Machine Learning in Gel-Based Additive Manufacturing: From Material Design to Process Optimization
Abstract
1. Introduction
2. Fundamentals of Gel Additive Manufacturing
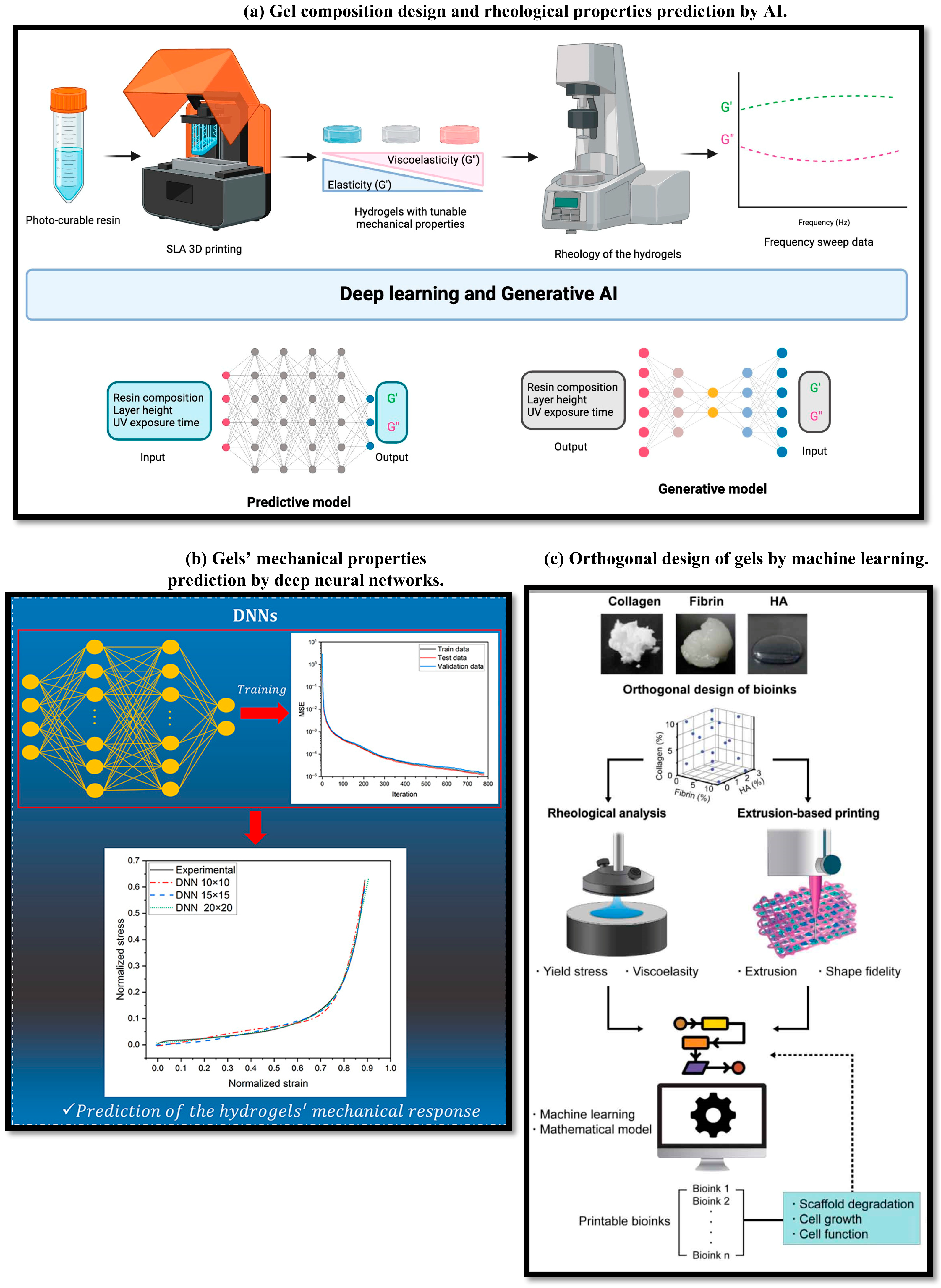
3. Machine Learning in Gels Material Design and 3D Printability
3.1. Accelerating Material Discovery and Formulation Design
| New Composition | Superior Properties | Machine Learning Algorithm | Ref. |
|---|---|---|---|
| Collagen gels | Predict molecular weight | Neural network | [137] |
| Mildly refined yellow pea ingredients | Improved gel stiffness | Neural network | [136] |
| CaO–SiO2–H2O C–S–H gels | Higher elastic moduli | Neural network, Gaussian Process (GP) | [138] |
| Polysaccharide colloids (KGM) | Better viscosity prediction | Extreme Gradient Boosting (XGB) | [139] |
| HAMA/GelMA hybrid hydrogels | Tunable viscosity | HydroThermo Multilayer Perceptron (MLP), Random Forest (RF) | [55] |
| Polyacrylamide hydrogels (PAA) | Predict G′, G″ or composition | Multilayer Perceptron (MLP), Variational Autoencoder (VAE), and Conditional Variational Autoencoder (CVAE) | [53] |
| Polyacrylamide (PAM) and organic crosslinkers | Better field screening | Logistic regression | [140] |
| Polysaccharide gels | Predict printability | SVR, Neural network, Convolutional neural network (CNN) | [50] |
| Atelocollagen, native collagen | Improved shape fidelity | Multiple regression | [51] |
| Hydrogel supercapacitor electrolytes | Higher capacitance, stability | SHAP (SHapley Additive exPlanations), tree models | [143] |
| Alginate/gelatin/TO-NFC bioink | Tunable viscosity, printable | Random forest | [141] |
| Photodegradable acrylic/methacrylic gels | Fast, tunable photodegradation | Bayesian optimization | [142] |
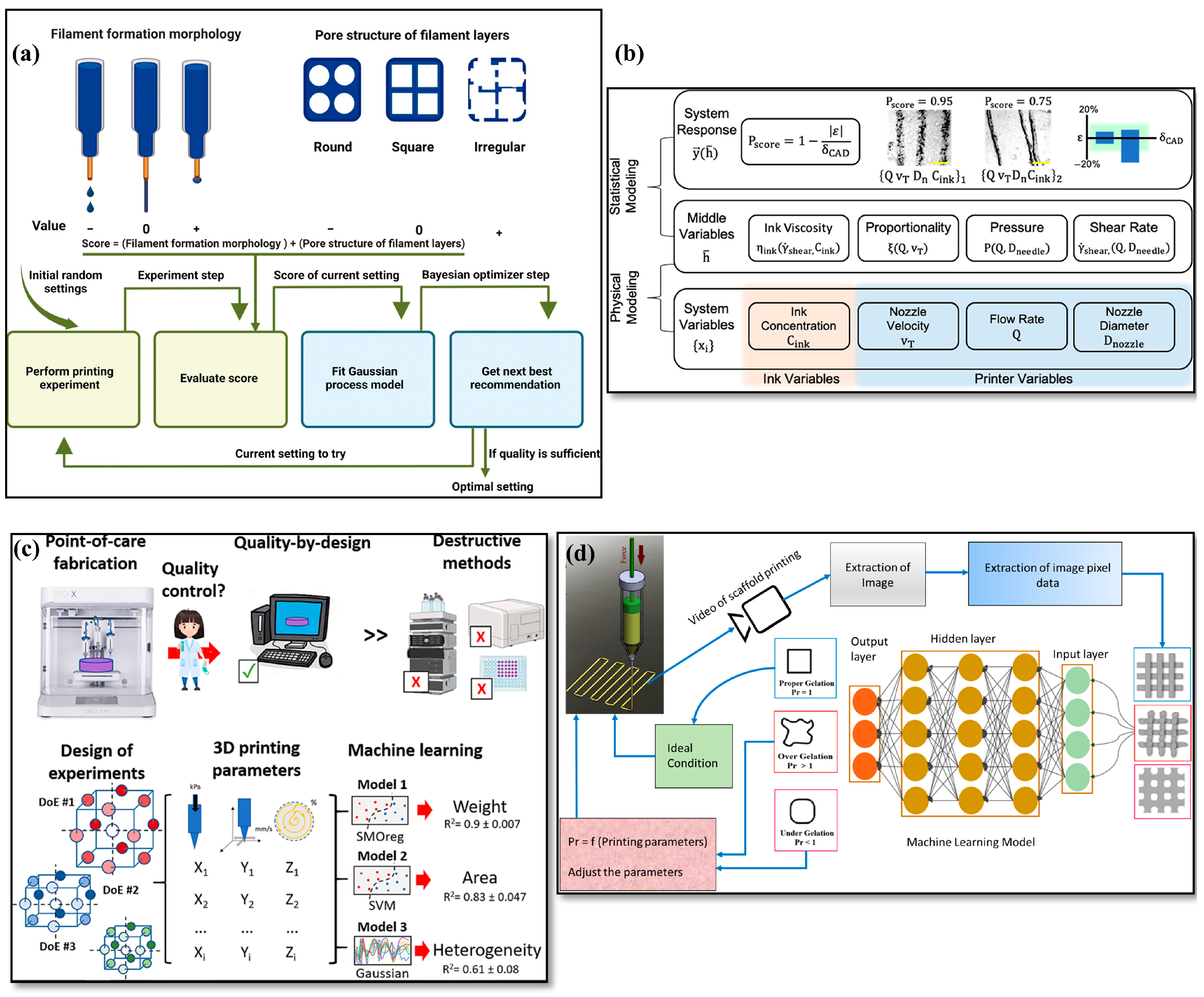
3.2. Enhancing Gels’ 3D Printability
4. AI-Driven Process Optimization in Gel Additive Manufacturing
4.1. Reasons to Use Machine Learning
4.2. Wide Availability of Resources
| Machine Learning | Description and Features | Application | |
|---|---|---|---|
| Open-source datasets | Mendeley [160] | Available from https://data.mendeley.com (accessed on 1 June 2025) | Datasets [171,172,173,174,175] |
| Google Dataset search | Available from https://datasetsearch.research.google.com (accessed on 1 June 2025) | Datasets [176,177] | |
| NIST [163] | Available from https://data.nist.gov (accessed on 1 June 2025) | Datasets [178] | |
| Zenodo [161] | Available from https://zenodo.org (accessed on 1 June 2025) | Datasets, journal papers, and formulations [179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211] | |
| Figshare [162] | Available from https://figshare.com (accessed on 1 June 2025) | Figures, videos, and datasets [212,213,214,215,216,217,218] | |
| AmeriGEOSS Community Platform DataHub [164] | Available from https://data.amerigeoss.org (accessed on 1 June 2025) | Patents, datasets, and project reports [219,220] | |
| Open-source packages | Scikit-learn [221] | Classical machine learning models (easy-to-use, general-purpose). Typical models: Support Vector Machine, Decision Tree, Random Forest, Logistic Regression, k-Nearest Neighbors, Principal Component Analysis, k-Means Clustering, Gradient Boosting Machine, Extreme Gradient Boosting (XGB), PCA. | Shrinkage [222], gel point [223], gelatin, pore size and stiffness [170], rheological properties [224], high elastic modulus and yield stress [51], simultaneously optimize material, formulation, and processing variables [165], high-fidelity [150], shear rate [225], compressive modulus, density, and porosity [226], printability from rheological measurements [166], high viscosity [149], storage and loss moduli, and hardness for extraordinary printability [227] |
| TensorFlow [228] | Deep learning and neural networks (high flexibility for research and production). Typical models: Convolutional Neural Network, Recurrent Neural Network, Deep Neural Network, Generative Adversarial Network, Long Short-Term Memory Network, Transformer Model. | Printing speed, printing pressure and infill percentage [151], and real-time videos monitoring [153,167] | |
| PyTorch [229] | Research, flexible deep learning model development, Convolutional Neural Network, Recurrent Neural Network, Transformer Model, Diffusion Model. | Light scattering compensation [168], monomer composition ratios [230], printability and scaffold quality [231], and material deposition temperature monitoring [154] | |
| Keras [232] | High-level API; excellent for quick development of deep learning models (uses TensorFlow backend). Convolutional Neural Network, Recurrent Neural Network, Deep Neural Network, Long Short-Term Memory Network. | Minimum extrusion pressure (MEP) and printed structure conformity (PSC) [169] | |
4.3. Predictive Process Optimization
4.4. Real-Time Quality Control and Autonomous Process Control
5. Research Limitations and Outlook
5.1. Limitations
5.2. Outlook
5.2.1. Real-Time Defect Detection with Multimodal Monitoring
5.2.2. Predictive Stimuli-Responsive 4D Gel Systems
5.2.3. AI Assisted In-Body Gel Printing
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Jiang, G.; Wang, G.; Zhou, J.; Zhang, Y.; Zhao, D. Promising Cellulose–Based Functional Gels for Advanced Biomedical Applications: A Review. Int. J. Biol. Macromol. 2024, 260, 129600. [Google Scholar] [CrossRef] [PubMed]
- Ferjaoui, Z.; López-Muñoz, R.; Akbari, S.; Chandad, F.; Mantovani, D.; Rouabhia, M.; Fanganiello, R.D. Design of Alginate/Gelatin Hydrogels for Biomedical Applications: Fine-Tuning Osteogenesis in Dental Pulp Stem Cells While Preserving Other Cell Behaviors. Biomedicines 2024, 12, 1510. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Patel, D.; Hickson, B.; DesRochers, J.; Hu, X. Recent Progress in Biopolymer-Based Hydrogel Materials for Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 1415. [Google Scholar] [CrossRef]
- Sajjadi, S.; Gholizadeh-Hashjin, A.; Shafizadeh, F.; Marefat, S.; Hamidi, S.; Farjami, A. Advancing Biomedicine with Gel-based Materials and Composites: A Comprehensive Review. J. Appl. Polym. Sci. 2023, 140, e54641. [Google Scholar] [CrossRef]
- Li, X.; Hu, R.; Xiong, Z.; Wang, D.; Zhang, Z.; Liu, C.; Zeng, X.; Chen, D.; Che, R.; Nie, X. Metal–Organic Gel Leading to Customized Magnetic-Coupling Engineering in Carbon Aerogels for Excellent Radar Stealth and Thermal Insulation Performances. Nano-Micro Lett. 2024, 16, 42. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, H.; Liu, D.; Chen, X.; Wang, D.; Dai, S.; Chen, F.; Xu, B.B. A Structural Gel Composite Enabled Robust Underwater Mechanosensing Strategy with High Sensitivity. Adv. Funct. Mater. 2022, 32, 2201396. [Google Scholar] [CrossRef]
- Deliormanlı, A.M. Novel Sol-Gel Inks for the Direct Writing of SiO2-Based Bioactive Glass Scaffolds for Tissue Engineering Applications. Silicon 2025, 17, 775–787. [Google Scholar] [CrossRef]
- Wu, J.; Yun, Z.; Song, W.; Yu, T.; Xue, W.; Liu, Q.; Sun, X. Highly Oriented Hydrogels for Tissue Regeneration: Design Strategies, Cellular Mechanisms, and Biomedical Applications. Theranostics 2024, 14, 1982. [Google Scholar] [CrossRef]
- Hu, S.; Zeng, C.; Jiang, Y.; Kong, W.; Zhu, M. Macrostructure and Microenvironment Biomimetic Hydrogel: Design, Properties, and Tissue Engineering Application. Chem. Mater. 2024, 36, 1054–1087. [Google Scholar] [CrossRef]
- Choi, S.; Lee, K.Y.; Kim, S.L.; MacQueen, L.A.; Chang, H.; Zimmerman, J.F.; Jin, Q.; Peters, M.M.; Ardoña, H.A.M.; Liu, X. Fibre-Infused Gel Scaffolds Guide Cardiomyocyte Alignment in 3D-Printed Ventricles. Nat. Mater. 2023, 22, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Revete, A.; Aparicio, A.; Cisterna, B.A.; Revete, J.; Luis, L.; Ibarra, E.; Segura González, E.A.; Molino, J.; Reginensi, D. Advancements in the Use of Hydrogels for Regenerative Medicine: Properties and Biomedical Applications. Int. J. Biomater. 2022, 3606765. [Google Scholar] [CrossRef]
- Zhang, S.; Kang, L.; Hu, S.; Hu, J.; Fu, Y.; Hu, Y.; Yang, X. Carboxymethyl Chitosan Microspheres Loaded Hyaluronic Acid/Gelatin Hydrogels for Controlled Drug Delivery and the Treatment of Inflammatory Bowel Disease. Int. J. Biol. Macromol. 2021, 167, 1598–1612. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bai, Z.; Zheng, M.; Yue, O.; Hou, M.; Cui, B.; Su, R.; Wei, C.; Liu, X. Engineered Gelatin-Based Conductive Hydrogels for Flexible Wearable Electronic Devices: Fundamentals and Recent Advances. J. Sci. Adv. Mater. Devices 2022, 7, 100451. [Google Scholar] [CrossRef]
- Bediaga-Bañeres, H.; Moreno-Benítez, I.; Arrasate, S.; Pérez-Álvarez, L.; Halder, A.K.; Cordeiro, M.N.D.; González-Díaz, H.; Vilas-Vilela, J.L. Artificial Intelligence-Driven Modeling for Hydrogel Three-Dimensional Printing: Computational and Experimental Cases of Study. Polymers 2025, 17, 121. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Xu, P.; Li, M.; Lu, W. Machine Learning for Perovskite Materials Design and Discovery. Npj Comput. Mater. 2021, 7, 23. [Google Scholar] [CrossRef]
- Vasudevan, R.; Pilania, G.; Balachandran, P.V. Machine Learning for Materials Design and Discovery. J. Appl. Phys. 2021, 129, 070401. [Google Scholar] [CrossRef]
- Ng, W.L.; Goh, G.L.; Goh, G.D.; Ten, J.S.J.; Yeong, W.Y. Progress and Opportunities for Machine Learning in Materials and Processes of Additive Manufacturing. Adv. Mater. 2024, 36, 2310006. [Google Scholar] [CrossRef]
- Tamir, T.S.; Xiong, G.; Fang, Q.; Yang, Y.; Shen, Z.; Zhou, M.; Jiang, J. Machine-Learning-Based Monitoring and Optimization of Processing Parameters in 3D Printing. Int. J. Comput. Integr. Manuf. 2023, 36, 1362–1378. [Google Scholar] [CrossRef]
- Mussel-Inspired Surface-Engineering of 3D Printed Scaffolds Employing Bedecked Transition Metal for Accelerated Bone Tissue Regeneration—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/abs/pii/S2772950825001360?via%3Dihub (accessed on 19 July 2025).
- Cheng, D.; Wei, C.; Huang, Y.; Zhang, Z.; Wang, D.; Liu, Z.; Newman, M.; Ma, T.; Chueh, Y.-H.; Zhang, X.; et al. Additive Manufacturing of Lithium Aluminosilicate Glass-Ceramic/Metal 3D Electronic Components via Multiple Material Laser Powder Bed Fusion. Addit. Manuf. 2022, 49, 102481. [Google Scholar] [CrossRef]
- Zhang, Z.; Mativenga, P.; Zhang, W.; Huang, S. Deep Learning-Driven Prediction of Mechanical Properties of 316L Stainless Steel Metallographic by Laser Powder Bed Fusion. Micromachines 2024, 15, 1167. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, C.; Lu, M. Fusion of Sentiment and Market Signals for Bitcoin Forecasting: A SentiStack Network Based on a Stacking LSTM Architecture. Big Data Cogn. Comput. 2025, 9, 161. [Google Scholar] [CrossRef]
- Wei, C.; Gu, H.; Zhang, Z.; Chueh, Y.-H.; Li, L. Laser and Arc-Based Methods for Additive Manufacturing of Multiple Material Components—From Design to Manufacture. In Additive Manufacturing Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2023; pp. 155–184. ISBN 978-3-527-83393-1. [Google Scholar]
- Zhang, T.; Liu, T.; Dutta, N.; Chen, Y.; Su, R.; Zhang, Z.; Wang, W.; Wang, C.C.L. Toolpath Generation for High Density Spatial Fiber Printing Guided by Principal Stresses. Compos. Part B Eng. 2025, 295, 112154. [Google Scholar] [CrossRef]
- Sha, Y.; Zhu, M.; Huang, K.; Zhang, Y.; Moissinac, F.; Zhang, Z.; Cheng, D.; Mativenga, P.; Liu, Z. Towards a New Avenue for Rapid Synthesis of Electrocatalytic Electrodes via Laser-Induced Hydrothermal Reaction for Water Splitting. Int. J. Extrem. Manuf. 2023, 6, 015502. [Google Scholar] [CrossRef]
- Zhu, M.; Ouyang, J.; Tian, Y.; Guo, W.; Zhang, Z.; Mativenga, P.; Li, L. Understanding Factors Affecting the Process Efficiency, Quality and Carbon Emission in Laser Drilling of CFRP Composite via Tailored Sequence Multiple-Ring Scanning. Compos. Part B Eng. 2024, 272, 111156. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Balabani, S.; Hirayama, R.; Huang, J. Machine Learning in Predicting Printable Biomaterial Formulations for Direct Ink Writing. Research 2023, 6, 0197. [Google Scholar] [CrossRef]
- Nadernezhad, A.; Groll, J. Machine Learning Reveals a General Understanding of Printability in Formulations Based on Rheology Additives. Adv. Sci. 2022, 9, 2202638. [Google Scholar] [CrossRef]
- Negut, I.; Bita, B. Exploring the Potential of Artificial Intelligence for Hydrogel Development—A Short Review. Gels 2023, 9, 845. [Google Scholar] [CrossRef]
- Li, Z.; Song, P.; Li, G.; Han, Y.; Ren, X.; Bai, L.; Su, J. AI Energized Hydrogel Design, Optimization and Application in Biomedicine. Mater. Today Bio 2024, 25, 101014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhou, X.; Fang, Y.; Xiong, Z.; Zhang, T. AI-Driven 3D Bioprinting for Regenerative Medicine: From Bench to Bedside. Bioact. Mater. 2025, 45, 201–230. [Google Scholar] [CrossRef] [PubMed]
- Sani, A.R.; Zolfagharian, A.; Kouzani, A.Z. Artificial Intelligence-augmented Additive Manufacturing: Insights on Closed-loop 3D Printing. Adv. Intell. Syst. 2024, 6, 2400102. [Google Scholar] [CrossRef]
- Shick, A.A.; Webber, C.M.; Kiarashi, N.; Weinberg, J.P.; Deoras, A.; Petrick, N.; Saha, A.; Diamond, M.C. Transparency of Artificial Intelligence/Machine Learning-Enabled Medical Devices. NPJ Digit. Med. 2024, 7, 21. [Google Scholar] [CrossRef]
- Lekadir, K.; Frangi, A.F.; Porras, A.R.; Glocker, B.; Cintas, C.; Langlotz, C.P.; Weicken, E.; Asselbergs, F.W.; Prior, F.; Collins, G.S. FUTURE-AI: International Consensus Guideline for Trustworthy and Deployable Artificial Intelligence in Healthcare. BMJ 2025, 388, e081554. [Google Scholar] [CrossRef]
- Bhuvaneswari, V.; Devarajan, B.; Rajeshkumar, L. Additive Manufacturing of Hydrogels: Process and Properties. Addit. Manuf. Nov. Mater. Process. Prop. Appl. 2024, 9, 295–318. [Google Scholar]
- Navaf, M.; Sunooj, K.V.; Aaliya, B.; Akhila, P.P.; Sudheesh, C.; Mir, S.A.; George, J. 4D Printing: A New Approach for Food Printing; Effect of Various Stimuli on 4D Printed Food Properties. A Comprehensive Review. Appl. Food Res. 2022, 2, 100150. [Google Scholar] [CrossRef]
- Zhou, Y.; Qin, R.; Chen, T.; Zhang, K.; Gui, J. 3D Bioprinting Modified Autologous Matrix-Induced Chondrogenesis (AMIC) Technique for Repair of Cartilage Defects. Mater. Des. 2021, 203, 109621. [Google Scholar] [CrossRef]
- Lee, J.M.; Sing, S.L.; Zhou, M.; Yeong, W.Y. 3D Bioprinting Processes: A Perspective on Classification and Terminology. Int. J. Bioprinting 2024, 4, 151. [Google Scholar] [CrossRef]
- Gonzalez-Gutierrez, J.; Cano, S.; Schuschnigg, S.; Kukla, C.; Sapkota, J.; Holzer, C. Additive Manufacturing of Metallic and Ceramic Components by the Material Extrusion of Highly-Filled Polymers: A Review and Future Perspectives. Materials 2018, 11, 840. [Google Scholar] [CrossRef]
- Foyt, D.A.; Norman, M.D.A.; Yu, T.T.L.; Gentleman, E. Exploiting Advanced Hydrogel Technologies to Address Key Challenges in Regenerative Medicine. Adv. Healthc. Mater. 2018, 7, 1700939. [Google Scholar] [CrossRef] [PubMed]
- Kotlarz, M.; Melo, P.; Ferreira, A.M.; Gentile, P.; Dalgarno, K. Cell Seeding via Bioprinted Hydrogels Supports Cell Migration into Porous Apatite-Wollastonite Bioceramic Scaffolds for Bone Tissue Engineering. Biomater. Adv. 2023, 153, 213532. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.-Y.; Weng, Y.-C.; Chen, C.-H.; Liao, Y.-C. Reactive Binder-Jet 3D Printing Process for Green Strength Enhancement. Addit. Manuf. 2023, 74, 103734. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B.; Khorasani, M. Additive Manufacturing Technologies; Springer International Publishing: Cham, Switzerland, 2021; ISBN 978-3-030-56126-0. [Google Scholar]
- Parab, N.D.; Barnes, J.E.; Zhao, C.; Cunningham, R.W.; Fezzaa, K.; Rollett, A.D.; Sun, T. Real Time Observation of Binder Jetting Printing Process Using High-Speed X-Ray Imaging. Sci. Rep. 2019, 9, 2499. [Google Scholar] [CrossRef]
- Zhou, T.; Song, Z.; Sundmacher, K. Big Data Creates New Opportunities for Materials Research: A Review on Methods and Applications of Machine Learning for Materials Design. Engineering 2019, 5, 1017–1026. [Google Scholar] [CrossRef]
- Ziaee, M.; Crane, N.B. Binder Jetting: A Review of Process, Materials, and Methods. Addit. Manuf. 2019, 28, 781–801. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, Z.; Hartley, W.D.; Sietins, J.M.; Williams, C.B.; Yu, H.Z. Unraveling Pore Evolution in Post-Processing of Binder Jetting Materials: X-Ray Computed Tomography, Computer Vision, and Machine Learning. Addit. Manuf. 2020, 34, 101183. [Google Scholar] [CrossRef]
- Lin, Z.; Qiu, X.; Cai, Z.; Li, J.; Zhao, Y.; Lin, X.; Zhang, J.; Hu, X.; Bai, H. High Internal Phase Emulsions Gel Ink for Direct-Ink-Writing 3D Printing of Liquid Metal. Nat. Commun. 2024, 15, 4806. [Google Scholar] [CrossRef]
- Wang, Y.; Willenbacher, N. Phase-Change-Enabled, Rapid, High-Resolution Direct Ink Writing of Soft Silicone. Adv. Mater. 2022, 34, 2109240. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Zhang, M.; Adhikari, B.; Li, C.; Lin, J. Indirect Prediction of the 3D Printability of Polysaccharide Gels Using Multiple Machine Learning (ML) Models. Int. J. Biol. Macromol. 2024, 280, 135769. [Google Scholar] [CrossRef]
- Lee, J.; Oh, S.J.; An, S.H.; Kim, W.-D.; Kim, S.-H. Machine Learning-Based Design Strategy for 3D Printable Bioink: Elastic Modulus and Yield Stress Determine Printability. Biofabrication 2020, 12, 035018. [Google Scholar] [CrossRef]
- Liu, H.; Xiao, S.; Tang, L.; Bao, E.; Li, E.; Yang, C.; Zhao, Z.; Sant, G.; Smedskjaer, M.M.; Guo, L.; et al. Predicting the Early-Stage Creep Dynamics of Gels from Their Static Structure by Machine Learning. Acta Mater. 2021, 210, 116817. [Google Scholar] [CrossRef]
- Mohammad, S.; Akand, R.; Cook, K.M.; Nilufar, S.; Chowdhury, F. Leveraging Deep Learning and Generative AI for Predicting Rheological Properties and Material Compositions of 3D Printed Polyacrylamide Hydrogels. Gels 2024, 10, 660. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wallmersperger, T.; Ehrenhofer, A. Application of Back Propagation Neural Networks and Random Forest Algorithms in Material Research of Hydrogels. PAMM 2023, 23, e202200278. [Google Scholar] [CrossRef]
- Deng, B.; Chen, S.; Lasaosa, F.L.; Xue, X.; Xuan, C.; Mao, H.; Cui, Y.; Gu, Z.; Doblare, M. Predicting Rheological Properties of HAMA/GelMA Hybrid Hydrogels via Machine Learning. J. Mech. Behav. Biomed. Mater. 2025, 168, 107005. [Google Scholar] [CrossRef]
- Zheng, Q.; Xie, B.; Xu, Z.; Wu, H. A Systematic Printability Study of Direct Ink Writing towards High-Resolution Rapid Manufacturing. Int. J. Extrem. Manuf. 2023, 5, 035002. [Google Scholar] [CrossRef]
- Pagac, M.; Hajnys, J.; Ma, Q.-P.; Jancar, L.; Jansa, J.; Stefek, P.; Mesicek, J. A Review of Vat Photopolymerization Technology: Materials, Applications, Challenges, and Future Trends of 3D Printing. Polymers 2021, 13, 598. [Google Scholar] [CrossRef] [PubMed]
- Raman, R.; Bhaduri, B.; Mir, M.; Shkumatov, A.; Lee, M.K.; Popescu, G.; Kong, H.; Bashir, R. High-Resolution Projection Microstereolithography for Patterning of Neovasculature. Adv. Healthc. Mater. 2016, 5, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Kowsari, K.; Akbari, S.; Wang, D.; Fang, N.X.; Ge, Q. High-Efficiency High-Resolution Multimaterial Fabrication for Digital Light Processing-Based Three-Dimensional Printing. 3D Print. Addit. Manuf. 2018, 5, 185–193. [Google Scholar] [CrossRef]
- Chen, L.; Fan, F.; Wang, P.; Liu, P.; Feng, B.; Duan, H. Vat Photopolymerization 3D Printing of High-Performance Ionic Organogel for Multifunctional Sensors. Mater. Today Commun. 2024, 41, 110580. [Google Scholar] [CrossRef]
- Chung, J.H.; Naficy, S.; Yue, Z.; Kapsa, R.; Quigley, A.; Moulton, S.E.; Wallace, G.G. Bio-Ink Properties and Printability for Extrusion Printing Living Cells. Biomater. Sci. 2013, 1, 763–773. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, M.; Yang, C. Dual Extrusion 3D Printing of Mashed Potatoes/Strawberry Juice Gel. LWT 2018, 96, 589–596. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Y.; Liu, C.; Regenstein, J.M.; Liu, X.; Zhou, P. Rheological and Mechanical Behavior of Milk Protein Composite Gel for Extrusion-Based 3D Food Printing. LWT 2019, 102, 338–346. [Google Scholar] [CrossRef]
- Zeng, X.; Chen, H.; Chen, L.; Zheng, B. Insights into the Relationship between Structure and Rheological Properties of Starch Gels in Hot-Extrusion 3D Printing. Food Chem. 2021, 342, 128362. [Google Scholar] [CrossRef]
- Nadgorny, M.; Xiao, Z.; Connal, L.A. 2D and 3D-Printing of Self-Healing Gels: Design and Extrusion of Self-Rolling Objects. Mol. Syst. Des. Eng. 2017, 2, 283–292. [Google Scholar] [CrossRef]
- Jin, Y.; Compaan, A.; Bhattacharjee, T.; Huang, Y. Granular Gel Support-Enabled Extrusion of Three-Dimensional Alginate and Cellular Structures. Biofabrication 2016, 8, 025016. [Google Scholar] [CrossRef] [PubMed]
- Menshutina, N.; Abramov, A.; Tsygankov, P.; Lovskaya, D. Extrusion-Based 3D Printing for Highly Porous Alginate Materials Production. Gels 2021, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Go, J.; Hart, A.J. Fast Desktop-Scale Extrusion Additive Manufacturing. Addit. Manuf. 2017, 18, 276–284. [Google Scholar] [CrossRef]
- Tian, S.; Stevens, R.; McInnes, B.T.; Lewinski, N.A. Machine Assisted Experimentation of Extrusion-Based Bioprinting Systems. Micromachines 2021, 12, 780. [Google Scholar] [CrossRef]
- Freeman, S.; Calabro, S.; Williams, R.; Jin, S.; Ye, K. Bioink Formulation and Machine Learning-Empowered Bioprinting Optimization. Front. Bioeng. Biotechnol. 2022, 10, 913579. [Google Scholar] [CrossRef]
- Masaeli, E.; Forster, V.; Picaud, S.; Karamali, F.; Nasr-Esfahani, M.H.; Marquette, C. Tissue Engineering of Retina through High Resolution 3-Dimensional Inkjet Bioprinting. Biofabrication 2020, 12, 025006. [Google Scholar] [CrossRef]
- Li, X.; Liu, B.; Pei, B.; Chen, J.; Zhou, D.; Peng, J.; Zhang, X.; Jia, W.; Xu, T. Inkjet Bioprinting of Biomaterials. Chem. Rev. 2020, 120, 10793–10833. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Song, J.; Song, B.; Lu, W.F. Multi-Objective Optimization Design through Machine Learning for Drop-on-Demand Bioprinting. Engineering 2019, 5, 586–593. [Google Scholar] [CrossRef]
- Bonatti, A.F.; Vozzi, G.; De Maria, C. Enhancing Quality Control in Bioprinting through Machine Learning. Biofabrication 2024, 16, 022001. [Google Scholar] [CrossRef]
- Shin, J.; Kang, R.; Hyun, K.; Li, Z.; Kumar, H.; Kim, K.; Park, S.S.; Kim, K. Machine Learning-Enhanced Optimization for High-Throughput Precision in Cellular Droplet Bioprinting. Adv. Sci. 2025, 12, 2412831. [Google Scholar] [CrossRef]
- Guillotin, B.; Souquet, A.; Catros, S.; Duocastella, M.; Pippenger, B.; Bellance, S.; Bareille, R.; Rémy, M.; Bordenave, L.; Amédée, J.; et al. Laser Assisted Bioprinting of Engineered Tissue with High Cell Density and Microscale Organization. Biomaterials 2010, 31, 7250–7256. [Google Scholar] [CrossRef]
- Dou, C.; Perez, V.; Qu, J.; Tsin, A.; Xu, B.; Li, J. A State-of-the-Art Review of Laser-Assisted Bioprinting and Its Future Research Trends. ChemBioEng. Rev. 2021, 8, 517–534. [Google Scholar] [CrossRef]
- Ventura, R.D. An Overview of Laser-Assisted Bioprinting (LAB) in Tissue Engineering Applications. Med. Lasers 2021, 10, 76–81. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D Bioprinting of Tissues and Organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Melchels, F.P.; Dhert, W.J.; Hutmacher, D.W.; Malda, J. Development and Characterisation of a New Bioink for Additive Tissue Manufacturing. J. Mater. Chem. B 2014, 2, 2282–2289. [Google Scholar] [CrossRef]
- Ren, X.; Shao, H.; Lin, T.; Zheng, H. 3D Gel-Printing—An Additive Manufacturing Method for Producing Complex Shape Parts. Mater. Des. 2016, 101, 80–87. [Google Scholar] [CrossRef]
- Russo Spena, S.; Grizzuti, N.; Tammaro, D. Linking Processing Parameters and Rheology to Optimize Additive Manufacturing of K-Carrageenan Gel Systems. Gels 2022, 8, 493. [Google Scholar] [CrossRef]
- Qin, B.; Zhong, Z. A Physics-Guided Machine Learning Model for Predicting Viscoelasticity of Solids at Large Deformation. Polymers 2024, 16, 3222. [Google Scholar] [CrossRef]
- John, T.; Mowbray, M.; Alalwyat, A.; Vousvoukis, M.; Martin, P.; Kowalski, A.; Fonte, C. Machine Learning for Viscoelastic Constitutive Model Identification and Parameterisation Using Large Amplitude Oscillatory Shear. Chem. Eng. Sci. 2024, 294, 120075. [Google Scholar] [CrossRef]
- Tytgat, L.; Van Damme, L.; Van Hoorick, J.; Declercq, H.; Thienpont, H.; Ottevaere, H.; Blondeel, P.; Dubruel, P.; Van Vlierberghe, S. Additive Manufacturing of Photo-Crosslinked Gelatin Scaffolds for Adipose Tissue Engineering. Acta Biomater. 2019, 94, 340–350. [Google Scholar] [CrossRef]
- Boluk, Y.; Lan, X.; Adesida, A. Additive Manufacturing by Gel-in-Gel Printing. Annu. Trans. Nord. Rheol. Soc. 2024, 32, 157–161. [Google Scholar] [CrossRef]
- Wang, P.; Winter, H.H.; Wagner, M.H.; Auhl, D. Gelation of PU Elastomers: Rheological Characterization for Liquid Additive Manufacturing. Rheol. Acta 2024, 63, 397–406. [Google Scholar] [CrossRef]
- Chimene, D.; Kaunas, R.; Gaharwar, A.K. Hydrogel Bioink Reinforcement for Additive Manufacturing: A Focused Review of Emerging Strategies. Adv. Mater. 2020, 32, 1902026. [Google Scholar] [CrossRef]
- Schwab, A.; Levato, R.; D’Este, M.; Piluso, S.; Eglin, D.; Malda, J. Printability and Shape Fidelity of Bioinks in 3D Bioprinting. Chem. Rev. 2020, 120, 11028–11055. [Google Scholar] [CrossRef]
- Warner, E.; Norton, I.; Mills, T. Comparing the Viscoelastic Properties of Gelatin and Different Concentrations of Kappa-Carrageenan Mixtures for Additive Manufacturing Applications. J. Food Eng. 2019, 246, 58–66. [Google Scholar] [CrossRef]
- Moura, D.; Pereira, R.F.; Goncalves, I.C. Recent Advances on Bioprinting of Hydrogels Containing Carbon Materials. Mater. Today Chem. 2022, 23, 100617. [Google Scholar] [CrossRef]
- Pereira, R.F.; Sousa, A.; Barrias, C.C.; Bártolo, P.J.; Granja, P.L. A Single-Component Hydrogel Bioink for Bioprinting of Bioengineered 3D Constructs for Dermal Tissue Engineering. Mater. Horiz. 2018, 5, 1100–1111. [Google Scholar] [CrossRef]
- Zehnder, T.; Sarker, B.; Boccaccini, A.R.; Detsch, R. Evaluation of an Alginate–Gelatine Crosslinked Hydrogel for Bioplotting. Biofabrication 2015, 7, 025001. [Google Scholar] [CrossRef] [PubMed]
- Ahlfeld, T.; Guduric, V.; Duin, S.; Akkineni, A.; Schütz, K.; Kilian, D.; Emmermacher, J.; Cubo-Mateo, N.; Dani, S.; Witzleben, M. v Methylcellulose–a Versatile Printing Material That Enables Biofabrication of Tissue Equivalents with High Shape Fidelity. Biomater. Sci. 2020, 8, 2102–2110. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tan, Y.J.; Kiran, R.; Tor, S.B.; Zhou, K. Submerged and Non-Submerged 3D Bioprinting Approaches for the Fabrication of Complex Structures with the Hydrogel Pair GelMA and Alginate/Methylcellulose. Addit. Manuf. 2021, 37, 101640. [Google Scholar] [CrossRef]
- Sakai, S.; Yoshii, A.; Sakurai, S.; Horii, K.; Nagasuna, O. Silk Fibroin Nanofibers: A Promising Ink Additive for Extrusion Three-Dimensional Bioprinting. Mater. Today Bio 2020, 8, 100078. [Google Scholar] [CrossRef] [PubMed]
- Hauptstein, J.; Forster, L.; Nadernezhad, A.; Horder, H.; Stahlhut, P.; Groll, J.; Blunk, T.; Teßmar, J. Bioink Platform Utilizing Dual-stage Crosslinking of Hyaluronic Acid Tailored for Chondrogenic Differentiation of Mesenchymal Stromal Cells. Macromol. Biosci. 2022, 22, 2100331. [Google Scholar] [CrossRef]
- Skardal, A.; Murphy, S.V.; Devarasetty, M.; Mead, I.; Kang, H.-W.; Seol, Y.-J.; Shrike Zhang, Y.; Shin, S.-R.; Zhao, L.; Aleman, J. Multi-Tissue Interactions in an Integrated Three-Tissue Organ-on-a-Chip Platform. Sci. Rep. 2017, 7, 8837. [Google Scholar] [CrossRef]
- Ouyang, L.; Highley, C.B.; Sun, W.; Burdick, J.A. A Generalizable Strategy for the 3D Bioprinting of Hydrogels from Nonviscous Photo-crosslinkable Inks. Adv. Mater. 2017, 29, 1604983. [Google Scholar] [CrossRef]
- Wang, Y.; Yue, H.; Liu, A.; Cui, Y.; Hou, Y.; Ni, X.; Pereira, R.F.; Huang, B.; Vyas, C.; Bartolo, P. Dual Crosslinkable Bioink for Direct and Embedded 3D Bioprinting at Physiological Temperature. Mater. Today 2025, 85, 1–16. [Google Scholar] [CrossRef]
- Ferris, C.J.; Gilmore, K.J.; Beirne, S.; McCallum, D.; Wallace, G.G. Bio-Ink for on-Demand Printing of Living Cells. Biomater. Sci. 2013, 1, 224–230. [Google Scholar] [CrossRef]
- Schwartz, R.; Malpica, M.; Thompson, G.L.; Miri, A.K. Cell Encapsulation in Gelatin Bioink Impairs 3D Bioprinting Resolution. J. Mech. Behav. Biomed. Mater. 2020, 103, 103524. [Google Scholar] [CrossRef]
- Billiet, T.; Gevaert, E.; De Schryver, T.; Cornelissen, M.; Dubruel, P. The 3D Printing of Gelatin Methacrylamide Cell-Laden Tissue-Engineered Constructs with High Cell Viability. Biomaterials 2014, 35, 49–62. [Google Scholar] [CrossRef]
- Petta, D.; Armiento, A.; Grijpma, D.; Alini, M.; Eglin, D.; D’este, M. 3D Bioprinting of a Hyaluronan Bioink through Enzymatic-and Visible Light-Crosslinking. Biofabrication 2018, 10, 044104. [Google Scholar] [CrossRef]
- Cengiz, N.; Gevrek, T.; Sanyal, R.; Sanyal, A. Orthogonal Thiol–Ene ‘Click’Reactions: A Powerful Combination for Fabrication and Functionalization of Patterned Hydrogels. Chem. Commun. 2017, 53, 8894–8897. [Google Scholar] [CrossRef]
- Lou, J.; Mooney, D.J. Chemical Strategies to Engineer Hydrogels for Cell Culture. Nat. Rev. Chem. 2022, 6, 726–744. [Google Scholar] [CrossRef]
- Lee, S.C.; Gillispie, G.; Prim, P.; Lee, S.J. Physical and Chemical Factors Influencing the Printability of Hydrogel-Based Extrusion Bioinks. Chem. Rev. 2020, 120, 10834–10886. [Google Scholar] [CrossRef]
- Yu, J.; Qiu, R.; Zheng, W.; Wang, K.; Liu, X.; Hu, Z.; Zhao, L. Multiphase Gel-Based Functional Inks in 3D Food Printing: A Review on Structural Design and Enhanced Bioaccessibility of Active Ingredients. Food Res. Int. 2025, 217, 116787. [Google Scholar] [CrossRef]
- Vadukoote, T.T.; Avestro, A.; Smith, D.K. 3D-Printing Multi-Component Multi-Domain Supramolecular Gels with Differential Conductivity. Angew. Chem. 2024, 136, e202409757. [Google Scholar] [CrossRef]
- Rutz, A.L.; Hyland, K.E.; Jakus, A.E.; Burghardt, W.R.; Shah, R.N. A Multi-Material Bioink Method for 3D Printing Tunable, Cell-Compatible Hydrogels. Adv. Mater. 2015, 27, 1607. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Yin, J.; Liu, P.; Li, Q.; Zhang, R.; Yang, H.; Zhou, H. Simultaneous Multi-Material Embedded Printing for 3D Heterogeneous Structures. Int. J. Extrem. Manuf. 2023, 5, 035001. [Google Scholar] [CrossRef]
- Brenneman, J.; Tansel, D.Z.; Fedder, G.K.; Panat, R. Interfacial Delamination and Delamination Mechanism Maps for 3D Printed Flexible Electrical Interconnects. Extrem. Mech. Lett. 2021, 43, 101199. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, Y.; Vyas, C.; Huang, B.; Bartolo, P. An Integrated Hybrid 3D Bioprinting of Heterogeneous and Zone-Specific Construct Resembling Structural and Biofunctional Properties of Osteochondral Tissue. Mater. Futur. 2025, 4, 025401. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, M.; Bhandari, B.; Liu, Y. Investigation on Lemon Juice Gel as Food Material for 3D Printing and Optimization of Printing Parameters. LWT 2018, 87, 67–76. [Google Scholar] [CrossRef]
- Díaz-Torres, E.; Rodríguez-Pombo, L.; Ong, J.J.; Basit, A.W.; Santoveña-Estévez, A.; Fariña, J.B.; Alvarez-Lorenzo, C.; Goyanes, A. Integrating Pressure Sensor Control into Semi-Solid Extrusion 3D Printing to Optimize Medicine Manufacturing. Int. J. Pharm. X 2022, 4, 100133. [Google Scholar] [CrossRef]
- Hajash, K.; Sparrman, B.; Guberan, C.; Laucks, J.; Tibbits, S. Large-Scale Rapid Liquid Printing. 3D Print. Addit. Manuf. 2017, 4, 123–132. [Google Scholar] [CrossRef]
- He, M.; Hou, Y.; Zhu, C.; He, M.; Jiang, Y.; Feng, G.; Liu, L.; Li, Y.; Chen, C.; Zhang, L. 3D-Printing Biodegradable PU/PAAM/Gel Hydrogel Scaffold with High Flexibility and Self-Adaptibility to Irregular Defects for Nonload-Bearing Bone Regeneration. Bioconjugate Chem. 2021, 32, 1915–1925. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; An, Y.; Li, M.; Zhao, X. Research on the Flow Behavior of Bio-Ink inside the Extrusion Nozzle during Printing. J. Appl. Phys. 2024, 136, 174701. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, G.; Xiao, Q.; Ji, A.; Feng, Y.; Zhu, G.; Zhou, L. 3D Printer Nozzle Structure Form Optimal Structural Analysis. Processes 2024, 12, 1482. [Google Scholar] [CrossRef]
- Jeon, O.; Lee, Y.B.; Jeong, H.; Lee, S.J.; Wells, D.; Alsberg, E. Individual Cell-Only Bioink and Photocurable Supporting Medium for 3D Printing and Generation of Engineered Tissues with Complex Geometries. Mater. Horiz. 2019, 6, 1625–1631. [Google Scholar] [CrossRef]
- Lian, L.; Zhou, C.; Tang, G.; Xie, M.; Wang, Z.; Luo, Z.; Japo, J.; Wang, D.; Zhou, J.; Wang, M. Uniaxial and Coaxial Vertical Embedded Extrusion Bioprinting. Adv. Healthc. Mater. 2022, 11, 2102411. [Google Scholar] [CrossRef]
- Lee, A.; Hudson, A.; Shiwarski, D.; Tashman, J.; Hinton, T.; Yerneni, S.; Bliley, J.; Campbell, P.; Feinberg, A. 3D Bioprinting of Collagen to Rebuild Components of the Human Heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef]
- Karvinen, J.; Kellomäki, M. Design Aspects and Characterization of Hydrogel-Based Bioinks for Extrusion-Based Bioprinting. Bioprinting 2023, 32, e00274. [Google Scholar] [CrossRef]
- Fu, Z.; Angeline, V.; Sun, W. Evaluation of Printing Parameters on 3D Extrusion Printing of Pluronic Hydrogels and Machine Learning Guided Parameter Recommendation. Int. J. Bioprinting 2021, 7, 434. [Google Scholar] [CrossRef]
- Sadeghzade, S.; Cao, J.; Yang, R.; Li, Y.; Li, Y.; Zhang, D.; Liu, J.; Yu, Z.; Fang, L.; Yuan, H. Highly Stretchable Alginate/Methylcellulose Hydrogels for 3D Bio-Printing: Photopolymerization Approach Enhancing Structural Integrity. Giant 2024, 18, 100280. [Google Scholar] [CrossRef]
- Jin, Y.; Zhao, D.; Huang, Y. Study of Extrudability and Standoff Distance Effect during Nanoclay-Enabled Direct Printing. Bio-Des. Manuf. 2018, 1, 123–134. [Google Scholar] [CrossRef]
- Xu, Q.; Jiang, J. Machine Learning for Polymer Swelling in Liquids. ACS Appl. Polym. Mater. 2020, 2, 3576–3586. [Google Scholar] [CrossRef]
- Targhi, E.K.; Niri, M.E.; Zitha, P.L. Design of Artificial Neural Network for Predicting the Reduction in Permeability of Porous Media as a Result of Polymer Gel Injection. Geoenergy Sci. Eng. 2023, 227, 211925. [Google Scholar] [CrossRef]
- Moreno-Guerra, J.A.; Gómez-Solís, C.; Alarcón, F.; Ríos-Orihuela, J.F.; Laurati, M.; Castañeda-Priego, R. Artificial Neural Network-Based Predictions of the Viscoelastic Properties of Polymer Matrix Composites. Phys. Fluids 2025, 37, 063104. [Google Scholar]
- Wang, Y.; Wallmersperger, T.; Ehrenhofer, A. Prediction of Hydrogel Swelling States Using Machine Learning Methods. Eng. Rep. 2024, 6, e12893. [Google Scholar] [CrossRef]
- Cadamuro, F.; Piazzoni, M.; Gamba, E.; Sonzongni, B.; Previdi, F.; Nicotra, F.; Ferramosca, A.; Russo, L. AI Tool for Prediction of ECM Mimics Hydrogel Formulations via Click Chemistry. Biomater. Adv. 2025, 175, 214323. [Google Scholar]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Xu, T.; Wang, J.; Zhao, S.; Chen, D.; Zhang, H.; Fang, Y.; Kong, N.; Zhou, Z.; Li, W.; Wang, H. Accelerating the Prediction and Discovery of Peptide Hydrogels with Human-in-the-Loop. Nat. Commun. 2023, 14, 3880. [Google Scholar] [CrossRef] [PubMed]
- Correa, S.; Grosskopf, A.K.; Lopez Hernandez, H.; Chan, D.; Yu, A.C.; Stapleton, L.M.; Appel, E.A. Translational Applications of Hydrogels. Chem. Rev. 2021, 121, 11385–11457. [Google Scholar] [CrossRef]
- Abdolazizi, K.P.; Linka, K.; Cyron, C.J. Viscoelastic Constitutive Artificial Neural Networks (vCANNs)–A Framework for Data-Driven Anisotropic Nonlinear Finite Viscoelasticity. J. Comput. Phys. 2024, 499, 112704. [Google Scholar] [CrossRef]
- Lie-Piang, A.; Garre, A.; Nissink, T.; Van Beek, N.; Van Der Padt, A.; Boom, R. Machine Learning to Quantify Techno-Functional Properties—A Case Study for Gel Stiffness with Pea Ingredients. Innov. Food Sci. Emerg. Technol. 2023, 83, 103242. [Google Scholar] [CrossRef]
- Núñez Carrero, K.C.; Velasco-Merino, C.; Asensio, M.; Guerrero, J.; Merino, J.C. Rheological Method for Determining the Molecular Weight of Collagen Gels by Using a Machine Learning Technique. Polymers 2022, 14, 3683. [Google Scholar] [CrossRef]
- Lyngdoh, G.A.; Li, H.; Zaki, M.; Krishnan, N.M.A.; Das, S. Elucidating the Constitutive Relationship of Calcium–Silicate–Hydrate Gel Using High Throughput Reactive Molecular Simulations and Machine Learning. Sci. Rep. 2020, 10, 21336. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hu, L.; Chen, S.; Ran, Y.; Pang, J.; Wu, S. Machine Learning Analysis for the Rheological Mechanism of Polysaccharide Colloids. J. Mol. Liq. 2025, 424, 127093. [Google Scholar] [CrossRef]
- Aldhaheri, M.; Wei, M.; Bai, B.; Alsaba, M. Development of Machine Learning Methodology for Polymer Gels Screening for Injection Wells. J. Pet. Sci. Eng. 2017, 151, 77–93. [Google Scholar] [CrossRef]
- Sarah, R.; Schimmelpfennig, K.; Rohauer, R.; Lewis, C.L.; Limon, S.M.; Habib, A. Characterization and Machine Learning-Driven Property Prediction of a Novel Hybrid Hydrogel Bioink Considering Extrusion-Based 3D Bioprinting. Gels 2025, 11, 45. [Google Scholar] [CrossRef]
- Seifermann, M.; Reiser, P.; Friederich, P.; Levkin, P.A. High-Throughput Synthesis and Machine Learning Assisted Design of Photodegradable Hydrogels. Small Methods 2023, 7, 2300553. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, S.; Hu, D.; Liu, J.; Zhang, Y.; Li, Z.; Su, Z.; Liang, D. Interpretable Machine Learning Analysis of Design Factors in Hydrogel Supercapacitors. Gels 2025, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Khalvandi, A.; Tayebi, L.; Kamarian, S.; Saber-Samandari, S.; Song, J. Data-Driven Supervised Machine Learning to Predict the Compressive Response of Porous PVA/Gelatin Hydrogels and in-Vitro Assessments: Employing Design of Experiments. Int. J. Biol. Macromol. 2023, 253, 126906. [Google Scholar] [CrossRef]
- Gillispie, G.; Prim, P.; Copus, J.; Fisher, J.; Mikos, A.G.; Yoo, J.J.; Atala, A.; Lee, S.J. Assessment Methodologies for Extrusion-Based Bioink Printability. Biofabrication 2020, 12, 022003. [Google Scholar] [CrossRef]
- Dahl, J.F.; Schlangen, M.; Jan Van Der Goot, A.; Corredig, M. Predicting Rheological Parameters of Food Biopolymer Mixtures Using Machine Learning. Food Hydrocoll. 2025, 160, 110786. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Singh, M.; Tong, Y.; Kucukdeger, E.; Yoon, H.Y.; Haring, A.P.; Roman, M.; Kong, Z.J.; Johnson, B.N. Rapid, Autonomous High-Throughput Characterization of Hydrogel Rheological Properties via Automated Sensing and Physics-Guided Machine Learning. Appl. Mater. Today 2023, 30, 101720. [Google Scholar] [CrossRef]
- Nunekpeku, X.; Zhang, W.; Gao, J.; Adade, S.Y.-S.S.; Li, H.; Chen, Q. Gel Strength Prediction in Ultrasonicated Chicken Mince: Fusing near-Infrared and Raman Spectroscopy Coupled with Deep Learning LSTM Algorithm. Food Control 2025, 168, 110916. [Google Scholar] [CrossRef]
- Hashemi, A.; Ezati, M.; Zumberg, I.; Vicar, T.; Chmelikova, L.; Cmiel, V.; Provaznik, V. Characterization and Optimization of a Biomaterial Ink Aided by Machine Learning-Assisted Parameter Suggestion. Mater. Today Commun. 2024, 40, 109777. [Google Scholar] [CrossRef]
- Bone, J.M.; Childs, C.M.; Menon, A.; Póczos, B.; Feinberg, A.W.; LeDuc, P.R.; Washburn, N.R. Hierarchical Machine Learning for High-Fidelity 3D Printed Biopolymers. ACS Biomater. Sci. Eng. 2020, 6, 7021–7031. [Google Scholar] [CrossRef]
- Bozorg, N.M.; Leclercq, M.; Lescot, T.; Bazin, M.; Gaudreault, N.; Dikpati, A.; Fortin, M.-A.; Droit, A.; Bertrand, N. Design of Experiment and Machine Learning Inform on the 3D Printing of Hydrogels for Biomedical Applications. Biomater. Adv. 2023, 153, 213533. [Google Scholar]
- Krishna, D.V.; Sankar, M.R. Machine Learning-Assisted Extrusion-Based 3D Bioprinting for Tissue Regeneration Applications. Ann. 3D Print. Med. 2023, 12, 100132. [Google Scholar] [CrossRef]
- Farhan Khan, M.; Alam, A.; Ateeb Siddiqui, M.; Saad Alam, M.; Rafat, Y.; Salik, N.; Al-Saidan, I. Real-Time Defect Detection in 3D Printing Using Machine Learning. Mater. Today Proc. 2021, 42, 521–528. [Google Scholar] [CrossRef]
- Liao, C.-Y.; Hsueh, T.-W. Deposition Width Prediction in Extrusion-Based Bioprinting Using Deep Learning with Thermal Imager Temperature Series. In Proceedings of the 2024 IEEE 20th International Conference on Automation Science and Engineering (CASE), Bari, Italy, 28 August–1 September 2024; IEEE: Piscataway, NJ, USA, 2024; pp. 2268–2273. [Google Scholar]
- Raccuglia, P.; Elbert, K.C.; Adler, P.D.; Falk, C.; Wenny, M.B.; Mollo, A.; Zeller, M.; Friedler, S.A.; Schrier, J.; Norquist, A.J. Machine-Learning-Assisted Materials Discovery Using Failed Experiments. Nature 2016, 533, 73–76. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, J.; Zhang, J.; Zhang, L.; Song, B.; Shi, Y. Mechanism of Simultaneous Improvement of Mechanical Performance and Conductivity of TiC/Cu Composites Prepared by Laser Powder Bed Fusion. J. Alloys Compd. 2024, 1002, 175281. [Google Scholar] [CrossRef]
- Full Article: Revisiting Kernel Logistic Regression Under the Random Utility Models Perspective. An Interpretable Machine-Learning Approach. Available online: https://www.tandfonline.com/doi/full/10.1080/19427867.2020.1861504?casa_token=all718V6ZsoAAAAA%3Ag1DrwVfwt6afuKxr8etYe9WqdyTrwqeGgJpurVLYnR4aDAAeQSJ6GssAdud4NfabKo07EHkLePyEXuA (accessed on 19 July 2025).
- Aria, M.; Cuccurullo, C.; Gnasso, A. A Comparison among Interpretative Proposals for Random Forests. Mach. Learn. Appl. 2021, 6, 100094. [Google Scholar] [CrossRef]
- Optimization and Interpretability Analysis of Machine Learning Methods for ZnO Colloid Particle Size Prediction|ACS Applied Nano Materials. Available online: https://pubs.acs.org/doi/full/10.1021/acsanm.4c05229?casa_token=EUqPzvWfbzwAAAAA%3AwU5lzmOxH_k1neyqT_0EJRphifmjJZ4FdFj9otX6DwQO4QM2NY0yeXl8wxRJmXSjFkiorxhlPgyIBleajQ (accessed on 19 July 2025).
- Swab, M. Mendeley Data. J. Can. Health Libr. Assoc. Assoc. Bibl. Santé Can. 2016, 37, 121–123. [Google Scholar] [CrossRef]
- Turner, G.; Culhane, M.; Delwar, I.; Sizemore, J. Data Archive-Zenodo. 2017. Available online: http://hdl.handle.net/10919/77614 (accessed on 10 May 2017).
- Singh, J. FigShare. J. Pharmacol. Pharmacother. 2011, 2, 138. [Google Scholar] [CrossRef]
- Plan, N.P.A. National Institute of Standards and Technology (NIST). Available online: https://www.nist.gov (accessed on 1 June 2025).
- Flores, A. AmeriGEOSS Projects; 2018. Available online: https://data.amerigeoss.org (accessed on 1 June 2025).
- Menon, A.; Póczos, B.; Feinberg, A.W.; Washburn, N.R. Optimization of Silicone 3D Printing with Hierarchical Machine Learning. 3D Print. Addit. Manuf. 2019, 6, 181–189. [Google Scholar] [CrossRef]
- Lu, Y.; Rai, R.; Nitin, N. Image-Based Assessment and Machine Learning-Enabled Prediction of Printability of Polysaccharides-Based Food Ink for 3D Printing. Food Res. Int. 2023, 173, 113384. [Google Scholar] [CrossRef]
- Kelly, D.; Sergis, V.; Ventura, I.; Blanco, L.; Mason, K.; Daly, A.C. Autonomous Control of Extrusion Bioprinting Using Convolutional Neural Networks. Adv. Funct. Mater. 2025, 2424553. [Google Scholar] [CrossRef]
- Madrid Wolff, J.A. Volumetric Additive Manufacturing in a Scattering Resin. Ph.D. Thesis, EPFL, Lausanne, Switzerland, 2023. [Google Scholar]
- Qavi, I.; Halder, S.; Tan, G. Optimization of Printability of Bioinks with Multi-Response Optimization (MRO) and Artificial Neural Networks (ANN). Prog. Addit. Manuf. 2025, 10, 3573–3598. [Google Scholar] [CrossRef]
- Ege, D.; Boccaccini, A.R. Investigating the Effect of Processing and Material Parameters of Alginate Dialdehyde-Gelatin (ADA-GEL)-Based Hydrogels on Stiffness by XGB Machine Learning Model. Bioengineering 2024, 11, 415. [Google Scholar] [CrossRef]
- Tanneberger, A.; Blomberg, R.; Bilosouva, G.; Ryan, A.; Magin, C. Supplemental Material: Engineered Hydrogel Biomaterials Facilitate Lung Progenitor Cell Differentiation from Induced Pluripotent Stem Cells. Bioengineering 2024, 11, 415. [Google Scholar]
- Zhang, C.; Fei, Y.; Li, M.; Li, J.; Tang, M.; Wang, G.; Li, J.; Wang, Y.; Ding, Y.; Peng, C. Chitosan-P407-PNIPAM Hydrogel Loaded with AgNPs/Lipid Complex for Antibacterial, Inflammation Regulation and Alveolar Bone Regeneration in Periodontitis Treatment. Int. J. Biol. Macromol. 2025, 307, 142080. [Google Scholar] [CrossRef]
- Megan, J.; Kartik, B.; Michael, P.; Grace, Z.L.; Kausik, S. Hydrogel Characterization Data; V1; Mendeley Data: London, UK, 2024. [Google Scholar] [CrossRef]
- Lulu, G.; Yani, Z.; Zili, L.; Jinpeng, S.; Fan, Y. SDS-PAGE Gels of GPR4–Gs/q, GPR68-Gs and GPR4apo-Anti BRIL Fab Complexes; V1; Mendeley Data: London, UK, 2024. [Google Scholar] [CrossRef]
- Usman, A.; Kong, Y.C.; Haotian, Z. Construction of Cold-Set Mickering Emulsion Gel Using Whey Protein Assembly Particles as Oil-Water Interfacial Stabilizer and Gelling Agent: Phase Stability, Nonlinear Rheology, and Tribology; V1; Mendeley Data: London, UK, 2025. [Google Scholar] [CrossRef]
- Smith, K.M.; Hsiao, L.C. Migration and Morphology of Colloidal Gel Clusters in Cylindrical Channel Flow. Langmuir 2021, 37, 10308–10318. [Google Scholar] [CrossRef]
- Babnigg, G.; Giometti, C.S. GELBANK: A Database of Annotated Two-dimensional Gel Electrophoresis Patterns of Biological Systems with Completed Genomes. Nucleic Acids Res. 2004, 32, D582–D585. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Romberg, S.K.; Lehrman, S.; Seppala, J.; Kotula, A.P. Identifying the Chemical Gel Point of Thermoset Composites Using Optimally Windowed Chirp Measurements; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2024. [Google Scholar] [CrossRef]
- Angelopoulou, A.; Efthimiadou, E.; Kordas, G. Dextran Modified pH Sensitive Silica Hydro-Xerogels as Promising Drug Delivery Scaffolds. Mater. Lett. 2012, 74, 50–53. [Google Scholar] [CrossRef]
- Efthimiadou, E.; Tapeinos, C.; Bilalis, P.; Kordas, G. New Approach in Synthesis, Characterization and Release Study of pH-Sensitive Polymeric Micelles, Based on PLA-Lys-b-PEGm, Conjugated with Doxorubicin. J. Nanoparticle Res. 2011, 13, 6725–6736. [Google Scholar] [CrossRef]
- Keshava, P.S.; Kumar, H.; Kumar, D. Effectiveness and Side Effects of Benzoyl Peroxide 2.5% Gel and Adapalene 0.1% Gel as Monotherapies and Combination Therapies for Face Acne. Int. J. Pharm. Clin. Res. 2023, 15, 2043–2048. [Google Scholar] [CrossRef]
- Braat, Q.J.S. Data of Graduation Project “Anisotropic Swelling of a Cylindrical Hydrogel”; V1, [Data Set]; Zenodo: Geneva, Switzerland, 2021. [Google Scholar] [CrossRef]
- Metaxa, A.-F.; Efthimiadou, E.K.; Boukos, N.; Kordas, G. Polysaccharides as a Source of Advanced Materials: Cellulose Hollow Microspheres for Drug Delivery in Cancer Therapy. J. Colloid Interface Sci. 2012, 384, 198–206. [Google Scholar] [CrossRef]
- Alnamoly, M.H.; Alzohairy, A.M.; El-Henawy, I.M. A Survey on Gel Images Analysis Software Tools. J. Intell. Syst. Internet Things 2020, 1, 48–60. [Google Scholar]
- Katke, C.; Korevaar, P.A.; Kaplan, C.N. Codes and Movies for: Diffusio-Phoretic Fast Swelling of Chemically Responsive Hydrogels; Zenodo: Geneva, Switzerland, 2024. [Google Scholar] [CrossRef]
- Bhillare, S.S. Formulation and Evaluation of Herbal Gel for Wound Healing. Int. J. Res. Publ. Rev. 2025, 6, 1698–1704. [Google Scholar]
- Cornish, K.; Scott, D.J.; Xie, W.; Mau, C.J.D.; Zheng, Y.F.; Liu, X.; Prestwich, G.D. Figure 11 in Unusual Subunits Are Directly Involved in Binding Substrates for Natural Rubber Biosynthesis in Multiple Plant Species. Phytochemistry 2018, 156, 55–72. [Google Scholar] [CrossRef]
- Yele, P.K. The Preliminary Phytochemical Screening and Evaluation of Herbal Antibacterial Gel of Ipomoea Carnea. Int. J. Pharm. Biol. Sci. IJPBS 2023, 13, 174–179. [Google Scholar] [CrossRef]
- Jassim, B.M.; Fadhil, A.A.; Eman, B.H. AL-Khedairy Gel in Vitro Release and Gel Rheogram 2023. Available online: https://zenodo.org/records/7578593 (accessed on 28 January 2023).
- Sukharev, Y.I.; Apalikova, I.Y.; Markov, B.A. New Principles for Investigation of Imperfect Crystallographic Forms of Colloid-Chemical Clusters. ИННОВАЦИОННЫЕ НАУЧНЫЕ ИССЛЕДОВАНИЯ 2023, 28, 4–28. [Google Scholar] [CrossRef]
- Khirodkra, K.; Rathod, P.; Jadhav, A.; Deshmukh, S. Formulation and Evaluation of Ashwagandha Gel Using for Treatment of Acne. Int. J. Pharm. Sci. 2025, 3, 3103–3111. [Google Scholar] [CrossRef]
- Dive, A.; Pandav, A.; Chougule, N. Preparation and Evaluation of Gel by Using Lemon Grass Oil and Eucalyptus Oil. Int. J. Pharm. Sci. 2025, 3, 3285–3291. [Google Scholar] [CrossRef]
- Sirisha, Y. Formulation and Evaluation of Chlorhexidine, Aloe Vera Gel Sodium Alginate Beads Incorporated Gel for Topical Application. Int. J. Pharm. Biol. Sci. IJPBS 2024, 14, 72–78. [Google Scholar] [CrossRef]
- Tomar, S.; Gorase, A.; Ware, P.; Jagzap, K.; Shinde, R. Formulation and Evaluation of Herbal Mouth Ulcer Gel by Utilizing Psidium Guajava and Jasminum Officinale. Int. J. Pharm. Sci. 2025, 3, 351–367. [Google Scholar] [CrossRef]
- Rathod, N.; Tikait, A.; Deshmukh, D.S.; Wankhade, S.; Rathod, A. Formulation and Evaluation of Guar Gum Gel for Wound Healing. Int. J. Pharm. Sci. 2025, 3, 1859–1866. [Google Scholar] [CrossRef]
- Manjula, T.K.; Nirosha, C.; Nandhini, D.; Revathi, V. Formulation and Evaluation of Fenugreek Gel for Anti Inflammatory Activity; Zenodo: Geneva, Switzerland, 2025. [Google Scholar]
- Semchenko, K.; Vyshnevska, L. Justification of the Gel Formers Selection in the Development of Oromucosal Drug in the Form of Troches. Ann. Mechnikov. Inst. 2019, 4, 42–44. [Google Scholar] [CrossRef]
- Mumtaj Begum, A.; Sibiraj, V.; Pharm, M.; Dhanalakshmi, V.; Kiruthiga, R.; Narmatha, J. Formulation and Evaluation of Thespesia Populnea Gel for Its Antiinflammatory and Analgesic Activity. Indo Am. J. Pharm. Res. 2025, 12, 401–413. [Google Scholar]
- Sa’ait, N.; Panit, G.; Abdullah, N.R.A.; Kastri, R.M.A.; Peter, D.J.A. Quick Gel. In APS Proceedings Volume 14; Zenodo: Geneva, Switzerland, 2024; pp. 157–160. [Google Scholar]
- Jassim, B.M.; Fadhil, A.A.; Eman, B.H. AL-Khedairy GEL RELEASE 2023. Available online: https://zenodo.org/records/7626659 (accessed on 9 February 2023).
- Garg, H. Gel Electrophoresis 2022. Available online: https://zenodo.org/records/14027618 (accessed on 1 December 2022).
- Parveen, N.; Saini, S.K. Formulation And Evaluation of Harbel Gel of Berberis Aristata for Anti-Psoriatic Activity. Int. J. Pharm. Sci. 2025, 3, 1446–1453. [Google Scholar] [CrossRef]
- RAO, M.R.A. Investigations on the Adsorptive Property of Silica Gel. Part II. Adsorptive Properties of Silica Gel Containing Residual Hydrogen Chloride. J. Indian Chem. Soc. 1935, 12, 340–342. [Google Scholar] [CrossRef]
- Gajubhai, P.H.; Jain, S.K.; Kankane, M. Development and in Vitro Characterization of the Proliposome Gel of Terbinafine Hydrochloride. World J. Biol. Pharm. Health Sci. 2024, 17, 198–214. [Google Scholar] [CrossRef]
- RAO, M.R.A. Investigations on the Adsorptive Property of Silica Gel. Part V. The Specific Grayity of Silica Gel under Various Liquids. J. Indian Chem. Soc. 1935, 12, 326–330. [Google Scholar] [CrossRef]
- Dangeti, A.; Jeeri, V.V.T.A.K.R.; Sarella, P.N.K.; Mogili, M. Natural Antibacterial Gel to Fight Tooth Decay: An in-Silico Modeling Approach. World J. Biol. Pharm. Health Sci. 2023, 14, 262–272. [Google Scholar] [CrossRef]
- Kovalyov, V.; Oliinyk, S.; Buryak, M.; Pul-Luzan, V.; Levachkova, Y.; Yakovenko, O. Study of the Antimicrobial Activity and Technological Properties of a Gel with Catalpa Bignonioides Extract and Dexpanthenol. Ann. Mechnikov’s Inst. 2024, 2, 55–60. [Google Scholar] [CrossRef]
- Madhulika; Gupta, M.K. Formulation and Development of Transferosomal Gel Incorporating with Chrysin for Topical Application. Indo Am. J. Pharm. Sci. 2023, 10, 170–176. [Google Scholar]
- Patil, D.R.; Patil, C.B.; Patil, A.N.; Pawar, D.S.P. An Review of the Innovative Method Used in Medicinal Gels. J. Adv. Res. Rev. Virol. Microbiol. 2024, 1, 1–12. [Google Scholar] [CrossRef]
- Tanmay Mangulkar, C.K.; Matre, R.; Miratkar, P.; Mirche, S.; Mitkari, R.; Mule, V.; Jadhav, L. A Review on Guava Leaf As A Herbal Gel. Int. J. Pharm. Sci. 2025, 3, 2738–2743. [Google Scholar] [CrossRef]
- Ruban, O.A.; Maslii, Y.S. Research on the Choice of Rational Concentration of the Gel Forming Agent in the Composition of Dental Gel. Ann. Mechnikov’s Inst. 2019, 2, 29–33. [Google Scholar] [CrossRef]
- Reay, S.L. Gel Uniformity Formed Gel. 2022. Available online: https://data.ncl.ac.uk/articles/dataset/Gel_uniformity_formed_gel/20552526/1 (accessed on 23 August 2022).
- Péden, R. Dreissena Rostriformis Bugensis 2DE Gels. 2024. Available online: https://figshare.com/articles/dataset/_i_Dreissena_rostriformis_bugensis_i_2DE_gels/25412314/1 (accessed on 14 March 2024).
- Zafer, M.Z. Gel Images Raw Data (Uncroped). 2024. Available online: https://figshare.com/articles/figure/Gel_Images_raw_data_uncroped_/28080665/1 (accessed on 23 December 2024).
- Harris, S.; Watt, R. 03-146 Gel Band Intensity.Xlsx. 2024. Available online: https://figshare.com/articles/dataset/03-146_Gel_Band_Intensity_xlsx/27499530/1 (accessed on 5 November 2024).
- Reay, S.L. Gel Uniformity 168 Hours Post Lysozyme Addition. 2022. Available online: https://data.ncl.ac.uk/articles/dataset/Gel_uniformity_168_hours_post_lysozyme_addition/20552532/1 (accessed on 23 August 2022).
- Li, R.; Wang, Z.; Huang, J.; He, S.; Peng, Y.; Wan, Y.; Ma, Q. Neutrophils Culture in Collagen Gel System. Front. Immunol. 2022, 13, 816037. [Google Scholar] [CrossRef]
- Xu, W.; Wang, T.; Wang, Y.; Wu, X.; Chen, Y.; Song, D.; Ci, Z.; Cao, Y.; Hua, Y.; Zhou, G.; et al. An Injectable Platform of Engineered Cartilage Gel and Gelatin Methacrylate to Promote Cartilage Regeneration. Front. Bioeng. Biotechnol. 2022, 10, 884036. [Google Scholar] [CrossRef]
- Seright, R.S.; Schrader, R.; II Hagstrom, J.; Wang, Y.; Al-Dahfeeri, A.; Gary, R.; Marin; Amaury; Lindquist, B. Conformance Improvement Using Gels. United States, 26 AD. Available online: https://www.osti.gov/biblio/824385 (accessed on 1 September 2003).
- Di Salvo, R. High Energy, Low Temperature Gelled Bi-Propellant Formulation. U.S. Patent 7,896,987, 1 March 2011. [Google Scholar]
- Varoquaux, G.; Buitinck, L.; Louppe, G.; Grisel, O.; Pedregosa, F.; Mueller, A. Scikit-Learn: Machine Learning without Learning the Machinery. GetMobile Mob. Comput. Commun. 2015, 19, 29–33. [Google Scholar] [CrossRef]
- Yamanishi, C.; Parigoris, E.; Takayama, S. Kinetic Analysis of Label-Free Microscale Collagen Gel Contraction Using Machine Learning-Aided Image Analysis. Front. Bioeng. Biotechnol. 2020, 8, 582602. [Google Scholar] [CrossRef]
- Gazo Hanna, E.; Younes, K.; Amine, S.; Roufayel, R. Exploring Gel-Point Identification in Epoxy Resin Using Rheology and Unsupervised Learning. Gels 2023, 9, 828. [Google Scholar] [CrossRef]
- Magzoub, M.I.; Kiran, R.; Salehi, S.; Hussein, I.A.; Nasser, M.S. Assessing the Relation between Mud Components and Rheology for Loss Circulation Prevention Using Polymeric Gels: A Machine Learning Approach. Energies 2021, 14, 1377. [Google Scholar] [CrossRef]
- Schwanekamp, T.; Engelhardt, L.; Reuber, M.; Beste, U. Additive Manufacturing of CrC-Enriched WC-Co Hybrid Carbide with Laser Based—Powder Bed Fusion. Int. J. Refract. Met. Hard Mater. 2025, 128, 107033. [Google Scholar] [CrossRef]
- Tafreshi, O.A.; Saadatnia, Z.; Ghaffari-Mosanenzadeh, S.; Okhovatian, S.; Park, C.B.; Naguib, H.E. Machine Learning-based Model for Predicting the Material Properties of Nanostructured Aerogels. SPE Polym. 2023, 4, 24–37. [Google Scholar] [CrossRef]
- Kong, Y.; Chen, J.; Guo, R.; Huang, Q. Regulation of Tapioca Starch 3D Printability by Yeast Protein: Rheological, Textural Evaluation, and Machine Learning Prediction. J. Food Eng. 2025, 387, 112341. [Google Scholar] [CrossRef]
- Developers, T. TensorFlow; Zenodo: Geneva, Switzerland, 2022. [Google Scholar]
- Imambi, S.; Prakash, K.B.; Kanagachidambaresan, G. PyTorch. In Programming with TensorFlow: Solution for edge computing applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 87–104. [Google Scholar]
- Jain, A.; Armstrong, C.D.; Joseph, V.R.; Ramprasad, R.; Qi, H.J. Machine-Guided Discovery of Acrylate Photopolymer Compositions. ACS Appl. Mater. Interfaces 2024, 16, 17992–18000. [Google Scholar] [CrossRef]
- Rafieyan, S.; Ansari, E.; Vasheghani-Farahani, E. A Practical Machine Learning Approach for Predicting the Quality of 3D (Bio) Printed Scaffolds. Biofabrication 2024, 16, 045014. [Google Scholar] [CrossRef] [PubMed]
- Gulli, A.; Pal, S. Deep Learning with Keras; Packt Publishing Ltd.: Birmingham, UK, 2017; ISBN 1-78712-903-9. [Google Scholar]
- De Ville, B. Decision Trees. Wiley Interdiscip. Rev. Comput. Stat. 2013, 5, 448–455. [Google Scholar] [CrossRef]
- Hearst, M.A.; Dumais, S.T.; Osuna, E.; Platt, J.; Scholkopf, B. Support Vector Machines. IEEE Intell. Syst. Their Appl. 1998, 13, 18–28. [Google Scholar] [CrossRef]
- Ketkar, N.; Moolayil, J.; Ketkar, N.; Moolayil, J. Convolutional Neural Networks. Deep. Learn. Python Learn. Best. Pract. Deep. Learn. Models PyTorch 2021, 197–242. [Google Scholar]
- Miikkulainen, R.; Liang, J.; Meyerson, E.; Rawal, A.; Fink, D.; Francon, O.; Raju, B.; Shahrzad, H.; Navruzyan, A.; Duffy, N. Evolving Deep Neural Networks. In Artificial Intelligence in the Age of Neural Networks and Brain Computing; Elsevier: Amsterdam, Netherlands, 2024; pp. 269–287. [Google Scholar]
- Allencherry, J.; Pradeep, N.; Shrivastava, R.; Joy, L.; Imbriacco, F.; Özel, T. Investigation of Hydrogel and Gelatin Bath Formulations for Extrusion-Based 3D Bioprinting Using Deep Learning. Procedia CIRP 2022, 110, 360–365. [Google Scholar] [CrossRef]
- Ege, D.; Arıcı, Ş. Investigation of Effect of Processing Parameters of 3D Printed NHS/EDC Crosslinked Carboxy Methyl Cellulose/Gelatin Hydrogels with Machine Learning Techniques. Mater. Res. Express 2024, 11, 045304. [Google Scholar] [CrossRef]
- Mukherjee, A.; Sarker, S.; Kumar, R.; Sahani, A.; Das, B. Optimization of Bio-Ink Using Machine Learning. In Artificial Intelligence in Tissue and Organ Regeneration; Elsevier: Amsterdam, The Netherlands, 2023; pp. 155–174. [Google Scholar]
- Ruberu, K.; Senadeera, M.; Rana, S.; Gupta, S.; Chung, J.; Yue, Z.; Venkatesh, S.; Wallace, G. Coupling Machine Learning with 3D Bioprinting to Fast Track Optimisation of Extrusion Printing. Appl. Mater. Today 2021, 22, 100914. [Google Scholar] [CrossRef]
- Yang, T.; Jin, Y.; Smith, L.M.; Dahotre, N.B.; Neogi, A. Real-Time in-Situ Ultrasound Monitoring of Soft Hydrogel 3D Printing with Subwavelength Resolution. Commun. Eng. 2024, 3, 162. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S. Machine Learning Techniques for Quality Assurance in Additive Manufacturing Processes. IJAMD 2024, 1, 21–40. [Google Scholar] [CrossRef]
- Sun, Z.; Lu, Y.; Zhao, Q.; Wu, J. A New Stereolithographic 3D Printing Strategy for Hydrogels with a Large Mechanical Tunability and Self-Weldability. Addit. Manuf. 2022, 50, 102563. [Google Scholar] [CrossRef]
- Go, K.; Kim, D.-M.; Lee, K.J. 3D Printable Hydrogel Filament with Functionalizable Moiety for In-Situ Flow-Based Sensor. Macromol. Res. 2024, 32, 467–473. [Google Scholar] [CrossRef]
- Bom, S.; Ribeiro, R.; Ribeiro, H.M.; Santos, C.; Marto, J. On the Progress of Hydrogel-Based 3D Printing: Correlating Rheological Properties with Printing Behaviour. Int. J. Pharm. 2022, 615, 121506. [Google Scholar] [CrossRef]
- Sun, W.; Webster-Wood, V. An Integrated Computer Vision System for Real-Time Monitoring and Control of Long-Fiber Embedded Hydrogel 3D Printing. Int. Conf. Addit. Manuf. Better World AMBW 2022 2022, 70, 376–381. [Google Scholar] [CrossRef]
- Bin Abu Sofian, A.D.A.; Lim, H.R.; Chew, K.W.; Show, P.L. Advancing 3D Printing through Integration of Machine Learning with Algae-Based Biopolymers. ChemBioEng. Rev. 2024, 11, 406–425. [Google Scholar] [CrossRef]
- Vanaei, S.; Vanaei, S.; Kanhonou, M.; Khelladi, S.; Tcharkhtchi, A.; Vanaei, H.R. Importance of Machine Learning in 3D Bioprinting. 3D Bioprinting Lab. Ind. 2024, 391–409. [Google Scholar]
- Gong, W.; Yao, H.-B.; Chen, T.; Xu, Y.; Fang, Y.; Zhang, H.-Y.; Li, B.-W.; Hu, J.-N. Smartphone Platform Based on Gelatin Methacryloyl (GelMA) Combined with Deep Learning Models for Real-Time Monitoring of Food Freshness. Talanta 2023, 253, 124057. [Google Scholar] [CrossRef]
- Lipskas, J.; Deep, K.; Yao, W. Robotic-Assisted 3D Bio-Printing for Repairing Bone and Cartilage Defects through a Minimally Invasive Approach. Sci. Rep. 2019, 9, 3746. [Google Scholar] [CrossRef]
- Li, X.; Lian, Q.; Li, D.; Xin, H.; Jia, S. Development of a Robotic Arm Based Hydrogel Additive Manufacturing System for In-Situ Printing. Appl. Sci. 2017, 7, 73. [Google Scholar] [CrossRef]
- Menghani, G. Efficient Deep Learning: A Survey on Making Deep Learning Models Smaller, Faster, and Better. ACM Comput. Surv. 2023, 55, 1–37. [Google Scholar] [CrossRef]
- Althati, C.; Tomar, M.; Shanmugam, L. Enhancing Data Integration and Management: The Role of AI and Machine Learning in Modern Data Platforms. J. Artif. Intell. Gen. Sci. JAIGS 2024, 2, 220–232. [Google Scholar] [CrossRef]
- Ramakers, R.; Anderson, F.; Grossman, T.; Fitzmaurice, G. Retrofab: A Design Tool for Retrofitting Physical Interfaces Using Actuators, Sensors and 3d Printing. In Proceedings of the 2016 CHI Conference on Human Factors in Computing Systems, San Jose, CA, USA, 7–12 May 2016; pp. 409–419. [Google Scholar]
- Pérez-Cortez, J.E.; Sánchez-Rodríguez, V.H.; Gallegos-Martínez, S.; Chuck-Hernández, C.; Rodriguez, C.A.; Álvarez, M.M.; Trujillo-de Santiago, G.; Vázquez-Lepe, E.; Martínez-López, J.I. Low-Cost Light-Based GelMA 3D Bioprinting via Retrofitting: Manufacturability Test and Cell Culture Assessment. Micromachines 2022, 14, 55. [Google Scholar] [CrossRef]
- Cortez, J.E.P.; Sánchez, V.H.; Vázquez-Lepe, E.; de Santiago, G.T.; Álvarez, M.M.; Martínez-López, J.I. Retrofitting of an Affordable 3D Printer: Towards a Material Efficient and Low-Cost Bioprinting System. Procedia CIRP 2022, 110, 150–155. [Google Scholar] [CrossRef]
- Eggert, S.; Kahl, M.; Kent, R.; Gaats, L.; Bock, N.; Meinert, C.; Hutmacher, D.W. An Open Source Technology Platform to Manufacture Hydrogel-Based 3D Culture Models in an Automated and Standardized Fashion. J. Vis. Exp. 2022, 181, e61261. [Google Scholar]
- Sun, W.; Schaffer, S.; Dai, K.; Yao, L.; Feinberg, A.; Webster-Wood, V. 3D Printing Hydrogel-Based Soft and Biohybrid Actuators: A Mini-Review on Fabrication Techniques, Applications, and Challenges. Front. Robot. AI 2021, 8, 673533. [Google Scholar] [CrossRef]
- Tafreshi, O.A.; Sheydaeian, E.; Ba Dughaish, M.A.S.; Ghaffari-Mosanenzadeh, S.; Saadatnia, Z.; Naguib, H.E. Additive Manufacturing of Aerogels: Recent Advancements and Innovations. Appl. Mater. Today 2025, 45, 102800. [Google Scholar] [CrossRef]
- Ahmed, K.; Shiblee, M.N.I.; Khosla, A.; Nagahara, L.; Thundat, T.; Furukawa, H. Recent Progresses in 4D Printing of Gel Materials. J. Electrochem. Soc. 2020, 167, 037563. [Google Scholar] [CrossRef]
- Shiblee, M.N.I.; Ahmed, K.; Kawakami, M.; Furukawa, H. 4D Printing of Shape-memory Hydrogels for Soft-robotic Functions. Adv. Mater. Technol. 2019, 4, 1900071. [Google Scholar] [CrossRef]
- Hann, S.Y.; Cui, H.; Nowicki, M.; Zhang, L.G. 4D Printing Soft Robotics for Biomedical Applications. Addit. Manuf. 2020, 36, 101567. [Google Scholar] [CrossRef]
- Davoodi, E.; Li, J.; Ma, X.; Najafabadi, A.H.; Yoo, J.; Lu, G.; Sani, E.S.; Lee, S.; Montazerian, H.; Kim, G.; et al. Imaging-Guided Deep Tissue in Vivo Sound Printing. Science 2025, 388, 616–623. [Google Scholar] [CrossRef]

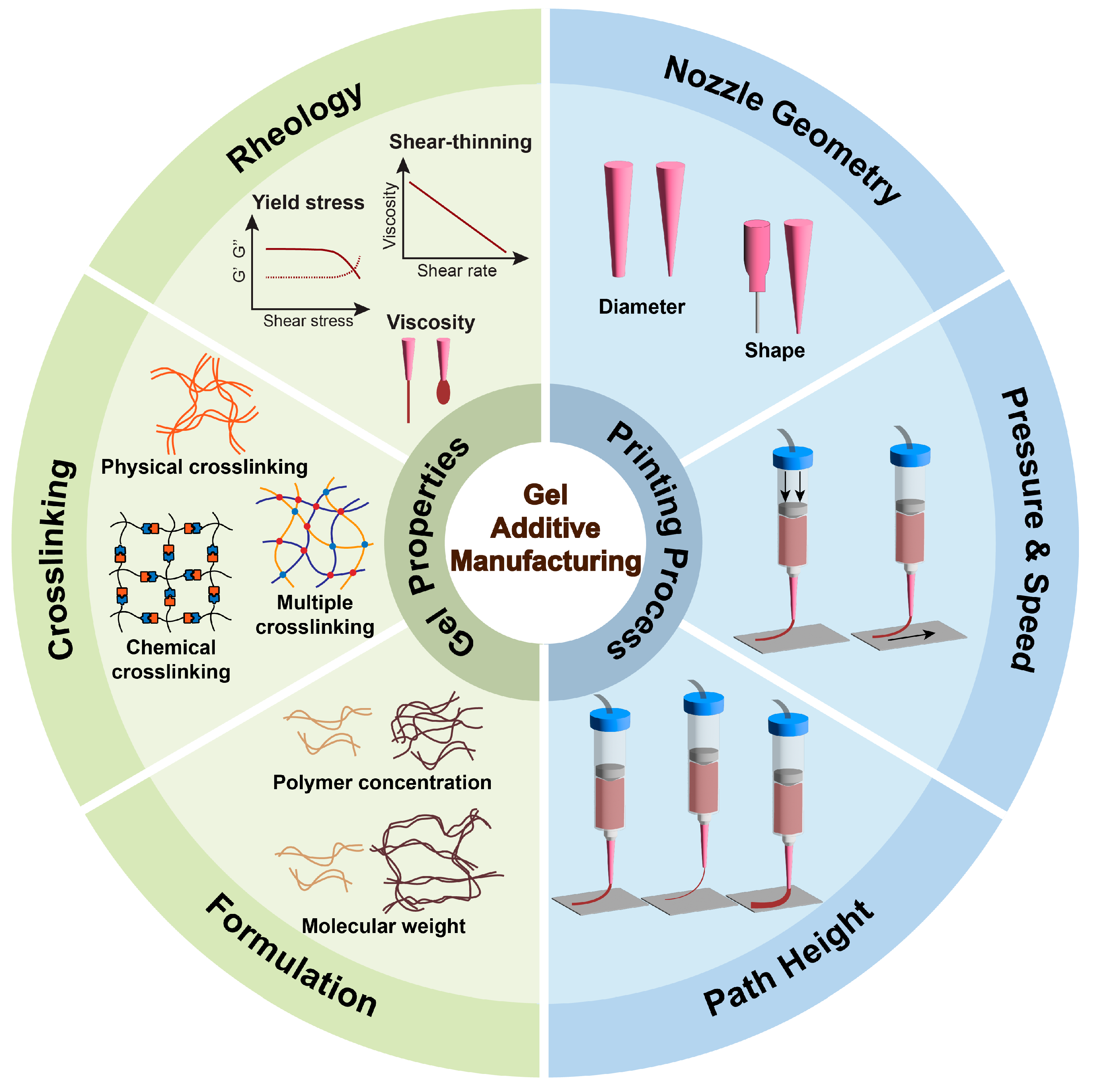
| Property Type | Key Feature | Effect on Printability | Machine Learning Methods |
|---|---|---|---|
| Rheological | Viscosity [50,55,137,139,146] | Determines ease of extrusion | Random forest, SHAP; gradient boosting |
| Storage modulus (G′), loss modulus (G″) [53,137,139,146,147] | Determines viscoelastic behavior and shape retention | Python 3.9 libraries (NumPy, Pandas, Scipy, and Sklearn) used for curve fitting, smoothing, and extrapolation for G′ and G″ curves; MLP (Multilayer Perceptron), VAE (Variational Autoencoder) | |
| Shear-thinning [50,55] | Enables smooth extrusion | Random forest | |
| Yield stress [50,55], critical stress [146] | Supports shape after printing and maintains printed shape | SHAP feature ranking; support vector machines | |
| Angular frequency (ω) [139] | Key parameters affecting viscosity | Decision tree (DT), random forest (RF), gradient boosting decision tree (GBDT), extreme gradient boosting (XGBoost) | |
| Mechanical | Elastic modulus [50,55] | Structural support post-printing and ensures structural integrity | Regression models; decision tree |
| Crosslinking potential [50] | Long-term stability | Data-driven formulation selection | |
| Surface tension [55] | Affects layer stacking | Neural networks | |
| Stiffness components [138] | Quantifies gel mechanical properties | Gaussian Process (GP) Regression, Neural Network (NN) | |
| Young’s modulus (Eu, kPa) [136] | Measures gel stiffness | Neural Network | |
| Gel strength (g × mm) [148] | Indicates firmness, quality | Long short-term memory network, Convolutional Neural Network | |
| Thermal Sensitivity | Denaturation temperature (Td) [137], gel point [147], max process temperature [146] | Sets upper limit for printing temperature to avoid degradation | SVM, random forest, extreme gradient boosting (XGB) |
| Water holding capacity (WHC) [146] | Influences structure formation | Random forest, decision tree | |
| Formulation | Concentration [53,55,136,139,146], molecular weight (MW) [139], H2O content (molar %) [138] | Modifies gel strength, key parameters affecting viscosity and mechanical properties | Logistic regression, decision tree, Neural Network |
| AM Methods | AM Process Parameter | How to Improve the Process | Machine Learning Methods | Ref |
|---|---|---|---|---|
| Extrusion bioprinting | Nozzle size, pressure | Optimize alginate formulation | Deep learning | [237] |
| Bioink composition | Control hydrogel rheology | Physics-informed ML | [147] | |
| NHS/EDC concentrations | Adjust crosslinking for flexibility | XGBoost, SHAP | [238] | |
| Nozzle speed, diameter | Improve viscosity, digestibility | ANN-GA, RSM | [149] | |
| Flow rate, nozzle design | Reduce trial-and-error | Decision trees | [239] | |
| Layer height, print speed | Bioink optimization | Multiscale ML, Big Data | [239] | |
| Bioink rheology | Multi-response optimization of printability | ANN, DOE, RSM | [169] | |
| Printing defects | Detect in real time, reduce waste | CNN, deep learning | [153] | |
| Ink composition, nozzle | Suggest ink formula and print settings | Bayesian optimization | [149] | |
| Bioink comp., shear rate | Predict viscosity, optimize formula | Random forest, DT, PF | [141] | |
| ADA-GEL, pore size | Tune stiffness for tissue engineering | XGBoost | [170] | |
| Print speed, flow rate, and nozzle, ink | Predict high-fidelity hydrogel prints | Hierarchical ML | [150] | |
| Bioink comp., temp, speed, and pressure | Minimize trial-and-error for printability | Bayesian optimization | [240] | |
| Path height, nozzle temp, and composition | Maximize print fidelity, minimize tests | SVM | [124] | |
| Vat photopolymerization | Monomer composition | Targeted property selection | Active learning, ML regression | [230] |
| Food printing | Starch/protein ratio | Predict printability and texture | PCA, SVM | [227] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Wang, Y.; Wang, W. Machine Learning in Gel-Based Additive Manufacturing: From Material Design to Process Optimization. Gels 2025, 11, 582. https://doi.org/10.3390/gels11080582
Zhang Z, Wang Y, Wang W. Machine Learning in Gel-Based Additive Manufacturing: From Material Design to Process Optimization. Gels. 2025; 11(8):582. https://doi.org/10.3390/gels11080582
Chicago/Turabian StyleZhang, Zhizhou, Yaxin Wang, and Weiguang Wang. 2025. "Machine Learning in Gel-Based Additive Manufacturing: From Material Design to Process Optimization" Gels 11, no. 8: 582. https://doi.org/10.3390/gels11080582
APA StyleZhang, Z., Wang, Y., & Wang, W. (2025). Machine Learning in Gel-Based Additive Manufacturing: From Material Design to Process Optimization. Gels, 11(8), 582. https://doi.org/10.3390/gels11080582






