Mechanisms of Covalent Bonds in Enhancing the Adsorption Stability of Clay–Polymer Gels in High-Temperature Environments
Abstract
1. Introduction
2. Results and Discussion
2.1. Thermal Stability Analysis of Silicone Polymers
2.2. Rheological and Filtration Performance Analysis
2.3. Shale Rolling Recovery of Organosilicon Polymer
2.4. Fourier Transform Infrared Spectroscopy (Ft-Ir) Spectroscopy Analysis
2.5. Effect of Different Temperatures on the Zeta Potential and Particle Size Distribution of the Drilling Fluid Gel System
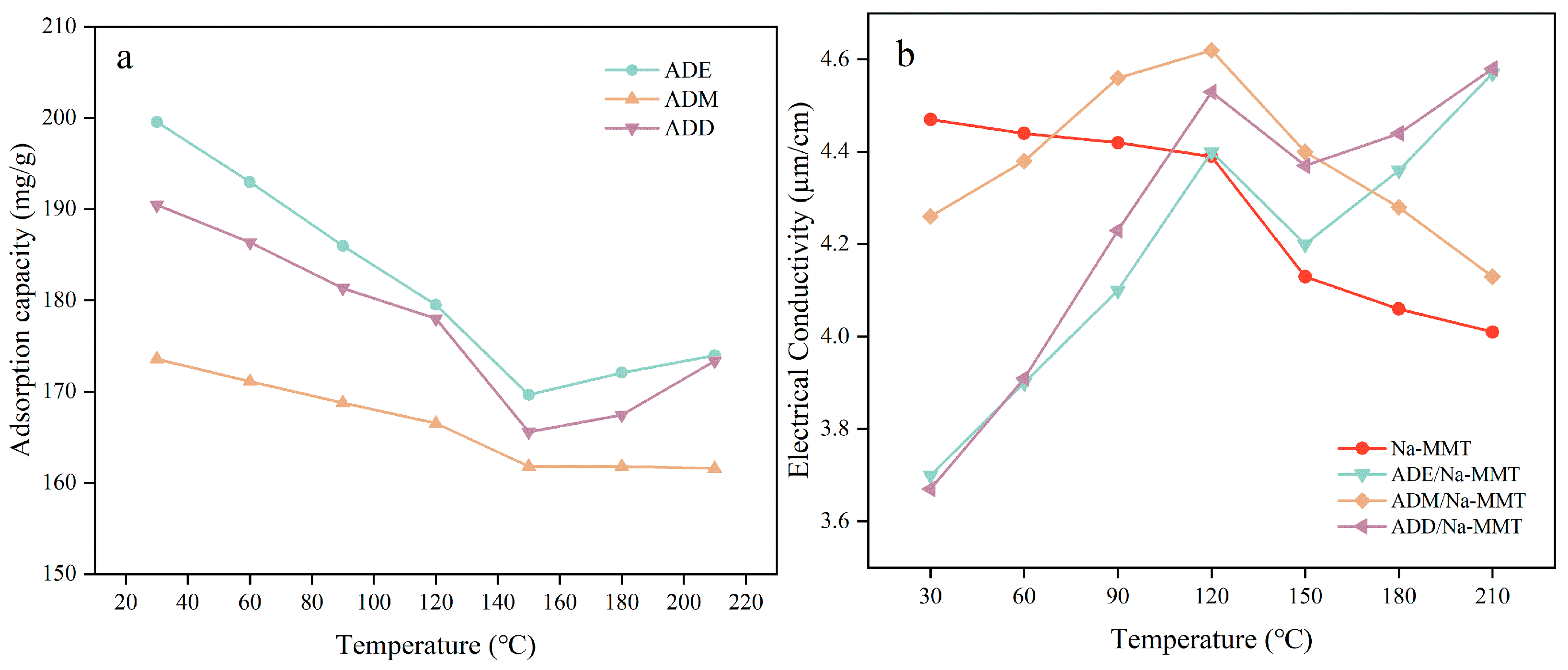
2.6. Effect of High Temperature on the Polymer Adsorption and Conductivity of the Polymer Drilling Fluid Gel System
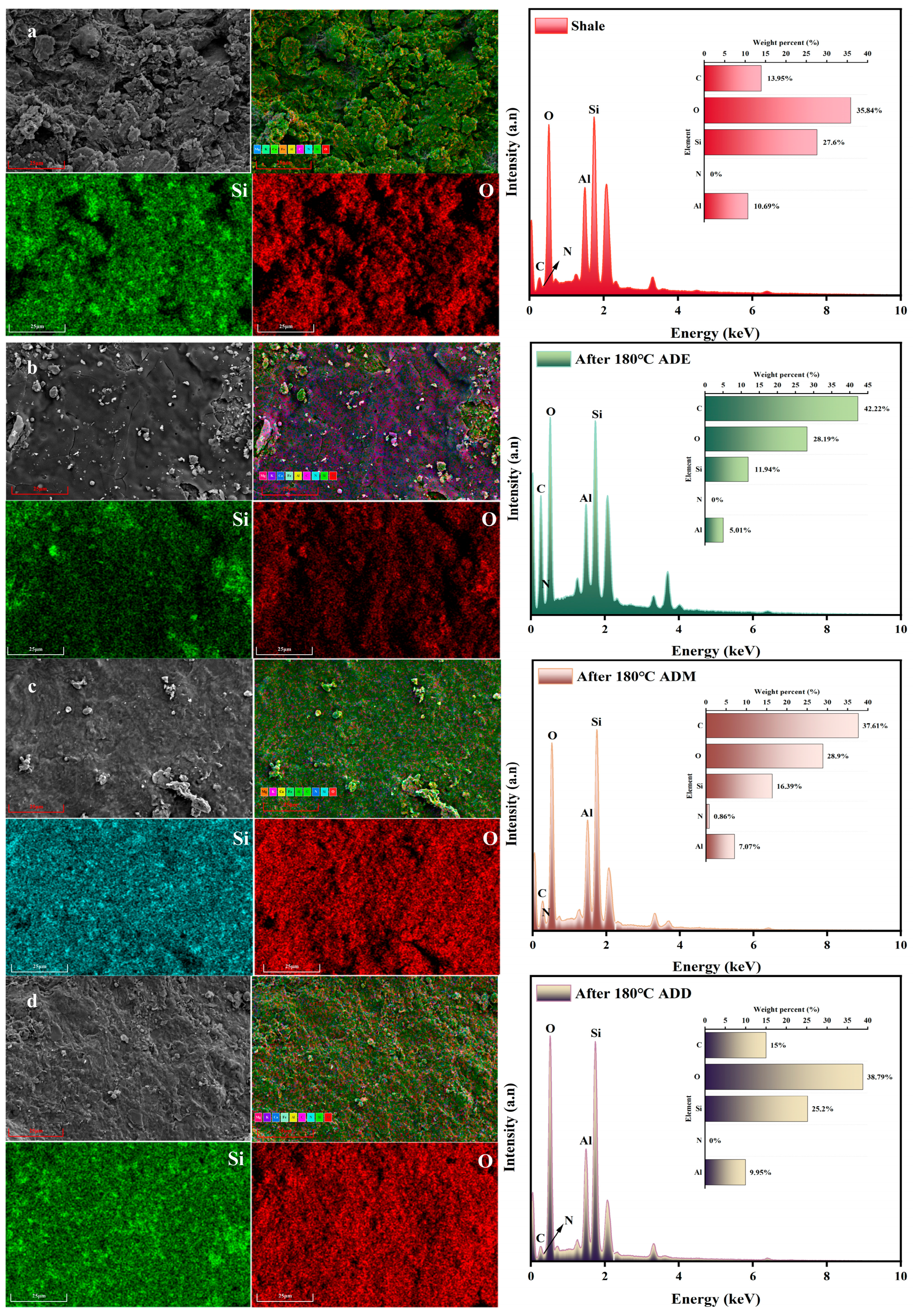
2.7. Effects of High Temperature on the Microscopic Morphology and Elemental Composition of the Polymer–Shale Surface Film
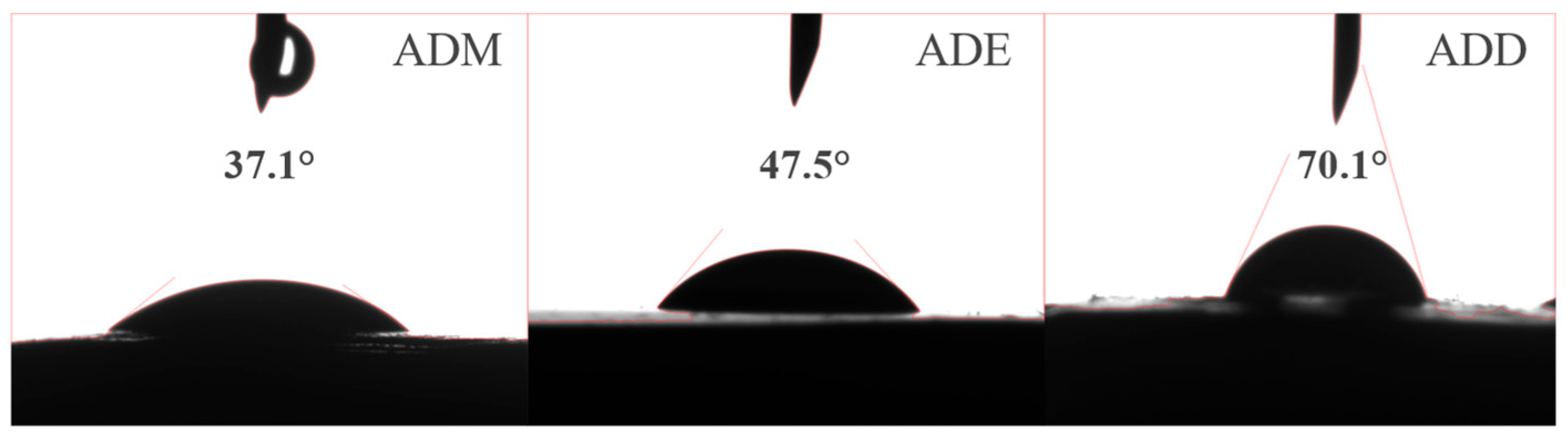
2.8. Wettability Analysis
3. Proposed Inhibition Mechanism
4. Conclusions
- (1)
- In this study, three organosilicon polymer inhibitors were successfully synthesized via soap-free emulsion polymerization. Experiments show that their rolling recovery rate at 210 °C was 30% higher than that of polyamine and KCl (exceeding 80%). After hydrolysis, the siloxane groups of the polymer generated Si-OH, which underwent dehydration condensation with Si-OH on the clay surface to form Si-O-Si covalent bonds. This resulted in a denser filter cake and a 53.3% reduction in fluid loss (decreasing to 14 mL), confirming that the covalent bond adsorption mechanism significantly enhanced the high-temperature stability of clay compared to traditional non-covalent bonds (hydrogen bonds/ionic bonds).
- (2)
- At room temperature, electrostatic adsorption occurred between the polymer and clay (the absolute value of zeta potential of the polymer drilling fluid gel system decreases by 44–50% after adding the polymer). The infrared spectrum of the polymer-clay mixed sample aged at 180 °C showed an increased peak of Si-O bonds, proving the formation of covalent bonds. This indicates that the adsorption between the polymer and clay shifted from low-temperature electrostatic adsorption to a synergistic effect of high-temperature electrostatic adsorption and covalent bond adsorption.
- (3)
- Experiments on conductivity and adsorption amount demonstrate that different substituent types affect the hydrolysis rate of siloxane groups, thereby determining the thermal stability of the covalent bonds formed between the polymer and clay at high temperatures: methoxy groups hydrolyzed rapidly, but were prone to self-polymerization and depletion at high temperatures, leading to bond disruption. Ethoxy groups maintained hydrolysis activity and continuously formed bonds at high temperatures. Methyl siloxyl groups with steric hindrance could inhibit the self-polymerization of methoxy groups, and the formed film exhibited certain hydrophobicity (contact angle: 70.1°).
- (4)
- In the future, drilling fluid conditioner types can be optimized: ADD-type polymers with steric hindrance are suitable for ultra-high temperature environments, combining inhibitory and fluid loss reduction properties. Meanwhile, introducing multifunctional groups (such as the synergy of amino and methoxy groups) can regulate hydrolysis timing and hydrophobicity, or compounding with nano-silica can enhance film strength to further improve high-temperature stability. Exploring biobased siloxane monomers to reduce synthesis costs and environmental toxicity will promote the development of green drilling fluid technology.
5. Experimental
5.1. Experimental Reagents
- (1)
- Main chemical reagents
- (2)
- Analysis of the mineral composition of shale rock fragments
5.2. Experimental Instruments
5.3. Preparation of Organosilicon Polymer
5.4. Thermogravimetric Analysis
5.5. Rheological Filtration Experiment
5.6. Evaluation of Rolling Recovery Performance
- R—rolling recovery rate of shale, %;
- M—weight of dried cuttings after aging, g.
5.7. Infrared Spectroscopy Analysis
5.8. Zeta Potential Experiment
5.9. Particle Size Experiment
5.10. Total Organic Carbon Analysis (TOC) Adsorption Experiment
- T—adsorption capacity, mg·g−1;
- CJ—polymer concentration, mg·L−1;
- CS—total organic carbon concentration in the supernatant, mg·L−1;
- ω—mass fraction of the carbon element in the polymer;
- CT—concentration of clay particles in the dispersion system, g·L−1.
5.11. Measuring the Degree of Hydrolysis by Conductivity
5.12. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-Ray Spectroscopy Analysis (EDS)
5.13. Contact Angle Experiment
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WBDFs | Water-based drilling fluids |
| SEM | Scanning electron microscopy |
| mm | Millimeter |
| TOC | Total organic carbon analysis |
| EDS | Energy-dispersive X-ray spectroscopy |
| Na-MMT | Sodium bentonite |
| AM | Acrylamide |
| DMC | Methacryloxyethyltrimethyl ammonium chloride |
| VTES | Triethoxysilane |
| VTMO | Trimethoxysilane |
| VMDS | Dimethoxymethylvinylsilane |
| ADE | Polymer of AM, DMC and VTES |
| ADM | Polymer of AM, DMC and VTMO |
| ADD | Polymer of AM, DMC and VMDS |
| ABIN | 2,2′-Azobis (2-methylpropionitrile) |
| AR | Analytical reagent |
References
- Shanafield, M.; Cook, P.G.; Simmons, C.T. Towards Quantifying the Likelihood of Water Resource Impacts from Unconventional Gas Development. Ground Water 2019, 57, 547–561. [Google Scholar] [CrossRef]
- Patel, P.R.; Kulkarni, M.N. Preliminary Results of Gps Studies for Monitoring Land Subsidence Over the Shallow Gas Reservoir in India. Surv. Rev. 2008, 40, 356–365. [Google Scholar] [CrossRef]
- Railsback, L.B. Depth and Nature of Giant Petroleum Discoveries Through Time as an Indicator of Resource Depletion. J. Ind. Ecol. 2013, 17, 345–351. [Google Scholar] [CrossRef]
- Wang, J.L.; Feng, J.X.; Bentley, Y.; Feng, L.Y.; Qu, H. A Review of Physical Supply and EROI of Fossil Fuels in China. Pet. Sci. 2017, 14, 806–821. [Google Scholar] [CrossRef]
- Li, Y.; Fu, J.; Hao, N.; Song, W.; Yu, L. Experimental Study on Unloading Failure Characteristics and Damage Evolution Rules of Deep Diorite Based on Triaxial Acoustic Emission Tests. Geosci. J. 2023, 27, 629–646. [Google Scholar] [CrossRef]
- Jiang, G.; Dong, T.; Cui, K.; He, Y.; Quan, X.; Yang, L.; Fu, Y. Research Status and Development Directions of Intelligent Drilling Fluid Technologies. Pet. Explor. Dev. 2022, 49, 660–670. [Google Scholar] [CrossRef]
- Zhang, Z.; Ai, Z.; Yang, L.; Zhang, Y.; Pang, X.; Yuan, Z.; Liu, Z.; Sun, J. Evaluation and Optimization of Cement Slurry Systems for Ultra-Deep Well Cementing at 220 °C. Materials 2024, 17, 5246. [Google Scholar] [CrossRef]
- Mu, L.; Ji, Z. Technologcial Progress and Development Directions of PetroChina Overseas Oil and Gas Exploration. Pet. Explor. Dev. 2019, 46, 1088–1099. [Google Scholar] [CrossRef]
- Liao, B.; Wang, J.; Han, X.; Wang, R.; Lv, K.; Bai, Y.; Jiang, H.; Shao, Z.; Wang, Y.; Sun, J. Microscopic Molecular Insights into Clathrate Methane Hydrates Dissociation in a Flowing System. Chem. Eng. J. 2022, 430, 133098. [Google Scholar] [CrossRef]
- Zhao, K.; Jia, C.; Li, Z.; Du, X.; Wang, Y.; Li, J.; Yao, Z.; Yao, J. Recent Advances and Future Perspectives in Carbon Capture, Transportation, Utilization, and Storage (CCTUS) Technologies: A Comprehensive Review. Fuel 2023, 351, 128913. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, C.; Bai, F.; Wang, W.; An, S.; Zhao, K.; Li, Z.; Li, J.; Sun, H. A Comprehensive Review of the Promising Clean Energy Carrier: Hydrogen Production, Transportation, Storage, and Utilization (HPTSU) Technologies. Fuel 2024, 355, 129455. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, B.; Kong, Z.; Sun, Z.; Qiu, L.; Wang, D. Oscillating Electric Field Effects on Adsorption of the Methane-Water System on Kaolinite Surface. Energy Fuel 2018, 32, 11440–11451. [Google Scholar] [CrossRef]
- Geng, Y.; Sun, J.; Wang, J.; Wang, R.; Yang, J.; Wang, Q.; Ni, X. Modified Nanopolystyrene as a Plugging Agent for Oil-Based Drilling Fluids Applied in Shale Formation. Energy Fuel 2021, 35, 16543–16552. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, Q.; Wang, J.; Long, G.; Cheng, H.; Shi, Y.; Sun, Q.; Jiang, H.; Abulimiti, Y.; Cao, Z.; et al. Hydrocarbon Accumulation Characteristics in Basement Reservoirs and Exploration Targets of Deep Basement Reservoirs in Onshore China. Pet. Explor. Dev. 2024, 51, 31–43. [Google Scholar] [CrossRef]
- Song, Z.; Ding, X.; Zhang, B.; Ge, B.; Tian, X.; Chen, X.; Ma, K.; Peng, H.; Wang, Y.; Yang, D. Dynamic Reconstruction of the Hydrocarbon Generation, Accumulation, and Evolution History in Ultra-Deeply-Buried Strata. Front. Earth Sci. 2022, 10, 927903. [Google Scholar] [CrossRef]
- Aitken, C.M.; Jones, D.M.; Larter, S.R. Anaerobic Hydrocarbon Biodegradation in Deep Subsurface Oil Reservoirs. Nature. 2004, 431, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Y.; Xie, Z.; Zhao, Y.; Zhang, C.; Sun, C.; Wu, G. The Advancement and Challenges of Seismic Techniques for Ultra-Deep Carbonate Reservoir Exploitation in the Tarim Basin of Northwestern China. Energies 2022, 15, 7653. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, R.; Chang, L.; Zhou, D.; Wu, Z.; Yan, G.; Shao, G.; Li, Y. Research and Application of EOR by Gas. Injection in Deep. Clastic Reservoir: Case Study on Tazhong 402 CIII Reservoir in Tarim Oilfield. Pet. Sci. Technol. 2024, 42, 1638–1655. [Google Scholar] [CrossRef]
- Zhu, Y.; He, Z.; Guo, X.; Li, L.; Omosanya, K.; Gao, J.; Tao, Z.; Lu, X. Multidisciplinary Insights into the Origin of Natural Gas from Hydrocarbon Generation and Charging History of Permian Dolomite Reservoir in Sichuan Basin. Pet. Sci. 2025, 22, 1428–1445. [Google Scholar] [CrossRef]
- Tian, W.; Wang, Q.; Liu, X.; Du, W.; Zhang, J.; Gang, C. Investigation of Dioscorea Opposutifolia L. as Green Lubricat in Water Based Drilling Fluids. Green Mater. 2022, 10, 169–175. [Google Scholar] [CrossRef]
- Huang, S.; Dong, T.; Jiang, G.; Yang, J.; Yang, X.; Wang, Q. Synthesis and Performance Evaluation of High-Temperature-Resistant Extreme-Pressure Lubricants for a Water-Based Drilling Fluid Gel System. Gels 2024, 10, 505. [Google Scholar] [CrossRef]
- Yan, C.; Deng, J.; Yu, B.; Li, W.; Chen, Z.; Hu, L.; Li, Y. Borehole Stability in High-Temperature Formations. Rock. Mech. Rock. Eng. 2014, 47, 2199–2209. [Google Scholar] [CrossRef]
- Yang, S.; Zhan, Q.; Pan, Y.; Wang, X.; Narimane, B. Research Progress on Low-Temperature Rheology of High-Performance Ocean Deepwater Drilling Fluids: An Overview. J. Pet. Sci. Eng. 2022, 218, 110978. [Google Scholar] [CrossRef]
- Chen, Y.; Li, W.; Wang, X.; Wu, P.; Wan, X.; Wang, Z.; Li, J.; Sun, X. A Numerical Simulation of the Effect of Drilling Fluid Rheology on Cutting Migration in Horizontal Wells at Different Drilling Fluid Temperatures. Processes 2024, 12, 2428. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Sun, J.; Wang, Z.; Dai, Z.; Sun, Y.; Zhang, T. A Novel Amphoteric Ion-Modified, Styrene-Based Nano-Microsphere and Its Application in Drilling Fluid. Materials 2023, 16, 6096. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Feng, Z.; Xi, B.; Wan, Z.; Yang, D.; Liang, W. Deformation and Instability Failure of Borehole at High Temperature and High Pressure in Hot Dry Rock Exploitation. Renew. Energy 2015, 77, 159–165. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, J.; Liu, J.; Lv, K.; Shao, Z.; Zhang, X.; Xu, Z.; Dai, Z.; Huang, N. Synthesis and Mechanism of Environmentally Friendly High Temperature and High Salt Resistant Lubricants. Pet. Sci. 2023, 20, 3110–3118. [Google Scholar] [CrossRef]
- Rana, A.; Arfaj, M.K.; Saleh, T.A. Advanced Developments in Shale Inhibitors for Oil Production with Low Environmental Footprints-A Review. Fuel 2019, 247, 237–249. [Google Scholar] [CrossRef]
- Xu, P.; Xiong, H.; Pu, X.; Liu, J.; Liu, X. Polymer Drilling Fluid with Micron-Grade Cenosphere for Deep Coal Seam. J. Chem. 2015, 2015, 967653. [Google Scholar] [CrossRef][Green Version]
- Liao, B.; Qiu, L.; Wang, D.; Bao, W.; Wei, Y.; Wang, Y. The Behaviour of Water on the Surface of Kaolinite with an Oscillating Electric Field. RSC Adv. 2019, 9, 21793–21803. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Sun, Y.; Lin, D.; Feng, X.; Wang, F. Insights into the High Temperature-Induced Failure Mechanism of Bentonite in Drilling Fluid. Chem. Eng. J. 2022, 445, 136680. [Google Scholar] [CrossRef]
- Vryzas, Z. Effect of Temperature on the Rheological Properties of Neat Aqueous Wyoming Sodium Bentonite Dispersions. Appl. Clay Sci. 2017, 136, 26–36. [Google Scholar] [CrossRef]
- Wang, G.; Jiang, G.; Yang, J.; Yang, L.; Li, X.; He, Y.; Chang, X. Novel N, N-Dimethylacrylamide Copolymer Containing Multiple Rigid Comonomers as a Filtrate Reducer in Water-Based Drilling Fluids and Mechanism Study. J. Appl. Polym. Sci. 2021, 138, e51001. [Google Scholar] [CrossRef]
- Liu, F.; Jiang, G.; Peng, S.; He, Y.; Wang, J. Amphoteric Polymer as an Anti-Calcium Contamination Fluid-Loss Additive in Water-Based Drilling Fluids. Energy Fuels 2016, 30, 7221–7228. [Google Scholar] [CrossRef]
- Xu, Y.; Yin, H.; Yuan, S.; Chen, Z. Film Morphology and Orientation of Amino Silicone Adsorbed onto Cellulose Substrate. Appl. Surf. Sci. 2009, 255, 8435–8442. [Google Scholar] [CrossRef]
- Krumpfer, J.W.; McCarthy, T.J. Rediscovering Silicones: “Unreactive” Silicones React with Inorganic Surfaces. Langmuir 2011, 27, 11514–11519. [Google Scholar] [CrossRef]
- Chu, Q.; Luo, P.; Zhao, Q.; Feng, J.; Kuang, X.; Wang, D. Application of a New Family of Organosilicon Quadripolymer as a Fluid Loss Additive for Drilling Fluid at High Temperature. J. Appl. Polym. Sci. 2013, 128, 28–40. [Google Scholar] [CrossRef]
- Chu, Q.; Lin, L.; Su, J. Amidocyanogen Silanol as a High-Temperature-Resistant Shale Inhibitor in Water-Based Drilling Fluid. Appl. Clay Sci. 2020, 184, 105396. [Google Scholar] [CrossRef]
- Chu, Q.; Su, J.; Lin, L. Inhibition Performance of Amidocyanogen Silanol in Water-Based Drilling Fluid. Appl. Clay Sci. 2020, 185, 105315. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, F.; Lv, K.; Chang, X. A Novel Film-Forming Silicone Polymer as Shale Inhibitor for Water-Based Drilling Fluids. e-Polymers 2019, 19, 574–578. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, J.; Dai, Z.; Chang, X.; Huang, X.; Liu, J.; Wang, Z.; Lv, K. Organosilicate Polymer as High Temperature Resistent Inhibitor for Water-Based Drilling Fluids. J. Polym. Res. 2020, 27, 107. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, J.; Li, Q.; Lv, K.; Wang, J.; Wang, Z. Mechanism of Organosilicate Polymer as High-Temperature Resistant Inhibitor in Water-Based Drilling Fluids. Colloids Surf. Physicochem. Eng. Asp. 2022, 641, 128489. [Google Scholar] [CrossRef]
- Graiver, D.; Farminer, K.W.; Narayan, R. A Review of the Fate and Effects of Silicones in the Environment. J. Polym. Environ. 2003, 11, 129–136. [Google Scholar] [CrossRef]
- Schlemmer, R.; Friedheim, J.E.; Growcock, F.B.; Bloys, J.B.; Headley, J.A.; Polnaszek, S.C. Chemical Osmosis, Shale, and Drilling Fluids. SPE Drill. Complet. 2003, 18, 318–331. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, H.; Fan, J.; Yin, X.; Liu, X.; Liu, Y.; Song, Q.; Li, H.; Ji, C. Optimization and Evaluation of an Environment-Friendly Water-Based Drilling Fluids for Shale Gas Horizontal Wells. Fresenius Environ. Bull. 2020, 29, 5479–5486. [Google Scholar]
- Zhang, L.; Jiang, G.; Tu, Y.; Cai, J.; Dou, B.; Zhang, J.; Ning, F. Experimental Research of Gas Hydrate Drilling Fluids Using Polyethylene Glycol as an Inhibitor. J. China Univ. Geosci. 2006, 17, 276–282. [Google Scholar] [CrossRef]
- Liu, W.; Hu, J.; Sun, Z.; Chu, H.; Li, X.; Sun, F. Research on Evaluation and Prevention of Hydrate Formation and Blockage Risk in Wellbore during Deepwater Gas Wells Drilling. J. Pet. Sci. Eng. 2019, 180, 668–680. [Google Scholar] [CrossRef]
- Shadizadeh, S.R.; Moslemizadeh, A.; Dezaki, A.S. A Novel Nonionic Surfactant for Inhibiting Shale Hydration. Appl. Clay Sci. 2015, 118, 74–86. [Google Scholar] [CrossRef]
- Hua, L.; Tian, Y.; Gui, Y.; Liu, W.; Wu, W. Semi-Analytical Study of Pile–Soil Interaction on a Permeable Pipe Pile Subjected to Rheological Consolidation of Clayey Soils. J. Int. J. Numer. Anal. Methods Geomech. 2025, 49, 1058–1074. [Google Scholar] [CrossRef]
- Yu, H.; Zhao, Z.; Taleghani, A.D.; Lian, Z.; Zhang, Q. Modeling thermal-induced wellhead growth through the lifecycle of a well. Geoenergy Sci. Eng. 2024, 241, 213098. [Google Scholar] [CrossRef]
- Su, Y.; Cui, Y.; Dupla, J.C.J.; Canou, J. Soil-water retention behaviour of fine/coarse soil mixture with varying coarse grain contents and fine soil dry densities. J. Can. Geotech J. 2022, 59, 291–299. [Google Scholar] [CrossRef]
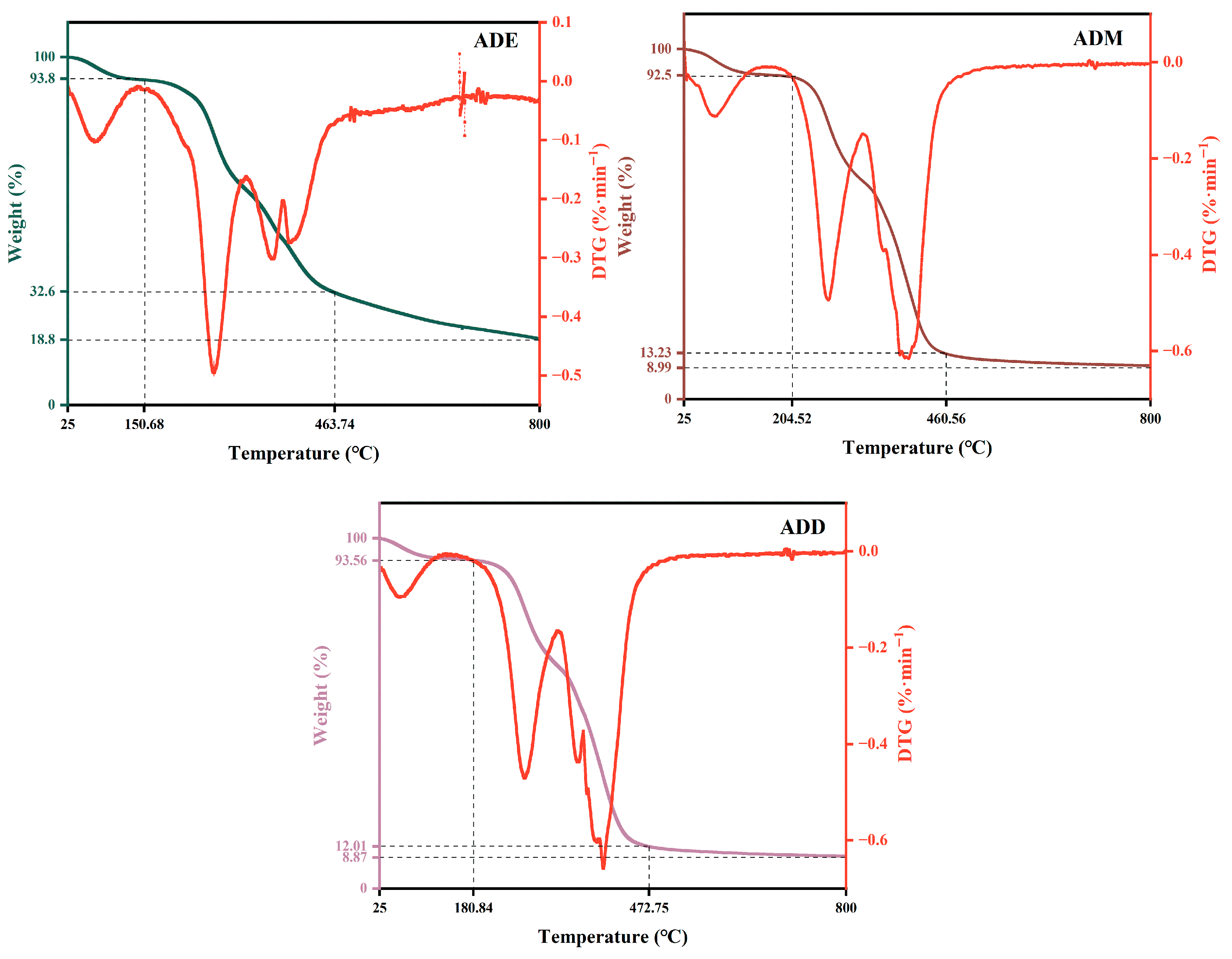
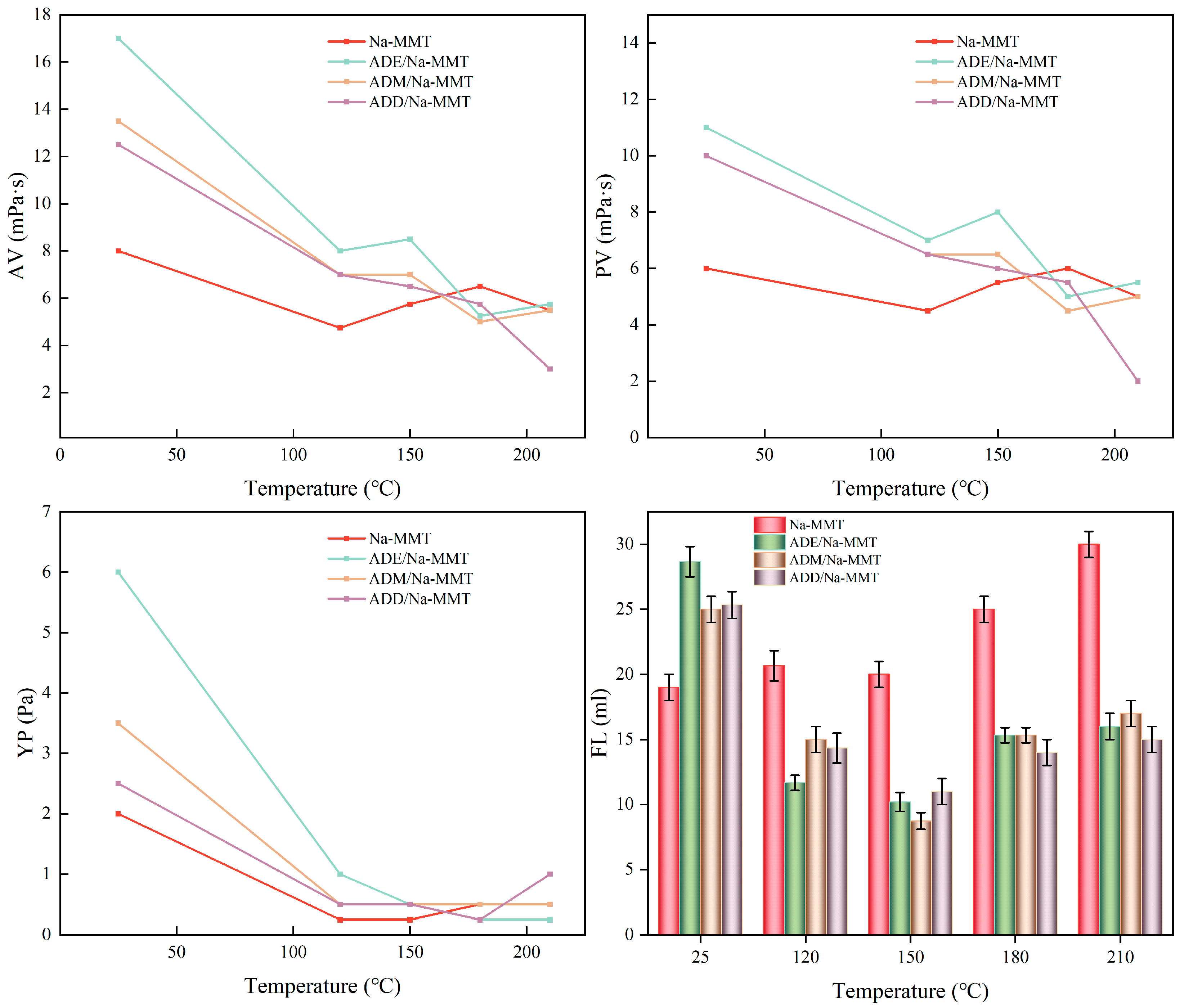
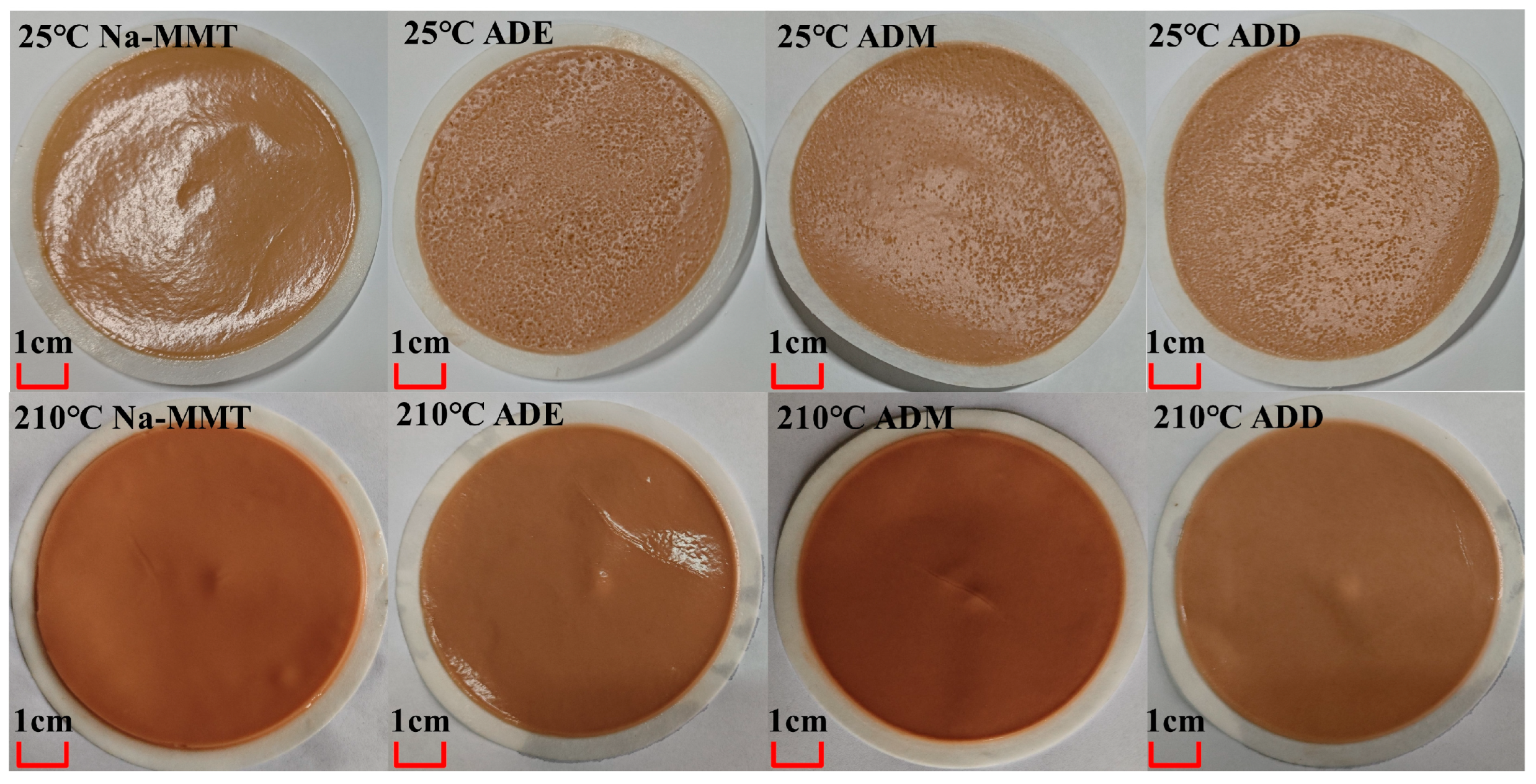


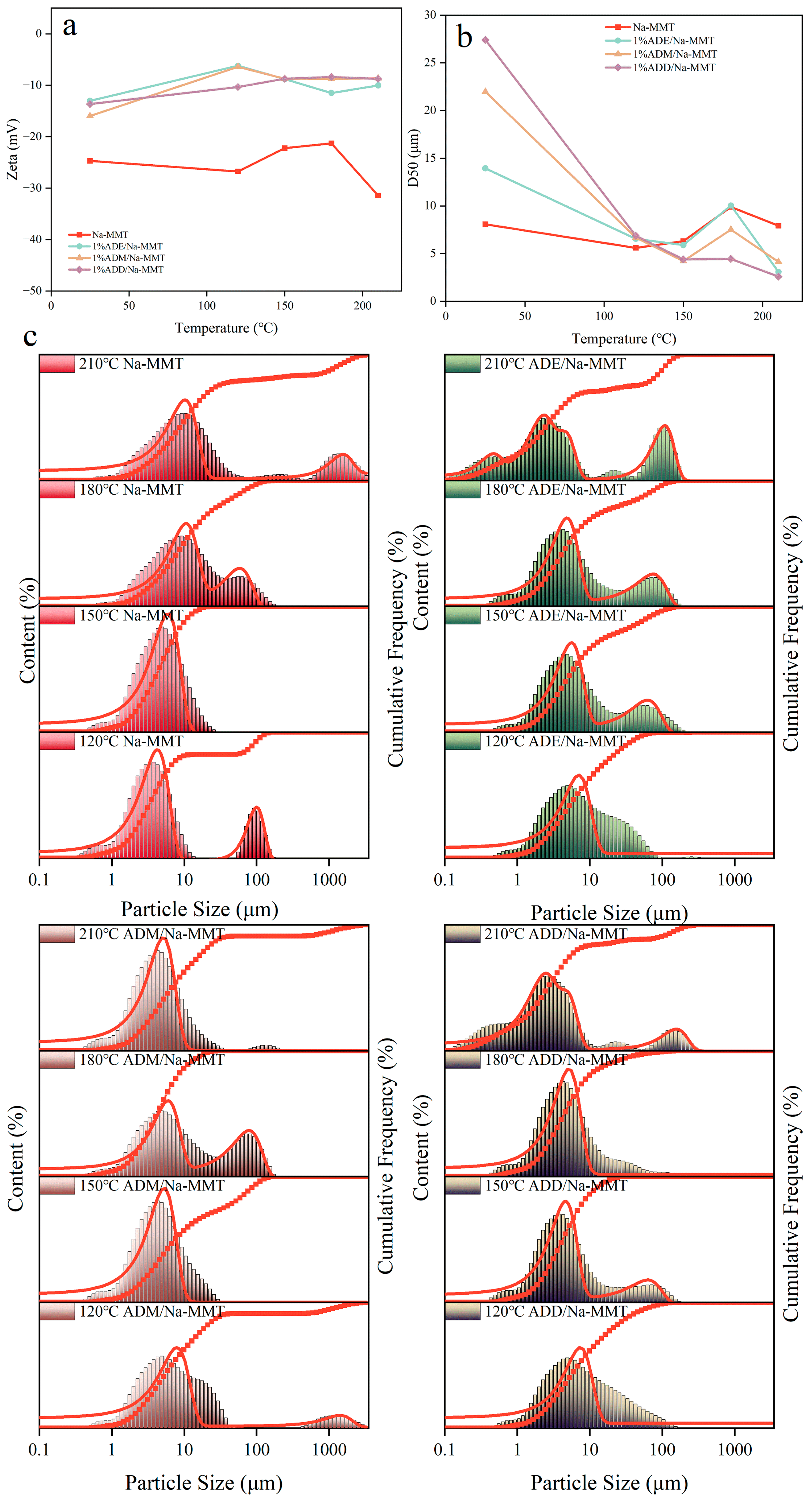

| Absorption Peak Position | Analysis | Intensity |
|---|---|---|
| 3400 cm−1 | N-H stretching vibration in AM | Strong |
| 2800–2975 cm−1 | C-H stretching vibration | Weak |
| 1630 cm−1 | Characteristic absorption peak of C=O stretching vibration | Strong |
| 1460 cm−1 | C-N stretching vibration in DMC | Weak |
| 1380 cm−1 | N-CH3 stretching vibration in DMC | Weak |
| 1155 cm−1 | C-O stretching vibration | Moderate |
| 1110 cm−1, 1112 cm−1 | Characteristic peaks of Si-O bonds in VTES, VTMO, VMDS | Moderate |
| Component | Content (wt%) | Clay Mineral Component | Content (wt%) |
|---|---|---|---|
| Quartz | 36.5 | Kaolinite | 1.3 |
| Potassium Feldspar | 4.3 | Chlorite | 8.6 |
| Sodium Feldspar | 4.4 | Illite | 13.7 |
| Calcite | 8.2 | Illite/Smectite Mixed Layer | 20.2 |
| Dolomite | 2.8 |
| Step | Added Reagents | Ratio | Stirring Speed (r/min) | Stirring Time (min) | Function |
|---|---|---|---|---|---|
| 1 | AM, DMC | 3:1 | 500 | 30 | Pre-dissolve the two monomers in deionized water to obtain Solution A. |
| 2 | C2H4O2, NaOH | 500 | 20 | Add saturated sodium hydroxide solution and glacial acetic acid to Solution A to adjust the pH value. | |
| 3 | Span 80, Tween 80 | 3:1 | 600 | 30 | Prepare the emulsifier. |
| 4 | VTES | 5% | 500 | 20 | Pre-dissolve the organosilicon monomer VTES in absolute ethanol to obtain organosilicon monomer Solution B. |
| 5 | ABIN | 0.5% | 200 | 20 | Transfer the emulsifier and Solution B to a three-necked flask equipped with a nitrogen inlet, condenser, and magnetic stirrer. Add ethanol (1 wt%) as a hydrolysis inhibitor and preheat the mixture to the reaction temperature. Then, introduce the initiator ABIN and carry out the reaction under constant temperature stirring to obtain polymer ADE. Replace the organosilicon monomer in Step 4 with VTMO and VMDS to obtain polymers ADM and ADD, respectively. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, F.; Dou, L.; Li, Y.; Wang, K.; Shi, Z.; Du, L.; Zhang, W.; Wang, Z. Mechanisms of Covalent Bonds in Enhancing the Adsorption Stability of Clay–Polymer Gels in High-Temperature Environments. Gels 2025, 11, 623. https://doi.org/10.3390/gels11080623
Wang Y, Zhang F, Dou L, Li Y, Wang K, Shi Z, Du L, Zhang W, Wang Z. Mechanisms of Covalent Bonds in Enhancing the Adsorption Stability of Clay–Polymer Gels in High-Temperature Environments. Gels. 2025; 11(8):623. https://doi.org/10.3390/gels11080623
Chicago/Turabian StyleWang, Yu, Fan Zhang, Liangbin Dou, Yutong Li, Kaiwen Wang, Zhengli Shi, Leyao Du, Wangyuan Zhang, and Zonglun Wang. 2025. "Mechanisms of Covalent Bonds in Enhancing the Adsorption Stability of Clay–Polymer Gels in High-Temperature Environments" Gels 11, no. 8: 623. https://doi.org/10.3390/gels11080623
APA StyleWang, Y., Zhang, F., Dou, L., Li, Y., Wang, K., Shi, Z., Du, L., Zhang, W., & Wang, Z. (2025). Mechanisms of Covalent Bonds in Enhancing the Adsorption Stability of Clay–Polymer Gels in High-Temperature Environments. Gels, 11(8), 623. https://doi.org/10.3390/gels11080623






