Tannic Acid-Enhanced Gelatin-Based Composite Hydrogel as a Candidate for Canine Periodontal Regeneration
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Characterization of Hydrogels
2.2. Cell Response to Tannic–Gelatin-Based Hydrogels
2.2.1. Cytotoxicity
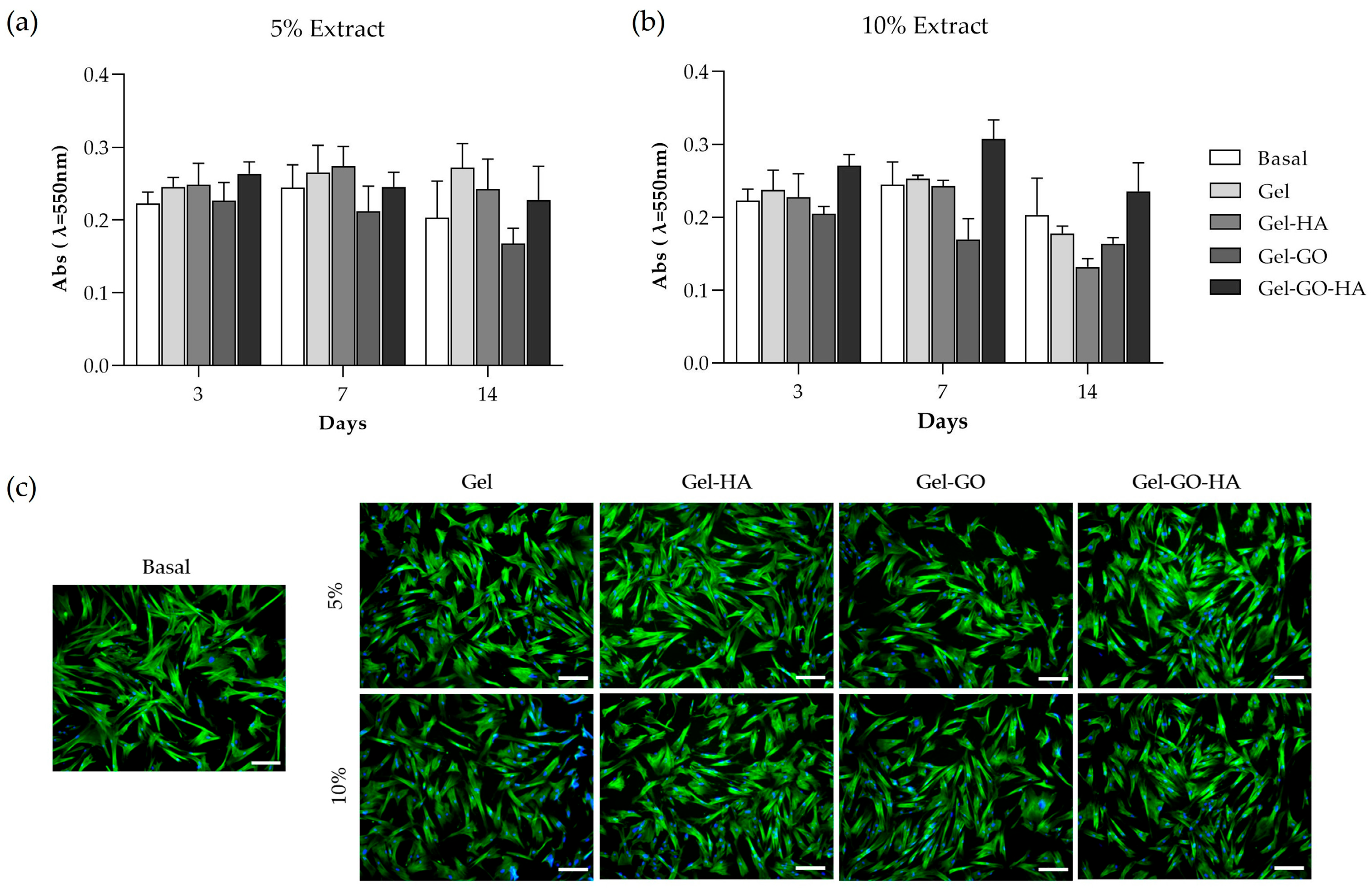
2.2.2. Metabolic Activity and Morphology of Canine PDL-Derived Cells
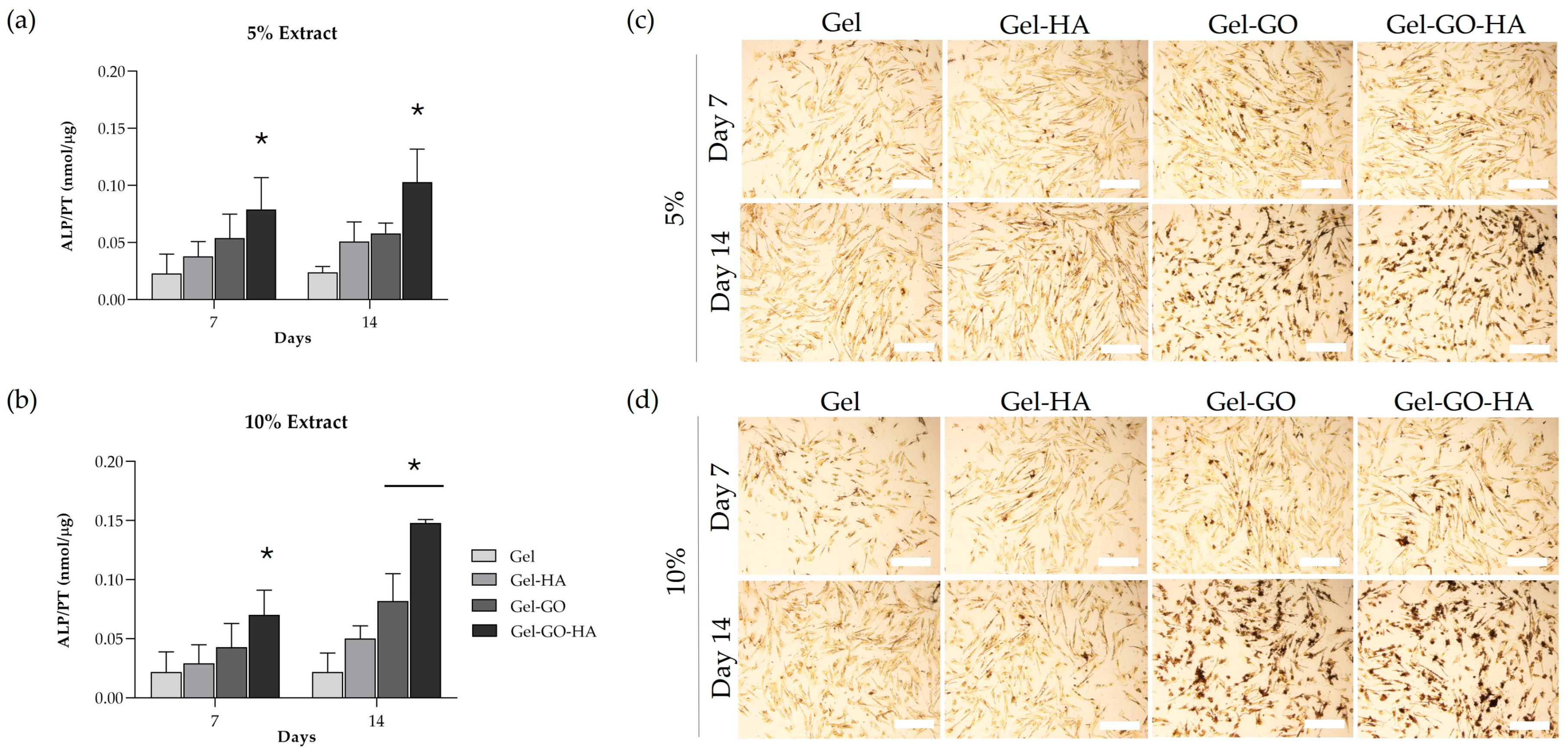
2.2.3. Osteogenic Response of Canine PDL-Derived Cells
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Production of Hydroxyapatite Nanoparticles
4.3. Preparation of Tannic Acid–Gelatin-Based Hydrogels’
4.4. Characterization of Tannic–Gelatin-Based Hydrogels
4.4.1. ATR-FTIR Spectroscopy
4.4.2. Scanning Electron Microscopy
4.4.3. Degradation Ratio
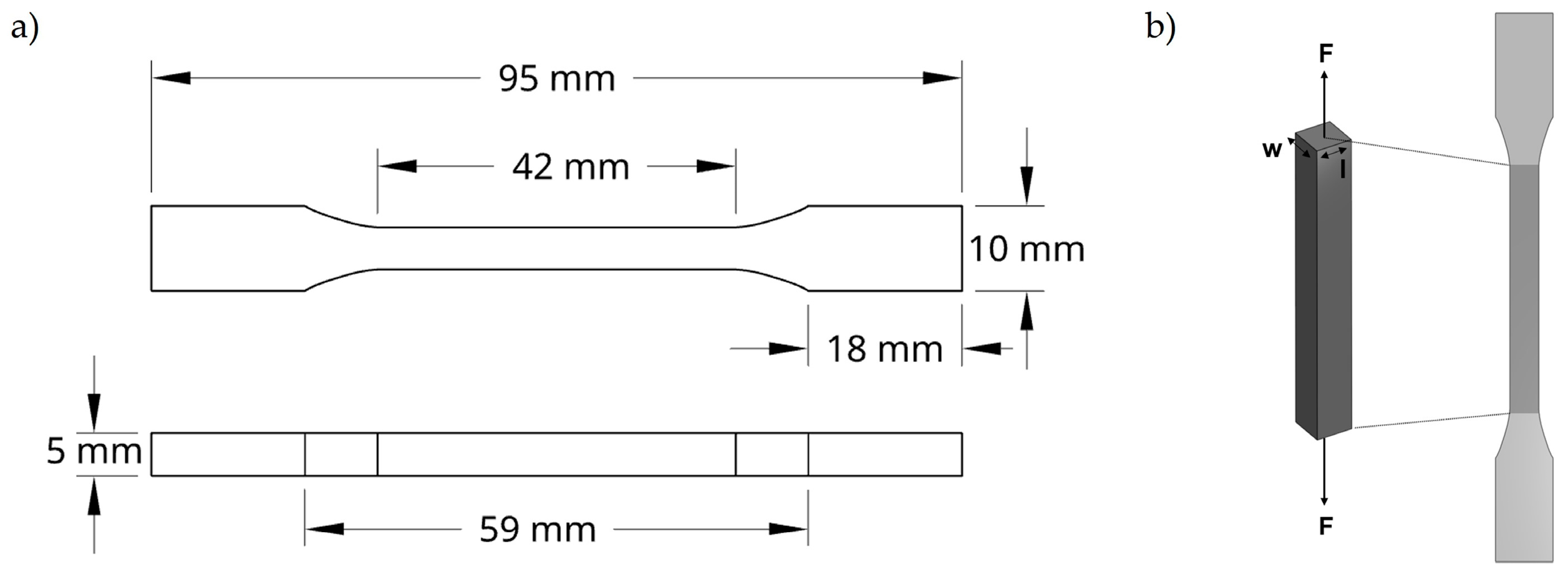
4.4.4. Tensile Tests
4.5. Biological Characterization of Tannic–Gelatin-Based Hydrogels
4.5.1. Cell Cultures
4.5.2. Viability/Proliferation (MTT Assay)
4.5.3. Total Protein Content and Alkaline Phosphatase Activity
4.5.4. Alkaline Phosphatase Histochemical Staining Assay
4.5.5. Immunocytochemical Staining Assay of F-Actin Cytoskeleton and Nucleus
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALP | Alkaline Phosphatase |
| ATR-FTIR | Attenuated total reflectance Fourier-transform infrared spectroscopy |
| BSA | Bovine Serum Albumin |
| DMSO | Dimethyl Sulfoxide |
| EDS | Energy-dispersive X-ray spectroscopy |
| FBS | Fetal Bovine Serum |
| GBR | Guided Bone Regeneration |
| GO | Graphene Oxide |
| GTR | Guided Tissue Regeneration |
| HA | Hydroxyapatite Nanoparticles |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| PBS | Phosphate-buffered Saline |
| PD | Periodontal Disease |
| PDL | Periodontal Ligament |
| p-NPP | p-Nitrophenyl Phosphate |
| PT | Total Protein Content |
| SEM | Scanning Electron Microscopy |
| TA | Tannic Acid |
References
- Cunha, E.; Tavares, L.; Oliveira, M. Revisiting Periodontal Disease in Dogs: How to Manage This New Old Problem? Antibiotics 2022, 11, 1729. [Google Scholar] [CrossRef]
- Bellows, J.; Berg, M.L.; Dennis, S.; Harvey, R.; Lobprise, H.B.; Snyder, C.J.; Stone, A.E.S.; Van de Wetering, A.G. 2019 AAHA Dental Care Guidelines for Dogs and Cats. J. Am. Anim. Hosp. Assoc. 2019, 55, 49–69. [Google Scholar] [CrossRef]
- Wadhawan, A.; Gowda, T.M.; Mehta, D.S. Gore-tex(®) versus resolut adapt(®) GTR membranes with perioglas(®) in periodontal regeneration. Contemp. Clin. Dent. 2012, 3, 406–411. [Google Scholar] [CrossRef]
- Aprile, P.; Letourneur, D.; Simon-Yarza, T. Membranes for Guided Bone Regeneration: A Road from Bench to Bedside. Adv. Healthc. Mater. 2020, 9, 2000707. [Google Scholar] [CrossRef]
- Abe, G.L.; Sasaki, J.I.; Tsuboi, R.; Kohno, T.; Kitagawa, H.; Imazato, S. Poly(lactic acid/caprolactone) bilayer membrane achieves bone regeneration through a prolonged barrier function. J. Biomed. Mater. Res. B Appl. Biomater. 2024, 112, e35365. [Google Scholar] [CrossRef]
- Pla, R.; Sanz-Esporrin, J.; Noguerol, F.; Vignoletti, F.; Gamarra, P.; Sanz, M. A Synthetic Bio-Absorbable Membrane in Guided Bone Regeneration in Dehiscence-Type Defects: An Experimental In Vivo Investigation in Dogs. Bioengineering 2023, 10, 841. [Google Scholar] [CrossRef]
- Gawor, J.P.; Strøm, P.; Nemec, A. Treatment of Naturally Occurring Periodontitis in Dogs with a New Bio-Absorbable Regenerative Matrix. Front. Vet. Sci. 2022, 9, 916171. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhou, X.; Cao, H.; Zhang, H.; Wang, D.; Guo, J.; Wang, J. Barrier membranes for periodontal guided bone regeneration: A potential therapeutic strategy. Front. Mater. 2023, 10, 1220420. [Google Scholar] [CrossRef]
- Calciolari, E.; Ravanetti, F.; Strange, A.; Mardas, N.; Bozec, L.; Cacchioli, A.; Kostomitsopoulos, N.; Donos, N. Degradation pattern of a porcine collagen membrane in an in vivo model of guided bone regeneration. J. Periodontal Res. 2018, 53, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Rothamel, D.; Schwarz, F.; Fienitz, T.; Smeets, R.; Dreiseidler, T.; Ritter, L.; Happe, A.; Zöller, J. Biocompatibility and biodegradation of a native porcine pericardium membrane: Results of in vitro and in vivo examinations. Int. J. Oral. Maxillofac. Implant. 2012, 27, 146–154. [Google Scholar]
- Lukin, I.; Erezuma, I.; Maeso, L.; Zarate, J.; Desimone, M.F.; Al-Tel, T.H.; Dolatshahi-Pirouz, A.; Orive, G. Progress in Gelatin as Biomaterial for Tissue Engineering. Pharmaceutics 2022, 14, 1177. [Google Scholar] [CrossRef]
- Mogha, P.; Iyer, S.; Majumder, A. Extracellular matrix protein gelatin provides higher expansion, reduces size heterogeneity, and maintains cell stiffness in a long-term culture of mesenchymal stem cells. Tissue Cell 2023, 80, 101969. [Google Scholar] [CrossRef]
- Milano, F.; Masi, A.; Madaghiele, M.; Sannino, A.; Salvatore, L.; Gallo, N. Current Trends in Gelatin-Based Drug Delivery Systems. Pharmaceutics 2023, 15, 1499. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Tong, X.; Conrad, B.; Yang, F. Injectable and in situ crosslinkable gelatin microribbon hydrogels for stem cell delivery and bone regeneration in vivo. Theranostics 2020, 10, 6035–6047. [Google Scholar] [CrossRef]
- Wu, E.; Huang, L.; Shen, Y.; Wei, Z.; Li, Y.; Wang, J.; Chen, Z. Application of gelatin-based composites in bone tissue engineering. Heliyon 2024, 10, e36258. [Google Scholar] [CrossRef] [PubMed]
- Purohit, S.D.; Singh, H.; Bhaskar, R.; Yadav, I.; Bhushan, S.; Gupta, M.K.; Kumar, A.; Mishra, N.C. Fabrication of Graphene Oxide and Nanohydroxyapatite Reinforced Gelatin–Alginate Nanocomposite Scaffold for Bone Tissue Regeneration. Front. Mater. 2020, 7, 250. [Google Scholar] [CrossRef]
- Zhihui, K.; Min, D. Application of Graphene Oxide-Based Hydrogels in Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2022, 8, 2849–2857. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.B.; Park, K.M.; Park, K.D. In situ graphene oxide-gelatin hydrogels with enhanced mechanical property for tissue adhesive and regeneration. Biochem. Biophys. Res. Commun. 2022, 592, 24–30. [Google Scholar] [CrossRef]
- Hermenean, A.; Codreanu, A.; Herman, H.; Balta, C.; Rosu, M.; Mihali, C.V.; Ivan, A.; Dinescu, S.; Ionita, M.; Costache, M. Chitosan-Graphene Oxide 3D scaffolds as Promising Tools for Bone Regeneration in Critical-Size Mouse Calvarial Defects. Sci. Rep. 2017, 7, 16641. [Google Scholar] [CrossRef]
- Kim, J.; Choi, K.S.; Kim, Y.; Lim, K.T.; Seonwoo, H.; Park, Y.; Kim, D.H.; Choung, P.H.; Cho, C.S.; Kim, S.Y.; et al. Bioactive effects of graphene oxide cell culture substratum on structure and function of human adipose-derived stem cells. J. Biomed. Mater. Res. A 2013, 101, 3520–3530. [Google Scholar] [CrossRef]
- Dinescu, S.; Ionita, M.; Pandele, A.M.; Galateanu, B.; Iovu, H.; Ardelean, A.; Costache, M.; Hermenean, A. In vitro cytocompatibility evaluation of chitosan/graphene oxide 3D scaffold composites designed for bone tissue engineering. Biomed. Mater. Eng. 2014, 24, 2249–2256. [Google Scholar] [CrossRef] [PubMed]
- Kashte, S.B.; Kadam, S.; Maffulli, N.; Potty, A.G.; Migliorini, F.; Gupta, A. Osteoinductive potential of graphene and graphene oxide for bone tissue engineering: A comparative study. J. Orthop. Surg. Res. 2024, 19, 527. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-W.; Song, J.-H.; Kim, H.-E. Nanofiber Generation of Gelatin–Hydroxyapatite Biomimetics for Guided Tissue Regeneration. Adv. Funct. Mater. 2005, 15, 1988–1994. [Google Scholar] [CrossRef]
- Shahi, S.; Sharifi, S.; Khalilov, R.; Dizaj, S.M.; Abdolahinia, E.D. Gelatin-hydroxyapatite Fibrous Nanocomposite for Regenerative Dentistry and bone Tissue Engineering. Open Dent. J. 2022, 16, e2208200. [Google Scholar] [CrossRef]
- Sharifi, S.; Samiei, M.; Abdolahinia, E.D.; Khalilov, R.; Shahi, S.; Dizaj, S.M. Gelatin-hydroxyapatite nano-fibers as promising scaffolds for guided tissue regeneration (GTR): Preparation, assessment of the physicochemical properties and the effect on mesenchymal stem cells. J. Adv. Periodontol. Implant. Dent. 2020, 12, 25–29. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, S.; Zhang, C.; Ikoma, T.; Guo, H.; Zhang, X.; Li, X.; Chen, W. Novel microsphere-packing synthesis, microstructure, formation mechanism and in vitro biocompatibility of porous gelatin/hydroxyapatite microsphere scaffolds. Ceram. Int. 2021, 47, 32187–32194. [Google Scholar] [CrossRef]
- Lian, H.; Zhang, L.; Meng, Z. Biomimetic hydroxyapatite/gelatin composites for bone tissue regeneration: Fabrication, characterization, and osteogenic differentiation in vitro. Mater. Des. 2018, 156, 381–388. [Google Scholar] [CrossRef]
- Byun, H.; Jang, G.N.; Hong, M.-H.; Yeo, J.; Shin, H.; Kim, W.J.; Shin, H. Biomimetic anti-inflammatory and osteogenic nanoparticles self-assembled with mineral ions and tannic acid for tissue engineering. Nano Converg. 2022, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Lin, J.; Luo, X.; Luo, G.; Zou, J.; Cai, L.; He, Y.; Qiu, X.; Xu, H. Tannic Acid-Programmed Hydroxyapatite Biomineralization Enables Bilayered Bone-Mimetic Hydrogels for Mandibular Regeneration. Chem. Mater. 2025, 37, 3853–3869. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Hwang, C.-H.; Kim, H.-E.; Jeong, S.-H. Enhancement of bio-stability and mechanical properties of hyaluronic acid hydrogels by tannic acid treatment. Carbohydr. Polym. 2018, 186, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Payne, D.E.; Martin, N.R.; Parzych, K.R.; Rickard, A.H.; Underwood, A.; Boles, B.R. Tannic acid inhibits Staphylococcus aureus surface colonization in an IsaA-dependent manner. Infect. Immun. 2013, 81, 496–504. [Google Scholar] [CrossRef]
- Yeo, J.; Lee, J.; Yoon, S.; Kim, W.J. Tannic acid-based nanogel as an efficient anti-inflammatory agent. Biomater. Sci. 2020, 8, 1148–1159. [Google Scholar] [CrossRef]
- Zheng, Y.; Liang, Y.; Zhang, D.; Sun, X.; Liang, L.; Li, J.; Liu, Y.N. Gelatin-Based Hydrogels Blended with Gellan as an Injectable Wound Dressing. ACS Omega 2018, 3, 4766–4775. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, H.; Yang, X.; Ma, Q. Tannic acid: A crosslinker leading to versatile functional polymeric networks: A review. RSC Adv. 2022, 12, 7689–7711. [Google Scholar] [CrossRef]
- Lee, J.; Kim, G. Calcium-Deficient Hydroxyapatite/Collagen/Platelet-Rich Plasma Scaffold with Controlled Release Function for Hard Tissue Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 278–289. [Google Scholar] [CrossRef]
- Yao, G.; Liu, X.; Zhang, G.; Han, Z.; Liu, H. Green synthesis of tannic acid functionalized graphene hydrogel to efficiently adsorb methylene blue. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126972. [Google Scholar] [CrossRef]
- Luo, J.; Lai, J.; Zhang, N.; Liu, Y.; Liu, R.; Liu, X. Tannic Acid Induced Self-Assembly of Three-Dimensional Graphene with Good Adsorption and Antibacterial Properties. ACS Sustain. Chem. Eng. 2016, 4, 1404–1413. [Google Scholar] [CrossRef]
- Anggresani, L.; Rahayu, A.; Perawati, S.; Yulianis, Y.; Sintia, U.; Setiawan, R. Synthesis and structure of hydroxyapatite/tannin composites. Polimery 2024, 69, 484–491. [Google Scholar] [CrossRef]
- Kong, W.; Du, Q.; Qu, Y.; Shao, C.; Chen, C.; Sun, J.; Mao, C.; Tang, R.; Gu, X. Tannic acid induces dentin biomineralization by crosslinking and surface modification. RSC Adv. 2022, 12, 3454–3464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Y.; Li, X.; Cao, Z.; Mo, Q.; Sheng, R.; Ling, C.; Chi, J.; Yao, Q.; Chen, J.; et al. Multifunctional polyphenol-based silk hydrogel alleviates oxidative stress and enhances endogenous regeneration of osteochondral defects. Mater. Today Bio 2022, 14, 100251. [Google Scholar] [CrossRef] [PubMed]
- Taheri, P.; Jahanmardi, R.; Koosha, M.; Abdi, S. Physical, mechanical and wound healing properties of chitosan/gelatin blend films containing tannic acid and/or bacterial nanocellulose. Int. J. Biol. Macromol. 2020, 154, 421–432. [Google Scholar] [CrossRef]
- Kim, N.E.; Park, S.; Kim, S.; Choi, J.H.; Kim, S.E.; Choe, S.H.; Kang, T.w.; Song, J.E.; Khang, G. Development of Gelatin-Based Shape-Memory Polymer Scaffolds with Fast Responsive Performance and Enhanced Mechanical Properties for Tissue Engineering Applications. ACS Omega 2023, 8, 6455–6462. [Google Scholar] [CrossRef]
- Santos, C.; Gomes, P.S.; Duarte, J.A.; Franke, R.P.; Almeida, M.M.; Costa, M.E.; Fernandes, M.H. Relevance of the sterilization-induced effects on the properties of different hydroxyapatite nanoparticles and assessment of the osteoblastic cell response. J. R. Soc. Interface 2012, 9, 3397–3410. [Google Scholar] [CrossRef]
- Camci-Unal, G.; Nichol, J.W.; Bae, H.; Tekin, H.; Bischoff, J.; Khademhosseini, A. Hydrogel surfaces to promote attachment and spreading of endothelial progenitor cells. J. Tissue Eng. Regen. Med. 2013, 7, 337–347. [Google Scholar] [CrossRef]
- Yu, Z.; Xiao, C.; Huang, Y.; Chen, M.; Wei, W.; Yang, X.; Zhou, H.; Bi, X.; Lu, L.; Ruan, J.; et al. Enhanced bioactivity and osteoinductivity of carboxymethyl chitosan/nanohydroxyapatite/graphene oxide nanocomposites. RSC Adv. 2018, 8, 17860–17877. [Google Scholar] [CrossRef] [PubMed]
- Sreekumaran, S.; Radhakrishnan, A.; Rauf, A.A.; Kurup, G.M. Nanohydroxyapatite incorporated photocrosslinked gelatin methacryloyl/poly(ethylene glycol)diacrylate hydrogel for bone tissue engineering. Prog. Biomater. 2021, 10, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Firouzi, N.; Baradar Khoshfetrat, A.; Kazemi, D. Enzymatically gellable gelatin improves nano-hydroxyapatite-alginate microcapsule characteristics for modular bone tissue formation. J. Biomed. Mater. Res. Part A 2020, 108, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.R.; Li, Y.C.; Jang, H.L.; Khoshakhlagh, P.; Akbari, M.; Nasajpour, A.; Zhang, Y.S.; Tamayol, A.; Khademhosseini, A. Graphene-based materials for tissue engineering. Adv. Drug Deliv. Rev. 2016, 105, 255–274. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Choe, G.; Park, J.; Lee, J.Y. Graphene oxide-incorporated hydrogels for biomedical applications. Polym. J. 2020, 52, 823–837. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef]
- Li, M.; Xiong, P.; Yan, F.; Li, S.; Ren, C.; Yin, Z.; Li, A.; Li, H.; Ji, X.; Zheng, Y.; et al. An overview of graphene-based hydroxyapatite composites for orthopedic applications. Bioact. Mater. 2018, 3, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Tang, Y. On mechanical properties of nanocomposite hydrogels: Searching for superior properties. Nano Mater. Sci. 2022, 4, 83–96. [Google Scholar] [CrossRef]
- Kim, D.; Lee, J.; Kim, G. Biomimetic gelatin/HA biocomposites with effective elastic properties and 3D-structural flexibility using a 3D-printing process. Addit. Manuf. 2020, 36, 101616. [Google Scholar] [CrossRef]
- Utech, S.; Boccaccini, A.R. A review of hydrogel-based composites for biomedical applications: Enhancement of hydrogel properties by addition of rigid inorganic fillers. J. Mater. Sci. 2016, 51, 271–310. [Google Scholar] [CrossRef]
- Guo, J.; Sun, W.; Kim, J.P.; Lu, X.; Li, Q.; Lin, M.; Mrowczynski, O.; Rizk, E.B.; Cheng, J.; Qian, G.; et al. Development of tannin-inspired antimicrobial bioadhesives. Acta Biomater. 2018, 72, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Bohidar, H.B. Effect of cationic size on gelation temperature and properties of gelatin hydrogels. Int. J. Biol. Macromol. 2005, 35, 81–88. [Google Scholar] [CrossRef]
- Pinho, L.C.; Queirós, J.A.; Santos, C.; Colaço, B.; Fernandes, M.H. Biomimetic In Vitro Model of Canine Periodontal Ligament. Int. J. Mol. Sci. 2024, 25, 12234. [Google Scholar] [CrossRef]
- Wang, M.O.; Etheridge, J.M.; Thompson, J.A.; Vorwald, C.E.; Dean, D.; Fisher, J.P. Evaluation of the in vitro cytotoxicity of cross-linked biomaterials. Biomacromolecules 2013, 14, 1321–1329. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for in Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Rosengren, A.; Faxius, L.; Tanaka, N.; Watanabe, M.; Bjursten, L.M. Comparison of implantation and cytotoxicity testing for initially toxic biomaterials. J. Biomed. Mater. Res. Part A 2005, 75A, 115–122. [Google Scholar] [CrossRef]
- Koch, F.; Meyer, N.; Valdec, S.; Jung, R.E.; Mathes, S.H. Development and application of a 3D periodontal in vitro model for the evaluation of fibrillar biomaterials. BMC Oral Health 2020, 20, 148. [Google Scholar] [CrossRef]
- Rosa, V.; Silikas, N.; Yu, B.; Dubey, N.; Sriram, G.; Zinelis, S.; Lima, A.F.; Bottino, M.C.; Ferreira, J.N.; Schmalz, G.; et al. Guidance on the assessment of biocompatibility of biomaterials: Fundamentals and testing considerations. Dent. Mater. 2024, 40, 1773–1785. [Google Scholar] [CrossRef]
- Hazen, M.J.; Freire, P.F.; Martín, J.P.; Peropadre, A.; Herrero, Ó.; López, L.; Labrador, V. The importance of microscopic analysis for accurate interpretation of chemical-induced cytotoxicity. Microsc. Sci. Technol. Appl. Educ. 2010, 3, 2145–2153. [Google Scholar]
- Vimalraj, S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene 2020, 754, 144855. [Google Scholar] [CrossRef] [PubMed]
- ISO 527-1:2019; Plastics—Determination of Tensile Properties—Part 1: General Principles. International Organization for Standardization: Geneva, Switzerland, 2019.
- Tanaka, K.; Iwasaki, K.; Feghali, K.E.; Komaki, M.; Ishikawa, I.; Izumi, Y. Comparison of characteristics of periodontal ligament cells obtained from outgrowth and enzyme-digested culture methods. Arch. Oral. Biol. 2011, 56, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Banyatworakul, P.; Osathanon, T.; Chumprasert, S.; Pavasant, P.; Pirarat, N. Responses of canine periodontal ligament cells to bubaline blood derived platelet rich fibrin in vitro. Sci. Rep. 2021, 11, 11409. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-12:2021; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. International Organization for Standardization: Geneva, Switzerland, 2021.

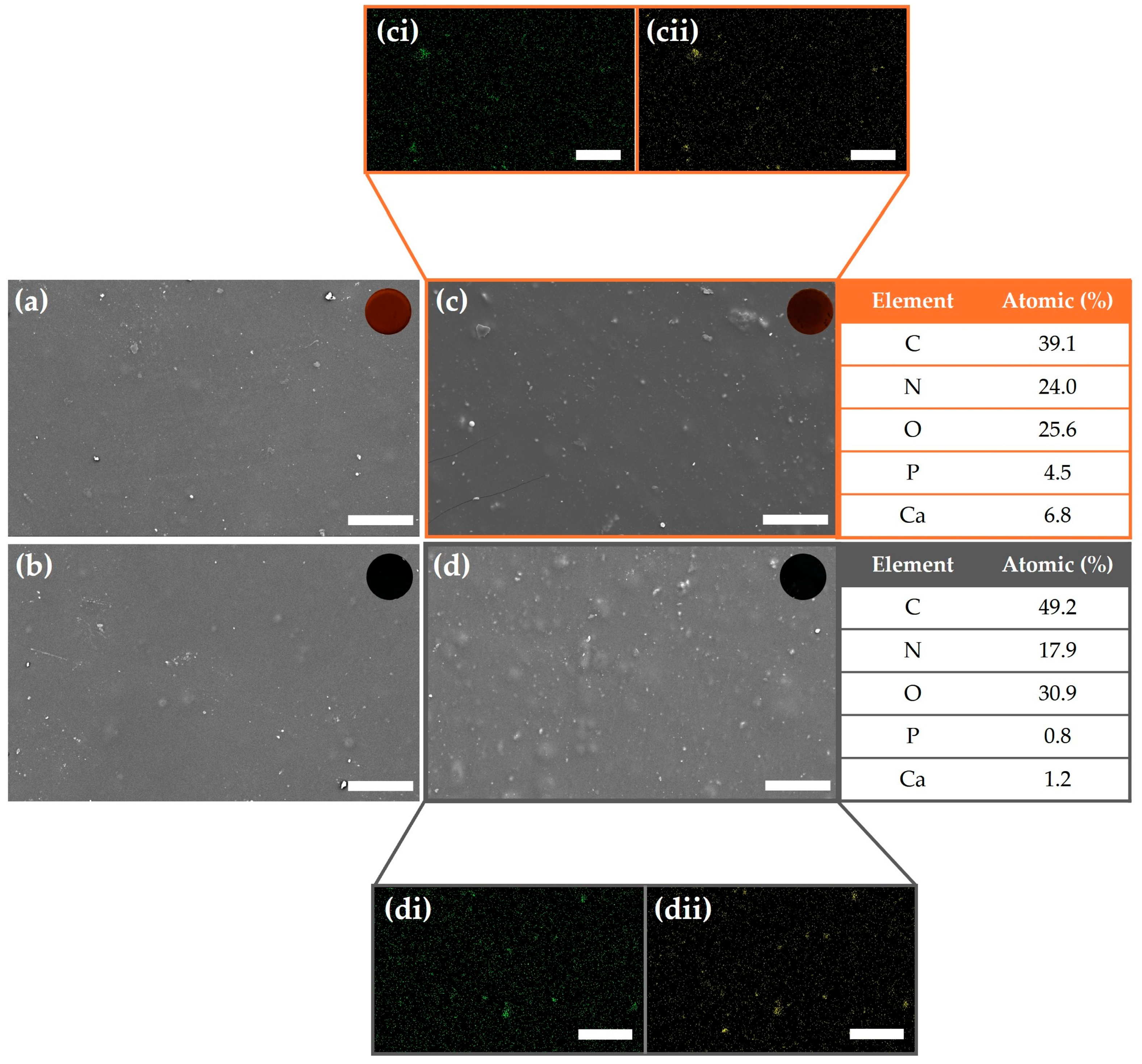
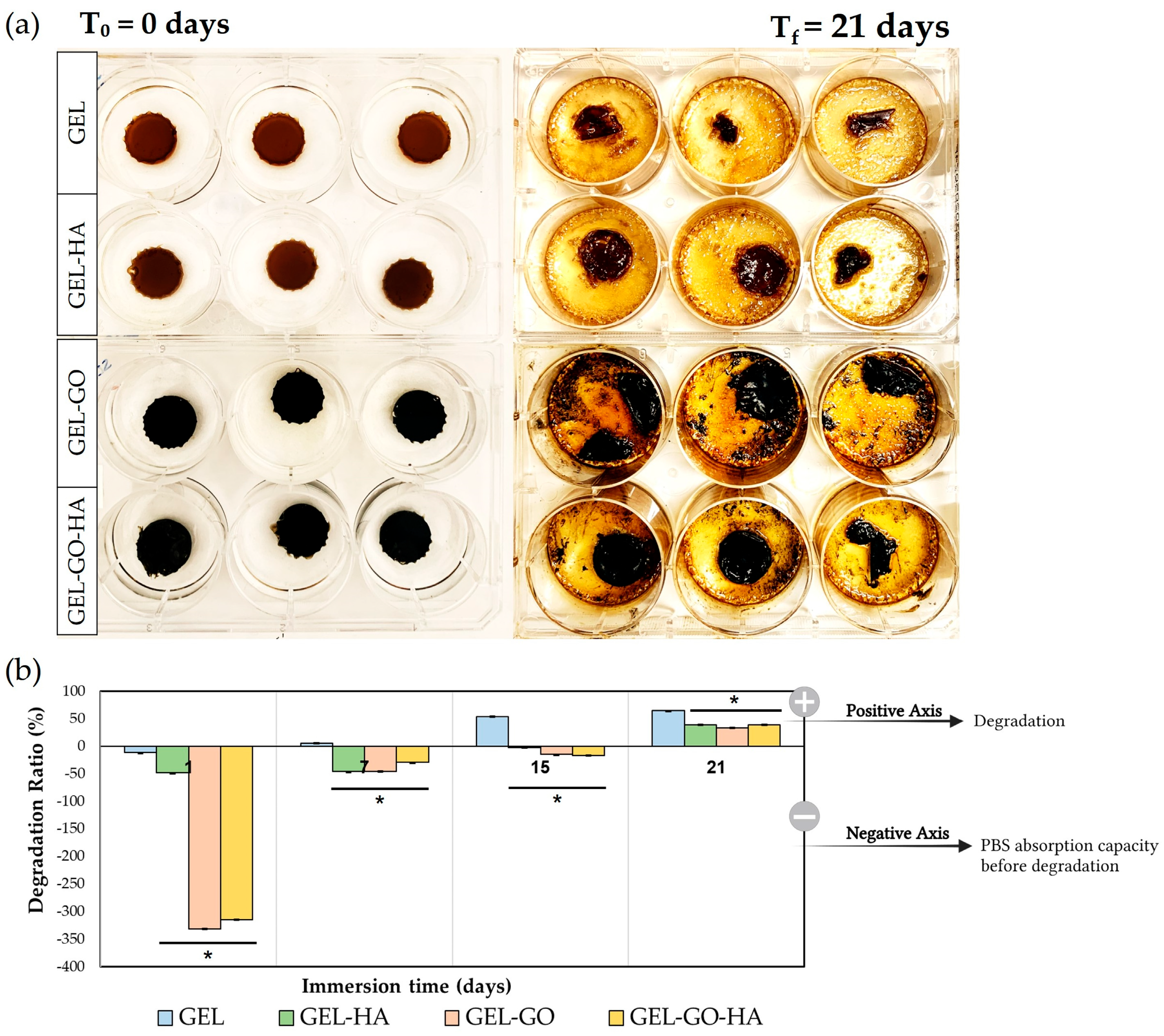
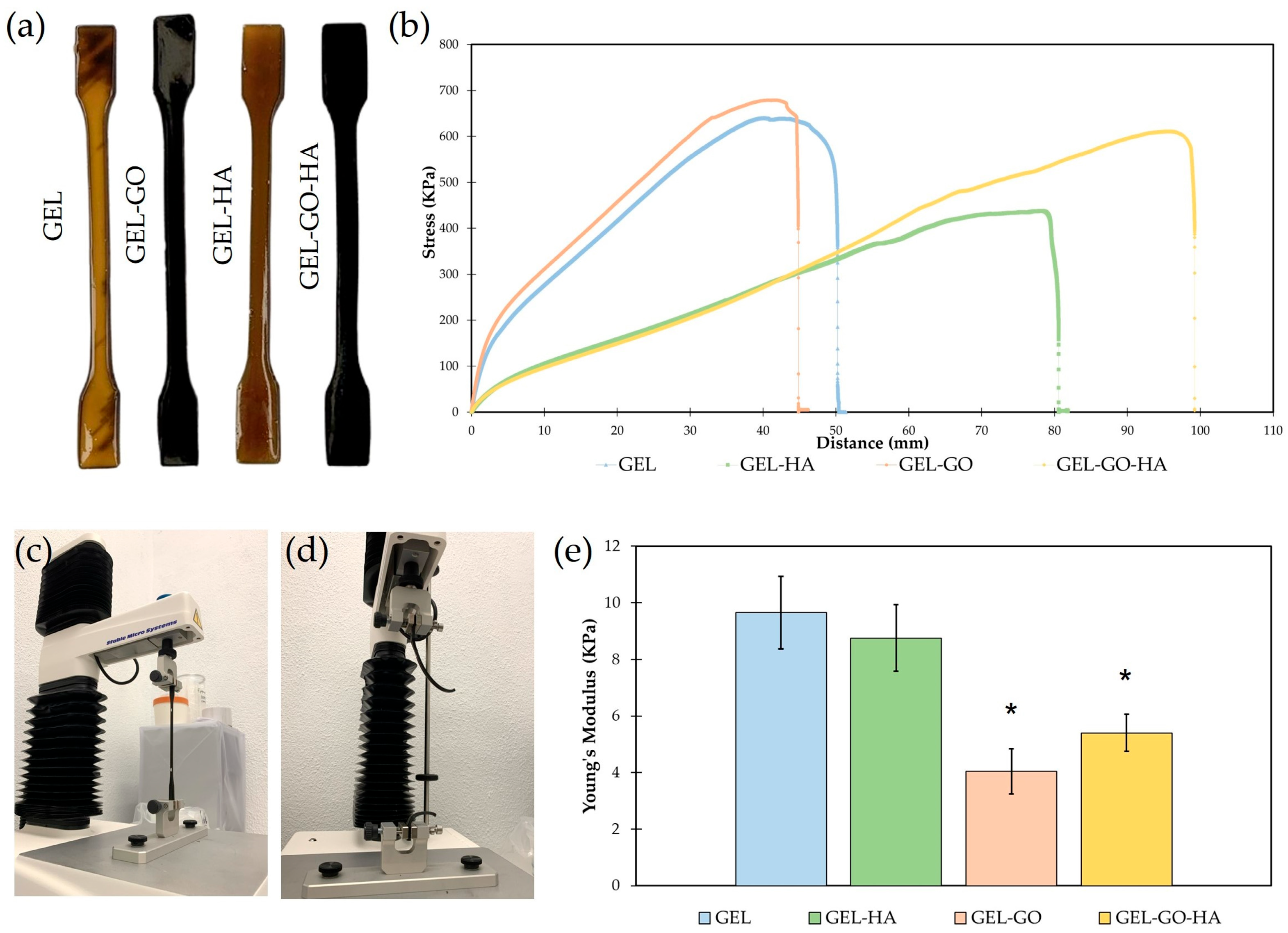
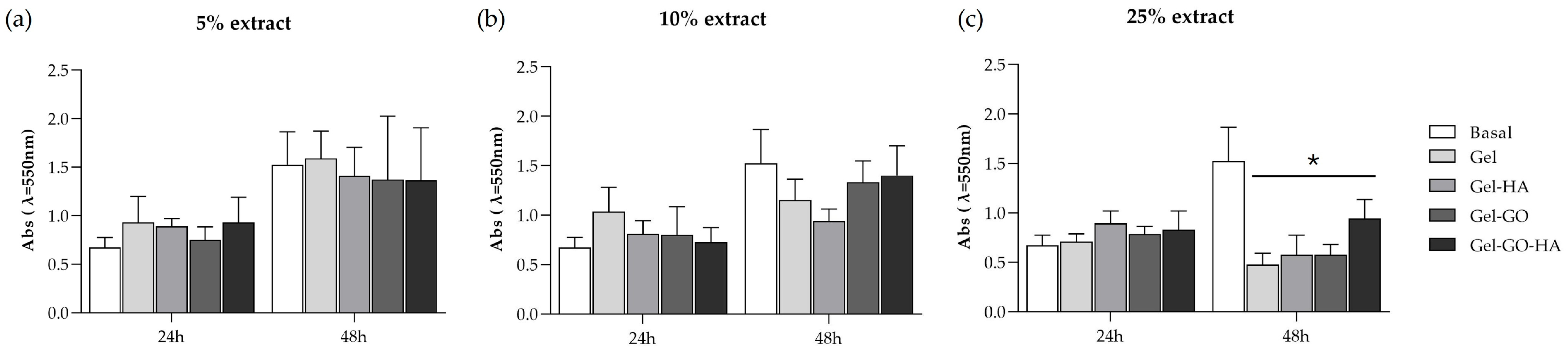
| Hydrogel | Gelatin (%) | Glycerin (%) | PVA (%) | Betaine (%) | TA (%) | GO (%) | HA NPs (%) | H2O (%) |
|---|---|---|---|---|---|---|---|---|
| TA–Gelatin (Gel) | 30 | 10 | 1.5 | 3 | 0.9 | - | - | 54.6 |
| Gel-GO | 30 | 10 | 1.5 | 3 | 0.9 | 7.5 | - | 47.1 |
| Gel-HA | 30 | 10 | 1.5 | 3 | 0.9 | - | 2 | 52.6 |
| Gel-GO-HA | 30 | 10 | 1.5 | 3 | 0.9 | 7.5 | 2 | 45.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinho, L.C.; Ferreira, M.; Graça, A.; Marto, J.; Colaço, B.; Fernandes, M.H.; Santos, C. Tannic Acid-Enhanced Gelatin-Based Composite Hydrogel as a Candidate for Canine Periodontal Regeneration. Gels 2025, 11, 650. https://doi.org/10.3390/gels11080650
Pinho LC, Ferreira M, Graça A, Marto J, Colaço B, Fernandes MH, Santos C. Tannic Acid-Enhanced Gelatin-Based Composite Hydrogel as a Candidate for Canine Periodontal Regeneration. Gels. 2025; 11(8):650. https://doi.org/10.3390/gels11080650
Chicago/Turabian StylePinho, Laura C., Marta Ferreira, Angélica Graça, Joana Marto, Bruno Colaço, Maria Helena Fernandes, and Catarina Santos. 2025. "Tannic Acid-Enhanced Gelatin-Based Composite Hydrogel as a Candidate for Canine Periodontal Regeneration" Gels 11, no. 8: 650. https://doi.org/10.3390/gels11080650
APA StylePinho, L. C., Ferreira, M., Graça, A., Marto, J., Colaço, B., Fernandes, M. H., & Santos, C. (2025). Tannic Acid-Enhanced Gelatin-Based Composite Hydrogel as a Candidate for Canine Periodontal Regeneration. Gels, 11(8), 650. https://doi.org/10.3390/gels11080650













