Formulation, Optimization, and Comprehensive Characterization of Topical Essential Oil-Loaded Anti-Acne Microemulgels
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Characteristics of Essential Oils
2.2. Solubility
2.3. Microbiology Pre-Formulation
2.4. Formulation and Optimization
HLB Values of Different Smix Ratios
2.5. Stability of Microemulsions
2.5.1. Kinetic Stability Findings
2.5.2. Thermodynamic Stability
2.6. Physicochemical Characteristics of Microemulsions
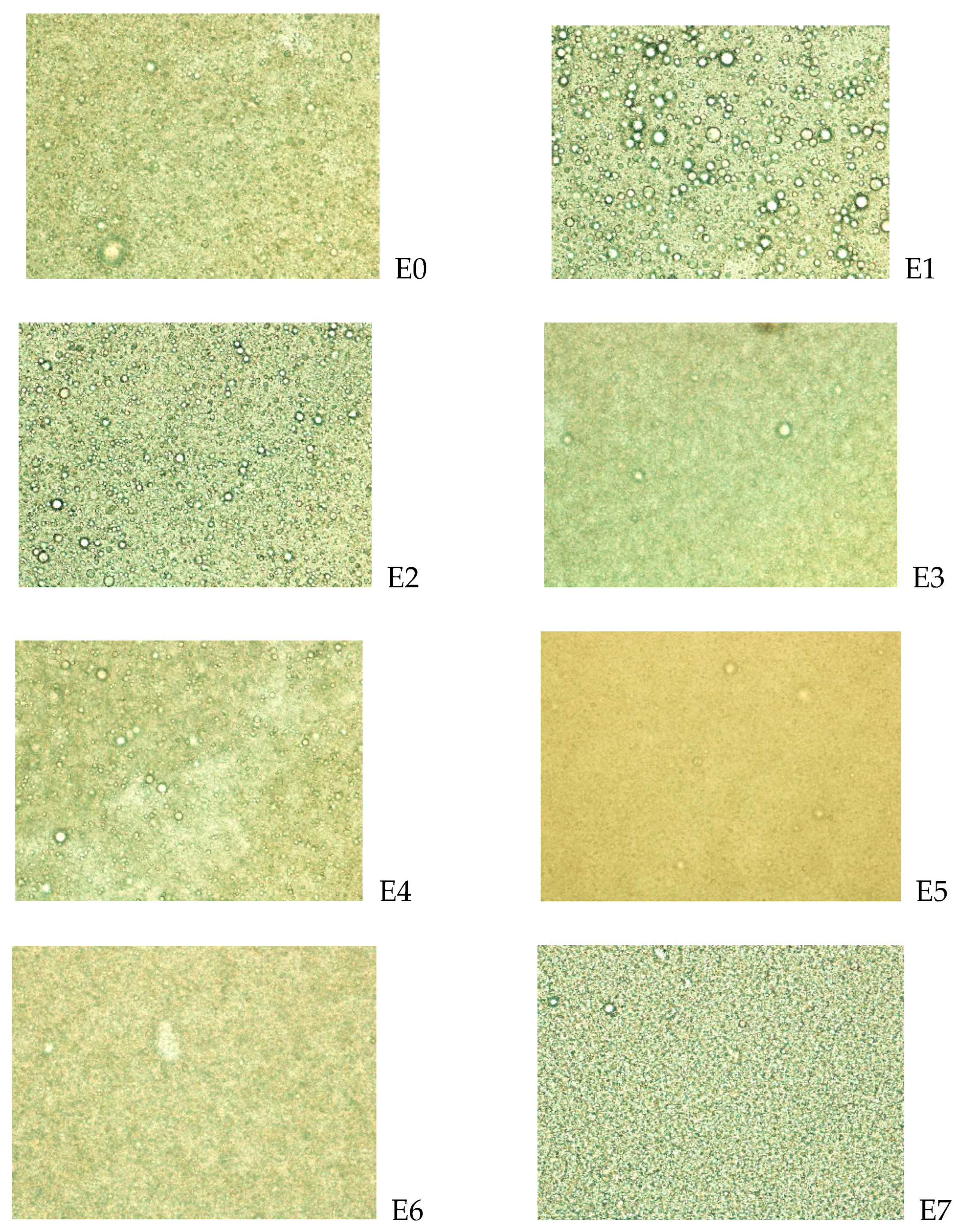
2.7. Microscopy of Microemulsions
2.8. Physicochemical Properties of Microemulgels
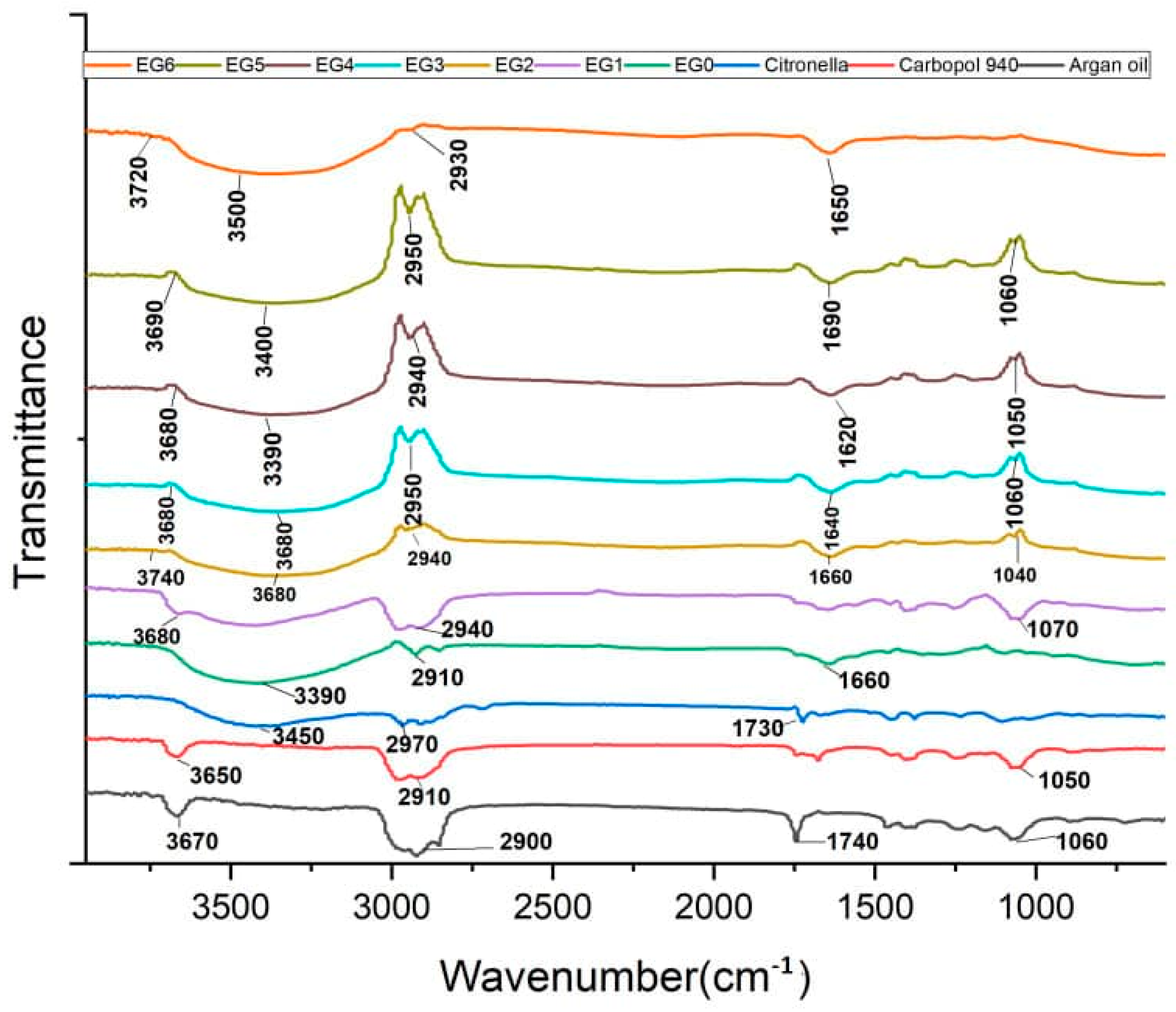
2.9. FTIR
2.10. DSC
2.11. Stability Testing of the Formulations
2.12. Antimicrobial Activities of the Microemulgel Formulations
2.13. Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Pre-Formulation
4.2.1. Physicochemical Characterization of Essential Oils
4.2.2. Microbiology Pre-Formulation Studies
- Essential oils
- Strain of Cutibacterium acnes
- Preparation of essential oil working solutions
- Serial dilution of solubilized EOs
4.3. Formulation Optimization
4.3.1. Selection of Components
4.3.2. Determination of HLB Values
4.3.3. Pseudo-Ternary Phase Diagram Construction
4.4. Stability of Emulsion
4.4.1. Kinetic Stability
4.4.2. Thermodynamic Stability Studies
4.5. Preparation of Microemulsion Loaded with Essential Oil
4.6. Physicochemical Properties of Microemulsions
4.6.1. Globule/Droplet Size
4.6.2. Refractive Index
4.6.3. Microemulsions’ Conductivity
4.6.4. Microemulsions’ Viscosity
4.7. Preparation of Emulgel
4.7.1. Preparation of the Gel
4.7.2. Incorporation of the Prepared Gel into Already Prepared Microemulsion
4.7.3. Optimization of the Emulgel Formulation
4.8. Physicochemical Properties of the Microemulgels
4.8.1. Physical Appearance
4.8.2. pH Determination
4.8.3. Microemulgels’ Conductivity
4.8.4. Microemulgels’ Viscosity
4.8.5. Spreadability
4.8.6. Extrudability
4.8.7. FTIR Analysis
4.8.8. Differential Scanning Calorimetry (DSC)
4.8.9. Physical Stability
4.9. Antimicrobial Activities of Microemulgels
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Zhang, T.C.; Yin, X.L.; Man, J.Y.; Yang, X.R.; Lu, M. Magnitude and Temporal Trend of Acne Vulgaris Burden in 204 Countries and Territories from 1990 to 2019: An Analysis from the Global Burden of Disease Study 2019. Br. J. Dermatol. 2022, 186, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Alshammrie, F.F.; Alshammari, R.; Alharbi, R.M.; Khan, F.H.; Alshammari, S.K. Epidemiology of Acne Vulgaris and Its Association with Lifestyle Among Adolescents and Young Adults in Hail, Kingdom of Saudi Arabia: A Community-Based Study. Cureus 2020, 12, e9277. [Google Scholar] [CrossRef]
- Ak, M. A Comprehensive Review of Acne Vulgaris. J. Clin. Pharm. 2019, 1, 17–44. [Google Scholar]
- Alanazi, M.S.; Hammad, S.M.; Mohamed, A.E. Prevalence and Psychological Impact of Acne Vulgaris among Female Secondary School Students in Arar City, Saudi Arabia, in 2018. Electron. Physician 2018, 10, 7224–7229. [Google Scholar] [CrossRef]
- Brüggemann, H.; Salar-Vidal, L.; Gollnick, H.P.M.; Lood, R. A Janus-Faced Bacterium: Host-Beneficial and -Detrimental Roles of Cutibacterium acnes. Front. Microbiol. 2021, 12, 673845. [Google Scholar] [CrossRef] [PubMed]
- Mongaret, C.; Velard, F.; Reffuveille, F. Cutibacterium acnes: The Urgent Need to Identify Diagnosis Markers. Infect. Immun. 2021, 89, 10–1128. [Google Scholar] [CrossRef]
- Mayslich, C.; Grange, P.A.; Dupin, N. Cutibacterium Acnes as an Opportunistic Pathogen: An Update of Its Virulence-Associated Factors. Microorganisms 2021, 9, 303. [Google Scholar] [CrossRef]
- Alkhawaja, E.; Hammadi, S.; Abdelmalek, M.; Mahasneh, N.; Alkhawaja, B.; Abdelmalek, S.M. Antibiotic Resistant Cutibacterium acnes among Acne Patients in Jordan: A Cross Sectional Sy. BMC Dermatol. 2020, 20, 17. [Google Scholar] [CrossRef]
- Skadins, I.; Zavorins, A.; Kroica, J.; Pavloviča, T.; Bruzgule, D.; Averjanova, T. Antibacterial Susceptibility Testing of Cutibacterium acnes in Acne Vulgaris Patients. Clin. Cosmet. Investig. Dermatol. 2021, 14, 671–677. [Google Scholar] [CrossRef]
- Dessinioti, C.; Katsambas, A.D. The Role of Propionibacterium Acnes in Acne Pathogenesis: Facts and Controversies. Clin. Dermatol. 2010, 28, 2–7. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Tanghetti, E.A. The Role of Inflammation in the Pathology of Acne. J. Clin. Aesthet. Dermatol. 2013, 6, 27–35. [Google Scholar]
- Cooper, A.J. Systematic Review of Propionibacterium Acnes Resistance to Systemic Antibiotics. Med. J. Aust. 1998, 169, 259–261. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Essential Oils and Their Constituents as Skin Penetration Enhancer for Transdermal Drug Delivery: A Review. J. Pharm. Pharmacol. 2015, 67, 473–485. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca Alternifolia (Tea Tree) Oil: A Review of Antimicrobial and Other Medicinal Properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef]
- Hammer, K.A. Treatment of Acne with Tea Tree Oil (Melaleuca) Products: A Review of Efficacy, Tolerability and Potential Modes of Action. Int. J. Antimicrob. Agents 2015, 45, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A. Monitoring of Antimicrobial Effect of GC-MS Standardized Melaleuca Alternifolia Oil (Tea Tree Oil) On Multidrug Resistant Uropathogens. IOSR J. Pharm. Biol. Sci. 2012, 2, 6–14. [Google Scholar] [CrossRef]
- Bassett, I.B.; Pannowitz, D.L.; Barnetson, R.S.C. A Comparative Study of Tea-Tree Oil versus Benzoylperoxide in the Treatment of Acne. Med. J. Aust. 1990, 153, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Shah, G.; Shri, R.; Panchal, V.; Sharma, N.; Singh, B.; Mann, A.S. Scientific Basis for the Therapeutic Use of Cymbopogon Citratus, Stapf (Lemon Grass). J. Adv. Pharm. Technol. Res. 2011, 2, 3–8. [Google Scholar] [CrossRef]
- Ali, M.M.; Yusuf, M.A.; Abdalaziz, M.N. GC-MS Analysis and Antimicrobial Screening of Essential Oil from Lemongrass (Cymbopogon Citratus). Int. J. Pharm. Chem 2017, 3, 72–76. [Google Scholar]
- Wei, A.; Shibamoto, T. Antioxidant Activities and Volatile Constituents of Various Essential Oils. J. Agric. Food Chem. 2007, 55, 1737–1742. [Google Scholar] [CrossRef]
- Lertsatitthanakorn, P. Effect of Citronella Oil on Time Kill Profile, Leakage and Morphological Changes of Propionibacterium acnes. J. Essent. Oil Res. 2010, 22, 270–274. [Google Scholar] [CrossRef]
- Hamzah, M.H.; Man, H.C.; Abidin, Z.Z.; Jamaludin, H. Comparison of Citronella Oil Extraction Methods from Cymbopogon nardus Grass by Ohmic-Heated Hydro-Distillation, Hydro-Distillation, and Steam Distillation. Bioresources 2013, 9, 256–272. [Google Scholar] [CrossRef][Green Version]
- Anwar, Y.; Siringoringo, V.S. Fractionation of Citronella Oil and Identification of Compounds by Gas Chromatography-Mass Spectrometry. Pharm. Sci. Res. 2020, 7, 138–144. [Google Scholar] [CrossRef]
- Haerussana, A.N.E.M.; Chairunnisa, H.F. Essential Oil Constituents and Pharmacognostic Evaluation of Java Citronella (Cymbopogon winterianus) Stem from Bandung, West Java, Indonesia. Open Access Maced. J. Med. Sci. 2022, 10, 1338–1346. [Google Scholar] [CrossRef]
- Han, X.; Parker, T.L. Lemongrass (Cymbopogon Flexuosus) Essential Oil Demonstrated Anti-Inflammatory Effect in Pre-Inflamed Human Dermal Fibroblasts. Biochim. Open 2017, 4, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Bayala, B.; Coulibaly, A.Y.; Djigma, F.W.; Nagalo, B.M.; Baron, S.; Figueredo, G.; Lobaccaro, J.-M.A.; Simpore, J. Chemical Composition, Antioxidant, Anti-Inflammatory and Antiproliferative Activities of the Essential Oil of Cymbopogon nardus, a Plant Used in Traditional Medicine. Biomol. Concepts 2020, 11, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Assmann, C.E.; Cadoná, F.C.; Bonadiman, B.D.S.R.; Dornelles, E.B.; Trevisan, G.; da Cruz, I.B.M. Tea Tree Oil Presents in vitro Antitumor Activity on Breast Cancer Cells without Cytotoxic Effects on Fibroblasts and on Peripheral Blood Mononuclear Cells. Biomed. Pharmacother. 2018, 103, 1253–1261. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Sobczak, M. Hydrogel-Based Active Substance Release Systems for Cosmetology and Dermatology Application: A Review. Pharmaceutics 2020, 12, 396. [Google Scholar] [CrossRef]
- Mehta, J.; Shah, C.N.; Upadhyay, U.M. Novel Topical Drug Delivery System: Microemulgel—A Review. Res. J. Pharm. Dos. Forms Technol. 2022, 14, 171–176. [Google Scholar] [CrossRef]
- Bunrathep, S.; Thongphasuk, P.; Settharaksa, S. Effect of an Essential Oil Blend of Citronella, Lemongrass, and Patchouli on Acne-Causing Bacteria. Songklanakarin J. Sci. Technol. 2024, 42, 1106–1112. [Google Scholar]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Prasad, H.; Batra, A.K. A Review on Formulation Approach to Enhance Oral Bioavailability of Poorly Soluble Drugs by Self Emulsifying Drug Delivery System. World J. Pharma. Res. 2014, 3, 1067–1084. [Google Scholar]
- Arancibia, C.; Garrido-Miranda, K.A.; Miller, R.; Smejkal, G.; Gross, V.; Lazarev, A. Theoretical and Experimental Determinations of the Hydrophilic–Lipophilic Balance (HLB) of Representative Oils and Lecithins. Colloids Interfaces 2024, 8, 21. [Google Scholar] [CrossRef]
- Yaghmur, A.; Aserin, A.; Mizrahi, Y.; Nerd, A.; Garti, N. Argan Oil-in-Water Emulsions: Preparation and Stabilization. J. Am. Oil Chem. Soc. 1999, 76, 15–18. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W.; Nahar, L.; Basar, N.; Sarker, S.D. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential Oils as Ecofriendly Biopesticides? Challenges and Constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, H.; Wang, J.; Li, F.; Wang, J.; Ma, X.; Li, J.; Huang, Y.; Liu, Z.; Zhang, L.; et al. Anti-Microbial Activity of Citronella (Cymbopogon citratus) Essential Oil Separation by Ultrasound Assisted Ohmic Heating Hydrodistillation. Ind. Crops Prod. 2022, 176, 114299. [Google Scholar] [CrossRef]
- Pazyar, N.; Yaghoobi, R.; Bagherani, N.; Kazerouni, A. A Review of Applications of Tea Tree Oil in Dermatology. Int. J. Dermatol. 2013, 52, 784–790. [Google Scholar] [CrossRef]
- Zekri, N.; Elazzouzi, H.; Ailli, A.; Gouruch, A.A.; Radi, F.Z.; El Belghiti, M.A.; Zair, T.; Nieto, G.; Centeno, J.A.; Lorenzo, J.M. Physicochemical Characterization and Antioxidant Properties of Essential Oils of M. pulegium (L.), M. suaveolens (Ehrh.) and M. spicata (L.) from Moroccan Middle-Atlas. Foods 2023, 12, 760. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Enshaieh, S.; Jooya, A.; Siadat, A.H.; Iraji, F. The Efficacy of 5% Topical Tea Tree Oil Gel in Mild to Moderate Acne Vulgaris: A Randomized, Double-Blind Placebo-Controlled Study. Indian J. Dermatol. Venereol. Leprol. 2007, 73, 22–25. [Google Scholar] [CrossRef]
- Lee, C.J.; Chen, L.W.; Chen, L.G.; Chang, T.L.; Huang, C.W.; Huang, M.C.; Wang, C.C. Correlations of the Components of Tea Tree Oil with Its Antibacterial Effects and Skin Irritation. J. Food Drug Anal. 2013, 21, 169–176. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Yang, S.-A.; Jeon, S.-K.; Lee, E.-J.; Im, N.-K.; Jung, J.-Y.; Lee, I.-S. Bioactivity and Chemical Composition of the Essential Oil of Tea Tree (Melaleuca Alternifolia). J. Life Sci. 2008, 18, 1644–1650. [Google Scholar] [CrossRef]
- Musakhanian, J.; Osborne, D.W.; Rodier, J.D. Skin Penetration and Permeation Properties of Transcutol® in Complex Formulations. AAPS PharmSciTech 2024, 25, 201. [Google Scholar] [CrossRef]
- Ande, S.N.; Sonone, K.B.; Bakal, R.L.; Ajmire, P.V.; Sawarkar, H.S. Role of Surfactant and Co-Surfactant in Microemulsion: A Review. Res. J. Pharm. Technol. 2022, 15, 4829–4834. [Google Scholar] [CrossRef]
- Otto, F.; van Hoogevest, P.; Syrowatka, F.; Heinl, V.; Neubert, R.H.H. Assessment of the Applicability of HLB Values for Natural Phospholipid Emulsifiers for Preparation of Stable Emulsions. Pharmazie 2020, 75, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Jhawat, V.; Gulia, M.; Sharma, A.K. Pseudoternary Phase Diagrams Used in Emulsion Preparation. In Chemoinformatics and Bioinformatics in the Pharmaceutical Sciences; Elsevier: Amsterdam, The Netherlands, 2021; pp. 455–481. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid Lipid Nanoparticles: Production, Characterization and Applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, F.; Zendehboudi, S. A Comprehensive Review on Emulsions and Emulsion Stability in Chemical and Energy Industries. Can. J. Chem. Eng. 2019, 97, 281–309. [Google Scholar] [CrossRef]
- Shakeel, F.; Baboota, S.; Ahuja, A.; Ali, J.; Faisal, M.S.; Shafiq, S. Stability Evaluation of Celecoxib Nanoemulsion Containing Tween 80. Thai J. Pharm. Sci. 2008, 32, 4–9. [Google Scholar] [CrossRef]
- Lawrence, M.J.; Rees, G.D. Microemulsion-Based Media as Novel Drug Delivery Systems. Adv. Drug Deliv. Rev. 2000, 45, 89–121. [Google Scholar] [CrossRef]
- Zhang, W.; Li, M.; Lin, M.; Luo, T.; Yao, C. Electrical Conductivity and Stability of O/W Emulsions. Acta Pet. Sin. 2008, 24, 592. [Google Scholar]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic Acid: A Key Molecule in Skin Aging. Dermatoendocrinology 2012, 4, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Said dos Santos, R.; Rosseto, H.C.; Bassi da Silva, J.; Vecchi, C.F.; Caetano, W.; Bruschi, M.L. The Effect of Carbomer 934P and Different Vegetable Oils on Physical Stability, Mechanical and Rheological Properties of Emulsion-Based Systems Containing Propolis. J. Mol. Liq. 2020, 307, 112969. [Google Scholar] [CrossRef]
- Kaltsa, O.; Alibade, A.; Batra, G.; Bozinou, E.; Makris, D.P.; Lalas, S.I. Fortification of Chocolate Using Moringa oleifera Extract Encapsulated in Microemulsions. OCL 2021, 28, 38. [Google Scholar] [CrossRef]
- Muhammed, S.A.; Al-Kinani, K.K. Formulation and in Vitro Evaluation of Meloxicam as a Self-Microemulsifying Drug Delivery System. F1000Research 2023, 12, 315. [Google Scholar] [CrossRef]
- Kola-Mustapha, A.T.; Khalid-Salako, F.A. Herbal Emulgels Incorporated with Cola millenii K. Schum Stem Bark Ethanol Extract Potentially for the Management of Rheumatoid Arthritis In-Vitro. Phytomedicine Plus 2021, 1, 100033. [Google Scholar] [CrossRef]





| Essential Oil | Color | Clarity | Form | Odor | Solubility | Density (g/mL) |
|---|---|---|---|---|---|---|
| C. witerianus | Light yellow | Clear | Liquid | Lemony | + | 0.892 |
| M. alternifolia | Colorless | Clear | Liquid | Pungent | + | 0.898 |
| C. flexuosus | Golden yellow | Clear | Liquid | Lemony | + | 0.984 |
| Essential Oil | MIC (v/v%) | MBC (v/v%) |
|---|---|---|
| Citronella | 0.078 | 0.125 |
| Tea tree | 0.016 | 1.000 |
| Lemongrass | 0.062 | 0.25 |
| Smix Ratio (PEG-40/Transcutol) | HLB Value |
|---|---|
| 0:1 | 4.20 |
| 1:1 | 9.60 |
| 1:2 | 7.76 |
| 1:3 | 6.90 |
| 1:4 | 6.36 |
| 2:1 | 11.45 |
| 3:1 | 12.30 |
| 4:1 | 12.84 |
| 1:0 | 15.00 |
| Microemulsion | 1000 rpm | 2000 rpm | 3000 rpm |
|---|---|---|---|
| AE1 | ✓ | ✓ | X |
| AE2 | ✓ | ✓ | ✓ |
| AE3 | X | X | X |
| AE4 | ✓ | ✓ | X |
| Microemulsion | Heating-Cooling | Centrifugation | Freeze–Thaw |
|---|---|---|---|
| AE1 | ✓ | X | X |
| AE2 | ✓ | ✓ | ✓ |
| AE3 | X | X | X |
| AE4 | X | X | X |
| Formulations | E0 | E1 | E2 | E3 | E4 | E5 | E6 | E7 |
|---|---|---|---|---|---|---|---|---|
| Droplet size + SD (μm) | 4.48 ± 2.59 | 4.74 ± 3.58 | 4.69 ± 2.79 | 8.28 ± 4.72 | 5.44 ± 2.73 | 6.38 ± 3.63 | 6.60 ± 1.98 | 6.77 ± 2.76 |
| Refractive index | 1.08 ± 0.12 | 1.07 ± 0.05 | 1.08 ± 0.07 | 1.07 ± 0.11 | 1.08 ± 0.04 | 1.07 ± 0.09 | 1.07 ± 0.14 | 1.07 ± 0.18 |
| Conductivity (µS/cm) | 105.3 ± 1.2 | 102.7 ± 2.3 | 106 ± 2.0 | 97.3 ± 2.5 | 103.3 ± 3.1 | 102.0 ± 3.0 | 94.3 ± 3.1 | 105.3 ± 1.2 |
| Viscosity (cP) | 22.0 ± 0.92 | 23.0 ± 1.27 | 22.0 ± 1.32 | 23.0 ± 1.59 | 23.0 ± 1.22 | 22.0 ± 1.02 | 23.0 ± 0.82 | 25.0 ± 1.15 |
| Formulations | EG0 | EG1 | EG2 | EG3 | EG4 | EG5 | EG6 | EG7 |
|---|---|---|---|---|---|---|---|---|
| Color | White | White | White | White | White | White | White | White |
| Texture | Smooth | Smooth | Smooth | Smooth | Smooth | Smooth | Smooth | Smooth |
| Odor | Nutty/earthy | Floral smell | Grassy smell | Citrus /pungent | Citrus /pungent | Grassy /floral | Lemon /pungent | Citrus /pungent |
| Homogeneity | Good | Good | Good | Good | Good | Good | Good | Good |
| pH | 5.00 ± 0.03 | 4.94 ± 0.12 | 4.93 ± 0.08 | 4.95 ± 0.06 | 4.84 ± 0.14 | 4.94 ± 0.18 | 4.97 ± 0.10 | 4.81 ± 0.08 |
| Conductivity (µS/cm) | 843.3 ± 9.2 | 750 ± 32.9 | 749.7 ± 18.2 | 755.3 ± 30.1 | 774.3 ± 12.9 | 754.0 ± 0.0 | 759.3 ± 7.0 | 840.7 ± 5.9 |
| Viscosity (cP) | 29.50 ± 0.83 | 30.20 ± 0.66 | 30.80 ± 0.72 | 29.80 ± 0.42 | 30.90 ± 1.04 | 30.50 ± 0.55 | 30.70 ± 0.45 | 31.13 ± 1.25 |
| Spreadability (cm2) | 9.8 ± 0.6 | 11.8 ± 0.5 | 11.6 ± 0.8 | 12.1 ± 1.0 | 11.9 ± 1.1 | 12.4 ± 0.6 | 12.1 ± 0.2 | 12.7 ± 0.8 |
| Extrudability (g/cm2) | 133.3 ± 3.6 | 142.3 ± 1.8 | 141.3 ± 2.4 | 144.3 ± 1.6 | 148.3 ± 4.3 | 146.3 ± 2.8 | 155.3 ± 4.3 | 166.7 ± 2.3 |
| Wavenumber (cm−1) | Functional Groups/Bonds | Vibrational Modes | Likely Sources in Sample | Found In |
|---|---|---|---|---|
| 3740–3650 | O–H (free hydroxyl) | Stretching | Water, phenols in EOs | All analytes, except citronella and EG0 |
| 3600–3390 | O–H (H-bonded hydroxyl, broad); N–H | Stretching | Carbopol, hyaluronic acid, triethanolamine, water | All analytes, except argan oil and carbopol |
| 2950–2900 | C–H (CH3, CH2) | Asymmetric/symmetric stretching | Argan oil, citronella, lemongrass, tea tree EOs, aliphatic EO chains, lipid chains, alkane groups | All analytes |
| 1740–1730 | C=O (ester/carboxylic acid) | Stretching | Argan oil esters, carbopol | Citronella and argan oils |
| 1690–1620 | C=O (aldehyde, ketone, amide I); C=C (alkene) or amide I | Stretching | Citral in citronella, hyaluronic acid backbone, EO ketones, terpenes (e.g., Limonene), hyaluronic acid | All EGs except EG2 |
| 1070–1040 | C–O (alcohols, ethers) | Stretching | EO alcohols (geraniol, citronellol), carbopol ester | All analytes except EG0 and EG6 |
| 1060–1050 | C–O–C (ether)/primary alcohol | Stretching | Argan oil, citronella, lemongrass EOs | EG5, EG4, EG3, carbopol, argan oil |
| Week | Color | Homogeneity/Phase Separation | pH | Centrifugation | Viscosity (cP) |
|---|---|---|---|---|---|
| 0 | White | Homogenous | 4.81 ± 0.08 | Stable | 31.13 ± 1.25 |
| 1 | White | Homogenous | 4.81 ± 0.08 | Stable | 31.90 ± 0.82 |
| 2 | White | Homogenous | 4.81 ± 0.08 | Stable | 32.22 ± 0.64 |
| 4 | White | Homogenous | 4.81 ± 0.08 | Stable | 33.93 ± 0.73 |
| 8 | White | Homogenous | 4.81 ± 0.08 | Stable | 34.02 ± 1.04 |
| 12 | White | Homogenous | 4.81 ± 0.08 | Stable | 36.42 ± 0.88 |
| Week | Color | Homogeneity/Phase Separation | pH | Centrifugation | Viscosity (cP) |
|---|---|---|---|---|---|
| 0 | White | Homogenous | 4.81 ± 0.08 | Stable | 31.13 ± 1.25 |
| 1 | White | Homogenous | 4.81 ± 0.08 | Stable | 31.45 ± 0.82 |
| 2 | White | Homogenous | 4.81 ± 0.08 | Stable | 31.62 ± 0.64 |
| 4 | White | Homogenous | 4.81 ± 0.08 | Stable | 31.93 ± 0.73 |
| 8 | White | Homogenous | 4.81 ± 0.08 | Stable | 32.02 ± 1.04 |
| 12 | White | Homogenous | 4.81 ± 0.08 | Stable | 32.42 ± 0.88 |
| Week | Color | Homogeneity/Phase Separation | pH | Centrifugation | Viscosity (cP) |
|---|---|---|---|---|---|
| 0 | White | Homogenous | 4.81 ± 0.08 | Stable | 31.13 ± 1.25 |
| 1 | White | Homogenous | 4.81 ± 0.08 | Stable | 30.96 ± 0.63 |
| 2 | White | Homogenous | 4.81 ± 0.08 | Stable | 29.42 ± 0.92 |
| 4 | White | Homogenous | 4.81 ± 0.08 | Stable | 27.58 ± 0.46 |
| 8 | Off-white | Heterogenous | 4.81 ± 0.08 | Unstable | 24.37 ± 0.78 |
| 12 | Off-white | Heterogenous | 4.81 ± 0.08 | Unstable | 20.22 ± 0.42 |
| Formulation | ZOI (mm) 1% Tween 80 | ZOI (mm) 5% Tween 80 | ZOI (mm) 10% Tween 80 |
|---|---|---|---|
| EG0 | 6 ± 0 | 6 ± 0 | 6 ± 0 |
| EG1 | 6 ± 0 | 6 ± 0 | 6 ± 0 |
| EG2 | 15 ± 0 | 15 ± 0 | 15 ± 0 |
| EG3 | 15 ± 0 | 20 ± 0 | 25 ± 0 |
| EG4 | 20 ± 0 | 23 ± 0 | 25 ± 0 |
| EG5 | 15 ± 0 | 23 ± 0 | 25 ± 0 |
| EG6 | 20 ± 0 | 20 ± 0 | 20 ± 0 |
| EG7 | 20 ± 0 | 20 ± 0 | 20 ± 0 |
| Type | Oil (%) | Smix (%) | Water (%) |
|---|---|---|---|
| AE1 | 5 | 10 | 85 |
| AE2 | 10 | 15 | 75 |
| AE3 | 15 | 20 | 65 |
| AE4 | 20 | 30 | 50 |
| Components | G1 (%) | G2 | G3 | G4 | G5 | G6 | G7 |
|---|---|---|---|---|---|---|---|
| Carbopol 940 | 1.0 | 1.5 | 2.0 | - | 1.0 | 1.5 | 2.0 |
| Hyaluronic acid | - | - | - | 1.0 | 1.0 | 1.0 | 1.0 |
| Germall™ plus | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Deionized water up to | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Components | Unloaded EG0 (%) | EG1 (%) | EG2 (%) | EG3 (%) | EG4 (%) | EG5 (%) | EG6 (%) | EG7 (%) |
|---|---|---|---|---|---|---|---|---|
| Citronella essential oil | - | 0.08 | - | - | 0.08 | - | 0.08 | 0.08 |
| Tea tree essential oil | - | - | 0.16 | - | 0.16 | 0.16 | - | 0.16 |
| Lemongrass essential oil | - | - | - | 0.63 | - | 0.63 | 0.63 | 0.63 |
| Argan oil | 10.0 | 9.92 | 9.84 | 9.37 | 9.76 | 9.21 | 9.29 | 9.13 |
| Carbopol 940 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Hyaluronic acid | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Germal plus | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Triethanolamine | qs | qs | qs | qs | qs | qs | qs | qs |
| Deionized water up to | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kola-Mustapha, A.T.; Raji, M.A.; Alzahrani, Y.A.; Binsaeed, N.H.; Adam, D.R.; Shameh, R.A.; Garaween, N.M.; Garaween, G. Formulation, Optimization, and Comprehensive Characterization of Topical Essential Oil-Loaded Anti-Acne Microemulgels. Gels 2025, 11, 612. https://doi.org/10.3390/gels11080612
Kola-Mustapha AT, Raji MA, Alzahrani YA, Binsaeed NH, Adam DR, Shameh RA, Garaween NM, Garaween G. Formulation, Optimization, and Comprehensive Characterization of Topical Essential Oil-Loaded Anti-Acne Microemulgels. Gels. 2025; 11(8):612. https://doi.org/10.3390/gels11080612
Chicago/Turabian StyleKola-Mustapha, Adeola Tawakalitu, Muhabat Adeola Raji, Yusra Abdulkarim Alzahrani, Noura Hatim Binsaeed, Doaa Rashed Adam, Ranim Abou Shameh, Noureldeen Mohammed Garaween, and Ghada Garaween. 2025. "Formulation, Optimization, and Comprehensive Characterization of Topical Essential Oil-Loaded Anti-Acne Microemulgels" Gels 11, no. 8: 612. https://doi.org/10.3390/gels11080612
APA StyleKola-Mustapha, A. T., Raji, M. A., Alzahrani, Y. A., Binsaeed, N. H., Adam, D. R., Shameh, R. A., Garaween, N. M., & Garaween, G. (2025). Formulation, Optimization, and Comprehensive Characterization of Topical Essential Oil-Loaded Anti-Acne Microemulgels. Gels, 11(8), 612. https://doi.org/10.3390/gels11080612







