A Succinoglycan-Riclin-Zinc-Phthalocyanine-Based Composite Hydrogel with Enhanced Photosensitive and Antibacterial Activity Targeting Biofilms
Abstract
1. Introduction
2. Results and Discussion
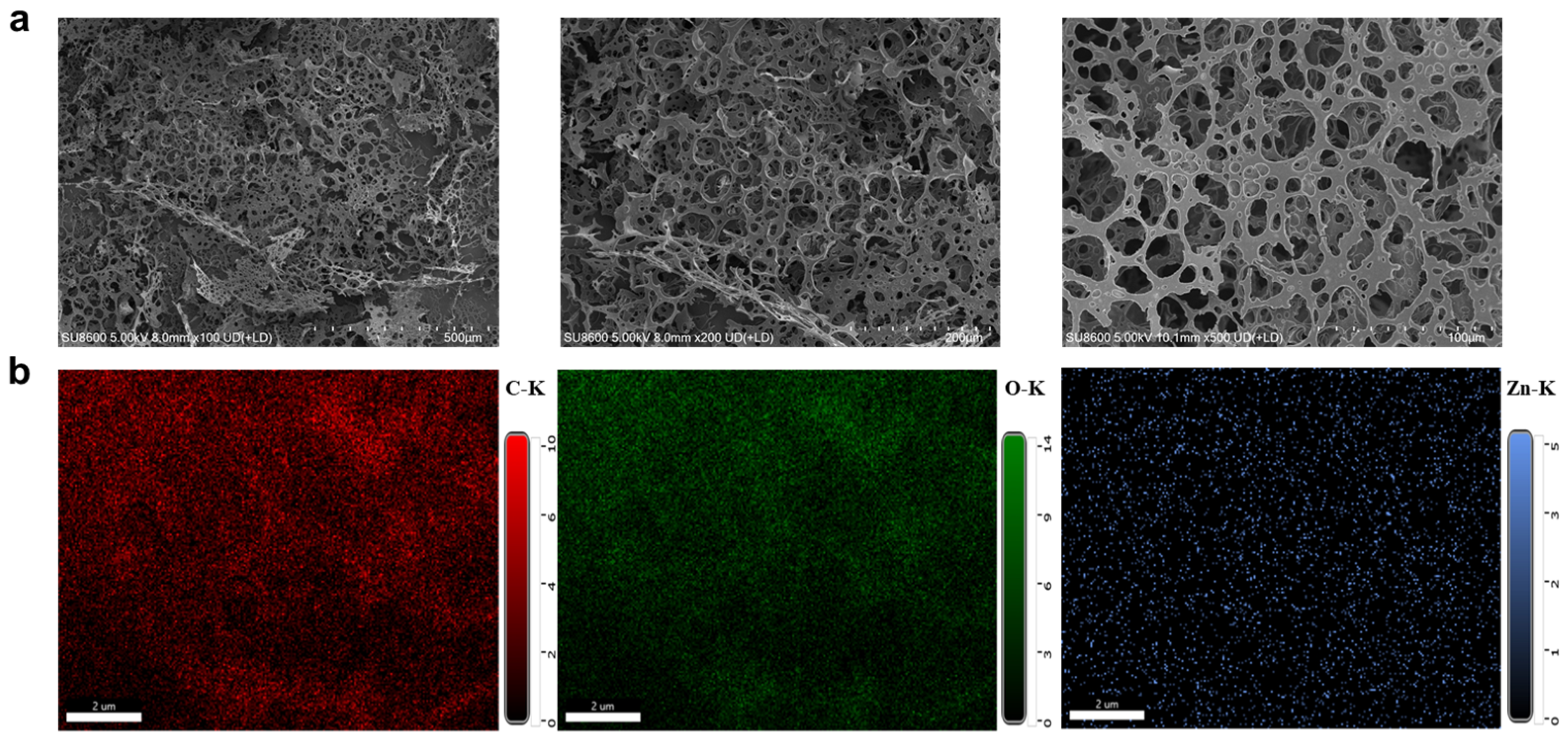
2.1. Synthesis and Characterization of RL-Zc Hydrogels
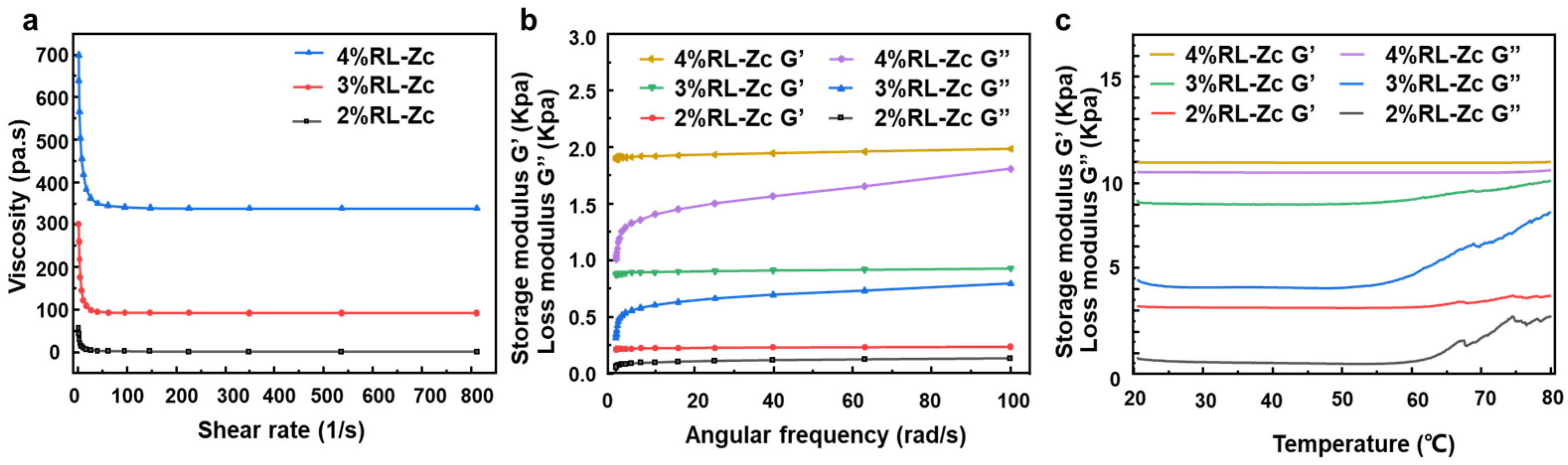
2.2. Rheology Performance of RL-Zc Hydrogels
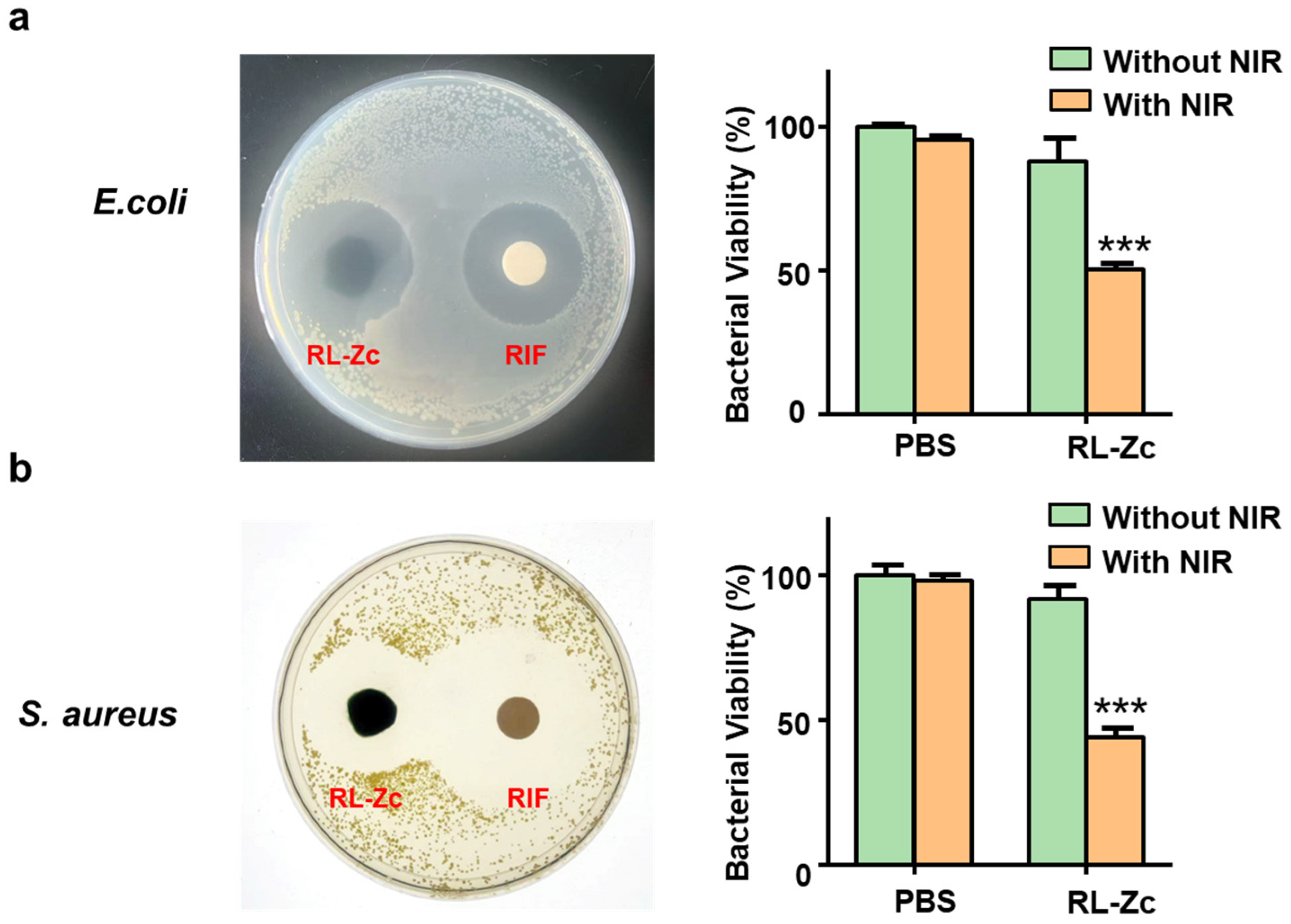
2.3. Antibacterial Activity of RL-Zc Hydrogels
2.4. Bacterial Disruption and Biofilm Penetration of RL-Zc Hydrogels
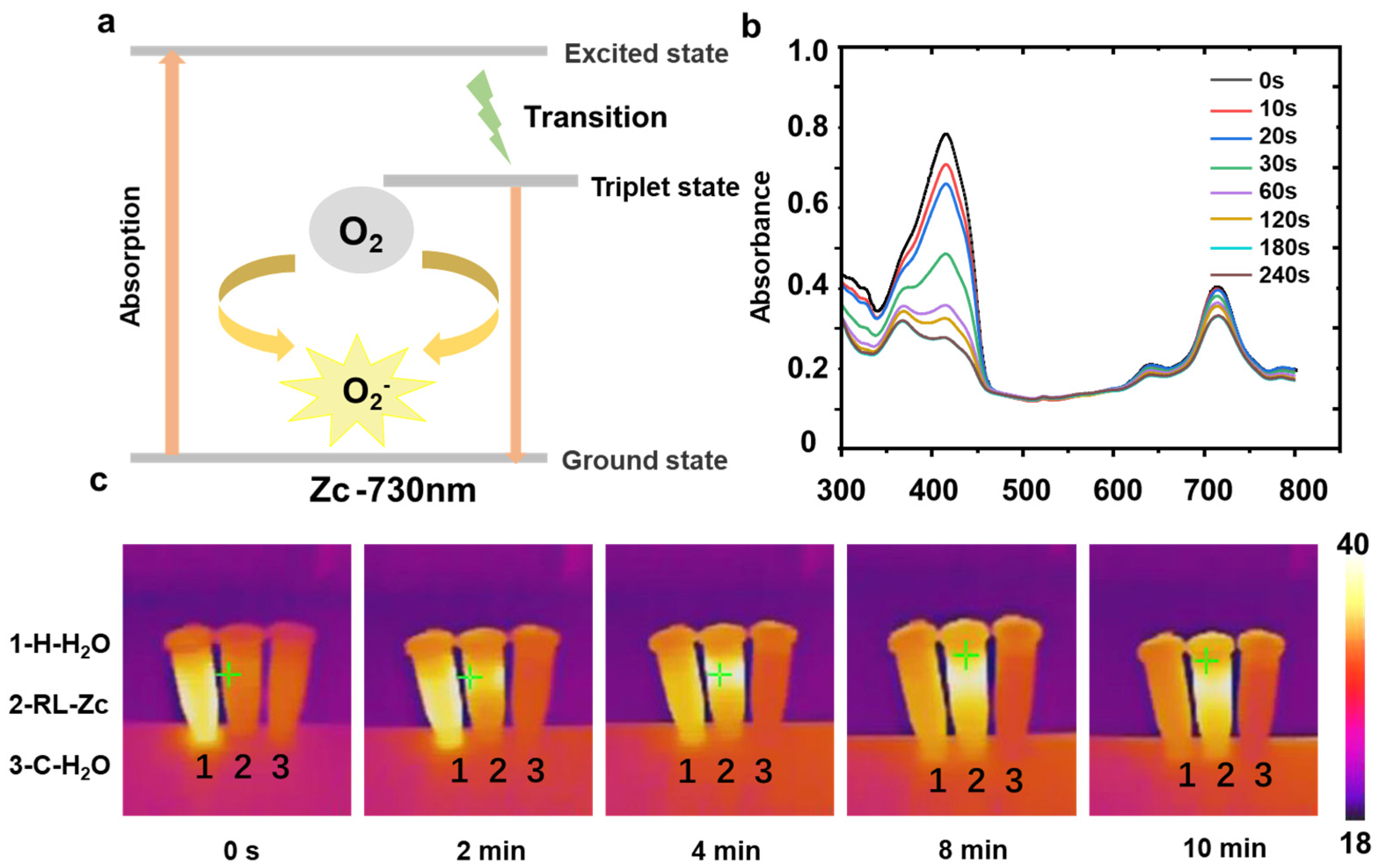
2.5. Photosensitive Property of RL-Zc Hydrogels
2.6. Metabolic Profiles of S. aureus Based on 1H NMR Technique
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Succinoglycan Riclin-Zinc Phthalocyanine (RL-Zc) Hydrogels
4.3. Fourier Transform Infrared Spectroscopy (FT-IR)
4.4. UV–Vis Spectroscopy
4.5. Scanning Electron Microscopy (SEM) Analysis
4.6. Zeta Potential Measurement
4.7. Rheology Analysis
4.8. Antibacterial Assay
4.8.1. Bacterial Culture
4.8.2. Bacterial Morphology
4.8.3. Bacterial Biofilm Assessment
4.8.4. PI Staining
4.9. Singlet Oxygen Generation
4.10. Photothermal Performance
4.11. 1H NMR-Based Metabolomics
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| RL | Riclin |
| Zc | Zinc phthalocyanine |
| PDT | Photodynamic therapy |
| PTT | Photothermal therapy |
| Pc | Phthalocyanine |
| PCA | Principal component analysis |
| PLS-DA | Partial least squares discriminant analysis |
| SEM | Scanning electron microscopy |
| FT-IR | Fourier transform infrared spectroscopy |
| NIR | Near-infrared irradiation |
| PI | Propidium iodide |
| RIF | Rifampicin |
References
- Sahayarayan, J.J.; Thiyagarajan, R.; Prathiviraj, R.; Tn, K.; Rajan, K.S.; Manivannan, P.; Balasubramanian, S.; Zainudin, M.H.M.; Alodaini, H.A.; Moubayed, N.M.; et al. Comparative genome analysis reveals putative and novel antimicrobial resistance genes common to the nosocomial infection pathogens. Microb. Pathog. 2024, 197, 107028. [Google Scholar] [CrossRef]
- Garg, S.S.; Dubey, R.; Sharma, S.; Vyas, A.; Gupta, J. Biological macromolecules-based nanoformulation in improving wound healing and bacterial biofilm-associated infection: A review. Int. J. Biol. Macromol. 2023, 247, 125636. [Google Scholar] [CrossRef]
- Barman, S.; Kurnaz, L.B.; Leighton, R.; Hossain, W.; Decho, A.W.; Tang, C. Intrinsic antimicrobial resistance: Molecular biomaterials to combat microbial biofilms and bacterial persisters. Biomaterials 2024, 311, 122690. [Google Scholar] [CrossRef]
- Jeyaraman, M.; Jayakumar, T.; Jeyaraman, N.; Nallakumarasamy, A.; Ramasubramanian, S.; Muthu, S.; Jain, V.K. Combating antimicrobial resistance in osteoarticular infections: Current strategies and future directions. J. Clin. Orthop. Trauma 2024, 58, 102791. [Google Scholar] [CrossRef]
- Lan, X.; Du, T.; Zhuo, J.; Wang, T.; Shu, R.; Li, Y.; Zhang, W.; Ji, Y.; Wang, Y.; Yue, X.; et al. Advances of biomacromolecule-based antibacterial hydrogels and their performance evaluation for wound healing: A review. Int. J. Biol. Macromol. 2024, 279, 135577. [Google Scholar] [CrossRef]
- Yu, J.; Liu, J.; Liu, Y.; Liu, S.; Su, Z.; Liang, D. Ag/TA@CNC Reinforced Hydrogel Dressing with Enhanced Adhesion and Antibacterial Activity. Gels 2025, 11, 591. [Google Scholar] [CrossRef]
- Gong, T.; Jiang, J.; Chen, C.; Lv, Y.; Cao, T.; Cao, P.; Zhan, Q. Temperature-responsive two-dimensional polydopamine hydrogel: Preparation, mechanisms, and applications in cancer treatment. Int. J. Biol. Macromol. 2024, 282, 136891. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Zhang, X.; Gao, J.; Yue, J.; Zhang, L.; Pan, G. Dual peptide-coordinated dynamic hydrogel with antibacterial and proangiogenic activities for infected skin wounds. Int. J. Biol. Macromol. 2025, 285, 138349. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Y.; Hu, S.; Chang, X.; Ding, Q.; Wang, K.; Chen, Y.; Zheng, J. Peroxidase-like copper-doped carbon-dots embedded in hydrogels for stimuli-responsive bacterial biofilm elimination and wound healing. Acta Biomater. 2025, 195, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.; Grenho, L.; Fernandes, M.H.; Lima, S.A.C. Photoactivated Curcumin-Loaded Lipid Nanoparticles in Hydrogel: A Cutting-Edge Intracanal Medicament for Advanced Endodontic Therapy. Gels 2025, 11, 308. [Google Scholar] [CrossRef]
- Zhu, J.; Cheng, H.; Zhang, Z.; Chen, K.; Zhang, Q.; Zhang, C.; Gao, W.; Zheng, Y. Antibacterial Hydrogels for Wound Dressing Applications: Current Status, Progress, Challenges, and Trends. Gels 2024, 10, 495. [Google Scholar] [CrossRef] [PubMed]
- Soleymani Eil Bakhtiari, S.; Bakhsheshi-Rad, H.R.; Karbasi, S.; Razzaghi, M.; Tavakoli, M.; Ismail, A.F.; Sharif, S.; RamaKrishna, S.; Chen, X.; Berto, F. 3-Dimensional Printing of Hydrogel-Based Nanocomposites: A Comprehensive Review on the Technology Description, Properties, and Applications. Adv. Eng. Mater. 2021, 23, 2100477. [Google Scholar] [CrossRef]
- Su, W.; Zhang, Y.; Chen, Y.; Huo, L.; Wen, F.; Cai, J.; Gong, Q.; Li, P. Intelligent response agarose/chitosan hydrogel wound dressing: Synergistic chemotherapy, PDT and PTT effects against bacterial infection. Int. J. Biol. Macromol. 2025, 298, 139927. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, M.; Ziental, D.; Biyiklioglu, Z.; Jozkowiak, M.; Baş, H.; Dlugaszewska, J.; Piotrowska-Kempisty, H.; Güzel, E.; Sobotta, L. Non-peripheral octasubstituted zinc(II) phthalocyanines bearing pyridinepropoxy substituents—Antibacterial, anticancer photodynamic and sonodynamic activity. J. Inorg. Biochem. 2025, 262, 112751. [Google Scholar] [CrossRef]
- Vohra, M.; Kour, A.; Kalia, N.P.; Kumar, M.; Sharma, S.; Jaglan, S.; Kamath, N.; Sharma, S. A comprehensive review of genomics, transcriptomics, proteomics, and metabolomic insights into the differentiation of Pseudomonas aeruginosa from the planktonic to biofilm state: A multi-omics approach. Int. J. Biol. Macromol. 2024, 257, 128563. [Google Scholar] [CrossRef]
- Wang, X.; Ding, J.; Chen, X.; Wang, S.; Chen, Z.; Chen, Y.; Zhang, G.; Liu, J.; Shi, T.; Song, J.; et al. Light-activated nanoclusters with tunable ROS for wound infection treatment. Bioact. Mater. 2024, 41, 385–399. [Google Scholar] [CrossRef]
- Chen, S.; Cheng, R.; Xu, X.; Kong, C.; Wang, L.; Fu, R.; Li, J.; Wang, S.; Zhang, J. The structure and flocculation characteristics of a novel exopolysaccharide from a Paenibacillus isolate. Carbohydr. Polym. 2022, 291, 119561. [Google Scholar] [CrossRef]
- Kim, K.; Jeong, J.-P.; Park, S.; Park, S.-I.; Jung, S. Enhanced physicochemical, rheological and antioxidant properties of highly succinylated succinoglycan exopolysaccharides obtained through succinic anhydride esterification reaction. Int. J. Biol. Macromol. 2025, 298, 140007. [Google Scholar] [CrossRef]
- Bayat, F.; Karimi, A.R.; Adimi, T. Design of nanostructure chitosan hydrogels for carrying zinc phthalocyanine as a photosensitizer and difloxacin as an antibacterial agent. Int. J. Biol. Macromol. 2020, 159, 598–606. [Google Scholar] [CrossRef]
- Yang, Y.; Zhuo, Y.; Zhu, C.; Zhang, H.; Wang, Y. Characterization of gelatin-oxidized riclin cryogels and their applications as reusable ice cubes in shrimp preservation. Food Res. Int. 2024, 192, 114766. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.P.; Kim, K.; Yoon, I.; Jang, S.; Jung, S. Multifunctional biodegradable films of caffeic acid–grafted succinoglycan and polyvinyl alcohol with enhanced antioxidant, antibacterial, and UV-shielding properties. Int. J. Biol. Macromol. 2025, 321, 146327. [Google Scholar] [CrossRef]
- Li, L.; Wang, S.; Chen, Y.; Dong, S.; Zhang, C.; Liao, L.; Zhang, W. Hydrogels mimicking the viscoelasticity of extracellular matrix for regenerative medicine: Design, application, and molecular mechanism. Chem. Eng. J. 2024, 498, 155206. [Google Scholar] [CrossRef]
- Jin, Y.; Zhao, B.; Guo, W.; Li, Y.; Min, J.; Miao, W. Penetration and photodynamic ablation of drug-resistant biofilm by cationic Iron oxide nanoparticles. J. Control. Release 2022, 348, 911–923. [Google Scholar] [CrossRef]
- Yao, W.; Wang, T.; Sun, W.; Zhang, X.; Xiong, H.; Yin, J.; Liu, L.; Liu, X.; Wang, X.; Jiang, H. Mature biofilm-sensitive lysozyme-grafted Bi-guanidine backbone porphyrin nanorods for deep penetration and double phototherapy. Biomaterials 2025, 323, 123431. [Google Scholar] [CrossRef] [PubMed]
- Hafezi, M.; Khorasani, S.N.; Khalili, S.; Sattari-Najafabadi, M.; Najarzadegan, M.; Neisiany, R.E. Shear thinning and self-healing behavior of an IPN nanocomposite based on gelatin methacryloyl/alginate/nano-clay for cartilage tissue engineering application. Eur. Polym. J. 2025, 226, 113761. [Google Scholar] [CrossRef]
- Choe, D.; Kim, Y.M.; Nam, J.E.; Nam, K.; Shin, C.S.; Roh, Y.H. Synthesis of high-strength microcrystalline cellulose hydrogel by viscosity adjustment. Carbohydr. Polym. 2018, 180, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Sheng, R.; Zhang, W.; Chen, J. Strong and tough hydrogels fabricated through molecular and structural engineering and their biomedical applications. Chem. Eng. J. 2025, 508, 160728. [Google Scholar] [CrossRef]
- Kim, S.; Jeong, D.; Lee, H.; Kim, D.; Jung, S. Succinoglycan dialdehyde-reinforced gelatin hydrogels with toughness and thermal stability. Int. J. Biol. Macromol. 2020, 149, 281–289. [Google Scholar] [CrossRef]
- Jiang, E.; He, C.; Xiao, X.; Dong, Y.; Gao, Y.; Chen, Z.; Wu, Y.; Song, W. The effects of α- and β-amino substituents of phthalocyanine on photophysical and nonlinear optical properties of graphene oxide-tetraamino zinc(II) phthalocyanine hybrids. Opt. Mater. 2017, 64, 193–202. [Google Scholar] [CrossRef]
- Repetowski, P.; Warszyńska, M.; Dąbrowski, J.M. NIR-activated multifunctional agents for the combined application in cancer imaging and therapy. Adv. Colloid Interface Sci. 2025, 336, 103356. [Google Scholar] [CrossRef]
- Ma, C.; Du, L.; Guo, Y.; Yang, X. A review of polysaccharide hydrogels as materials for skin repair and wound dressing: Construction, functionalization and challenges. Int. J. Biol. Macromol. 2024, 280, 135838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Lu, S.; Zhang, S.; Wang, R.; Gan, D.; Liu, P.; Shen, J. Bacterial microenvironment-responsive antibacterial, adhesive, and injectable oxidized dextran-based hydrogel for chronic diabetic wound healing. Int. J. Biol. Macromol. 2025, 309, 143095. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.-D.; Li, S.-L.; Huang, Z.-L.; Zhang, L.; Liu, H.; Zheng, B.-Y.; Ke, M.-R.; Huang, J.-D. A non-aggregated zinc(II) phthalocyanine with hexadeca cations for antitumor and antibacterial photodynamic therapies. J. Photochem. Photobiol. B 2020, 213, 112086. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Chen, S.; Ge, W.; Zhao, Y.; Xu, X.; Wang, S.; Zhang, J. Riclin-Capped Silver Nanoparticles as an Antibacterial and Anti-Inflammatory Wound Dressing. Int. J. Nanomed. 2022, 17, 2629–2641. [Google Scholar] [CrossRef]
- Carrasco-Rojas, J.; Sandoval, F.I.; Schuh, C.M.A.P.; Lagos, C.F.; Morales, J.O.; Arriagada, F.; Ortiz, A.C. NLC-Based Rifampicin Delivery System: Development and Characterization for Improved Drug Performance Against Staphylococcus aureus. Pharmaceutics 2025, 17, 799. [Google Scholar] [CrossRef]
- Yalazan, H.; Ömeroğlu, I. Determination of photophysical and photochemical features of novel peripheral and non-peripheral zinc(II) phthalocyanines containing Schiff base for photodynamic therapy. J. Mol. Struct. 2025, 1348, 143443. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, X.; Wang, H.; Zhang, P. Structure-property relationships of main-group element phthalocyanines in photo/sono-driven cancer therapy. Coord. Chem. Rev. 2025, 544, 216969. [Google Scholar] [CrossRef]
- Wu, S.; Huang, Y.; Yan, J.; Li, Y.; Wang, J.; Yang, Y.Y.; Yuan, P.; Ding, X. Bacterial Outer Membrane-Coated Mesoporous Silica Nanoparticles for Targeted Delivery of Antibiotic Rifampicin against Gram-Negative Bacterial Infection In Vivo. Adv. Funct. Mater. 2021, 31, 2103442. [Google Scholar] [CrossRef]
- Du, Y.; Chu, G.; Yu, R.; Cui, R.; Wang, Y.; Mai, Y.; Guan, M.; Xu, F.; Zhou, Y. Hyperbranched polyphthalocyanine micelles with dual PTT/PDT functions for bacteria eradication under an NIR window. Chem. Commun. 2023, 59, 14169–14172. [Google Scholar] [CrossRef]
- Mandal, S.; Hazra, C.; Joshi, N.; Kumar, P.; Sen, R.; Das, S.; Das, K. Development and performance evaluation of chitosan hydrogel based ZnO/CuO antibacterial and antialgal nanocoating for the control of biofouling in cultural heritage stones. Constr. Build. Mater. 2025, 489, 142236. [Google Scholar] [CrossRef]
- Zhao, G.; Usui, M.L.; Lippman, S.I.; James, G.A.; Stewart, P.S.; Fleckman, P.; Olerud, J.E. Biofilms and Inflammation in Chronic Wounds. Adv. Wound Care 2013, 2, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.-L.; Cheng, K.; Li, Y.; Zhong, Z.-T.; Hou, X.-L.; Song, L.-B.; Zhang, F.; Wang, J.-H.; Zhao, Y.-D.; Xu, Q.-R. The eradication of biofilm for therapy of bacterial infected chronic wound based on pH-responsive micelle of antimicrobial peptide derived biodegradable microneedle patch. Chem. Eng. J. 2023, 462, 142222. [Google Scholar] [CrossRef]
- Wang, W.; Ping, Y.; Zhang, L.; Feng, J.; Zhang, W.; Sun, J.; Wang, J. Visible light-trigged photodynamic antibacterial film with mild temperature enhancement for long-term preservation of perishable products. Chem. Eng. J. 2025, 503, 158457. [Google Scholar] [CrossRef]
- Novikova, I.N.; Potapova, E.V.; Dremin, V.V.; Dunaev, A.V.; Abramov, A.Y. Laser-induced singlet oxygen selectively triggers oscillatory mitochondrial permeability transition and apoptosis in melanoma cell lines. Life Sci. 2022, 304, 120720. [Google Scholar] [CrossRef]
- Cabral, F.V.; Xu, Q.; Greer, A.; Lyons, A.M.; Hasan, T. Superhydrophobic Dressing for Singlet Oxygen Delivery in Antimicrobial Photodynamic Therapy against Multidrug-Resistant Bacterial Biofilms. ACS Appl. Bio. Mater. 2024, 7, 6175–6185. [Google Scholar] [CrossRef]
- Sarker, S.R.; Yamazaki, T.; Sou, K.; Takemura, I.; Kurita, Y.; Nomura, K.; Ichimura, M.; Suzuki, T.; Kai, A.; Araki, T.; et al. Harnessing Dye-Induced Local Heating in Lipid Membranes: A Path to Near-Infrared Light-Modulated Artificial Synaptic Vesicles. ACS Nano 2025, 19, 28768–28783. [Google Scholar] [CrossRef]
- Misba, L.; Zaidi, S.; Khan, A.U. Efficacy of photodynamic therapy against Streptococcus mutans biofilm: Role of singlet oxygen. J. Photochem. Photobiol. B 2018, 183, 16–21. [Google Scholar] [CrossRef]
- Xu, F.; Cui, T.; Long, Z.; Shen, H.; Xie, H.; Liu, H.; Xie, J.; Yang, K.; Tao, Y. Multifunctional Silk Fibroin Nanocarriers Loaded with Chlorin e6 for PDT/PTT Synergistic Elimination of Drug-Resistant Bacteria and Biofilms. ACS Infect. Dis. 2025, 11, 1707–1718. [Google Scholar] [CrossRef]
- Huang, X.; Li, C.; Zhang, C.; Xiang, Y.; Yu, Z.; Li, P.; Bai, B.; Wen, Z.; Hou, T. Repurposing Etalocib suppresses multidrug-resistant Staphylococcus aureus by disrupting the bacterial membrane. BMC Microbiol. 2025, 25, 472. [Google Scholar] [CrossRef]
- Wang, X.-N.; Liu, J.-Q.; Ji, W.-L.; Huo, Z.-L.; Liu, L.-F.; Zheng, J.-Y. Characterization of trimethylamine metabolic pathways using pseudo-targeted metabolomics. J. Pharm. Biomed. Anal. 2025, 258, 116737. [Google Scholar] [CrossRef]
- Ho, K.-L.; Chung, Y.-C.; Lin, Y.-H.; Tseng, C.-P. Biofiltration of trimethylamine, dimethylamine, and methylamine by immobilized Paracoccus sp. CP2 and Arthrobacter sp. CP1. Chemosphere 2008, 72, 250–256. [Google Scholar] [CrossRef]
- Pereira, A.R.; Gomes, I.B.; Simões, M. Choline-based ionic liquids for planktonic and biofilm growth control of Bacillus cereus and Pseudomonas fluorescens. J. Mol. Liq. 2022, 346, 117077. [Google Scholar] [CrossRef]
- Alirezaei, M.; Khoshdel, Z.; Dezfoulian, O.; Rashidipour, M.; Taghadosi, V. Beneficial antioxidant properties of betaine against oxidative stress mediated by levodopa/benserazide in the brain of rats. J. Physiol. Sci. 2015, 65, 243–252. [Google Scholar] [CrossRef]
- Petrosyan, A.; Ghazaryan, V.; Giester, G.; Fleck, M.; Tylczyński, Z.; Wiesner, M. Halogenides of dimethylglycine in comparison with respective salts of glycine, sarcosine and betaine. J. Mol. Struct. 2018, 1158, 106–121. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, W.; Sun, J.; Ma, Q. Metabolomics revealed the antibacterial mechanism of carnosic acid against Shewanella putrefaciens. Food Biosci. 2025, 68, 106767. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, X.; Zhao, Y.; Ge, W.; Ding, Z.; Liu, J.; Wang, L.; Xu, X.; Zhang, J. Anti-tumor activity and immunogenicity of a succinoglycan riclin. Carbohydr. Polym. 2021, 255, 117370. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Shi, H.; Ma, K.; Shen, H.; Li, B.; Shen, S.; Wang, X.; Jin, Y. Hybrid nanoparticles for drug delivery and bioimaging: Mesoporous silica nanoparticles functionalized with carboxyl groups and a near-infrared fluorescent dye. J. Colloid Interface Sci. 2013, 395, 306–314. [Google Scholar] [CrossRef]
- Han, Z.-R.; Wang, Y.-F.; Liu, X.; Wu, J.-D.; Cao, H.; Zhao, X.; Chai, W.-G.; Yu, G.-L. Fluorescent Labeling of Several Glycosaminoglycans and Their Interaction with Anti-Chondroitin Sulfate Antibody. Chin. J. Anal. Chem. 2011, 39, 1352–1357. [Google Scholar] [CrossRef]



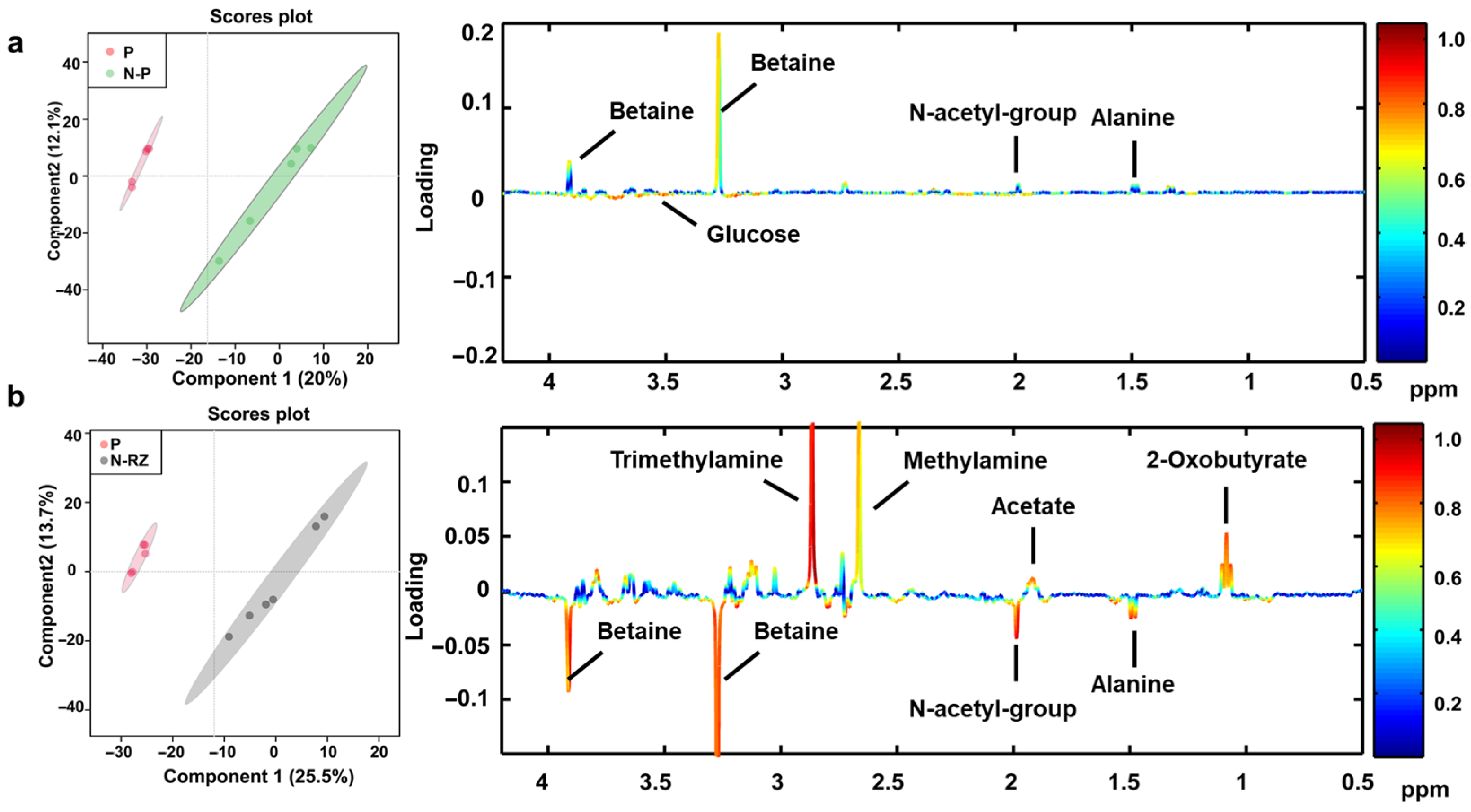
| No. | Metabolite | Assignments | N-P/P | N-RL/P | ||
|---|---|---|---|---|---|---|
| FC 1 | p-Value 2 | FC | p-Value | |||
| 1 | Isoleucine | δ-CH3, γ-CH3, α-CH | 1.33 | 0.32 | ** | |
| 2 | Leucine | δ-CH3, δ-CH3, α-CH | 1.24 | 0.58 | * | |
| 3 | Valine | γ-CH3, γ-CH3, α-CH | 1.24 | 0.21 | *** | |
| 4 | 2-Oxobutyrate | CH3, CH2 | 1.4 | 14.8 | ** | |
| 5 | Lactate | CH3 | 1.32 | 1.01 | ||
| 6 | Alanine | CH3, CH | 1.37 | * | 0.52 | *** |
| 7 | Lysine | δ-CH2, β-CH2, ε-CH2, α-CH | 0.95 | 1.15 | ||
| 8 | Acetate | CH3 | 1.03 | 3.45 | ** | |
| 9 | N-Acetyl group | CH3, CH2 | 1.37 | * | 0.38 | *** |
| 10 | Glutamate | β-CH2, γ-CH2, α-CH | 1.05 | 1.12 | ||
| 11 | Glutamine | βCH2, γCH2, αCH | 0.99 | 0.46 | ** | |
| 12 | Succinate | CH2 | 1.18 | 0.86 | ||
| 13 | Methylamine | CH3 | 0.71 | 18.11 | * | |
| 14 | Sarcosine | CH3 | 1.35 | * | 0.59 | ** |
| 15 | Dimethylamine | CH3 | 1.3 | 3.71 | ** | |
| 16 | Trimethylamine | CH3 | 1.12 | 17.73 | *** | |
| 17 | Creatine | CH3, CH2 | 1.21 | 1.89 | * | |
| 18 | Choline | CH3 | 0.6 | 3.01 | ** | |
| 19 | Betaine | CH2, CH3 | 1.37 | * | 0.65 | ** |
| 20 | Myo-inositol | CH3 | 1.15 | 1.42 | * | |
| 21 | Glycine | CH2 | 1.13 | 1.53 | ||
| 22 | Glucose | CH, CH, CH | 0.85 | ** | 1.17 | * |
| 23 | Phenylalanine | CH=CH | 1.23 | 1.3 | ||
| 24 | Formate | CH | 1.06 | 1.72 | ||
 . 2 p-Values were calculated based on a parametric Student’s t-test (dependent on the conformity to the normal distribution). * p < 0.05, ** p < 0.01, *** p < 0.001.
. 2 p-Values were calculated based on a parametric Student’s t-test (dependent on the conformity to the normal distribution). * p < 0.05, ** p < 0.01, *** p < 0.001.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zhang, H.; Zhang, X.; Shen, S.; Wu, B.; Peng, D.; Yin, J.; Wang, Y. A Succinoglycan-Riclin-Zinc-Phthalocyanine-Based Composite Hydrogel with Enhanced Photosensitive and Antibacterial Activity Targeting Biofilms. Gels 2025, 11, 672. https://doi.org/10.3390/gels11080672
Yang Y, Zhang H, Zhang X, Shen S, Wu B, Peng D, Yin J, Wang Y. A Succinoglycan-Riclin-Zinc-Phthalocyanine-Based Composite Hydrogel with Enhanced Photosensitive and Antibacterial Activity Targeting Biofilms. Gels. 2025; 11(8):672. https://doi.org/10.3390/gels11080672
Chicago/Turabian StyleYang, Yunxia, Hongmei Zhang, Xueqing Zhang, Shuyan Shen, Baojuan Wu, Dexin Peng, Jie Yin, and Yanqing Wang. 2025. "A Succinoglycan-Riclin-Zinc-Phthalocyanine-Based Composite Hydrogel with Enhanced Photosensitive and Antibacterial Activity Targeting Biofilms" Gels 11, no. 8: 672. https://doi.org/10.3390/gels11080672
APA StyleYang, Y., Zhang, H., Zhang, X., Shen, S., Wu, B., Peng, D., Yin, J., & Wang, Y. (2025). A Succinoglycan-Riclin-Zinc-Phthalocyanine-Based Composite Hydrogel with Enhanced Photosensitive and Antibacterial Activity Targeting Biofilms. Gels, 11(8), 672. https://doi.org/10.3390/gels11080672





