Evaluation and Experiment of High-Strength Temperature- and Salt-Resistant Gel System
Abstract
1. Introduction
2. Results and Discussion
2.1. Analysis and Evaluation of Gel System
2.1.1. Two-Factor Interaction in the Response Surface
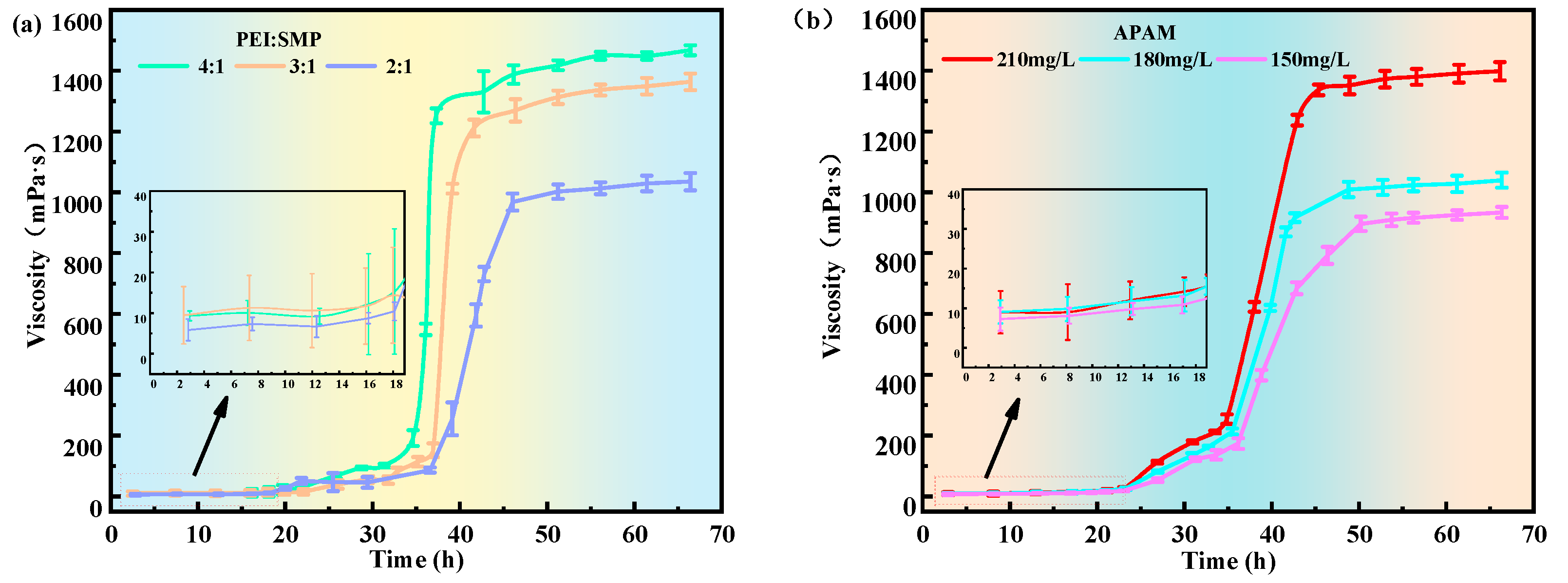
2.1.2. Time–Viscosity Relationship Fitting
2.2. Test and Characterization of Gel System
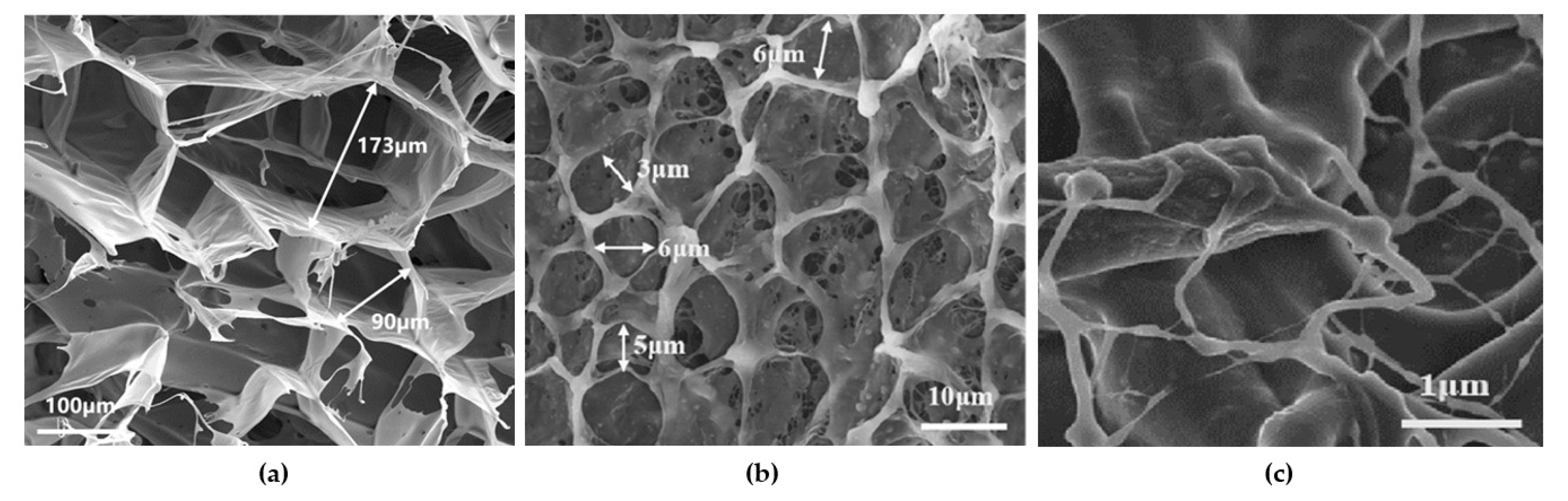
2.2.1. SEM Electron Microscope Image Analysis
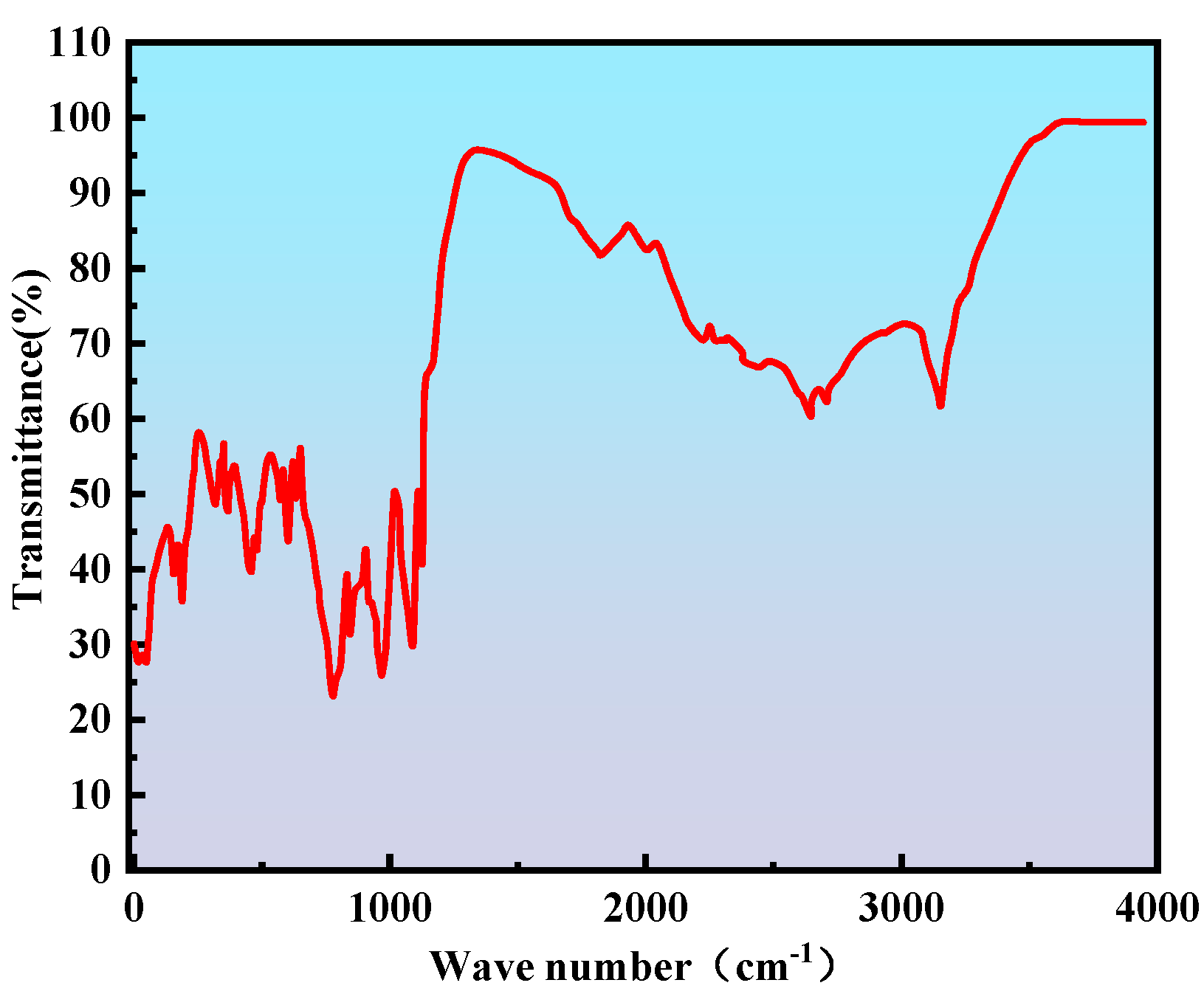
2.2.2. Infrared Spectroscopy Analysis
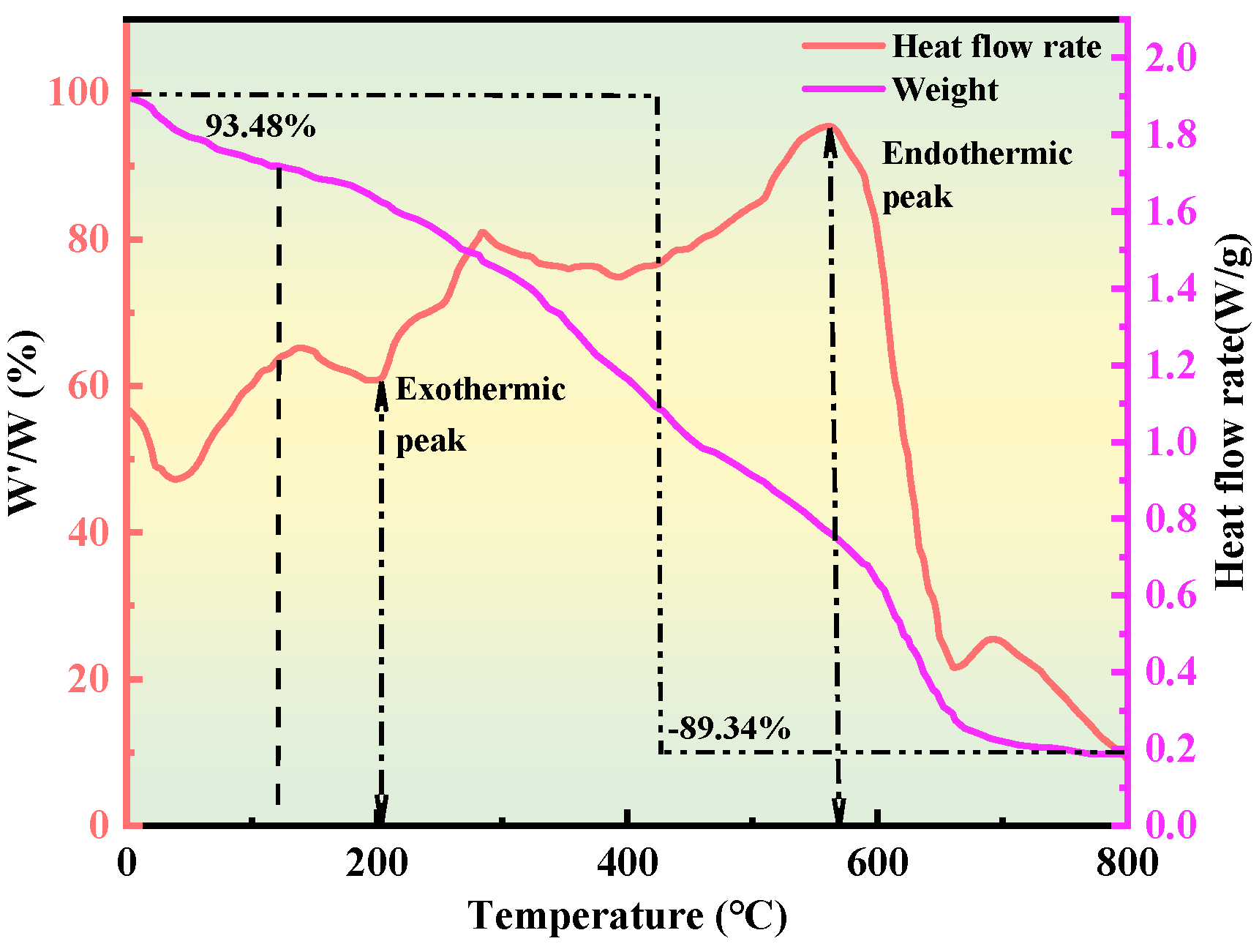
2.2.3. Thermogravimetric Analysis
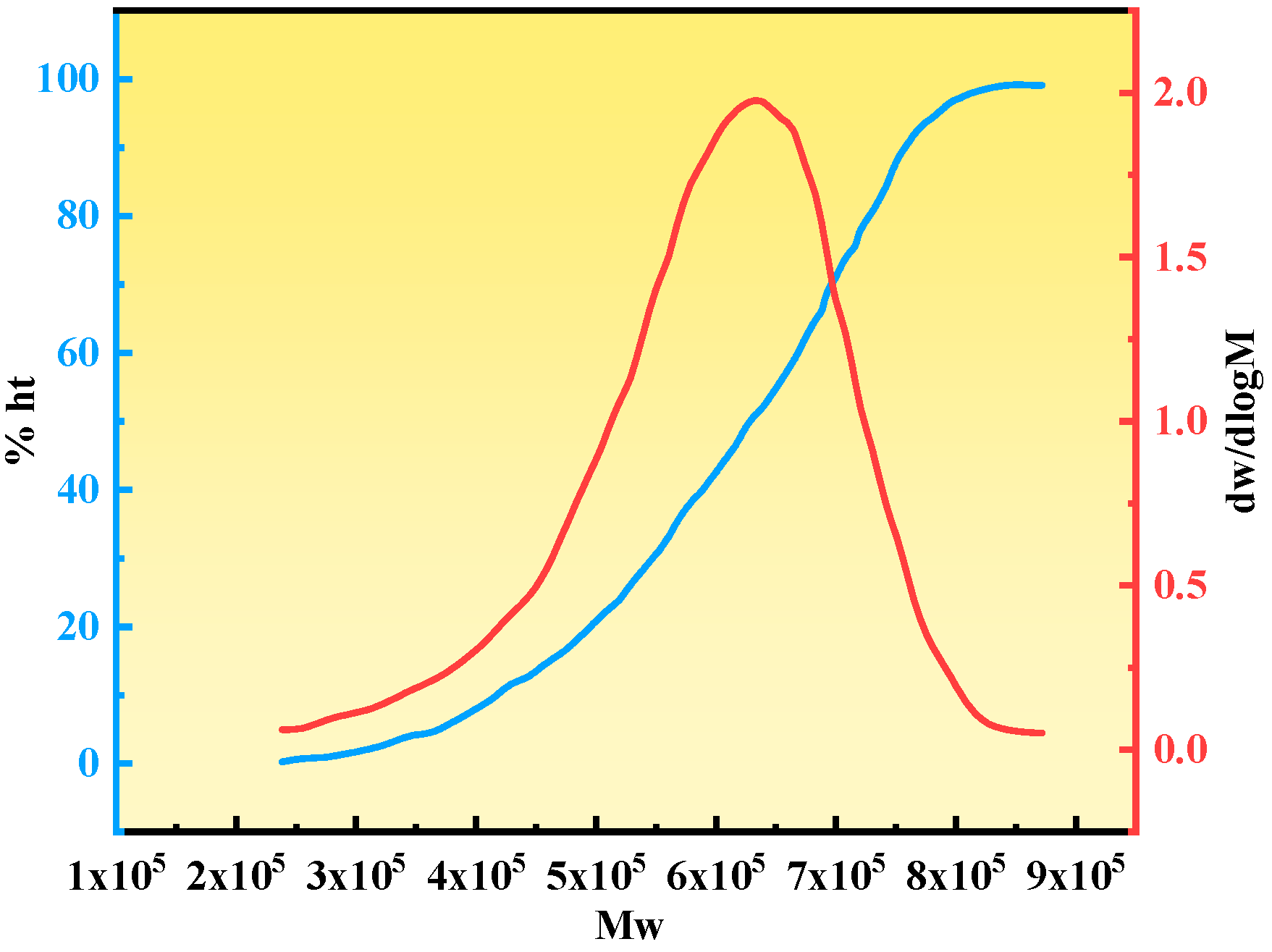
2.2.4. Molecular Weight Analysis
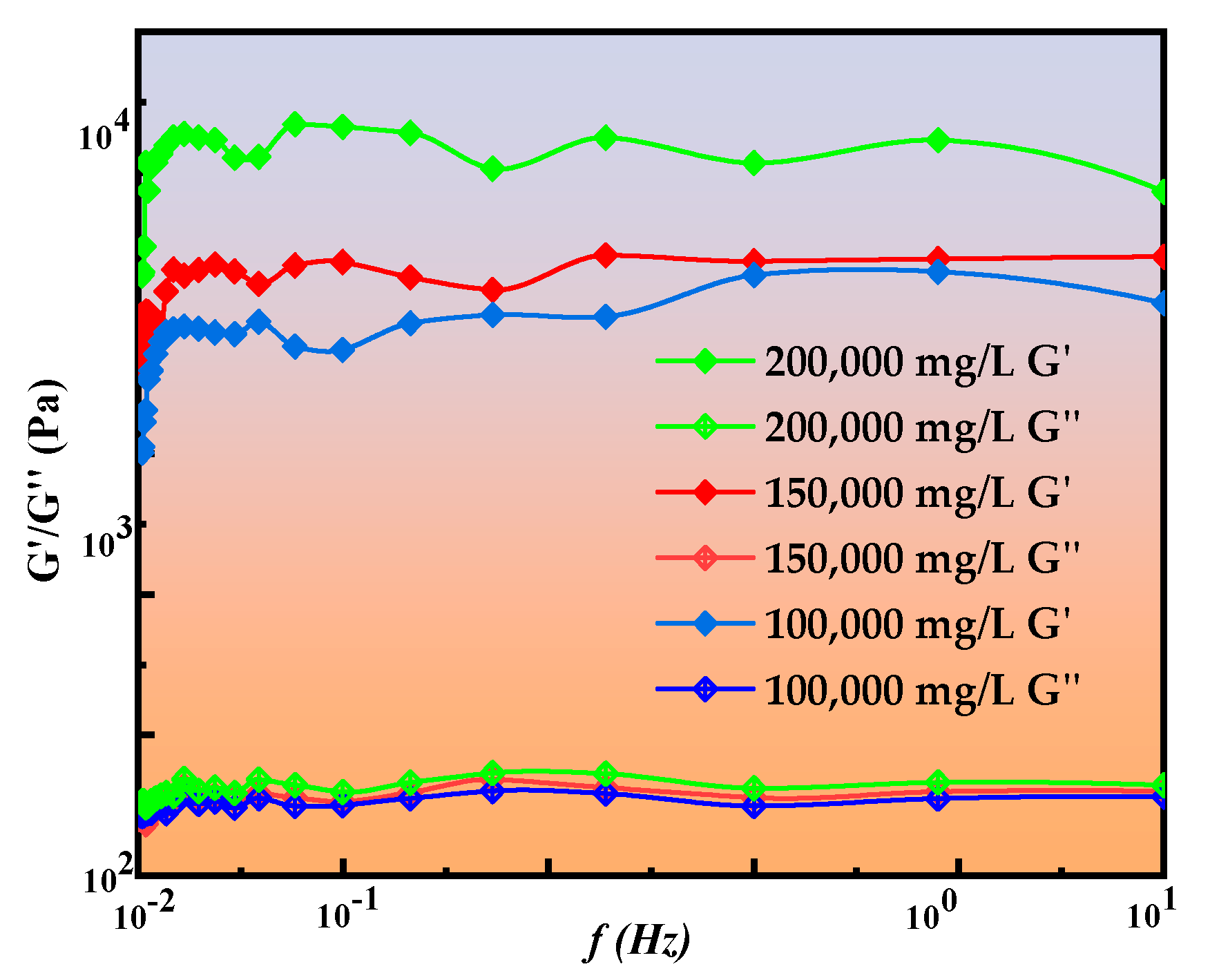
2.2.5. Rheological Curve Analysis
2.2.6. Nuclear Magnetic Resonance Curve Analysis
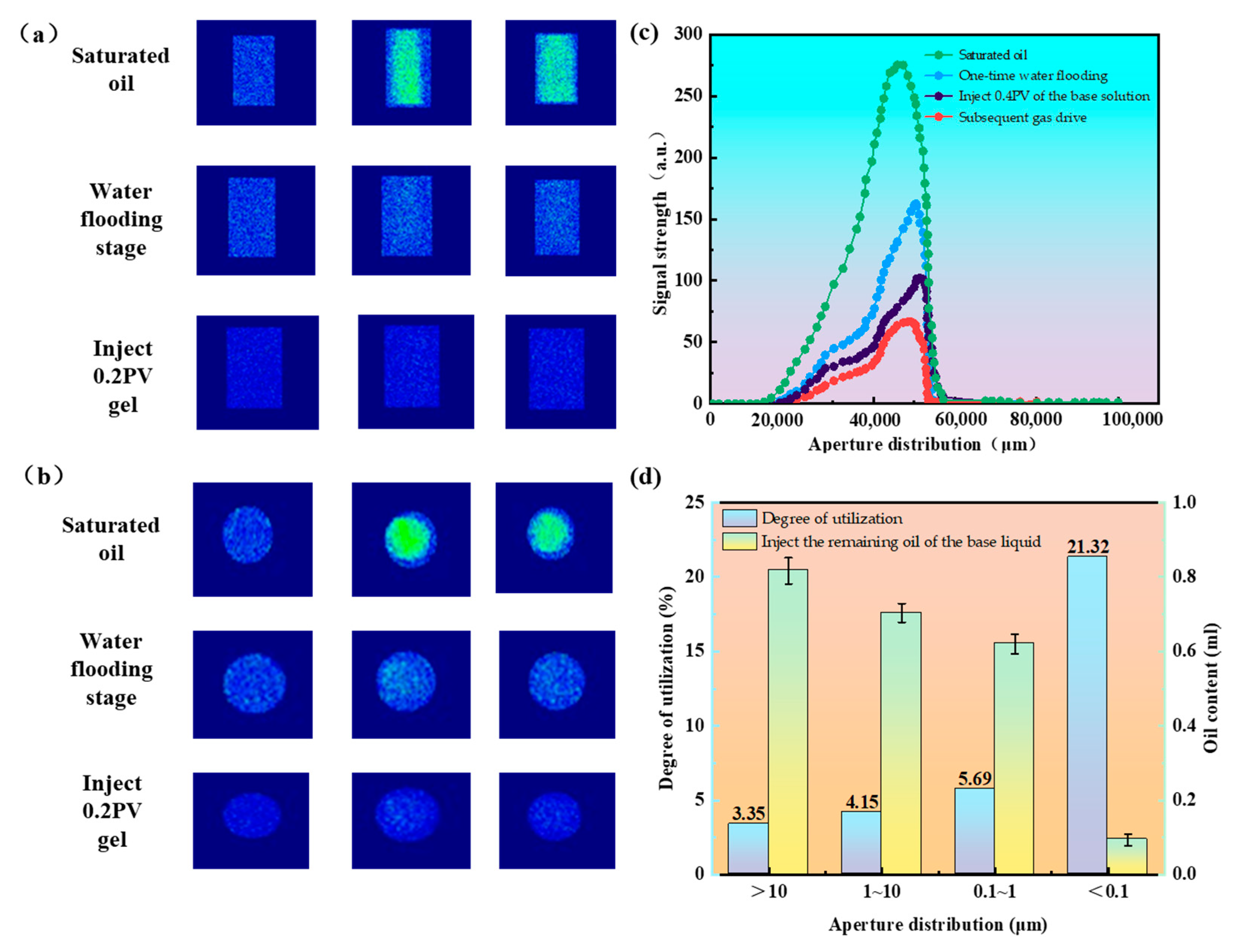
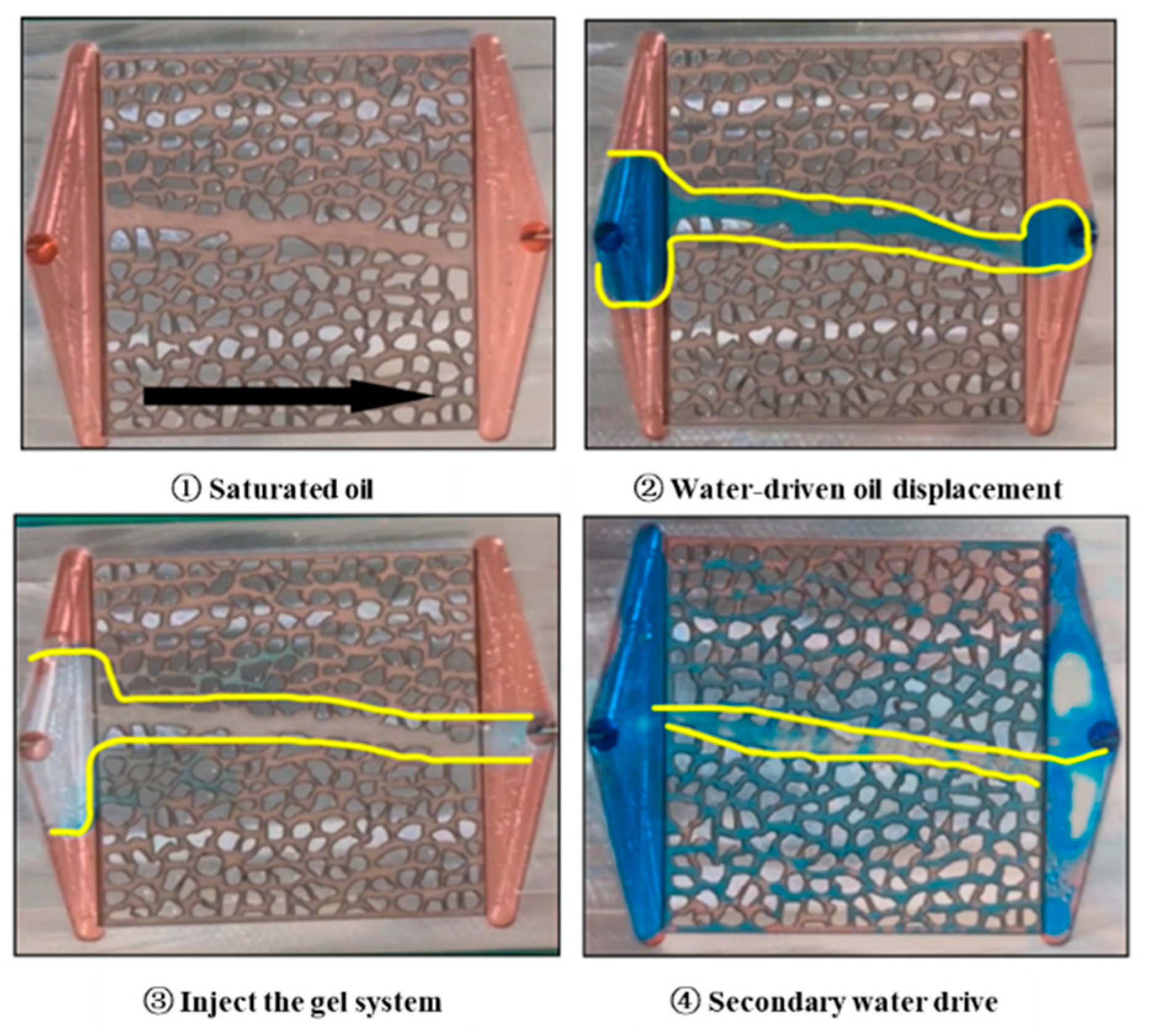
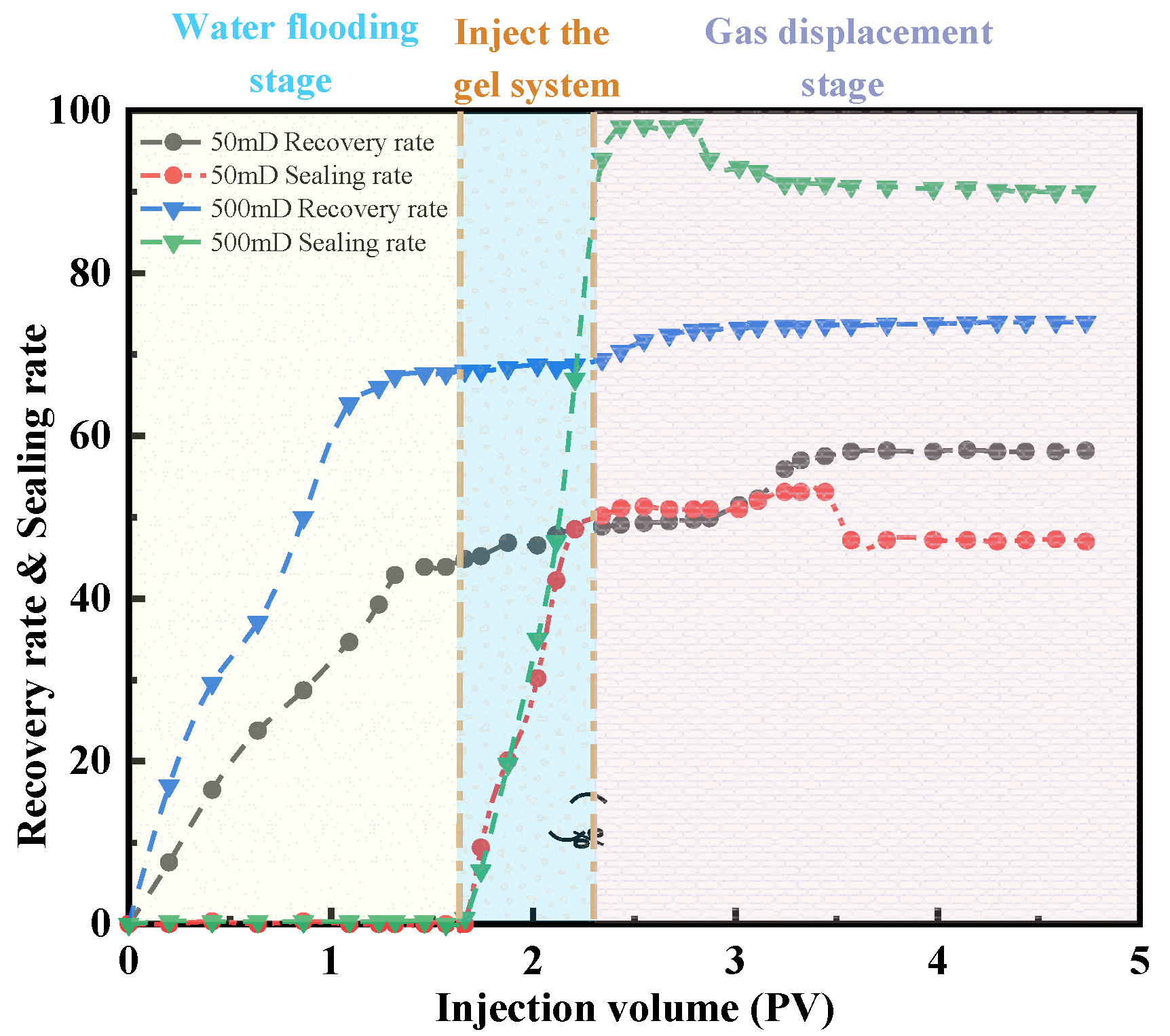
2.3. Analysis of Core Oil Displacement Experimental Curves
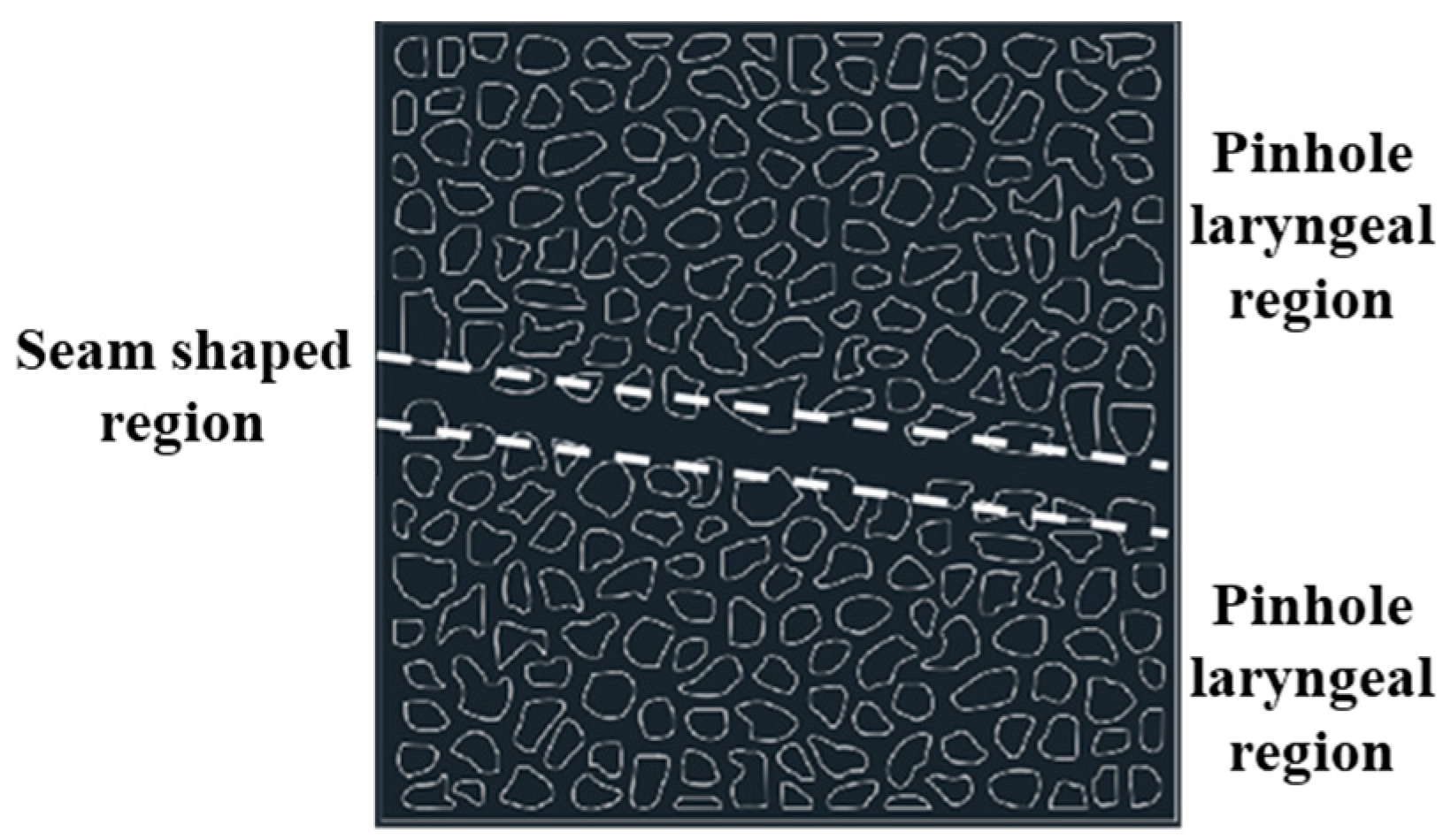
2.3.1. Microscopic Effects
2.3.2. Parallel Experiment of Two Tubes
3. Conclusions
4. Materials and Methods
4.1. Materials and Instruments
4.2. Gel Preparation Process
4.3. Optimize the Optimal Reaction Conditions
4.3.1. The Response Surface Method Is Used to Analyze the Interaction Between the Two Factors
4.3.2. Time–Viscosity Relationship
4.4. Evaluation and Analysis of Gel System
4.4.1. SEM Electron Microscope
4.4.2. Infrared Spectrum
4.4.3. Thermogravimetric Analysis Method and Principle
4.4.4. Molecular Weight Analysis Method and Principle
4.4.5. Rheological Experiment
4.4.6. Magnetic Resonance Testing
4.5. Double-Tube Parallel Core Experiment
4.5.1. Gel-Sealing Mechanism and Microscopic Visualization Experiment
4.5.2. Parallel Displacement Experiment
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Zhong, L.; Wang, C.; Li, S.; Yuan, X.; Liu, Y.; Meng, X.; Zou, J.; Wang, Q. Investigation of a high temperature gel system for application in saline oil and gas reservoirs for profile modification. J. Pet. Sci. Eng. 2020, 195, 107852. [Google Scholar] [CrossRef]
- Zhai, Y.; Fang, Z.; Feng, J.; Sun, C.; Deng, W.; Wen, Y. Development and performance evaluation of re-crosslinkable preformed particle gel under high temperature and high salt conditions. Polymer 2024, 296, 126824. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, J.; Zhang, L.; Yang, L.; Li, Y.; Wang, J.; Fan, H. A novel approach to investigating the influencing factors and mechanism of re-crosslinking performance of re-crosslinkable preformed particle gels. Geoenergy Sci. Eng. 2025, 247, 213679. [Google Scholar] [CrossRef]
- Peng, Y.; Ye, J.; Li, Y.; Chen, Y.; Li, Z.; Zhang, D. Development and Performance Evaluation of Novel Self-Degradable Preformed Particulate Gels with High-Temperature and High-Salinity Resistance. SPE J. 2025, 30, 1105–1115. [Google Scholar] [CrossRef]
- Xin, H.; Fang, B.; Yu, L.; Lu, Y.; Xu, K.; Li, K. Rheological performance of high-temperature-resistant, salt-resistant fracturing fluid gel based on organic-zirconium-crosslinked HPAM. Gels 2023, 9, 151. [Google Scholar] [CrossRef]
- Xu, B.; Wang, Y. Profile control performance and field application of preformed particle gel in low-permeability fractured reservoir. J. Pet. Explor. Prod. Technol. 2021, 11, 477–482. [Google Scholar] [CrossRef]
- Deng, J.; Lian, H.; Zhuang, Y.; Zhao, H.; Wang, Z.; Tian, Y.; Lin, C.; Yuan, H.; Han, M.; Lu, G.; et al. Synthesis and performance evaluation of multi-crosslinked preformed particle gels with ultra-high strength and high-temperature and high-salinity resistance for conformance control. Fuel 2024, 357, 130027. [Google Scholar] [CrossRef]
- Guo, H.; Ge, J.; Xu, Y.; Lv, Q.; Li, Z.; Zhou, D.; Tao, Z. Preparation and Mechanism of Stability for High-Temperature and High-Salinity Gels. SPE J. 2022, 27, 3565–3578. [Google Scholar] [CrossRef]
- Ge, J.; Guo, H.; Zhang, T.; Zhou, D.; Xu, Y.; Lü, Q. Development of temperature and salinity resistant phenolic gel and its performance regulation mechanism. Acta Pet. Sin. 2022, 43, 1145. [Google Scholar]
- Zhou, G.; Zhang, X.; Yan, W.; Qiu, Z. Synthesis, Characteristics, and Field Applications of High-Temperature and Salt-Resistant Polymer Gel Tackifier. Gels 2025, 11, 378. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Bai, B.; Zhou, E.; Pu, J.; Schuman, T.P. Experimental Evaluation of Oxidizing Breakers for a Polyacrylamide-Based Re-Crosslinkable Preformed Particle Gel. Energy Fuels 2019, 33, 5001–5010. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, L.; Cao, Z.; Hao, T.; Liu, Y.; Wu, W. High-temperature performance and cross-linking mechanism of different types of gel systems in saline environment. J. Appl. Polym. Sci. 2022, 139, 51452. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, H.; Zhang, L.; Huang, Y.; Zhao, M.; Dai, C. Development of Novel Delayed Swelling Polymer Gel Particles with Salt Resistance for Enhanced In-Depth Permeability Control. SPE J. 2024, 29, 2060–2075. [Google Scholar] [CrossRef]
- Shu, Z.; Qi, Y.; Luo, P. Research and performance evaluation of modified nano-silica gel plugging agent. J. Appl. Polym. Sci. 2023, 140, e53873. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, L.; Luo, P.; Li, D. Synthesis and evaluation of delayed anti-high temperature gel plugging agent. Front. Energy Res. 2022, 10, 1003473. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, H.; Djouonkep, L.D.W.; Lv, J.; Xie, B. Preparation, Evaluation and Field Application of Thermally Induced Crosslinked Polymer Gel Leakage Plugging Agent. J. Polym. Environ. 2024, 32, 5677–5688. [Google Scholar] [CrossRef]
- Fang, J.; Sun, J.; Feng, X.; Pan, L.; Bai, Y.; Yang, J. Research and Development of a High-Temperature-Resistant, Gel-Breaking Chemical Gel Plugging Agent and Evaluation of Its Physicochemical Properties. Gels 2025, 11, 350. [Google Scholar] [CrossRef]
- Liu, J.; Fu, H.; Luo, Z.; Chen, W.; Liu, F.; Zhao, M. Preparation and performance of pH-temperature responsive low-damage gel temporary plugging agent. Colloids Surf. A Physicochem. Eng. Asp. 2023, 662, 130990. [Google Scholar] [CrossRef]
- Fan, H.; Pu, W.; Du, D.; Xu, M.; Chen, L.; Xu, L.; Jin, F. Experimental study on the plugging and profile control of preformed particle gels in high temperature and salinity reservoir. J. Appl. Polym. Sci. 2023, 140, 14. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, W.; Zhou, Q.; Fu, C.; He, S. Preparation and Experimental Study of a Low-Initial-Viscosity Gel Plugging Agent. ACS Omega 2020, 5, 15715–15727. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-K.; Hou, J.-R.; Wu, W.-P.; Qu, M.; Liang, T.; Zhong, W.-X.; Wen, Y.-C.; Sun, H.-T.; Pan, Y.-N. A novel profile modification HPF-Co gel satisfied with fractured low permeability reservoirs in high temperature and high salinity. Pet. Sci. 2024, 21, 683–693. [Google Scholar] [CrossRef]
- Liu, F.; Yin, D.; Sun, J.; Luo, X.; Huang, X. Preparation and Characterization of Temperature-Sensitive Gel Plugging Agent. Gels 2024, 10, 742. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Liu, J.; Liu, S.; Yuan, S.; Cai, W.; Jiang, H. A gel system with long-term high-temperature stability for deep profile modification of high-temperature oil and gas reservoirs. J. Appl. Polym. Sci. 2023, 140, e54733. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, G.; Xiang, C. Synthesis and Properties of a Gel Agent with a High Salt Resistance for Use in Weak-Gel-Type Water-Based Drilling Fluid. Arab. J. Sci. Eng. 2022, 47, 12045–12055. [Google Scholar] [CrossRef]
- Zou, H.; Wang, Y.; Xu, Y.; Li, J.; Wu, L.; Su, G.; Yu, X.; Yang, H. Synthesis and Performance Study of Self-Degradable Gel Plugging Agents Suitable for Medium- and Low-Temperature Reservoirs. ACS Omega 2024, 9, 33702–33709. [Google Scholar] [CrossRef]
- Wang, H.; Yang, C.; Zhang, Y.; Wang, C. Preparation and Effect of CO2 Response Gel for Plugging Low-Permeability Reservoirs. Gels 2024, 10, 449. [Google Scholar] [CrossRef]
- Jia, H.; Ren, Q. Evidence of the Gelation Acceleration Mechanism of HPAM Gel with Ammonium Salt at Ultralow Temperature by SEM Study. SPE Prod. Oper. 2016, 31, 238–246. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, R.; Sha, X.; Liu, G.; Wang, X.-Y.; Lin, R.-S.; Tong, Y. Strength development and thermal stability analysis of carbonation-cured red mud-based cementitious materials. Constr. Build. Mater. 2025, 470, 140724. [Google Scholar] [CrossRef]
- Papp, D.; Carlström, G.; Nylander, T.; Sandahl, M.; Turner, C. A Complementary Multitechnique Approach to Assess the Bias in Molecular Weight Determination of Lignin by Derivatization-Free Gel Permeation Chromatography. Anal. Chem. 2024, 96, 10612–10619. [Google Scholar] [CrossRef]
- Liu, M.; Ge, J.; Wu, Q.; Ma, A.; Li, J.; Zhang, G.; Fan, H.; Jiang, P.; Pei, H. Study on temperature-resistance and salt-tolerance aluminum gel and its rheological analysis. Colloid Polym. Sci. 2024, 302, 665–677. [Google Scholar] [CrossRef]
- Deng, Z.; Liu, M.; Qin, J.; Sun, H.; Zhang, H.; Zhi, K.; Zhu, D. Mechanism study of water control and oil recovery improvement by polymer gels based on nuclear magnetic resonance. J. Pet. Sci. Eng. 2022, 209, 109881. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Wang, Z.; Li, H.; Zhao, Y.; Pan, Y.; Liu, Y.; Yuan, W.; Hou, J. Evaluation of Profile Control and Oil Displacement Effect of Starch Gel and Nano-MoS2 Combination System in High-Temperature Heterogeneous Reservoir. Gels 2024, 10, 127. [Google Scholar] [CrossRef] [PubMed]
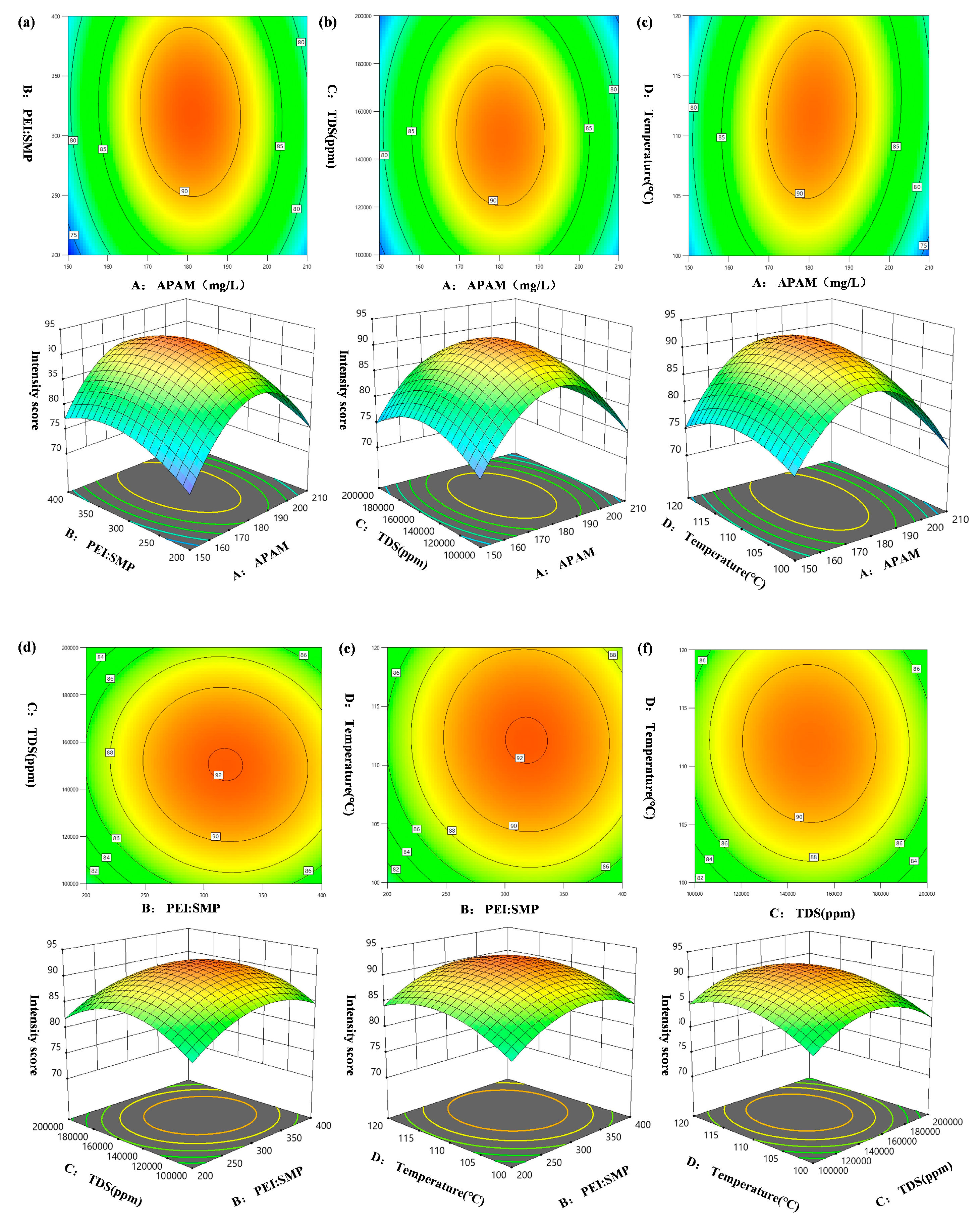

| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 1201.88 | 14 | 85.85 | 9.13 | <0.0001 |
| A | 4.08 | 1 | 4.08 | 3.54 | 0.0793 |
| B | 33.33 | 1 | 33.33 | 0.4341 | 0.5200 |
| C | 0.7500 | 1 | 0.7500 | 0.797 | 0.7815 |
| D | 21.33 | 1 | 21.33 | 2.27 | 0.1528 |
| AB | 2.25 | 1 | 2.25 | 0.2392 | 0.6318 |
| AC | 1.00 | 1 | 1.00 | 0.1063 | 0.7489 |
| AD | 9.00 | 1 | 9.00 | 0.9569 | 0.3435 |
| BC | 1.00 | 1 | 1.00 | 0.1063 | 0.7489 |
| BD | 0.2500 | 1 | 0.2500 | 0.0266 | 0.8727 |
| CD | 0.2500 | 1 | 0.2500 | 0.0266 | 0.8727 |
| A2 | 1015.05 | 1 | 1015.05 | 107.92 | 0.0023 |
| B2 | 126.30 | 1 | 126.30 | 13.43 | <0.0001 |
| C2 | 165.76 | 1 | 165.76 | 17.62 | 0.0008 |
| D2 | 86.01 | 1 | 86.01 | 9.14 | 0.0085 |
| Residual | 141.08 | 15 | 9.14 | / | / |
| Items not drafted | 131.08 | 10 | 13.11 | 6.55 | 0.0855 |
| Pure error | 0.1280 | 5 | 0.0520 | / | / |
| Total sum | 1332.97 | 29 | / | / | / |
| Level | Factor | |||
|---|---|---|---|---|
| A. APAM (mg/L) | B. PEI:SMP | C. TDS (ppm) | D. Temperature (°C) | |
| −1 | 150 | 2:1 | 100,000 | 100 |
| 0 | 180 | 3:1 | 150,000 | 110 |
| 1 | 210 | 4:1 | 200,000 | 120 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Xiao, D.; Wang, J.; Liang, T. Evaluation and Experiment of High-Strength Temperature- and Salt-Resistant Gel System. Gels 2025, 11, 669. https://doi.org/10.3390/gels11080669
Yang C, Xiao D, Wang J, Liang T. Evaluation and Experiment of High-Strength Temperature- and Salt-Resistant Gel System. Gels. 2025; 11(8):669. https://doi.org/10.3390/gels11080669
Chicago/Turabian StyleYang, Changhua, Di Xiao, Jun Wang, and Tuo Liang. 2025. "Evaluation and Experiment of High-Strength Temperature- and Salt-Resistant Gel System" Gels 11, no. 8: 669. https://doi.org/10.3390/gels11080669
APA StyleYang, C., Xiao, D., Wang, J., & Liang, T. (2025). Evaluation and Experiment of High-Strength Temperature- and Salt-Resistant Gel System. Gels, 11(8), 669. https://doi.org/10.3390/gels11080669





