Sol–Gel-Synthesized Metal Oxide Nanostructures: Advancements and Prospects for Spintronic Applications—A Comprehensive Review
Abstract
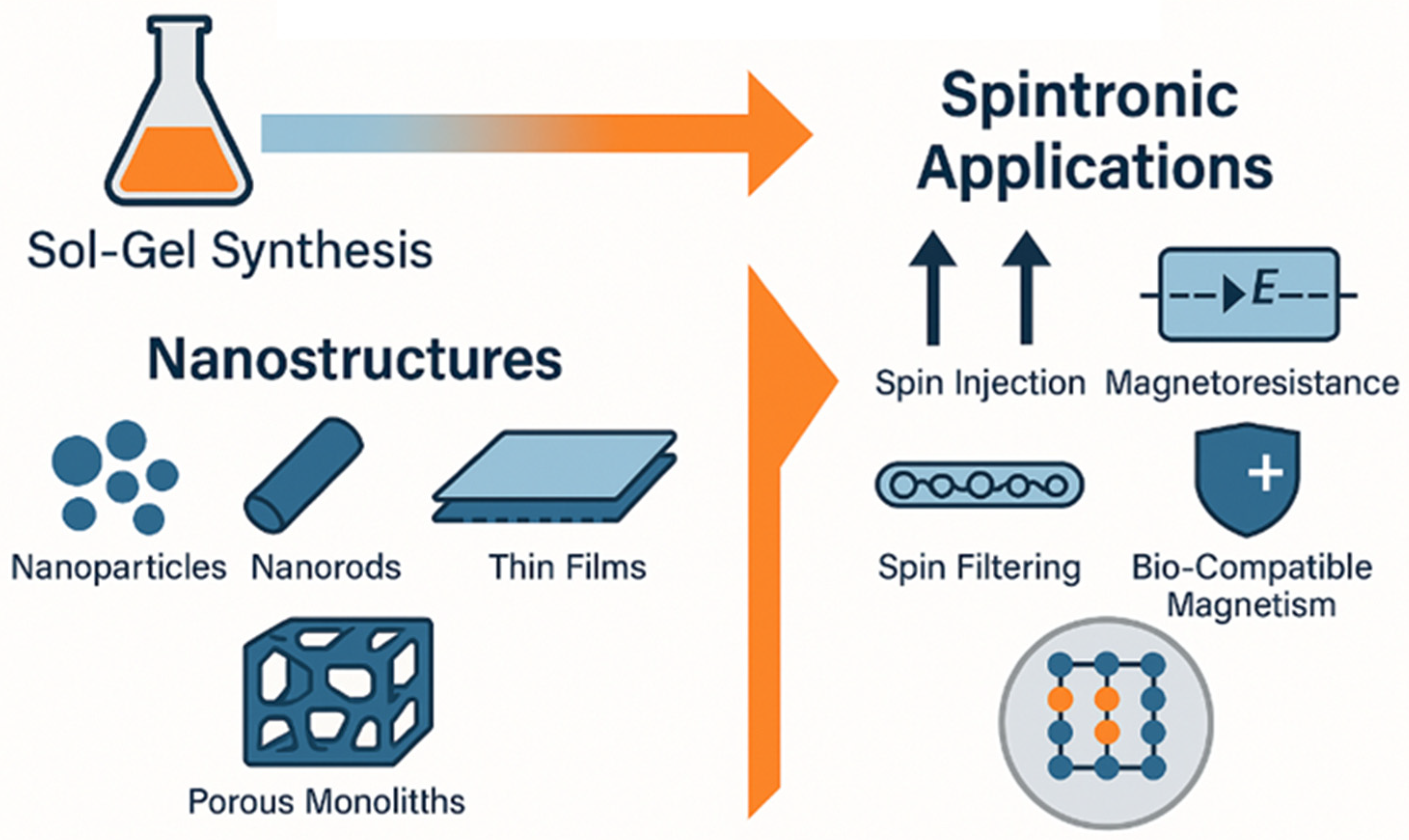
1. Introduction
2. Sol–Gel Synthesis of Magnetic Oxide Nanostructures
2.1. Overview and Motivation
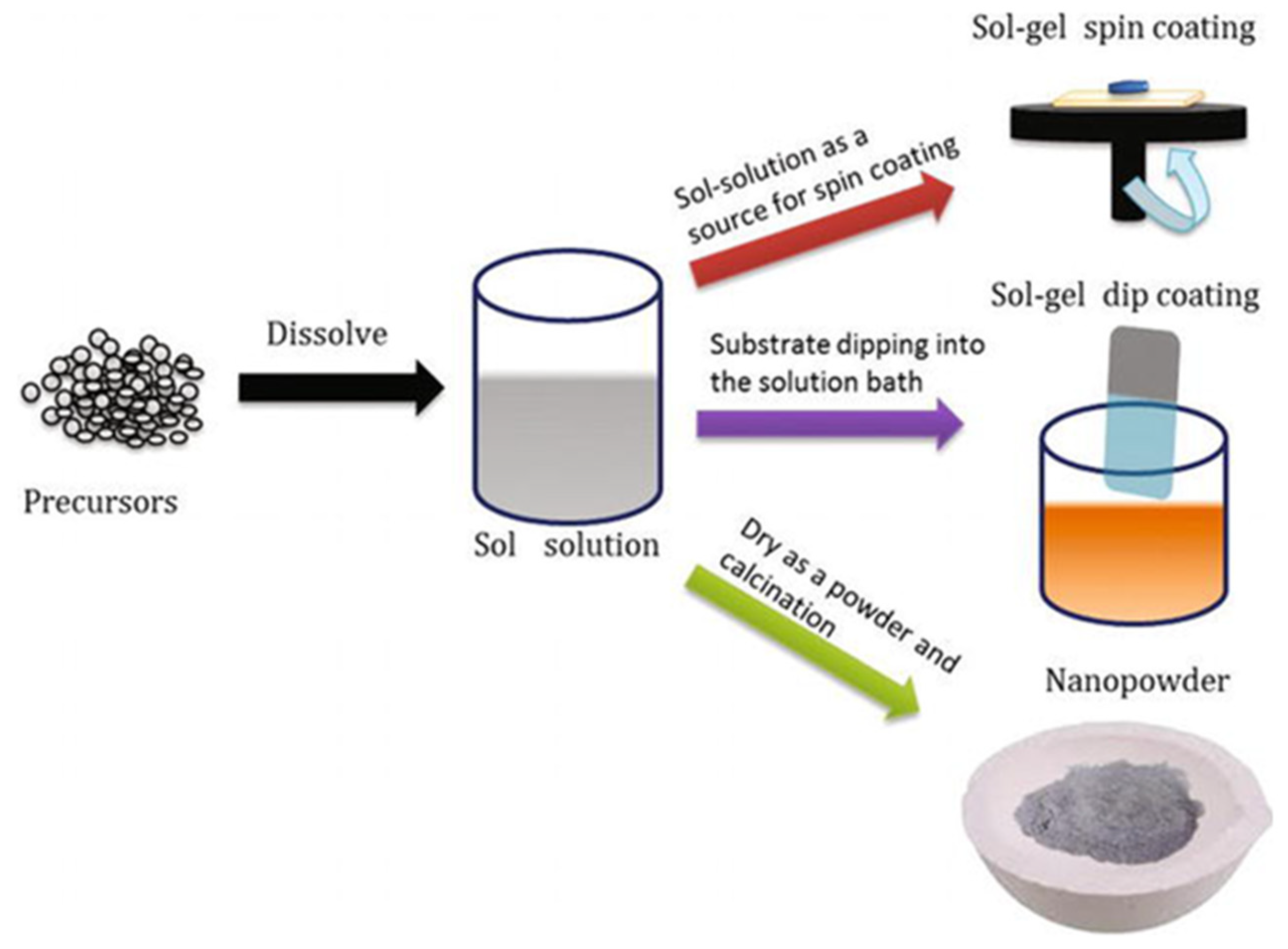
2.2. Fundamentals of the Sol–Gel Process
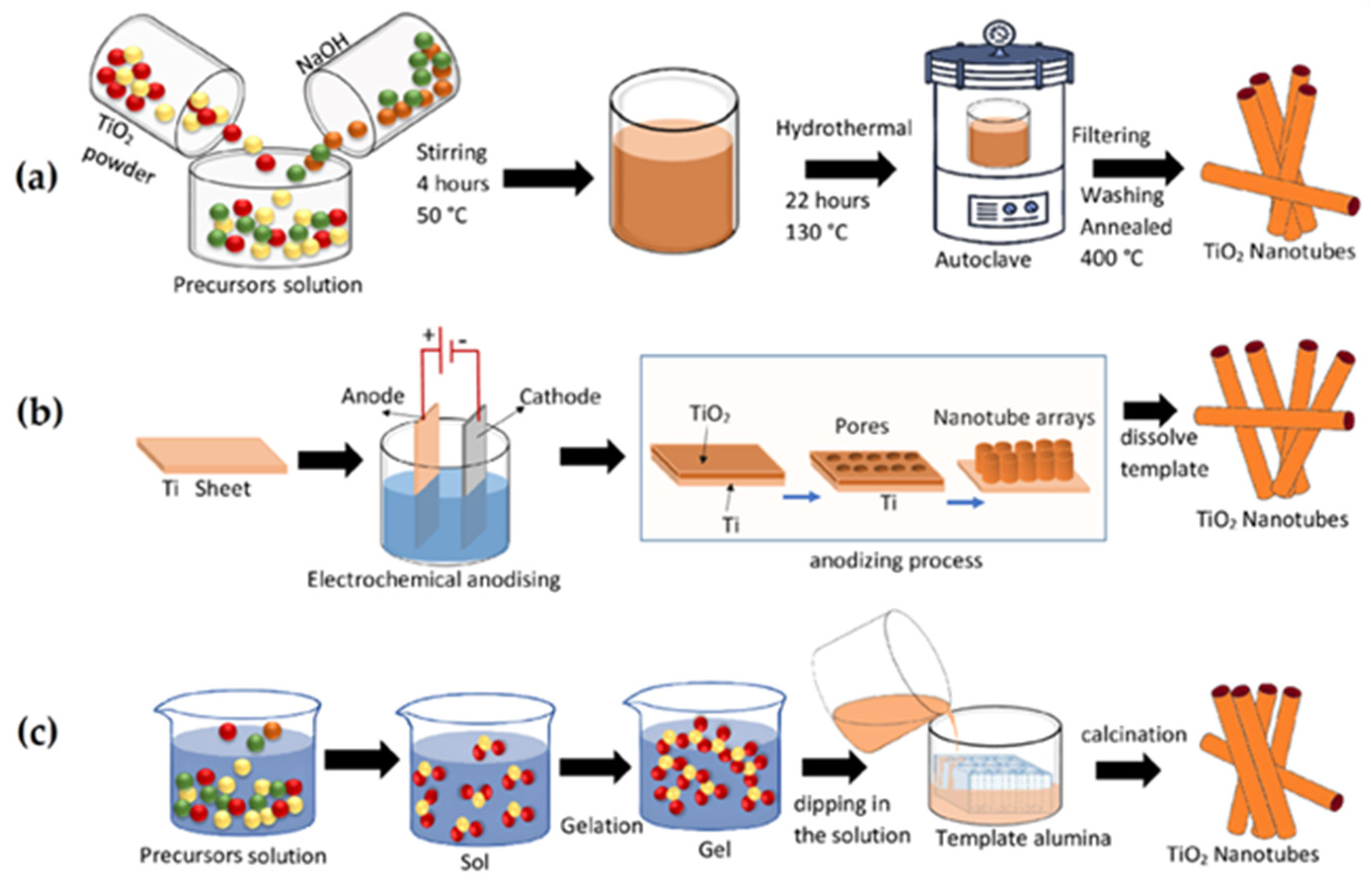
2.3. Sol–Gel Variants and Techniques for Magnetic Oxides
2.4. Examples of Sol–Gel Derived Magnetic Oxide Systems
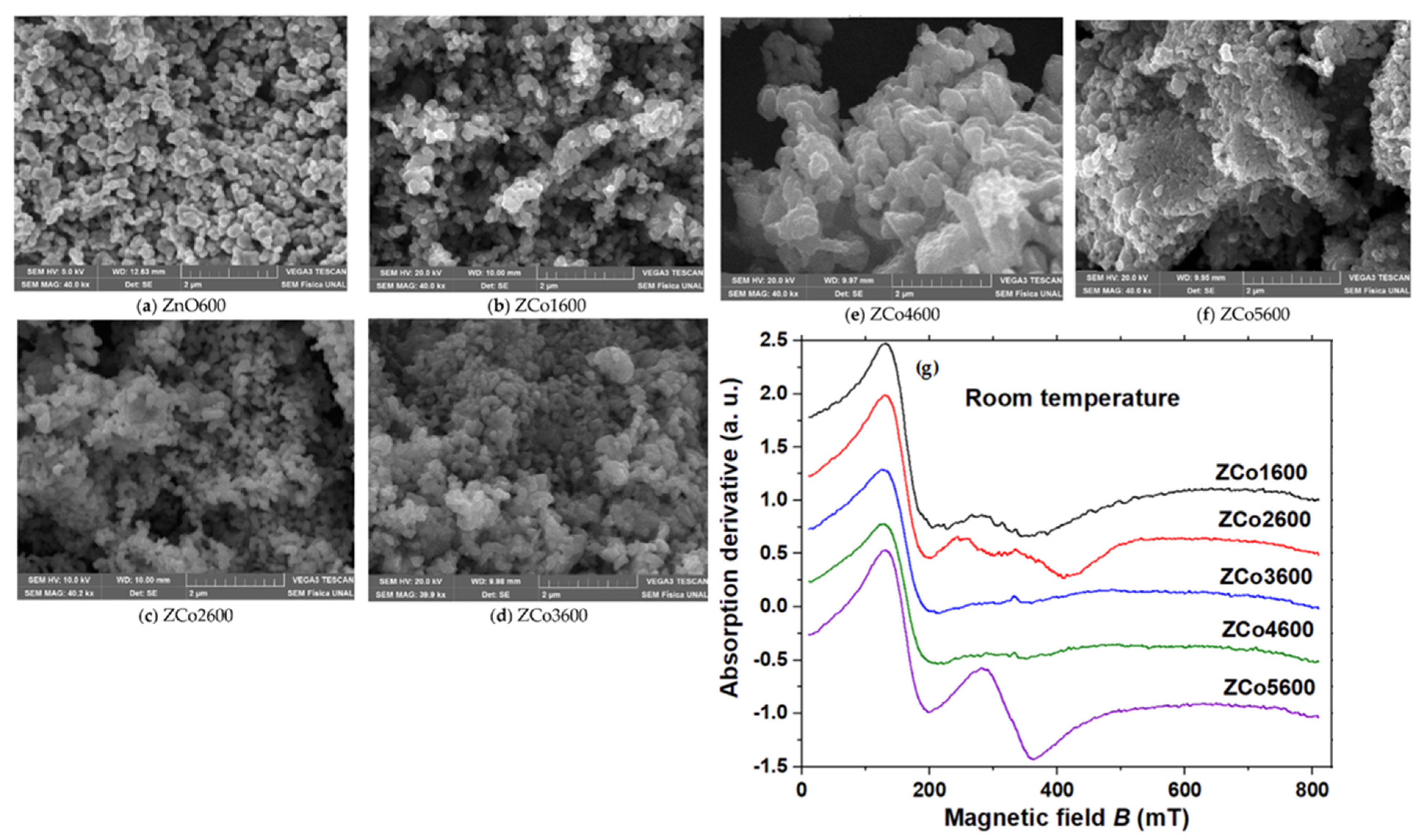
2.4.1. Co-Doped ZnO (Zn1−xCoxO)
2.4.2. La1−xSrxMnO3 (LSMO)
2.4.3. Fe3O4 (Magnetite) Nanoparticles
2.4.4. NiFe2O4 and CoFe2O4 Ferrites
2.4.5. Mn-Doped TiO2
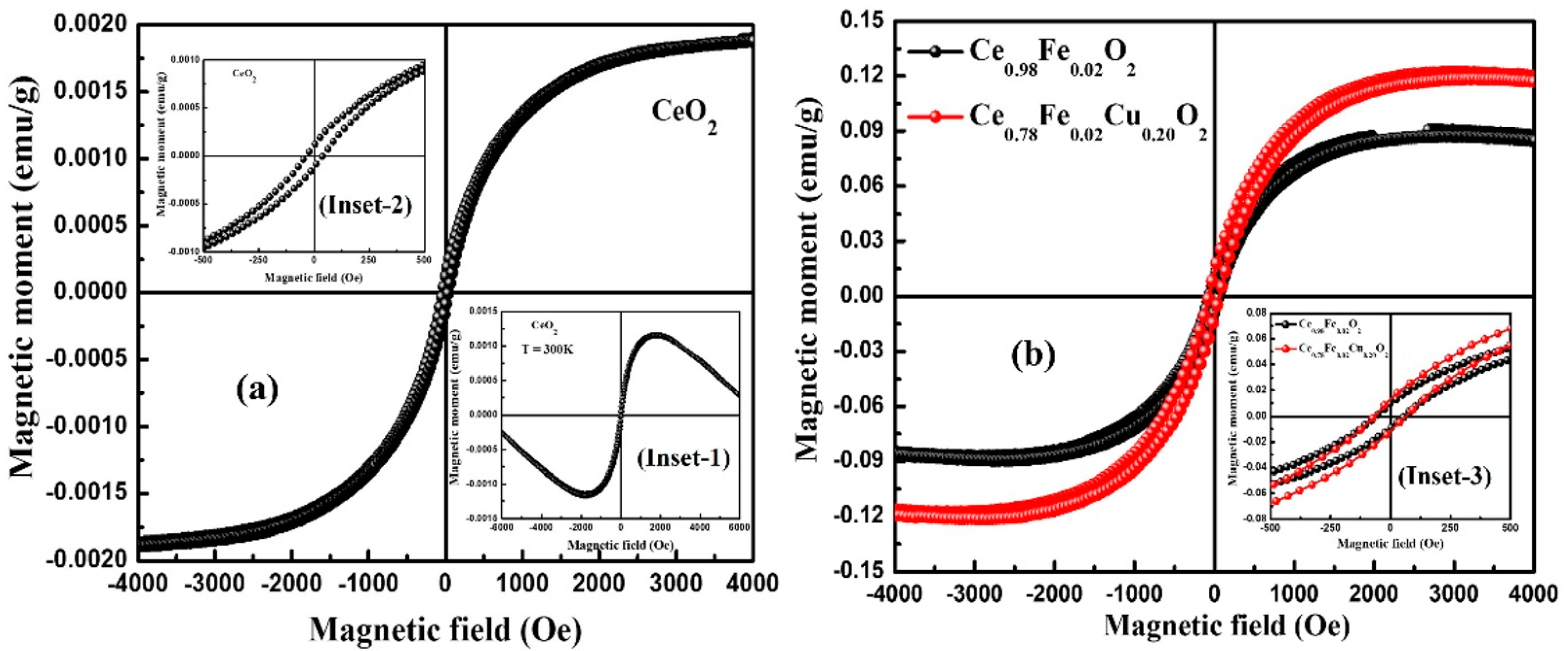
2.4.6. CeO2 Doped with Fe or Co
2.4.7. Mechanistic Insights and Critical Perspectives
2.5. Processing Parameters and Their Effects
Effect of Sol–Gel Parameters on Magnetic Properties
2.6. Challenges and Considerations
3. Recent Advances and Applications in Spintronics
3.1. Dilute Magnetic Oxides and Room-Temperature Ferromagnetism
3.2. Spin-Dependent Transport and Magnetoresistance
3.3. Multifunctional Nanostructures for Spintronics
3.4. Nanostructure Morphology and Dimensional Effects
4. Outlook and Future Directions
Integration with 2D, Topological, and Hybrid Spintronic Systems
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kharat, P.B.; Somvanshi, S.B.; Mopari, A.M.; Mahadik, M.M. Emerging trends and future prospects in magnetic nanomaterials research. In Materials Science for Future Applications; CRC Press: Boca Raton, FL, USA, 2025; pp. 334–361. [Google Scholar]
- Mallick, P. Band-gap engineering of ceramic coatings. In Advanced Ceramic Coatings: Fundamentals, Manufacturing, and Classification; Elsevier: Amsterdam, The Netherlands, 2023; p. 39. [Google Scholar]
- Pisano, R.; Durlo, A. Nanosciences and nanotechnologies: A scientific–historical introductory. In Nanosciences and Nanotechnologies; Springer: Cham, Switzerland, 2025; p. 1. [Google Scholar]
- Zhang, C.; Li, X.; Jiang, L.; Tang, D.; Xu, H.; Zhao, P.; Fu, J.; Zhou, Q.; Chen, Y. 3D printing of functional magnetic materials: From design to applications. Adv. Funct. Mater. 2021, 31, 2102777. [Google Scholar] [CrossRef]
- Gutfleisch, O.; Willard, M.A.; Brück, E.; Chen, C.H.; Sankar, S.G.; Liu, J.P. Magnetic materials and devices for the 21st century: Stronger, lighter, and more energy efficient. Adv. Mater. 2011, 23, 821–842. [Google Scholar] [CrossRef]
- Gong, C.; Zhang, X. Two-dimensional magnetic crystals and emergent heterostructure devices. Science 2019, 363, eaav4450. [Google Scholar] [CrossRef]
- Ye, D.; Huang, Y.; Zong, Y.; Gao, H. PO-SRPP: A decentralized pivoting path planning method for self-reconfigurable satellites. IEEE Trans. Ind. Electron. 2024, 71, 14318–14327. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, L.; Guo, Q.; Zhao, S. Quantitative precision second-order temporal transformation based pose control for spacecraft proximity operations. IEEE Trans. Aerosp. Electron. Syst. 2024, 61, 1931–1941. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.; Yan, X. Two-dimensional material-based devices for in-sensor computing. Npj Unconv. Comput. 2025, 2, 19. [Google Scholar] [CrossRef]
- Sort, J. Beyond storage: The expanding role of magnetism in intelligent computing. APL Mater. 2025, 13, 070401. [Google Scholar] [CrossRef]
- Zhang, H.H.; Chao, J.B.; Wang, Y.W.; Liu, Y.; Yao, H.M.; Zhao, Z.P. 5G base station antenna array with heatsink radome. IEEE Trans. Antennas Propag. 2024, 72, 2270–2278. [Google Scholar] [CrossRef]
- Zhao, L.C.; Zhong, Y.T.; Li, J.X.; Jiang, C.L.; Xiao, M.F.; Yang, D.; Liao, X.Y. Preparation of Cyclocarya paliurus sugar-free jelly. Sci. Technol. Food Ind. 2020, 41, 234–239. [Google Scholar]
- Zhao, L.-C.; Li, W.; He, Y.-F.; Li, X.-H.; Wang, Z.; Liu, W.-C.; Zheng, Y.-N.; Liang, J. Fast and Sensitive LC-DAD-ESI/MS Method for Analysis of Saikosaponins c, a, and d from the Roots of Bupleurum falcatum (Sandaochaihu). Molecules 2011, 16, 1533–1543. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Zhu, Y.Y.; Qian, S.G.; Hou, K.P. Types and space distribution characteristics of debris flow disasters along China-Pakistan Highway. Electron. J. Geotech. Eng. 2016, 21, 191–200. [Google Scholar]
- Yang, Z.Q.; Niu, X.; Hou, K.; Liang, W.; Guo, Y. Prediction model on maximum potential pollution range of debris flows generated in tailings dam break. Electron. J. Geotech. Eng. 2015, 20, 4363–4369. [Google Scholar]
- Gao, J. Friction coefficient estimation of the clutch in automatic transmission based on improved persistent excitation condition. In Proceedings of the 2017 Chinese Automation Congress (CAC), Jinan, China, 20–22 October 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 1–6. [Google Scholar]
- Wu, Z.; Zhang, L.; Wang, Y.; Chen, J. Preliminary studies on diagnostic cast of peptic ulcer based on saliva proteome and bioinformatics. In Proceedings of the 2011 4th International Conference on Biomedical Engineering and Informatics (BMEI), Shanghai, China, 15–17 October 2011; IEEE: Piscataway, NJ, USA, 2011; Volume 3, pp. 1720–1724. [Google Scholar]
- Liu, Q.; Ren, H.; Wei, Q.; Li, M. Multifunctional chiral halide perovskites: Advancing chiro-optics, chiro-optoelectronics, and spintronics. Adv. Sci. 2025, e09155. [Google Scholar] [CrossRef]
- Sundaram, G.A.; Muniyandi, G.R.; Ethiraj, J.; Parimelazhagan, V.; Kumar, A.S.K. Introduction and advancements in room-temperature ferromagnetic metal oxide semiconductors for enhanced photocatalytic performance. ChemEngineering 2024, 8, 36. [Google Scholar] [CrossRef]
- Bibes, M.; Villegas, J.E.; Barthélémy, A. Ultrathin oxide films and interfaces for electronics and spintronics. Adv. Phys. 2011, 60, 5–84. [Google Scholar] [CrossRef]
- Chen, S.; Yuan, S.; Hou, Z.; Tang, Y.; Zhang, J.; Wang, T.; Li, K.; Zhao, W.; Liu, X.; Chen, L.; et al. Recent progress on topological structures in ferroic thin films and heterostructures. Adv. Mater. 2021, 33, 2000857. [Google Scholar] [CrossRef]
- Almeida, R.M.; Gonçalves, M.C. Sol–gel process and products. Encycl. Glass Sci. Technol. Hist. Cult. 2021, 23, 969–979. [Google Scholar]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.-M.; Mahapatra, C.; Kim, H.-W.; Knowles, J.C. Sol–gel-based materials for biomedical applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef]
- Soltani, S.; Hjiri, M.; Aldukhayel, A.M.; Essid, M.; Jbeli, A.; Althumairi, N.A. Effects of silver doping at the A-site on the structure, surface morphology, and magnetic behavior of La1−xAgxSrMn2O5+δ (x = 0.1 and 0.2). RSC Adv. 2025, 15, 22616–22628. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, M.; Li, Y.; Jia, X.; Zhang, Y. Effects of Tiantai I on the activity of central cholinergic system in mice with spontaneous Alzheimer disease. Chin. J. Tissue Eng. Res. 2006, 10, 163–165. [Google Scholar]
- Wu, Z.; Li, M.; Jia, X.; Zhang, Y. Changes of neuronal nitric oxide synthase in relevant cerebral regions in spontaneous senile dementia model and regulation of Tiantai I. Chin. J. Tissue Eng. Res. 2005, 9, 244–247. [Google Scholar]
- Wu, Z.; Li, M.; Jia, X.; Zhang, Y. Influence of Tiantai No. 1 Recipe on learning and memory function of spontaneous Alzheimer disease models. Chin. J. Tissue Eng. Res. 2005, 9, 180–181. [Google Scholar]
- Wu, Z.; Li, M.; Huang, A.C.; Jia, X.; Li, Y.; Chen, M. Effects of serum containing natural cerebrolysin on glucose-regulated protein 78 and CCAAT enhancer-binding protein homologous protein expression in neuronal PC12 cells following tunicamycin-induced endoplasmic reticulum stress. Neural Regen. Res. 2009, 4, 92–97. [Google Scholar]
- Wu, Z.Z.; Huang, A.C.; de Vellis, J.; Li, Y.H. Effect of Tiantai No. 1 on β-amyloid-induced neurotoxicity and NF-κB and cAMP responsive element-binding protein. Neural Regen. Res. 2008, 3, 286–292. [Google Scholar]
- Wu, Z.; Cao, M.; Wang, J.; Zhang, X.; Wu, A. Study on saliva proteome and bioinformatics in patients with chronic gastritis. In Proceedings of the 2011 IEEE International Symposium on IT in Medicine and Education, Cuangzhou, China, 30 August–2 September 2011; IEEE: Piscataway, NJ, USA, 2011; Volume 1, pp. 285–289. [Google Scholar]
- Soltani, S.; Hjiri, M.; Ahmed, N.I.A.; Jbeli, A.; Aldukhayel, A.M.; Althumairi, N.A. Metal halide perovskites for energy applications: Recent advances, challenges, and future perspectives. RSC Adv. 2025, 15, 21811–21837. [Google Scholar] [CrossRef]
- Parida, P.; Patra, J.; Singh, V.R.; Verma, V.K. Dilute magnetic semiconductors and its applications—An overview. Springer Proc. Phys. 2024, 413, 181–220. [Google Scholar] [CrossRef]
- Tian, G.; Zhao, C.; Zhang, X.; Mu, W.; Jiang, Y.; Wei, X.; Zhao, M.; Shi, Z.; Jin, Y.; Si, J.; et al. Evidence-Based traditional Chinese medicine research: Two decades of development, its impact, and breakthrough. J. Evid.-Based Med. 2021, 14, 65–74. [Google Scholar] [CrossRef]
- Tian, G.; Qian, K.; Li, X.; Sun, M.; Jiang, H.; Qiu, W.; Xie, X.; Zhao, Z.; Huang, L.; Luo, S.; et al. Can a holistic view facilitate the development of intelligent traditional Chinese medicine? A survey. IEEE Trans. Comput. Soc. Syst. 2023, 10, 700–713. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, Y.; Zhang, M.; He, K.; Tian, G. Causal association between circulating inflammatory markers and sciatica development: A Mendelian randomization study. Front. Neurol. 2024, 15, 1380719. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Xia, H.; Xia, G.Y.; Li, X.Y.; Zhao, C.; Zhang, X.T.; Lin, S.; Tian, G.H. Effect of Siegesbeckiae Herba in treating chronic pain. Zhongguo Zhong Yao Za Zhi 2020, 45, 1851–1858. [Google Scholar]
- Lin, Y.; Li, X.; Huang, L.; Xie, X.; Luo, T.; Tian, G. Acupuncture combined with Chinese herbal medicine for discogenic low back pain: Protocol for a multicentre, randomised controlled trial. BMJ Open 2024, 14, e088898. [Google Scholar] [CrossRef]
- Yang, J.J. Analysis of clinical medication characteristics of zedoary turmeric oil injection based on real world data. Chin. J. New Drugs 2023, 32, 547–552. [Google Scholar]
- Lv, S.; Liu, H.; Wang, F.; Liu, X.; Peng, M.; Wei, Y.; Li, C. Effect of axial misalignment on the microstructure, mechanical, and corrosion properties of magnetically impelled arc butt welding joint. Mater. Today Commun. 2024, 40, 109866. [Google Scholar] [CrossRef]
- Ravikumar, D.; Jeyakumar, S.C.; Vinita, V.S.; Usharani, S.; Dhas, S.S.J.; Kumar, D.S.; Almansour, A.I.; Biju, C.S. An overview of the optical and magnetic properties of Ba0.6Cd0.4TiO3 micro rods prepared by the facile sol–gel approach for spintronic applications. J. Electroceram. 2024, 52, 314–325. [Google Scholar] [CrossRef]
- Dai, Q.Q.; Li, X.Y.; Xia, G.Y.; Xia, H.; Wei, X.H.; Shang, H.C.; Lin, S.; Tian, G.H. Research progress on animal models of chronic pain and its application in traditional Chinese medicine research. Zhongguo Zhong Yao Za Zhi 2020, 45, 5866–5876. [Google Scholar] [PubMed]
- Xiang, D.; He, D.; Sun, H.; Gao, P.; Zhang, J.; Ling, J. HCMPE-Net: An unsupervised network for underwater image restoration with multi-parameter estimation based on homology constraint. Opt. Laser Technol. 2025, 186, 112616. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, F.; Qian, J.; Fujita, H.; Yu, J.; Zeng, K.; Chen, E. CrowdFPN: Crowd counting via scale-enhanced and location-aware feature pyramid network. Appl. Intell. 2025, 55, 359. [Google Scholar] [CrossRef]
- Guan, Y.; Cui, Z.; Zhou, W. Reconstruction in off-axis digital holography based on hybrid clustering and the fractional Fourier transform. Opt. Laser Technol. 2025, 186, 112622. [Google Scholar] [CrossRef]
- Rahman, M.M.; Awaltanova, E.; Amri, A.; Altarawneh, M.; Hossain, M.A.; Zhao, X.; Liew, W.Y.H.; Minakshi, M.; Yin, C.-Y.; Veder, J.-P.; et al. A holistic analysis of surface, chemical bonding states and mechanical properties of sol–gel synthesized CoZn-oxide coatings complemented by finite element modeling. Ceram. Int. 2019, 45, 10882–10898. [Google Scholar] [CrossRef]
- Hou, Y.; Tang, K.; Wang, J.; Xie, D.; Zhang, H. Assortative mating on blood type: Evidence from one million Chinese pregnancies. Proc. Natl. Acad. Sci. USA 2022, 119, e2209643119. [Google Scholar] [CrossRef]
- Huang, Y.; Hong, T.; Chang, X.; Ma, T. Travel and regional development: A quantitative analysis of China. J. Reg. Sci. 2025, 1–35. [Google Scholar] [CrossRef]
- Yu, Z.; Ning, Z.; Zhang, H.; Yang, H.; Chang, S.J. A generalized Faustmann model with multiple carbon pools. For. Policy Econ. 2024, 169, 103363. [Google Scholar] [CrossRef]
- Xiang, X.; Liu, H.; Zheng, C.; Jiang, N.; Huang, F.; Zhou, Q. Flavor profile of 4-isothiocyanato-1-butene in microwave rapeseed oil and its anti-inflammatory properties in vitro. J. Agric. Food Chem. 2025, 73, 10520–10530. [Google Scholar] [CrossRef]
- Yu, Z.; Ning, Z.; Chang, W.Y.; Chang, S.J.; Yang, H. Optimal harvest decisions for the management of carbon sequestration forests under price uncertainty and risk preferences. For. Policy Econ. 2023, 151, 102957. [Google Scholar] [CrossRef]
- Ding, L.N.; Hu, Y.H.; Li, T.; Li, M.; Li, Y.T.; Wu, Y.Z.; Cao, J.; Tan, X.L. A GDSL motif-containing lipase modulates Sclerotinia sclerotiorum resistance in Brassica napus. Plant Physiol. 2024, 196, 2973–2988. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Z.; Wang, J.; Hu, Y.H.; Sun, Q.S.; Geng, R.; Ding, L.N. Antimicrobial peptides: Classification, mechanism, and application in plant disease resistance. Probiotics Antimicrob. Proteins 2025, 17, 1432–1446. [Google Scholar] [CrossRef]
- Chen, Z.; Pu, Q.; Zhu, L.; Zhou, W. Creep behaviour between resilient wheels and rails in a metro system. Veh. Syst. Dyn. 2025, 1–21. [Google Scholar] [CrossRef]
- Fan, Q.; Lu, Q.; Yang, X. Spatiotemporal assessment of recreation ecosystem service flow from green spaces in Zhengzhou’s main urban area. Humanit. Soc. Sci. Commun. 2025, 12, 97. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, J.; Jiang, X.; Shi, T.; Jin, X.; Ren, Y.; Ji, K.; Xin, Z.; Zhang, Z.; Yin, C.; et al. MoS2 Nanozyme–Chlorella Hydrogels: Pioneering a Hepatocellular Carcinoma Integrative Therapy. Adv. Funct. Mater. 2025, 35, 2417125. [Google Scholar] [CrossRef]
- Du, J.; Liu, H.; Wang, F.; Bao, W.; Li, H. Solidification microstructure reconstruction and its effects on phase transformation, grain boundary transformation mechanism, and mechanical properties of TC4 alloy welded joint. Metall. Mater. Trans. A 2024, 55, 1193–1206. [Google Scholar] [CrossRef]
- Afreen, S.; Sajjanar, S.M. Advances in solid-state physics: A review of recent developments and emerging trends. J. Sci. Res. Technol. 2018, 3. Available online: https://www.jsrtjournal.com/index.php/JSRT/article/view/175 (accessed on 15 March 2025).
- Feng, G.; Jiang, W.; Liu, J.; Zhang, Q.; Wu, Q.; Miao, L. A novel green nonaqueous sol-gel process for preparation of partially stabilized zirconia nanopowder. Process. Appl. Ceram. 2017, 11, 220–224. [Google Scholar] [CrossRef]
- Feng, G.; Jiang, F.; Jiang, W.; Liu, J.; Zhang, Q.; Wu, Q.; Hu, Q.; Miao, L.; Liang, J. Low-temperature preparation of novel stabilized aluminum titanate ceramic fibers via nonhydrolytic sol-gel method through linear self-assembly of precursors. Ceram. Int. 2019, 45, 18704–18709. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, W.H.; Liu, J.M.; Feng, G.; Xu, G.R. Low temperature synthesis of ultrafine Al2TiO5; powders by hydrolytic sol-gel method. Mater. Sci. Forum 2016, 848, 473–477. [Google Scholar] [CrossRef]
- Jiang, W.H.; Hu, Z.; Liu, J.M. Study on low-temperature synthesis of iron-stabilized aluminum titanate via non-hydrolytic sol-gel method. J. Syn. Cryst. 2011, 40, 465–469. [Google Scholar]
- Fu, K.L.; Jiang, W.H.; Liu, J.M.; Feng, G.; Pan, M. Study on mullite whiskers preparation via non-hydrolytic sol-gel process combined with molten salt method. Mater. Sci. Forum 2016, 848, 278–283. [Google Scholar] [CrossRef]
- Bao, Y.; Jiang, W.H.; Feng, G.; Liu, J.M.; Wu, Q. Low Temperature Preparation of Aluminum Titanate Film via Sol-Gel Method. Adv. Mater. Res. 2014, 936, 238–242. [Google Scholar] [CrossRef]
- Wei, H.Y.; Jiang, W.H.; Lin, J. Comparative research on the synthesis of aluminum titanate powders by nonhydrolytic and hydrolytic sol-gel method. J. Inorg. Mater. 2009, 24, 199–203. [Google Scholar] [CrossRef]
- Jiang, W.; Wei, H.; Feng, G.; Zhou, Y.; Yu, Y. Effect of oxygen donor alcohols on low temperature nonhydrolytic sol-gel synthesis of aluminum titanate. J. Chin. Ceram. Soc. 2008, 36, 11–16. [Google Scholar]
- Feng, G.; Jiang, W.; Liu, J.; Li, C.; Zhang, Q.; Miao, L.; Wu, Q. Synthesis and luminescence properties of Al2O3@ YAG: Ce core–shell yellow phosphor for white LED application. Ceram. Int. 2018, 44, 8435–8439. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, S.Y.; Yin, Y.; Zheng, N.; Ali, A.; Younis, M.; Ruan, S.; Zeng, Y.J. Emerging ferromagnetic materials for electrical spin injection: Towards semiconductor spintronics. Npj Spintron 2025, 3, 10. [Google Scholar] [CrossRef]
- Al-Douri, Y.; Ameri, M. Physical studies of spintronics-based Heusler alloys. Crit. Rev. Solid State Mater. Sci. 2025, 50, 189–238. [Google Scholar] [CrossRef]
- Li, H.; Zhu, M.; Guo, Z.; Li, G.; Shang, J.; Yang, Y.; Feng, Y.; Lu, Y.; Zhang, Q.; Wang, S.; et al. Spintronics in GaN-Based Semiconductors: Research Progress, Challenges and Perspectives. Adv. Mater. Technol. 2025, 10, 2401017. [Google Scholar] [CrossRef]
- Ishchenko, O.; Rogé, V.; Lamblin, G.; Lenoble, D.; Fechete, I. TiO2, ZnO, and SnO2-based metal oxides for photocatalytic applications: Principles and development. Comptes Rendus Chim. 2021, 24, 103–124. [Google Scholar] [CrossRef]
- Landström, A. Nanostructured Metal Oxide Semiconductors for Functional Applications. Doctor’s Thesis, Luleå University of Technology, Luleå, Sweden, 2021. [Google Scholar]
- Nassar, K.I.; Slimi, M.; Rammeh, N.; Bouhamed, A.; Njeh, A.; Kanoun, O. Investigation of AC electrical conductivity and dielectric properties of BiBaFeZnO6 double perovskite oxides. J. Mater. Sci. Mater. Electron. 2021, 32, 24050–24057. [Google Scholar] [CrossRef]
- Soltani, S.; Ahmed, N.I.; Jbeli, A.; Aldukhayel, A.M.; Althumairi, N.A. Recent advances in sol–gel synthesis of oxide perovskites for energy and functional applications: A mini review. J. Sol-Gel Sci. Technol. 2025, 115, 663–687. [Google Scholar] [CrossRef]
- Iben Nassar, K.; Slimi, M.; Rammeh, N.; Soreto Teixeira, S.; Graça, M.P.F. Structural and electrical properties of double perovskite oxide LaPbFeTiO6 synthesized by a sol–gel process. Appl. Phys. A 2021, 127, 940. [Google Scholar] [CrossRef]
- Mohamed, M.; Nassar, K.I.; Mohamed, M.; Rammeh, N.; Graça, M.P.F. Effects of partial Li-substitution on structural, electrical and dielectric properties in La1-xLixSrMn2O5+δ (x = 0.05, 0.10 and 0.15) brownmillerite oxides. J. Mol. Struct. 2022, 1258, 132658. [Google Scholar] [CrossRef]
- Nassar, K.I.; Rammeh, N.; Teixeira, S.S.; Graça, M.P.F. Effect of Pr substitution in the A site on the structural, dielectric and magnetic properties of double perovskite La2NiMnO6. Appl. Phys. A 2022, 128, 373. [Google Scholar] [CrossRef]
- Giannou, R. Metal-Oxide Thin Film Transistors by Sol-Gel Method. Master’s Thesis, University of Ioannina, Ioannina, Greece, 2025. [Google Scholar]
- Benamara, M.; Nassar, K.I.; Essid, M.; Frick, S.; Rugmini, R.; Sekhar, K.C.; Silva, J.P. Visible light-driven removal of Rhodamine B using indium-doped zinc oxide prepared by sol–gel method. J. Sol-Gel Sci. Technol. 2024, 111, 553–565. [Google Scholar] [CrossRef]
- Tayari, F.; Benamara, M.; Lal, M.; Essid, M.; Thakur, P.; Kumar, D.; Teixeira, S.S.; Graça, M.P.F.; Nassar, K.I. Exploring Enhanced Structural and Dielectric Properties in Ag-Doped Sr(NiNb)0.5O3 Perovskite Ceramic for Advanced Energy Storage. Ceramics 2024, 7, 958–974. [Google Scholar] [CrossRef]
- Thakur, P.; Nassar, K.I.; Kumar, D.; Kumar, P.; Sharma, P.; Tirth, V.; Alosaimy, A.S.; Algahtani, A.; Essidh, M.; Lal, M. Tailoring of structural, morphological, electrical, and magnetic properties of LaMn1−xFexO3 ceramics. RSC Adv. 2024, 14, 23592–23605. [Google Scholar] [CrossRef]
- Nunocha, P.; Bongkarn, T.; Eiad-ua, A.; Channei, D.; Suriwong, T. Efficient and novel synthesis of Nb-doped SrTiO3 nanoparticles via microwave-assisted sol-gel auto-combustion. Opt. Mater. 2025, 159, 116665. [Google Scholar] [CrossRef]
- Tayari, F.; Teixeira, S.S.; Graça, M.P.F.; Essid, M.; Nassar, K.I. Investigating structural, dielectric, and electrical characteristics of sol–gel synthesized perovskite ceramic Bi0.7Ba0.3(FeTi)0.5O3. J. Sol-Gel Sci. Technol. 2024, 112, 601–613. [Google Scholar] [CrossRef]
- Dhahri, R.; Benamara, M.; Nassar, K.I.; Elkenany, E.B.; Al-Syadi, A.M. Zinc oxide-based sensor prepared by modified sol–gel route for detection of low concentrations of ethanol, methanol, acetone, and formaldehyde. Semicond. Sci. Technol. 2024, 39, 115021. [Google Scholar] [CrossRef]
- Thakur, P.; Kishore, K.; Kumar, R.; Pathak, D.; Nassar, K.I.; Lal, M. Structural, morphological, dielectric, and magnetic properties of CoFe2O4 ceramics at different sintering temperatures. J. Mater. Sci. Mater. Electron. 2024, 35, 1932. [Google Scholar] [CrossRef]
- Dhahri, R.; Tayari, F.; Albargi, H.B.; Elkenany, E.B.; Al-Syadi, A.M.; Sharma, N.; Lal, M.; Nassar, K.I. Comprehensive analysis of structural, dielectric, and electrical properties of sol–gel synthesized Ba-doped bismuth ferric titanate perovskite nanoparticles. J. Mater. Sci. Mater. Electron. 2024, 35, 1972. [Google Scholar] [CrossRef]
- Dhahri, R.; Benamara, M.; Bouzidi, S.; Moussa, S.B.; Alzahrani, A.Y.; Nassar, K.I.; Zahmouli, N.; Elkenany, E.B.; Al-Syadi, A.M. Effect of Gd doping on the microstructure and electrical characteristics of Maghemite (γ-Fe2O3) ceramics. J. Sol-Gel Sci. Technol. 2025, 113, 225–242. [Google Scholar] [CrossRef]
- Dhahri, R.; Albargi, H.B.; Jbeli, A.; Elkenany, E.B.; Althumairi, N.A.; Al-Syadi, A.M.; Sharma, N.; Lal, M.; Nassar, K.I. Enhanced electrical and magnetic properties of barium manganese titanium oxide perovskite ceramics synthesized by solid-state reaction. J. Mater. Sci. Mater. Electron. 2025, 36, 30. [Google Scholar] [CrossRef]
- Dhahri, R.; Tayari, F.; Haouas, A.; Alathlawi, H.J.; Albargi, H.B.; Elkenany, E.B.; Al-Syadi, A.M.; Sharma, N.; Lal, M.; Nassar, K.I. Crystal structural characteristics and optical and electrical properties of Bi-doped (Ba0.8Sr0.2)(Ti0.85Zr0.15)O3 perovskite ceramics. J. Mater. Sci. Mater. Electron. 2025, 36, 331. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, M.; Kishore, K.; Pathak, D.; Batoo, K.M.; Qaisar, T.; Ibrahim, A.A.; Kumar, D.; Nassar, K.I.; Lal, M. Influence of low sintering temperature on the structural, morphological, and dielectric properties of (Bi0.4Ba0.6)Na0.5TiO3 ceramics. J. Mater. Sci. Mater. Electron. 2025, 36, 392. [Google Scholar] [CrossRef]
- Tayari, F.; Teixeira, S.S.; Graca, M.P.F.; Nassar, K.I. Progress and developments in the fabrication and characterization of metal halide perovskites for photovoltaic applications. Nanomaterials 2025, 15, 613. [Google Scholar] [CrossRef] [PubMed]
- Tayari, F.; Nassar, K.I.; Carvalho, J.P.; Teixeira, S.S.; Hammami, I.; Gavinho, S.R.; Graça, M.P.F.; Valente, M.A. Sol–Gel Synthesis and Comprehensive Study of Structural, Electrical, and Magnetic Properties of BiBaO3 Perovskite. Gels 2025, 11, 450. [Google Scholar] [CrossRef] [PubMed]
- Tayari, F.; Teixeira, S.S.; Graca, M.P.F.; Nassar, K.I. A comprehensive review of recent advances in perovskite materials: Electrical, dielectric, and magnetic properties. Inorganics 2025, 13, 67. [Google Scholar] [CrossRef]
- Iben Nassar, K.; Slimi, M.; Rammeh, N.; Teixeira, S.S.; Graça, M.P.F. Impedance spectroscopic analysis and electrical proprieties in SrAgNiMnO6 double perovskite ceramic. J. Mater. Sci. Mater. Electron. 2022, 33, 20134–20143. [Google Scholar] [CrossRef]
- Iben Nassar, K.; Tayari, F.; Slimi, M.; Rammeh, N.; Njeh, A.; Soreto Teixeira, S.; Graça, M.P.F. Studies on structural, dielectric behavior, and electrical properties of BiBaNiNbO6 double perovskite for electronic applications. J. Mater. Sci. Mater. Electron. 2022, 33, 27147–27157. [Google Scholar] [CrossRef]
- Mohamed, M.; Mohamed, M.; Iben Nassar, K.; Rammeh, N. Effect of the partial substitution of K on the structural, magnetic and magnetocaloric properties of brownmillerite oxides La1−xKxSrMn2O5+δ (0.1 ≤ x ≤ 0.3). Indian J. Phys. 2023, 97, 1717–1725. [Google Scholar] [CrossRef]
- Nassar, K.I.; Rammeh, N. Study of structural, dielectric, and electrical properties of the LaBaFe1.2Mn0.8O6 double perovskite. Indian J. Phys. 2023, 97, 1749–1757. [Google Scholar] [CrossRef]
- Nassar, K.I.; Tayari, F.; Slimi, M.; Rammeh, N.; Njeh, A.; Teixeira, S.S.; Graça, M.P.F. Temperature-and frequency-dependent dielectric and impedance spectroscopy of double perovskite oxide BiBa0.5Ag0.5Ni2O6 electronic material. J. Mater. Sci. Mater. Electron. 2023, 34, 216. [Google Scholar] [CrossRef]
- Tayari, F.; Nassar, K.I.; Maalem, M.B.; Teixeira, S.S.; Graça, M.P.F. Structural, morphology, Raman spectroscopy, magnetic and electrical proprieties of Ba-Ni0.5Mn0.25Fe0.25O3 ceramic for electronic applications. Indian J. Phys. 2023, 97, 3545–3555. [Google Scholar] [CrossRef]
- Ahmer, M.F.; Ullah, Q.; Uddin, M.K. Magnetic metal oxide assisted conducting polymer nanocomposites as eco-friendly electrode materials for supercapacitor applications: A review. J. Polym. Eng. 2025, 45, 1–41. [Google Scholar] [CrossRef]
- Benamara, M.; Nassar, K.I.; Soltani, S.; Kallekh, A.; Dhahri, R.; Dahman, H.; El Mir, L. Light-enhanced electrical behavior of a Au/Al-doped ZnO/p-Si/Al heterostructure: Insights from impedance and current–voltage analysis. RSC Adv. 2023, 13, 28632–28641. [Google Scholar] [CrossRef]
- Liao, H.; Ma, H.; Meng, H.; Kang, N.; Wang, L. Ropinirole suppresses LPS-induced periodontal inflammation by in-hibiting the NAT10 in an ac4C-dependent manner. BMC Oral Health 2024, 24, 510. [Google Scholar] [CrossRef]
- Benamara, M.; Iben Nassar, K.; Rivero-Antúnez, P.; Essid, M.; Soreto Teixeira, S.; Zhao, S.; Serrà, A.; Esquivias, L. Study of electrical and dielectric behaviors of copper-doped zinc oxide ceramic prepared by spark plasma sintering for electronic device applications. Nanomaterials 2024, 14, 402. [Google Scholar] [CrossRef]
- Faouzia, T.; Kais, I.N.; Benamara, M.; Essid, M. Sol–gel synthesized (Bi0.5Ba0.5Ag)0.5(NiMn)0.5O3 perovskite ceramic: An exploration of its structural characteristics, dielectric properties and electrical conductivity. Ceram. Int. 2024, 50, 11207–11215. [Google Scholar]
- Tayari, F.; Iben Nassar, K.; Algessair, S.; Hjiri, M.; Benamara, M. Investigating Fe-doped Ba0.67Ni0.33Mn1−xFexO3 (x = 0, 0.2) ceramics: Insights into electrical and dielectric behaviors. RSC Adv. 2024, 14, 12561–12573. [Google Scholar] [CrossRef]
- Sharma, B. Perovskites in the Womb of Science: Roadmap to Global Market. In Contemporary Advancements in Materials Technology; Apple Academic Press: New York, NY, USA, 2025; pp. 87–104. [Google Scholar]
- Thiagarajan, S.; Sanmugam, A.; Vikraman, D. Facile methodology of sol-gel synthesis for metal oxide nanostructures. In Recent Applications in Sol-Gel Synthesis; IntechOpen: London, UK, 2017; pp. 1–17. [Google Scholar]
- Younis, M.; Abdullah, M.; Dai, S.; Iqbal, M.A.; Tang, W.; Sohail, M.T.; Atiq, S.; Chang, H.; Zeng, Y.J. Magnetoresistance in 2D Magnetic Materials: From Fundamentals to Applications. Adv. Funct. Mater. 2025, 35, 2417282. [Google Scholar] [CrossRef]
- Yu, H.; Zeng, H.; Zhang, Y.; Liu, Y.; ShangGuan, W.; Zhang, X.; Zhang, Z.; Zhang, Y. Two-Dimensional Layered Topological Semimetals for Advanced Electronics and Optoelectronics. Adv. Funct. Mater. 2025, 35, 2412913. [Google Scholar] [CrossRef]
- Ilosvai, A.M.; Dojcsak, D.; Váradi, C.; Nagy, M.; Kristály, F.; Fiser, B.; Viskolcz, B.; Vanyorek, L. Sonochemical combined synthesis of nickel ferrite and cobalt ferrite magnetic nanoparticles and their application in glycan analysis. Int. J. Mol. Sci. 2022, 23, 5081. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Kumar, P.S.; Hemavathy, R.V.; Jeevanantham, S.; Jawahar, M.J.; Neshaanthini, J.P.; Saravanan, R. A review on synthesis methods and recent applications of nanomaterial in wastewater treatment: Challenges and future perspectives. Chemosphere 2022, 307, 135713. [Google Scholar] [CrossRef] [PubMed]
- Navas, D.; Fuentes, S.; Castro-Alvarez, A.; Chavez-Angel, E. Review on sol-gel synthesis of perovskite and oxide nanomaterials. Gels 2021, 7, 275. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, M.; Li, D.; Wu, R.; Lin, R.; Yang, C. An AUV-enabled dockable platform for long-term dynamic and static monitoring of marine pastures. IEEE J. Ocean. Eng. 2024, 50, 276–293. [Google Scholar] [CrossRef]
- Abidin, M.Z.U.; Ikram, M.; Moeen, S.; Nazir, G.; Kanoun, M.B.; Goumri-Said, S. A comprehensive review on the synthesis of ferrite nanomaterials via bottom-up and top-down approaches: Advantages, disadvantages, characterizations and computational insights. Coord. Chem. Rev. 2024, 520, 216158. [Google Scholar] [CrossRef]
- Leng, K.; Xia, W.; Tang, Q.; Yang, L.; Xie, Y.; Wu, Z.; Yi, K.; Zhu, X. Structural, dielectric, magnetic and optical properties of double perovskite oxide Sm2NiMnO6 nanoparticles synthesized by a sol−gel process. Nanotechnology 2021, 32, 285703. [Google Scholar] [CrossRef] [PubMed]
- Borlaf, M.; Moreno, R. Colloidal sol-gel: A powerful low-temperature aqueous synthesis route of nanosized powders and suspensions. Open Ceram. 2021, 8, 100200. [Google Scholar] [CrossRef]
- Bera, S.; Udayabhanu, G.; Narayan, R.; Rout, T.K. Sol–gel process for anticorrosion coatings. J. Res. Updates Polym. Sci. 2013, 2, 209–231. [Google Scholar] [CrossRef]
- Devesa, S. Comparative Overview of Metal Oxide Nanoparticle Synthesis Methods: Conventional Sol-Gel Versus Green Approaches. In Sol-Gel—A Versatile Wide Technol; IntechOpen: London, UK, 2025. [Google Scholar]
- Myasoedova, T.N.; Kalusulingam, R.; Mikhailova, T.S. Sol-gel materials for electrochemical applications: Recent advances. Coatings 2022, 12, 1625. [Google Scholar] [CrossRef]
- Smith, D.K. Supramolecular gels–a panorama of low-molecular-weight gelators from ancient origins to next-generation technologies. Soft Matter 2024, 20, 10–70. [Google Scholar] [CrossRef]
- Şahin, İ.; Özbakır, Y.; Inönü, Z.; Ulker, Z.; Erkey, C. Kinetics of supercritical drying of gels. Gels 2017, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhu, M.; Li, Y. Sol–gel research in China: A brief history and recent research trends in synthesis of sol–gel derived materials and their applications. J. Sol-Gel Sci. Technol. 2023, 106, 406–421. [Google Scholar] [CrossRef]
- Adul-Rasool, A.A.; Athair, D.M.; Zaidan, H.K.; Rheima, A.M.; Al-Sharify, Z.T.; Mohammed, S.H. 0, 1, 2, 3D nanostructures, Types of bulk nanostructured materials, and drug nanocrystals: An overview. Cancer Treat. Res. Commun. 2024, 40, 100834. [Google Scholar]
- Kindikah, I.; Nnyia, M.O.; Daniel, S.; Odebiyi, O.S.; Iliya, M. 2D Binary Transition Metal Iodides: Advances in Synthesis and Device Applications. Nano Trends 2025, 10, 100105. [Google Scholar] [CrossRef]
- Rahimi, H.; Ghasemi, A.; Mozaffarinia, R.; Tavoosi, M. Magnetic properties and magnetization reversal mechanism of Nd-Fe-B nanoparticles synthesized by a sol-gel method. J. Magn. Magn. Mater. 2017, 444, 111–118. [Google Scholar] [CrossRef]
- Gaweł, B.; Gaweł, K.; Øye, G. Sol-Gel Synthesis of Non-Silica Monolithic Materials. Materials 2010, 3, 2815–2833. [Google Scholar] [CrossRef]
- Kessler, V.G. Metal Alkoxides as Models for Metal Oxides—The Concept Revisited. J. Sol-Gel Sci. Technol. 2024, 112, 502–511. [Google Scholar] [CrossRef]
- Ghaani, M.R.; Mohtasebi, A.M.; Tajeri, R.; Marashi, P. A Comparison of the Role of the Chelating Agent on the Structure of Lithium Conducting Solid Electrolyte Li1.4Al0.4Ti1.6(PO4)3: Pechini vs. Modified Pechini-Type Methods. Batteries 2020, 6, 48. [Google Scholar] [CrossRef]
- Dimesso, L. Pechini Processes: An Alternate Approach of the Sol-Gel Method, Preparation, Properties, and Applications. In Handbook of Sol-Gel Science and Technology; Springer: Cham, Switzerland, 2018; pp. 1067–1088. [Google Scholar]
- Bhagwat, V.R.; Humbe, A.V.; More, S.D.; Jadhav, K.M. Sol-Gel Auto Combustion Synthesis and Characterizations of Cobalt Ferrite Nanoparticles: Different Fuels Approach. Mater. Sci. Eng. B 2019, 248, 114388. [Google Scholar] [CrossRef]
- Tangcharoen, T. Calcination Temperature-Dependent Structural, Optical, and Photoluminescence Properties of Mg-Al Bimetallic Oxide Prepared by Sol-Gel Auto Combustion Method. J. Magn. Alloys 2025, 13, 2884–2899. [Google Scholar] [CrossRef]
- Kimling, M.C.; Caruso, R.A. Sol–Gel Synthesis of Hierarchically Porous TiO2 Beads Using Calcium Alginate Beads as Sacrificial Templates. J. Mater. Chem. 2012, 22, 4073–4082. [Google Scholar] [CrossRef]
- Estévez Ruiz, E.P.; López Lago, J.; Thirumuruganandham, S.P. Experimental studies on TiO2 NT with metal dopants through co-precipitation, sol–gel, hydrothermal scheme and corresponding computational molecular evaluations. Materials 2023, 16, 3076. [Google Scholar] [CrossRef]
- Naziba, A.T.; Nafisa, M.T.; Sultana, R.; Ehsan, M.F.; Tareq, A.R.; Rashid, R.; Das, H.; Ullah, A.K.M.A.; Kibria, A.K.M.F. Structural, Optical, and Magnetic Properties of Co-Doped ZnO Nanorods: Advancements in Room Temperature Ferromagnetic Behavior for Spintronic Applications. J. Magn. Magn. Mater. 2024, 593, 171836. [Google Scholar] [CrossRef]
- Luo, Z.; Rong, P.; Yang, Z.; Zhang, J.; Zou, X.; Yu, Q. Preparation and Application of Co-Doped Zinc Oxide: A Review. Molecules 2024, 29, 3373. [Google Scholar] [CrossRef] [PubMed]
- Birajdar, S.D.; Khirade, P.P.; Bhagwat, V.R.; Humbe, A.V.; Jadhav, K.M. Synthesis, Structural, Morphological, Optical and Magnetic Properties of Zn1−xCoxO (0 ≤ x ≤ 0.36) Nanoparticles Synthesized by Sol-Gel Auto Combustion Method. J. Alloys Compd. 2016, 683, 513–526. [Google Scholar] [CrossRef]
- Acosta-Humánez, M.F.; Magon, C.J.; Montes-Vides, L.; Jiménez, J.; Almanza, O. Structural, Magnetic, Optical and Photocatalytic Properties of Co-Doped ZnO Nanocrystals. Int. J. Mol. Sci. 2025, 26, 2117. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.J.; Lau, L.N.; Lim, K.P.; Hon, X.T.; Daud, N.A.A.; Kechik, M.M.A.; Chen, S.K.; Shabdin, M.K.B.; Shaari, A.H.; Miryala, M. Characterisations of La-Sr-Mn-O (LSMO) Thin Film Fabricated by RF Sputtering. Coatings 2023, 13, 541. [Google Scholar] [CrossRef]
- Guo, L.; Bo, L.; Li, Y.; Jiang, Z.; Tian, Y.; Li, X. Sr Doping Effect on the Structure Property and NO Oxidation Performance of Dual-Site Doped Perovskite La(Sr)Co(Fe)O3. Solid State Sci. 2021, 113, 106519. [Google Scholar] [CrossRef]
- Smari, M.; Moisiuc, M.V.; Al-Haik, M.Y.; Astefanoaei, I.; Stancu, A.; Shelkovyi, F.; Gimaev, R.; Piashova, J.; Zverev, V.; Haik, Y. Tunable Magnetic Heating in La0.51Sr0.49MnO3 and La0.51Dy0.045Sr0.445MnO3 Nanoparticles: Frequency- and Amplitude-Dependent Behavior. Nanomaterials 2025, 15, 642. [Google Scholar] [CrossRef]
- Jesus, J.; Regadas, J.; Costa, B.; Carvalho, J.; Pádua, A.; Henriques, C.; Soares, P.I.P.; Gavinho, S.; Valente, M.A.; Graça, M.P.F.; et al. Green Sol–Gel Synthesis of Iron Oxide Nanoparticles for Magnetic Hyperthermia Applications. Pharmaceutics 2024, 16, 1578. [Google Scholar] [CrossRef]
- Nguyen, M.D.; Tran, H.V.; Xu, S.; Lee, T.R. Fe3O4 Nanoparticles: Structures, Synthesis, Magnetic Properties, Surface Functionalization, and Emerging Applications. Appl. Sci. 2021, 11, 11301. [Google Scholar] [CrossRef]
- Ganapathe, L.S.; Mohamed, M.A.; Mohamad Yunus, R.; Berhanuddin, D.D. Magnetite (Fe3O4) Nanoparticles in Biomedical Application: From Synthesis to Surface Functionalisation. Magnetochemistry 2020, 6, 68. [Google Scholar] [CrossRef]
- Guragain, D.; Rai, B.K.; Yoon, S.; Poudel, T.P.; Bhandari, S.C.; Mishra, S.R. Effect of Terbium Ion Substitution in Inverse Spinel Nickel Ferrite: Structural and Magnetic Study. Magnetochemistry 2020, 6, 14. [Google Scholar] [CrossRef]
- Naik, P.R.; Rajashekara, V.A.; Mudhulu, S.; Channegowda, M. Uranium Removal from Water by Adsorption: A Detailed Characterisation and Parametric Study Using Nickel Ferrite (NiFe2O4) Nanoadsorbent. Environ. Pollut. Bioavailab. 2025, 37, 2515293. [Google Scholar] [CrossRef]
- Sharma, P.; Solanki, M.B.; Thakor, S.; Jain, P.; Zapadiya, A.; Shah, D.V.; Vaja, C. Comprehensive Study of Spinel Nanoparticles Using Experimental, Theoretical and Machine Learning Methods. Phys. B Condens. Matter 2025, 699, 416820. [Google Scholar] [CrossRef]
- Boussafel, H.; Sedrati, C.; Alleg, S. Green Synthesis of NiFe2O4, CoFe2O4, and Ni0.5Co0.5Fe2O4 by Sol–Gel Autocombustion Method Using Olive Leaf Extract as Fuel. Appl. Phys. A 2024, 130, 374. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Oxygen Vacancy and Dopant Concentration Dependent Magnetic Properties of Mn Doped TiO2 Nanoparticle. Curr. Appl. Phys. 2013, 13, 1025–1031. [Google Scholar] [CrossRef]
- Adedokun, O.; Sivaprakash, P.; Ajani, A.S.; Bello, I.T.; Arumugam, S. Structural, Optical and Magnetic Studies of Sol-Gel Synthesized Mg-Doped Pure Anatase TiO2 Nanoparticles for Spintronic and Optoelectronics Applications. Phys. B Condens. Matter 2023, 667, 415199. [Google Scholar] [CrossRef]
- Tsega, M. Structural and Optical Properties of Mn-Doped Anatase-Rutile TiO2 Nanoparticles. Indian J. Phys. 2025, 99, 177–189. [Google Scholar] [CrossRef]
- Kumaran, C.; Baskaran, I.; Sathyaseelan, B.; Senthilnathan, K.; Manikandan, E.; Sambasivam, S. Effect of Doping of Iron on Structural, Optical and Magnetic Properties of CeO2 Nanoparticles. Chem. Phys. Lett. 2022, 808, 140110. [Google Scholar] [CrossRef]
- Kumar, S.; Ahmed, F.; Ahmad, N.; Shaalan, N.M.; Kumar, R.; Alshoaibi, A.; Arshi, N.; Dalela, S.; Alvi, P.A.; Kumari, K. Influence of Fe and Cu Co-Doping on Structural, Magnetic and Electrochemical Properties of CeO2 Nanoparticles. Materials 2022, 15, 4119. [Google Scholar] [CrossRef] [PubMed]
- Ozugurlu, E. Cd-Doped ZnO Nanoparticles: An Experimental and First-Principles DFT Studies. J. Alloys Compd. 2021, 861, 158620. [Google Scholar] [CrossRef]
- Muskan, A.; Singh, R.K.; Kumar, N.; Kumar, P.; Monalisa. Analogous Behaviour of Nd3+ Rare Earth Substituted Tuned Structural, Stability, Magnetic and Ferroelectric Properties of CoFe2O4 Ferrite Nanomaterials for Its Multifunctional Application, Synthesized Using Green Approach. J. Mater. Sci. Mater. Electron. 2024, 35, 1597. [Google Scholar] [CrossRef]
- Bokov, D.; Jalil, A.T.; Chupradit, S.; Suksatan, W.; Ansari, M.J.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by Sol-Gel Method: Synthesis and Application. Adv. Mater. Sci. Eng. 2021, 2021, 5102014. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Q.; Hong, J.; Dong, Z.; Wang, J. Degradation of Bisphenol A by Persulfate Activation via Oxygen Vacancy-Rich CoFe2O4–x. Chemosphere 2019, 221, 412–422. [Google Scholar] [CrossRef]
- Yang, K.; Li, C.; Zhu, Q.; Wang, H.; Qi, J. Rich Oxygen Vacancies in Bimetallic MnCo2O4.5 Spheres for Enhancing Lean Methane Catalytic Oxidation. Nanomaterials 2025, 15, 524. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Chen, P.-C. P-Type Spinel ZnCo2O4 Thin Films Prepared Using Sol-Gel Process. Appl. Surf. Sci. 2020, 505, 144460. [Google Scholar] [CrossRef]
- Riaz, S.; Shah, S.M.H.; Akbar, A.; Atiq, S.; Naseem, S. Effect of Mn Doping on Structural, Dielectric and Magnetic Properties of BiFeO3 Thin Films. J. Sol-Gel Sci. Technol. 2015, 74, 329–339. [Google Scholar] [CrossRef]
- Yang, N.; Orgiani, P.; Di Bartolomeo, E.; Foglietti, V.; Torelli, P.; Ievlev, A.V.; Rossi, G.; Licoccia, S.; Balestrino, G.; Kalinin, S.V.; et al. Effects of Dopant Ionic Radius on Cerium Reduction in Epitaxial Cerium Oxide Thin Films. J. Phys. Chem. C 2017, 121, 8841–8849. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Brahma, S.; Wu, S.; Huang, J.L.; Lee, A.C. Crystal Structure Evolution of Piezoelectric Fe-Doped ZnO Film by Magnetron Co-Sputtering Technique. Condens. Matter 2025, 10, 6. [Google Scholar] [CrossRef]
- Brand, E.; Rosendal, V.; Wu, Y.; Tran, T.; Palliotto, A.; Maznichenko, I.V.; Ostanin, S.; Esposito, V.; Ernst, A.; Zhou, S.; et al. Defect-Induced Magnetic Symmetry Breaking in Oxide Materials. Appl. Phys. Rev. 2025, 12, 1. [Google Scholar] [CrossRef]
- Groppo, E.; Rojas-Buzo, S.; Bordiga, S. The Role of In Situ/Operando IR Spectroscopy in Unraveling Adsorbate-Induced Structural Changes in Heterogeneous Catalysis. Chem. Rev. 2023, 123, 12135–12169. [Google Scholar] [CrossRef]
- Han, Y.; Lao, B.; Zheng, X.; Li, S.; Li, R.W.; Wang, Z. Transition Metal Oxides: A New Frontier in Spintronics Driven by Novel Quantum States and Efficient Charge-Spin Interconversion. Front. Mater. 2024, 11, 1444769. [Google Scholar] [CrossRef]
- Sinha, S.; Singh, M.; Singh, R. ZnO Based Diluted Magnetic Semiconductors for Spintronic Device Applications: A Review. Int. J. Emerg. Res. Manag. Technol. 2015, 4, 16–20. [Google Scholar]
- Gupta, A.; Zhang, R.; Kumar, P.; Kumar, V.; Kumar, A. Nano-Structured Dilute Magnetic Semiconductors for Efficient Spintronics at Room Temperature. Magnetochemistry 2020, 6, 15. [Google Scholar] [CrossRef]
- Sundaram, G.A.; Kanniah, R.; Karthikeyan, V. Tuning the Magnetic and Photocatalytic Properties of Wide Bandgap Metal Oxide. In Updates on Titanium Dioxide; IntechOpen: London, UK, 2023; p. 133. [Google Scholar]
- Yıldırım, O. Effect of Microstructure on the Magnetic Properties of Transition Metal Implanted TiO2 Films. Ph.D. Thesis, Technische Universität Dresden, Dresden, Germany, 2016. [Google Scholar]
- Yi, X.; Zhao, R.; Lin, Y. The impact of nighttime car body lighting on pedestrians’ distraction: A virtual real-ity simulation based on bottom-up attention mechanism. Saf. Sci. 2024, 180, 106633. [Google Scholar] [CrossRef]
- Zhu, X. Methods for the Syntheses of Perovskite Magnetic Nanomagnets. In Fundamentals of Low Dimensional Magnets; CRC Press: Boca Raton, FL, USA, 2022; pp. 125–160. [Google Scholar]
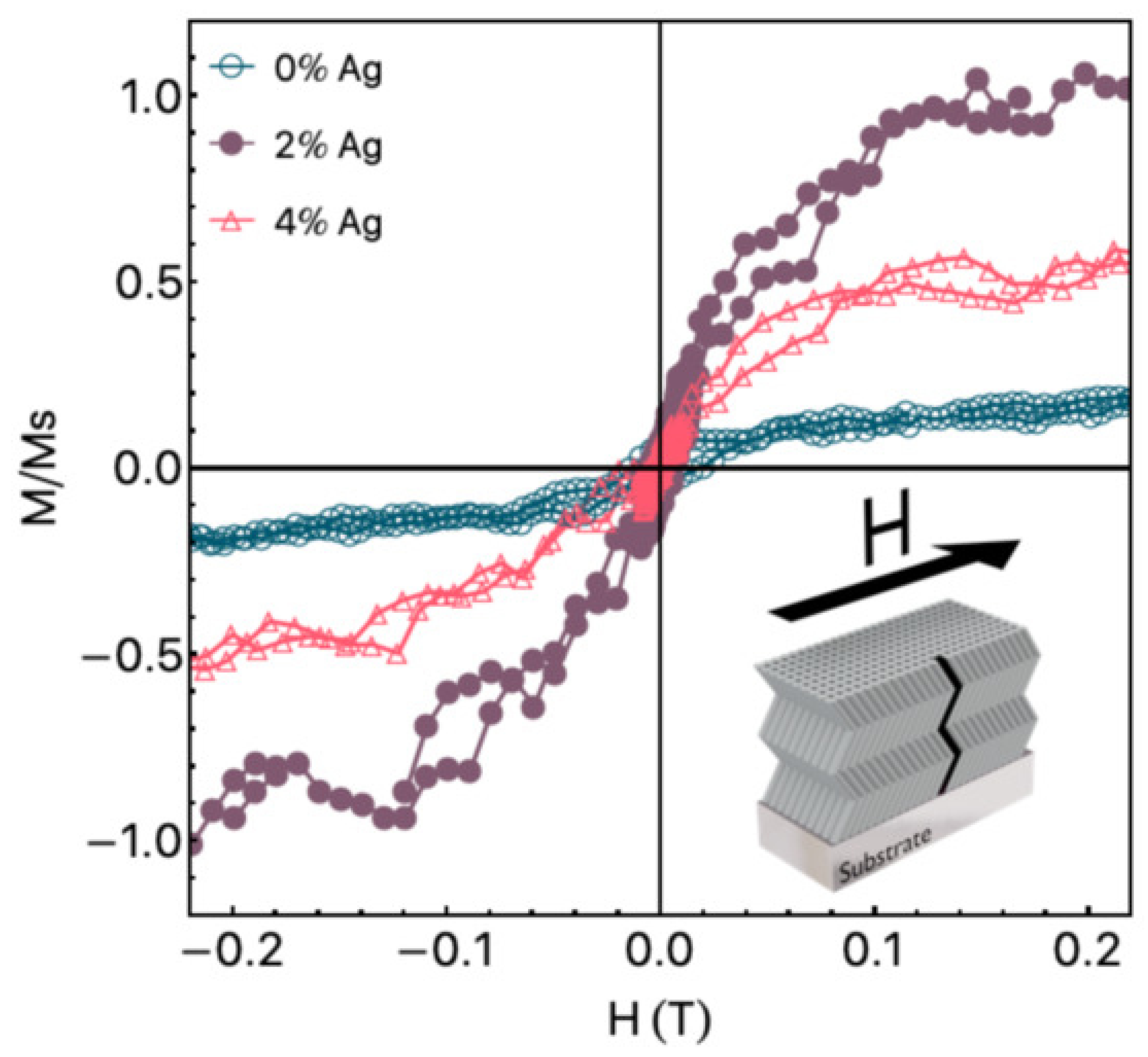
- Correa, M.A.; Ferreira, A.; Tromer, R.M.; Machado, L.D.; Gamino, M.; França Junior, S.A.N.; Bohn, F.; Vaz, F. Improving the Room-Temperature Ferromagnetism in ZnO and Low-Doped ZnO: Ag Films Using GLAD Sputtering. Materials 2021, 14, 5337. [Google Scholar] [CrossRef]
- Xie, Q.; Yu, H.; Yuan, H.; Han, G.; Chen, X.; Liu, Z. Enhanced Magnetic Properties and Thermal Conductivity of FESICR Soft Magnetic Composite with Al2O3 Insulation Layer Prepared by Sol-Gel Process. Metals 2023, 13, 813. [Google Scholar] [CrossRef]
- Ghosh, N.; Deb, D.; Dey, P. Optically Tunable Magnetoresistance Properties in La0.7Sr0.3MnO3-Glass Nanocomposites: A Step towards Optospintronics. J. Mater. Sci. Mater. Electron. 2024, 35, 2223. [Google Scholar] [CrossRef]
- Elilarassi, R.; Chandrasekaran, G. Optical, Electrical and Ferromagnetic Studies of ZnO: Fe Diluted Magnetic Semiconductor Nanoparticles for Spintronic Applications. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 186, 120–131. [Google Scholar] [CrossRef]
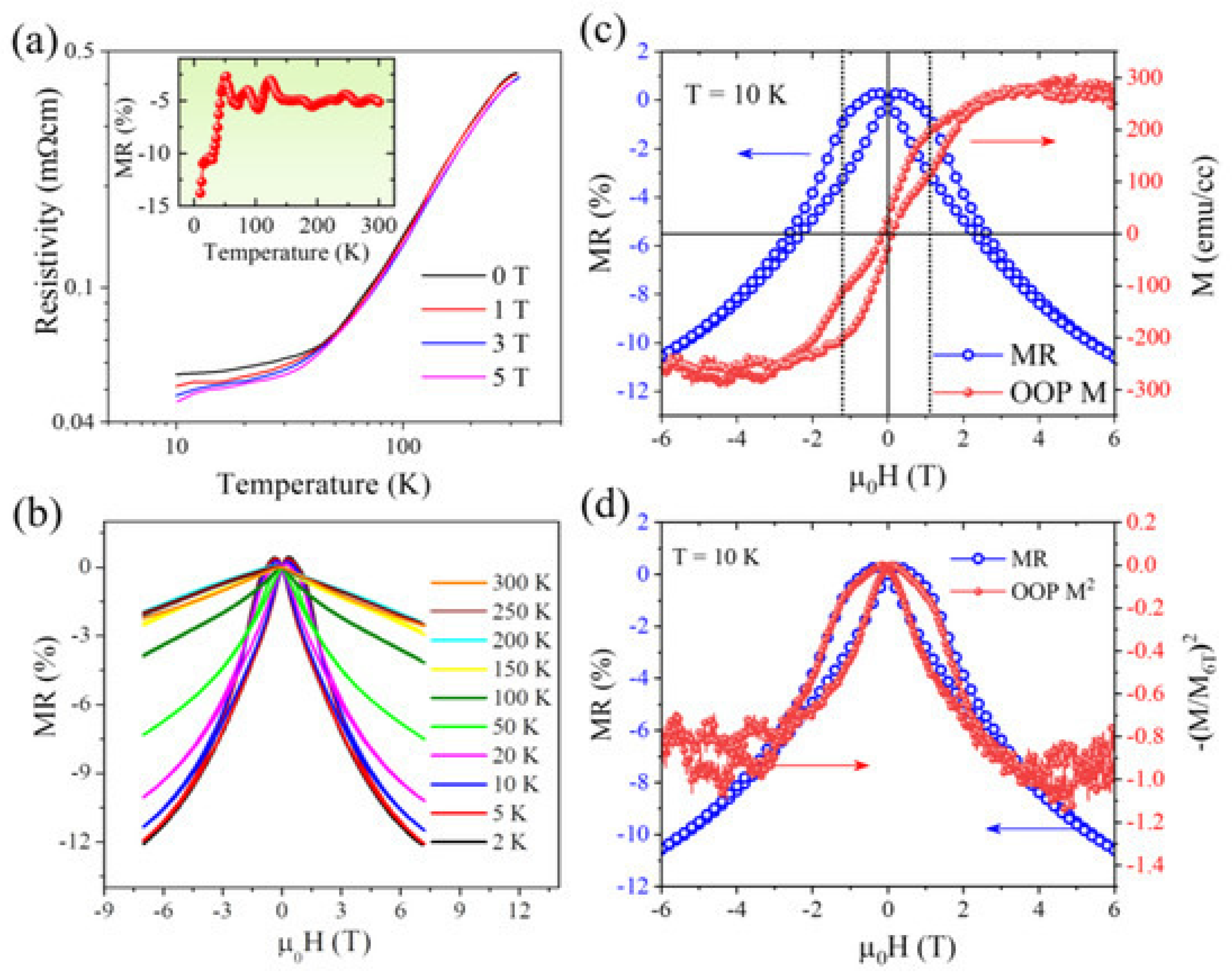
- Muchharla, B.; Madhogaria, R.P.; Detellem, D.; Hung, C.-M.; Chanda, A.; Mudiyanselage, N.W.Y.A.Y.; Duong, A.T.; Trinh, M.-T.; Witanachchi, S.; Phan, M.-H. Intergranular Spin Dependent Tunneling Dominated Magnetoresistance in Helimagnetic Manganese Phosphide Thin Films. Nanomaterials 2023, 13, 1478. [Google Scholar] [CrossRef]
- Vlăduț, C.M.; Anastasescu, C.; Preda, S.; Mocioiu, O.C.; Petrescu, S.; Pandele-Cusu, J.; Culita, D.; Bratan, V.; Balint, I.; Zaharescu, M. Mn-Doped ZnO Nanopowders Prepared by Sol–Gel and Microwave-Assisted Sol–Gel Methods and Their Photocatalytic Properties. Beilstein J. Nanotechnol. 2024, 15, 1283–1296. [Google Scholar] [CrossRef] [PubMed]
- Lonardi, L.; Lew-Tong, C.; Miranda, B.D.; Sahukari, B.; Poddar, H.; Satheesh, K.; Chadha, U. Toward a Circular Nanotechnology for Biofuels: Integrating Sustainable Synthesis, Recovery, and Performance Optimization. arXiv 2025, arXiv:2506.17548. [Google Scholar] [CrossRef]
- Elsheikh, N.Y.; Shams, M.S.; Arais, A.A.; Battisha, I.K. Structural, Morphological, and Magnetic Study of Transition Metals Co-Doped ZnFeO Nanocomposites Prepared by Sol-Gel Method for Spintronic Applications. Ceram. Int. 2025, 51, 16099–16108. [Google Scholar] [CrossRef]
- Vashisht, A.; Gupta, I.; Singla, S. 4 Piezoelectric. In Nano-Dimensional Zinc Oxide for Energy Harvesting, Sensing, and Environmental Remediation; Chapman and Hall/CRC: Boca Raton, FL, USA, 2025; p. 61. [Google Scholar]

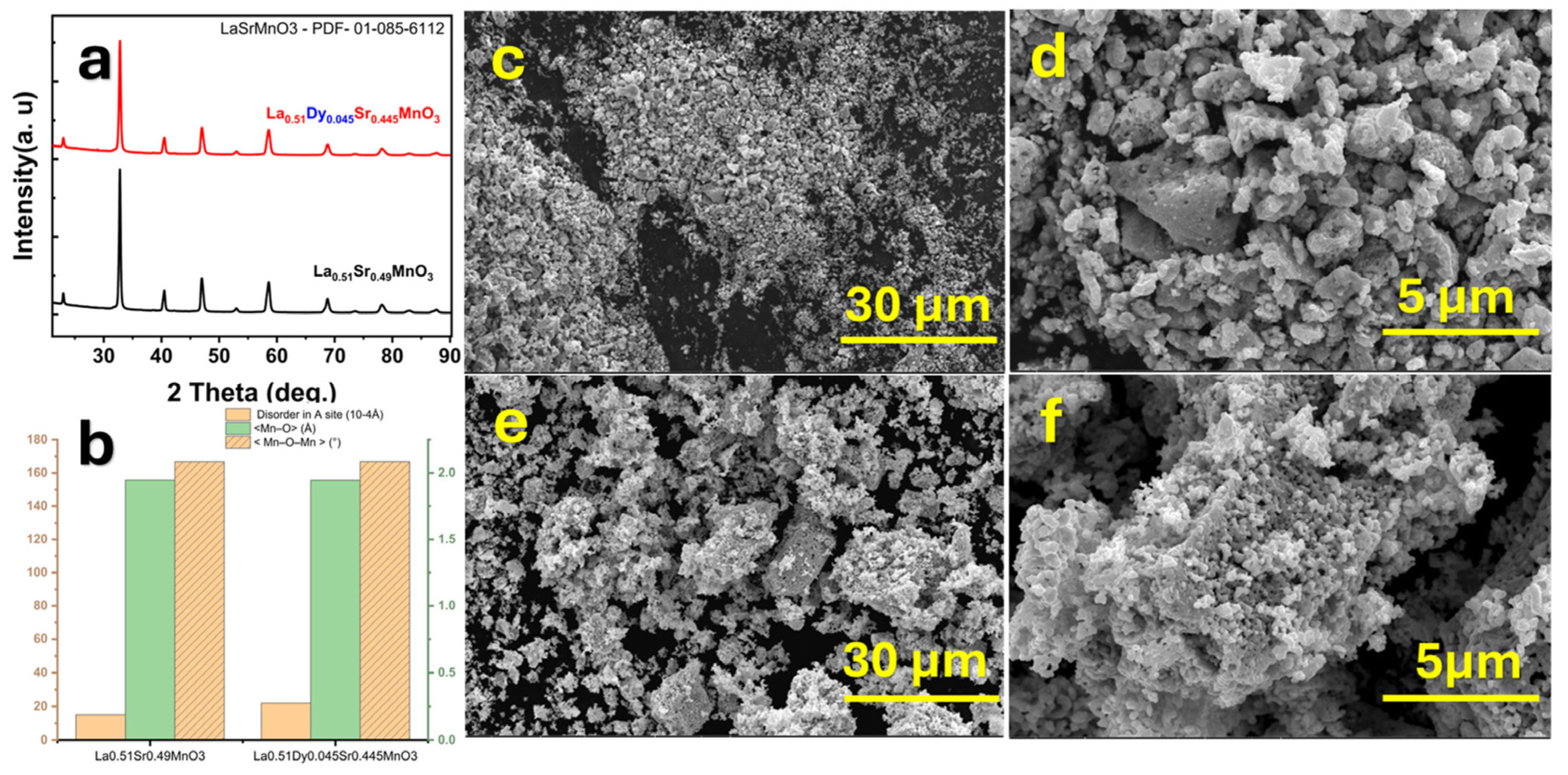
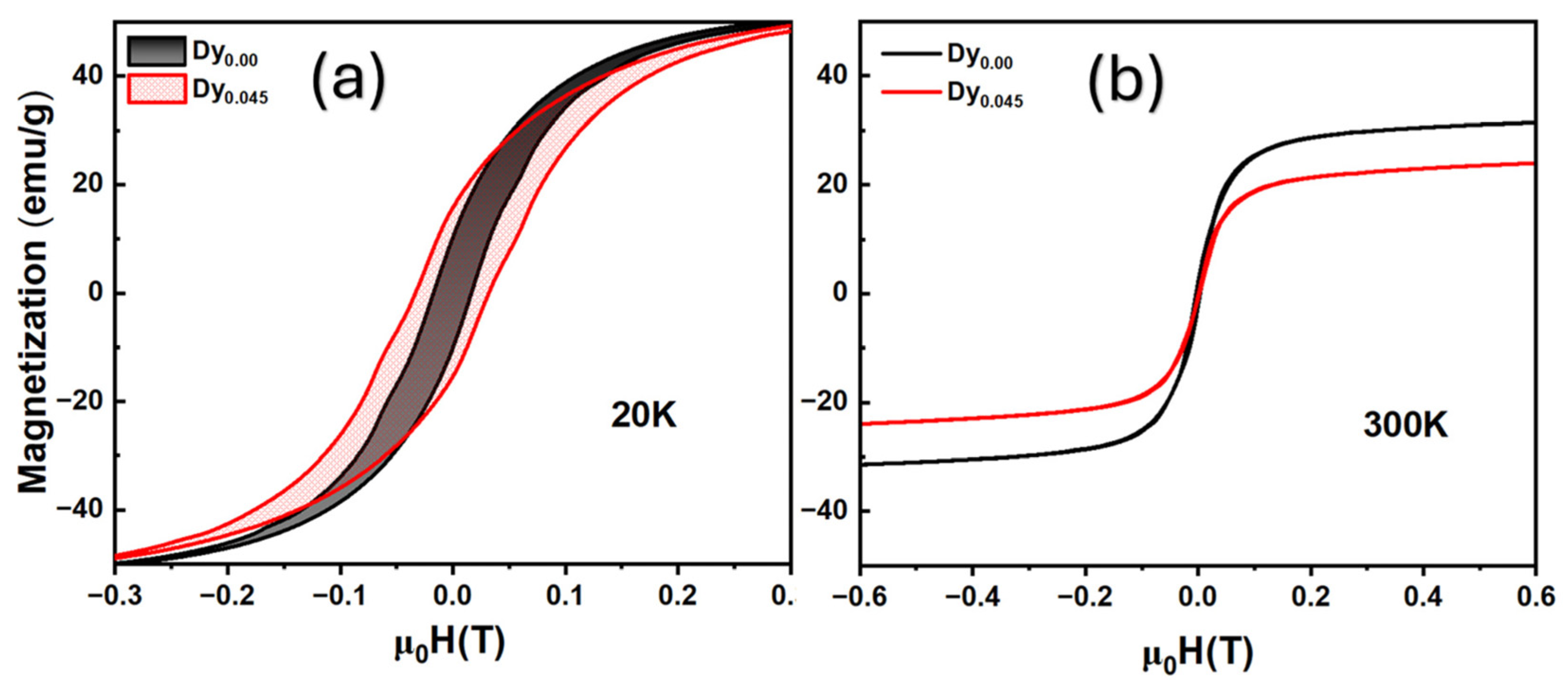
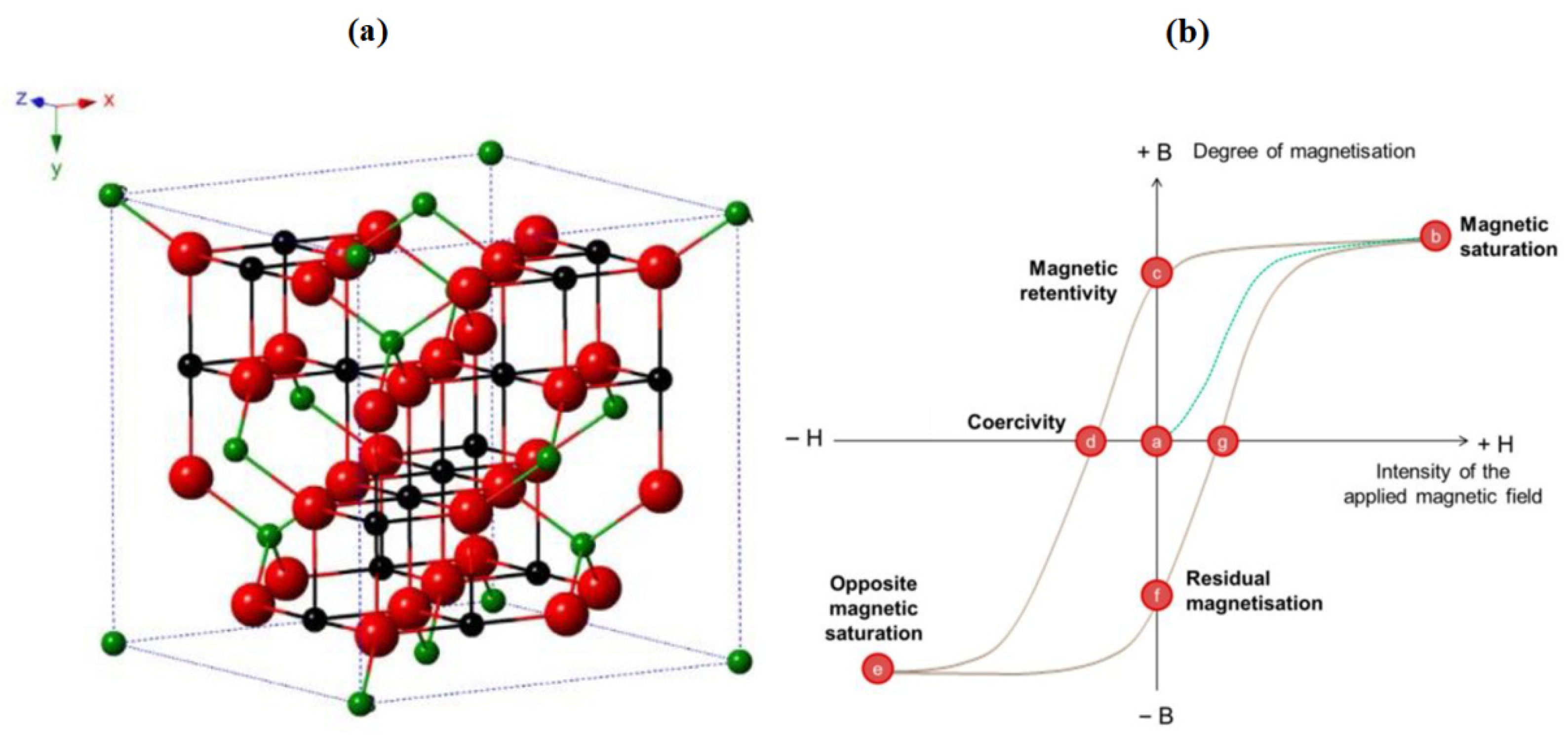


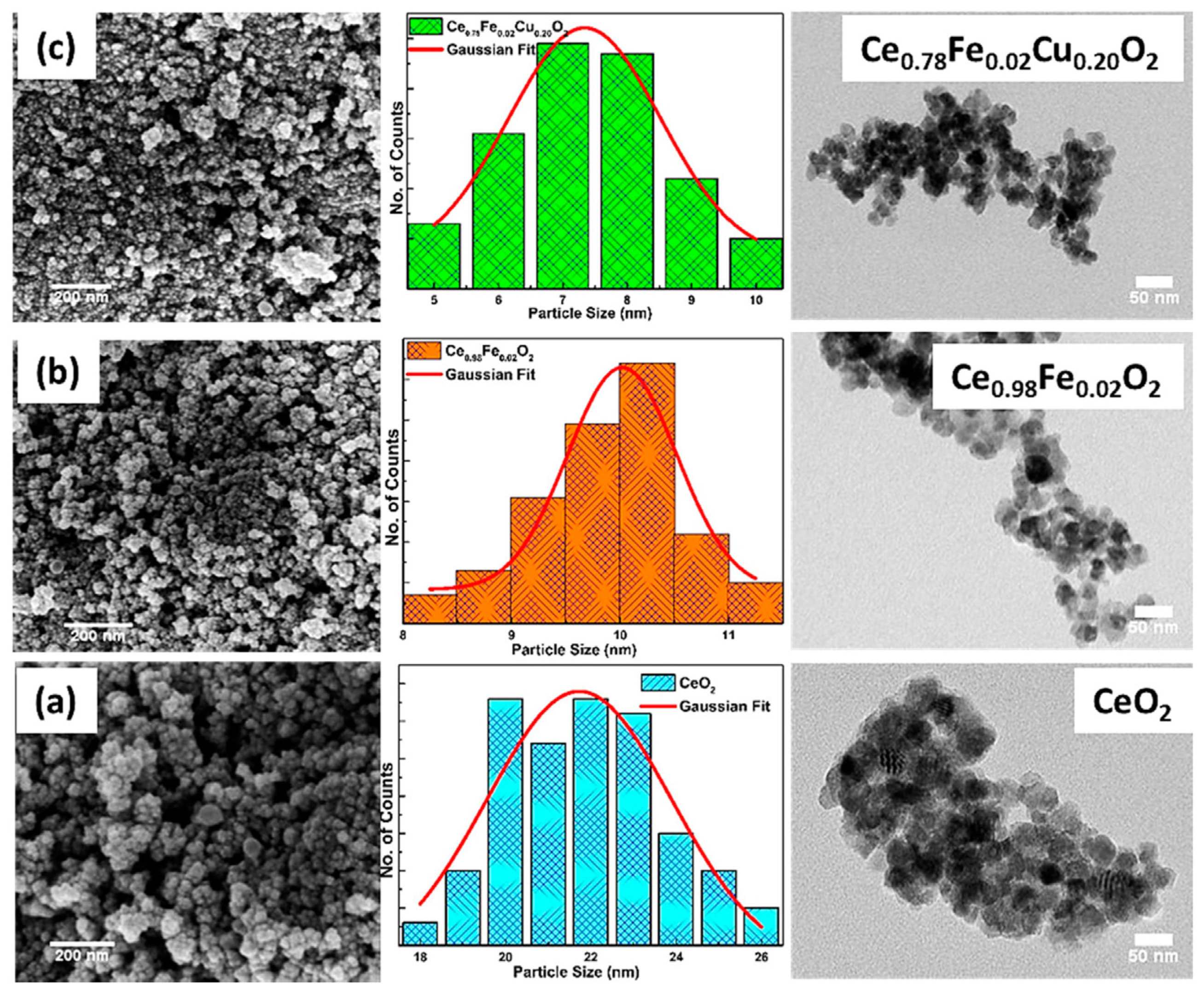
| Aspect | Advantages | Disadvantages | Spintronic Relevance |
|---|---|---|---|
| Temperature | Low-temperature processing (<600 °C) | May require post-annealing for full crystallization | Enables compatibility with flexible substrates and CMOS platforms |
| Dopant Control | Atomic-scale mixing ensures uniform dopant distribution | Dopant clustering or segregation may occur | Crucial for tuning magnetic uniformity and room-temperature FM |
| Scalability | Scalable for large-area coatings and batch synthesis | Film uniformity over large substrates is still challenging | Allows wafer-scale or device-scale fabrication of spintronic layers |
| Cost | Uses inexpensive precursors and low-energy processing | Some alkoxides can be air/moisture-sensitive and expensive | Reduces fabrication cost for practical spintronic devices |
| Versatility | Applicable to a wide range of oxides, morphologies, composites | Limited to materials that form stable sol–gel networks | Enables multifunctionality (e.g., magneto-optic, magnetoelectric) |
| Defect Engineering | Facilitates oxygen vacancy control and defect-induced magnetism | Difficult to isolate intrinsic from extrinsic magnetic effects | Key for inducing spin polarization via oxygen vacancies |
| Phase Purity | High compositional homogeneity reduces unwanted phases | Secondary phases (e.g., metallic clusters) may still form | Prevents spurious magnetic signals in spintronic measurements |
| Device Compatibility | Amenable to deposition on silicon, glass, flexible substrates | Drying, shrinkage, and cracking can hinder film quality | Critical for integration into memory and sensor architectures |
| Method | Temperature (°C) | Dopant Control | Phase Purity | Scalability | Spintronic Relevance | Cost |
|---|---|---|---|---|---|---|
| Sol–Gel | 400–600 | High (molecular level) | High | High | Good (DMS, ferrites, thin films) | Low |
| Hydrothermal | 100–250 | Moderate | High | Moderate | Moderate (nanorods, hierarchical) | Moderate |
| Solid-State | >1000 | Low | Low | High | Low (poor dopant uniformity) | Low |
| CVD | 600–1000 | High | High | Low | High (thin films, precise layering) | High |
| Oxide System | Dopants | Sol–Gel Variant | Morphology | Magnetic Behavior | Spintronic Relevance |
|---|---|---|---|---|---|
| ZnO | Co2+ | Alkoxide, nitrate-based | Nanoparticles (10–20 nm) | Room-temperature ferromagnetism | DMS, spin filters, photocatalysis |
| TiO2 | Mn, Mg | Citrate–nitrate, alkoxide | Thin films, nanocrystals | Defect-mediated FM | Spin filters, magneto-optics |
| La1−xSrxMnO3 | Dy3+ | Pechini (polymeric gel) | Porous nanoparticles | Double-exchange FM | Colossal MR, tunnel junctions |
| NiFe2O4, CoFe2O4 | Ni2+, Co2+ | Green auto-combustion | Porous spinel powders | Tunable anisotropy, exchange bias | Spin valves, high-frequency devices |
| CeO2 | Fe3+, Cu2+ | Citrate-assisted sol–gel | Ultra-fine particles (5–10 nm) | F-center exchange FM | Redox-active spintronic devices |
| Parameter | Effect on Magnetic Behavior |
|---|---|
| Calcination temperature | Influences crystallite size, phase formation, and defect structure |
| Dopant concentration | Controls magnetic ordering, dopant distribution, and potential secondary phases |
| pH of solution | Affects particle size distribution and gel homogeneity |
| Atmosphere (air, N2, O2) | Modulates oxidation state, oxygen vacancies, and defect-induced magnetism |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nassar, K.I.; Teixeira, S.S.; Graça, M.P.F. Sol–Gel-Synthesized Metal Oxide Nanostructures: Advancements and Prospects for Spintronic Applications—A Comprehensive Review. Gels 2025, 11, 657. https://doi.org/10.3390/gels11080657
Nassar KI, Teixeira SS, Graça MPF. Sol–Gel-Synthesized Metal Oxide Nanostructures: Advancements and Prospects for Spintronic Applications—A Comprehensive Review. Gels. 2025; 11(8):657. https://doi.org/10.3390/gels11080657
Chicago/Turabian StyleNassar, Kais Iben, Sílvia Soreto Teixeira, and Manuel P. F. Graça. 2025. "Sol–Gel-Synthesized Metal Oxide Nanostructures: Advancements and Prospects for Spintronic Applications—A Comprehensive Review" Gels 11, no. 8: 657. https://doi.org/10.3390/gels11080657
APA StyleNassar, K. I., Teixeira, S. S., & Graça, M. P. F. (2025). Sol–Gel-Synthesized Metal Oxide Nanostructures: Advancements and Prospects for Spintronic Applications—A Comprehensive Review. Gels, 11(8), 657. https://doi.org/10.3390/gels11080657








