Self-Healing, Electroconductive Hydrogels for Wound Healing Applications
Abstract
1. Introduction
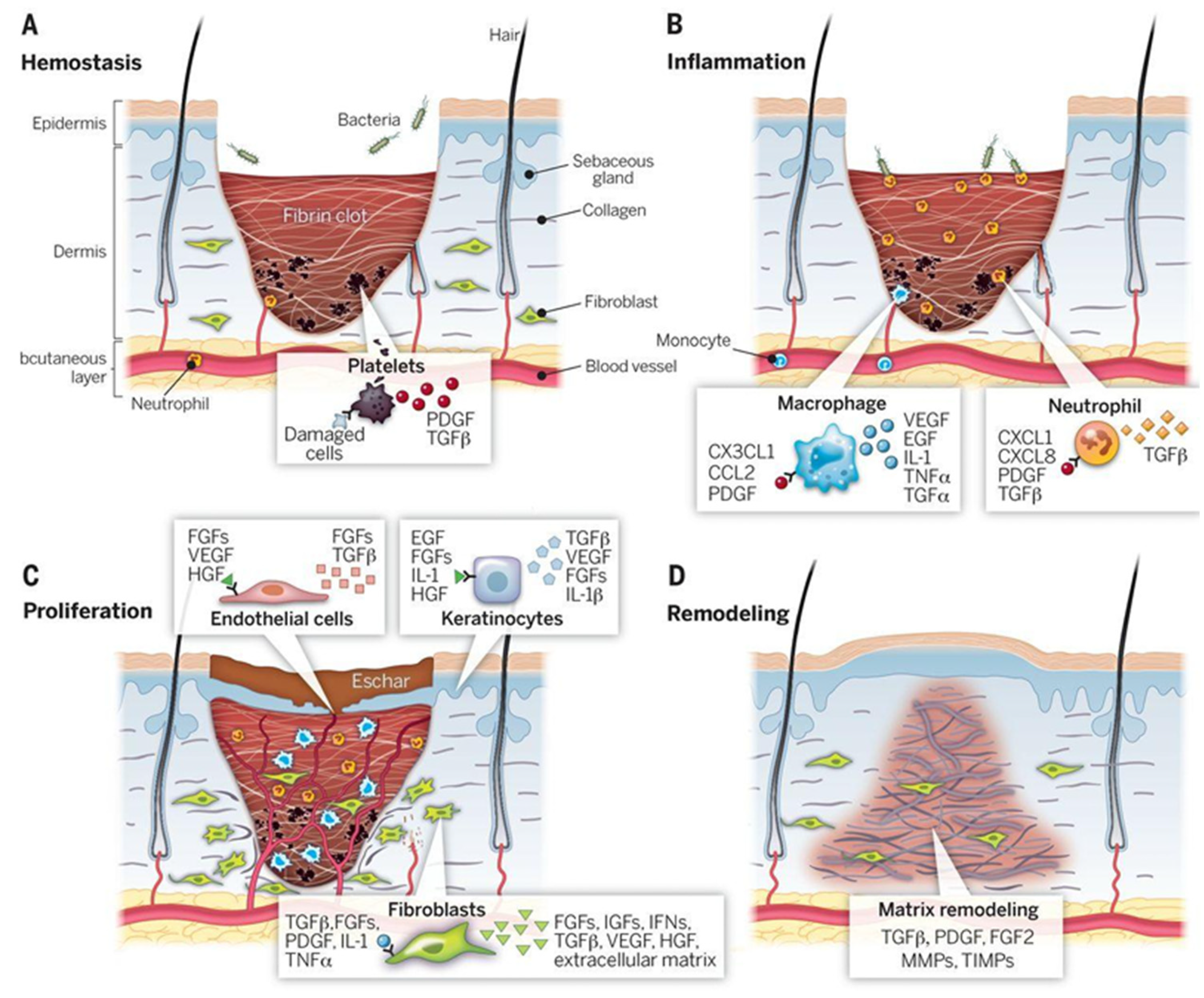
2. Wound Healing Biology
3. Self-Healing Hydrogels: Mechanisms
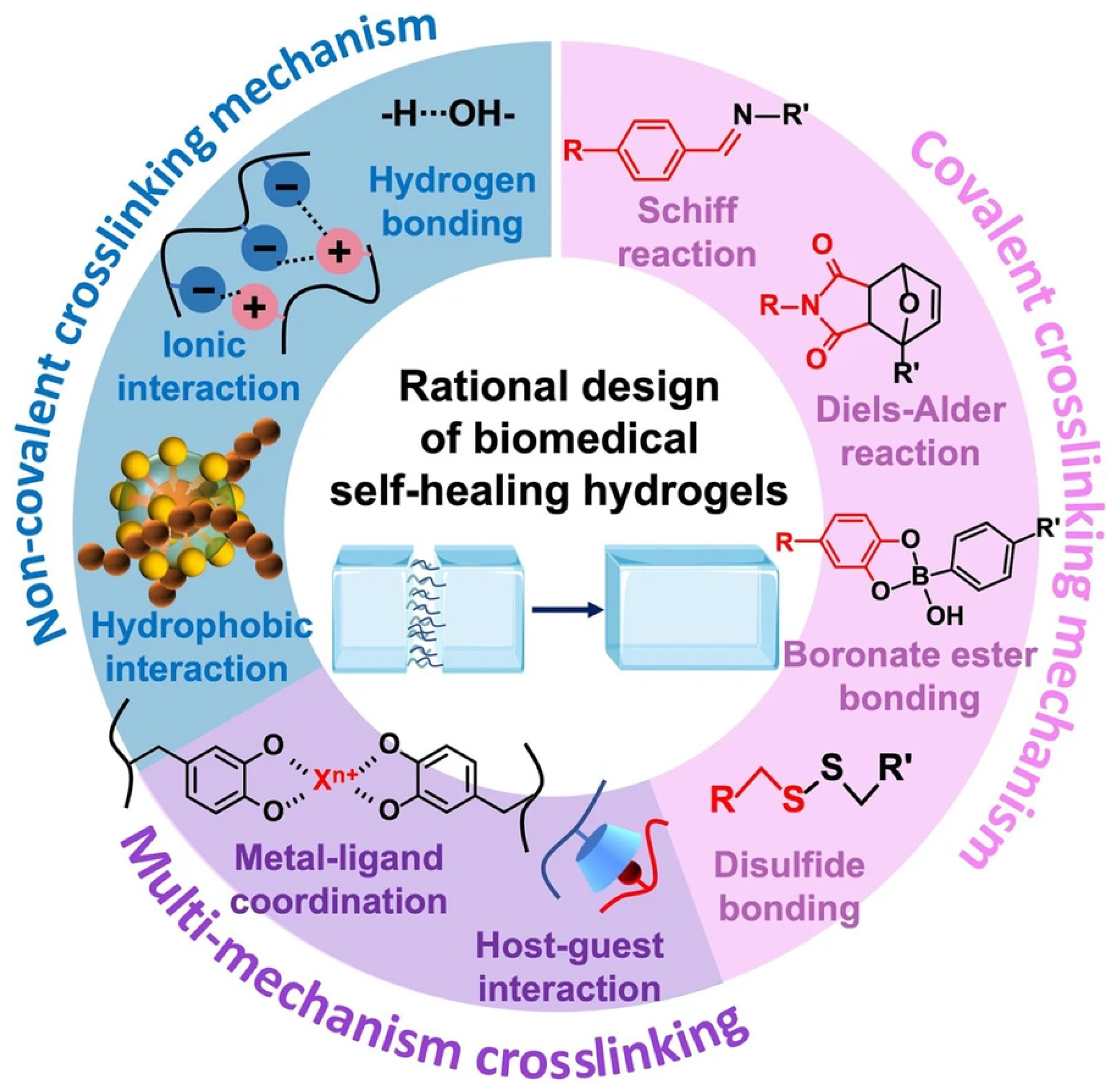
3.1. Dynamic Covalent Bonding
3.2. Ionic Interaction
3.3. Hydrogen Bonds
3.4. Hydrophobic Interaction
3.5. Metal Coordination Interaction
3.6. Host–Guest Interaction
4. Designing Electroconductive Hydrogels for Wound Healing
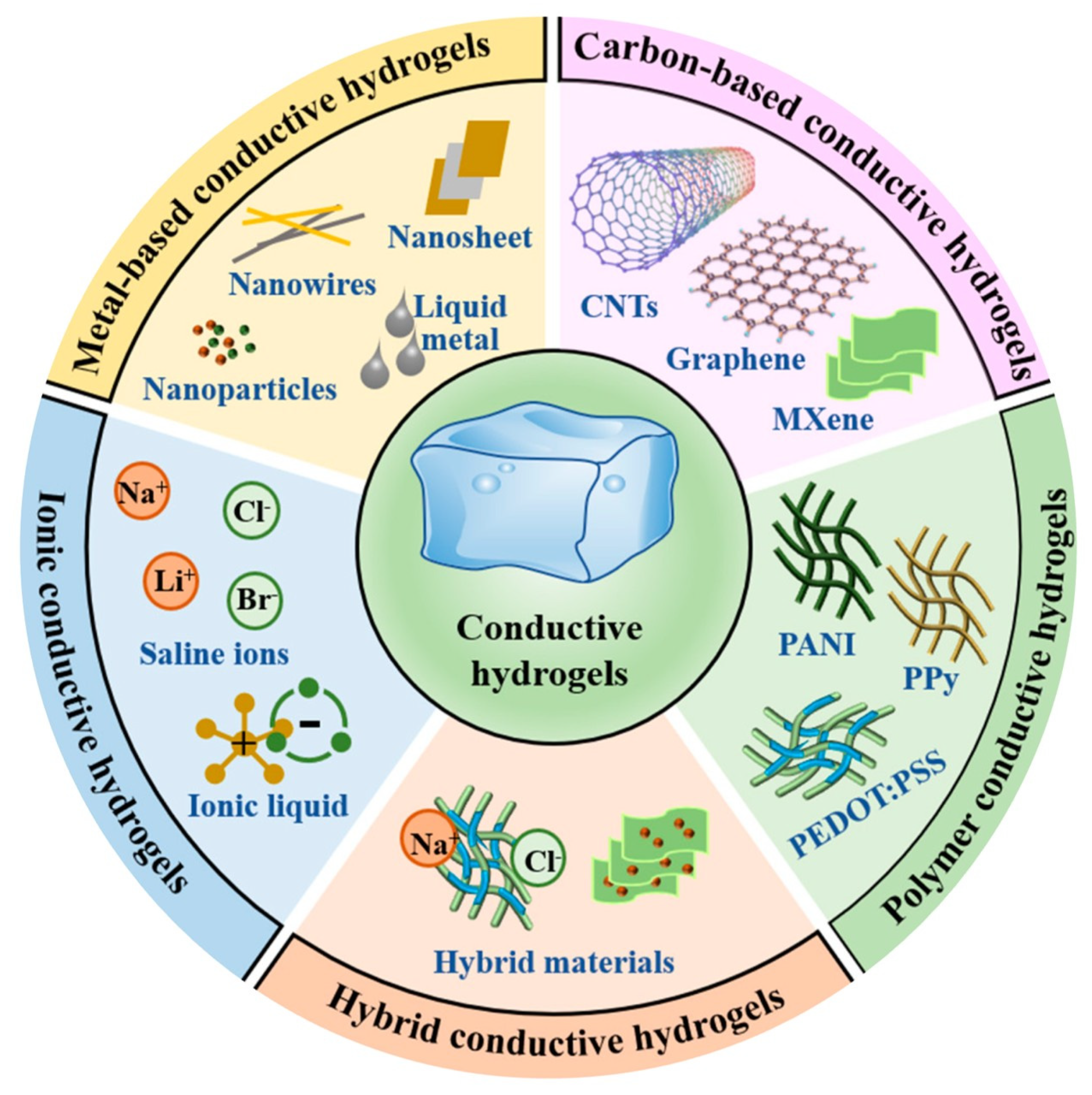
4.1. Conducting Polymers
4.2. Carbon Nanomaterials
4.3. Noble Metal Nanomaterials
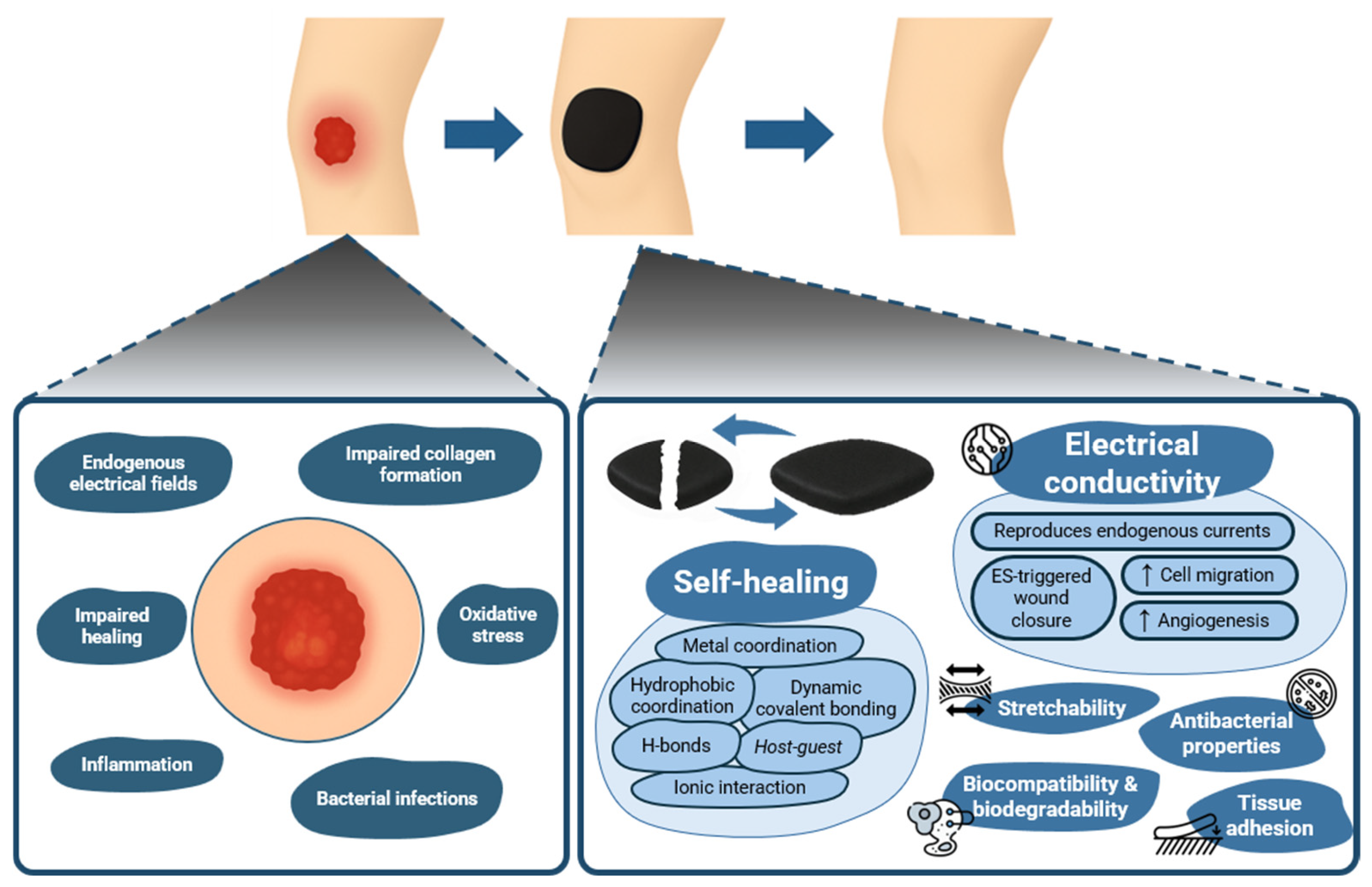
5. Self-Healing, Electroconductive Hydrogels: Wound Healing Evidence
6. Translational Challenges and Future Steps
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3SPMA | 3-sulfopropyl methacrylate |
| AC | Alternating Current |
| AD | Adamantane |
| AI | Artificial Intelligence |
| Ag | Silver |
| Alg-PBA | Phenylboronic acid-grafted Sodium Alginate |
| Au | Gold |
| BC | Bacterial Cellulose |
| bFGF | Basic Fibroblast Growth Factor |
| CD | Cyclodextrin |
| CMCS | Carboxymethyl Chitosan |
| CNTs | Carbon Nanotubes |
| CPs | Conducting Polymers |
| CS | Chitosan |
| DLP | Digital Light Processing |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| DTT | Dithiothreitol |
| ECG | Electrocardiogram |
| EDOT | 3,4-Ethylenedioxythiophene |
| EGF | Epidermal Growth Factor |
| EMG | Electromyogram |
| EMT | Epithelial-to-Mesenchymal Transition |
| ES | Electrical Stimulation |
| FGF | Fibroblast Growth Factor |
| FN1 | Fibronectin 1 |
| GG | Guar Gum |
| GMP | Good Manufacturing Practice |
| GQDs | Graphene Quantum Dots |
| GelMA | Methacrylated Gelatin |
| HDF | Human Dermal Fibroblast |
| HRP | Horseradish Peroxidase |
| HUVEC | Human Umbilical Vein Endothelial Cell |
| IL | Interleukin |
| MDCK | Madin–Darby Canine Kidney |
| MMP9 | Matrix Metalloproteinase 9 |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MTMS | Methyltrimethoxysilane |
| MWCNTs | Multi-Walled Carbon Nanotubes |
| NIR | Near Infrared |
| NPs | Nanoparticles |
| NRs | Nanorods |
| NT3 | Neurotrophin 3 |
| OA | Oxidized Alginate |
| PA | Poly(thiophene-3-acetic acid) |
| PAA | Poly(acrylic acid) |
| PACPH | Polydopamine/AgNPs/Cellulose Nanocrystals/Polypyrrole Hydrogel |
| PAM | Polyacrylamide |
| PANI | Polyaniline |
| PBA | Phenylboronic Acid |
| PBS | Phosphate-Buffered Saline |
| PDA | Polydopamine |
| PEDOT | Poly(3,4-ethylenedioxythiophene) |
| PSS | Polystyrene Sulfonate |
| PEG | Poly(ethylene glycol) |
| PEGDA | Poly(ethylene glycol) Diacrylate |
| PEGDE | Poly(ethylene glycol) Diglycidyl Ether |
| PEGS | Polyethylene Glycol-co-poly(glycerol sebacate) |
| PNIPAm | Poly(N-isopropylacrylamide) |
| PPy | Polypyrrole |
| PrGO | Polydopamine-modified Reduced Graphene Oxide |
| rGO | Reduced Graphene Oxide |
| ROS | Reactive Oxygen Species |
| RT | Room Temperature |
| SA | Sodium Alginate |
| SBMA | Sulfobetaine Methacrylate |
| SNR | Signal-to-Noise Ratio |
| TA | Tannic Acid |
| TCH | Tetracycline Hydrochloride |
| TGA | Thioglycolic Acid |
| TNF-α | Tumor Necrosis Factor Alpha |
| TP | Tea Polyphenol |
| UV | Ultraviolet |
| VEGF | Vascular Endothelial Growth Factor |
| XG | Xanthan Gum |
| ZnCS | Zinc-chelated Chitosan |
References
- Yazdi, S.J.M.; Baqersad, J. Mechanical Modeling and Characterization of Human Skin: A Review. J. Biomech. 2022, 130, 110864. [Google Scholar] [CrossRef] [PubMed]
- Peña, O.A.; Martin, P. Cellular and Molecular Mechanisms of Skin Wound Healing. Nat. Rev. Mol. Cell Biol. 2024, 25, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. Wound Healing: Cellular Mechanisms and Pathological Outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Y.; Ji, S.-F.; Fu, X.-B.; Jiang, Y.-F.; Sun, X.-Y. Biomaterial-Based Mechanical Regulation Facilitates Scarless Wound Healing with Functional Skin Appendage Regeneration. Mil. Med. Res. 2024, 11, 13. [Google Scholar] [CrossRef]
- Hassanshahi, A.; Moradzad, M.; Ghalamkari, S.; Fadaei, M.; Cowin, A.J.; Hassanshahi, M. Macrophage-Mediated Inflammation in Skin Wound Healing. Cells 2022, 11, 2953. [Google Scholar] [CrossRef]
- Falanga, V.; Isseroff, R.R.; Soulika, A.M.; Romanelli, M.; Margolis, D.; Kapp, S.; Granick, M.; Harding, K. Chronic Wounds. Nat. Rev. Dis. Primer 2022, 8, 50. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Ghomi, E.R.; Niazi, M.; Ramakrishna, S. The Evolution of Wound Dressings: From Traditional to Smart Dressings. Polym. Adv. Technol. 2023, 34, 520–530. [Google Scholar] [CrossRef]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel Wound Dressings for Bioactive Treatment of Acute and Chronic Wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Almeida, D.; Sanjuan-Alberte, P.; Silva, J.C.; Ferreira, F.C. 3D (Bio)Printing of Magnetic Hydrogels: Formulation and Applications in Tissue Engineering. Int. J. Bioprinting 2023, 10, 0965. [Google Scholar] [CrossRef]
- Gounden, V.; Singh, M. Hydrogels and Wound Healing: Current and Future Prospects. Gels 2024, 10, 43. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, N.; Ma, M. Electroconductive Hydrogels for Biomedical Applications. WIREs Nanomed. Nanobiotechnol. 2019, 11, e1568. [Google Scholar] [CrossRef] [PubMed]
- Rogers, Z.J.; Zeevi, M.P.; Koppes, R.; Bencherif, S.A. Electroconductive Hydrogels for Tissue Engineering: Current Status and Future Perspectives. Bioelectricity 2020, 2, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, L.; Zheng, W.; Yang, G.; Jiang, X. Rapid Fabrication of Self-Healing, Conductive, and Injectable Gel as Dressings for Healing Wounds in Stretchable Parts of the Body. Adv. Funct. Mater. 2020, 30, 2002370. [Google Scholar] [CrossRef]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound Dressings—A Review. BioMedicine 2015, 5, 22. [Google Scholar] [CrossRef]
- Abd-ElSalam, H.-A.H.; Refaeey, O.A.; Waked, K.G.; Elsherbiny, K.A.; Aleam, A.M.; Ibrahim, M.Q.; Farag, M.H.; Nasef, A.M.; ElMeshad, A.N. A Review Exploring the Wound-Healing Activity of Self-Healing Hydrogels: Fabrication, Characterization, Mechanism, and Biomedical Applications. J. Clust. Sci. 2024, 35, 2019–2037. [Google Scholar] [CrossRef]
- Talebian, S.; Mehrali, M.; Taebnia, N.; Pennisi, C.P.; Kadumudi, F.B.; Foroughi, J.; Hasany, M.; Nikkhah, M.; Akbari, M.; Orive, G.; et al. Self-Healing Hydrogels: The Next Paradigm Shift in Tissue Engineering? Adv. Sci. 2019, 6, 1801664. [Google Scholar] [CrossRef]
- Jia, N.; Yang, J.; Liu, J.; Zhang, J. Electric Field: A Key Signal in Wound Healing. Chin. J. Plast. Reconstr. Surg. 2021, 3, 95–102. [Google Scholar] [CrossRef]
- Mamun, A.A.; Shao, C.; Geng, P.; Wang, S.; Xiao, J. Recent Advances in Molecular Mechanisms of Skin Wound Healing and Its Treatments. Front. Immunol. 2024, 15, 1395479. [Google Scholar] [CrossRef]
- Lu, C.; Kolbenschlag, J.; Nüssler, A.K.; Ehnert, S.; McCaig, C.D.; Čebron, U.; Daigeler, A.; Prahm, C. Direct Current Electrical Fields Improve Experimental Wound Healing by Activation of Cytokine Secretion and Erk1/2 Pathway Stimulation. Life 2021, 11, 1195. [Google Scholar] [CrossRef]
- Raja, R. Wound Re-Epithelialization: Modulating Kerationcyte Migration in Wound Healing. Front. Biosci. 2007, 12, 2849. [Google Scholar] [CrossRef]
- Yan, T.; Jiang, X.; Guo, X.; Chen, W.; Tang, D.; Zhang, J.; Zhang, X.; Zhang, D.; Zhang, Q.; Jia, J.; et al. Electric Field-Induced Suppression of PTEN Drives Epithelial-to-Mesenchymal Transition via mTORC1 Activation. J. Dermatol. Sci. 2017, 85, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; An, R.; Zhao, J.; Qiu, M.; Wang, Z.; Ren, H.; Yu, D.; Zhu, X. Self-Healing Hydrogels: Mechanisms and Biomedical Applications. MedComm 2025, 6, e70181. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, J.; Ji, T.; Xue, Y.; Zhao, L.; Zhao, K.; Jia, B.; Wang, B.; Wang, J.; Zhang, S.; et al. Self-Healing Hydrogel Bioelectronics. Adv. Mater. 2024, 36, 2306350. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Chen, Y.; Yu, M.; Hou, M.; Gong, G.; Tan, H.; Li, N.; Xu, J. NIR Light-Induced Rapid Self-Healing Hydrogel toward Multifunctional Applications in Sensing. Nano Energy 2023, 107, 108119. [Google Scholar] [CrossRef]
- de Luna, M.S.; Marturano, V.; Manganelli, M.; Santillo, C.; Ambrogi, V.; Filippone, G.; Cerruti, P. Light-Responsive and Self-Healing Behavior of Azobenzene-Based Supramolecular Hydrogels. J. Colloid Interface Sci. 2020, 568, 16–24. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, D.; Zhou, Y.; Zhang, Q.; Wu, S. Controlling Properties and Functions of Polymer Gels Using Photochemical Reactions. Macromol. Rapid Commun. 2022, 43, 2100703. [Google Scholar] [CrossRef]
- Habault, D.; Zhang, H.; Zhao, Y. Light-Triggered Self-Healing and Shape-Memory Polymers. Chem. Soc. Rev. 2013, 42, 7244. [Google Scholar] [CrossRef]
- Huang, W.-C.; Ali, F.; Zhao, J.; Rhee, K.; Mou, C.; Bettinger, C.J. Ultrasound-Mediated Self-Healing Hydrogels Based on Tunable Metal–Organic Bonding. Biomacromolecules 2017, 18, 1162–1171. [Google Scholar] [CrossRef]
- Amaral, A.J.R.; Pasparakis, G. Stimuli Responsive Self-Healing Polymers: Gels, Elastomers and Membranes. Polym. Chem. 2017, 8, 6464–6484. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, C.; Li, Y.; Wang, K.; Wang, X.; Wei, Y.; Tao, L. Synthesis of an Injectable, Self-Healable and Dual Responsive Hydrogel for Drug Delivery and 3D Cell Cultivation. Polym. Chem. 2017, 8, 537–544. [Google Scholar] [CrossRef]
- Deng, C.C.; Brooks, W.L.A.; Abboud, K.A.; Sumerlin, B.S. Boronic Acid-Based Hydrogels Undergo Self-Healing at Neutral and Acidic pH. ACS Macro Lett. 2015, 4, 220–224. [Google Scholar] [CrossRef]
- Park, D.-J.; Kim, S.-C.; Jang, J.-B.; Lee, B.; Lee, S.; Ryu, B.; Je, J.-Y.; Park, W.S.; Jung, W.-K. Multifunctional Hydrogel Dressing Based on Fish Gelatin/Oxidized Hyaluronate for Promoting Diabetic Wound Healing. J. Mater. Chem. B 2024, 12, 4451–4466. [Google Scholar] [CrossRef]
- Xu, J.; Hsu, S. Self-Healing Hydrogel as an Injectable Implant: Translation in Brain Diseases. J. Biomed. Sci. 2023, 30, 43. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cheng, F.; Wei, X.; Yi, X.; Tang, S.; Wang, Z.; Zhang, Y.S.; He, J.; Huang, Y. Injectable, Self-Healing, Antibacterial, and Hemostatic N,O-Carboxymethyl Chitosan/Oxidized Chondroitin Sulfate Composite Hydrogel for Wound Dressing. Mater. Sci. Eng. C 2021, 118, 111324. [Google Scholar] [CrossRef] [PubMed]
- Muir, V.G.; Burdick, J.A. Chemically Modified Biopolymers for the Formation of Biomedical Hydrogels. Chem. Rev. 2021, 121, 10908–10949. [Google Scholar] [CrossRef]
- He, L.; Szopinski, D.; Wu, Y.; Luinstra, G.A.; Theato, P. Toward Self-Healing Hydrogels Using One-Pot Thiol–Ene Click and Borax-Diol Chemistry. ACS Macro Lett. 2015, 4, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial Anti-Oxidant Electroactive Injectable Hydrogel as Self-Healing Wound Dressing with Hemostasis and Adhesiveness for Cutaneous Wound Healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef]
- Wang, C.; Liang, C.; Wang, R.; Yao, X.; Guo, P.; Yuan, W.; Liu, Y.; Song, Y.; Li, Z.; Xie, X. The Fabrication of a Highly Efficient Self-Healing Hydrogel from Natural Biopolymers Loaded with Exosomes for the Synergistic Promotion of Severe Wound Healing. Biomater. Sci. 2020, 8, 313–324. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Fu, Y.; Wei, Y.; Zhao, L.; Tao, L. Self-Adapting Hydrogel to Improve the Therapeutic Effect in Wound-Healing. ACS Appl. Mater. Interfaces 2018, 10, 26046–26055. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial Adhesive Injectable Hydrogels with Rapid Self-Healing, Extensibility and Compressibility as Wound Dressing for Joints Skin Wound Healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Sapuła, P.; Bialik-Wąs, K.; Malarz, K. Are Natural Compounds a Promising Alternative to Synthetic Cross-Linking Agents in the Preparation of Hydrogels? Pharmaceutics 2023, 15, 253. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cheng, J.; Ran, L.; Yu, K.; Lu, B.; Lan, G.; Dai, F.; Lu, F. An Injectable Self-Healing Hydrogel with Adhesive and Antibacterial Properties Effectively Promotes Wound Healing. Carbohydr. Polym. 2018, 201, 522–531. [Google Scholar] [CrossRef]
- Li, W.; Wang, B.; Zhang, M.; Wu, Z.; Wei, J.; Jiang, Y.; Sheng, N.; Liang, Q.; Zhang, D.; Chen, S. All-Natural Injectable Hydrogel with Self-Healing and Antibacterial Properties for Wound Dressing. Cellulose 2020, 27, 2637–2650. [Google Scholar] [CrossRef]
- Wang, H.; Lu, B.; Zhou, J.; Lai, J.; Zheng, X.; Guo, S.-Z.; Zhang, L.-M. Biobased Physicochemical Reversible Dual-Cross-Linked Hydrogel: Self-Healing, Antibacterial, Antioxidant, and Hemostatic Properties for Diabetic Wound Healing. Biomacromolecules 2025, 26, 2637–2653. [Google Scholar] [CrossRef]
- Basu, S.; Pacelli, S.; Paul, A. Self-Healing DNA-Based Injectable Hydrogels with Reversible Covalent Linkages for Controlled Drug Delivery. Acta Biomater. 2020, 105, 159–169. [Google Scholar] [CrossRef]
- Lei, J.; Li, X.; Wang, S.; Yuan, L.; Ge, L.; Li, D.; Mu, C. Facile Fabrication of Biocompatible Gelatin-Based Self-Healing Hydrogels. ACS Appl. Polym. Mater. 2019, 1, 1350–1358. [Google Scholar] [CrossRef]
- Chen, M.; Tian, J.; Liu, Y.; Cao, H.; Li, R.; Wang, J.; Wu, J.; Zhang, Q. Dynamic Covalent Constructed Self-Healing Hydrogel for Sequential Delivery of Antibacterial Agent and Growth Factor in Wound Healing. Chem. Eng. J. 2019, 373, 413–424. [Google Scholar] [CrossRef]
- Li, Q.; Liu, C.; Wen, J.; Wu, Y.; Shan, Y.; Liao, J. The Design, Mechanism and Biomedical Application of Self-Healing Hydrogels. Chin. Chem. Lett. 2017, 28, 1857–1874. [Google Scholar] [CrossRef]
- Yang, Y.; Urban, M.W. Self-Healing Polymeric Materials. Chem. Soc. Rev. 2013, 42, 7446. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, R.; Zhao, X.; Zhang, Y.; Tam, A.; Yan, Y.; Shen, H.; Zhang, Y.S.; Qi, J.; Feng, Y.; et al. An Injectable Self-Healing Coordinative Hydrogel with Antibacterial and Angiogenic Properties for Diabetic Skin Wound Repair. NPG Asia Mater. 2019, 11, 3. [Google Scholar] [CrossRef]
- Morozova, S.M. Recent Advances in Hydrogels via Diels–Alder Crosslinking: Design and Applications. Gels 2023, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Cao, X.; Li, Y.; Zeng, L.; Zhu, J.; Wang, G.; Chen, X. Diels–Alder Crosslinked HA/PEG Hydrogels with High Elasticity and Fatigue Resistance for Cell Encapsulation and Articular Cartilage Tissue Repair. Polym Chem 2014, 5, 5116–5123. [Google Scholar] [CrossRef]
- Ilochonwu, B.C.; van der Lugt, S.A.; Annala, A.; Di Marco, G.; Sampon, T.; Siepmann, J.; Siepmann, F.; Hennink, W.E.; Vermonden, T. Thermo-Responsive Diels-Alder Stabilized Hydrogels for Ocular Drug Delivery of a Corticosteroid and an Anti-VEGF Fab Fragment. J. Control. Release 2023, 361, 334–349. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, W.; Wu, D.; Nagao, M.; Hall, D.G.; Thundat, T.; Narain, R. Injectable Self-Healing Zwitterionic Hydrogels Based on Dynamic Benzoxaborole–Sugar Interactions with Tunable Mechanical Properties. Biomacromolecules 2018, 19, 596–605. [Google Scholar] [CrossRef]
- Wu, D.; Wang, W.; Diaz-Dussan, D.; Peng, Y.-Y.; Chen, Y.; Narain, R.; Hall, D.G. In Situ Forming, Dual-Crosslink Network, Self-Healing Hydrogel Enabled by a Bioorthogonal Nopoldiol–Benzoxaborolate Click Reaction with a Wide pH Range. Chem. Mater. 2019, 31, 4092–4102. [Google Scholar] [CrossRef]
- Amaral, A.J.R.; Emamzadeh, M.; Pasparakis, G. Transiently Malleable Multi-Healable Hydrogel Nanocomposites Based on Responsive Boronic Acid Copolymers. Polym. Chem. 2018, 9, 525–537. [Google Scholar] [CrossRef]
- Liang, Y.; Li, M.; Yang, Y.; Qiao, L.; Xu, H.; Guo, B. pH/Glucose Dual Responsive Metformin Release Hydrogel Dressings with Adhesion and Self-Healing via Dual-Dynamic Bonding for Athletic Diabetic Foot Wound Healing. ACS Nano 2022, 16, 3194–3207. [Google Scholar] [CrossRef]
- Chen, Y.; Diaz-Dussan, D.; Wu, D.; Wang, W.; Peng, Y.-Y.; Asha, A.B.; Hall, D.G.; Ishihara, K.; Narain, R. Bioinspired Self-Healing Hydrogel Based on Benzoxaborole-Catechol Dynamic Covalent Chemistry for 3D Cell Encapsulation. ACS Macro Lett. 2018, 7, 904–908. [Google Scholar] [CrossRef]
- Zhou, L.; Dai, C.; Fan, L.; Jiang, Y.; Liu, C.; Zhou, Z.; Guan, P.; Tian, Y.; Xing, J.; Li, X.; et al. Injectable Self-Healing Natural Biopolymer-Based Hydrogel Adhesive with Thermoresponsive Reversible Adhesion for Minimally Invasive Surgery. Adv. Funct. Mater. 2021, 31, 2007457. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, Y. Boronic Acid-Containing Hydrogels: Synthesis and Their Applications. Chem. Soc. Rev. 2013, 42, 8106. [Google Scholar] [CrossRef]
- Ding, X.; Li, G.; Zhang, P.; Jin, E.; Xiao, C.; Chen, X. Injectable Self-Healing Hydrogel Wound Dressing with Cysteine-Specific On-Demand Dissolution Property Based on Tandem Dynamic Covalent Bonds. Adv. Funct. Mater. 2021, 31, 2011230. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, Z.; Liu, K.; Ji, X.; Fatehi, P.; Chen, J. A Cellulose Nanofibril-Reinforced Hydrogel with Robust Mechanical, Self-Healing, pH-Responsive and Antibacterial Characteristics for Wound Dressing Applications. J. Nanobiotechnol. 2022, 20, 312. [Google Scholar] [CrossRef]
- Wei, H.; Jing, H.; Cheng, C.; Liu, Y.; Hao, J. A Biomimetic One-Stone-Two-Birds Hydrogel with Electroconductive, Photothermally Antibacterial and Bioadhesive Properties for Skin Tissue Regeneration and Mechanosensation Restoration. Adv. Funct. Mater. 2025, 35, 2417280. [Google Scholar] [CrossRef]
- Qiao, L.; Liang, Y.; Chen, J.; Huang, Y.; Alsareii, S.A.; Alamri, A.M.; Harraz, F.A.; Guo, B. Antibacterial Conductive Self-Healing Hydrogel Wound Dressing with Dual Dynamic Bonds Promotes Infected Wound Healing. Bioact. Mater. 2023, 30, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liang, Y.; He, J.; Zhang, H.; Guo, B. Two-Pronged Strategy of Biomechanically Active and Biochemically Multifunctional Hydrogel Wound Dressing to Accelerate Wound Closure and Wound Healing. Chem. Mater. 2020, 32, 9937–9953. [Google Scholar] [CrossRef]
- Ali, A.; Govindharaj, M.; Fatma, B.; Alshehhi, K.H.; Islayem, D.; Alsaafeen, N.B.; Pappa, A.M.; Pitsalidis, C. In Situ Development of Self-Healing, Injectable, Glucose and pH-Responsive Electroconductive Composite Hydrogels. Adv. Compos. Hybrid Mater. 2025, 8, 270. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, J.; Wei, Z.; Zhang, B.; Weng, X. Advances and Progress in Self-Healing Hydrogel and Its Application in Regenerative Medicine. Materials 2023, 16, 1215. [Google Scholar] [CrossRef]
- Wu, H.-D.; Yang, J.-C.; Tsai, T.; Ji, D.-Y.; Chang, W.-J.; Chen, C.-C.; Lee, S.-Y. Development of a Chitosan–Polyglutamate Based Injectable Polyelectrolyte Complex Scaffold. Carbohydr. Polym. 2011, 85, 318–324. [Google Scholar] [CrossRef]
- Phadke, A.; Zhang, C.; Arman, B.; Hsu, C.-C.; Mashelkar, R.A.; Lele, A.K.; Tauber, M.J.; Arya, G.; Varghese, S. Rapid Self-Healing Hydrogels. Proc. Natl. Acad. Sci. USA 2012, 109, 4383–4388. [Google Scholar] [CrossRef]
- Ye, X.; Li, X.; Shen, Y.; Chang, G.; Yang, J.; Gu, Z. Self-Healing pH-Sensitive Cytosine- and Guanosine-Modified Hyaluronic Acid Hydrogels via Hydrogen Bonding. Polymer 2017, 108, 348–360. [Google Scholar] [CrossRef]
- Cheng, R.; Xu, M.; Zhang, X.; Jiang, J.; Zhang, Q.; Zhao, Y. Hydrogen Bonding Enables Polymer Hydrogels with pH-Induced Reversible Dynamic Responsive Behaviors. Angew. Chem. Int. Ed. 2023, 62, e202302900. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, M.; Zhao, Z. Facile Fabrication of Self-Healing, Injectable and Antimicrobial Cationic Guar Gum Hydrogel Dressings Driven by Hydrogen Bonds. Carbohydr. Polym. 2023, 310, 120723. [Google Scholar] [CrossRef]
- Appel, E.A.; Tibbitt, M.W.; Webber, M.J.; Mattix, B.A.; Veiseh, O.; Langer, R. Self-Assembled Hydrogels Utilizing Polymer–Nanoparticle Interactions. Nat. Commun. 2015, 6, 6295. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Nkwantabisah, S.; Gillmor, J.R.; Switalski, S.C.; Slater, G.L. An Autonomous Self-healing Hydrogel Based on Surfactant-free Hydrophobic Association. J. Appl. Polym. Sci. 2017, 134, 44800. [Google Scholar] [CrossRef]
- Chang, X.; Geng, Y.; Cao, H.; Zhou, J.; Tian, Y.; Shan, G.; Bao, Y.; Wu, Z.L.; Pan, P. Dual-Crosslink Physical Hydrogels with High Toughness Based on Synergistic Hydrogen Bonding and Hydrophobic Interactions. Macromol. Rapid Commun. 2018, 39, 1700806. [Google Scholar] [CrossRef] [PubMed]
- Algi, M.P.; Okay, O. Highly Stretchable Self-Healing Poly(N,N-Dimethylacrylamide) Hydrogels. Eur. Polym. J. 2014, 59, 113–121. [Google Scholar] [CrossRef]
- Gulyuz, U.; Okay, O. Self-Healing Poly(Acrylic Acid) Hydrogels: Effect of Surfactant. Macromol. Symp. 2015, 358, 232–238. [Google Scholar] [CrossRef]
- Holten-Andersen, N.; Harrington, M.J.; Birkedal, H.; Lee, B.P.; Messersmith, P.B.; Lee, K.Y.C.; Waite, J.H. pH-Induced Metal-Ligand Cross-Links Inspired by Mussel Yield Self-Healing Polymer Networks with near-Covalent Elastic Moduli. Proc. Natl. Acad. Sci. USA 2011, 108, 2651–2655. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Z.; Huang, Y.; Yu, R.; Guo, B. Dual-Dynamic-Bond Cross-Linked Antibacterial Adhesive Hydrogel Sealants with On-Demand Removability for Post-Wound-Closure and Infected Wound Healing. ACS Nano 2021, 15, 7078–7093. [Google Scholar] [CrossRef]
- Deng, K.; Huang, Q.; Yan, X.; Dai, Y.; Zhao, J.; Xiong, X.; Wang, H.; Chen, X.; Chen, P.; Liu, L. Facile Fabrication of a Novel, Photodetachable Salecan-Based Hydrogel Dressing with Self-Healing, Injectable, and Antibacterial Properties Based on Metal Coordination. Int. J. Biol. Macromol. 2024, 264, 130551. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Guan, L.; He, W.; Yin, W.; Ye, D.; Gao, J.; Wang, M.; Pan, G. Bioactive Metal Ion-Coordinated Dynamic Hydrogel with Antibacterial, Immunomodulatory, and Angiogenic Activities for Infected Wound Repair. ACS Appl. Mater. Interfaces 2024, 16, 32104–32117. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jerca, V.V.; Hoogenboom, R. Self-Healing Metallo-Supramolecular Hydrogel Based on Specific Ni 2+ Coordination Interactions of Poly(Ethylene Glycol) with Bistriazole Pyridine Ligands in the Main Chain. Macromol. Rapid Commun. 2020, 41, 1900457. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gao, C.; Jia, P.; Song, L.; Kang, J.; Han, M.; Yu, W.; Nian, R. Novel in Situ and Rapid Self-Gelation Recombinant Collagen-like Protein Hydrogel for Wound Regeneration: Mediated by Metal Coordination Crosslinking and Reinforced by Electro-Oxidized Tea Polyphenols. Biofabrication 2025, 17, 015027. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zhang, T.; Li, H.-N.; Cong, H.-P.; Antonietti, M.; Yu, S.-H. Dynamic Au-Thiolate Interaction Induced Rapid Self-Healing Nanocomposite Hydrogels with Remarkable Mechanical Behaviors. Chem 2017, 3, 691–705. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, Y.; Xie, Q.; Fan, C.; Hilborn, J.; Dai, J.; Ossipov, D.A. Moldable Hyaluronan Hydrogel Enabled by Dynamic Metal–Bisphosphonate Coordination Chemistry for Wound Healing. Adv. Healthc. Mater. 2018, 7, 1700973. [Google Scholar] [CrossRef]
- Zeng, L.; Song, M.; Gu, J.; Xu, Z.; Xue, B.; Li, Y.; Cao, Y. A Highly Stretchable, Tough, Fast Self-Healing Hydrogel Based on Peptide–Metal Ion Coordination. Biomimetics 2019, 4, 36. [Google Scholar] [CrossRef]
- Zhang, K.; Yuan, W.; Wei, K.; Yang, B.; Chen, X.; Li, Z.; Zhang, Z.; Bian, L. Highly Dynamic Nanocomposite Hydrogels Self-Assembled by Metal Ion-Ligand Coordination. Small 2019, 15, 1900242. [Google Scholar] [CrossRef]
- Deng, Z.; Guo, Y.; Zhao, X.; Ma, P.X.; Guo, B. Multifunctional Stimuli-Responsive Hydrogels with Self-Healing, High Conductivity, and Rapid Recovery through Host–Guest Interactions. Chem. Mater. 2018, 30, 1729–1742. [Google Scholar] [CrossRef]
- Zhang, B.; He, J.; Shi, M.; Liang, Y.; Guo, B. Injectable Self-Healing Supramolecular Hydrogels with Conductivity and Photo-Thermal Antibacterial Activity to Enhance Complete Skin Regeneration. Chem. Eng. J. 2020, 400, 125994. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Z.; Li, M.; Yuan, Y.; Wang, W.; Zhang, L.; Wan, P. Mussel-Inspired Self-Healing Adhesive MXene Hydrogel for Epidermal Electronics. Device 2024, 2, 100253. [Google Scholar] [CrossRef]
- Khan, A.; Rehman, W.; Alanazi, M.M.; Khan, Y.; Rasheed, L.; Saboor, A.; Iqbal, S. Development of Novel Multifunctional Electroactive, Self-Healing, and Tissue Adhesive Scaffold to Accelerate Cutaneous Wound Healing and Hemostatic Materials. ACS Omega 2023, 8, 39110–39134. [Google Scholar] [CrossRef]
- Zhao, N.; Yuan, W. Functionally Integrated Bioglass Microspheres-Composited Double-Network Hydrogel with Good Tissue Adhesion and Electrical Conductivity for Efficient Wound Treatment and Health Detection. Compos. Part B Eng. 2022, 242, 110095. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, C.; Xu, Y.; Chen, J.; Ning, N.; Yang, Z.; Guo, Y.; Hu, X.; Wang, Y. Highly Stretchable and Conductive Self-Healing Hydrogels for Temperature and Strain Sensing and Chronic Wound Treatment. ACS Appl. Mater. Interfaces 2020, 12, 40990–40999. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Y.; Lian, L.; Liu, K.; Lu, M.; Chen, Y.; Zhang, L.; Zhang, X.; Wan, P. Flexible Accelerated-Wound-Healing Antibacterial MXene-Based Epidermic Sensor for Intelligent Wearable Human-Machine Interaction. Adv. Funct. Mater. 2022, 32, 2208141. [Google Scholar] [CrossRef]
- Liu, N.; Ma, H.; Li, M.; Qin, R.; Li, P. Electroconductive Hydrogels for Bioelectronics: Challenges and Opportunities. FlexMat 2024, 1, 269–301. [Google Scholar] [CrossRef]
- Wang, J.; Yang, B.; Jiang, Z.; Liu, Y.; Zhou, L.; Liu, Z.; Tang, L. Recent Advances of Conductive Hydrogels for Flexible Electronics. Electron. Mater. 2024, 5, 101–131. [Google Scholar] [CrossRef]
- Liang, Y.; Qiao, L.; Qiao, B.; Guo, B. Conductive Hydrogels for Tissue Repair. Chem. Sci. 2023, 14, 3091–3116. [Google Scholar] [CrossRef]
- Calderón Moreno, J.M.; Chelu, M.; Popa, M. Eco-Friendly Conductive Hydrogels: Towards Green Wearable Electronics. Gels 2025, 11, 220. [Google Scholar] [CrossRef]
- Li, Y.; Tan, S.; Zhang, X.; Li, Z.; Cai, J.; Liu, Y. Design Strategies and Emerging Applications of Conductive Hydrogels in Wearable Sensing. Gels 2025, 11, 258. [Google Scholar] [CrossRef]
- Guo, X.; Facchetti, A. The Journey of Conducting Polymers from Discovery to Application. Nat. Mater. 2020, 19, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Sazcı, O.; Uğraşkan, V.; Hazar, A.B.Y. Conductive Polymers for Medical Applications. In Handbook of Polymers in Medicine; Elsevier: Amsterdam, The Netherlands, 2023; pp. 305–325. ISBN 9780128237977. [Google Scholar]
- Dias, D.; Resina, L.; Ferreira, F.C.; Sanjuan-Alberte, P.; Esteves, T. Synthesis Strategies and Cancer Therapy Applications of PEDOT Nanoparticles. Mater. Adv. 2024, 5, 7561–7583. [Google Scholar] [CrossRef]
- Talikowska, M.; Fu, X.; Lisak, G. Application of Conducting Polymers to Wound Care and Skin Tissue Engineering: A Review. Biosens. Bioelectron. 2019, 135, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.P.; Anis, A. Conducting Polymer Hydrogels. In Polymeric Gels; Elsevier: Amsterdam, The Netherlands, 2018; pp. 467–486. ISBN 9780081021798. [Google Scholar]
- Sun, Z.; Ou, Q.; Dong, C.; Zhou, J.; Hu, H.; Li, C.; Huang, Z. Conducting Polymer Hydrogels Based on Supramolecular Strategies for Wearable Sensors. Exploration 2024, 4, 20220167. [Google Scholar] [CrossRef]
- Lee, J.-J.; Ng, H.Y.; Lin, Y.-H.; Liu, E.-W.; Lin, T.-J.; Chiu, H.-T.; Ho, X.-R.; Yang, H.-A.; Shie, M.-Y. The 3D Printed Conductive Grooved Topography Hydrogel Combined with Electrical Stimulation for Synergistically Enhancing Wound Healing of Dermal Fibroblast Cells. Biomater. Adv. 2022, 142, 213132. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, X.; You, T.; Zhao, B.; Dong, L.; Huang, C.; Zhou, X.; Xing, M.; Qian, W.; Luo, G. 3D Printing-Based Hydrogel Dressings for Wound Healing. Adv. Sci. 2024, 11, 2404580. [Google Scholar] [CrossRef]
- Li, Y.; Peng, W.; Dong, Y.; Fan, B.; Qian, W.; Ji, X.; Lu, X.; Gan, D.; Liu, P. Mussel-Inspired PEDOT-Incorporated Gelatin-Based Conductive Hydrogel with Flexibility and Electroactivity to Accelerate Wound Healing In Vitro. ACS Appl. Polym. Mater. 2023, 5, 4233–4243. [Google Scholar] [CrossRef]
- Gan, D.; Han, L.; Wang, M.; Xing, W.; Xu, T.; Zhang, H.; Wang, K.; Fang, L.; Lu, X. Conductive and Tough Hydrogels Based on Biopolymer Molecular Templates for Controlling in Situ Formation of Polypyrrole Nanorods. ACS Appl. Mater. Interfaces 2018, 10, 36218–36228. [Google Scholar] [CrossRef]
- Tang, L.; Xie, S.; Wang, D.; Wei, Y.; Ji, X.; Wang, Y.; Zhao, N.; Mou, Z.; Li, B.; Sun, W.R.; et al. Astragalus Polysaccharide/Carboxymethyl Chitosan/Sodium Alginate Based Electroconductive Hydrogels for Diabetic Wound Healing and Muscle Function Assessment. Carbohydr. Polym. 2025, 350, 123058. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Zhang, J.; Hu, X.; Yang, Z.; Guo, Y.; Wang, Y. In-Situ Doping of a Conductive Hydrogel with Low Protein Absorption and Bacterial Adhesion for Electrical Stimulation of Chronic Wounds. Acta Biomater. 2019, 89, 217–226. [Google Scholar] [CrossRef]
- Guan, L.; Ou, X.; Wang, Z.; Li, X.; Feng, Y.; Yang, X.; Qu, W.; Yang, B.; Lin, Q. Electrical Stimulation-Based Conductive Hydrogel for Immunoregulation, Neuroregeneration and Rapid Angiogenesis in Diabetic Wound Repair. Sci. China Mater. 2023, 66, 1237–1248. [Google Scholar] [CrossRef]
- Lin, X.; Yang, X.; Li, P.; Xu, Z.; Zhao, L.; Mu, C.; Li, D.; Ge, L. Antibacterial Conductive Collagen-Based Hydrogels for Accelerated Full-Thickness Wound Healing. ACS Appl. Mater. Interfaces 2023, 15, 22817–22829. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Hui, F.; Tian, T.; Yan, R.; Xin, J.; Zhao, X.; Jiang, Y.; Zhang, Z.; Kuang, Y.; Li, N.; et al. A Novel Conductive Antibacterial Nanocomposite Hydrogel Dressing for Healing of Severely Infected Wounds. Front. Chem. 2021, 9, 787886. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Shen, L.; Lu, Y.; Hu, C.; Liang, Z.; Long, L.; Ning, N.; Chen, J.; Guo, Y.; Yang, Z.; et al. Intrinsic Antibacterial and Conductive Hydrogels Based on the Distinct Bactericidal Effect of Polyaniline for Infected Chronic Wound Healing. ACS Appl. Mater. Interfaces 2021, 13, 52308–52320. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, N.; Elumalai, K.; Manickam, S.; Bakthavatchalam, G.; Tamilselvan, P. Carbon Nanomaterials: Revolutionizing Biomedical Applications with Promising Potential. Nano Mater. Sci. 2024, in press. [Google Scholar] [CrossRef]
- Stocco, T.; Zhang, T.; Dimitrov, E.; Ghosh, A.; da Silva, A.; Melo, W.; Tsumura, W.; Silva, A.; Sousa, G.; Viana, B.; et al. Carbon Nanomaterial-Based Hydrogels as Scaffolds in Tissue Engineering: A Comprehensive Review. Int. J. Nanomed. 2023, 18, 6153–6183. [Google Scholar] [CrossRef]
- Fan, Z.; Liu, B.; Wang, J.; Zhang, S.; Lin, Q.; Gong, P.; Ma, L.; Yang, S. A Novel Wound Dressing Based on Ag/Graphene Polymer Hydrogel: Effectively Kill Bacteria and Accelerate Wound Healing. Adv. Funct. Mater. 2014, 24, 3933–3943. [Google Scholar] [CrossRef]
- Zmejkoski, D.Z.; Marković, Z.M.; Mitić, D.D.; Zdravković, N.M.; Kozyrovska, N.O.; Bugárová, N.; Marković, B.M.T. Antibacterial Composite Hydrogels of Graphene Quantum Dots and Bacterial Cellulose Accelerate Wound Healing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 1796–1805. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Han, Y.; Guo, B. Mussel-Inspired, Antibacterial, Conductive, Antioxidant, Injectable Composite Hydrogel Wound Dressing to Promote the Regeneration of Infected Skin. J. Colloid Interface Sci. 2019, 556, 514–528. [Google Scholar] [CrossRef]
- Ravanbakhsh, H.; Bao, G.; Mongeau, L. Carbon Nanotubes Promote Cell Migration in Hydrogels. Sci. Rep. 2020, 10, 2543. [Google Scholar] [CrossRef]
- Wang, J.; He, J.; Zhou, R.; Zeng, R.; Guan, S.; Yang, X.; Liu, Z.; Liu, Y.; Zhu, X.; Liao, Q.; et al. Accelerated Diabetic Wound Healing via Electrical and Oxidative Microenvironment Regulation by MXene Nanosheet-Based Hydrogel Dressings. ACS Appl. Nano Mater. 2025, 8, 5466–5480. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, Z.; Li, N.; Zhang, J.; Li, M.; Han, L.; Cheng, R.; Shen, Z.; Han, D.; Sang, S. CS/Gel/MWCNTs Conductive Scaffolds Assisted by Electrical Stimulus for Skin Tissue Engineering. Biotechnol. Bioeng. 2025, 122, 2259–2272. [Google Scholar] [CrossRef]
- Liu, D.; Bi, S.; Wang, H.; Gu, J.; Wang, S. Polydopamine Interface-Modulated MXene-Based Conductive Antibacterial Hydrogels for on-Skin Health Monitoring and Diabetic Wound Healing. Compos. Part A Appl. Sci. Manuf. 2024, 180, 108065. [Google Scholar] [CrossRef]
- Gao, C.; Song, S.; Lv, Y.; Huang, J.; Zhang, Z. Recent Development of Conductive Hydrogels for Tissue Engineering: Review and Perspective. Macromol. Biosci. 2022, 22, 2200051. [Google Scholar] [CrossRef] [PubMed]
- Min, J.H.; Patel, M.; Koh, W.-G. Incorporation of Conductive Materials into Hydrogels for Tissue Engineering Applications. Polymers 2018, 10, 1078. [Google Scholar] [CrossRef]
- Zheng, W.; Yang, W.; Wei, W.; Liu, Z.; Tremblay, P.; Zhang, T. An Electroconductive and Antibacterial Adhesive Nanocomposite Hydrogel for High-Performance Skin Wound Healing. Adv. Healthc. Mater. 2024, 13, 2303138. [Google Scholar] [CrossRef]
- Hu, X.Q.; Zhu, J.Z.; Hao, Z.; Tang, L.; Sun, J.; Sun, W.R.; Hu, J.; Wang, P.Y.; Basmadji, N.P.; Pedraz, J.L.; et al. Renewable Electroconductive Hydrogels for Accelerated Diabetic Wound Healing and Motion Monitoring. Biomacromolecules 2024, 25, 3566–3582. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Hikmat, S.; Ghith, D.A.; Hajeer, M.; Hamadneh, L.; Qattan, D.; Khalil, E.A. Gold Nanoparticles Loaded into Polymeric Hydrogel for Wound Healing in Rats: Effect of Nanoparticles’ Shape and Surface Modification. Int. J. Pharm. 2019, 565, 174–186. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, X.; Yue, O.; Hou, M.; Zhang, H.; Beyer, S.; Blocki, A.M.; Wang, Q.; Gong, G.; Liu, X.; et al. Skin-Inspired Gelatin-Based Flexible Bio-Electronic Hydrogel for Wound Healing Promotion and Motion Sensing. Biomaterials 2021, 276, 121026. [Google Scholar] [CrossRef]
- Ren, D.; Zhang, Y.; Du, B.; Wang, L.; Gong, M.; Zhu, W. An Antibacterial, Conductive Nanocomposite Hydrogel Coupled with Electrical Stimulation for Accelerated Wound Healing. Int. J. Nanomed. 2024, 19, 4495–4513. [Google Scholar] [CrossRef]
- Ayreen, Z.; Khatoon, U.; Kirti, A.; Sinha, A.; Gupta, A.; Lenka, S.S.; Yadav, A.; Mohanty, R.; Naser, S.S.; Mishra, R.; et al. Perilous Paradigm of Graphene Oxide and Its Derivatives in Biomedical Applications: Insight to Immunocompatibility. Biomed. Pharmacother. 2024, 176, 116842. [Google Scholar] [CrossRef]
- Brumberg, V.; Astrelina, T.; Malivanova, T.; Samoilov, A. Modern Wound Dressings: Hydrogel Dressings. Biomedicines 2021, 9, 1235. [Google Scholar] [CrossRef]
- Ribeiro, M.; Simões, M.; Vitorino, C.; Mascarenhas-Melo, F. Hydrogels in Cutaneous Wound Healing: Insights into Characterization, Properties, Formulation and Therapeutic Potential. Gels 2024, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Han, Y.; Yang, L.; Kankala, R.K.; Wang, S.; Chen, A.; Fu, C. Conductive Hydrogels: Intelligent Dressings for Monitoring and Healing Chronic Wounds. Regen. Biomater. 2025, 12, rbae127. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Wei, L.; Yang, Z.; Liu, X.; Ma, H.; Zhao, J.; Liu, L.; Wang, L.; Chen, R.; Cheng, Y. Hydrogel Wound Dressings Accelerating Healing Process of Wounds in Movable Parts. Int. J. Mol. Sci. 2024, 25, 6610. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, P.; Xie, J.; Tang, Z.; Fu, J.; Ning, Y.; Zhong, Q.; Wang, D.; Lei, M.; Mai, H.; et al. A Multifunctional Injectable, Self-Healing, and Adhesive Hydrogel-Based Wound Dressing Stimulated Diabetic Wound Healing with Combined Reactive Oxygen Species Scavenging, Hyperglycemia Reducing, and Bacteria-Killing Abilities. J. Nanobiotechnol. 2024, 22, 444. [Google Scholar] [CrossRef] [PubMed]
- Alberts, A.; Tudorache, D.-I.; Niculescu, A.-G.; Grumezescu, A.M. Advancements in Wound Dressing Materials: Highlighting Recent Progress in Hydrogels, Foams, and Antimicrobial Dressings. Gels 2025, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhang, M.; Chong, C.-M.; Lin, D.; Chen, S.; Zhen, Y.; Ding, H.; Zhong, H.-J. Recent Advances in the 3D Printing of Conductive Hydrogels for Sensor Applications: A Review. Polymers 2024, 16, 2131. [Google Scholar] [CrossRef]
- Vafa, Z.J.; Zare, E.N.; Eslam, M.R.F.; Makvandi, P. Development of Ecofriendly, Biodegradable Electrically Conductive Double-Layer Bio-Hydrogel Nanocomposite for Sustainable Medical Device Applications. Adv. Compos. Hybrid Mater. 2025, 8, 174. [Google Scholar] [CrossRef]
- Nie, L.; Wei, Q.; Li, J.; Deng, Y.; He, X.; Gao, X.; Ma, X.; Liu, S.; Sun, Y.; Jiang, G.; et al. Fabrication and Desired Properties of Conductive Hydrogel Dressings for Wound Healing. RSC Adv. 2023, 13, 8502–8522. [Google Scholar] [CrossRef]
- Sui, B.; Liu, X.; Sun, J. Biodistribution, Inter-/Intra-Cellular Localization and Respiratory Dysfunction Induced by Ti3C2 Nanosheets: Involvement of Surfactant Protein down-Regulation in Alveolar Epithelial Cells. J. Hazard. Mater. 2021, 402, 123562. [Google Scholar] [CrossRef]
- Sosa, S.; Tubaro, A.; Carlin, M.; Ponti, C.; Vázquez, E.; Prato, M.; Pelin, M. Assessment of Skin Sensitization Properties of Few-Layer Graphene and Graphene Oxide through the Local Lymph Node Assay (OECD TG 442B). NanoImpact 2023, 29, 100448. [Google Scholar] [CrossRef]
- Guo, B.; Glavas, L.; Albertsson, A.-C. Biodegradable and Electrically Conducting Polymers for Biomedical Applications. Prog. Polym. Sci. 2013, 38, 1263–1286. [Google Scholar] [CrossRef]
- Jadoun, S.; Riaz, U.; Budhiraja, V. Biodegradable Conducting Polymeric Materials for Biomedical Applications: A Review. Med. Devices Sens. 2021, 4, e10141. [Google Scholar] [CrossRef]
- Luo, R.; Dai, J.; Zhang, J.; Li, Z. Accelerated Skin Wound Healing by Electrical Stimulation. Adv. Healthc. Mater. 2021, 10, 2100557. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, M.; Rahman, E.; Powner, M.B.; Triantis, I.F. Making Sense of Electrical Stimulation: A Meta-Analysis for Wound Healing. Ann. Biomed. Eng. 2024, 52, 153–177. [Google Scholar] [CrossRef]
- Rizzo, D.; Javidi, D.; Werpachowski, N.; Frasier, K.; Maher, B.; Huh, Y.; Shah, S.; Althobaiti, R.F. Advancing Wound Healing Using Cutaneous Bioelectronic Interfaces for Real-Time Monitoring and Electrical Stimulation. Dermis 2025, 5, 36. [Google Scholar] [CrossRef]
- Chen, M.; Liu, H.; Chen, X.; Kang, L.; Yao, X.; Tan, L.; Zhu, W.; Yu, J.; Qin, X.; Wu, D. A Novel Multifunction of Wearable Ionic Conductive Hydrogel Sensor for Promoting Infected Wound Healing. Appl. Mater. Today 2024, 39, 102298. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, M.; Xu, T.; Zhang, X. Multifunctional Hydrogel as Wound Dressing for Intelligent Wound Monitoring. Chem. Eng. J. 2022, 433, 134625. [Google Scholar] [CrossRef]
- Wang, L.; Xu, T.; Zhang, X. Multifunctional Conductive Hydrogel-Based Flexible Wearable Sensors. TrAC Trends Anal. Chem. 2021, 134, 116130. [Google Scholar] [CrossRef]
- She, Y.; Liu, H.; Yuan, H.; Li, Y.; Liu, X.; Liu, R.; Wang, M.; Wang, T.; Wang, L.; Liu, M.; et al. Artificial Intelligence-Assisted Conductive Hydrogel Dressings for Refractory Wounds Monitoring. Nano-Micro Lett. 2025, 17, 319. [Google Scholar] [CrossRef] [PubMed]
- Finster, R.; Sankaran, P.; Bihar, E. Computational and AI-Driven Design of Hydrogels for Bioelectronic Applications. Adv. Electron. Mater. 2025, 2400763. [Google Scholar] [CrossRef]
- Damiati, L.A.; Alsudir, S.A.; Mohammed, R.Y.; Majrashi, M.A.; Albrahim, S.H.; algethami, A.; Alghamdi, F.O.; Alamari, H.A.; Alzaydi, M.M. 4D Printing in Skin Tissue Engineering: A Revolutionary Approach to Enhance Wound Healing and Combat Infections. Bioprinting 2025, 45, e00386. [Google Scholar] [CrossRef]
- Naghib, S.M.; Hosseini, S.N.; Beigi, A. 3D 4D Printing of Chitosan-Based Scaffolds for Wound Dressing Applications. Carbohydr. Polym. Technol. Appl. 2024, 8, 100594. [Google Scholar] [CrossRef]
- Lu, Z.; Cui, J.; Liu, F.; Liang, C.; Feng, S.; Sun, Y.; Gao, W.; Guo, Y.; Zhang, B.; Huang, W. A 4D Printed Adhesive, Thermo-Contractile, and Degradable Hydrogel for Diabetic Wound Healing. Adv. Healthc. Mater. 2024, 13, e2303499. [Google Scholar] [CrossRef] [PubMed]
- Flynn, K.; Mahmoud, N.N.; Sharifi, S.; Gould, L.J.; Mahmoudi, M. Chronic Wound Healing Models. ACS Pharmacol. Transl. Sci. 2023, 6, 783–801. [Google Scholar] [CrossRef]
| Hydrogel Composition | Conductive Components | Self-Healing Mechanism | Physico-Chemical Performance | Biological Outcomes | Ref. |
|---|---|---|---|---|---|
| QCS-PANI (QCSP) and PEGS-FA | PANI | Dynamic Schiff base bonding between QCSP amines and PEGS-FA aldehydes; network reinforced by π–π stacking and electrostatic interactions | Conductivity: optimal 0.237 S·m−1 (QCSP3/PEGS-FA1.5) Gelation: 86–374 s; G’: 58–368 Pa; Swelling: 170–200%; Pore size: 107–204 µm; Critical strain: 250%; Thixotropic; Excellent antioxidant activity (>84% DPPH scavenging) improved with PANI; Adhesiveness: up to 4.9 kPa | In vitro: >95% viability (L929), non-hemolytic, >99% kill (E. coli, S. aureus); In vivo: mouse full-thickness wound model; 10% better contraction at day 10 vs. Tegaderm™; ↑ collagen, granulation, EGF, TGF-β, VEGF; ↓ inflammation | [38] |
| QCS-β-CD, QCS-AD, GO-β-CD | GO-CD | Dynamic host–guest interactions (β-CD and AD) + hydrogen bonding between QCS-β-CD, QCS-AD, GO-β-CD | Conductivity: 0.07–0.11 S·m−1 (GO4); Swelling ratio ~113%; Adhesive strength: 130 Pa; Shear-thinning and injectable; Photothermal ΔT: 20.1°C (10 min, 808 nm, 1.4 W/cm2); Network recovered within 14 s after strain-induced collapse | In vitro: L929 fibroblast proliferation ↑ with rGO; Hemolysis < 5%; Antibacterial: 95% E. coli death (2 h), 100% S. aureus and MRSA; NIR photothermal effect: complete kill in 10 min. In vivo (mice full-thickness wounds): 98.3% wound closure (Day 14); ↑ collagen, VEGF, epidermal/granulation tissue thickness; ↓ IL-6 | [90] |
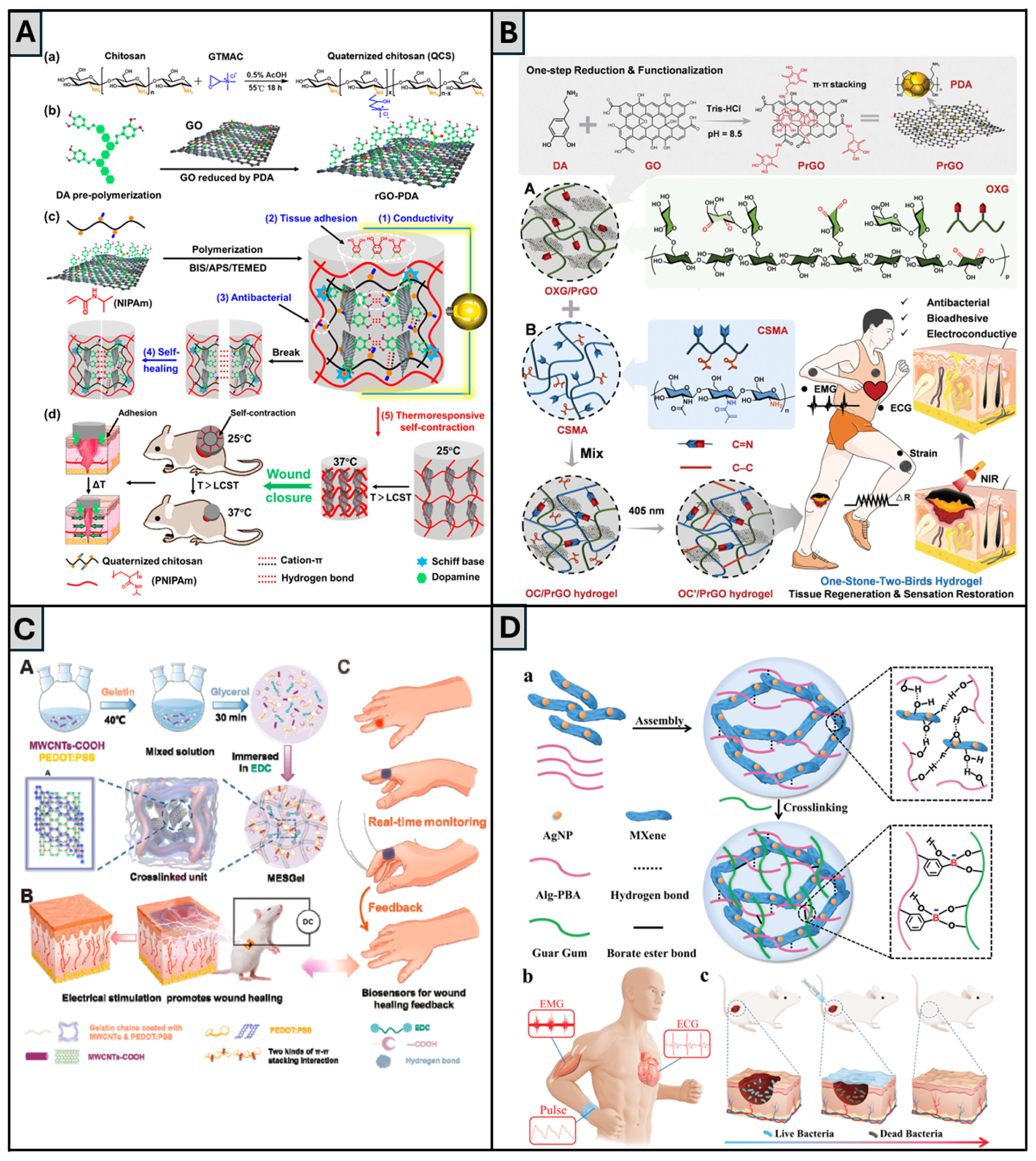
| QCS, rGO-PDA, PNIPAm, BIS, APS/TEMED | rGO-PDA | Dynamic Schiff base bonds between PDA and QCS; cation–π interactions; non-covalent interactions between catechol (PDA) groups; hydrogel reforms after strain cycles and incubation at 25 °C for 2 h | Self-healing strain threshold: 611%; Recovery of G′ and G″ after 1000% strain; Conductivity: up to 0.56 S·m−1 with rGO-PDA 4 mg/mL; Swelling ratio (25 °C): 436–588%, decreases to 205–302% at 37 °C; Tensile stress: up to 148.9 kPa after 60 min self-contraction at 37 °C; Adhesion to porcine skin: 9.68 kPa | In vitro L929 fibroblasts. Antibacterial: >85% killing (E. coli, S. aureus); 100% kill under NIR (10 min); DPPH scavenging ~97.8%; L929 viability: 82–93%; hemolysis < 2%; Doxy release: 93% over 10 days at 37 °C; In vivo: full-thickness skin defect in mice; comparison with commercial Tegaderm. Contraction-assisted closure: wound size reduced to 49 ± 6% on day 7; Histology: ↑ COL (76%), ↑ granulation, ↑ CD31, ↓ IL-6, ↑ hair follicles (865%) | [66] |
| OXG (oxidized xyloglucan), CSMA, PrGO | PDA-modified rGO nanosheets (PrGO) | Dynamic Schiff base bonds between aldehyde groups (OXG) and amine groups (CSMA) | Conductivity: 0.17 S·m−1; Modulus: from ~100 Pa (dynamic) to 20 kPa (after UV-triggered crosslinking); Adhesive strength to porcine skin: 4.6 kPa; NIR-induced photothermal conversion: ΔT ≈ 20.2 °C; Max surface temp: ~47 °C; DPPH scavenging: 77.8%; Potential to detect motion in different joints (knee, elbow, finger) | In vitro: HUVECs—↑ viability (106% at 24 h), ↑ migration (scratch assay), Hemolysis ratio < 5%; In vivo: full-thickness wound in ICR mice—Day 5: 83.9% closure (vs. 63.0% control), Day 21: full re-epithelialization, ↑ granulation (199 μm), ↑ COL (81%), ↑ immune regulation (↑ Th17/Th1/Th2 pathways) Hydrogel-treated group showed faster healing rate (84% vs. 63%) and a more native-like epidermal layer | [64] |
| PEGDA (matrix), DTT (crosslinker), Borax (catalyst) | AuNPs, PEDOT:PSS and/or MXene | Thiol-acrylate Michael addition forms covalent PEGDA–DTT network; DTT provides –SH groups; Borax facilitates reversible boronate ester bonds with DTT diols | 3D-printable, injectable; Stretchability ~210%; G′ up to 417.5 Pa (P:P/MX-HG); Conductivity (S·m−1): 2.36 (pHG), 159.3 (AuNP-HG), 229.0 (P:P-HG), 229.5 (MX-HG), 283.6 (P:P/MX-HG); Adhesiveness to skin; Self-healed hydrogels (within 30 min at 37°C) retain G′ and conductivity; pH responsive, adequate for drug release in infected wound conditions. | In Vitro, HDF-Ad cells: ↑ viability over 7 days; Scratch wound assay: full closure at 24 h with pHG, TCH-HG; slower initial closure for P:P/MX-HG; Antibacterial effect (inhibition zones): 22.3 mm (E. coli), 20.8 mm (P. aeruginosa); | [67] |
| Gelatin with EDC + PEDOT:PSS + MWCNTs-COOH (MESGel) | PEDOT:PSS and MWCNTs-COOH | Hydrogen bonding, electrostatic interactions, and π–π stacking among gelatin, PEDOT:PSS, and MWCNTs-COOH | Conductivity: 0.71 S·m−1 (2.0 mL PEDOT:PSS); Rapid self-repair (60% in 2 min; ~100% in 10 min); Stability retained post self-healing; Tensile strain: 425% (pre)/375% (post-healing); Compressive strain: 71%/65%; Swelling ratio > 4.5; Thermal denaturation temp: 65.1°C; Shear-thinning; Broad viscoelastic range; Degradation: 92.3% in 3 wks; Potential to detect motion in different joints (wrist, elbow, finger) | In vitro: CHL cells—viability > 111.3%, CPI: 63.7 (vs. 59.1 MESGel), enhanced by ES; In vivo: rat full-thickness skin model—>90% wound closure (day 10), ↑ collagen, granulation, re-epithelialization, PDGF, VEGF; high sensor performance (ΔR/R0 > 40%) and 100 ms response time in joint motion monitoring | [131] |
| CMCS, Alg-PBA, TP, MXene nanosheets | MXene nanosheets | Dynamic borate ester bonds between -OH groups (CMCS, TP) and PBA (Alg-PBA); supramolecular interactions (hydrogen bonding, π–π stacking, cation–π) between CMCS, TP, MXene, and Alg-PBA | Conductivity: improved with MXene, but excessive MXene content led to decreased sensitivity to bending; Stretchability: up to 300%; Adhesion strength: 32.76 kPa (plastic), 18.74 kPa (porcine skin); Self-healing: seconds (ambient conditions); Injectable; Degradation: ~21 days in PBS; Sensing: GF = 0.79 (vs. 0.26 without MXene), unchanged in the cut-and-healed hydrogel; Detected swallowing movements, finger and elbow bending and wrist pulse, as well as ECG and EMG signals | In vitro: L929 fibroblasts—no cytotoxicity (72 h); Hemolysis: minimal (comparable to PBS); Antibacterial: 93.06% (S. aureus), 96.30% (E. coli). In vivo: Mouse liver hemorrhage and tail amputation models—↓ blood loss (30.66 mg (liver) vs. 446.64 mg (control), 18.76 mg (tail) vs. 265.54 mg (control)); EMG and ECG signals monitored effectively during motion; SNR (EMG): 29.7 dB vs. 16.7 dB (commercial electrode) | [91] |
| GelMA, Ti3C2 MXene, V-Os (collagen-binding antimicrobial peptide) | Ti3C2 MXene | Physical self-healing through reversible supramolecular interactions between GelMA chains, Ti3C2 MXene nanosheets, and the collagen-binding peptide V-Os | Conductivity: 0.7 mS m−1; Tensile 0.193 MPa; Porosity 89%; Swelling 351%; Injectable | In vitro: NIH3T3—↑proliferation and adhesion under ES (100 Hz, 200 mV), ↑COL-I and VEGF expression; Bacterial survival ≈ 0%; In vivo: full-thickness rat wound model—wound closure 94% (day 11, ES); ↓ TNF-α, ↑ CD31 angiogenesis, ↑ COL-I; ↓ scar tissue | [132] |
| PVA (matrix), GO/Ag/TGA nanocomposites, PANI (optional shell) | GO/Ag/TGA; with or without PANI coating (GATP-PVA) | Hydrogen bonding between PVA chains; TGA introduces thiol and carboxyl groups that enhance hydrogen bonding and interfacial adhesion; In GATP-PVA, disulfide bonding and π–π interactions with PANI shell further stabilize network | Shear-thinning and thixotropic behaviors; Tensile strength ~1.1 MPa; Conductivity (ionic): 0.138 S·m−1 (GATP-PVA); Self-healing demonstrated via oscillatory rheology over 3 break–heal cycles with minimal G′ loss; Strong tissue adhesion attributed to TGA-functionalized GO; | In vitro L929 fibroblasts: ↑ viability vs. pristine PVA; ROS scavenging properties In vivo mouse wound model: ~90% wound contraction at day 15 with GATP-PVA; Histology: denser collagen, ↑ vascularization, ↑ reepithelialization; Hemostatic: ↓ liver bleeding volume in GATP group; Antibacterial: strong activity vs. S. aureus, P. aeruginosa, K. pneumoniae | [92] |
| OSD, CMC, Fe3+, and PDA coated poly(thiophene-3-acetic acid)) | PDA-coated poly(thiophene-3-acetic acid) (PA) | Dual dynamic bonding: (1) Schiff base between aldehyde (OSD) and amino groups (CMC); (2) Metal coordination between catechol (OSD/PA) and Fe3+ | Swelling ratio: 240% (OSD/CMC/Fe/PA5); Degradation: ~63 h; Conductivity: 7.2 × 10−2 S·m−1 (PA5); ΔT (photothermal): 25°C @10 min NIR; G′: 120.9 Pa (PA5); Adhesive strength > 5 kPa (pigskin test) | In vitro: L929 cells (viability ≥ 80%); Antibacterial: E. coli and MRSA (99% in 5–10 min NIR); In vivo: mouse full-thickness MRSA-infected wound model; wound closure 97.02% (Day 14, PA3 + NIR); ↓ TNF-α, ↑ VEGF and angiogenesis | [65] |
| XG, OSA-DA, fucoidan, and 45S5 bioglass microspheres | Ca2+ and Si4+ ions released from 45S5 bioglass | Dynamic Schiff base bonds between dopamine amines and OSA aldehydes; enhanced by hydrogen bonding (XG), Ca2+ chelation (OSA), and π–π stacking from catechol groups | Conductivity: 0.65 S·m−1 (vs. 0.4 S·m−1 in groups without bioglass); Adhesion: >12 kPa on porcine skin; Swelling ~350% at 48 h; Shear-thinning; G’ recovery after 200% strain; Porous 3D structure; Fucoidan release sustained > 120 h | In vitro: NIH 3T3 proliferation (>300% RGR), migration (scratch and Transwell), hemolysis < 2%, antibacterial (E. coli: 40%, S. aureus: 32% inhibition); In vivo: SD rat full-thickness wound model, 98% wound closure by day 9, ↑ VEGF/CD31/collagen, ↓ TNF-α | [93] |
| GG-based slime (GS) (matrix) | PEDOT:PSS | Dynamic intermolecular hydrogen bonding between –OH groups in CG chains; PEDOT:PSS electrostatically interacts with CG quaternary ammonium groups; gel reforms within 30 min at RT without external stimulus. | Injectable; Stretchability up to 500% (vs. 150% for GG alone); Conductivity: 0.22 S·m−1 (vs. 0.104 S·m−1 in CG alone); Both hydrogels showed thixotropic behavior | No cytotoxicity in MDCK, HF, and 3T3 cells; hemolysis < 5%; In vitro wound healing: closure rate 71% (PPGS) vs. 57% (GG) on day 7; In vivo (rat, dorsum, and occiput wounds): Histology: ↑ granulation tissue, 76% COL deposition (vs. 56% CG, 20% control); ↓ inflammation, ↑ hair follicle regeneration. | [14] |
| PVA, Zn2+-functionalized CS (ZnCS), CS-PPy, borax (crosslinker) | CS-PPy | Combination of reversible di-diol complexation (PVA-borax), Zn2+-CS coordination bonds, and hydrogen bonding | Conductivity: 116 S·m−1; Tensile stretchability: >3500%; Self-healing time: 10 s; Stable after autoclaving and freezing; LED circuit reconnection validated | In vitro: fibroblasts—no cytotoxicity; Hemolysis < 2%; Antimicrobial against S. aureus and P. aeruginosa In vivo: diabetic infected rat model—↑ wound closure in PCPZ + ES (3 V, 1 h/day); ↑ COL deposition, re-epithelialization, and mature blood vessels on day 21 | [94] |
| GG, Alg-PBA, AgNPs-coated MXene nanosheets, NaOH | AgNPs/MXene nanosheets | Dynamic crosslinking between –OH in GG and PBA groups in Alg-PBA; supramolecular interactions among AgNPs/MXene, GG, and Alg-PBA | Tensile strain: 166.67% (original) vs. 165.28% (healed); Break strength recovery: ~95%; Shear-thinning; Injectable; Degrades in 45 days in PBS (pH 7.2); 3D porous structure with high hydrophilicity | In vitro: L929 fibroblasts—no cytotoxicity; Antibacterial efficacy: 77.78% (S. aureus), 85.82% (E. coli); Epidermic sensor detects wrist/finger bending, swallowing, ECG, EMG (SNR: 17.8 dB); In vivo: murine full-thickness infected wound (8 mm)—wound closure 98.16% (day 12) vs. 80.5% (control); ↓ inflammation, ↑ collagen, ↑ vascularization | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, D.; Dias, D.; Ferreira, F.C.; Esteves, T. Self-Healing, Electroconductive Hydrogels for Wound Healing Applications. Gels 2025, 11, 619. https://doi.org/10.3390/gels11080619
Almeida D, Dias D, Ferreira FC, Esteves T. Self-Healing, Electroconductive Hydrogels for Wound Healing Applications. Gels. 2025; 11(8):619. https://doi.org/10.3390/gels11080619
Chicago/Turabian StyleAlmeida, Duarte, Diogo Dias, Frederico Castelo Ferreira, and Teresa Esteves. 2025. "Self-Healing, Electroconductive Hydrogels for Wound Healing Applications" Gels 11, no. 8: 619. https://doi.org/10.3390/gels11080619
APA StyleAlmeida, D., Dias, D., Ferreira, F. C., & Esteves, T. (2025). Self-Healing, Electroconductive Hydrogels for Wound Healing Applications. Gels, 11(8), 619. https://doi.org/10.3390/gels11080619








