formulation for Burn Wound Healing. Chem. Asian J. 2022, 17, e202200396. [Google Scholar] [CrossRef]
- Gokce, E.H.; Tuncay Tanrıverdi, S.; Eroglu, I.; Tsapis, N.; Gokce, G.; Tekmen, I.; Fattal, E.; Ozer, O. Wound Healing Effects of Collagen-Laminin Dermal Matrix Impregnated with Resveratrol Loaded Hyaluronic Acid-DPPC Microparticles in Diabetic Rats. Eur. J. Pharm. Biopharm. 2017, 119, 17–27. [Google Scholar] [CrossRef]
- Ioniță, A.C. In vitro effects of some synthesized aminoacetanilide N’-substituted on human leukocytes separated from peripheral blood. Farmacia 2019, 67, 684–690. [Google Scholar] [CrossRef]
- Li, Y.; Fu, R.; Zhu, C.; Fan, D. An Antibacterial Bilayer Hydrogel Modified by Tannic Acid with Oxidation Resistance and Adhesiveness to Accelerate Wound Repair. Colloids Surf. B Biointerfaces 2021, 205, 111869. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Suo, H.; Xie, Y.; Wang, K.; Wang, H.; Hou, Z.; Gao, Y.; Zhang, L.; Tao, J.; Jiang, H.; et al. Dopamine-Substituted Multidomain Peptide Hydrogel with Inherent Antimicrobial Activity and Antioxidant Capability for Infected Wound Healing. ACS Appl. Mater. Interfaces 2021, 13, 29380–29391. [Google Scholar] [CrossRef] [PubMed]
- Ehterami, A.; Salehi, M.; Farzamfar, S.; Samadian, H.; Vaez, A.; Ghorbani, S.; Ai, J.; Sahrapeyma, H. Chitosan/Alginate Hydrogels Containing Alpha-Tocopherol for Wound Healing in Rat Model. J. Drug Deliv. Sci. Technol. 2019, 51, 204–213. [Google Scholar] [CrossRef]
- Yahia, E.A.; El-Sharkawey, A.E.; Bayoumi, M.M. Quantitative Evaluation of Diabetic Foot Wound Healing Using Hydrogel Nanosilver Based Dressing Vs. Traditional Dressing: A Prospective Randomized Control Study. Pak. J. Med. Health Sci. 2021, 15, 1571–1574. [Google Scholar] [CrossRef]
- Verdú-Soriano, J.; Casado-Díaz, A.; de Cristino-Espinar, M.; Luna-Morales, S.; Dios-Guerra, C.; Moreno-Moreno, P.; Dorado, G.; Quesada-Gómez, J.M.; Rodríguez-Mañas, L.; Lázaro-Martínez, J.L. Hard-to-Heal Wound Healing: Superiority of Hydrogel EHO-85 (Containing Olea Europaea Leaf Extract) vs. a Standard Hydrogel. A Randomized Controlled Trial. Gels 2023, 9, 962. [Google Scholar] [CrossRef]
- dos Santos, T.M.; de Sousa, D.S.F.; Di Piero, K.C.; Todeschini, A.R.; Dias, W.B.; Oliveira, C.A.; dos Santos, E.P.; Monteiro, M.S.d.S.B.; Gomes, M.K.; Moura, C.d.R.; et al. Development and Clinical Application of Hydrogel Formulations Containing Papain and Urea for Wound Healing. Braz. J. Pharm. Sci. 2023, 59, e201090. [Google Scholar] [CrossRef]
- Barbosa, M.; Carvalho, V.; Paggiaro, A. Hydrogel Enriched With Sodium Alginate and Vitamins A and E for Diabetic Foot Ulcer: A Randomized Controlled Trial. Wounds 2022, 34, 229–235. [Google Scholar] [CrossRef]
- Della Pepa, G.; Lombardi, G.; Gianfrancesco, S.; Piccolo, R.; Chirico, G.; Pellegrino, M.; Santella, L.; Tecce, N.; Volpicelli, A.; Sollo, E.; et al. Triticum Vulgare Extract and Polyhexanide (Fitostimoline® Hydrogel/Fitostimoline® Plus Gauze) versus Saline Gauze Dressing in Patients with Diabetic Foot Ulcers: Results of a Randomized Controlled Trial. J. Clin. Med. 2023, 12, 3596. [Google Scholar] [CrossRef]
- Edmonds, M. Apligraf in the Treatment of Neuropathic Diabetic Foot Ulcers. Int. J. Low. Extrem. Wounds 2009, 8, 11–18. [Google Scholar] [CrossRef]
- Elsharkawi, M.; Ghoneim, B.; Westby, D.; Jones, D.; Tawfick, W.; Walsh, S.R. Adipose-Derived Stem Cells in Patients with Venous Ulcers: Systematic Review. Vascular 2023, 31, 989–993. [Google Scholar] [CrossRef]
- Kim, Y.H.; Shim, H.S.; Lee, J.; Kim, S.W. A Prospective Randomized Controlled Multicenter Clinical Trial Comparing Paste-Type Acellular Dermal Matrix to Standard Care for the Treatment of Chronic Wounds. J. Clin. Med. 2022, 11, 2203. [Google Scholar] [CrossRef] [PubMed]
- Clegg, J.R.; Adebowale, K.; Zhao, Z.; Mitragotri, S. Hydrogels in the Clinic: An Update. Bioeng. Transl. Med. 2024, 9, e10680. [Google Scholar] [CrossRef] [PubMed]
- Vernerey, F.J.; Lalitha Sridhar, S.; Muralidharan, A.; Bryant, S.J. Mechanics of 3D Cell–Hydrogel Interactions: Experiments, Models, and Mechanisms. Chem. Rev. 2021, 121, 11085–11148. [Google Scholar] [CrossRef] [PubMed]
- Richbourg, N.; Wechsler, M.E.; Rodriguez-Cruz, J.J.; Peppas, N.A. Model-Based Modular Hydrogel Design. Nat. Rev. Bioeng. 2024, 2, 575–587. [Google Scholar] [CrossRef]
- Li, Z.; Song, P.; Li, G.; Han, Y.; Ren, X.; Bai, L.; Su, J. AI Energized Hydrogel Design, Optimization and Application in Biomedicine. Mater. Today Bio 2024, 25, 101014. [Google Scholar] [CrossRef] [PubMed]
- Islamkulov, M.; Karakuş, S.; Özeroğlu, C. Design Artificial Intelligence-Based Optimization and Swelling Behavior of Novel Crosslinked Polymeric Network Hydrogels Based on Acrylamide-2-Hydroxyethyl Methacrylate and Acrylamide-N-Isopropylacrylamide. Colloid. Polym. Sci. 2023, 301, 259–272. [Google Scholar] [CrossRef]
- Rippon, M.G.; Fleming, L.; Chen, T.; Rogers, A.A.; Ousey, K. Artificial Intelligence in Wound Care: Diagnosis, Assessment and Treatment of Hard-to-Heal Wounds: A Narrative Review. J. Wound Care 2024, 33, 229–242. [Google Scholar] [CrossRef]
- Chen, M.-Y. Progress in the Application of Artificial Intelligence in Skin Wound Assessment and Prediction of Healing Time. Am. J. Transl. Res. 2024, 16, 2765–2776. [Google Scholar] [CrossRef]
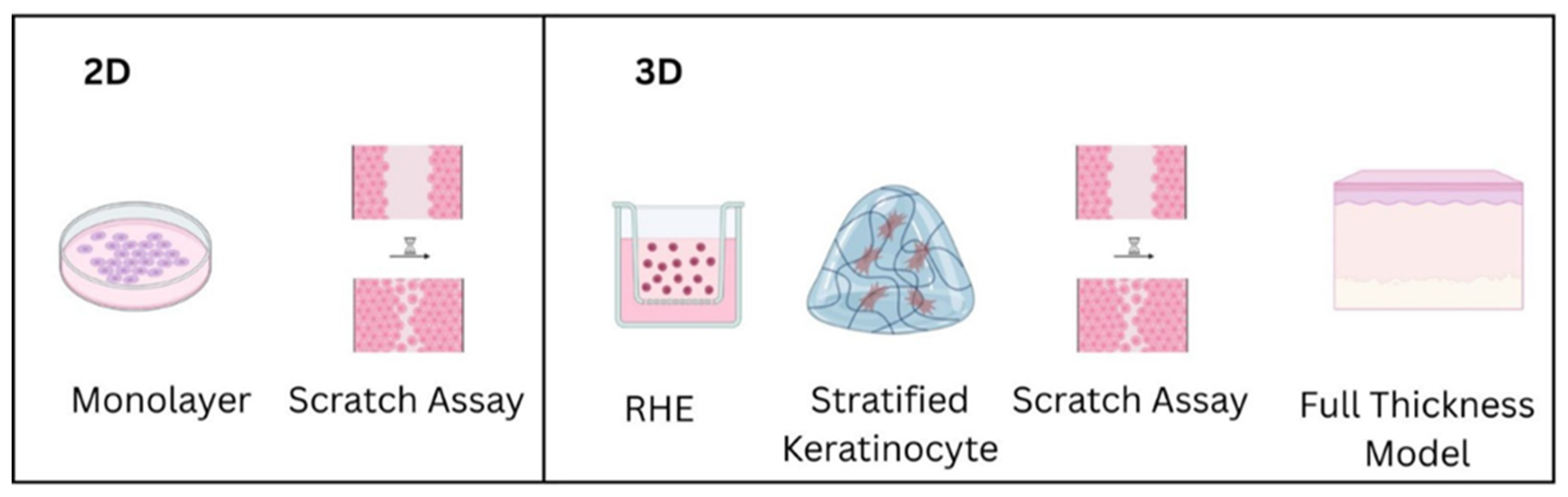
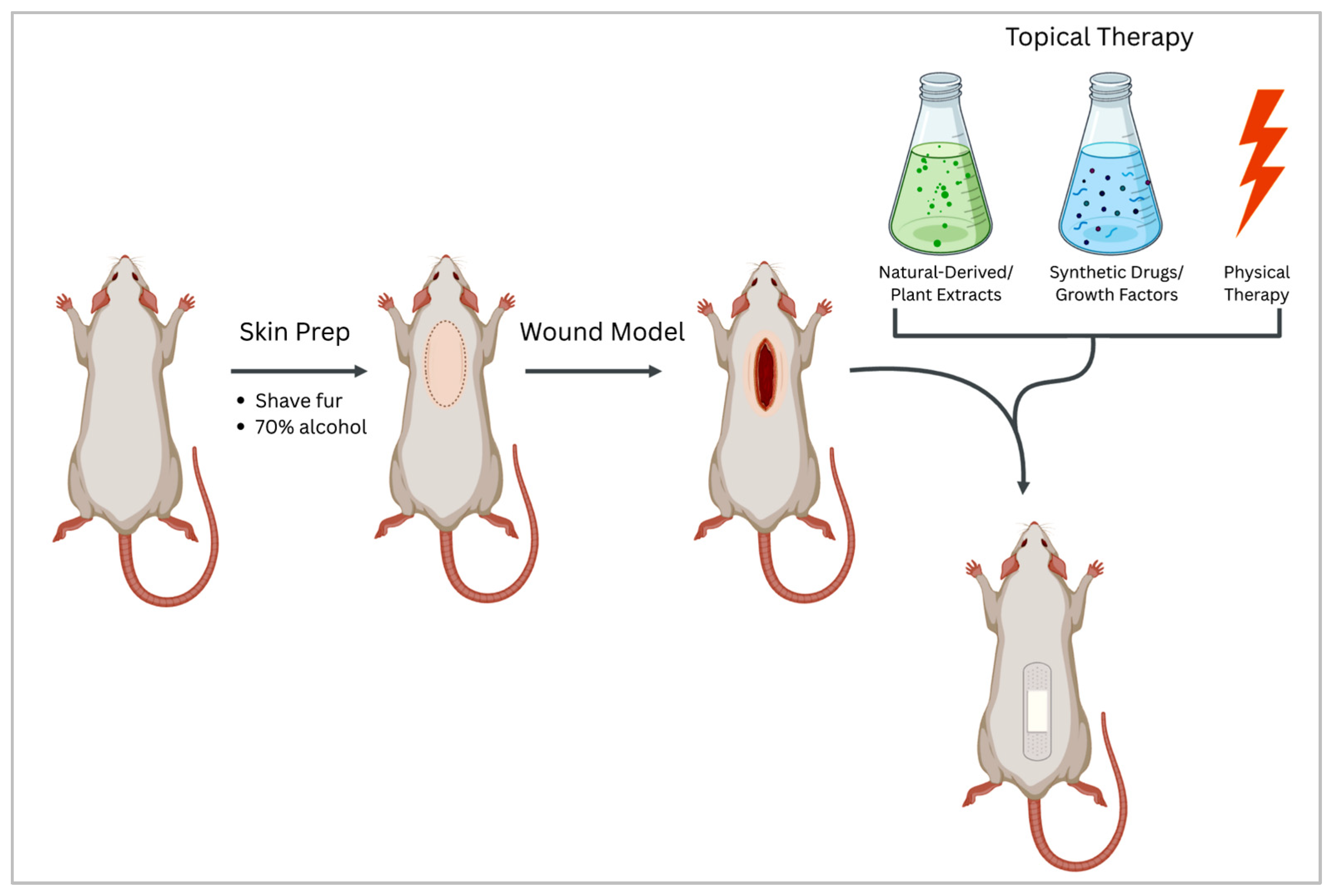
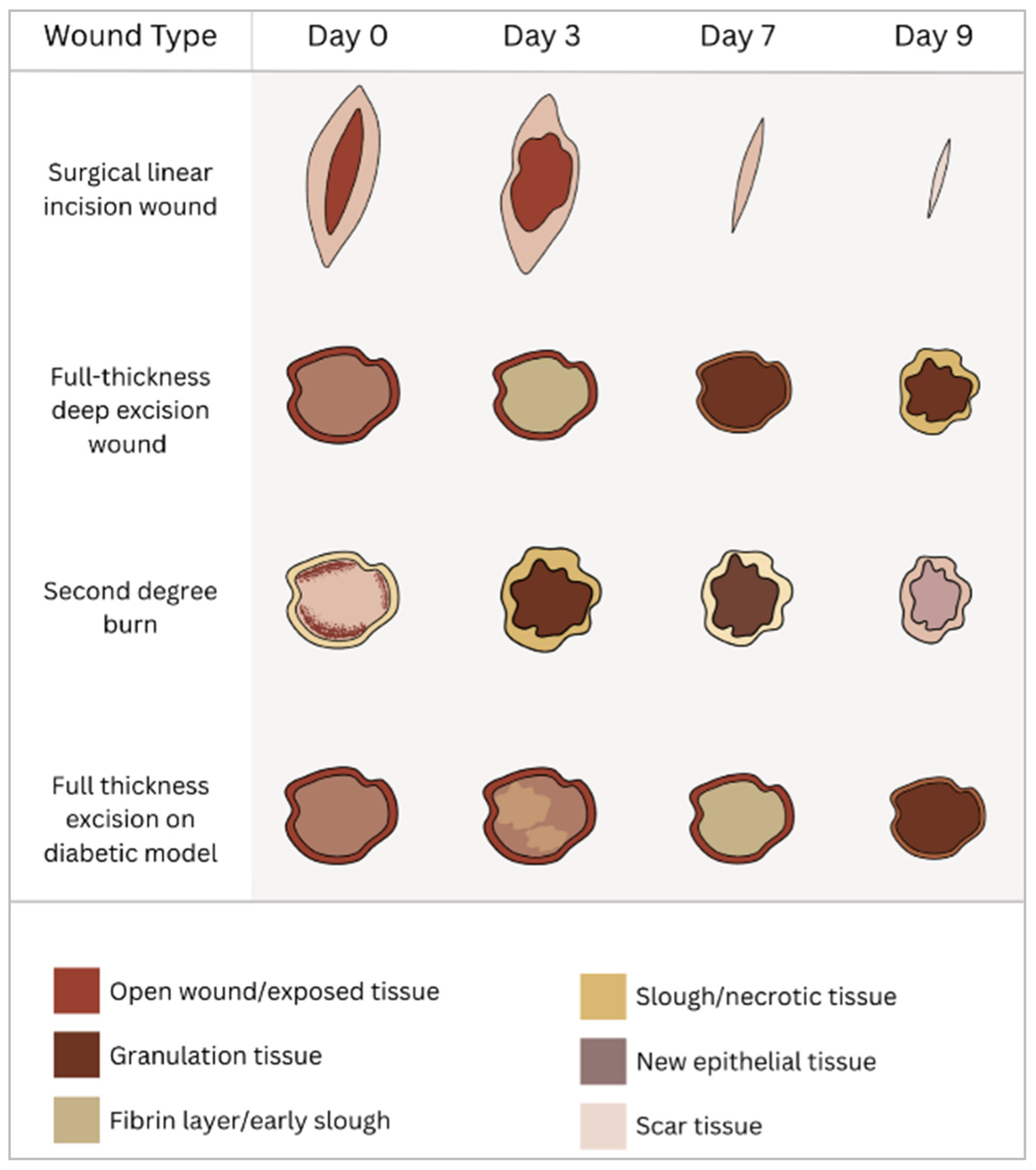
| Model Type | Characteristics | Advantages | Limitations | Typical Applications |
|---|---|---|---|---|
| Monolayer Culture [152,153,154] | single layer of cells on a flat surface | simple, cost-effective, and easy to analyze | limited cell–cell and ECM interactions | basic research, drug screening |
| Scratch Assay [152,155,156] | mechanical wound in monolayer | reproducible, widely used | not representative of tissue architecture | migration studies, wound closure assays |
| Transwell Assay [131,152,157] | migration through a porous membrane | quantitative, adaptable | no structural tissue context | chemotaxis, migration analysis |
| Co-culture System (2D) [127,131] | multiple cell types in a monolayer setup | intercellular communication, easy setup | hard to dissect specific pathways | interaction studies, signaling analysis |
| Process | 2D Models | 3D Models |
|---|---|---|
| Inflammation | inducible by pro-inflammatory stimuli assessment: inflammatory markers +: reproducible | layered cells with immune-like behavior assessment: inflammatory markers +: better simulate real inflammatory responses |
| Oxidative Stress | inducible by prooxidant stimuli | −: source identification of the marker, donor/time-dependent |
| Cell migration | assessment: imaging/oxidative stress markers +: reproducible | central hypoxia increases ROS +: reflects wound zone stress, assessment: oxidative stress markers −: source identification of the marker, donor/time dependent |
| Collagen Synthesis | scratch assay shows cell migration assessment: keratinocyte or fibroblast movement over time analyzed by imaging software, in dynamics | reconstructed skin models with epidermis with or without dermis −: difficult wound creation −: assessment by harvesting, HP imaging for re-epithelialization at different time points, and epithelial barrier function tests |
| Apoptosis | fibroblast cultures assessment: protein output/imaging | −: collagen deposited in scaffold or matrix assessment: imaging/biochemical assays |
| No. | Model/Species | Treatment | Main Results |
|---|---|---|---|
| 1 | FTEW dorsal area—rabbits | Nanoemulsion hydrogel with Hypericum perforatum extract | Upregulated growth factors, increased vessel growth, and anti-microbial properties [172] |
| 2 | FTEW dorsal area—rats | Rutin-loaded zein gel | Promoted movement of cells towards the wound site, increased inflammation [173] |
| 3 | FTEW dorsal area—rats | Sodium thiosulfate gel | Increased fibroblast movement towards the wound bed, increased antioxidant enzymes [174] |
| 4 | FTEW dorsal area—mice | Gel containing P. russeliana extract | Low antioxidant effects, anti-inflammatory activity, promoted collagen formation, analgesic and pro-angiogenesis properties [175] |
| 5 | FTEW dorsal area—rats | Alginate hydrogel loaded with cerium oxide nanoparticles | Accelerated wound repair by reducing oxidative stress and inflammation, faster tissue recovery [169] |
| 6 | FTEW dorsal area—rats | Cream containing Astragalus floccosus extract | Accelerated wound healing by increased fibroblast proliferation, collagen synthesis, and re-epithelialization [176] |
| 7 | FTEW dorsal neck area—on Sprague Dawley rats | Sinomenine alkaloid solution extracted from Sinomenum Acutum | Faster wound closure with increased fibroblast proliferation and collagen deposition, antioxidant and anti-inflammatory effects [177] |
| 8 | FTEW (bilaterally punched dorsum wound) on C57BL/6 mice | mouse mesenchymal stem cells from hair follicles/dermal fibroblasts/growth factors | Reduced hypertrophic scarring and enhanced wound healing/combination therapy of MSCs, fibroblasts, and growth factors was the most effective [178] |
| 9 | FTEW (punched dorsum wound-6 mm) on rats | Sildenafil cream | Faster wound closure, reduced oxidative stress [179] |
| 10 | FTEW on rat and diabetic mouse, rat random flap, partial-thickness thermal injury on guinea pig | Systemic therapy with angiotensin (1–7) | Faster wound healing, increased cell proliferation without hypertensive effect, promoted regeneration, and flap survival [180] |
| 11 | SIW on rats and rabbits | Aloe ferox miller and Aloe Arborescens Miller whole leaf extracts | Faster healing, reduced inflammation, and antibacterial and antifungal activity with no skin toxicity [181] |
| 12 | FTEW dorsal area—BALB/c mice | Alchemilla vulgaris and Mimosa extract mixture | Rapid wound repair through improved skin re-epithelialization, higher cell proliferation, collagen synthesis, angiogenesis, and skin appendages [182] |
| 13 | FTEW dorsolateral flanks—Wistar rats | Ointment containing honey and Ageratum conyzoides leaf extract | Increased wound contraction, faster healing, and Ageratum extract reduced inflammation but increased fibrosis despite lower fibroblast count [183] |
| 14 | FTEW dorsal area—mice | chitosan patch with doxycycline/zinc/selenium nanoparticles | Anti-inflammatory and hemostatic effects, enhanced blood vessel formation [184] |
| 15 | Circular full-thickness skin excisions on a rat’s scalp | Chitosan membrane | Accelerated healing, anti-inflammatory effects by reduced leukocyte counts, and increased IL-4 levels [185] |
| 16 | FTEW dorsal area—diabetic Wistar rats | N-acetyl cysteine local and systemic | Reduced wound size and oxidative stress by both topical and systemic administration [186] |
| 17 | FTEW dorsal area—diabetic Wistar rats | Paeoniflorin-loaded hyaluronic acid hydrogel | Accelerated wound repair by anti-inflammatory effects mediated by modulation of macrophage populations (M1 towards M2 phenotype), enhanced angiogenesis, and collagen production [187] |
| 18 | FTEW dorsal area—nude mice | Heparinized adipose-derived scaffolds enriched with growth factors | Improved re-epithelialization, angiogenesis, and skin appendage regeneration through enhanced fibroblast migration and blood vessel growth [188] |
| 19 | FTEW dorsal area—mice | injection of mesenchymal Stem cell-derived extracellular vesicles/umbilical cord blood-derived extracellular vesicles in the wound margins | Enhanced wound healing by increased tissue growth and reduced scar formation [189] |
| 20 | FTEW dorsal area—rats | Neodymium–yttrium–aluminum garnet (Nd:YAG) pulsed high-intensity laser | Improved wound healing by increasing fibroblast proliferation, collagen synthesis, and thickness of the granular layer [190] |
| 21 | SI dorsal area—KO mice (double deletion of IL-10 and IL-4 genes) | Interleukin-10, local application | Improved wound healing, reduced inflammation, and enhanced scarring quality [191] |
| 22 | FTEW dorsal area—mice | Extracellular matrix/stromal vascular fraction gel conditioned medium | Faster wound closure, higher collagen deposition, increased growth factor secretion, and higher fibroblast and keratinocyte count and activity [192] |
| 23 | FTEW dorsal area—mice | Encapsulated Spirulina protein hydrolysates with nanoliposomes | Increased wound closure in mice by boosting fibroblast growth, skin regrowth, and enhanced markers of angiogenesis and collagen deposition [193] |
| 24 | FTEW dorsal area—mice | Silk protein-biomaterial wound dressings with epidermal growth factor and silver sulfadiazine | Better wound closure rate, less scarring, and collagen production [194] |
| 25 | FTEW dorsal area—C57 diabetic mice | Intermittent fasting and pulsed radiofrequency energy | Combined therapy exhibited antioxidant effects and induced faster wound healing by boosting cell migration, angiogenesis, and nerve growth [195] |
| 26 | Full-thickness SIW dorsal area—albino guinea pigs | Pulsed electrical stimulation | Both anodal and cathodal electrical stimulation boosted wound recovery in guinea pigs, improving closure and scar strength, regardless of polarity sequence [196] |
| Model Type | Characteristics | Advantages | Limitations | Typical Applications |
|---|---|---|---|---|
| 3D Tissue Constructs [152] | multiple cell layers in the ECM | better tissue architecture, more realistic | higher complexity, longer setup | advanced research, tissue engineering |
| Co-culture System (3D) [155,156] | different cell types embedded in or layered within a 3D matrix | mimics natural skin structure and function | technically demanding | ECM remodeling, inflammatory studies |
| Hydrogel-based Models [30,131,158] | cells in/around gels like collagen or Matrigel | ECM simulation, tunable stiffness | batch variability, limited duration | ECM interaction, scaffold testing |
| Organotypic Culture [158] | stratified epidermis + fibroblast-populated dermis | closest to in vivo skin | time-consuming, resource-intensive | preclinical drug testing, epidermal healing |
| Explant Culture [159,160] | full-thickness skin cultured ex vivo | preserves native structure | short-term viability, donor variability | re-epithelialization, tissue remodeling |
| Microfluidic Skin-on-a-Chip [156,158] | skin model in a microfluidic device with a controlled environment | real-time imaging, dynamic flow conditions | expensive, specialized equipment needed | high-resolution studies, mechanistic modeling |
| Bioprinted Skin Constructs [161] | layered printing of cells and ECM components | reproducible, customizable architecture | high cost, needs specialized skills | personalized modeling, drug/cosmetic testing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldea, I.; Grosu, I.G.; Ghafury, S.; Golat, C.; Doubali, D.; Vestemean, A.-M.; Cedorge, A.N.; Florian, I.; Yiannoulatos, M.; Wajahat, M.M.; et al. Biological Models for Evaluating Hydrogel-Based Formulations in Wound Healing. Gels 2025, 11, 705. https://doi.org/10.3390/gels11090705
Baldea I, Grosu IG, Ghafury S, Golat C, Doubali D, Vestemean A-M, Cedorge AN, Florian I, Yiannoulatos M, Wajahat MM, et al. Biological Models for Evaluating Hydrogel-Based Formulations in Wound Healing. Gels. 2025; 11(9):705. https://doi.org/10.3390/gels11090705
Chicago/Turabian StyleBaldea, Ioana, Ioana Georgeta Grosu, Sahar Ghafury, Cristian Golat, Doriane Doubali, Ana-Maria Vestemean, Aris Nicolas Cedorge, Ilinca Florian, Michael Yiannoulatos, Muhammad Mudassir Wajahat, and et al. 2025. "Biological Models for Evaluating Hydrogel-Based Formulations in Wound Healing" Gels 11, no. 9: 705. https://doi.org/10.3390/gels11090705
APA StyleBaldea, I., Grosu, I. G., Ghafury, S., Golat, C., Doubali, D., Vestemean, A.-M., Cedorge, A. N., Florian, I., Yiannoulatos, M., Wajahat, M. M., Silli, L. R., Stavrou, T., & Mitrea, D. R. (2025). Biological Models for Evaluating Hydrogel-Based Formulations in Wound Healing. Gels, 11(9), 705. https://doi.org/10.3390/gels11090705







