Antibacterial Composites Based on Alginate/Egg White and ZnO Nanoparticles with the Addition of Essential Oils
Abstract
1. Introduction
2. Results and Discussion
2.1. ZnO Nanoparticles
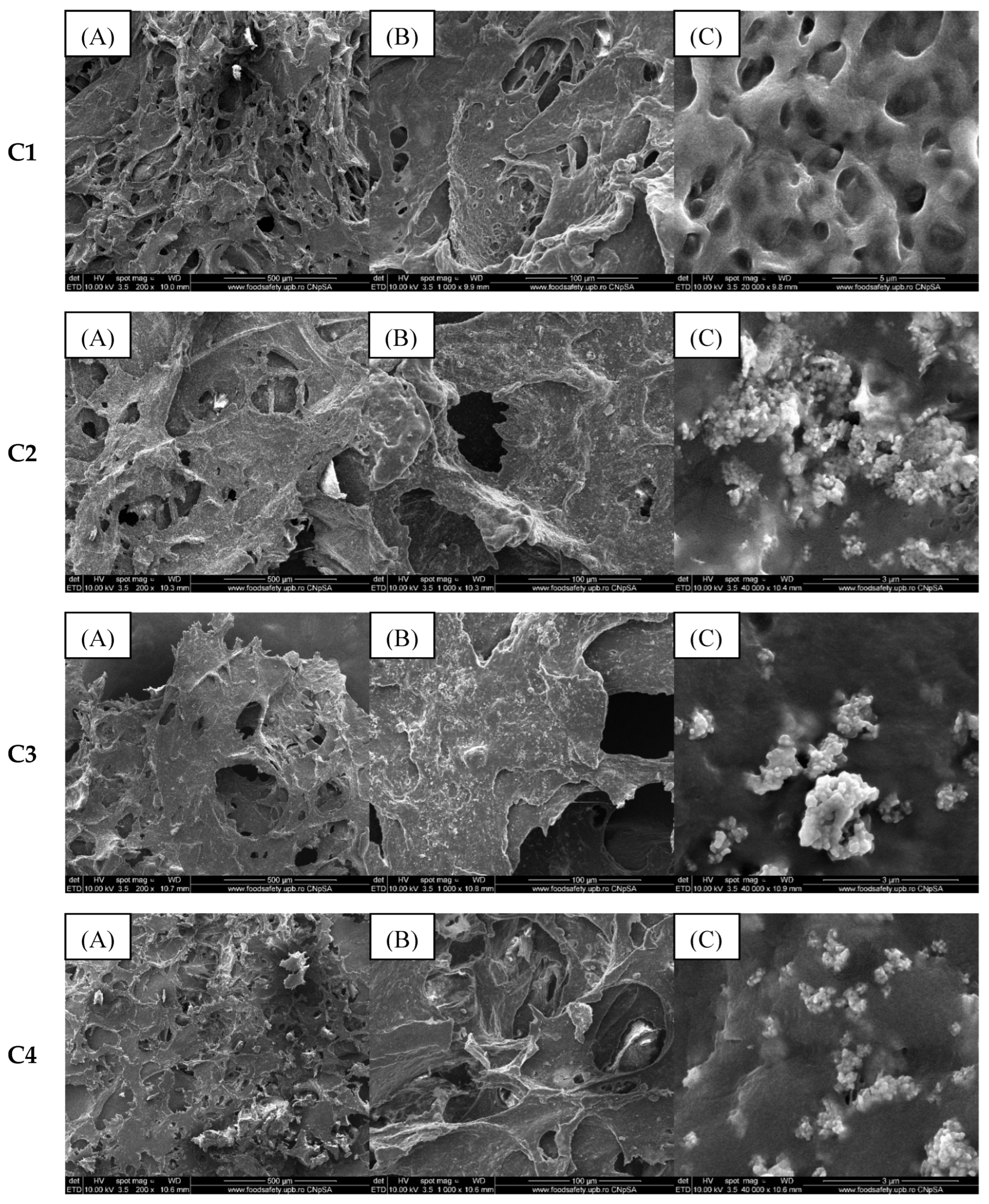
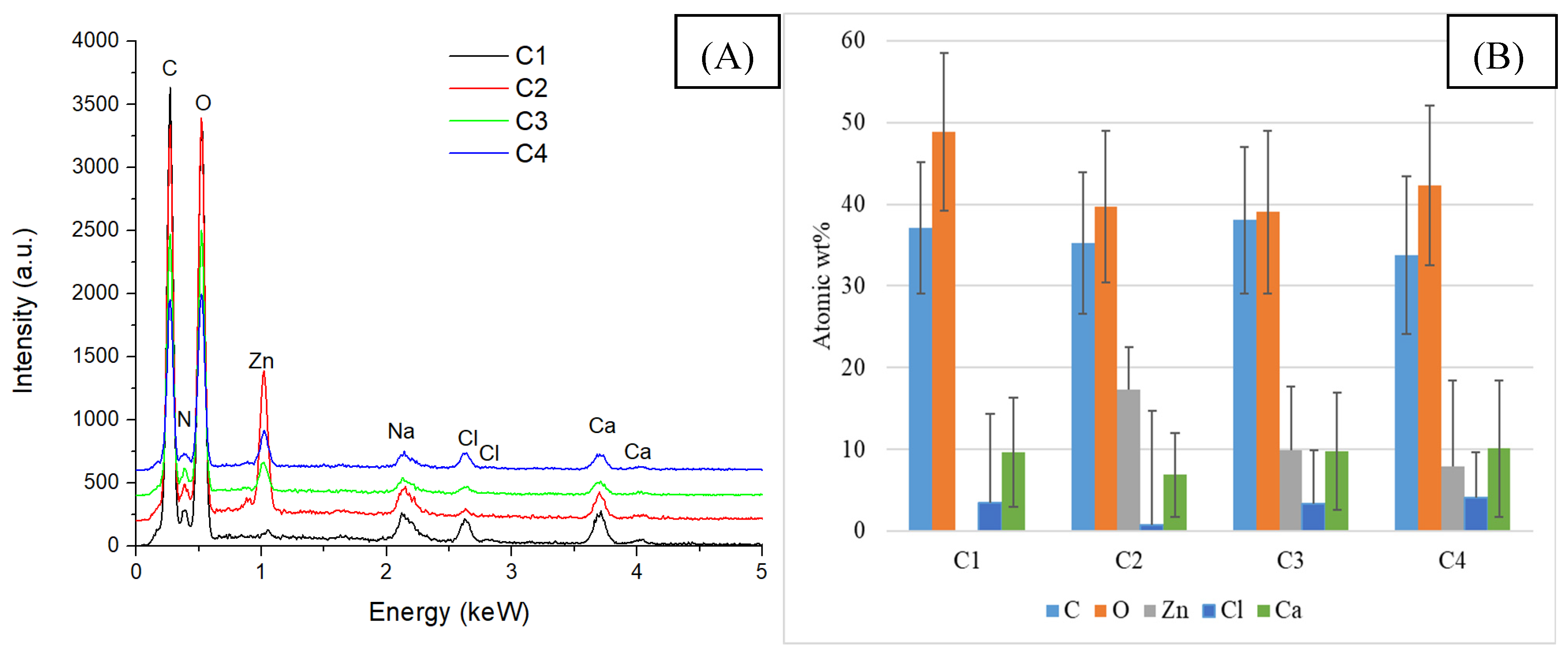
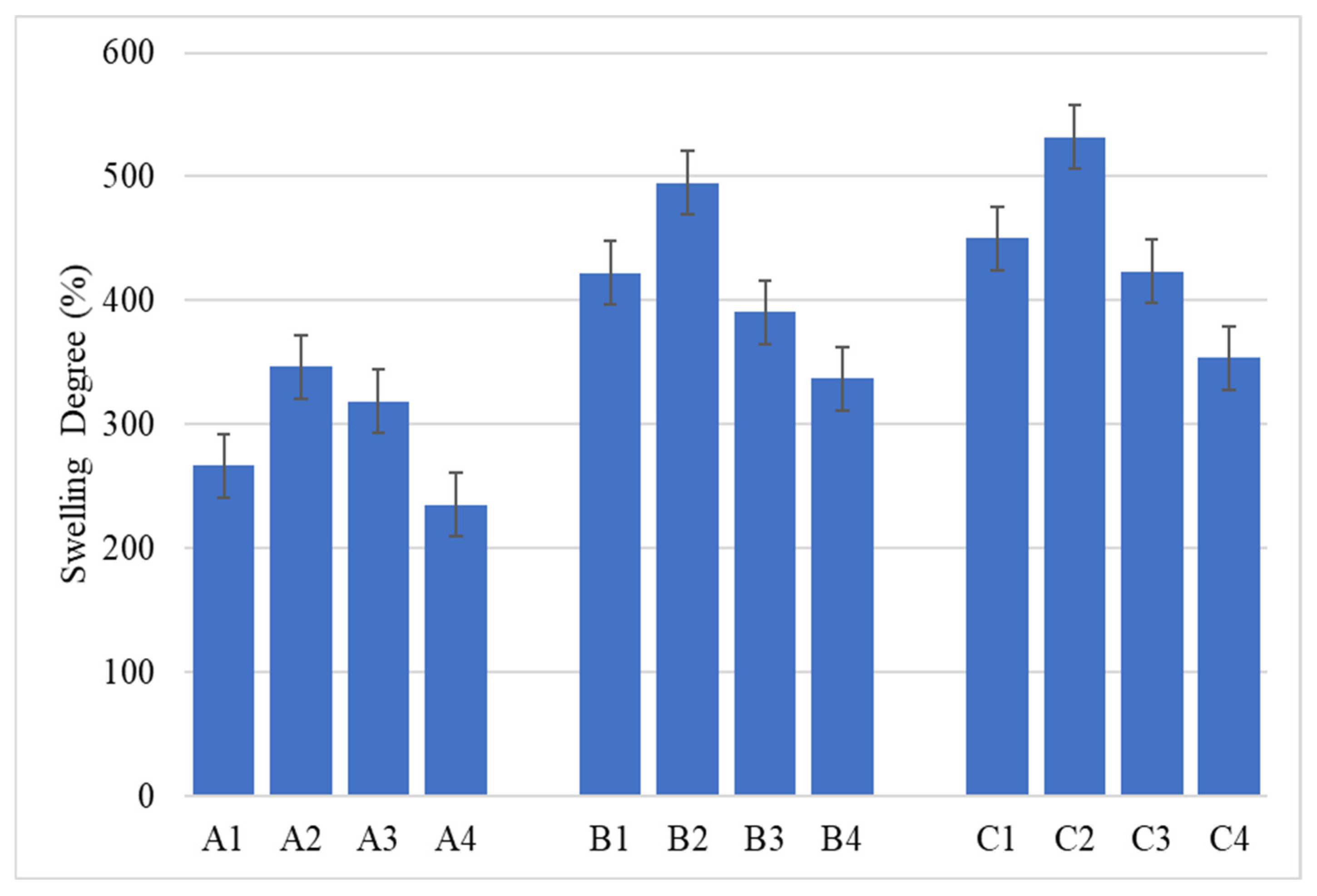
2.2. Alginate/Egg White–ZnO Composite
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Experimental Procedure
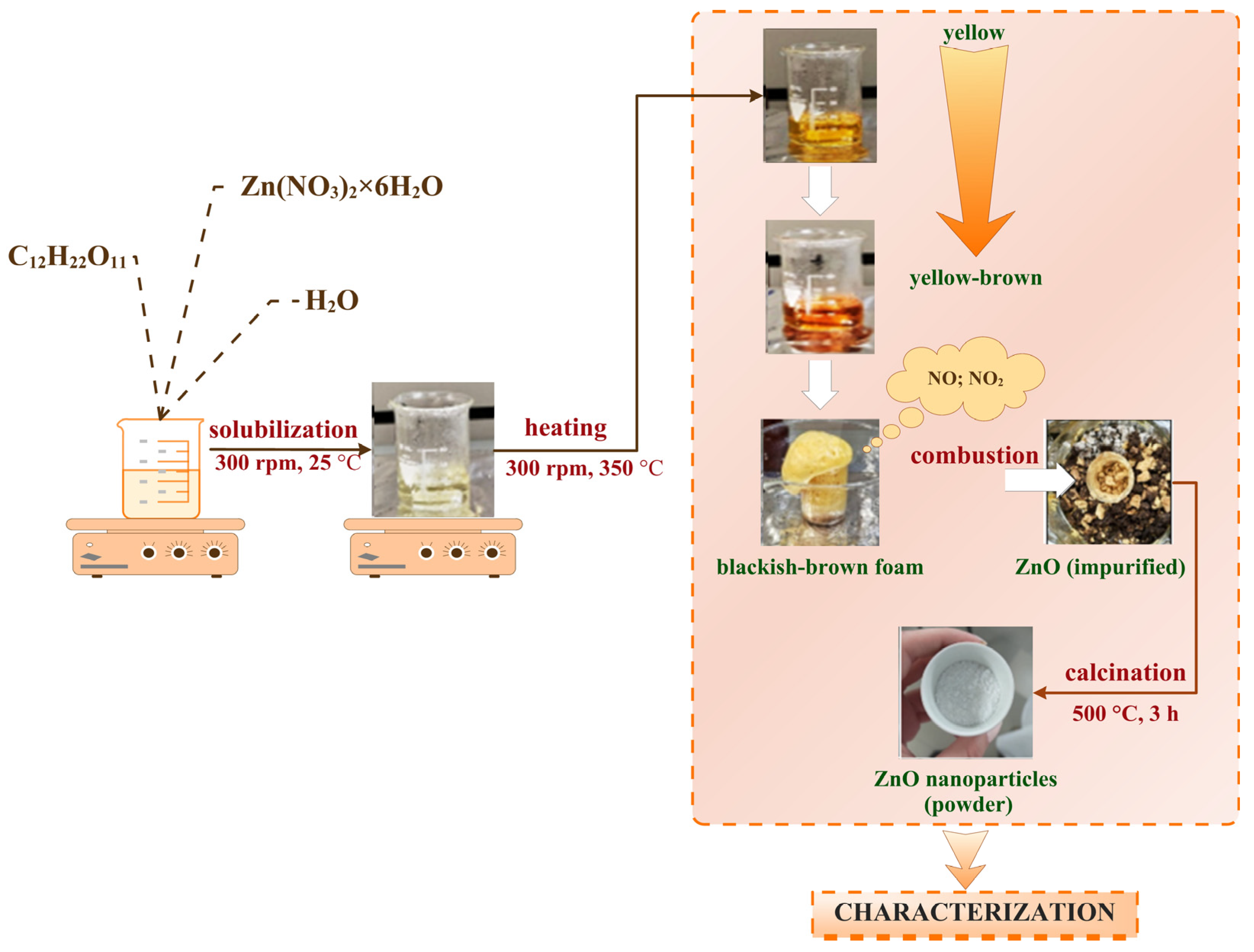
4.2.1. Synthesis of ZnO Nanoparticles
4.2.2. Development of Alginate/Egg White–Zinc Oxide Composite
4.3. Characterization Methods
4.4. Microbiological Activity
4.5. Cell Viability Determination
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bueno, J.; Demirci, F.; Baser, K.H.C. Antimicrobial Strategies in Novel Drug Delivery Systems. In The Microbiology of Skin, Soft Tissue, Bone and Joint Infections; Elsevier: Amsterdam, The Netherlands, 2017; pp. 271–286. [Google Scholar] [CrossRef]
- Linz, M.S.; Mattappallil, A.; Finkel, D.; Parker, D. Clinical Impact of Staphylococcus aureus Skin and Soft Tissue Infections. Antibiotics 2023, 12, 557. [Google Scholar] [CrossRef] [PubMed]
- Mihai, M.M.; Preda, M.; Lungu, I.; Gestal, M.C.; Popa, M.I.; Holban, A.M. Nanocoatings for Chronic Wound Repair—Modulation of Microbial Colonization and Biofilm Formation. Int. J. Mol. Sci. 2018, 19, 1179. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, M.; Niranjan, R.; Thangam, R.; Madhan, B.; Pandiyarasan, V.; Ramachandran, C.; Oh, D.-H.; Venkatasubbu, G.D. Investigations on the antimicrobial activity and wound healing potential of ZnO nanoparticles. Appl. Surf. Sci. 2019, 479, 1169–1177. [Google Scholar] [CrossRef]
- Bessa, L.J.; Fazii, P.; Di Giulio, M.; Cellini, L. Bacterial isolates from infected wounds and their antibiotic susceptibility pattern: Some remarks about wound infection. Int. Wound J. 2015, 12, 47–52. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Joorabloo, A.; Adeli, H.; Milan, P.B.; Amoupour, M. Enhanced antimicrobial and full-thickness wound healing efficiency of hydrogels loaded with heparinized ZnO nanoparticles: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2021, 166, 200–212. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Title 21—Food and Drugs, Chapter I—Food and Drug Administration Department of Health and Human Services, Subchapter B—Food for Human Consumption, Part 182 Substances Generally Recognized as Safe; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2024. [Google Scholar]
- Spoială, A.; Ilie, C.-I.; Trușcă, R.-D.; Oprea, O.-C.; Surdu, V.-A.; Vasile, B.Ș.; Ficai, A.; Ficai, D.; Andronescu, E.; Dițu, L.-M. Zinc Oxide Nanoparticles for Water Purification. Materials 2021, 14, 4747. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.-E.; Jin, H.-E. Antimicrobial Activity of Zinc Oxide Nano/Microparticles and Their Combinations against Pathogenic Microorganisms for Biomedical Applications: From Physicochemical Characteristics to Pharmacological Aspects. Nanomaterials 2021, 11, 263. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial Properties of ZnO Nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Rahman, A.U.; Tajuddin; Husen, A. Properties of Zinc Oxide Nanoparticles and Their Activity Against Microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef]
- Stoica, A.E.; Bîrcă, A.C.; Pițigoi, M.L.; Grumezescu, A.M.; Vasile, B.Ș.; Iordache, F.; Ficai, A.; Andronescu, E. Nanostructured Zinc Oxide for Wound Dressings. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2024, 86, 161–174. [Google Scholar]
- Zudyte, B.; Luksiene, Z. Visible light-activated ZnO nanoparticles for microbial control of wheat crop. J. Photochem. Photobiol. B 2021, 219, 112206. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Han, Y.; Cui, M.; Tey, H.L.; Wang, L.; Xu, C. ZnO nanoparticles as an antimicrobial tissue adhesive for skin wound closure. J. Mater. Chem. B 2017, 5, 4535–4541. [Google Scholar] [CrossRef] [PubMed]
- Rayyif, S.M.I.; Mohammed, H.B.; Curuțiu, C.; Bîrcă, A.C.; Grumezescu, A.M.; Vasile, B.Ș.; Dițu, L.M.; Lazăr, V.; Chifiriuc, M.C.; Mihăescu, G.; et al. ZnO Nanoparticles-Modified Dressings to Inhibit Wound Pathogens. Materials 2021, 14, 3084. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Espitia, P.J.P.; Otoni, C.G.; Soares, N.F.F. Zinc Oxide Nanoparticles for Food Packaging Applications. In Antimicrobial Food Packaging; Elsevier: Amsterdam, The Netherlands, 2016; pp. 425–431. [Google Scholar] [CrossRef]
- Pushpalatha, C.; Suresh, J.; Gayathri, V.; Sowmya, S.; Augustine, D.; Alamoudi, A.; Zidane, B.; Albar, N.H.M.; Patil, S. Zinc Oxide Nanoparticles: A Review on Its Applications in Dentistry. Front. Bioeng. Biotechnol. 2022, 10, 917990. [Google Scholar] [CrossRef]
- Hatamie, A.; Khan, A.; Golabi, M.; Turner, A.P.F.; Beni, V.; Mak, W.C.; Sadollahkhani, A.; Alnoor, H.; Zargar, B.; Bano, S.; et al. Zinc Oxide Nanostructure-Modified Textile and Its Application to Biosensing, Photocatalysis, and as Antibacterial Material. Langmuir 2015, 31, 10913–10921. [Google Scholar] [CrossRef]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; de Moraes Ruy Sapata, V.; Lopes, P.R.M.; de Moraes, P.B.; Montagnolli, R.N.; Ferreira, H.; Bidoia, E.D. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar] [CrossRef]
- Álvarez-Chimal, R.; García-Pérez, V.I.; Álvarez-Pérez, M.A.; Tavera-Hernández, R.; Reyes-Carmona, L.; Martínez-Hernández, M.; Arenas-Alatorre, J.Á. Influence of the particle size on the antibacterial activity of green synthesized zinc oxide nanoparticles using Dysphania ambrosioides extract, supported by molecular docking analysis. Arab. J. Chem. 2022, 15, 103804. [Google Scholar] [CrossRef]
- Asadpoor, M.; Ithakisiou, G.-N.; van Putten, J.P.M.; Pieters, R.J.; Folkerts, G.; Braber, S. Antimicrobial Activities of Alginate and Chitosan Oligosaccharides Against Staphylococcus aureus and Group B Streptococcus. Front. Microbiol. 2021, 12, 700605. [Google Scholar] [CrossRef]
- Sayin, S.; Depci, T.; Naz, M.; Sezer, S.; Karaaslan, M.G. Characterization and evaluation of the antimicrobial properties of algal alginate; a potential natural protective for cosmetics. J. Res. Pharm. 2022, 26, 198–209. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, X. Alginate hydrogel dressings for advanced wound management. Int. J. Biol. Macromol. 2020, 162, 1414–1428. [Google Scholar] [CrossRef] [PubMed]
- Jalili-Firoozinezhad, S.; Rajabi-Zeleti, S.; Mohammadi, P.; Gaudiello, E.; Bonakdar, S.; Solati-Hashjin, M.; Marsano, A.; Aghdami, N.; Scherberich, A.; Baharvand, H.; et al. Facile Fabrication of Egg White Macroporous Sponges for Tissue Regeneration. Adv. Healthc. Mater. 2015, 4, 2281–2290. [Google Scholar] [CrossRef]
- Aiyelabegan, H.T.; Zaidi, S.S.Z.; Fanuel, S.; Eatemadi, A.; Ebadi, M.T.K.; Sadroddiny, E. Albumin-based biomaterial for lung tissue engineering applications. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 853–861. [Google Scholar] [CrossRef]
- Jalili-Firoozinezhad, S.; Filippi, M.; Mohabatpour, F.; Letourneur, D.; Scherberich, A. Chicken egg white: Hatching of a new old biomaterial. Mater. Today 2020, 40, 193–214. [Google Scholar] [CrossRef]
- Jahani, S.; Ashrafizadeh, H.; Babai, K.; Siahpoosh, A.; Cheraghian, B. Effect of ointment-based egg white on healing of second- degree wound in burn patients: A triple-blind randomized clinical trial study. Avicenna J. Phytomed 2019, 9, 260–270. [Google Scholar] [PubMed]
- Deyno, S.; Mtewa, A.G.; Abebe, A.; Hymete, A.; Makonnen, E.; Bazira, J.; Alele, P.E. Essential oils as topical anti-infective agents: A systematic review and meta-analysis. Complement. Ther. Med. 2019, 47, 102224. [Google Scholar] [CrossRef]
- Khajavi, R.; Abbasipour, M.; Barzi, M.G.; Rashidi, A.; Rahimi, M.K.; Mirzababa, H.H. Eucalyptus Essential Oil-Doped Alginate Fibers as a Potent Antibacterial Wound Dressing. Adv. Polym. Technol. 2014, 33, 1–7. [Google Scholar] [CrossRef]
- Travassos, A.R.; Claes, L.; Boey, L.; Drieghe, J.; Goossens, A. Non-fragrance allergens in specific cosmetic products. Contact Dermat. 2011, 65, 276–285. [Google Scholar] [CrossRef] [PubMed]
- de Groot, A.C.; Schmidt, E. Chemical Composition of and Contact Allergy to Essential Oils. In Essential Oils; Routledge: London, UK, 2021; pp. 45–925. [Google Scholar] [CrossRef]
- Mori, H.-M.; Kawanami, H.; Kawahata, H.; Aoki, M. Wound healing potential of lavender oil by acceleration of granulation and wound contraction through induction of TGF-β in a rat model. BMC Complement. Altern. Med. 2016, 16, 144. [Google Scholar] [CrossRef]
- Huang, J.; You, X.; Xin, P.; Gu, Z.; Chen, C.; Wu, J. Egg white as a natural and safe biomaterial for enhanced cancer therapy. Chin. Chem. Lett. 2021, 32, 1737–1742. [Google Scholar] [CrossRef]
- M, A.M.; D, U.; Ashwin, B.M.; Yardily, A.; Dennison, M.S. Microwave-assisted green synthesized ZnO nanoparticles: An experimental and computational investigation. Discov. Appl. Sci. 2025, 7, 177. [Google Scholar] [CrossRef]
- Wang, H.; Ren, C.; Bai, W.; Tang, Z.; Lei, H.; Tian, H.; Du, G. Eucalyptus oil encapsulated within calcium-crosslinked sodium alginate for natural wood preservatives against fungi and termite. Ind. Crops Prod. 2025, 225, 120468. [Google Scholar] [CrossRef]
- Rusu, A.G.; Niță, L.E.; Roșca, I.; Croitoriu, A.; Ghilan, A.; Mititelu-Tarțău, L.; Grigoraș, A.V.; Crețu, B.E.B.; Chiriac, A.P. Alginate-Based Hydrogels Enriched with Lavender Essential Oil: Evaluation of Physicochemical Properties, Antimicrobial Activity, and In Vivo Biocompatibility. Pharmaceutics 2023, 15, 2608. [Google Scholar] [CrossRef]
- Sánchez, E.C.; García, M.T.; Pereira, J.; Oliveira, F.; Craveiro, R.; Paiva, A.; Gracia, I.; García-Vargas, J.M.; Duarte, A.R.C. Alginate–Chitosan Membranes for the Encapsulation of Lavender Essential Oil and Development of Biomedical Applications Related to Wound Healing. Molecules 2023, 28, 3689. [Google Scholar] [CrossRef] [PubMed]
- Cleetus, C.M.; Primo, F.A.; Fregoso, G.; Raveendran, N.L.; Noveron, J.C.; Spencer, C.T.; Ramana, C.V.; Joddar, B. Alginate Hydrogels with Embedded ZnO Nanoparticles for Wound Healing Therapy. Int. J. Nanomed. Vol. 2020, 15, 5097–5111. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Wang, Y.; Shi, F.; Nie, Y.; Liu, T.; Song, K. Effects of concentration variation on the physical properties of alginate-based substrates and cell behavior in culture. Int. J. Biol. Macromol. 2019, 128, 184–195. [Google Scholar] [CrossRef]
- Jayakumar, R.; Kumar, P.S.; Mohandas, A.; Lakshmanan, V.-K.; Biswas, R. Exploration of alginate hydrogel/nano zinc oxide composite bandages for infected wounds. Int. J. Nanomed. 2015, 10, 53–66. [Google Scholar] [CrossRef]
- Trandafilović, L.V.; Božanić, D.K.; Dimitrijević-Branković, S.; Luyt, A.S.; Djoković, V. Fabrication and antibacterial properties of ZnO–alginate nanocomposites. Carbohydr. Polym. 2012, 88, 263–269. [Google Scholar] [CrossRef]
- Francis, G.L. Albumin and mammalian cell culture: Implications for biotechnology applications. Cytotechnology 2010, 62, 1–16. [Google Scholar] [CrossRef]
- Cascone, M.G.; Rosellini, E.; Maltinti, S.; Baldassare, A.; Lazzeri, L. Cell laden alginate/albumin hydrogel fibers for potential skin tissue engineering applications. Biomed. Eng. 2018, 30, 1850045. [Google Scholar] [CrossRef]
- Yuan, H.; Zheng, X.; Liu, W.; Zhang, H.; Shao, J.; Yao, J.; Mao, C.; Hui, J.; Fan, D. A novel bovine serum albumin and sodium alginate hydrogel scaffold doped with hydroxyapatite nanowires for cartilage defects repair. Colloids Surf. B Biointerfaces 2020, 192, 111041. [Google Scholar] [CrossRef] [PubMed]
- Meng, R.; Zhu, H.; Deng, P.; Li, M.; Ji, Q.; He, H.; Jin, L.; Wang, B. Research progress on albumin-based hydrogels: Properties, preparation methods, types and its application for antitumor-drug delivery and tissue engineering. Front. Bioeng. Biotechnol. 2023, 11, 1137145. [Google Scholar] [CrossRef]
- Mendes, L.P.; Delgado, J.M.F.; Costa, A.D.A.; Vieira, M.S.; Benfica, P.L.; Lima, E.M.; Valadares, M.C. Biodegradable nanoparticles designed for drug delivery: The number of nanoparticles impacts on cytotoxicity. Toxicol. Vitr. 2015, 29, 1268–1274. [Google Scholar] [CrossRef] [PubMed]
- Miastkowska, M.; Kantyka, T.; Bielecka, E.; Kałucka, U.; Kamińska, M.; Kucharska, M.; Kilanowicz, A.; Cudzik, D.; Cudzik, K. Enhanced biological activity of a novel preparation of lavandula angustifolia essential oil. Molecules 2021, 26, 2458. [Google Scholar] [CrossRef]
- Islam, M.T.; Dominguez, A.; Alvarado-Tenorio, B.; Bernal, R.A.; Montes, M.O.; Noveron, J.C. Sucrose-Mediated Fast Synthesis of Zinc Oxide Nanoparticles for the Photocatalytic Degradation of Organic Pollutants in Water. ACS Omega 2019, 4, 6560–6572. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes inbioactive glass-ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- Asmat-Campos, D.; Rojas-Jaimes, J.; de la Cruz, M.S.; de Oca-Vasquez, G.M. Enhanced antimicrobial efficacy of biogenic ZnO nanoparticles through UV-B activation: A novel approach for textile garment. Heliyon 2024, 10, e25580. [Google Scholar] [CrossRef] [PubMed]
- Haddada, E.B.; Karkouch, I.; Hamraoui, K.; Faris, N.; Tabbene, O.; Horchani, K.; Ferhi, M. Highly efficient antibacterial activity in the dark and under UV illumination of ZnO nanoplates dispersed in water. Emergent Mater. 2023, 6, 1503–1517. [Google Scholar] [CrossRef]
- Jin, S.E.; Hwang, W.; Lee, H.J.; Jin, H.-E. Dual UV irradiation-based metal oxide nanoparticles for enhanced antimicrobial activity in Escherichia coli and M13 bacteriophage. Int. J. Nanomed. 2017, 12, 8057–8070. [Google Scholar] [CrossRef]
- Dolete, G.; Ilie, C.-I.; Chircov, C.; Purcăreanu, B.; Motelica, L.; Moroșan, A.; Oprea, O.C.; Ficai, D.; Andronescu, E.; Dițu, L.-M. Synergistic Antimicrobial Activity of Magnetite and Vancomycin-Loaded Mesoporous Silica Embedded in Alginate Films. Gels 2023, 9, 295. [Google Scholar] [CrossRef]
- Lemnaru, G.-M.; Truşcă, R.D.; Ilie, C.-I.; Țiplea, R.E.; Ficai, D.; Oprea, O.; Stoica-Guzun, A.; Ficai, A.; Dițu, L.-M. Antibacterial Activity of Bacterial Cellulose Loaded with Bacitracin and Amoxicillin: In Vitro Studies. Molecules 2020, 25, 4069. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, CLSI Supplement M100, 33rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2023. [Google Scholar]
- Chircov, C.; Bîrcă, A.C.; Dănciulescu, L.A.; Neacșu, I.A.; Oprea, O.C.; Trușcă, R.D.; Andronescu, E. Usnic Acid-Loaded Magnetite Nanoparticles—A Comparative Study between Synthesis Methods. Molecules 2023, 28, 5198. [Google Scholar] [CrossRef] [PubMed]


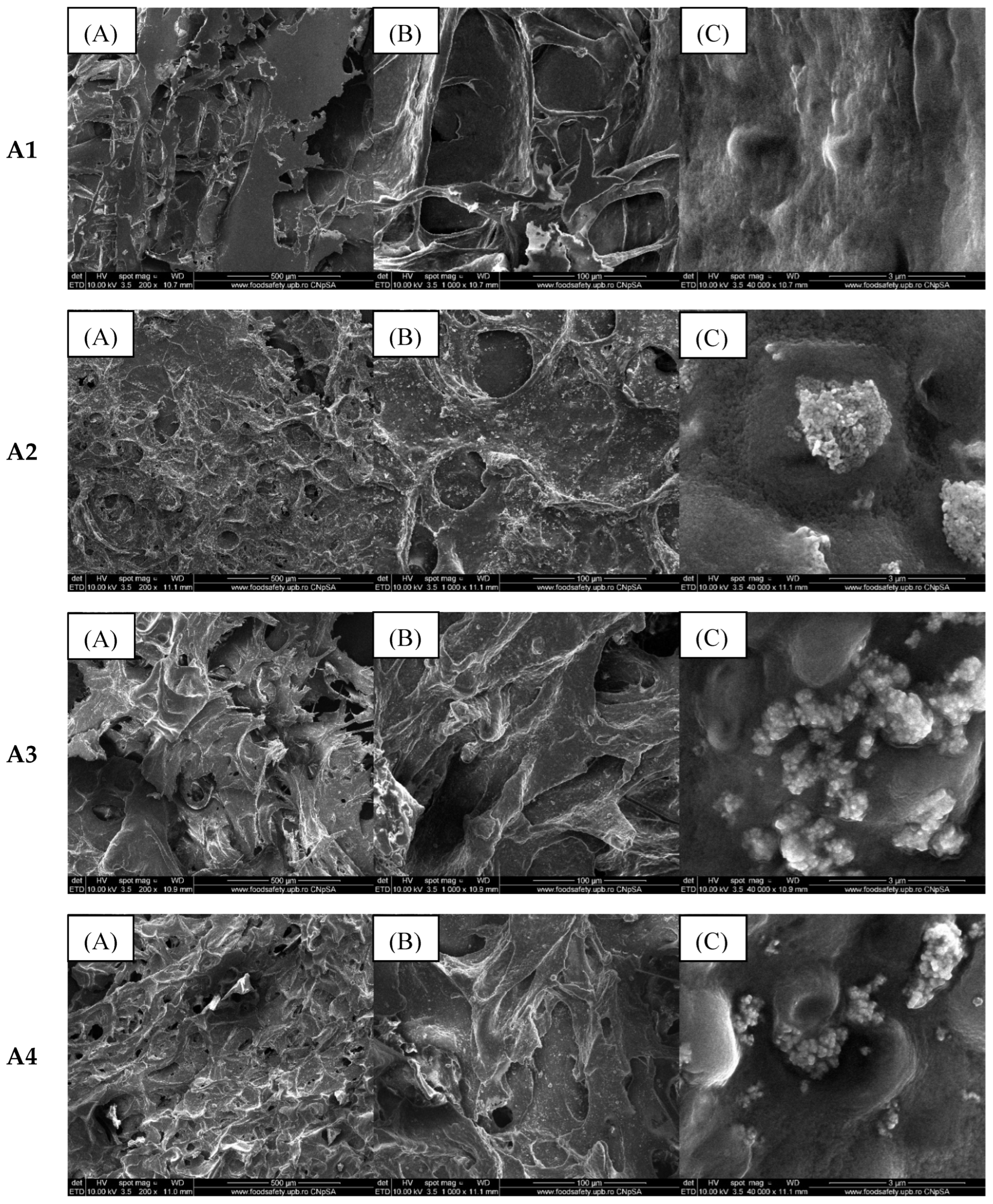
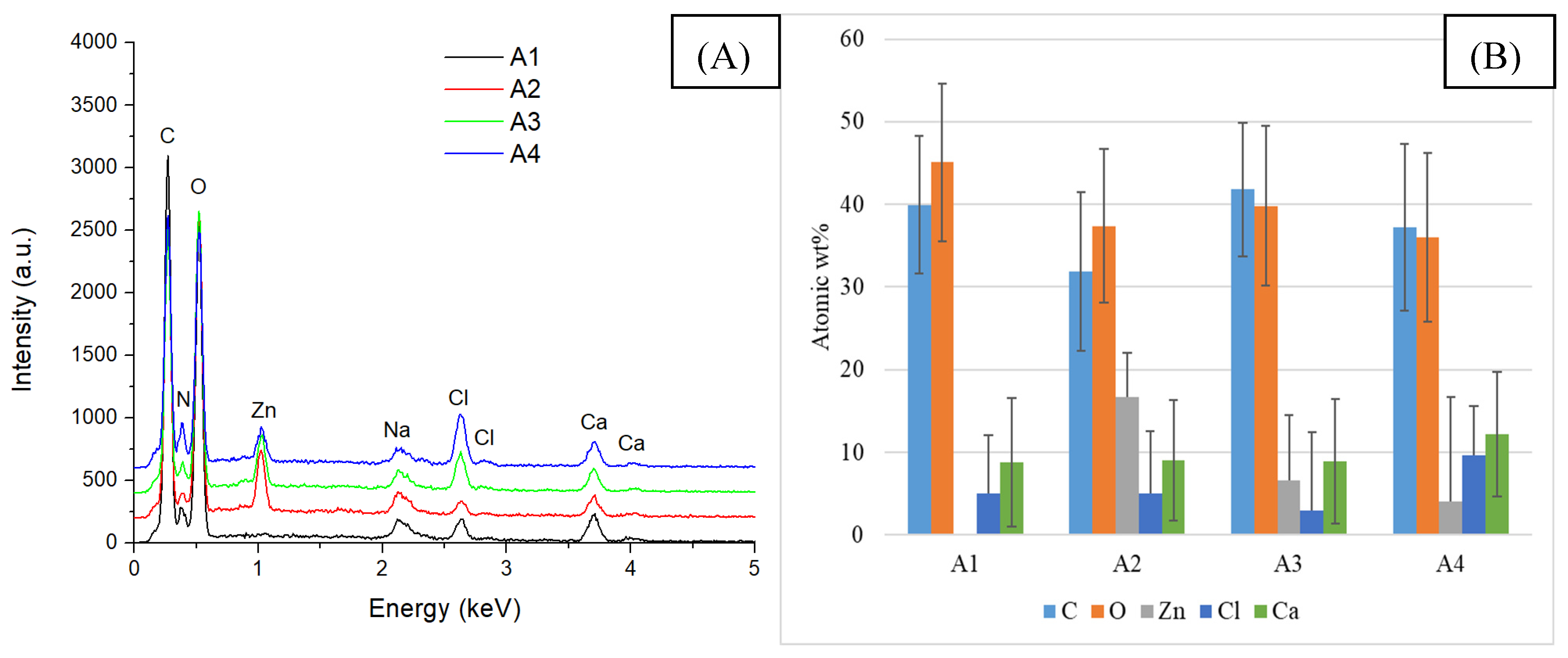

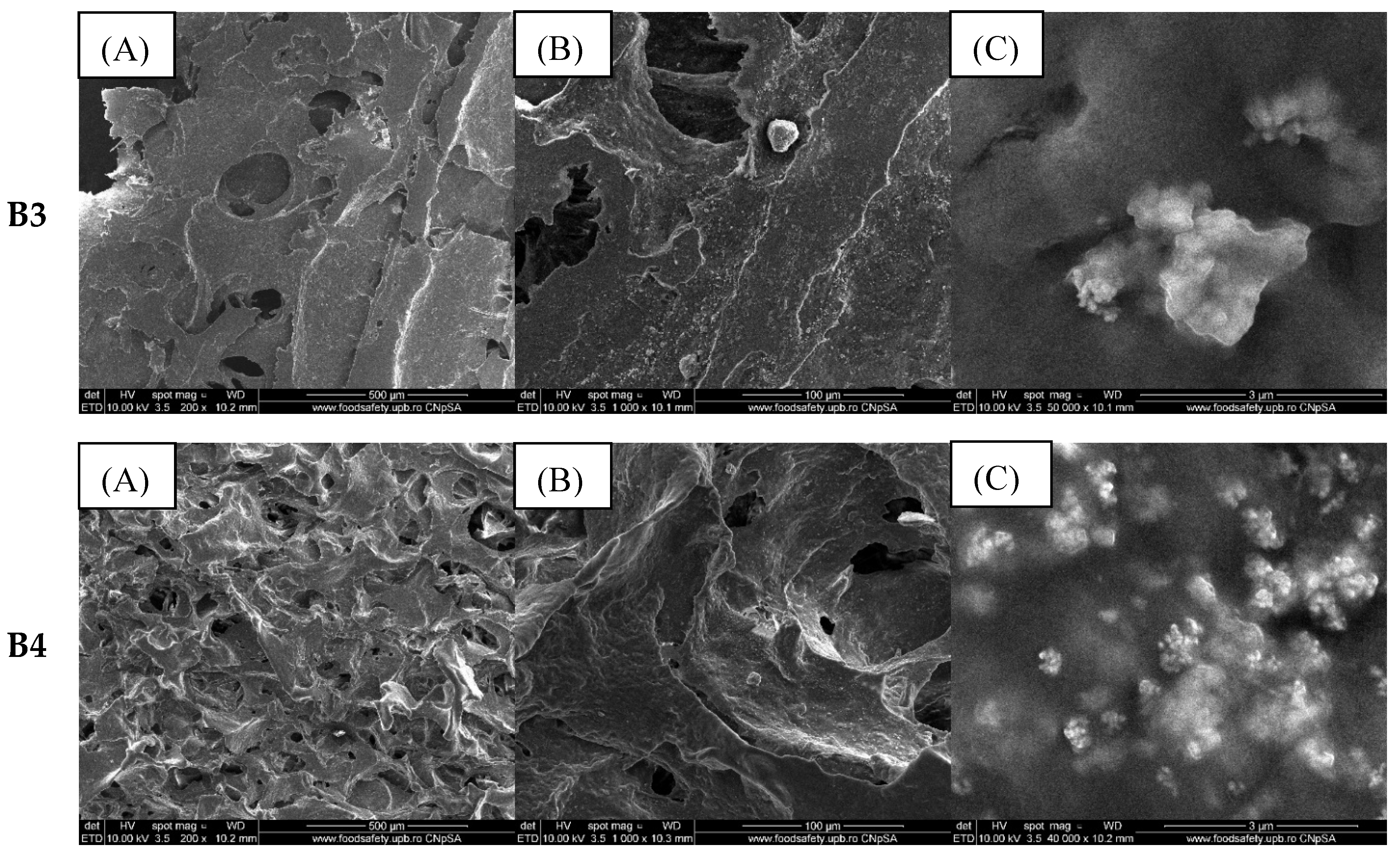
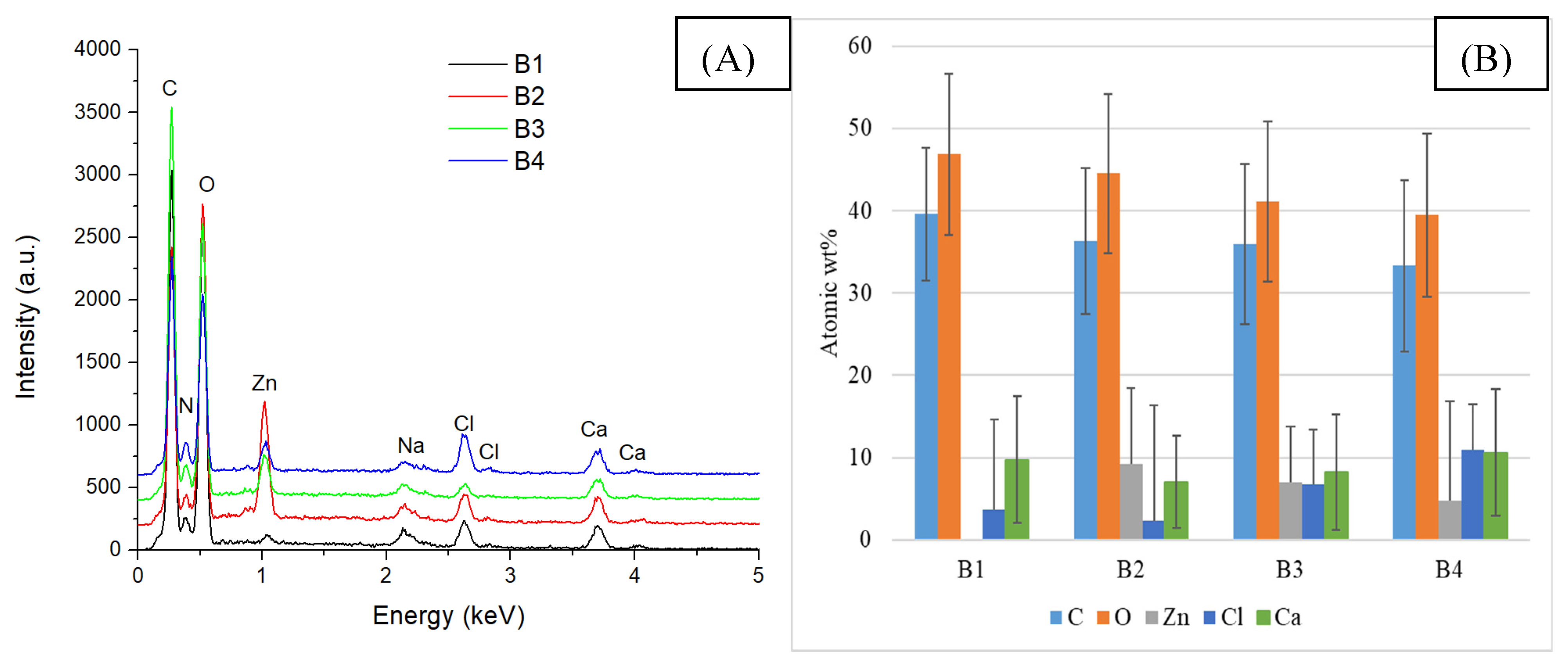
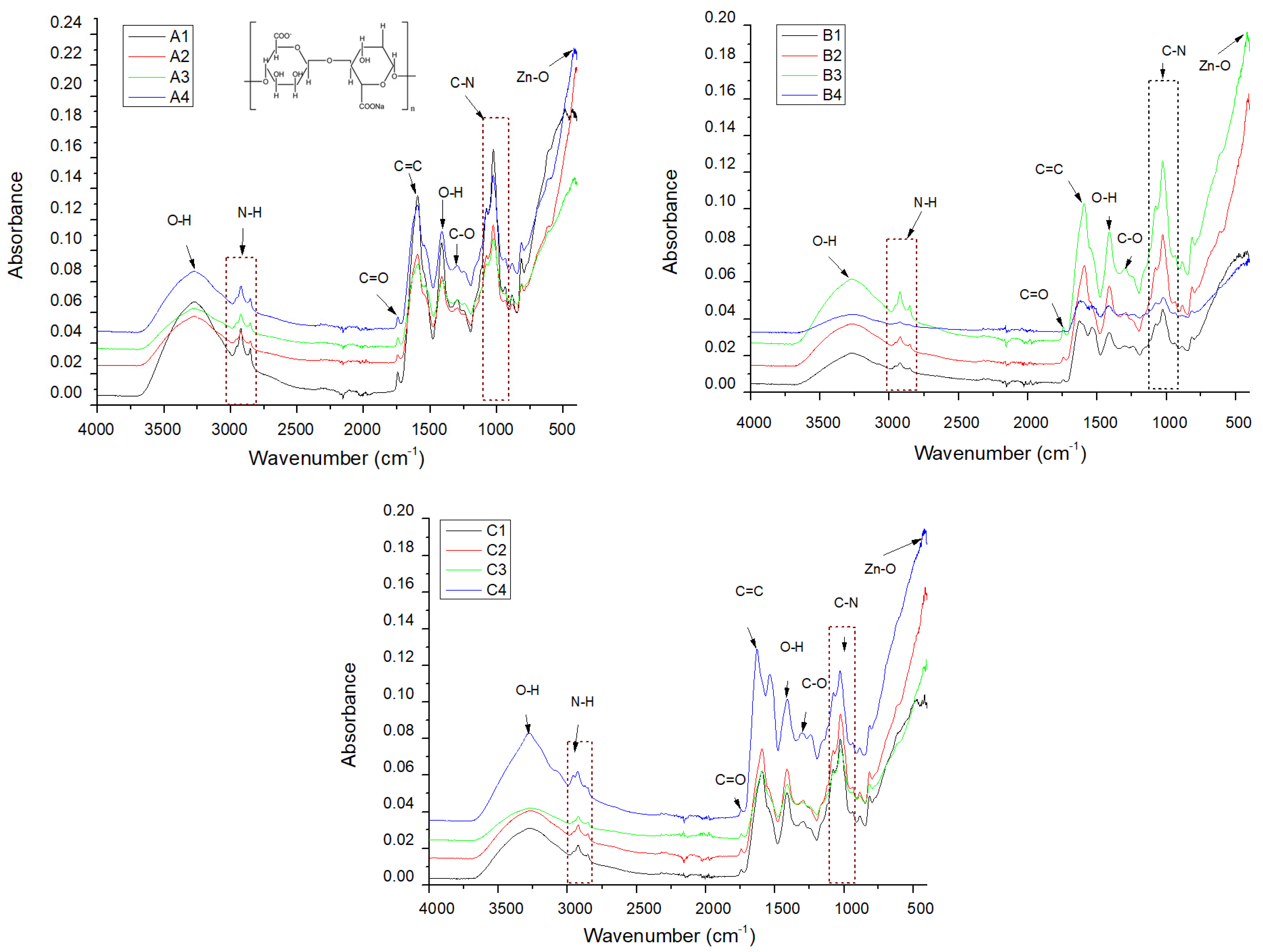

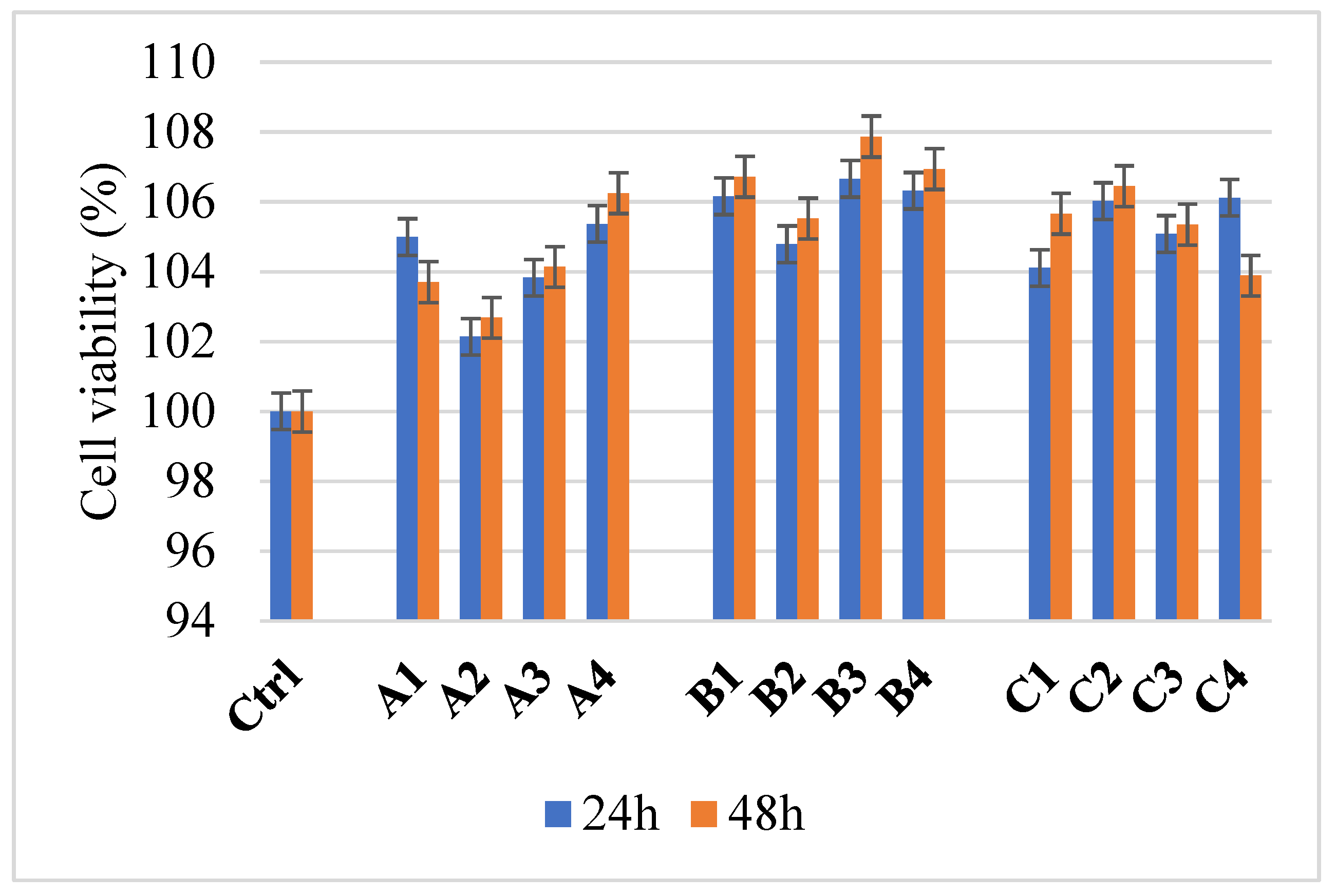
| Sample/Assignment | A1/B1/C1 | A2/B2/C2 | A3/B3/C3 | A4/B4/C4 |
|---|---|---|---|---|
| O–H bond | 3272/3277/3270 | 3270/3270/3271 | 3269/3270/3268 | 3273/3272/3279 |
| N–H stretch | 2924/2923/2925 | 2924/2922/2923 | 2924/2923/2925 | 2923/2923/2926 |
| C=O stretch | 1745/1743/1745 | 1744/1744/1745 | 1745/1745/1744 | 1744/1745/1746 |
| C=C | 1595/1597/1593 | 1595/1593/1593 | 1593/1591/1592 | 1596/1595/1630 |
| O–H | 1413/1412/1412 | 1412/1411/1411 | 1410/1412/1412 | 1413/1409/1409 |
| C–N | 1027/1025/1025 | 1027/1026/1026 | 1026/1025/1026 | 1028/1024/1027 |
| Zn–O | /-/- | 416/417/413 | 417/418/426 | 417/421/421 |
| Composite Name | Egg White (mL) | Alginate (%) | ZnO (%) | Essential Oil Type |
|---|---|---|---|---|
| A1 | 100 | 2 | 0 | - |
| A2 | 0.5 | - | ||
| A3 | 0.5 | Lavender | ||
| A4 | 0.5 | Eucalyptus | ||
| B1 | 100 | 3 | 0 | - |
| B2 | 0.5 | - | ||
| B3 | 0.5 | Lavender | ||
| B4 | 0.5 | Eucalyptus | ||
| C1 | 100 | 4 | 0 | - |
| C2 | 0.5 | - | ||
| C3 | 0.5 | Lavender | ||
| C4 | 0.5 | Eucalyptus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicoară, A.-I.; Anton, A.V.; Trușcă, R.D.; Bîrcă, A.C.; Ilie, C.-I.; Dițu, L.-M. Antibacterial Composites Based on Alginate/Egg White and ZnO Nanoparticles with the Addition of Essential Oils. Gels 2025, 11, 459. https://doi.org/10.3390/gels11060459
Nicoară A-I, Anton AV, Trușcă RD, Bîrcă AC, Ilie C-I, Dițu L-M. Antibacterial Composites Based on Alginate/Egg White and ZnO Nanoparticles with the Addition of Essential Oils. Gels. 2025; 11(6):459. https://doi.org/10.3390/gels11060459
Chicago/Turabian StyleNicoară, Adrian-Ionuț, Adelina Valentina Anton, Roxana Doina Trușcă, Alexandra Cătălina Bîrcă, Cornelia-Ioana Ilie, and Lia-Mara Dițu. 2025. "Antibacterial Composites Based on Alginate/Egg White and ZnO Nanoparticles with the Addition of Essential Oils" Gels 11, no. 6: 459. https://doi.org/10.3390/gels11060459
APA StyleNicoară, A.-I., Anton, A. V., Trușcă, R. D., Bîrcă, A. C., Ilie, C.-I., & Dițu, L.-M. (2025). Antibacterial Composites Based on Alginate/Egg White and ZnO Nanoparticles with the Addition of Essential Oils. Gels, 11(6), 459. https://doi.org/10.3390/gels11060459










