Chitosan–Glycerol Injectable Hydrogel for Intratumoral Delivery of Macromolecules
Abstract
1. Introduction
2. Results and Discussion
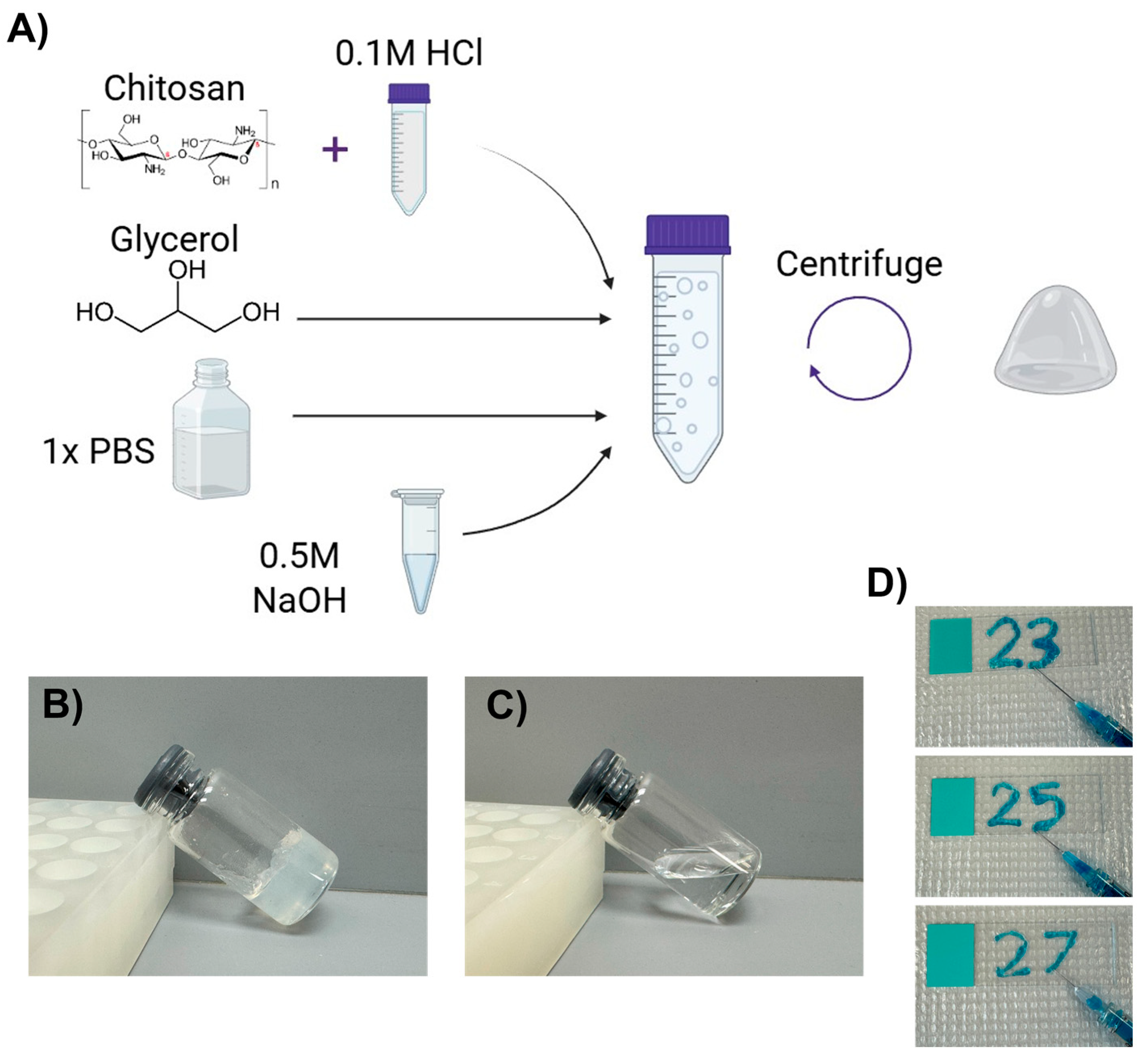
2.1. A Novel Chitosan–Glycerol Hydrogel Is Created and Parameterized
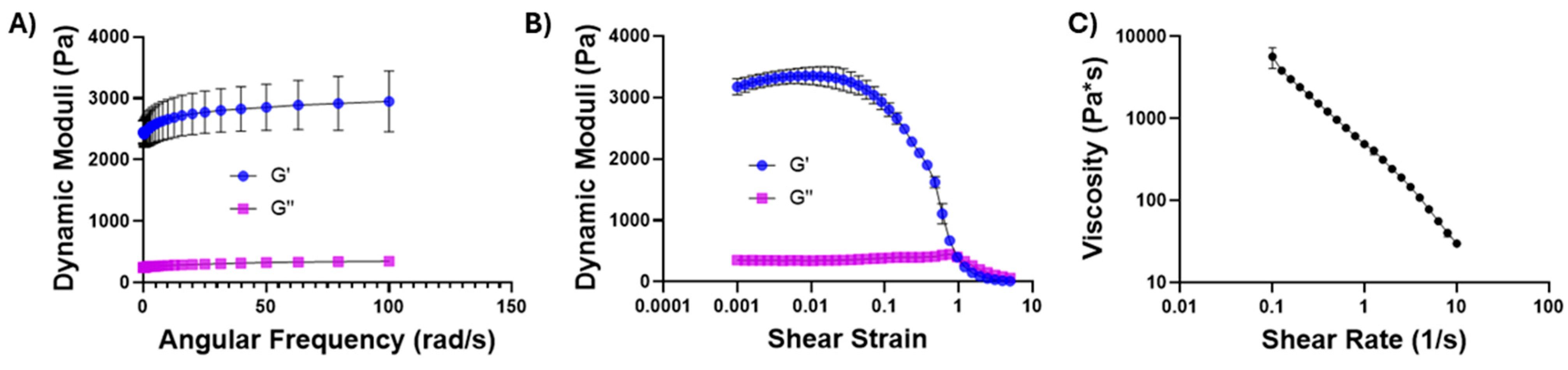
2.2. Chitosan–Glycerol Hydrogel Displays Shear-Thinning Behavior
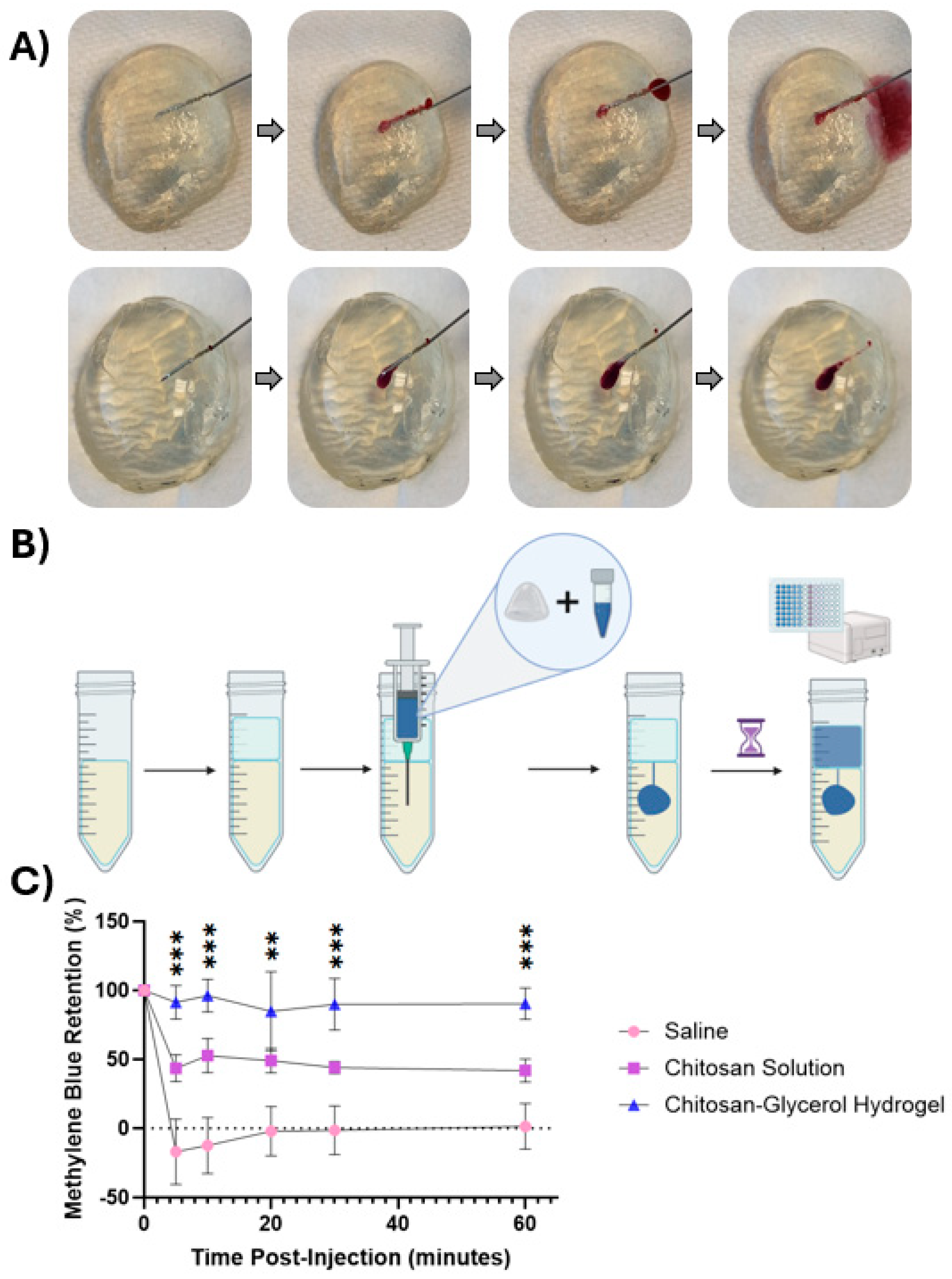
2.3. Chitosan–Glycerol Hydrogel Resists Leakage from Injection Site
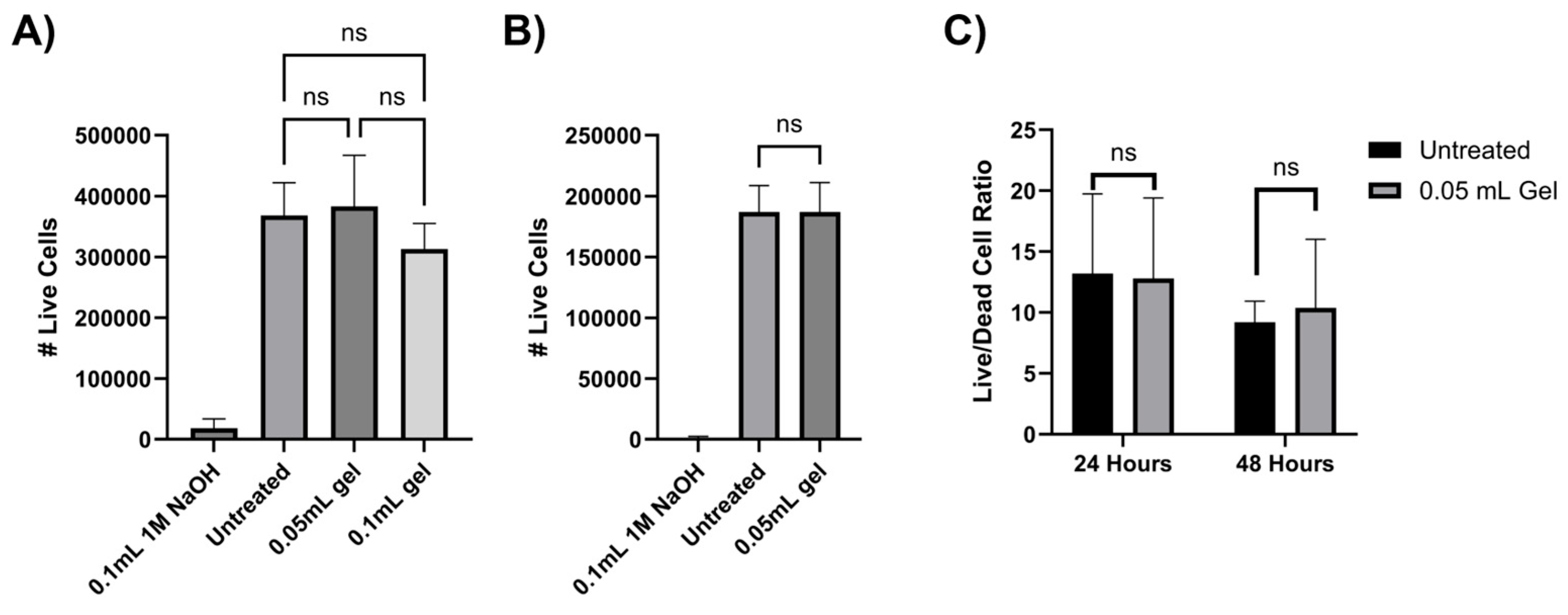
2.4. Chitosan–Glycerol Hydrogel Is Nontoxic
2.5. Hydrogel Displays Varied Release Profiles by Molecular Weight and Saturation
3. Conclusions
4. Materials and Methods
4.1. Materials and Reagents
4.2. Hydrogel Creation
4.3. Hydrogel Parameterization Testing
4.4. Mechanical Property Characterization
4.5. Injection Retention Testing
4.6. Cell Viability for Biocompatibility
4.7. Release Profiles
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keam, S.; Turner, N.; Kugeratski, F.G.; Rico, R.; Colunga-Minutti, J.; Poojary, R.; Alekseev, S.; Patel, A.B.; Li, Y.J.; Sheshadri, A.; et al. Toxicity in the era of immune checkpoint inhibitor therapy. Front. Immunol. 2024, 15, 1447021. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.J.; Weber, J.S. Immune-related toxicities of checkpoint inhibitors: Mechanisms and mitigation strategies. Nat. Rev. Drug Discov. 2022, 21, 495–508. [Google Scholar] [CrossRef]
- Melero, I.; Castanon, E.; Alvarez, M.; Champiat, S.; Marabelle, A. Intratumoural administration and tumour tissue targeting of cancer immunotherapies. Nat. Rev. Clin. Oncol. 2021, 18, 558–576. [Google Scholar] [CrossRef]
- Chang, H.-P.; Le, H.K.; Shah, D.K. Pharmacokinetics and Pharmacodynamics of Antibody-Drug Conjugates Administered via Subcutaneous and Intratumoral Routes. Pharmaceutics 2023, 15, 1132. [Google Scholar] [CrossRef]
- Andtbacka, R.H.I.; Ross, M.; Puzanov, I.; Milhem, M.; Collichio, F.; Delman, K.A.; Amatruda, T.; Zager, J.S.; Cranmer, L.; Hsueh, E.; et al. Patterns of Clinical Response with Talimogene Laherparepvec (T-VEC) in Patients with Melanoma Treated in the OPTiM Phase III Clinical Trial. Ann. Surg. Oncol. 2016, 23, 4169–4177. [Google Scholar] [CrossRef]
- Zaharoff, D.A.; Hance, K.W.; Rogers, C.J.; Schlom, J.; Greiner, J.W. Intratumoral immunotherapy of established solid tumors with chitosan/IL-12. J. Immunother. 2010, 33, 697–705. [Google Scholar] [CrossRef]
- Francis, D.M.; Manspeaker, M.P.; Schudel, A.; Sestito, L.F.; O’mElia, M.J.; Kissick, H.T.; Pollack, B.P.; Waller, E.K.; Thomas, S.N. Blockade of immune checkpoints in lymph nodes through locoregional delivery augments cancer immunotherapy. Sci. Transl. Med. 2020, 12, eaay3575. [Google Scholar] [CrossRef]
- Johnson, E.E.; Lum, H.D.; Rakhmilevich, A.L.; Schmidt, B.E.; Furlong, M.; Buhtoiarov, I.N.; Hank, J.A.; Raubitschek, A.; Colcher, D.; Reisfeld, R.A.; et al. Intratumoral immunocytokine treatment results in enhanced antitumor effects. Cancer Immunol. Immunother. 2008, 57, 1891–1902. [Google Scholar] [CrossRef]
- Hong, W.X.; Haebe, S.; Lee, A.S.; Westphalen, C.B.; Norton, J.A.; Jiang, W.; Levy, R. Intratumoral Immunotherapy for Early-stage Solid Tumors. Clin. Cancer Res. 2020, 26, 3091–3099. [Google Scholar] [CrossRef] [PubMed]
- Mori, V.; DuComb, E.A.; Wagner, S.; Khan, F.; Bates, J.H.T.; Kinsey, C.M. Visualizing intratumoral injections in lung tumors by endobronchial ultrasound. J. Cancer 2023, 14, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Sheth, R.A.; Murthy, R.; Hong, D.S.; Patel, S.; Overman, M.J.; Diab, A.; Hwu, P.; Tam, A. Assessment of Image-Guided Intratumoral Delivery of Immunotherapeutics in Patients with Cancer. JAMA Netw. Open 2020, 3, e207911. [Google Scholar] [CrossRef] [PubMed]
- Munoz, N.M.; Williams, M.; Dixon, K.; Dupuis, C.; McWatters, A.; Avritscher, R.; Manrique, S.Z.; McHugh, K.; Murthy, R.; Tam, A.; et al. Influence of injection technique, drug formulation and tumor microenvironment on intratumoral immunotherapy delivery and efficacy. J. Immunother. Cancer 2021, 9, e001800. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Nafo, W. Hydrogel biomaterials for drug delivery: Mechanisms, design, and Drugs. Hydrogels Tradit. Innov. Platf. Mult. Appl. 2023. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, X.; Wu, Y.; Gao, J. Locally injectable hydrogels for tumor immunotherapy. Gels 2021, 7, 224. [Google Scholar] [CrossRef]
- Far, B.F.; Isfahani, A.A.; Nasiriyan, E.; Pourmolaei, A.; Mahmoudvand, G.; Rouzbahani, A.K.; Amin, M.N.; Naimi-Jamal, M.R. An updated review on advances in hydrogel-based nanoparticles for liver cancer treatment. Livers 2023, 3, 161–189. [Google Scholar] [CrossRef]
- Grosskopf, A.K.; Labanieh, L.; Klysz, D.D.; Roth, G.A.; Xu, P.; Adebowale, O.; Gale, E.C.; Jons, C.K.; Klich, J.H.; Yan, J.; et al. Delivery of car-T cells in a transient injectable stimulatory hydrogel niche improves treatment of solid tumors. Sci. Adv. 2022, 8, eabn8264. [Google Scholar] [CrossRef]
- Erfani, A.; Diaz, A.E.; Doyle, P.S. Hydrogel-enabled, Local Administration and combinatorial delivery of immunotherapies for cancer treatment. Mater. Today 2023, 65, 227–243. [Google Scholar] [CrossRef]
- Zheng, H.; Li, M.; Wu, L.; Liu, W.; Liu, Y.; Gao, J.; Lu, Z. Progress in the application of hydrogels in immunotherapy of gastrointestinal tumors. Drug Deliv. 2023, 30, 2161670. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Geng, Y.; Yue, B.; Lo, P.-C.; Huang, J.; Jin, H. Injectable hydrogel as a unique platform for antitumor therapy targeting immunosuppressive tumor microenvironment. Front. Immunol. 2022, 12, 832942. [Google Scholar] [CrossRef]
- Clegg, J.R.; Adebowale, K.; Zhao, Z.; Mitragotri, S. Hydrogels in the clinic: An update. Bioeng. Transl. Med. 2024, 9, e10680. [Google Scholar] [CrossRef]
- Choi, H.; Choi, W.-S.; Jeong, J.-O. A Review of Advanced Hydrogel Applications for Tissue Engineering and Drug Delivery Systems as Biomaterials. Gels 2024, 10, 693. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, F.; Zeng, Y.; Lin, L.; Yu, H.; Zhang, S.; Yang, W. Hydrogel systems for spatiotemporal controlled delivery of immunomodulators: Engineering the tumor immune microenvironment for enhanced cancer immunotherapy. Front. Cell Dev. Biol. 2024, 12, 1514595. [Google Scholar] [CrossRef] [PubMed]
- Sepe, F.; Valentino, A.; Marcolongo, L.; Petillo, O.; Conte, R.; Margarucci, S.; Peluso, G.; Calarco, A. Marine-Derived Polysaccharide Hydrogels as Delivery Platforms for Natural Bioactive Compounds. Int. J. Mol. Sci. 2025, 26, 764. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An overview of its properties and applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan based hydrogels: Characteristics and pharmaceutical applications. Res. Pharm. Sci. 2015, 10, 1–16. [Google Scholar]
- Li, Y.; Zhang, Y.; Wei, Y.; Tao, L. Preparation of Chitosan-based Injectable Hydrogels and Its Application in 3D Cell Culture. J. Vis. Exp. 2017, 127, 56253. [Google Scholar]
- Shariatinia, Z.; Jalali, A.M. Chitosan-based hydrogels: Preparation, properties and applications. Int. J. Biol. Macromol. 2018, 115, 194–220. [Google Scholar] [CrossRef] [PubMed]
- Zaharoff, D.A.; Rogers, C.J.; Hance, K.W.; Schlom, J.; Greiner, J.W. Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination. Vaccine 2007, 25, 2085–2094. [Google Scholar] [CrossRef]
- Koppolu, B.P.; Smith, S.G.; Ravindranathan, S.; Jayanthi, S.; Kumar, T.K.S.; Zaharoff, D.A. Controlling chitosan-based encapsulation for protein and vaccine delivery. Biomaterials 2014, 35, 4382–4389. [Google Scholar] [CrossRef]
- Ogawa, K.; Yui, T.; Okuyama, K. Three D structures of chitosan. Int. J. Bio. Macromol. 2004, 34, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chilakamarry, C.R.; Sakinah, A.M.M.; Zularisam, A.W.; Pandey, A. Glycerol waste to value added products and its potential applications. Syst. Microbiol. Biomanuf. 2021, 1, 378–396. [Google Scholar] [CrossRef]
- Tarique, J.; Sapuan, S.M.; Khalina, A. Effect of glycerol plasticizer loading on the physical, mechanical, thermal, and barrier properties of arrowroot (Maranta arundinacea) starch biopolymers. Sci. Rep. 2021, 11, 13900. [Google Scholar] [CrossRef]
- Hamano, T.; Mitsuhashi, Y.; Aoki, N.; Yamamoto, S.; Tsuji, S.; Ito, Y.; Oji, Y. Determination of glycerol in foods by high-performance liquid chromatography with Fluorescence Detection. J. Chromatogr. A 1991, 541, 265–272. [Google Scholar] [CrossRef]
- Stout, E.I.; McKessor, A. Glycerin-Based Hydrogel for Infection Control. Adv. Wound Care 2012, 1, 48–51. [Google Scholar] [CrossRef]
- Samadi, A.; Azandeh, S.; Orazizadeh, M.; Bayati, V.; Rafienia, M.; Karami, M.A. Fabrication and Characterization of Glycerol/Chitosan/Polyvinyl Alcohol-Based Transparent Hydrogel Films Loaded with Silver Nanoparticles for Antibacterial Wound Dressing Applications. Adv. Biomed. Res. 2021, 10, 4. [Google Scholar] [CrossRef]
- Domján, A.; Bajdik, J.; Pintye-Hódi, K. Understanding of the Plasticizing Effects of Glycerol and PEG 400 on Chitosan Films Using Solid-State NMR Spectroscopy. Macromolecules 2009, 42, 4667–4673. [Google Scholar] [CrossRef]
- Uman, S.; Dhand, A.; Burdick, J.A. Recent advances in shear-thinning and self-healing hydrogels for biomedical applications. J. Appl. Polym. Sci. 2019, 137, 48668. [Google Scholar] [CrossRef]
- Guo, M.; Wu, Y.; Xue, S.; Xia, Y.; Yang, X.; Dzenis, Y.; Li, Z.; Lei, W.; Smith, A.T.; Sun, L. A highly stretchable, ultra-tough, remarkably tolerant, and robust self-healing glycerol-hydrogel for a dual-responsive soft actuator. J. Mater. Chem. A 2019, 7, 25969–25977. [Google Scholar] [CrossRef]
- Ramesan, V.S.; Jain, S. Chitosan-glycerol gel as barrier formulation for metal allergy. ACS Omega 2019, 4, 5900–5903. [Google Scholar] [CrossRef]
- Debandi, M.V.; Bernal, C.R.; Francois, N. Development of Biodegradable Films Based on Chitosan/Glycerol Blends Suitable for Biomedical Applications. J. Tissue Sci. Eng. 2016, 7, 1–9. [Google Scholar]
- Epure, V.; Griffon, M.; Pollet, E.; Avérous, L. Structure and properties of glycerol-plasticized chitosan obtained by mechanical kneading. Carbohydr. Polym. 2011, 83, 947–952. [Google Scholar] [CrossRef]
- Kobrin, R.L.; Mantooth, S.M.; Zaharoff, D.A. Abstract 3188: Chitosan-glycerol injectable gel for intratumoral retention of immunotherapeutics. Cancer Res. 2024, 84 (Suppl. S6), 3188. [Google Scholar] [CrossRef]
- Bhuiyan, M.H.; Clarkson, A.N.; Ali, M.A. Optimization of thermoresponsive chitosan/β-glycerophosphate hydrogels for injectable neural tissue engineering application. Colloids Surf. B Biointerfaces 2023, 224, 113193. [Google Scholar] [CrossRef]
- Saravanan, S.; Vimalraj, S.; Thanikaivelan, P.; Banudevi, S.; Manivasagam, G. A review on injectable chitosan/beta glycerophosphate hydrogels for bone tissue regeneration. Int. J. Biol. Macromol. 2019, 121, 38–54. [Google Scholar] [CrossRef]
- Qian, J.; Wang, X.; Chen, Y.; Mo, C.; Liang, C.; Guo, H. The correlation of molecule weight of chitosan oligomers with the corresponding viscosity and antibacterial activity. Carbohydr. Res. 2023, 530, 108860. [Google Scholar] [CrossRef]
- Karydis-Messinis, A.; Moschovas, D.; Markou, M.; Gkantzou, E.; Vasileiadis, A.; Tsirka, K.; Gioti, C.; Vasilopoulos, K.C.; Bagli, E.; Murphy, C.; et al. Development, physicochemical characterization and in vitro evaluation of chitosan-fish gelatin-glycerol hydrogel membranes for wound treatment applications. Carbohydr. Polym. Technol. Appl. 2023, 6, 100338. [Google Scholar] [CrossRef]
- Chircov, C.; Bejenaru, I.T.; Nicoară, A.I.; Bîrcă, A.C.; Oprea, O.C.; Tihăuan, B. Chitosan-Dextran-Glycerol Hydrogels Loaded with Iron Oxide Nanoparticles for Wound Dressing Applications. Pharmaceutics 2022, 14, 2620. [Google Scholar] [CrossRef]
- Kocak, F.Z.; Yar, M.; Rehman, I.U. Hydroxyapatite-Integrated, Heparin- and Glycerol-Functionalized Chitosan-Based Injectable Hydrogels with Improved Mechanical and Proangiogenic Performance. Int. J. Mol. Sci 2022, 23, 5370. [Google Scholar] [CrossRef]
- Mantooth, S.M.; Hancock, A.M.; Thompson, P.M.; Varghese PJ, G.; Meritet, D.M.; Vrabel, M.R.; Hu, J.; Zaharoff, D.A. Characterization of an injectable chitosan hydrogel for the tunable, localized delivery of immunotherapeutics. ACS Biomater. Sci. Eng. 2024, 10, 905–920. [Google Scholar] [CrossRef] [PubMed]
- Jalalvandi, E.; Shavandi, A. In situ-forming and ph-responsive hydrogel based on chitosan for vaginal delivery of therapeutic agents. J. Mater. Sci. Mater. Med. 2018, 29, 158. [Google Scholar] [CrossRef]
- Lee, J.S.; Nah, H.; Moon, H.-J.; Lee, S.J.; Heo, D.N.; Kwon, I.K. Controllable delivery system: A temperature and ph-responsive injectable hydrogel from Succinylated Chitosan. Appl. Surf. Sci. 2020, 528, 146812. [Google Scholar] [CrossRef]
- Bertsch, P.; Diba, M.; Mooney, D.J.; Leeuwenburgh, S.C.G. Self-Healing Injectable Hydrogels for Tissue Regeneration. Chem. Rev. 2023, 123, 834–873. [Google Scholar] [CrossRef]
- Devi, V.K.A.; Shyam, R.; Palaniappan, A.; Jaiswal, A.K.; Oh, T.-H.; Nathanael, A.J. Self-Healing Hydrogels: Preparation, Mechanism and Advancement in Biomedical Applications. Polymers 2021, 13, 3782. [Google Scholar] [CrossRef]
- Qin, J.; Sun, M.; Hu, W.; Cheng, J.; Fan, Z.; Du, J. Stimuli-responsive hydrogels for cancer immunotherapy. Polym. Chem. 2023, 14, 793–802. [Google Scholar] [CrossRef]
- Andrade, F.; Roca-Melendres, M.M.; Durán-Lara, E.F.; Rafael, D.; Schwartz, S., Jr. Stimuli-Responsive Hydrogels for Cancer Treatment: The Role of pH, Light, Ionic Strength and Magnetic Field. Cancers 2021, 13, 1164. [Google Scholar] [CrossRef] [PubMed]
- Costantini, C.; Bellet, M.M.; Pariano, M.; Renga, G.; Stincardini, C.; Goldstein, A.L.; Garaci, E.; Romani, L. A Reappraisal of Thymosin Alpha1 in Cancer Therapy. Front. Oncol. 2019, 9, 873. [Google Scholar] [CrossRef] [PubMed]
- Gmunder, H.; Lesslauer, W. A 45-KDA human t-cell membrane glycoprotein functions in the regulation of cell proliferative responses. Eur. J. Biochem. 1984, 142, 153–160. [Google Scholar] [CrossRef]
- Rybchenko, V.S.; Aliev, T.K.; Panina, A.A.; Kirpichnikov, M.P.; Dolgikh, D.A. Targeted Cytokine Delivery for Cancer Treatment: Engineering and Biological Effects. Pharmaceutics 2023, 15, 336. [Google Scholar] [CrossRef]
- Kang, J.; Sun, T.; Zhang, Y. Immunotherapeutic progress and application of bispecific antibody in cancer. Front. Immunol. 2022, 13, 1020003. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, A.; Georgiou, L.; Christodoulou, T.; Panayioyou, N.; Loannides, C.; Zamboglou, N.; Damianou, C. MR relaxation times of agar-based tissue-mimicking phantoms. J. Appl. Clin. Med. Phys. 2022, 23, e13533. [Google Scholar] [CrossRef] [PubMed]
- Sofokleous, P.; Damianou, C. High-quality Agar and Polyacrylamide Tumor-mimicking Phantom Models for Magnetic Resonance-guided Focused Ultrasound Applications. J. Med. Ultrasound 2023, 32, 121–133. [Google Scholar] [CrossRef]
- Kim, M.; Im, S.; Park, I.; Kim, D.; Kim, E.S.; Joseph, J.; Yoon, J. Fabrication of agar-based tissue-mimicking phantom for the technical evaluation of biomedical optical imaging systems. Curr. Appl. Phys. 2024, 61, 80–85. [Google Scholar] [CrossRef]
- Elisei, R.C.; Grauar, F.; Szold, A.; Melzer, A.; Moldovan, C.V.; Motrescu, M.; Mois, E.; Popa, C.; Pisla, D.; Vasida, C.; et al. Gelatin-Based Liver Phantoms for Training Purposes: A Cookbook Approach. J. Clin. Med. 2024, 13, 3440. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.A.; Van Berckel, P.V.; Lai, M.; Dankelman, J.; Hendriks, B.H.W. Tissue-mimicking phantom materials with tunable optical properties suitable for assessment of diffuse reflectance spectroscopy during electrosurgery. Biomed. Opt. Express 2022, 13, 2616–2643. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobrin, R.L.; Mantooth, S.M.; Mulry, A.L.; Zaharoff, D.J.; Zaharoff, D.A. Chitosan–Glycerol Injectable Hydrogel for Intratumoral Delivery of Macromolecules. Gels 2025, 11, 607. https://doi.org/10.3390/gels11080607
Kobrin RL, Mantooth SM, Mulry AL, Zaharoff DJ, Zaharoff DA. Chitosan–Glycerol Injectable Hydrogel for Intratumoral Delivery of Macromolecules. Gels. 2025; 11(8):607. https://doi.org/10.3390/gels11080607
Chicago/Turabian StyleKobrin, Robert L., Siena M. Mantooth, Abigail L. Mulry, Desmond J. Zaharoff, and David A. Zaharoff. 2025. "Chitosan–Glycerol Injectable Hydrogel for Intratumoral Delivery of Macromolecules" Gels 11, no. 8: 607. https://doi.org/10.3390/gels11080607
APA StyleKobrin, R. L., Mantooth, S. M., Mulry, A. L., Zaharoff, D. J., & Zaharoff, D. A. (2025). Chitosan–Glycerol Injectable Hydrogel for Intratumoral Delivery of Macromolecules. Gels, 11(8), 607. https://doi.org/10.3390/gels11080607







