Sol–Gel Synthesized CuFe2O4-Modified Biochar Derived from Tea Waste for Efficient Ni(II) Removal: Adsorption, Regeneration, and ANN Modeling
Abstract
1. Introduction
2. Results and Discussion
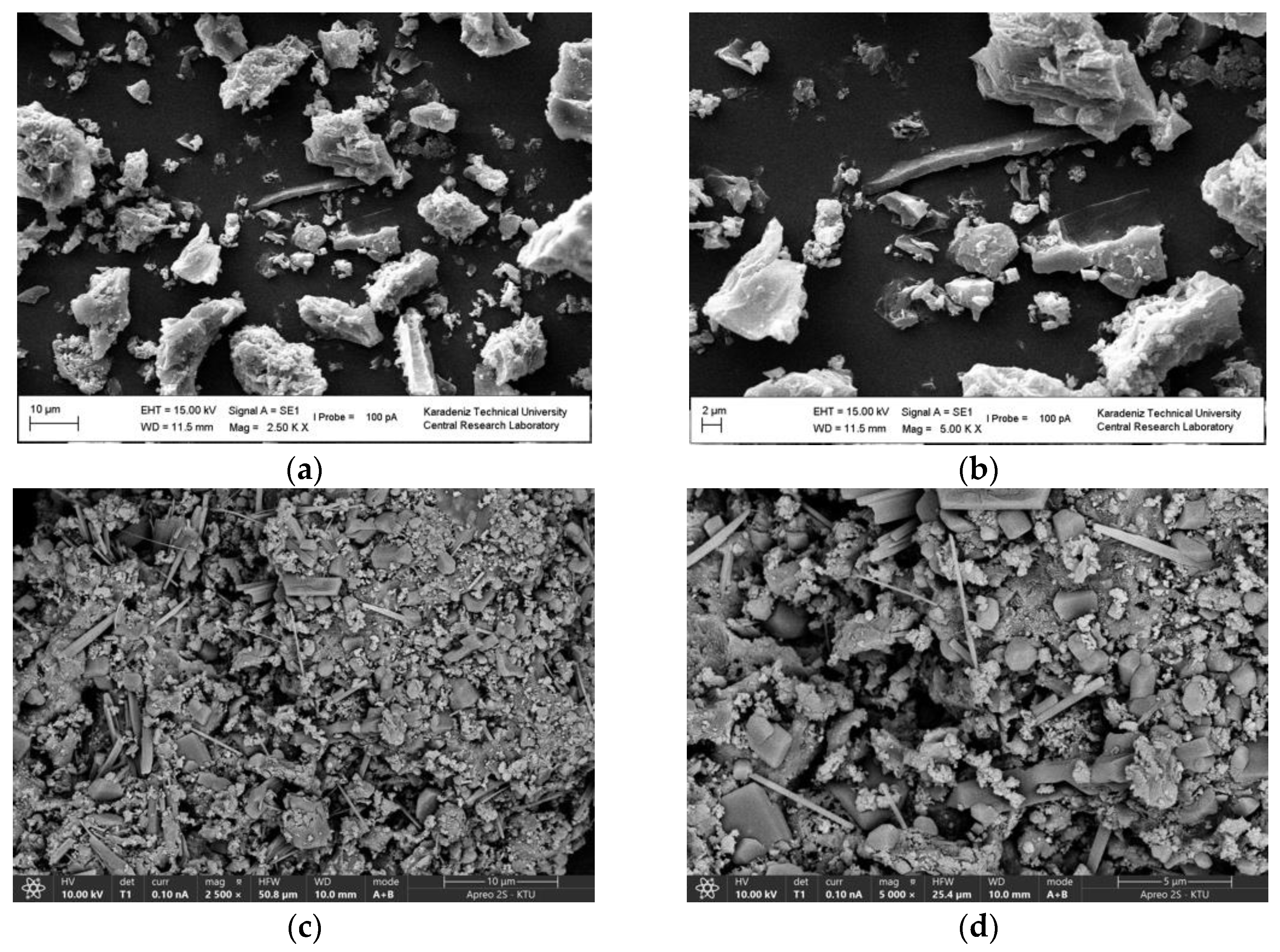
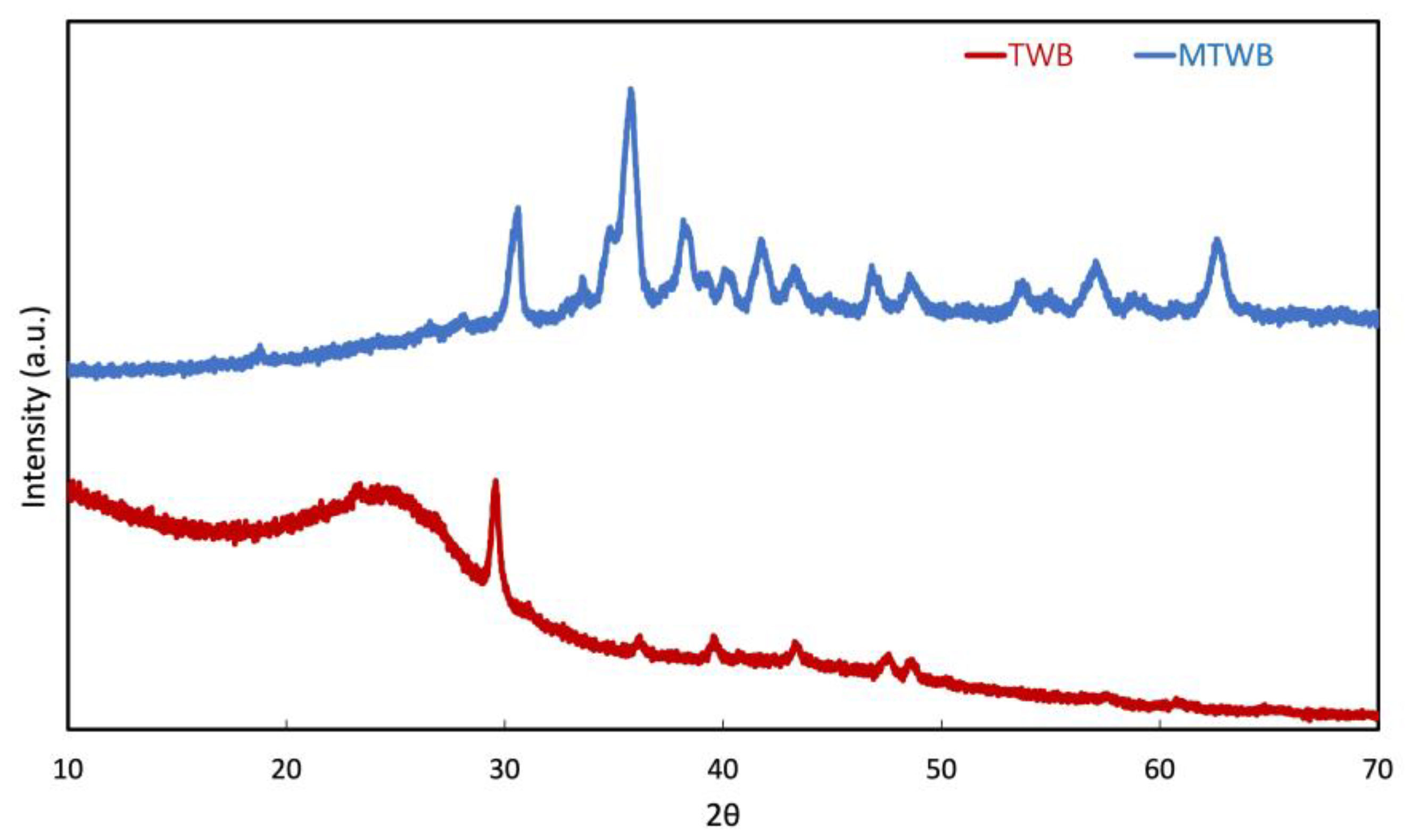
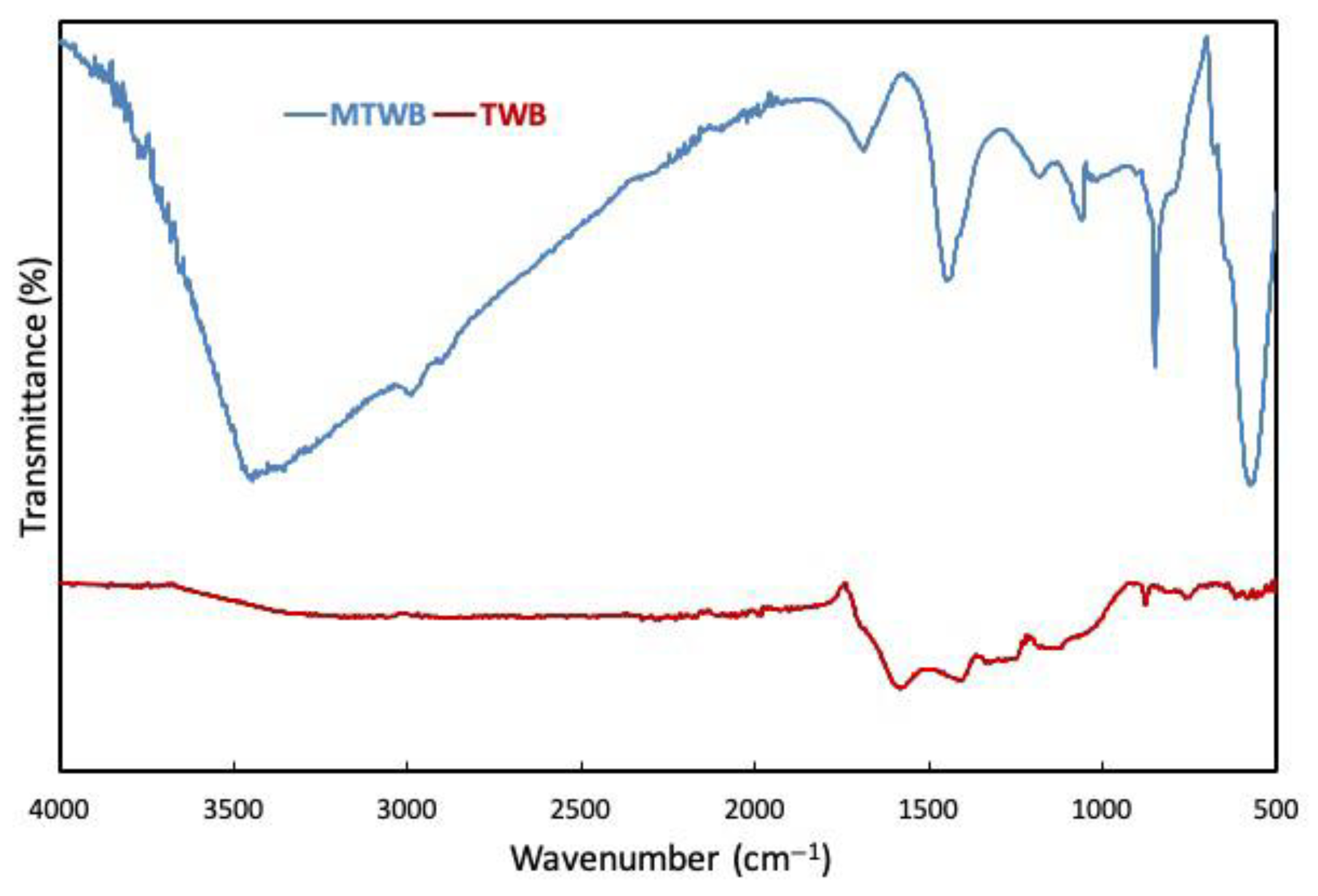
2.1. Characterization
2.2. Impact of Parameters and Evaluation of Data
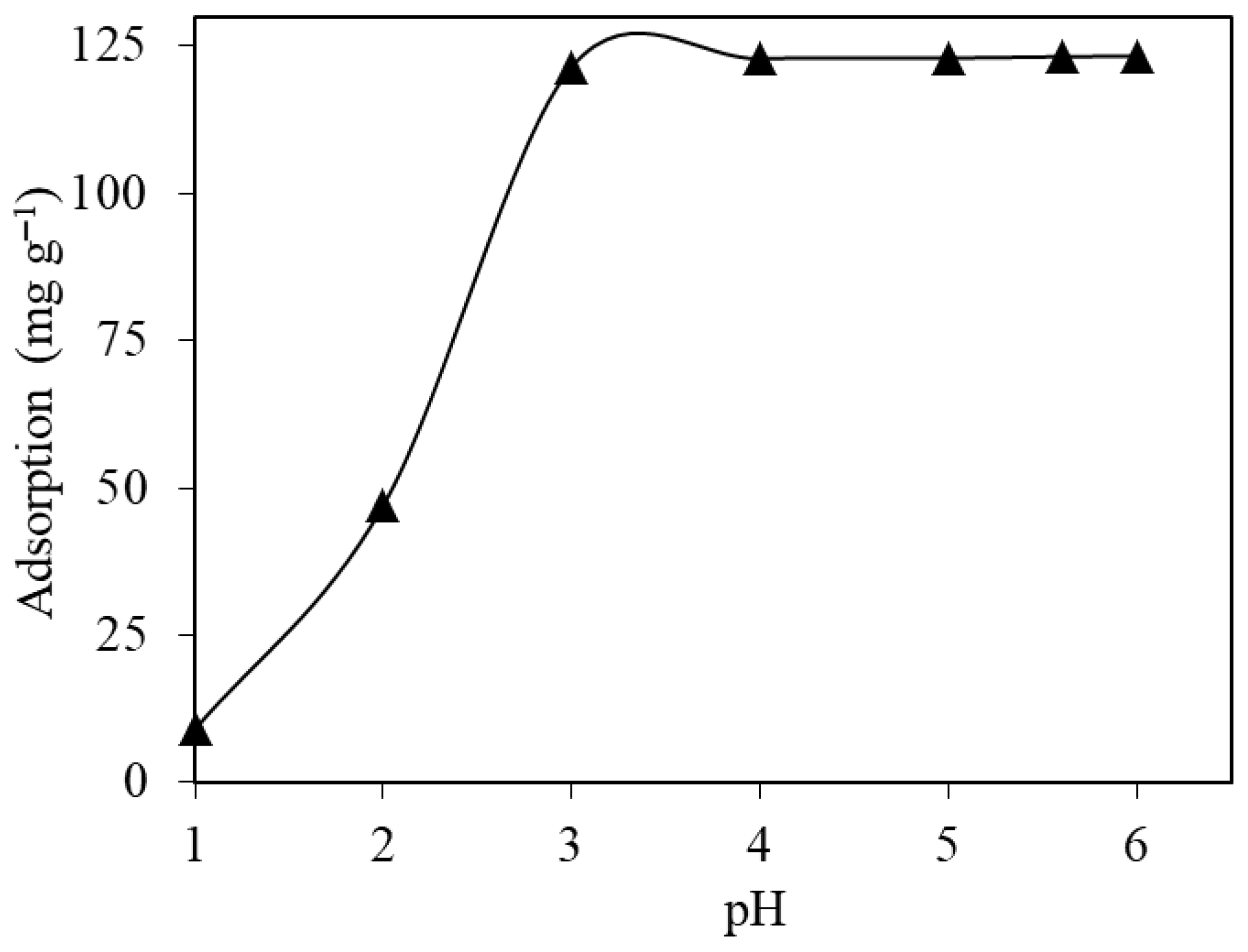
2.2.1. Initial Solution pH
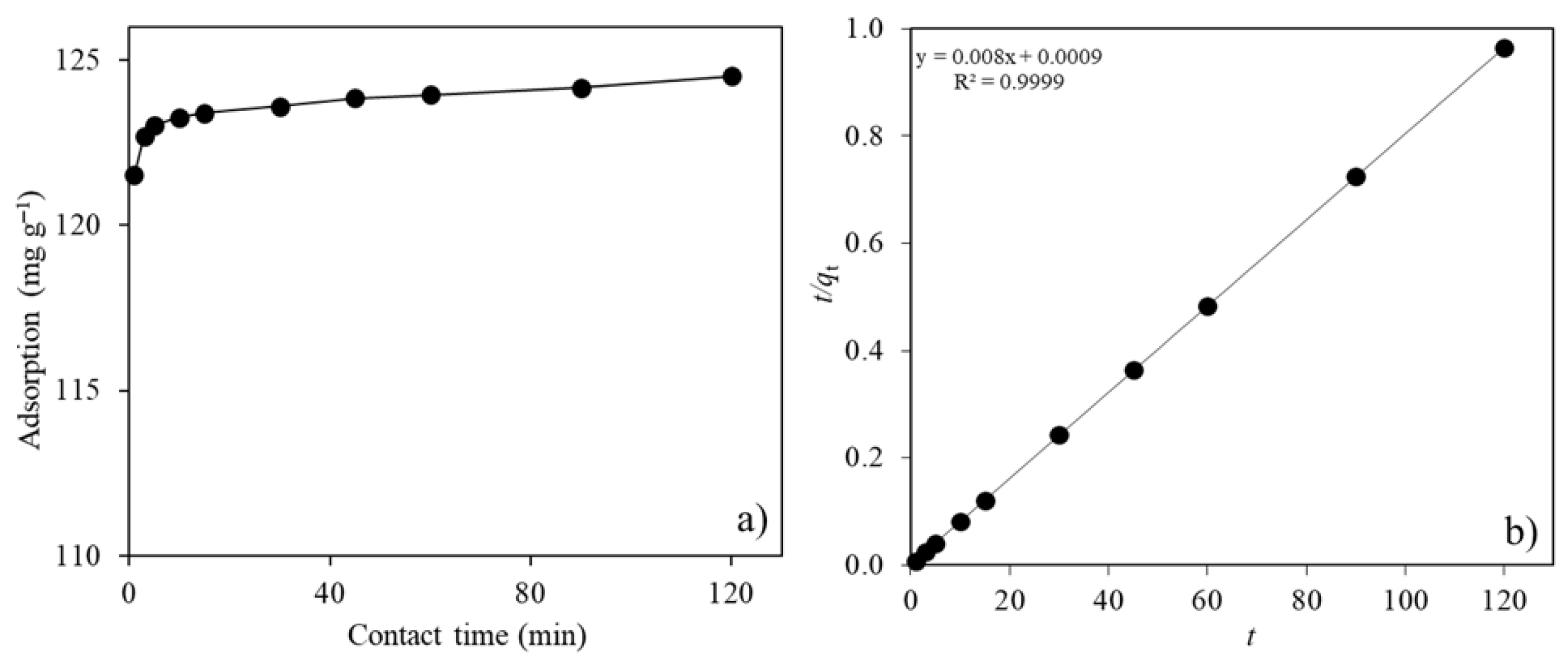
2.2.2. Contact Time
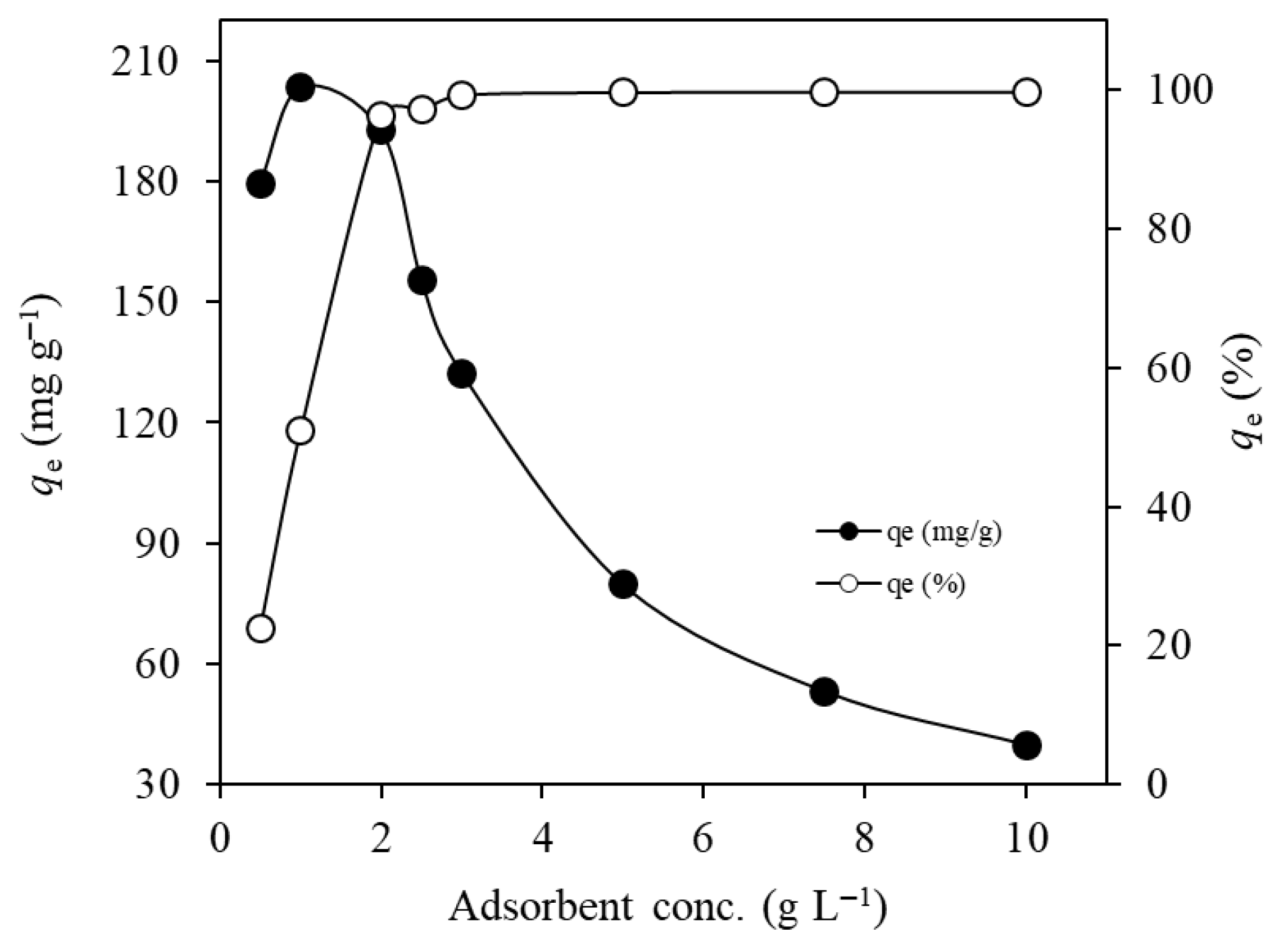
2.2.3. Adsorbent Dosage
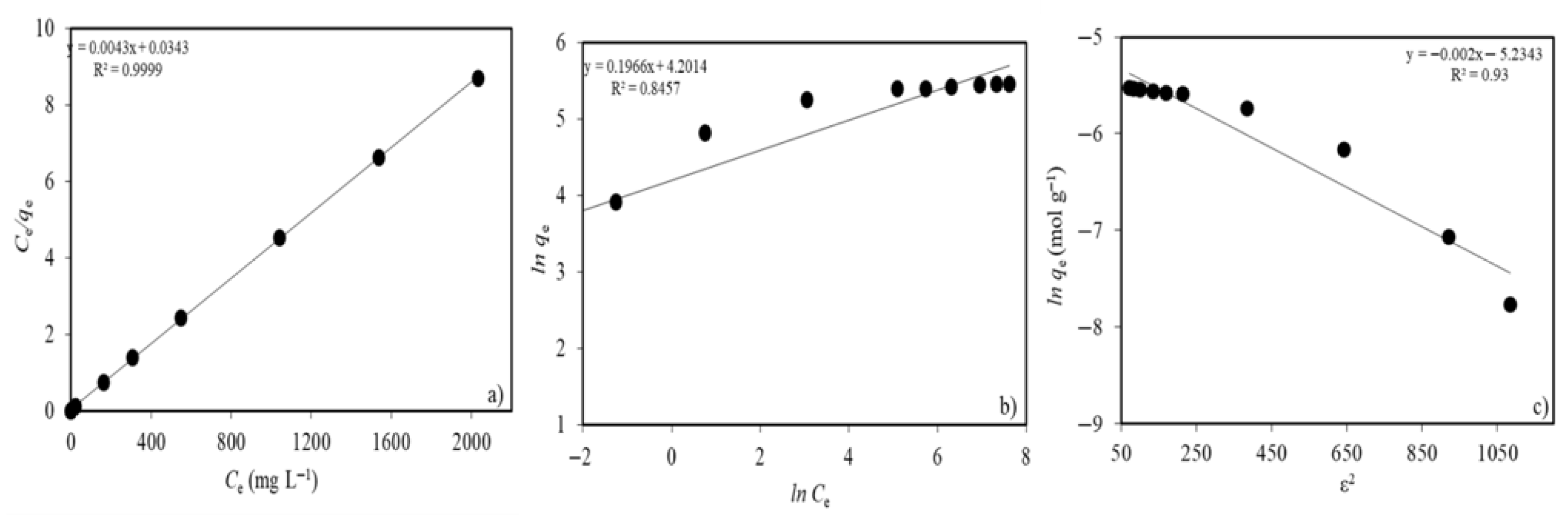
2.2.4. Ni(II) Concentration and Adsorption Isotherms
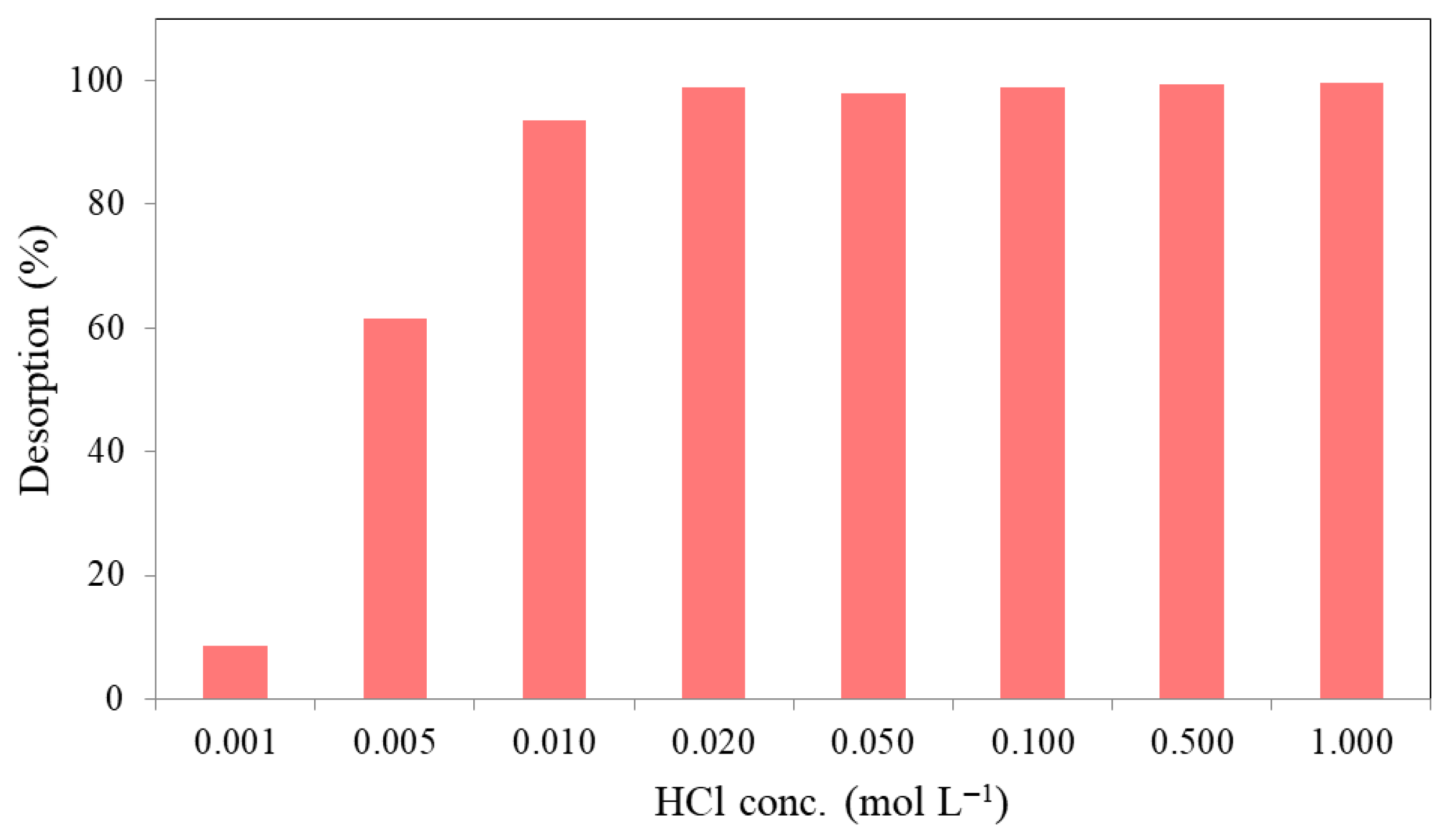
2.2.5. Desorption
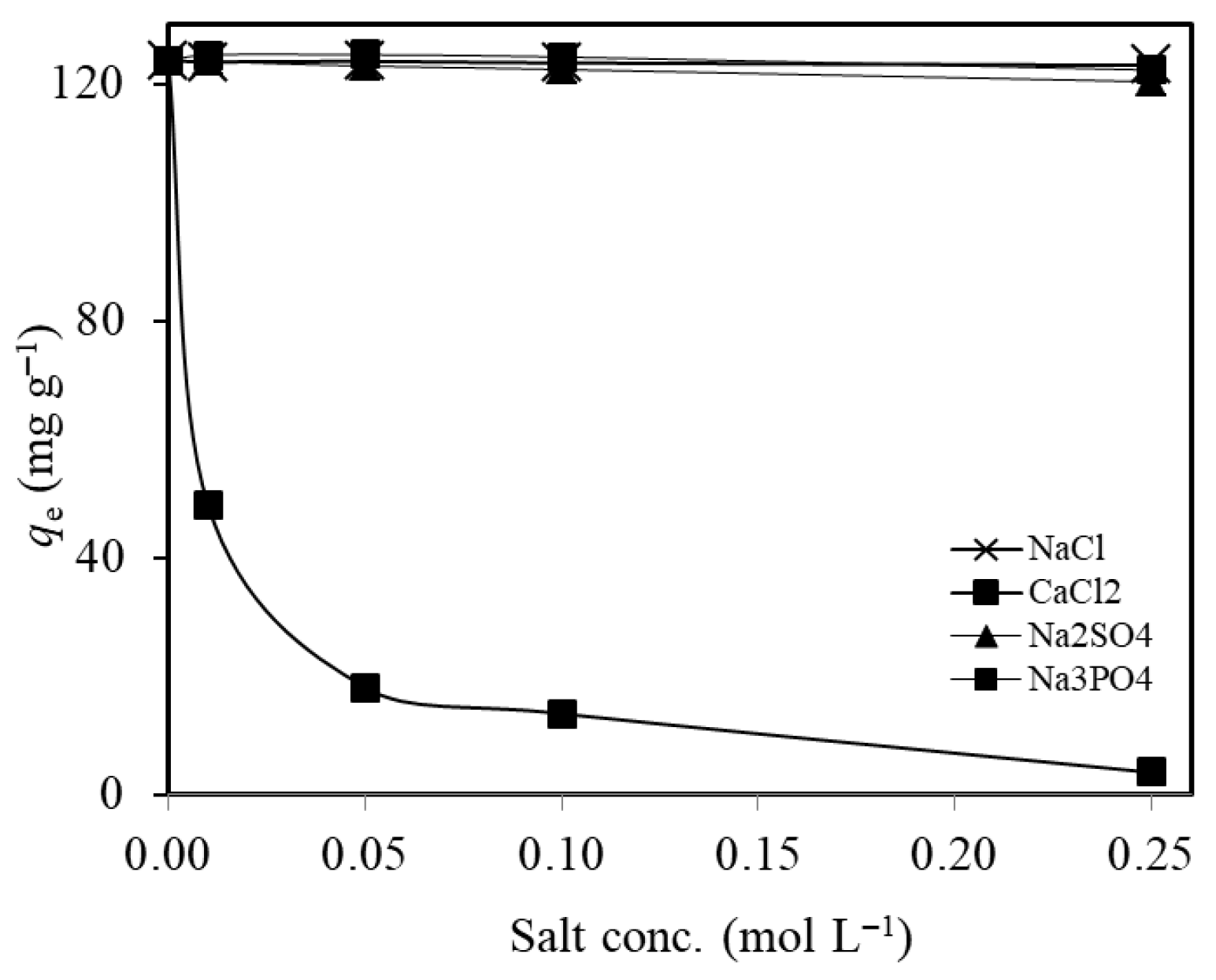
2.2.6. Effect of Co-Existing Ions and Real Wastewater Application
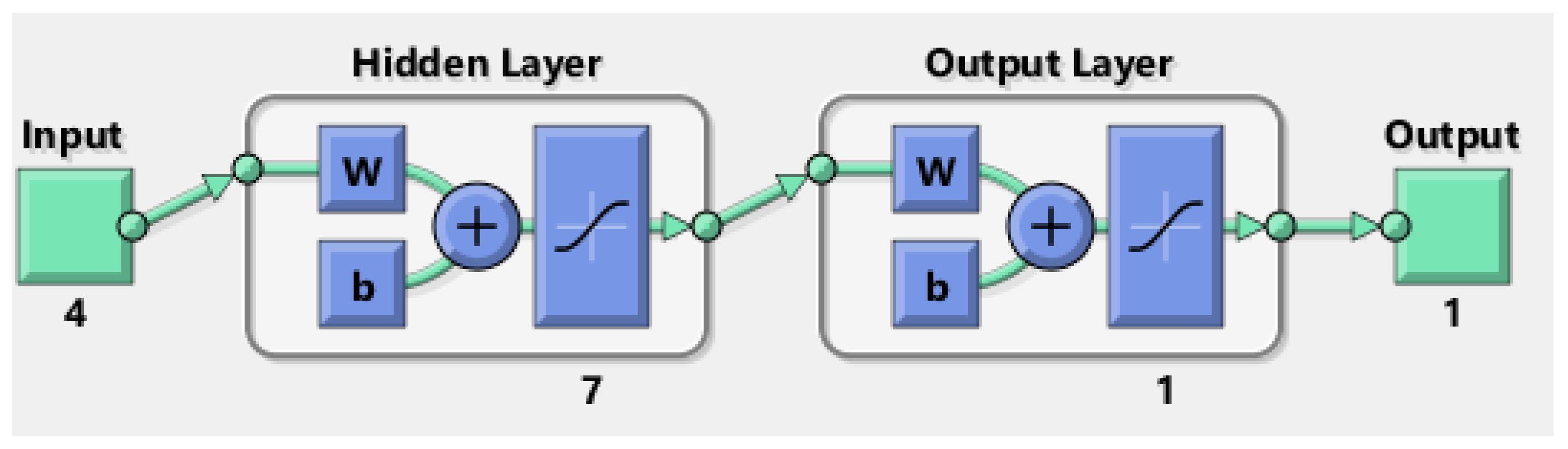
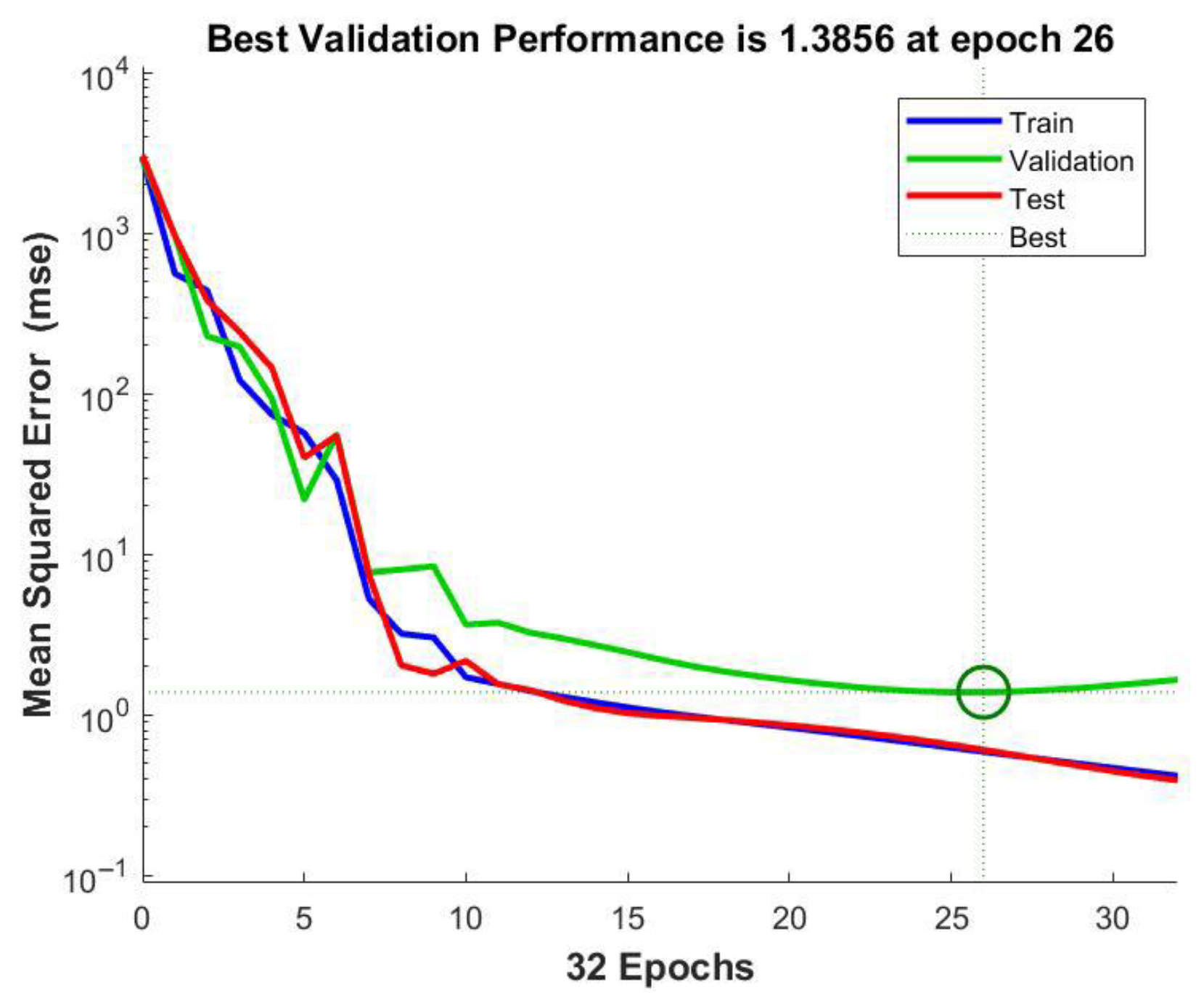
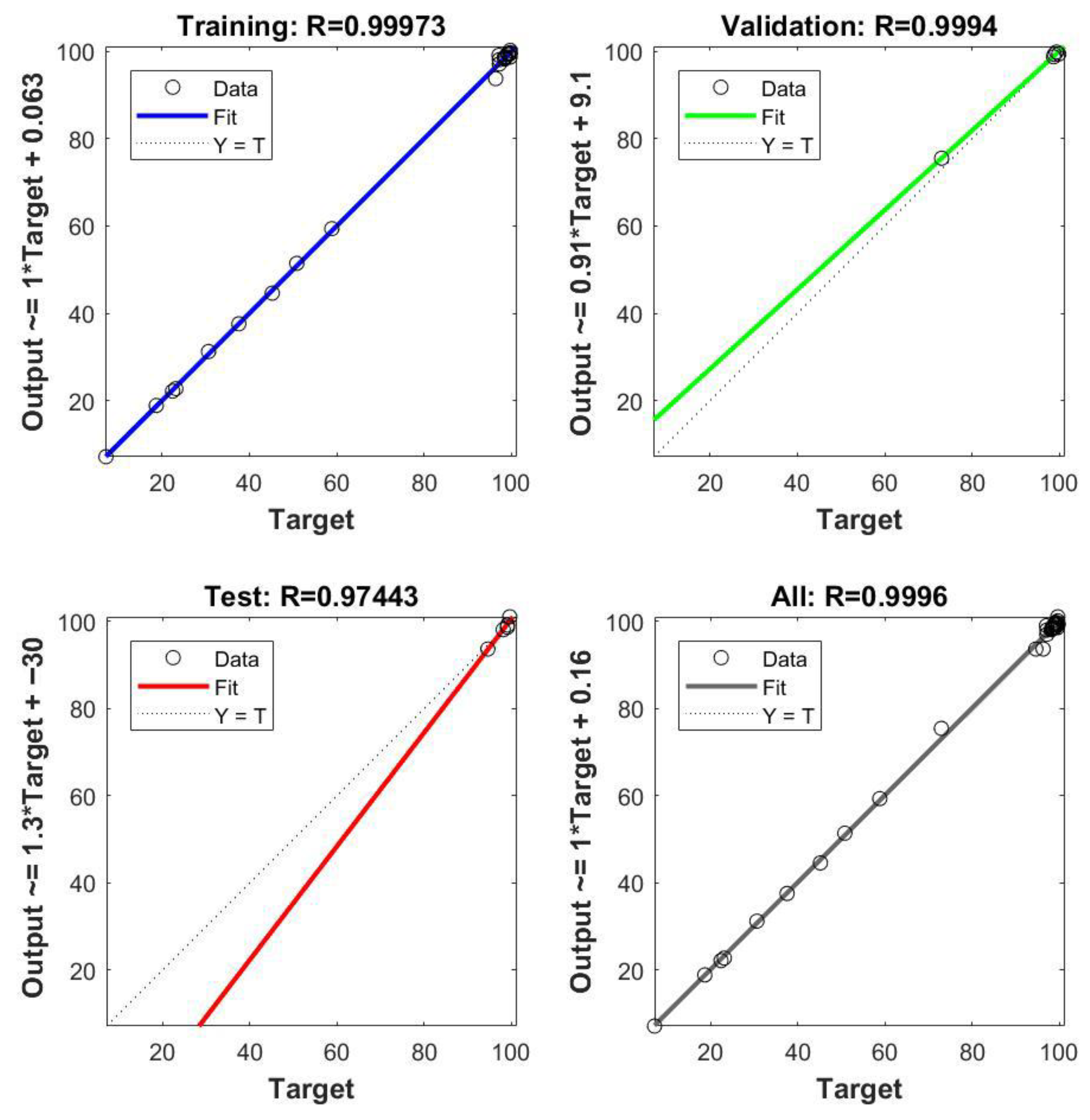
2.2.7. ANN Modeling
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Apparatus
4.2. Preparation of the Adsorbent Material
4.2.1. Preparation of TWB
4.2.2. Sol–Gel Synthesis of CuFe2O4-Modified Brewed Tea Waste
↓
9CuFe2O4 (s) + 120 CO2 (g) + 80 H2O (l) + 36 N2 (g)
4.3. Batch Method
4.4. Impacts of Parameters and Evaluation of Data
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamai-Amara, H.; Abou-Saleh, H.; Al-Ghouti, M.A.; Crovella, S.; Saadaoui, I.; Soubra, L. Microalgae potential to protect from heavy metals-induced carcinogenicity. Algal Res. 2024, 78, 103411. [Google Scholar] [CrossRef]
- Hamai-Amara, H.; Saadaoui, I.; Cherif, M.; Da’ana, D.A.; Soubra, L.; Al-Ghouti, M.A. Evidencing nickel biosorption capacity of cyanobacteria Chroococcidiopsis sp.: Potential metallo-protective agents. BMC Chem. 2025, 19, 59. [Google Scholar] [CrossRef] [PubMed]
- Khalaj, M.; Ghashang, M. An investigation of the mechanical properties and adsorption potentials of Fe2O3@SiO2-L-cysteine-cellulose system. Sci. Rep. 2025, 15, 8413. [Google Scholar] [CrossRef] [PubMed]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. Npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Liu, C.; Lin, J.; Chen, H.; Wang, W.; Yang, Y. Comparative Study of Biochar Modified with Different Functional Groups for Efficient Removal of Pb(II) and Ni(II). Int. J. Environ. Res. Public Health 2022, 19, 11163. [Google Scholar] [CrossRef]
- John, M.M.; Benettayeb, A.; Belkacem, M.; Mitchel, C.R.; Brahim, M.H.; Benettayeb, I.; Haddou, B.; Al-Farraj, S.; Alkahtane, A.A.; Ghosh, S.; et al. An overview on the key advantages and limitations of batch and dynamic modes of biosorption of metal ions. Chemosphere 2024, 357, 142051. [Google Scholar] [CrossRef]
- Munshi, A.M.; Alamrani, N.A.; Alessa, H.; Aljohani, M.; Ibarhiam, S.F.; Saad, F.A.; Al-Qahtani, S.D.; El-Metwaly, N.M. Thiophene functionalized cellulose immobilized with metal organic framework for removal of heavy metals. Cellulose 2023, 30, 7235–7250. [Google Scholar] [CrossRef]
- Dhaouadi, F.; Sellaoui, L.; Reynel-Ávila, H.E.; Landín-Sandoval, V.; Mendoza-Castillo, D.I.; Jaime-Leal, J.E.; Lima, E.C.; Bonilla-Petriciolet, A.; Lamine, A.B. Adsorption mechanism of Zn2+, Ni2+, Cd2+, and Cu2+ ions by carbon-based adsorbents: Interpretation of the adsorption isotherms via physical modelling. Environ. Sci. Pollut. Res. 2021, 28, 30943–30954. [Google Scholar] [CrossRef]
- Packirisamy, V.; Ariyamuthu, R.; Thangavelu, K.; Murugesan, V.V.; Rugmangathan, J. Banana peel biomass–derived activated carbon for nickel ion removal and energy storage applications. Biomass Convers. Biorefinery 2025. [Google Scholar] [CrossRef]
- Oyebola, E.O.; Hefnawy, M.; Ofudje, E.A.; El Gamal, A.; Akande, J.A.; Emran, T.B. Nickel ions biosorption onto sawmill wood waste products: Kinetics, equilibrium, and thermodynamic investigations. BioResources 2025, 20, 3024–3046. [Google Scholar] [CrossRef]
- Cao, D.; Niu, R.; Mo, G.; Deng, H.; Liu, R.; Liu, J.; Fan, J. Adsorption properties and competitive adsorption mechanism exhibited by carbon-nanotube-modified biochar for removal of crude oil and Ni(II) pollutants from water. Ecotoxicol. Environ. Saf. 2025, 290, 117557. [Google Scholar] [CrossRef]
- Babu, P.; Sivakumar, V.M.; Thirumarimurugan, M. Efficient removal of Cu(II) and Ni(II) ions from aqueous solutions using reduced graphene oxide/bentonite clay/ZnO nanocomposites: Kinetics, mechanisms, and adsorption performance. Glob. NEST J. 2024, 27, 06605. [Google Scholar]
- Chien, M.; Chen, S.; Huang, K.; Moja, T.N.; Hwang, S. Cell Morphology, Material Property and Ni(II) Adsorption of Microcellular Injection-Molded Polystyrene Reinforced with Graphene Nanoparticles. Polymers 2025, 17, 189. [Google Scholar] [CrossRef]
- Msimango, N.M.; Makhanya, F.M.; Ntola, P.; Qwabe, L.Q. Remediation of Ni(II) using sugarcane bagasse and its chemically modified derivatives: A comparison of linear and non-linear kinetic and isotherm models. Sep. Sci. Technol. 2025, 60, 899–917. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Alyasi, H.; Karamshahi, F.; Simson, S.; Tongb, Y.; Kochkodan, V.; Lawler, J. Nickel removal from synthetic wastewater by novel zeolite-doped magnesium- iron- and zinc-oxide nanocomposites by hydrothermal-calcination technique. Sci. Rep. 2024, 14, 30954. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Shirazian, S.; Soltani, R.; Zare, M.H. Bio-originated mesosilicate SBA-15: Synthesis, characterization, and application for heavy metal removal. NPJ Clean Water 2024, 7, 49. [Google Scholar] [CrossRef]
- Manjuladevi, M.; Anitha, R.; Manonmani, S. Kinetic study on adsorption of Cr(VI), Ni(II), Cd(II) and Pb(II) ions from aqueous solutions using activated carbon prepared from Cucumis melo peel. Appl. Water Sci. 2018, 8, 36. [Google Scholar] [CrossRef]
- Elbar, D.; Rahaly, H.; Guiedeui, A. Kinetics of Experimental Adsorption of Nickel Metal by Activated Carbon. J. Min. Sci. 2022, 58, 144–150. [Google Scholar] [CrossRef]
- Iamsaard, K.; Weng, C.H.; Yen, L.T.; Tzeng, J.-H.; Poonpakdee, C.; Lin, Y.-T. Adsorption of metal on pineapple leaf biochar: Key affecting factors, mechanism identification, and regeneration evaluation. Bioresour. Technol. 2022, 344, 126131. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K.; Phan, C. Synthesis and characterization of slow pyrolysis pine cone bio-char in the removal of organic and inorganic pollutants from aqueous solution by adsorption: Kinetic, equilibrium, mechanism and thermodynamic. Bioresour. Technol. 2017, 246, 76–81. [Google Scholar] [CrossRef]
- Khalid, S.; Chaudhary, M.N.; Nazir, R.; Ahmad, S.R.; Hussain, N.; Ayub, Y.; Ibrar, M. Biochar supported metallo-inorganic nanocomposite: A green approach for decontamination of heavy metals from water. PLoS ONE 2023, 18, e0289069. [Google Scholar] [CrossRef]
- Abdul, G.; Zhu, X.; Chen, B. Structural characteristics of biochar-graphene nanosheet composites and their adsorption performance for phthalic acid esters. Chem. Eng. J. 2017, 319, 9–20. [Google Scholar] [CrossRef]
- Liu, H.; Liang, S.; Gao, J.; Ngo, H.H.; Guo, W.; Guo, Z.; Li, Y. Development of biochars from pyrolysis of lotus stalks for Ni(II) sorption: Using zinc borate as flame retardant. J. Anal. Appl. Pyrolysis 2014, 107, 336–341. [Google Scholar] [CrossRef]
- Isaac, R.; Siddiqui, S. Sequestration of Ni(II) and Cu(II) using FeSO4 modified Zea mays husk magnetic biochar: Isotherm, kinetics, thermodynamic studies and RSM. J. Hazard. Mater. Adv. 2022, 8, 100162. [Google Scholar] [CrossRef]
- Amin, M.; Chetpattananondh, P. Biochar from extracted marine Chlorella sp. residue for high efficiency adsorption with ultrasonication to remove Cr(VI), Zn(II) and Ni(II). Bioresour. Technol. 2019, 289, 121578. [Google Scholar] [CrossRef] [PubMed]
- Weidner, E.; Karbassiyazdi, E.; Altaee, A.; Jesionowski, T.; Ciesielczyk, F. Hybrid Metal Oxide/Biochar Materials for Wastewater Treatment Technology: A Review. ACS Omega 2022, 7, 27062–27078. [Google Scholar] [CrossRef]
- Lapeñas, L.A.; Peña-Bahamonde, J.; Faria, L.U.S.; Luna, M.D.G.; Rodrigues, D.F. Removing heavy metal ions from wastewater by Chlorella sorokiniana coupled to manganese-doped magnetic ferrite nanoparticles. JHM Lett. 2023, 4, 100082. [Google Scholar] [CrossRef]
- Masunga, N.; Mmelesi, O.K.; Kefeni, K.K.; Mamba, B.B. Recent advances in copper ferrite nanoparticles and nanocomposites synthesis, magnetic properties and application in water treatment: Review. J. Environ. Chem. Eng. 2019, 7, 103179. [Google Scholar] [CrossRef]
- Hou, H.; Xu, G.; Tan, S.; Xiang, S. Effects of solvents on the synthesis and infrared radiation emissivity of CuFe2O4 spinels. J. Alloys Compd. 2018, 763, 736–741. [Google Scholar] [CrossRef]
- Masunga, N.; Mamba, B.B.; Getahun, Y.W.; El-Gendy, A.A.; Kefeni, K.K. Synthesis of single-phase superparamagnetic copper ferrite nanoparticles using an optimized coprecipitation method. Mater. Sci. Eng. B 2021, 272, 115368. [Google Scholar] [CrossRef]
- Kohli, S.; McCurdy, P.R.; Johnson, D.C.; Das, J.; Prieto, A.L.; Rithner, C.D.; Fisher, E.R. Template-assisted chemical vapor deposited spinel Ferrite nanotubes. J. Phys. Chem. C 2010, 114, 19557–19561. [Google Scholar] [CrossRef]
- Karuppaiya, D.; Dinesh, A.; Krishnan, Y.; Babu, K.; Sivadasan, S.; Sureshkumar, K.; Sakkaraiyan, S.; Sinthuja, S.; Radhakrishnan, K.; Elumalai, S.; et al. Synthesis of CuFe2O4 Nanoparticles by Microwave Combustion Method: Structural, Morphological, Optical and Photocatalytic Properties. Semiconductors 2024, 58, 874–881. [Google Scholar] [CrossRef]
- Goya, G.F.; Rechenberg, H.R.; Jiang, J.Z. Structural and magnetic properties of ball milled copper ferrite. J. Appl. Phys. 1998, 84, 1101–1108. [Google Scholar] [CrossRef]
- Sartale, S.D.; Lokhande, C.D.; Muller, M. Electrochemical synthesis of nanocrystalline CuFe2O4 thin films from non-aqueous (ethylene glycol) medium. Mater. Chem. Phys. 2003, 80, 120–128. [Google Scholar] [CrossRef]
- Samikannu, K.; Ahmad, A.F.; Ismail, I.; Aspanut, Z.; Majid, W.H.A. Sol–Gel Combustion Synthesis and Study the High Frequency Dielectric Properties of Copper Ferrite Nanoparticles. J. Clust. Sci. 2023, 34, 2633–2641. [Google Scholar] [CrossRef]
- Ismael, M.; Wark, M. A simple sol–gel method for the synthesis of Pt co-catalyzed spinel-type CuFe2O4 for hydrogen production; the role of crystallinity and band gap energy. Fuel 2024, 359, 130429. [Google Scholar] [CrossRef]
- Selima, S.S.; Khairy, M.; Mousa, M.A. Comparative studies on the impact of synthesis methods on structural, optical, magnetic and catalytic properties of CuFe2O4. Ceram. Int. 2019, 45, 6535–6540. [Google Scholar] [CrossRef]
- Patil, P.N.; Kumar, S.; Anshu, A.; Jali, V.M.; Sahoo, B. Green synthesis of meso-porous CuFe2O4 nanoparticles through aloe-vera assisted sol–gel auto-combustion method. J. Mater. Sci. Mater. Electron. 2025, 36, 825. [Google Scholar] [CrossRef]
- Ng, I.-S.; Wu, X.; Yang, X.; Xie, Y.; Lu, Y.; Chen, C. Synergistic effect of Trichoderma reesei cellulases on agricultural tea waste for adsorption of heavy metal Cr(VI). Bioresour. Technol. 2013, 145, 297–301. [Google Scholar] [CrossRef]
- Pal, D.; Maiti, S.K. Abatement of cadmium (Cd) contamination in sediment using tea waste biochar through meso-microcosm study. J. Clean. Prod. 2019, 212, 986–996. [Google Scholar] [CrossRef]
- Alardhi, S.M.; Fiyadh, S.S.; Salman, A.D.; Adelikhah, M. Prediction of methyl orange dye (MO) adsorption using activated carbon with an artificial neural network optimization modeling. Heliyon 2023, 9, e12888. [Google Scholar] [CrossRef]
- Jiao, M.; Jacquemin, J.; Zhang, R.; Zhao, N.; Liu, H. The Prediction of Cu(II) Adsorption Capacity of Modified Pomelo Peels Using the PSO-ANN Model. Molecules 2023, 28, 6957. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Patel, A.K.; Singhania, R.R.; Chen, C.-W.; Saratale, R.G.; Dong, C.-D. Enhanced copper (II) bioremediation from wastewater using nano magnetite (Fe3O4) modified biochar of Ascophyllum nodosum. Bioresour. Technol. 2023, 388, 129654. [Google Scholar] [CrossRef] [PubMed]
- Ervine, M.; Mangwandi, C. Evaluation of magnetic teawaste-based biochar particles for removal of cadmium from aqueous solutions. Particuology 2025, 99, 92–105. [Google Scholar] [CrossRef]
- Al-Wasidi, A.S.; Shah, R.K.; Abdelrahman, E.A.; Mabrouk, E.-S.M. Facile Synthesis of CuFe2O4 Nanoparticles for Efficient Removal of Acid Blue 113 and Malachite Green Dyes from Aqueous Media. Inorganics 2024, 12, 143. [Google Scholar] [CrossRef]
- Labidi, N.S.; Djebaili, A. Studies of The Mechanism of Polyvinyl Alcohol Adsorption on The Calcite/Water Interface in The Presence of Sodium Oleate. J. Miner. Mater. Charact. Eng. 2008, 7, 147–161. [Google Scholar] [CrossRef]
- Zhao, T.; Ma, X.; Cai, H.; Ma, Z.; Liang, H. Study on the Adsorption of CuFe2O4-Loaded Corncob Biochar for Pb(II). Molecules 2020, 25, 3456. [Google Scholar] [CrossRef]
- Aboulsoud, Y.I.E. Biosorptive removal of fluoride from wastewater using tea domestic waste biochar. Environ. Dev. Sustain. 2024, 27, 16451–16468. [Google Scholar] [CrossRef]
- Khurshid, H.; Mustafa, M.R.U.; Rashid, U.; Isa, M.H.; Ho, Y.C.; Shah, M.M. Adsorptive removal of COD from produced water using tea waste biochar. Environ. Technol. Innov. 2021, 23, 101563. [Google Scholar] [CrossRef]
- Sultan, M.Z.; Jamil, Y.; Javed, Y.; Sarfraz, R.A. Synthesis, Characterization, and Study of Thermal Response of Cu-Doped Fe3O4 Nanoparticles. J. Supercond. Nov. Magn. 2021, 34, 3209–3221. [Google Scholar] [CrossRef]
- Stojanović, J.; Milojević-Rakić, M.; Bajuk-Bogdanović, D.; Ranđelović, D.; Sokić, M.; Otašević, B.; Malenović, A.; Ležaić, A.J.; Protić, A. Chemometrically-aided general approach to novel adsorbents studies: Case study on the adsorption of pharmaceuticals by the carbonized Ailanthus altissima leaves. Heliyon 2024, 10, e34841. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substance. Kung. Sven. Veten. Hand. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Kinetic models for the sorption of dye from aqueous solution by wood. J. Environ. Sci. Health B 1998, 76, 183–191. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. ASCE 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Markandeya, S.A.; Shukla, S.P.; Mohan, D.; Singh, N.B.; Bhargava, D.S.; Shukla, R.; Pandey, G.; Yadav, V.P.; Kisku, G.C. Adsorptive capacity of sawdust for the adsorption of MB dye and designing of two-stage batch adsorber. Cogent Environ. Sci. 2015, 1, 1075856. [Google Scholar] [CrossRef]
- Ozdes, D.; Duran, C. Equilibrium, kinetics, and thermodynamic evaluation of mercury (II) removal from aqueous solutions by moss (Homalothecium sericeum) biomass. Environ. Prog. Sustain. Energy 2015, 34, 1620–1628. [Google Scholar] [CrossRef]
- Crini, G.; Peindy, H.N.; Gimbert, F.; Robert, C. Removal of C.I. Basic Green 4 (Malachite Green) from aqueous solutions by adsorption using cyclodextrin-based adsorbent: Kinetic and equilibrium studies. Sep. Purif. Technol. 2007, 53, 97–110. [Google Scholar] [CrossRef]
- Ngah, W.S.W.; Hanafiah, M.A.K.M. Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: A review. Bioresour. Technol. 2008, 99, 3935–3948. [Google Scholar] [CrossRef]
- Simón, D.; Palet, C.; Cristóbal, A. Cadmium Removal by Adsorption on Biochars Derived from Wood Industry and Craft Beer Production Wastes. Water 2024, 16, 1905. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Jock, A.A.; Oboh, I.; Inyang, U.E.; Ganchok, L.P.; Adeku, O. Chromium and nickel metal ions removal from contaminated water using Nigerian bentonite clay. Water Pract. Technol. 2021, 16, 825–836. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Uber die adsorption in losungen. Z. Für Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Radushkevich, L.V. Equation of the characteristics curve of activated charcoal. Chem. Zent. 1947, 1, 875. [Google Scholar]
- Tran, H.N.; You, S.-J.; Chao, H.-P. Thermodynamic parameters of cadmium adsorption onto orange peel calculated from various methods: A comparison study. J. Environ. Chem. Eng. 2016, 4, 2671–2682. [Google Scholar] [CrossRef]
- Sun, Y.; Yue, Q.; Gao, B.; Gao, Y.; Xu, X.; Li, Q.; Wang, Y. Adsorption and cosorption of ciprofloxacin and Ni(II) on activated carbon-mechanism study. J. Taiwan. Inst. Chem. Eng. 2014, 45, 681–688. [Google Scholar] [CrossRef]
- Ahmadi, M.; Kouhgardi, E.; Ramavandi, B. Physico-chemical study of dew melon peel biochar for chromium attenuation from simulated and actual wastewaters. Korean J. Chem. Eng. 2016, 33, 2589–2601. [Google Scholar] [CrossRef]
- Xue, Y.; Kamali, M.; Kakavandi, B.; Costa, M.E.V.; Thompson, I.P.; Huang, W.; Appels, L.; Dewil, R. Activation of peracetic acid by a magnetic biochar-ferrospinel AFe2O4 (A = Cu, Co, or Mn) nanocomposite for the degradation of carbamazepine—A comparative and mechanistic study. Chem. Eng. J. 2024, 490, 151932. [Google Scholar] [CrossRef]

| qe exp (mg g−1) | 124.5 |
| Pseudo-first order model | |
| k1 (min−1) | −0.0199 |
| qe cal (mg g−1) | 1.81 |
| R2 | 0.8818 |
| Pseudo-second order model | |
| k2 (g mg−1 min−1) | 0.071 |
| qe cal (mg g−1) | 125.0 |
| R2 | 0.9999 |
| Intraparticle diffusion model | |
| kid,1 (mg g−1 min−1/2) | 1.26 |
| R2 | 0.9596 |
| kid,2 (mg g−1 min−1/2) | 0.14 |
| R2 | 0.9949 |
| C | 122.2 |
| Langmuir isotherm model | |
| qmax (mg g−1) | 232.6 |
| b (L mg−1) | 0.125 |
| R2 | 0.9999 |
| Freundlich isotherm model | |
| Kf (mg g−1) | 66.8 |
| n | 5.1 |
| R2 | 0.8457 |
| D-R isotherm model | |
| qm (mg g−1) | 17.7 |
| β (mol2 kJ−2) | −0.002 |
| E (kJ mol−1) | 15.8 |
| R2 | 0.9300 |
| Adsorbent | qmax (mg g−1) | Optimum Conditions | Reference | ||
|---|---|---|---|---|---|
| pH | Dosage | Time | |||
| Banana peel activated carbon | 41.53 | 7 | - | 60 min | [9] |
| Acid activated sawmill wood waste products | 76.342 | 5.0 | 1.2 g/L | 100 min | [10] |
| Unactivated (raw) sawmill wood waste products | 68.752 | 5.0 | 1.0 g/L | 120 min | |
| Carbon-nanotube-modified biochar (CNT3-CBC800) | 32.87 | 6 | 1.0 g/L | 6 h | [11] |
| Reduced graphene oxide/bentonite | 252.41 | 10 | - | 80 min | [12] |
| Reduced graphe[ne oxide/bentonite/1%ZnO | 185 | 10 | - | 70 min | |
| Reduced graphene oxide/bentonite/3%ZnO | 125.03 | 10 | - | 70 min | |
| Reduced graphene oxide/bentonite/5%ZnO | 96.60 | 10 | - | 70 min | |
| Sugarcane bagasse | 53.9 | 6 | - | 1 h | [14] |
| Extracted cellulose | 37.0 | 6 | - | 1 h | |
| Carboxymethyl cellulose | 152.8 | 6 | - | 1 h | |
| Pineapple leaf biochar | 44.88 | 5.0 | 2.0 g/L | 15 min | [19] |
| MnFe2O4 doped hydroxyapatite/kaolinite/biochar | 204.680 | 7.0 | 2.0 g/L | 50 min | [21] |
| MnFe2O4 doped hydroxyapatite/vermiculite/biochar | 230.340 | 7.0 | 2.0 g/L | 30 min | |
| Biochar of Zea mays husk modified by FeSO4 | 22.53 | 6 | - | 90 min | [24] |
| Marine Chlorella sp. biochar (ultrasonication adsorption) | 27.45 | 7 | 0.5 g/L | 15 min | [25] |
| Marine Chlorella sp. biochar (conventional adsorption) | 24.76 | 7 | 0.5 g/L | 80 min | |
| A. donax Linn activated carbon | 8.614 | 6.0 | 4.0 g/L | 1 h | [67] |
| MTWB | 232.6 | 5.6 | 1.0 g/L | 15 min | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duran, C.; Ozeken, S.T.; Seker, S.; Ozdes, D. Sol–Gel Synthesized CuFe2O4-Modified Biochar Derived from Tea Waste for Efficient Ni(II) Removal: Adsorption, Regeneration, and ANN Modeling. Gels 2025, 11, 628. https://doi.org/10.3390/gels11080628
Duran C, Ozeken ST, Seker S, Ozdes D. Sol–Gel Synthesized CuFe2O4-Modified Biochar Derived from Tea Waste for Efficient Ni(II) Removal: Adsorption, Regeneration, and ANN Modeling. Gels. 2025; 11(8):628. https://doi.org/10.3390/gels11080628
Chicago/Turabian StyleDuran, Celal, Sengul Tugba Ozeken, Serdal Seker, and Duygu Ozdes. 2025. "Sol–Gel Synthesized CuFe2O4-Modified Biochar Derived from Tea Waste for Efficient Ni(II) Removal: Adsorption, Regeneration, and ANN Modeling" Gels 11, no. 8: 628. https://doi.org/10.3390/gels11080628
APA StyleDuran, C., Ozeken, S. T., Seker, S., & Ozdes, D. (2025). Sol–Gel Synthesized CuFe2O4-Modified Biochar Derived from Tea Waste for Efficient Ni(II) Removal: Adsorption, Regeneration, and ANN Modeling. Gels, 11(8), 628. https://doi.org/10.3390/gels11080628








